Abstract
Few studies have investigated the association between ectopic fat from different depots and cardiovascular risk scores and their components in the same population, and none have investigated these relationships in South Asians. In a cross-sectional analysis of 796 participants in the Mediators of Atherosclerosis in South Asians Living in America (MASALA) Study who had measurements of visceral, subcutaneous, pericardial, hepatic, and intermuscular fat from abdominal and cardiac CT scans, we used linear regression to determine the associations of one standard deviation difference in each ectopic fat depot with Pooled Cohort Risk Score and its components. Pericardial and visceral fat were more strongly associated with Pooled Cohort Risk Score [3.1 % (95% CI: 2.5 to 3.7) and 2.7% (2.1 to 3.3) respectively] and components than intermuscular fat [2.3% (1.7 to 3.0)]; subcutaneous fat was inversely associated [−2.6%, (−3.2 to 1.9)] and hepatic fat attenuation was not linearly associated with Pooled Cohort Risk Score when mutually adjusted [−0.3% (−0.9 to 0.4)]. Associations for risk factor components differed by fat depot. In conclusion, subcutaneous and hepatic fat may have different functions than fat stored in other depots in South Asians. Determining whether these relationships are heterogeneous by race may help elucidate the mechanisms underlying CVD disparities.
Keywords: Cardiovascular Disease, Risk Score, Ectopic Fat, Visceral Fat, Cardiovascular Disease Risk Factors
INTRODUCTION
Visceral fat is associated with cardiovascular disease (CVD) risk and mortality,1–5 where subcutaneous fat has even been suggested to play a protective role in cardiometabolic risk.6–10 The growing understanding that the function and effects of fat are heterogeneous highlights the need to understand the role of ectopic fat stored in different depots,11–13 such as pericardial, hepatic, and intermuscular fat.14–17 Importantly, since so few studies have temporally concordant measurements from multiple fat compartments beyond visceral and subcutaneous fat,1,18,19 the mutual relationship of these fat stores with CVD risk is remains unknown. Determining which of these scenarios is best supported by the evidence is particularly important for the cardiometabolic health of South Asians. A pathway of increased CVD risk has been outlined for this group through higher levels of visceral fat.20 To address these issues, we hypothesized that fat from different ectopic depots would be differentially associated with the American College of Cardiology/American Heart Association Atherosclerotic Cardiovascular Disease Pooled Cohort Risk Score (AKA: ASCVD Risk Score) and its components in a cross-sectional analysis of the Mediators of Atherosclerosis in South Asians Living in America (MASALA) Study.21 Specifically, we hypothesized that subcutaneous fat would be inversely associated with CVD risk, but fat from all other depots would be positively associated with the Pooled Cohort Risk Score.
METHODS
MASALA is a 2-site longitudinal cohort study of 906 self-identified South Asians in the United States, free of CVD who weighed ≤ 300 lbs, and were enrolled from 2010 to 2013.22 After excluding those with any missing ectopic fat measurements (n=97) and those missing CVD risk factors needed to calculate the Pooled Cohort Risk Score (n=13), we included 796 participants in this analysis. All participants provided written informed consent and MASALA protocols were approved by review boards at each participating institution.
To measure ectopic fat depots, trained radiology technicians conducted standardized abdominal computed tomography (CT) protocols.22 Lateral scout image of the spine was used to establish the position between the L4 and L5 vertebrae for measurement of visceral, subcutaneous, and intermuscular fat mass using the Medical Image Processing, Analysis, and Visualization software. Visceral fat was defined by area on the slice within the body cavity and appropriate density in Hounsfiled Units (HU). Subcutaneous fat was defined as area outside the body cavity and intermuscular fat was calculated by combining extramyocellular fat from the muscles in the abdomen. Non-contrast cardiac-gated CT scans were used to measure pericardial fat volume and hepatic fat attenuation.17,23 Pericardial fat was also distinguished by density from the entire heart using volume analysis software (GE Healthcare, Waukesha, WI), and included epicardial and paracardial fat in and around the pericardium. Higher hepatic fat was quantified as the inverse of the hepatic fat attenuation.
