Abstract
Objective
We estimated and compared the risk of clinically identified acquired drug resistance under i) immediate initiation (the currently recommended ART initiation strategy), ii) initiation with CD4<500, and iii) initiation with CD4<350 cells/mm3.
Design
Cohort study based on routinely collected data from the HIV-CAUSAL Collaboration.
Methods
For each individual, baseline was the earliest time when all eligibility criteria (ART-naïve, AIDS-free, and others) were met after 1999. Acquired drug resistance was defined using the Stanford classification as resistance to any antiretroviral drug that was clinically identified at least 6 months after ART initiation. We used the parametric g-formula to adjust for time-varying (CD4 count, HIV-RNA, AIDS, ART regimen and drug resistance testing) and baseline (calendar period, mode of acquisition, sex, age, geographical origin, ethnicity and cohort) characteristics.
Results
In 50,981 eligible individuals, 10% had CD4 count>500 at baseline, and 63% initiated ART during follow-up. Of 2,672 tests for acquired drug resistance, 794 found resistance. The estimated 7-year risk (95% CI) of acquired drug resistance was 3.2% (2.8,3.5) for immediate initiation, 3.1% (2.7,3.3) for initiation with CD4<500, and 2.8% (2.5,3.0) for initiation with CD4<350 cells/mm3. In analyses restricted to individuals with baseline in 2005–2015, the corresponding estimates were 1.9% (1.8, 2.5), 1.9% (1.7,2.4) and 1.8% (1.7,2.2).
Conclusions
Our findings suggest that the risk of acquired drug resistance is very low, especially in recent calendar periods, and that immediate ART initiation only slightly increases the risk. It is unlikely that drug resistance will jeopardize the proven benefits of immediate ART initiation.
Keywords: HIV, drug resistance, when to start, parametric g-formula, comparative effectiveness
Introduction
Immediate initiation of antiretroviral therapy (ART) upon diagnosis of HIV infection results in lower risk of serious clinical events1,2 and virus transmission when compared with delayed ART initiation3,4. Therefore international clinical guidelines now recommend immediate ART initiation for all HIV-positive individuals, regardless of CD4 count.5–8 A potential concern is that the more prolonged exposure associated with immediate initiation9 can increase the risk of acquiring drug resistance, which decreases virus susceptibility to specific antiretroviral drugs or drug classes.
Estimates of the long-term risk of acquired drug resistance under immediate ART initiation are needed to fully understand the public health impact of the current guidelines for ART initiation and to estimate the number of individuals in need of second line therapy. These estimates, however, will need to be obtained from observational studies because the follow-up of the completed randomized trials was relatively short 1,2 and no other large randomized trials are planned. While several observational studies found that ART initiation at high CD4 counts was associated with a lower risk of drug resistance, 10–13 these studies did not adjust for time-varying confounders and used the date of ART initiation, rather than the date of entry into care, as the time origin.
Here we estimate and compare the 7-year risks of acquired drug resistance under immediate ART initiation and the previously recommended CD4 count-based initiation strategies. To do so, we use data on HIV-positive individuals receiving HIV care in 5 European countries.
Methods
Study population
The HIV-CAUSAL Collaboration is a consortium of prospective HIV cohorts from Europe and the Americas. All cohorts record routinely collected data in clinical practice within settings with universal access to care. Data collected include patient characteristics (age, sex, geographical origin, and transmission category), use of ART (type of regimes and dates of start and discontinuation), CD4 cell counts, and plasma HIV-RNA, AIDS-defining conditions, and deaths. Each cohort submits data in a standardized format (http://www.hicdep.org/) to the coordinating center. Ethics approval was granted by the ethics committees of each of the participating cohorts according to country-specific regulations.
The analyses presented here are based on data pooled in September 2015 and were conducted on 6 cohorts within the Collaboration that contributed data on genotypic drug resistance testing conducted as part of routine clinical care (AMACS from Greece, AIDS Therapy Evaluation in the Netherlands (ATHENA), CoRIS from Spain, Swiss HIV Cohort Study, UK CHIC/UK HIV Drug Resistance Database, and UK Register of HIV Seroconverters from the United Kingdom). The analyses were restricted to individuals who met the following eligibility criteria after 1999: age ≥18 years and CD4 cell count and HIV-RNA measurements within 3 months of each other while AIDS-free and ART-naïve. Because in the early antiretroviral era ART combinations were suboptimal and the drug resistance testing uncommon, we also restricted to individuals who entered a cohort on or after January 1, 2000. Follow-up started at baseline, defined as the earliest date that all eligibility criteria were met, and ended at the earliest of detection of acquired drug resistance, death, 12 months after the most recent HIV-RNA or CD4 count laboratory measurement, cohort-specific administrative censoring, date of pregnancy when known, or initiation of an antiretroviral therapy combination not defined as ART (see below).
Treatment strategies
We defined initiation of combined ART as initiation of combination of antiretroviral drugs including at least two nucleoside reverse transcriptase inhibitors plus either one or more protease inhibitors, one nonnucleoside reverse transcriptase inhibitor, one entry/fusion inhibitor, or one integrase inhibitor. We compared the following strategies: i) immediate ART initiation within 3 months of baseline, ii) initiation within 3 months of a CD4 count <500 cells/mm3 or an AIDS diagnosis, and iii) initiation within 3 months of a CD4 cell count <350 cells/mm3 or an AIDS diagnosis14.
