Summary
A fungal protease (Alp1/Asp f13) from Aspergillus fumigatus was detected in the airways of subjects with asthma but not controls, which correlated strongly with disease severity, respiratory dysfunction, and steroid use.
Keywords: Asthma, molds, Aspergillus fumigatus, protease, allergen, bronchial smooth muscle
To the Editor
Although fungi are ubiquitous in the environment and respiratory exposure to airborne spores occurs on a daily basis, sensitization and disease due to such exposure is relatively infrequent. Aspergillus fumigatus (Af) has been linked to severe, uncontrolled asthma. A potentially unique phenotype, termed “severe asthma with fungal sensitization” (SAFS) is thought to describe approximately 20–25% of individuals with uncontrolled, persistent disease.1 Previously, we detected a known allergen serine protease from Af, Alkaline protease 1 (Alp1), in the bronchial submucosa of subjects with mild/moderate asthma but not healthy controls and in allergen-challenged but not naïve mice.2 We determined that Alp1 disrupted airway smooth muscle (ASM)-extracellular matrix (ECM) interactions through its protease activity, which augmented Ca2+ signaling and RhoA activation. Based on these initial findings, we hypothesized that: 1) Alp1 protease contributes the pathogenesis of asthma in some patients; 2) Alp1 abundance in the airways correlates with disease severity.
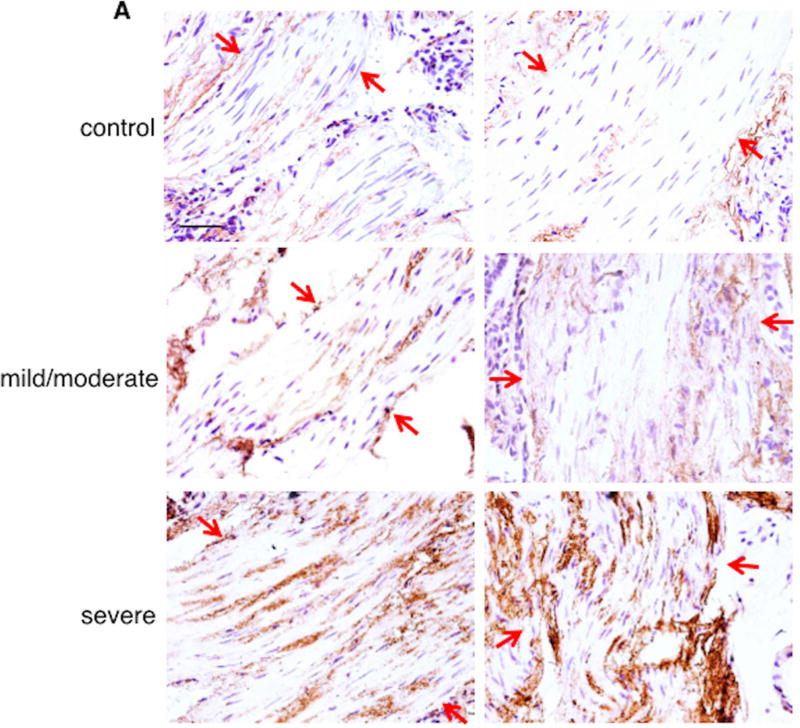
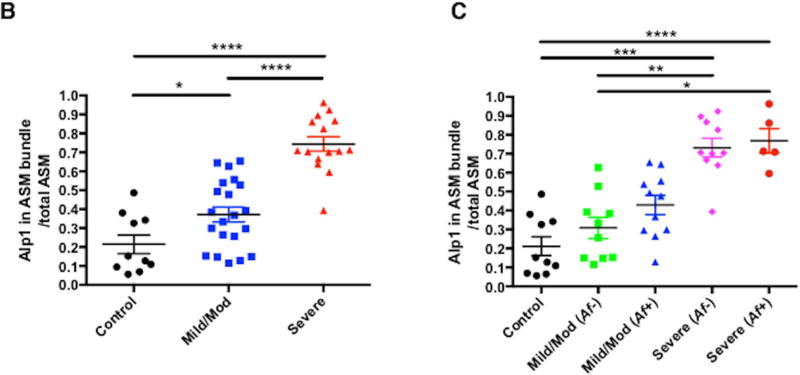
To further delineate the role of Alp1 in the lower respiratory tract in asthma, we evaluated Alp1 immunoreactivity in airway sections from 36 asthmatics and 10 healthy controls and in induced sputum supernatants from another 29 asthmatics and 10 healthy controls. The demographics of these subjects are described in Tables E1 and E2 in this Letter’s Online Repository. There were an approximately equal number of male/female subjects, and the median age was 34 years (range 18–68 years). The group with severe asthma was significantly older than the healthy control group (median age 49, range 20–68 years; p=0.01). Subjects with asthma were classified as mild/moderate or severe based on GINA criteria. Immunostaining of airway sections with anti-Alp 1 antisera detected Alp1 immunoreactivity in bronchial smooth muscle bundles of lungs of subjects with asthma but not in healthy subjects, and Alp1 quantities in the bronchial smooth muscle layer increased progressively with asthma severity (Figure 1A–B). Little to no staining of was observed when airway sections from subjects with severe asthma were incubated with non-specific rabbit antisera (Figure E1). Surprisingly, Alp1 abundance was roughly equivalent in patients with and without sensitivity to Af (Figure 1C). We also observed similar patterns of Alp1 staining within the bronchial epithelium (Figure E2).