Pooled Cohort Risk Score was calculated from 11 components measured using a standardized protocol.21 Age, sex, smoking status, education level and family income, medical history, and medication use were self-reported at the enrollment interview.22 BMI was calculated from weight and height measured with balance-beam scale and stadiometer, respectively. Blood pressure, total and HDL cholesterol, and fasting glucose were measured using standardized measurements and protocols.22
We categorized the Pooled Cohort Risk Score into 3 groups: <7.5%, 7.5% to 10% and ≥10%. We then described the MASALA participants by calculating means and standard errors for baseline characteristics for each of the 3 groups, and used the Cuzick non-parametric test for trend across the groups. We calculated Pearson correlations between risk scores, ectopic fat measures, body mass index (BMI), and waist circumference.
We used multivariable linear regression to determine the associations between each ectopic fat depot and the Pooled Cohort Risk Score. We used likelihood ratio tests and R2 values to investigate best fitting models including quadratic terms to assess non-linearity. To compare the strength of associations for different depots and assess whether mutual adjustment of different fat depots produces different results, specifically focusing on mutual adjustment for visceral and subcutaneous fat, we estimated the difference in risk score for a one standard deviation difference of fat from each depot. We used likelihood ratio tests and R2 values to determine whether the addition of other fat depots to the visceral fat model improved the model fit for Pooled Cohort Risk Scores. Since a higher level of hepatic fat is indicated by lower hepatic fat attenuation, where useful to improve the interpretation and comparability of results, we inverted the hepatic fat attenuation values by multiplying the measured values by −1.
We similarly assessed the association between ectopic fat and each binary risk score component using multivariable logistic regression, adjusting for socioeconomic factors. As in the risk score analysis, we continued to characterize the fat depots by using a one standard deviation difference in order to compare the strength of the associations between different fat depots.
We also assessed the continuous risk associations for each fat depot separately by creating graphs of the Lowess curves for each ectopic fat variable with the Pooled Cohort Risk Score. We also conducted a number of sensitivity analyses to assess additional adjustment for education and income, the inclusion of BMI or waist circumference, and heterogeneity by age and sex. We included BMI and waist circumference specifically to determine whether ectopic fat depots provide additional explanatory value beyond the use of anthropometric measures. All analyses were conducted using Stata 14 (StataCorp. College Station 2009).
RESULTS