Clinical guidelines recommend drug resistance testing at all episodes of virological failure in ART treated people5–8,15. However, in clinical practice these tests are not always performed. Therefore, to ensure that our estimates reflect the real-world frequency of both drug resistance testing and clinically identified acquired drug resistance, none of the strategies (i)-(iii) imposed a drug resistance test at virological failure. In sensitivity analyses (see below), we considered an alternative strategy that imposed a drug resistance test at each episode of virological failure.
Drug resistance
The outcome was clinically identified, acquired drug resistance up to 7 years after baseline. Acquired drug resistance was defined as predicted intermediate or high level resistance to any of the following antiretroviral drugs in use during the study period: atazanavir, darunavir, fosamprenavir, indinavir, lopinavir, nelfinavir, saquinavir, tipranavir (protease inhibitors - PI); lamivudine, emtricitabine, abacavir, didanosine, tenofovir, stavudine, zidovudine (nucleoside reverse transcriptase inhibitors - NRTI); nevirapine, efavirenz, etravirine, rilpivirine (non-nucleoside reverse transcriptase inhibitors - NNRTI). Resistance to integrase inhibitors was not examined because it was collected only in one cohort and even in this cohort it was very rare.16 Predicted resistance was derived using the Genotypic Resistance Interpretation Algorithm, version 7.0 (HIVdb Program, Stanford University, http://hivdb.stanford.edu). To minimize the inclusion of transmitted drug resistance in the outcome definition, mutations conferring resistance to a drug class the person had never taken or genotypic tests on blood samples collected within 6 months after ART initiation were not considered as acquired drug resistance.
Statistical methods
Because standard statistical methods cannot appropriately adjust for time-varying confounders affected by prior treatment 17,18, we used the parametric g-formula to adjust for the time-varying confounders CD4 cell count, HIV-RNA level, AIDS, drug resistance testing, and treatment class (NNRTI versus non-NNRTI based regime) as well as for time-fixed confounders measured at baseline.
The parametric g-formula, a generalization of standardization for time-varying treatments and confounders, 18–20 estimates the risk of drug resistance that would have been observed if all patients in the study had adhered to a particular treatment initiation strategy and none had been lost to follow-up, under the assumptions of no residual confounding, no measurement error, and no model misspecification 19,21. The estimation procedure for the HIV-CAUSAL Collaboration has been described elsewhere 20. Briefly, the procedure has two steps. First, parametric regression models are used to estimate the joint distribution of the outcome, treatment and time-varying covariates conditional on previous treatment and covariate history. Second, a Monte Carlo simulation using the above estimates is run to simulate the distribution of the post-baseline outcomes and time-varying covariates separately under each ART initiation strategy.
For the first step, we fit separate binary logistic regression models for time-varying indicators of genotypic testing, detection of an acquired drug resistance after a test, death, AIDS-defining condition, ART initiation, measurement of CD4 cell count, measurement of HIV RNA, and linear regression models for CD4 cell count and HIV-RNA on the natural logarithm scale. All regression models included as covariates the most recent value of these time-varying variables using cubic splines, time since last CD4 count and HIV-RNA measurements, and the following baseline variables: CD4 cell count (<100, 100–199,200–349,350–499, ≥500 cells/mm3), HIV-RNA level (<10000,10000–100000, >100000 copies/mL), age (<35,35–49,≥50 years), sex, mode of HIV acquisition (heterosexual, homo/bisexual, injecting drug users, or other/unknown), calendar year (2000–2004, 2005–2010, 2011–2015), testing for transmitted drug resistance (i.e., testing done before ART initiation and within 12 months of baseline), geographical origin (Western countries, sub-Saharan Africa, other, unknown), ethnicity (white, black, unknown) and cohort. All models also included an interaction term for number of months since ART initiation. The g-formula estimates of risk in the presence of competing risks (death) can be interpreted as an extension of the sub-distribution cumulative incidence function to the setting of time-varying treatments and confounders.19 We used a nonparametric bootstrap procedure based on 500 samples to obtain percentile-based 95% confidence intervals (CIs).
Like all regression-based methods, the parametric g-formula relies on correct model specification. To explore the validity of our parametric assumptions, we compared the observed means of the outcome and time-varying covariates with those predicted by our models. The time-varying means predicted by our models under observed ART initiation were similar to the observed means in the original data (Appendix Figure). All analyses were conducted with the publicly available SAS macro GFORMULA (http://www.hsph.harvard.edu/causal/software/).
We conducted subgroup analyses in individuals with baseline CD4>500 cells/mm3 (because treatment effectiveness may depend on the initial CD4 count), individuals with baseline date at or after 1/1/2005 (because some study participants started antiretroviral combinations different from those in current use), and individuals originating from Sub-Saharan Africa (because patterns of acquired drug resistance and testing might differ by geographical origin22 due to different HIV subtype and patterns of treatment adherence).
Sensitivity analyses
The uptake of drug resistance testing was relatively low in our cohorts, even though HIV guidelines during the study period recommended testing at every episode of virological failure. While this low uptake is consistent with previous reports from high-income countries23, it may be partly explained by under-ascertainment of drug resistance testing in our cohorts. We therefore conducted two analyses to explore the sensitivity of our results to under-ascertainment. First, we estimated the 7-year risk of acquired drug resistance if a drug resistance test had been conducted at each episode of virological failure. Second, we estimated the 7-year risk of drug resistance under the assumption that acquired drug resistance was present at every episode of virological failure. Virological failure was defined as the second of two consecutive HIV-RNA≥400 copies/mL measured at least 6 months after ART initiation7 preceded by a HIV-RNA≤50 copies/mL.
