SUMMARY
Active smoking in stable COPD subjects significantly increased eosinophil accumulation in the distal airspaces, but not in sputum or peripheral blood. Our findings support the need to investigate this cell-type as a potential driver of COPD symptomatology and progression.
Keywords: BAL, Cigarette smoke, COPD, Eosinophil, Human
To the Editor
Novel therapies for chronic obstructive pulmonary disease (COPD) are urgently needed. Eosinophilic inflammation is an appealing target, as blood or sputum eosinophils in stable COPD may predict responses to systemic or inhaled corticosteroid (ICS) therapy 1. Titrating steroid therapy in the stable state based on sputum eosinophils reduced severe exacerbations 2 and has been recommended for clinical practice 3. However, the prevalence of eosinophilic inflammation in COPD and its uniformity between systemic and lung compartments remain incompletely defined. Controversy exists on whether sputum analysis (reflecting large airway events) is required, or if blood eosinophilia can suffice, based on the strong correlation between the two found by one group 4. Thus, better understanding of eosinophils in COPD is needed.
The Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) (ClinicalTrials.gov NCT01969344T4), which recently completed enrolling 2,981 participants, provides a unique opportunity to address these controversies 5. This analysis was performed on a subset (n = 139) of SPIROMICS participants who agreed to a bronchoscopy sub-study, which used multicolor flow cytometry to identify leukocyte subsets and to define their activation states. All investigations were conducted according to principles of the Declaration of Helsinki. The protocol was approved by the institutional review boards of the eight participating clinical centers. Briefly, participants underwent sputum induction, then 2–4 weeks later, returned for a bronchoscopy visit at which we collected peripheral blood and bronchoalveolar lavage (BAL). Sputum, blood, and BAL samples were stained on the day of collection at the clinical centers, then fixed and shipped to a central laboratory for flow cytometry analysis. Additional details can be found in the Online Supplement. We categorized participants as never-smokers (NS); current smokers (CS-NAO) and former smokers (FS-NAO) with no airflow obstruction; and current smokers (CS-COPD) and former smokers (FS-COPD) with COPD (Table 1).
Table 1.
Subject characteristics
| Group | NS | FS-NAO | CS-NAO | FS-COPD | CS-COPD | p value |
|---|---|---|---|---|---|---|
| Subjects, n | 21 | 29 | 30 | 37 | 22 | |
| Sex ratio, M/F | 8/13 | 13/16 | 16/14 | 24/13 | 12/10 | 0.32 |
| Age, years (SD) | 52 (8) | 63 (8) | 51 (6) | 65 (7) | 60 (7) | <0.0001 |
| Smoking, pack-years (SD) | 0 (0) | 39 (16) | 35 (12) | 51 (24) | 53 (20) | 0.0007 * |
| FEV1, % pred. (SD) | 100 (0) | 102 (12) | 97 (13) | 79 (19) | 78 (17) | <0.0001 |
| FEV1/FVC (SD) | 0.82 (0.06) | 0.76 (0.06) | 0.76 (0.05) | 0.58 (0.09) | 0.58 (0.10) | <0.0001 |
| ICS 3 use (yes/no) | 1/20 | 1/28 | 3/26 ** | 15/22 | 6/16 | 0.0002 |
Data are presented as mean (SD) except for sex ratios and ICS use; M, male; F, female;
former smoker defined as having quit for more than six months;
ICS, inhaled corticosteroids. NS, never-smokers; FS-NAO, former smokers, no airflow obstruction; CS-NAO, current smokers, no airflow obstruction; FS-COPD, former smokers with COPD; CS-COPD, current smokers with COPD. One-way ANOVA with Holm-Sidak post-hoc testing was used to determine significant differences between groups.
NS group omitted, no single group statistically different by post-hoc testing.
data missing from one subject.
Using our described staining protocol and flow cytometric gating 6, we identified eosinophils as CD45+, CCR3+, CD16− cells with high FSC and SSC (Supplemental Fig. 1). When grouping subject by smoking status and COPD disease status, we found no differences between eosinophil percentages in peripheral blood (Fig. 1A) or sputum (Fig. 1B). By contrast, BAL eosinophils were significantly increased as a percentage of all CD45+ cells in current smokers with COPD, relative to other groups (Fig. 1C). However, percentage of BAL eosinophils did not correlate significantly in any sample type with FEV1% predicted or imaging variables (percent emphysema or Pi10) (not shown). IgE levels were largely within the normal range [geometric mean 46.7 IU/mL (95% CI 35.3, 60.2)]. Log10-transformed IgE levels neither differed between groups nor correlated with eosinophil percentages in any of the three sample types, whether among all subjects or only the CS-COPD group (not shown). We found no correlation of BAL eosinophil percentages with plasma levels of the CCR3 ligands CCL5, CCL8 and CCL24 (not shown), and did not measure CCL11 or IL-5.
Figure 1. Active smoking significantly increases BAL eosinophil percentages in COPD.