Participants with higher Pooled Cohort Risk Scores were more likely to be older, male, have income below $75,000, be current smokers, drink alcohol, have lower subcutaneous fat, but higher waist circumference, visceral, pericardial, hepatic, and intermuscular fat (Table 1). The following sets of variables were highly correlated: visceral fat, pericardial fat, with waist circumference; and BMI with subcutaneous fat (Supplemental Table 1).
Table 1.
Baseline characteristics (Mean (SE)) of 796 MASALA participants by Atherosclerotic Cardiovascular Disease Pooled Cohort Risk Score
| Variable
|
Atherosclerotic Cardiovascular Disease Risk Score
|
P-value for Trend
|
||
|---|---|---|---|---|
| <7.5% | 7.5–10% | ≥10% | ||
| (n=535) | (n=53) | (n=208) | ||
| Age (years) | 50.7 (0.29) | 59.4 (0.79) | 66.1 (0.45) | <0.001 |
| Women | 59 (2.1%) | 26 (6.1%) | 19 (2.7%) | <0.001 |
| Born in India | 83 (1.6%) | 77 (5.8%) | 86 (2.4%) | 0.40 |
| Gross Family Income ≥ $75,000 | 79 (1.7%) | 72 (6.2%) | 62 (3.4%) | <0.001 |
| BS or higher | 89 (1.4%) | 92 (3.7%) | 88 (2.3%) | 0.70 |
| Current alcohol | 28 (2.0%) | 40 (6.8%) | 41 (3.4%) | 0.001 |
| Exercise ≥ 1314 MET–min/week | 39 (2.1%) | 42 (6.8%) | 40 (3.4%) | 0.73 |
| Current smoker | 10 (1.3%) | 19 (5.4%) | 22 (2.9%) | <0.001 |
| Diabetes | 17 (1.6%) | 38 (6.7%) | 48 (3.5%) | <0.001 |
| Diabetes Medication Use | 7.9 (1.2%) | 13 (4.7%) | 39 (3.4%) | <0.001 |
| Hypertension | 28 (1.9%) | 49 (6.9%) | 72 (3.1%) | <0.001 |
| Total Cholesterol (mg/dL) | 191 (1.5) | 181 (5.0) | 182 (2.8) | 0.001 |
| Lipid lowering medication use | 126 (24%) | 14 (26%) | 95 (46%) | <0.001 |
| High Cholesterol | 144 (27%) | 15 (28%) | 103 (50%) | <0.001 |
| High-density lipoprotein (mg/dL) | 51.1 (0.60) | 47.9 (1.92) | 48.5 (0.86) | 0.013 |
| Low high-density lipoprotein | 195 (36%) | 30 (57%) | 116 (56%) | <0.001 |
| Body Mass Index (kg/m2) | 25.9 (0.17) | 27.3 (1.02) | 25.7 (2.64) | 0.75 |
| Waist Circumference (cm) | 91.2 (0.42) | 96.0 (1.29) | 95.4 (0.70) | <0.001 |
| Visceral Fat (cm2) | 122 (2.11) | 144 (7.52) | 160 (4.47) | <0.001 |
| Subcutaneous Fat (cm2) | 242 (4.13) | 227 (11.4) | 222 (6.35) | 0.001 |
| Hepatic Fat Attenuation (Inverted HU) | −55.3 (0.48) | −53.5 (1.47) | −53.5 (0.62) | 0.001 |
| Pericardial Fat (cm3) | 51.5 (1.05) | 65.5 (3.87) | 74.3 (2.34) | <0.001 |
| Intermuscular abdominal Fat (cm2) | 19.9 (0.32) | 22.3 (1.55) | 24.2 (0.69) | <0.001 |
All fat depots were significantly associated with Pooled Cohort Risk Score (Table 2), with the strongest association for pericardial fat, followed closely by visceral fat, and then by intermuscular fat. Subcutaneous fat was inversely associated with risk and hepatic fat attenuation estimates were reduced and became non-significant when mutually adjusted for other depots. Hepatic fat also explained ≤ 1% of the variance in risk and was the only fat depot with a non-significant likelihood ratio test. The full model with all 5 fat depots explained 18% of the variance in Pooled Cohort Risk Score. While the distribution of risk scores was approximately log normal, results using log transformation produced nearly identical results (data not shown).