Figure 1. Alp1 is present in human airways and correlates with asthma severity.
(A) Airway sections were immunostained with anti-Alp1Ab (representative images from 10–21 subjects/group (original magnification, 40×, bar=50 µm). Arrows delineate smooth muscle areas. (B–C) Alp1 staining in ASM increases with asthma severity (B) but not sensitivity to Af (C). Quantified using ImageJ and represented as Alp1+ ASM per total ASM area. (A) *p=0.04, ****p<0.0001, one way ANOVA. (B)*,**,***,****p=0.01; 0.004; 0.0008; <0.0001, Kruskal Wallis.
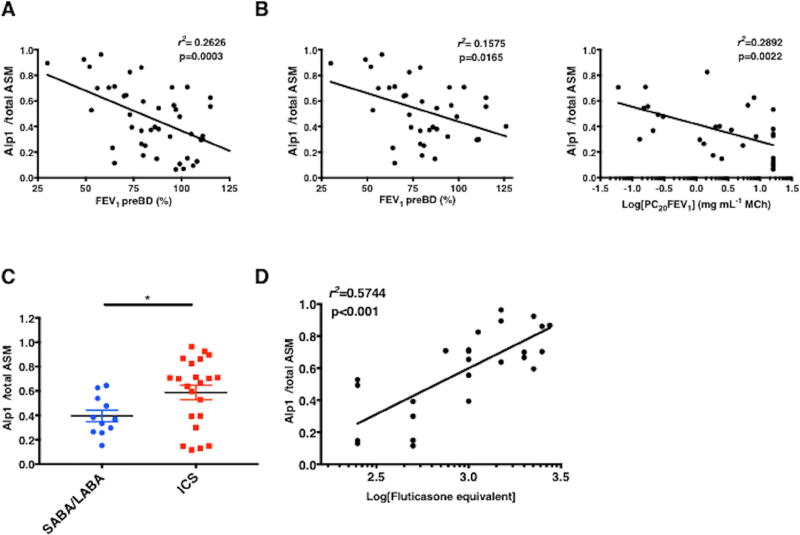
Alp1 immunoreactivity in ASM negatively correlated with pre-bronchodilator FEV1 (analysis of the cohort as a whole or restricted to the asthma group only) and PC20 (the provocative concentration of inhaled methacholine inducing a 20% decrease in FEV1) (Figure 2A–B). Alp1 immunostaining in airways from patients receiving short or long acting β-agonists only (SABA/LABA, respectively) was significantly less than in the airways of patients requiring LABA plus inhaled corticosteroids (ICS), and Alp1 quantities correlated with ICS requirement (fluticasone equivalent dose) (Figure 2C–D).
Figure 2. Alp1 immunostaining in ASM correlates with lung functional impairment.
(A–C) Correlation between Alp1 staining and FEV1 pre-bronchodilator (BD) in the entire cohort (A) or among subjects with asthma (B); Alp1 correlation between Log(PC20FEV1) (C). Alp1 immunostaining in airways of subjects with asthma treated with ICS or SABA/LABA (*p=0.03, Mann-Whitney). (D) Correlation between Alp1 staining in airways and ICS requirement [Log(fluticasone equivalent)].
Because we previously detected Alp1 in bronchoalveolar lavage fluid (BALF) of Af sensitized and challenged mice, but not naïve mice, by immunoblotting, we determined whether it could also be measured non-invasively in sputum from human subjects by immunoblotting. We detected a ~33 kDa band corresponding to the protease purified from commercial Af allergen extracts or from supernatants of Af cultures (Figure E3A left panel). In sputum samples from subjects with asthma, but not healthy controls, we observed a additional band at ~42–45 kDa, potentially representing an unprocessed precursor3 as detection of either band was strongly reduced or eliminated when blots were incubated with Alp1 antibody preadsorbed with antigen (Figure E3A right panel). This band was present only in sputum from subjects with asthma but not controls (Figure E3B). To determine whether Alp1 quantities in induced sputum correlated with disease severity, we generated a standard curve based on quantitative immunoblotting of defined amounts of purified Alp1 (Figure E4A). However, Alp1 quantities were similar in sputum from patients with mild/moderate and severe asthma (Figure E3B). Alp1 quantities were significantly higher in sputum from patients with Af sensitivity than those without, regardless of clinical severity (Figure E4C). Alp1 levels were also significantly correlated with sputum neutrophil, but not eosinophil counts (Figure E4D and data not shown). We did not detect Alp1 in sera from healthy subjects or patients with asthma. Collectively, these results suggest that Alp1 protease may contribute to severe asthma through two distinct mechanisms: 1) allergenicity; and 2) proteolytic destruction of lung tissue, which could promote influx of neutrophils into the airway lumen.