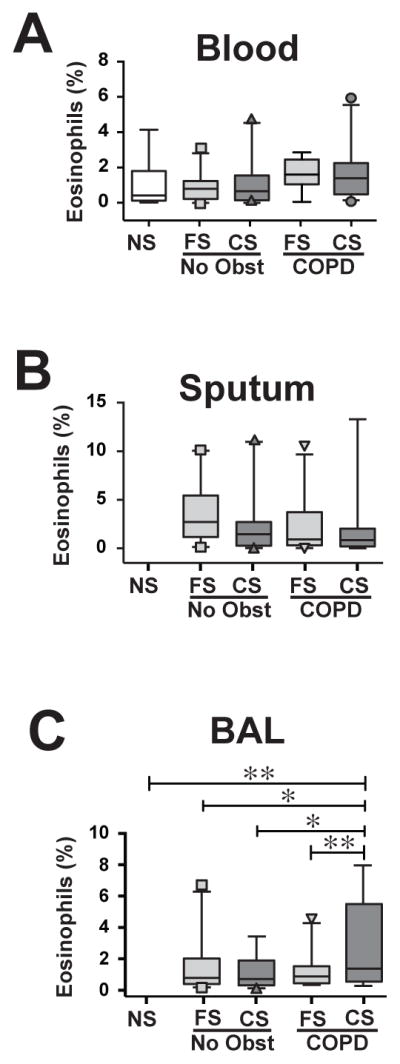
Flow cytometric analysis of eosinophils in (A) blood (n=97), (B) sputum (n=94) and (C) BAL (n=91). NS, never-smokers; FS-NAO, former smokers, no airflow obstruction; CS-NAO, current smokers, no airflow obstruction; FS-COPD, former smokers with COPD; CS-COPD, current smokers with COPD. Box and whiskers plot showing median ± interquartile range, 5th and 95th percentiles, with outliers shown as symbols. *, p<0.05; **, p<0.01; ANOVA with Holm-Sidak post-hoc testing.
To better understand the correlation between current smoking and elevated BAL eosinophil percentages in COPD, we performed multivariate modeling. We adjusted for demographics (age, sex, African-American race), smoking intensity (per 10 pack-year exposure), obstruction and smoking status (never vs. former vs. current), chronic bronchitis, histories of asthma or gastroesophageal reflux, and ICS use. We also controlled for the presence of self-reported eye/nose allergies (defined as both any diagnosis and current diagnosis, with positive response [presence of allergy symptoms] reported by 18.5% of participants) and self-reported seasonal allergies (positive response [presence of allergy symptoms] reported by 13.3% of participants). Results showed a significant association between eosinophil percentage in BAL with current smoking plus COPD, with a 2.5 fold increase in eosinophil percentage in smokers with COPD (β = 2.5; 95% CI 1.0, 3.9).
To gain insights into eosinophil activation states in various compartments, we examined cell-surface expression of the adhesion molecules CD11b (Clone CBRM1/5, which recognizes an activation-specific epitope), CD34, and CD49d; the activation receptor CD69; and CD125, the IL-5 receptor alpha chain. Within a given subject group, every receptor showed significant differences between sample types in percentages of positive eosinophils (Supplemental Table 1, column p values). By contrast, within sample types, only CD125 in BAL differed between groups (significantly lower in CS-COPD) (Supplemental Table 1, row p values).
Accordingly, we analyzed eosinophil receptor-positivity in the three sample types regardless of subject groups (Supplemental Fig. 2). Relative to blood, significantly more eosinophils were positive for CD11b, CD34 and CD69 in BAL and for CD34 and CD69 in sputum. CD69 may contribute to intrapulmonary eosinophil retention as shown for lung-resident T memory cells 7, although for both cell types, CD69 might be up-regulated by the lung environment but not causing retention.
Finally, we examined the magnitude of receptor expression by mean fluorescence intensity (MFI) (Supplemental Table 2 & Supplemental Fig. 3A), which, as with the percent of eosinophils expressing a receptor, differed significantly between sample types for every receptor. In terms of subject comparisons, there were no significant differences in expression of CD34, CD69 or CD125 by BAL eosinophils, but expression of CD11b was significantly greater in FS-NAO relative to FS-COPD (Supplemental Fig. 3B). Moreover, expression of CD49d was significantly higher in BAL eosinophils of CS-COPD than in groups other than CS-NAO, which was also elevated relative to FS-COPD (but not to other groups) (Supplemental Fig. 3B).
This analysis demonstrates that active smoking increases steady-state localization of eosinophils to the distal lung in COPD, relative to smokers (current or former) without airflow obstruction and to never-smokers. This interaction was not observed in FS-COPD, implying that smoking reversibly impacts eosinophil recruitment or retention (or both). Eosinophilic inflammation was compartmentalized, as eosinophils as a percentage of all leukocytes in BAL showed no correlation with results in blood or in sputum (data not shown). These data extend two previous studies that used cytospin differential counts to enumerate BAL eosinophils. Both found, as we did, that induced sputum results neither differed between groups nor correlated with BAL percentages 8, 9. Our results are also congruent with those of Wen and colleagues, who found increased BAL eosinophils in current smokers with COPD, relative to ex-smokers with COPD 9.