Table 2.
Linear regression (beta estimates (95% confidence intervals)) for Atherosclerotic Cardiovascular Disease Pooled Cohort Risk Score (%) for one standard deviation difference in ectopic fat in MASALA (Bold indicates estimates are significantly different from zero at the p<0.05 level)
| Model | Visceral Fat | Subcutaneous Fat | Pericardial Fat | Hepatic Fat* | Intermuscular Fat |
|---|---|---|---|---|---|
| SD=56 cm2 | SD=94 cm2 | SD=30 cm3 | SD=11 HU | SD=9 cm2 | |
| Unadjusted† | 2.71 (2.09–3.34) | −0.94 (−1.59– −0.29) | 3.09 (2.46–3.71) | 0.87 (0.22–1.52) | 2.31 (1.68–2.95) |
| Adjusted for Visceral Fat‡ | -- | −1.82 (−2.46– −1.19) | 2.24 (1.40–3.09) | −0.47 (−1.17–0.24) | 1.29 (0.58–2.00) |
| Adjusted for Subcutaneous Fat | 3.22 (2.58–3.85) | - | 3.65 (3.01–4.29) | 1.06 (0.41–1.72) | 3.23 (2.56–3.91) |
| Adjusted for Pericardial Fat | 1.22 (0.39–2.06) | −1.95 (−2.58– −1.32) | - | −0.26 (−0.92–0.40) | 1.16 (0.46–1.85) |
| Adjusted for Hepatic Fat | 2.93 (2.22–3.63) | −1.12 (−1.77– −0.46) | 3.18 (2.51–3.86) | - | 2.24 (1.60–2.88) |
| Adjusted for Muscle Fat | 2.08 (1.37–2.79) | −2.25 (−2.92– −1.58) | 2.54 (1.84–3.25) | 0.61 (−0.03–1.24) | - |
| Full model: Complete Mutual Adjustment | 1.13 (0.25–2.01) | −2.58 (−3.23– −1.92) | 2.30 (1.46–3.14) | −0.25 (−0.92–0.43) | 1.79 (1.05–2.53) |
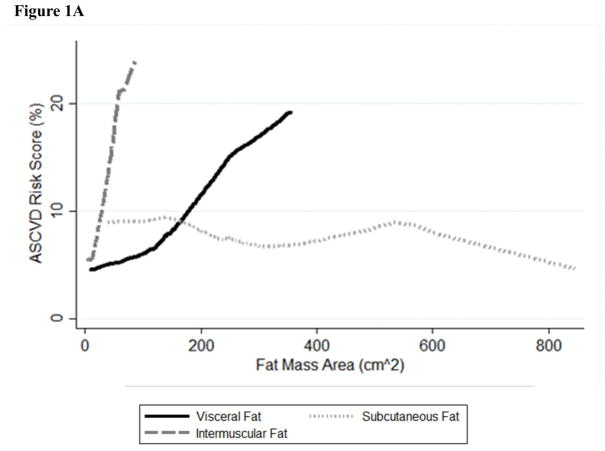
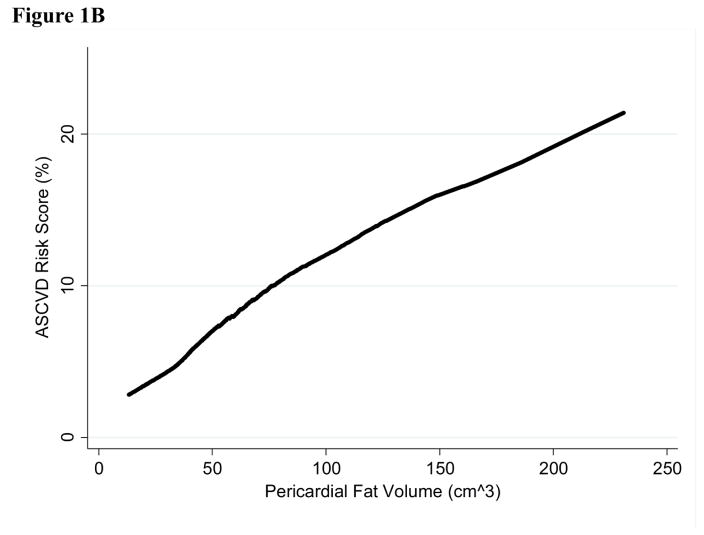
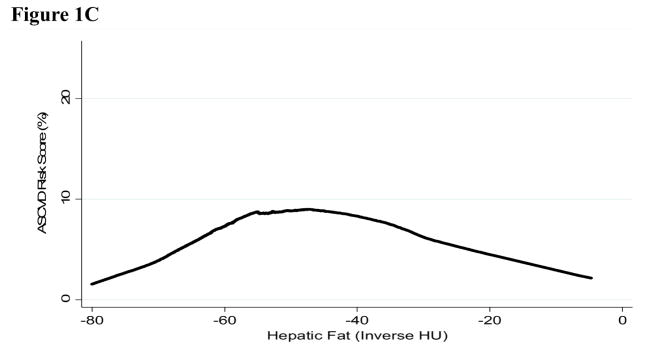
No fat depots exhibited evidence of non-linearity in their relationship with Pooled Cohort Risk Score (p>0.10), except for hepatic fat (p<0.001). The inverted U-shape seen for hepatic fat attenuation in Figure 1 is unusual in biological relationships, and this specific relationship may explain why hepatic fat is inversely associated with Pooled Cohort Risk Score in the linear model and non-significant when adjusted for other fat depots in Table 2. When the curvature for hepatic fat is accounted for, the estimate for hepatic fat is inverse and greatly increased (−6.30% (−9.88 to −2.73)), and the estimates for non-linearity and model fit are also significant (p<0.001). The inflection point of the curve is close to the cut-point for fatty liver (hepatic attenuation <40 HU), but including fatty liver in the model did not significantly improve model fit (p=0.20). Including alcohol consumption in the model did not alter the results (not shown).
Figure 1. Lowess curves for Atherosclerotic Cardiovascular Disease Pooled Cohort Risk Score by one standard deviation difference in ectopic fat depot in 796 MASALA participants A. Visceral, subcutaneous, and intermuscular fat B. Pericardial fat C. Hepatic fat.
* Hepatic fat values have been inverted so high HU indicates higher level of fat in the liver.