Our estimates would be confounded if the decision to test for drug resistance depended on treatment adherence, a type of information that is usually not captured in HIV cohort data. We therefore compared the estimates with and without adjustment for time-varying, self-reported adherence (never missed a dose in the previous four weeks versus missed at least one dose) in the Swiss HIV Cohort Study, the only cohort collecting this information longitudinally24,25.
Finally, in order to examine the impact of heterogeneity by cohort in the patterns of drug resistance testing and the collection of genotyping resistance, we reran the analyses excluding each of the 6 cohorts one at a time.
Results
Table 1 shows the baseline characteristics of the 50,981 eligible individuals: 80% were men, 71% started follow-up after 2004; median [IQR] CD4 cell count and age at baseline were 405 [256, 580] cells/mm3 and 35 [29, 42] years, respectively. 18,002 (25%) individuals had a CD4 count> 500 cells/mm3 at baseline. A total of 22,161 individuals (43%) were tested for transmitted drug resistance while ART-naïve and within 12 months of baseline; transmitted drug resistance was detected in 6.1% of these tests.
Table 1.
Baseline characteristics of the 50,981 included individuals, HIV-CAUSAL Collaboration 2000–2015.
| Baseline characteristics | Eligible | Initiators of ART (%) | Median [IQR] follow-up time, years | ||
|---|---|---|---|---|---|
| CD4 count, cells/mm3 | <100 | 4021 (8%) | 86 | 3.8 | [1.9, 6.8] |
| 100 – 200 | 4820 (9%) | 86 | 4 | [2.0, 6.8] | |
| 200 – 350 | 11581 (23%) | 76 | 3.9 | [2.0, 6.5] | |
| 350 – 500 | 12557 (25%) | 60 | 3.7 | [1.9, 6.0] | |
| >500 | 18002 (35%) | 44 | 3.3 | [1.7, 5.6] | |
| HIV-RNA, copies/mL | <10000 | 14491 (28%) | 48 | 3.5 | [1.8, 5.9] |
| 10000 – 100000 | 22670 (44%) | 64 | 3.8 | [1.9, 6.3] | |
| >100000 | 13820 (27%) | 77 | 3.7 | [1.8, 6.4] | |
| Sex | Male | 40933 (80%) | 63 | 3.7 | [1.9, 6.2] |
| Female | 10048 (20%) | 62 | 3.5 | [1.8, 6.3] | |
| Age, years | < 35 | 25001 (41%) | 57 | 3.3 | [1.8, 5.8] |
| 35 – 50 | 21015 (10%) | 67 | 3.9 | [2.1, 6.5] | |
| >50 | 4965 (31%) | 75 | 3.7 | [2.0, 6.3] | |
| Mode of HIV acquisition | Heterosexual | 15743 (31%) | 65 | 3.7 | [1.8, 6.3] |
| Homo/bi-sexual | 29482 (58%) | 63 | 3.8 | [2.0, 6.3] | |
| Injection drug-use | 2279 (4%) | 54 | 2.5 | [1.3, 5.4] | |
| Other/unknown | 3477 (7%) | 59 | 3.3 | [1.8, 5.5] | |
| Calendar year | 2000–2004 | 14894 (29%) | 61 | 6.7 | [3.3, 9.8] |
| 2005–2009 | 19254 (38%) | 67 | 4.5 | [2.7, 6.0] | |
| 2010 – 2015 | 16833 (33%) | 59 | 1.9 | [1.2, 2.8] | |
| Region of origin | Western countries | 19751 (39%) | 68 | 3.8 | [2.0, 6.3] |
| Sub-saharan Africa | 2233 (4%) | 66 | 3.7 | [1.8, 6.2] | |
| Rest of the world | 4407 (9%) | 61 | 3.3 | [1.8, 5.8] | |
| Unknown | 24590 (48%) | 59 | 3.6 | [1.8, 6.3] | |
| Ethnicity | White | 20714 (41%) | 63 | 3.9 | [2.0, 6.7] |
| Black | 7991 (16%) | 59 | 3.4 | [1.8, 5.9] | |
| Other/unknown | 22276 (44%) | 64 | 3.5 | [1.8, 5.9] | |
| Testing for transmitted drug resistance | No | 28820 (56%) | 63 | 3.4 | [1.8, 6.5] |
| Yes | 22161 (43%) | 63 | 3.8 | [2.1, 6.0] | |
|
| |||||
| Overall | 50981 (100%) | 62 | 3.7 | [1.9, 6.5] | |
During a follow-up of 204,914 person-years, 31,969 (63%) individuals initiated ART at a median CD4 count of 270 [177, 369] and 5 [1, 20] months after baseline. Of these, 3207 (10%) initiated ART with CD4 count>500 cells/mm3. The most common initial ART combinations were NNRTI plus 2 NRTI (64%) and boosted PI plus 2 NRTI (29%). Initial combinations containing INI plus 2 NRTI, unboosted PI plus 2 NRTI and 3 NRTI were uncommon (3%, 3% and 1%, respectively). The presence of acquired drug resistance was tested for in 2,672 samples with median [IQR] HIV-RNA of 2,475 [236, 33,434] copies/mL. Factors associated with higher rates of testing were female sex, heterosexual HIV transmission group, injecting-drug use and younger age at baseline (Table 2). The rate of drug resistance testing declined steadily over time. Similar associations and trends by baseline characteristics and calendar period were found for the rates of detection of drug resistance and of virological failure (Table 2). Cohorts differed in the rates of drug resistance testing and of virological failure (Appendix Table 1).