Several observations support a pathogenic contribution of fungal colonization of the airways to severe asthma. Chronic fungal exposure is linked to asthma exacerbations in both children and adults.4 In experimental models of asthma in mice, chronic respiratory inoculation with fungi induces a more severe asthma phenotype than inhalation of other allergens such as house dust mite (HDM) through IL-33-dependent yet steroid-independent mechanisms.5 Alp1 is the major serine protease secreted by Af, and serine proteinase activity is crucial for Af to induce allergic airway inflammation and AHR.6, 7 Here we found equivalent Alp1 quantities in the airways regardless of Af sensitivity and no correlation between sputum Alp1 and eosinophils, suggesting that an IgE/type 2 response to Af is not strictly required for the pathogenicity of Alp1 in asthma. Fungal protease allergens may exert direct pathogenic effects on epithelial barrier function by disrupting cell-cell junctions, inducing cytoskeletal rearrangements, and promoting cytokine (e.g. IL-8) secretion.8, 9 Alp1 in sputum correlated well with sputum neutrophils, suggesting that it might increase epithelial secretion of neutrophil chemoattractants and thereby actively promote neutrophil migration to the airway lumen.
Alp1 may persist in the airways of patients with severe asthma due to impaired mucociliary clearance mechanisms, i.e. asthma-induced alteration of epithelium morphology and/or corticosteroid use. Alp1 also activates mucin gene expression in airway epithelial cells, which could further impede fungal clearance. Because our study was retrospective and cross-sectional, we could not address the utility of Alp1 measurements to evaluate longitudinal parameters including disease control and progression, or responses to specific treatments. However, Alp1 could be explored as a biomarker for endotype classification and/or surrogate for assessment of therapeutic interventions as it is easily identified in the airways from subjects with asthma but is virtually undetectable in samples from healthy controls, and it strongly correlates with hard functional endpoints (FEV1, PC20).
Supplementary Material
Acknowledgments
Funding sources
This research was supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, NIH.
Abbreviations used
- Af
Aspergillus fumigatus
- Alp1
Alkaline protease 1
- SAFS
severe asthma with fungal sensitization
- AHR
airway hyperresponsiveness
- ABPA
allergic bronchopulmonary aspergillosis
- ECM
extracellular matrix
- IHC
immunohistochemistry
- BALF
bronchoalveolar lavage fluid
- ICS
inhaled corticosteroid
- GINA
Global Initiative for Asthma
- SABA
short acting β-agonist
- LABA
long acting β-agonist
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors declare no conflicts of interest.
References
- 1.Denning DW, Pashley C, Hartl D, Wardlaw A, Godet C, Del Giacco S, et al. Fungal allergy in asthma-state of the art and research needs. Clin Transl Allergy. 2014;4:14. doi: 10.1186/2045-7022-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balenga NA, Klichinsky M, Xie Z, Chan EC, Zhao M, Jude J, et al. A fungal protease allergen provokes airway hyper-responsiveness in asthma. Nat Commun. 2015;6:6763. doi: 10.1038/ncomms7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behnsen J, Lessing F, Schindler S, Wartenberg D, Jacobsen ID, Thoen M, et al. Secreted Aspergillus fumigatus protease Alp1 degrades human complement proteins C3, C4, and C5. Infect Immun. 2010;78:3585–94. doi: 10.1128/IAI.01353-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal R. Severe asthma with fungal sensitization. Curr Allergy Asthma Rep. 2011;11:403–13. doi: 10.1007/s11882-011-0217-4. [DOI] [PubMed] [Google Scholar]
- 5.Castanhinha S, Sherburn R, Walker S, Gupta A, Bossley CJ, Buckley J, et al. Pediatric severe asthma with fungal sensitization is mediated by steroid-resistant IL-33. J Allergy Clin Immunol. 2015;136:312–22. e7. doi: 10.1016/j.jaci.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Namvar S, Warn P, Farnell E, Bromley M, Fraczek M, Bowyer P, et al. Aspergillus fumigatus proteases, Asp f 5 and Asp f 13, are essential for airway inflammation and remodelling in a murine inhalation model. Clin Exp Allergy. 2015;45:982–93. doi: 10.1111/cea.12426. [DOI] [PubMed] [Google Scholar]
- 7.Millien VO, Lu W, Shaw J, Yuan X, Mak G, Roberts L, et al. Cleavage of fibrinogen by proteinases elicits allergic responses through Toll-like receptor 4. Science. 2013;341:792–6. doi: 10.1126/science.1240342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kauffman HF, Tomee JF, van de Riet MA, Timmerman AJ, Borger P. Protease-dependent activation of epithelial cells by fungal allergens leads to morphologic changes and cytokine production. J Allergy Clin Immunol. 2000;105:1185–93. doi: 10.1067/mai.2000.106210. [DOI] [PubMed] [Google Scholar]
- 9.Borger P, Koeter GH, Timmerman JA, Vellenga E, Tomee JF, Kauffman HF. Proteases from Aspergillus fumigatus induce interleukin (IL)-6 and IL-8 production in airway epithelial cell lines by transcriptional mechanisms. J Infect Dis. 1999;180:1267–74. doi: 10.1086/315027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.