** Standard deviation for each fat depot is: visceral fat 56cm2, subcutaneous fat 94cm2, pericardial fat 30cm3, hepatic fat 11HU, and intermuscular fat 9cm2
Sensitivity analyses investigating further adjustments for potential confounders found relatively similar results (Supplemental Table 2). Including BMI in the model resulted in an inverse and significant estimate for BMI (−0.34 kg/m2 (−0.54 to −0.15)) and a significant likelihood ratio test for its inclusion (p<0.001) with all ectopic fat depots, except hepatic fat, remaining significant at the p<0.005 level. The inclusion of waist circumference (0.06 cm (−0.03 to 0.15)) strongly attenuated the estimate for visceral fat, but the estimates for the other depots were similar and the likelihood ratio test for the inclusion of waist circumference was not statistically significant (p=0.20). While the relationship differed significantly by age only for pericardial and hepatic fat, estimates were generally attenuated for the older age group with the exception of a much stronger relationship for hepatic fat (Supplemental Table 2). Estimates were weaker overall in the subgroup analysis by sex, but with significantly stronger relationships for subcutaneous and intermuscular fat for men.
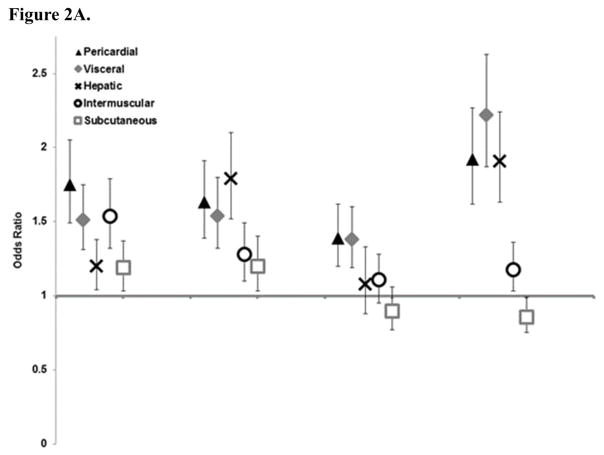
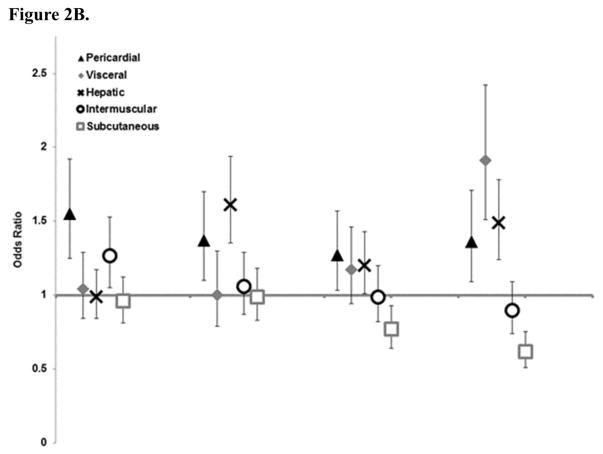
Pericardial fat had the strongest estimates overall for risk factor components (Figure 2), followed by visceral fat and intermuscular fat; however, visceral and intermuscular fat estimates were almost fully attenuated in the full model except for hypertension and intermuscular fat, and low HDL cholesterol. Estimates for hepatic fat differed by risk factor component with significant associations for diabetes and low HDL cholesterol. Estimates for subcutaneous fat were weaker, but with mutual adjustment remained significant for total and HDL cholesterol.
Figure 2. Association of Atherosclerotic Cardiovascular Disease Pooled Cohort Risk Score components (odds ratios and 95% confidence intervals) by one standard deviation of ectopic fat A. Univariate B. Mutually adjusted for other fat depots.
* Hepatic fat values have been inverted so high HU indicates higher level of fat in the liver.
** Standard deviation for each fat depot is: visceral fat 56cm2, subcutaneous fat 94cm2, pericardial fat 30cm3, hepatic fat 11HU, and intermuscular fat 9cm2
*** For Figure 2A all odds ratios are statistically significant at the p<0.05 level except for hepatic and intermuscular fat with high cholesterol. For Figure 2B only the following estimates are statistically significant: Hypertension with pericardial and intermuscular fat; Diabetes with pericardial and hepatic fat; High cholesterol with pericardia fat and hepatic fat; and Low HDL with pericardial, visceral, hepatic, and subcutaneous fat.