Table 2.
Tests for acquired drug resistance and number of episodes of virological failure* and corresponding rates per 1000 person-years 6 months after ART initiation. HIV-CAUSAL Collaboration 2000–2015.
| Baseline characteristics | Tests for acquired drug resistance | Tests detecting acquired drug resistance | Virological failure episodes* | ||||
|---|---|---|---|---|---|---|---|
| N | Tests/1000 person-years | N | Tests/1000 person-years | N | Episodes/1000 person-years | ||
| Overall | 2672 | 20.1 | 794 | 6 | 1874 | 14.1 | |
|
| |||||||
| CD4 count, cells/mm3 | <100 | 449 | 28.9 | 207 | 13.3 | 227 | 14.8 |
| 100 – 200 | 433 | 23.5 | 138 | 7.5 | 254 | 13.8 | |
| 200 – 350 | 750 | 19.3 | 216 | 5.6 | 546 | 14.1 | |
| 350 – 500 | 487 | 15.8 | 111 | 3.6 | 403 | 13 | |
| >500 | 553 | 19.1 | 122 | 4.2 | 404 | 15.3 | |
| HIV-RNA, copies/mL | <10000 | 435 | 16 | 111 | 4.1 | 425 | 15.6 |
| 10000 – 100000 | 1117 | 18.4 | 338 | 5.6 | 803 | 13.2 | |
| >100000 | 1120 | 25 | 345 | 7.7 | 646 | 14.4 | |
| Sex | Male | 1959 | 18.1 | 574 | 5.3 | 1300 | 12 |
| Female | 713 | 29 | 220 | 9 | 574 | 23.4 | |
| Age, years | 18 – 35 | 1318 | 23.9 | 382 | 6.9 | 953 | 17.3 |
| 35 – 50 | 1136 | 18.3 | 351 | 5.7 | 790 | 12.8 | |
| >50 | 218 | 14 | 61 | 3.9 | 131 | 8.4 | |
| Mode of HIV acquisition | Heterosexual | 1087 | 25.8 | 367 | 8.7 | 778 | 18.7 |
| Homo/bi-sexual | 1326 | 16.9 | 337 | 4.3 | 864 | 11 | |
| Injection drug-use | 127 | 30.1 | 36 | 8.5 | 120 | 28.5 | |
| Other/unknown | 132 | 17 | 54 | 7 | 102 | 13.2 | |
| Calendar year | 2000–2004 | 1403 | 24 | 486 | 8.3 | 1104 | 18.9 |
| 2005–2009 | 1069 | 19 | 264 | 4.7 | 609 | 10.8 | |
| 2010 – 2015 | 200 | 11.1 | 44 | 2.4 | 162 | 9 | |
| Region of origin | Western countries | 524 | 9.2 | 181 | 3.2 | 602 | 10.6 |
| Sub-Saharan Africa | 159 | 28.2 | 65 | 11.5 | 128 | 22.7 | |
| Rest of the world | 137 | 13.6 | 55 | 5.4 | 109 | 10.8 | |
| Unknown | 1852 | 30.9 | 493 | 8.2 | 1035 | 17.2 | |
| Ethnicity | White | 1288 | 22.1 | 312 | 5.4 | 849 | 14.6 |
| Black | 727 | 38.1 | 245 | 12.8 | 461 | 24.2 | |
| Other/unknown | 657 | 11.9 | 237 | 4.3 | 564 | 10.2 | |
| Testing for transmitted drug resistance | No | 1478 | 19 | 508 | 6.6 | 1200 | 15.5 |
| Yes | 1194 | 11.1 | 286 | 5.2 | 674 | 12.2 | |
Virological failure was defined as the second of two consecutive HIV-RNA≥400 copies/mL preceded by HIV-RNA≤50 copies/mL.
There were 1874 episodes of virological failure, of which 617 (33%) were followed by a drug resistance test within 12 months. The proportion of episodes of virological failure followed by a test increased from 20% in 2000–2004 to 37% and 36% in 2005–2009 and 2010–2015, respectively. Resistance to any drug was detected in 794 (30%) samples. Resistance to NNRTI, NRTI and boosted PI was detected in 512 (19%), 576 (22%), and 67 (3%) samples, respectively.
The observed risk of acquired drug resistance was 2.7% at 7 years after baseline. The estimated 7-year risk (95% CI) of acquired drug resistance was 3.2% (2.8,3.5) for immediate ART initiation, 3.1% (2.7,3.3) for initiation at CD4<500 cells/mm3, and 2.8% (2.5,3.1) for initiation at CD4<350 cells/mm3. Compared with immediate initiation, the risk difference (95% CI) was −0.13% (−0.22, −0.06) under initiation at CD4<500 cells/mm3, and −0.37% (−0.52, −0.22) under initiation at CD4<350 cells/mm3 (Figure and Table 3).
Figure.