DISCUSSION
Among a South Asian sample, higher pericardial fat volume, visceral fat area, and intermuscular fat area were significantly associated with higher Pooled Cohort Risk Score, while higher subcutaneous fat was significantly associated with lower risk. The relationship with hepatic fat was non-linear. Associations for ectopic fat differed by each CVD risk factor component in the Pooled Cohort Risk Score.
Excess adiposity stored centrally has long been known to be a more specific predictor of cardiometabolic risk compared to fat stored peripherally;24,25 however, the relative contributions of fat from different depots to cardiometabolic risk remains unknown. Mutual adjustment of fat from multiple depots is rare because few studies have ectopic fat measured from multiple depots at the same time point in the same participants.1,18,19 Our findings are generally consistent with prior literature showing that visceral fat is positively associated with CVD risk independent of BMI.25–27 Our results are also generally consistent with prior evidence about the association between pericardial fat and CVD risk;18,19 however, the relative contribution of pericardial and visceral fat in our results adds to inconsistent prior evidence.18,19,27 Whether pericardial fat has a stronger association with CVD risk than visceral fat may depend on factors yet to be fully investigated including the relative quantities of fat in these depots, their relative contributions to development of type 2 diabetes, and the population being studied.
It has been suggested that subcutaneous fat may be protective against CVD,6–8 which is consistent with our results; however, most studies of CVD events have shown no association with subcutaneous fat.1–5 One promising theory is that subcutaneous capacity allows for the benign storage of excess adiposity, and only when this capacity is exceeded and fat is placed in other depots does this excess becomes pathological.9,10 This concept has been used specifically to explain higher CVD risk in South Asians compared to other groups, suggesting that this ethnic group has lower subcutaneous capacity and therefore develops higher levels of ectopic fat at a lower BMI.20 New methods to appropriately test this theory of subcutaneous capacity are needed and it is possible that with further study we will need to re-conceptualize subcutaneous fat.
Our results show that hepatic fat is either non-linearly related or not an independent risk factor for CVD. Additional evidence and/or biological justification that this is the true shape of the association between hepatic fat and CVD risk is needed before concluding that these estimates are valid. While there is growing interest in using hepatic fat to predict CVD risk, very few studies have investigated hepatic and visceral fat together.28,29 Hepatic fat may also play a different role in CVD risk than other ectopic fat, given the high level of within person variability of hepatic fat in the short term.30 Compared to the relative stability of ectopic fat in other depots over time, the high variability of hepatic fat may also indicate a less direct association with CVD risk. While fat in all ectopic compartments may increase CVD risk, this may be due to differential impacts on risk score components with hepatic fat contributing disproportionately to diabetes risk, pericardial fat to hypertension, and visceral and subcutaneous fat to HDL.
One important finding from our work is that estimates for all fat depots are independent from BMI. As a surrogate measurement of total fat mass, BMI may account for variance orthogonal from fat stored in ectopic depots. Waist circumference is thought to be a more specific marker of ectopic fat and visceral fat explicitly. Our results showing that estimates for visceral fat are attenuated by the inclusion of waist circumference agree with this premise, indicating that waist circumference may be a decent proxy for visceral fat in South Asian populations when the use of CT scans is impractical.
This study has a number of limitations. CVD outcomes are not yet available and the Pooled Cohort Risk Score has not been validated in the South Asian American population and therefore may not appropriately account for differences observed in this group. Second, the MASALA study excluded participants with body weight ≥300 lbs due to CT scanner weight limitations, thereby limiting the ability to assess pathology from the highest levels of obesity. Lastly, the inclusion of age and sex in the risk score definitions limits the analytic options for investigating heterogeneity, and this study is not powered to detect interaction. This study also has a number of strengths. The primary strength is the direct measurement with CT of ectopic fat from a comprehensive group of fat depots measured concurrently. This unique resource allowed us to compare and mutually adjust for fat in different depots. The MASALA study also offers the opportunity to study a unique population. Given the current concern that groups from Asia may have higher cardiometabolic risk at the same level of BMI than non-Hispanic Whites, the ability to assess the associations between directly measured ectopic fat and atherosclerotic CVD risk in South Asians offers novel insight into this topic.
Higher visceral, pericardial, and intermuscular fat are strongly and significantly associated with higher Pooled Cohort Risk Score in South Asian Americans. Subcutaneous fat is inversely associated with risk, but more work is needed to determine if subcutaneous fat is itself protective or if another theory such as subcutaneous capacity is better supported. The relationship between hepatic fat and CVD risk may differ from fat stored in other compartments; these discrepancies might be ascribed to differences in either measurement or function. This study provides early evidence for a more comprehensive understanding of the role ectopic fat plays in the development of atherosclerotic CVD risk.
Supplementary Material
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MASALA study for their valuable contributions.
Funding: AS was supported by NIH training grant 5T32DK007418-34 and the Wilsey Family Foundation. The MASALA study was supported by Grant Number R01HL093009 from the National Heart, Lung, And Blood Institute and the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 RR024131. The body composition measurements were supported by grant K24HL112827. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health. The funders had no role in the design, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit it for publication.
Footnotes
Disclosures: The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. JACC. 2013;62:921–925. doi: 10.1016/j.jacc.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujimoto WY, Bergstrom RW, Boyko EJ, Chen KW, Leonetti DL, Newell-Morris L, Shofer JB, Wahl PW. Visceral adiposity and incident coronary heart disease in Japanese-American men. The 10-year follow-up results of the Seattle Japanese-American Community Diabetes Study. Diabetes care. 1999;22:1808–1812. doi: 10.2337/diacare.22.11.1808. [DOI] [PubMed] [Google Scholar]
- 3.Katzmarzyk PT, Mire E, Bouchard C. Abdominal obesity and mortality: The Pennington Center Longitudinal Study. Nutr Diabetes. 2012;2:e42. doi: 10.1038/nutd.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNeely MJ, Shofer JB, Leonetti DL, Fujimoto WY, Boyko EJ. Associations among visceral fat, all-cause mortality, and obesity-related mortality in Japanese Americans. Diabetes care. 2012;35:296–298. doi: 10.2337/dc11-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicklas BJ, Penninx BW, Cesari M, Kritchevsky SB, Newman AB, Kanaya AM, Pahor M, Jingzhong D, Harris TB. Association of visceral adipose tissue with incident myocardial infarction in older men and women: the Health, Aging and Body Composition Study. Am J Epidemiol. 2004;160:741–749. doi: 10.1093/aje/kwh281. [DOI] [PubMed] [Google Scholar]
- 6.Golan R, Shelef I, Rudich A, Gepner Y, Shemesh E, Chassidim Y, Harman-Boehm I, Henkin Y, Schwarzfuchs D, Avraham SB, Witkow S, Liberty IF, Tangi-Rosental O, Sarusi B, Stampfer MJ, Shai I. Abdominal superficial subcutaneous fat: a putative distinct protective fat subdepot in type 2 diabetes. Diabetes Care. 2012;35:640–647. doi: 10.2337/dc11-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porter SA, Massaro JM, Hoffmann U, Vasan RS, O’donnel CJ, Fox CS. Abdominal Subcutaneous Adipose Tissue: A Protective Fat Depot? Diabetes Care. 2009;32:1068–1075. doi: 10.2337/dc08-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential Fat Deposition in Subcutaneous Versus Visceral Depots Is Associated with Insulin Sensitivity. J Clin Endocrinol Metab. 2011;96:E1756–E1760. doi: 10.1210/jc.2011-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demerath EW, Sun SS, Rogers N, Lee M, Reed D, Choh AC, Couch W, Czerwinski SA, Chumlea WC, Siervogel RM, Towne B. Anatomical patterning of visceral adipose tissue: race, sex, and age variation. Obesity. 2007;15:2984–2993. doi: 10.1038/oby.2007.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Despres JP. Is visceral obesity the cause of the metabolic syndrome? Ann Med. 2006;38:52–63. doi: 10.1080/07853890500383895. [DOI] [PubMed] [Google Scholar]