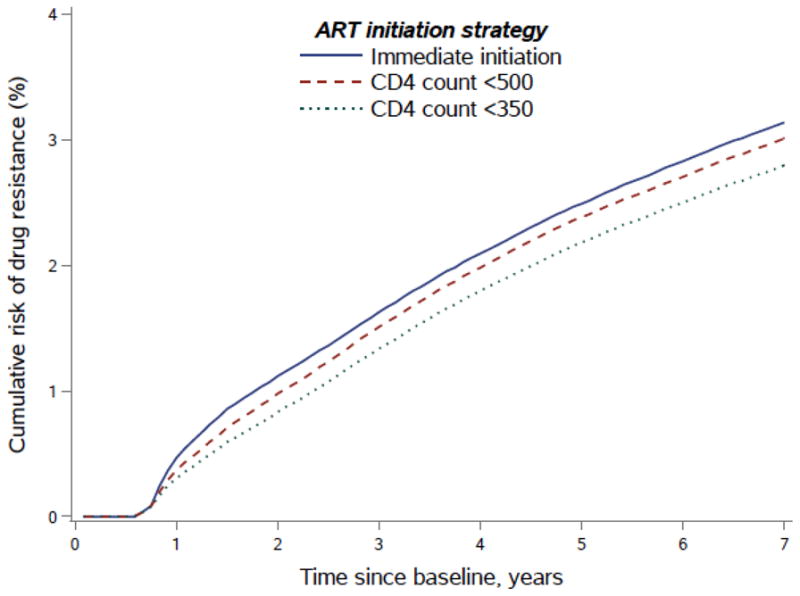
Risk of acquired drug resistance up to 7 years after baseline by ART initiation strategy. HIV-CAUSAL Collaboration 2000–2015.
Table 3.
Seven-year risk of acquired drug resistance and risk difference by ART initiation strategy and inclusion criteria. HIV-CAUSAL Collaboration 2000–2015.
| Inclusion criteria | ART initiation strategy | Risk at 7 years, % (95% CI) | Risk difference (95% CI) |
|---|---|---|---|
| All patients (N=50,981) | Immediate | 3.2 (2.8,3.5) | 0 (Ref.) |
| <500 cells/mm3 | 3.1 (2.7,3.3) | −0.13 (−0.22, −0.06) | |
| <350 cells/mm3 | 2.8 (2.5,3.0) | −0.37 (−0.52, −0.22) | |
|
| |||
| Baseline in calendar years 2005–2015 (N=36,087) | Immediate | 1.9 (1.8,2.5) | 0 (Ref.) |
| <500 cells/mm3 | 1.9 (1.7,2.4) | −0.08 (−0.21,0.04) | |
| <350 cells/mm3 | 1.8 (1.7,2.2) | −0.15 (−0.39,0.02) | |
|
| |||
| Baseline CD4 count>500 cells/mm3 (N= 18,002) | Immediate | 1.6 (1.2,2.3) | 0 (Ref.) |
| <500 cells/mm3 | 1.9 (1.4,2.4) | 0.22 (−0.18,0.48) | |
| <350 cells/mm3 | 1.6 (1.2,2.1) | −0.08 (−0.62,0.36) | |
|
| |||
| Individuals with Sub-Saharan Africa as region of origin (N=2233) | Immediate | 6.5 (4.2,9.3) | 0 (Ref.) |
| <500 cells/mm3 | 6.2 (4.2,9.1) | −0.27 (−0.85,0.11) | |
| <350 cells/mm3 | 5.6 (3.7,8.7) | −0.90 (−1.67,0.00) | |
When restricting the analyses to individuals with baseline in 2005–2015, the estimated 7-year risk (95% CI) of acquired resistance was 1.9% (1.8, 2.5) for immediate ART initiation, 1.9% (1.7,2.4) for initiation at CD4<500 cells/mm3, and 1.8% (1.7,2.2) for initiation at CD4<350 cells/mm3. When the analyses were restricted to individuals with baseline CD4>500 cells/mm3 the 7-year risk estimates were 1.6% (1.2,2.3), 1.9% (1.4,2.4) and 1.6% (1.2,2.1) for immediate initiation and initiation with CD4<500 and CD4<350 cells/mm3, respectively. Under immediate ART initiation the estimated 7-year risk of acquired drug resistance was 4.4% (3.8,5.0) when we imposed a drug resistance test at each episode of virological failure, and 10.9% (10.1,11.4) when we assumed that every instance of virological failure was due to acquired drug resistance (Table 4). Results did not materially change in the subgroup analyses sequentially excluding each of the cohorts (Appendix Table 2). In the Swiss HIV Cohort Study data, the estimates of risk under all strategies adjusting for self-reported adherence were similar to the estimates not adjusting for adherence (risk difference <0.4%).
Table 4.
Sensitivity analyses to examine the role of potential underascertainment of drug resistance testing. Estimated 7-year risk of acquired drug resistance and risk difference by ART initiation strategy. HIV-CAUSAL Collaboration 2000–2015.
| Outcome | ART initiation strategy | Risk at 7 years, % (95% CI) | Risk difference (95% CI) |
|---|---|---|---|
| Acquired drug resistance under the observed data | Immediate | 3.2 (2.8,3.5) | 0 (Ref.) |
| <500 cells/mm3 | 3.1 (2.7,3.3) | −0.13 (−0.22, −0.06) | |
| <350 cells/mm3 | 2.8 (2.5,3.0) | −0.37 (−0.52, −0.22) | |
|
| |||
| Acquired drug resistance assuming a drug resistance test at every virological failure* episode | Immediate | 4.4 (3.8,5.0) | 0 (Ref.) |
| <500 cells/mm3 | 3.8 (3.3,4.3) | −0.60 (−0.71, −0.44) | |
| <350 cells/mm3 | 3.1 (2.8,3.6) | −1.27 (−1.47, −0.99) | |
|
| |||
| Virological failure* episode | Immediate | 10.9 (10.1,11.4) | 0 (Ref.) |
| <500 cells/mm3 | 9.3 (8.6,9.8) | −1.58 (−1.74, −1.39) | |
| <350 cells/mm3 | 7.5 (6.9,7.8) | −3.42 (−3.69, −3.07) | |
Virological failure was defined as the second of two consecutive HIV-RNA≥400 copies/mL preceded by HIV-RNA≤50 cells/mm3.