- 11.Kershaw EE, Flier JS. Adipose Tissue as an Endocrine Organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 12.Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc. 2001;60:329–339. doi: 10.1079/pns200194. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Musani SK, Bidulescu A, Carr JJ, Wilson JG, Taylor HA, Fox CS. Fatty liver, abdominal adipose tissue and atherosclerotic calcification in African Americans: the Jackson Heart Study. Atherosclerosis. 2012;224:521–525. doi: 10.1016/j.atherosclerosis.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miljkovic I, Kuipers AL, Cauley JA, Prasad T, Lee CG, Ensrud KE, Cawthon PM, Hoffman AR, Dam TT, Gordon CL, Zmuda JM. Greater Skeletal Muscle Fat Infiltration Is Associated With Higher All-Cause and Cardiovascular Mortality in Older Men. J Gerontol A Biol Sci Med Sci. 2015;70:1133–1140. doi: 10.1093/gerona/glv027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, Kawahito Y, Yoshida N, Suetsugu A, Kato T, Okuda J, Ida K, Yoshikawa T. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13:1579–1584. doi: 10.3748/wjg.v13.i10.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pisto P, Santaniemi M, Bloigu R, Ukkola O, Kesaniemi YA. Fatty liver predicts the risk for cardiovascular events in middle-aged population: a population-based cohort study. BMJ open. 2014;4:e004973. doi: 10.1136/bmjopen-2014-004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding J, Hsu FC, Harris TB, Liu Y, Kritchevsky SB, Szklo M, Ouyang P, Espeland MA, Lohman KK, Criqui MH, Allison M, Bluemke DA, Carr JJ. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J of Clin Nutr. 2009;90:499–504. doi: 10.3945/ajcn.2008.27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Fox CS, Hickson D, Sarpong D, Ekunwe L, May WD, Hundley GW, Carr JJ, Taylor HA. Pericardial adipose tissue, atherosclerosis, and cardiovascular disease risk factors: the Jackson heart study. Diabetes care. 2010;33:1635–1639. doi: 10.2337/dc10-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, O’Donnell CJ, Fox CS. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 20.Sniderman AD, Bhopal R, Prabhakaran D, Sarrafzadegan N, Tchernof A. Why might South Asians be so susceptible to central obesity and its atherogenic consequences? The adipose tissue overflow hypothesis. Int J Epidemiol. 2007;36:220–225. doi: 10.1093/ije/dyl245. [DOI] [PubMed] [Google Scholar]
- 21.Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith J, Sidney C, Sorlie P, Stone NJ, Wilson PWF. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 22.Kanaya AM, Kandula N, Herrington D, Budoff MJ, Hulley S, Vittinghoff E, Liu K. Mediators of Atherosclerosis in South Asians Living in America (MASALA) Study: Objectives, Methods, and Cohort Description. Clin Cardiol. 2013;36:712–720. doi: 10.1002/clc.22219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alman AC, Jacobs DR, Jr, Lewis CE, Snell-Bergeon JK, Carnethon MR, Terry JG, Goff DC, Jr, Ding J, Carr JJ. Higher pericardial adiposity is associated with prevalent diabetes: The Coronary Artery Risk Development in Young Adults study. Nutr Metab Cardiovasc Dis. 2016;26:326–332. doi: 10.1016/j.numecd.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Fox CS, Hickson DA, May WD, Hairston KG, Carr JJ, Taylor HA. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. Journal Clin Endocrinol Metab. 2010;95:5419–5426. doi: 10.1210/jc.2010-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abraham TM, Pedley A, Massaro JM, Hoffmann U, Fox CS. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation. 2015;132:1639–1647. doi: 10.1161/CIRCULATIONAHA.114.015000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, O’Donnell CJ, Fox CS, Hoffmann U. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30:850–856. doi: 10.1093/eurheartj/ehn573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Fox CS, Hickson D, Bidulescu A, Carr JJ, Taylor HA. Fatty liver, abdominal visceral fat, and cardiometabolic risk factors: the Jackson Heart Study. Arterioscler Thromb Vasc Biol. 2011;31:2715–2722. doi: 10.1161/ATVBAHA.111.234062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westerbacka J, Cornér A, Tiikkainen M, Tamminen M, Vehkavaara S, Häkkinen A-M, Fredriksson J, Yki-Järvinen H. Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: implications for sex differences in markers of cardiovascular risk. Diabetologia. 2004;47:1360–1369. doi: 10.1007/s00125-004-1460-1. [DOI] [PubMed] [Google Scholar]
- 30.Thomas EL, Fitzpatrick JA, Malik SJ, Taylor-Robinson SD, Bell JD. Whole body fat: content and distribution. Prog Nucl Magn Reson Spectrosc. 2013;73:56–80. doi: 10.1016/j.pnmrs.2013.04.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.