Discussion
In HIV-positive individuals receiving routine clinical care in Europe, we estimated that the risk of acquired drug resistance was similar under immediate and delayed ART initiation. Compared with ART initiation with CD4<500 cells/mm3 or AIDS and CD4<350 cells/mm3 or AIDS, immediate ART initiation increased the 7-year risk of acquired drug resistance by only 0.13% and 0.37%, respectively. The estimated 7-year risk of clinically identified acquired drug resistance was approximately 3% under all ART initiation strategies. These risks and risk differences were even lower in individuals with initial CD4 count>500 cells/mm3 and individuals who entered the study after 2004.
To our knowledge, this is the first study to estimate the public health impact of immediate ART initiation on the risk of acquired drug resistance. Previous studies found that higher CD4 count at ART initiation was associated with lower risk of resistance10–13. Unlike in these studies, we quantified the risk of drug resistance since baseline, a proxy of entry into HIV care, rather than since ART initiation. Our estimates of risk take into account that i) immediate initiation implies longer exposure to ART and thus increased chance of drug resistance and ii) the risk of acquiring drug resistance before ART initiation is zero. Our findings indicate that it is unlikely that the clinical benefits of early ART initiation demonstrated by randomized clinical trials1,2 and observational studies 26–30 will be lessened by development of acquired drug resistance. In high-income countries, the need for second line treatments due to the development of acquired drug resistance will increase only slightly under the new ART initiation guidelines5–8. In particular, our findings indicate that in a hypothetical cohort of 1000 patients, immediate initiation would imply only 4 additional cases in need of second line treatment over a 7 year period compared to initiation with CD4<350 cells/mm3 or AIDS. Moreover, because of the increasing use of antiretroviral treatments associated with low drug resistance such as integrase inhibitors and new generation PI, which were underrepresented in our data, we expect this increase to be even lower in the longer term. Further, the low risk of acquired drug resistance after 2004 indicates that acquired drug resistance is becoming a rare phenomenon in Europe, though higher among individuals born in Sub-Saharan Africa. This is compatible with previous findings from the Swiss HIV Cohort Study22,31 and generalizes them to other European countries with similar access to health care.
As in previous studies aimed at estimating the comparative effectiveness of immediate ART initiation27, we analyzed the data both including all patients regardless of initial CD4 count (to estimate the public health impact) and including only patients with high CD4 count at baseline (to estimate the effectiveness in this particular subgroup). The low risk of drug resistance in the subgroup of individuals with baseline CD4 cell count >500 cells/mm3 alleviates the concern that individuals who are diagnosed with HIV while asymptomatic might be less likely to adhere to treatment.
International and national HIV guidelines recommend testing for transmitted drug resistance in all ART-naïve individuals and testing for acquired drug resistance in ART-treated people after all episodes of virological failure. Despite these recommendations only 43% of the people included in our study were known to be tested for transmitted drug resistance and only 33% of virological failure episodes were known to be followed by an acquired drug resistance test. Our estimates were, however, robust under different scenarios of potential under-ascertainment of acquired drug resistance.
The main strengths of our study are the large sample size of over 50,000 individuals and the setting in HIV clinics in Europe that are considered representative of routine clinical practice32. Care should be taken with generalizing our results to resource-limited settings because of the differences in the HIV epidemics, and availability of viral load monitoring, of drug resistance testing facilities and of second and third line therapy.
This study has several limitations. First, the validity of our estimates relies on the assumption that all factors that influenced the decision to initiate ART as well as the risk of developing drug resistance were adjusted for. We expect this assumption to approximately hold because we adjusted for the most important factors used to decide whether to initiate ART such as CD4 count, HIV-RNA and AIDS. Second, our methods require all models to be correctly specified. This condition cannot be guaranteed, but it seems plausible because our models resulted in simulated data sets with distributions of outcome and time-varying covariates similar to those in the original data. Third, we could not estimate the long-term effect of immediate initiation because approximately only 25% of included patients had follow-up longer than 7 years. Fourth, given that only half of the patients were genotyped before ART initiation, some resistance-associated mutations may, in fact, have been transmitted. It is, therefore, possible that our estimates of risk of acquired drug resistance are somewhat pessimistic. Finally, only a small proportion of patients initiated treatment with integrase inhibitors, an ART regimen currently recommended as first line therapy, therefore we could not appropriately assess drug resistance for this drug class. However, according to recent reports, drug resistance to integrase inhibitors is very rare and integrase inhibitors-treated patients exhibit low rates of virological failure16,31.
In conclusion, the risk of acquired drug resistance after immediate ART initiation, the currently recommended strategy for ART initiation, is very small and not materially larger than if treatment is deferred. Therefore, it is unlikely that the benefits of immediate ART initiation will be compromised by development of acquired drug resistance in high-income countries and that immediate initiation will imply a substantial increase in need of second line treatments. Continual efforts should be made to monitor trends in transmitted drug resistance and acquired drug resistance in high- and low-income countries.
Supplementary Material
Summary.
We estimated and compared the risk of acquired drug resistance under immediate initiation (the currently recommended ART initiation strategy) and CD4 count-based strategies. Immediate initiation increased the risk of drug resistance, but the magnitude of the change were very small.
Acknowledgments
Principal contributions made by the authors.
Data collection: Heiner Bucher, Huldrych Günthard, T. Sonia Boender, David Dunn, Matthias Egger, Federico Garcia, Tracy R. Glass, David Hawkins, Sophie Jose, Susana Monge, Santiago Moreno, Dimitrios Paraskevis, Andrew Phillips, Kholoud Porter, Peter Reiss, Caroline Sabin, Alexandra Scherrer, Marie-Paule Schneider, Ard van Sighem, Metallidis Simeon, Georgia Vourli; Study design: Sara Lodi; Statistical analyses: Sara Lodi, Roger Logan; Interpretation of results: All authors; Read and approved the manuscript: All authors; Drafted the manuscript: Sara Lodi, Miguel Hernan. Sara Lodi is the guarantor.
Funding
This work was supported by NIH grant R01 AI102634. Sara Lodi is funded by Harvard University CFAR grant P30 AI060354.
Footnotes
Conflicts of interest
HF Günthard reports receipt of unrestricted research grants from Gilead Sciences and Roche, fees for data and safety monitoring board membership from Merck; consulting/advisory board membership fees from Gilead Sciences; and travel reimbursement from Gilead, Bristol-Myers Squibb, and Janssen. HC Bucher or his institution has received honorarium, support to attend conferences or unrestricted research grants from Gilead Sciences, BMS, Viiv Healthcare, Janssen, Abbvie, MSD in the last 3 years preceding the submission date of this manuscript. A Phillips has received payment for invited presentations from Gilead Sciences. S Jose received speakers fees from Gilead. C Sabin received funding from Gilead Sciences, ViiV Healthcare and Janssen-Cilag for the membership of Data Safety and Monitoring Boards, Advisory Boards, Speaker Panels and for the preparation of educational materials. A van Sighem reports grants from European Centre for Disease Prevention and Control, personal fees from ViiV Healthcare, personal fees from Gilead Sciences, personal fees from Janssen-Cilag, outside the submitted work. Peter Reiss through his institution received independent scientific grant support from Gilead Sciences, Janssen Pharmaceuticals Inc, Merck & Co, Bristol-Myers Squibb and ViiV Healthcare; he has served on a scientific advisory board for Gilead Sciences and a data safety monitoring committee for Janssen Pharmaceuticals Inc; he chaired a scientific symposium by ViiV Healthcare, for which his institution has received remuneration. D Paraskevis has received research grants from Gilead Sciences, GlaxoSmithKline travel grants from Gilead Sciences, GlaxoSmithKline Janssen, and participated to advisory boards of Gilead Sciences and Merck. K.Porter received personal fees from ViiV healthcare. No other conflicts to report.
References
- 1.Temprano ANRS Study Group. A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa. N Engl J Med. 2015;373(9):808–22. doi: 10.1056/NEJMoa1507198. [DOI] [PubMed] [Google Scholar]
- 2.Insight Start Study Group. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373(9):795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. The New England journal of medicine. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodger AJ, Cambiano V, Bruun T, et al. Sexual Activity Without Condoms and Risk of HIV Transmission in Serodifferent Couples When the HIV-Positive Partner Is Using Suppressive Antiretroviral Therapy. JAMA. 2016;316(2):171–81. doi: 10.1001/jama.2016.5148. [DOI] [PubMed] [Google Scholar]
- 5.Panel on Antiretroviral Guidelines for Adults and Adolescents. [accessed 30 November 2016];Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2016 http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 6.European AIDS clinical society (EACS) [accessed 30 November 2016];European guidelines for treatment of HIV infected adults in Europe. 2016 http://www.eacsociety.org/files/guidelines_8.1-english.pdf.
- 7.World Health Organization (WHO) [accessed 30 November 2016];Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. 2016 http://apps.who.int/iris/bitstream/10665/246200/1/9789241511124-eng.pdf?ua=1. [PubMed]
- 8.Gunthard HF, Saag MS, Benson CA, et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2016 Recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316(2):191–210. doi: 10.1001/jama.2016.8900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strategies for Management of Antiretroviral Therapy Study G. El-Sadr WM, Lundgren J, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355(22):2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 10.Uy J, Armon C, Buchacz K, Wood K, Brooks JT Investigators H. Initiation of HAART at higher CD4 cell counts is associated with a lower frequency of antiretroviral drug resistance mutations at virologic failure. J Acquir Immune Defic Syndr. 2009;51(4):450–3. doi: 10.1097/QAI.0b013e3181acb630. [DOI] [PubMed] [Google Scholar]
- 11.UK Collaborative Group on HIV Drug Resistance UK CHIC Study Group. Long-term probability of detecting drug-resistant HIV in treatment-naive patients initiating combination antiretroviral therapy. Clin Infect Dis. 2010;50(9):1275–85. doi: 10.1086/651684. [DOI] [PubMed] [Google Scholar]
- 12.Harrigan PR, Hogg RS, Dong WW, et al. Predictors of HIV drug-resistance mutations in a large antiretroviral-naive cohort initiating triple antiretroviral therapy. J Infect Dis. 2005;191(3):339–47. doi: 10.1086/427192. [DOI] [PubMed] [Google Scholar]
- 13.Lodi S, Phillips A, Fidler S, et al. Role of HIV infection duration and CD4 cell level at initiation of combination anti-retroviral therapy on risk of failure. PLoS One. 2013;8(9):e75608. doi: 10.1371/journal.pone.0075608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ancelle-Park R. Expanded European AIDS case definition. Lancet. 1993;341(8842):441. doi: 10.1016/0140-6736(93)93040-8. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch MS, Gunthard HF, Schapiro JM, et al. Antiretroviral drug resistance testing in adult HIV-1 infection: 2008 recommendations of an International AIDS Society-USA panel. Clin Infect Dis. 2008;47(2):266–85. doi: 10.1086/589297. [DOI] [PubMed] [Google Scholar]
- 16.Scherrer AU, Yang WL, Kouyos RD, et al. Successful Prevention of Transmission of Integrase Resistance in the Swiss HIV Cohort Study. J Infect Dis. 2016;214(3):399–402. doi: 10.1093/infdis/jiw165. [DOI] [PubMed] [Google Scholar]
- 17.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 18.Robins J, Hernan M. Estimation of the causal effects of time-varying exposures. In: Fitzmaurice GDM, Verbeke G, Molenberghs G, editors. Advances in longitudinal data analysis. Boca Raton: Chapman and Hall/CRC Press; 2009. pp. 553–99. [Google Scholar]
- 19.Taubman SL, Robins JM, Mittleman MA, Hernan MA. Intervening on risk factors for coronary heart disease: an application of the parametric g-formula. Int J Epidemiol. 2009;38(6):1599–611. doi: 10.1093/ije/dyp192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young JG, Cain LE, Robins JM, O’Reilly EJ, Hernan MA. Comparative effectiveness of dynamic treatment regimes: an application of the parametric g-formula. Stat Biosci. 2011;3(1):119–43. doi: 10.1007/s12561-011-9040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robins JM. A new approach to causal inference in mortality studies with a sustained exposure period: application to the healthy worker survivor effect. Mathematical Modelling. 1986;7(9–12):1393–512. [Google Scholar]
- 22.Staehelin C, Keiser O, Calmy A, et al. Longer term clinical and virological outcome of sub-Saharan African participants on antiretroviral treatment in the Swiss HIV Cohort Study. J Acquir Immune Defic Syndr. 2012;59(1):79–85. doi: 10.1097/QAI.0b013e318236be70. [DOI] [PubMed] [Google Scholar]
- 23.Eyawo O, Fernandes KA, Brandson EK, et al. Suboptimal use of HIV drug resistance testing in a universal health-care setting. AIDS Care. 2011;23(1):42–51. doi: 10.1080/09540121.2010.498871. [DOI] [PubMed] [Google Scholar]
- 24.Glass TR, Sterne JA, Schneider MP, et al. Self-reported nonadherence to antiretroviral therapy as a predictor of viral failure and mortality. AIDS. 2015;29(16):2195–200. doi: 10.1097/QAD.0000000000000782. [DOI] [PubMed] [Google Scholar]
- 25.von Wyl V, Klimkait T, Yerly S, et al. Adherence as a predictor of the development of class-specific resistance mutations: the Swiss HIV Cohort Study. PLoS One. 2013;8(10):e77691. doi: 10.1371/journal.pone.0077691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cain LE, Logan R, Robins JM, et al. When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries: an observational study. Ann Intern Med. 2011;154(8):509–15. doi: 10.1059/0003-4819-154-8-201104190-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edwards JK, Cole SR, Westreich D, et al. Age at Entry Into Care, Timing of Antiretroviral Therapy Initiation, and 10-Year Mortality Among HIV-Seropositive Adults in the United States. Clin Infect Dis. 2015;61(7):1189–95. doi: 10.1093/cid/civ463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lodi S, Phillips A, Logan R, et al. Comparative effectiveness of strategies for antiretroviral treatment initiation in HIV-positive individuals in high-income countries: an observational cohort study of immediate universal treatment versus CD4-based initiation. Lancet HIV. 2015;2(8):e335–e43. doi: 10.1016/S2352-3018(15)00108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sterne JA, May M, Costagliola D, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373(9672):1352–63. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Writing Committee for the Cascade Collaboration. Timing of HAART initiation and clinical outcomes in human immunodeficiency virus type 1 seroconverters. Arch Intern Med. 2011;171(17):1560–9. doi: 10.1001/archinternmed.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scherrer AU, von Wyl V, Yang WL, et al. Emergence of Acquired HIV-1 Drug Resistance Almost Stopped in Switzerland: A 15-Year Prospective Cohort Analysis. Clin Infect Dis. 2016;62(10):1310–7. doi: 10.1093/cid/ciw128. [DOI] [PubMed] [Google Scholar]
- 32.Touloumi G. Assessing the representativeness of European HIV cohorts participants as compared to HIV Surveillance data- an ECDC Project. HepHIV 2017 Conference: HIV and Viral Hepatitis: Challenges of Timely Testing and Care; Malta. 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