Strengths of our study include its size, inclusion of extensively phenotyped subjects at multiple clinical centers, and rigorous analytic plan, using a single flow cytometry instrument and analysis by a limited number of scientists blinded to clinical data. Limitations of our entire immunophenotyping study include the absence of viability staining at the time of flow acquisition and the duration (usually 2–4 weeks) between sputum and bronchoscopy visits. Smoking status was determined by self-report, albeit in temporal proximity to the bronchoscopy visit, but was not verified by objective measurements. Finally, subjects who agreed to in the bronchoscopy sub-study were self-selected and may not be representative of the general population.
Supplementary Material
Acknowledgments
SOURCES OF SUPPORT
SPIROMICS was supported by contracts from the NIH/NHLBI (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C HHSN268200900019C, HHSN268200900020C), which were supplemented by contributions made through the Foundation for the NIH from AstraZeneca; Bellerophon Therapeutics; Boehringer-Ingelheim Pharmaceuticals, Inc; Chiesi Farmaceutici SpA; Forest Research Institute, Inc; GSK; Grifols Therapeutics, Inc; Ikaria, Inc; Nycomed GmbH; Takeda Pharmaceutical Company; Novartis Pharmaceuticals Corporation; Regeneron Pharmaceuticals, Inc; and Sanofi. Additional support came from the Department of Veterans Affairs through Merit Review Awards I01 BX001389 (CMF) and I01 CX000911 (JLC) and the NIH/NHLBI through Grants K2 HL128936 and R01 HL122438-S1 (CHM).
ABBREVIATIONS
- BAL
bronchoalveolar lavage
- CI
confidence interval
- COPD
Chronic obstructive pulmonary disease
- ICS
inhaled corticosteroids
- SPIROMICS
Subpopulations and Intermediate Outcomes in COPD Study
- NS
never-smoker(s)
- CS-NAO
current smoker(s) with no airflow obstruction
- FS-NAO
former smoker(s) with no airflow obstruction
- CS-COPD
current smoker(s) with COPD
- FS-COPD
former smoker(s) with COPD
Footnotes
AUTHORS’ CONTRIBUTIONS:
PGW designed the overall bronchoscopy sub-study; CMF, NEA, & JLC designed the Immunophenotyping protocol; MKH, CBC, NNH, NP, & MZ assisted with sample collection; SXL, AJH, VRS & CMF performed experiments; EEC, DJC, &WKO assisted with collection and dissemination of participant data; CHM, JLC, CMF analyzed data; RGB, ERB, SAC, CBC, CMD, ATH, EAH, RJK, FJM, DAM, RP, & SIR assisted with data analysis and interpretation; CHM & CMF wrote the first draft, and all Authors participated in revisions and approved the final version.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ernst P, Saad N, Suissa S. Inhaled corticosteroids in COPD: the clinical evidence. Eur Respir J. 2015;45:525–37. doi: 10.1183/09031936.00128914. [DOI] [PubMed] [Google Scholar]
- 2.Siva R, Green RH, Brightling CE, Shelley M, Hargadon B, McKenna S, et al. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur Respir J. 2007;29:906–13. doi: 10.1183/09031936.00146306. [DOI] [PubMed] [Google Scholar]
- 3.McDonald VM, Higgins I, Wood LG, Gibson PG. Multidimensional assessment and tailored interventions for COPD: respiratory utopia or common sense? Thorax. 2013;68:691–4. doi: 10.1136/thoraxjnl-2012-202646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bafadhel M, McKenna S, Terry S, Mistry V, Reid C, Haldar P, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184:662–71. doi: 10.1164/rccm.201104-0597OC. [DOI] [PubMed] [Google Scholar]
- 5.Couper D, Lavange LM, Han M, Barr RG, Bleecker E, Hoffman EA, et al. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) Thorax. 2013;69:491–4. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman CM, Crudgington S, Stolberg VR, Brown JP, Sonstein J, Alexis NE, et al. Design of a multi-center immunophenotyping analysis of peripheral blood, sputum and bronchoalveolar lavage fluid in the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS) J Transl Med. 2015;13:19. doi: 10.1186/s12967-014-0374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark RA. Resident memory T cells in human health and disease. Sci Transl Med. 2015;7:269rv1. doi: 10.1126/scitranslmed.3010641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutgers SR, Timens W, Kaufmann HF, van der Mark TW, Koeter GH, Postma DS. Comparison of induced sputum with bronchial wash, bronchoalveolar lavage and bronchial biopsies in COPD. Eur Respir J. 2000;15:109–15. doi: 10.1183/09031936.00.15110900. [DOI] [PubMed] [Google Scholar]
- 9.Wen Y, Reid DW, Zhang D, Ward C, Wood-Baker R, Walters EH. Assessment of airway inflammation using sputum, BAL, and endobronchial biopsies in current and ex-smokers with established COPD. Int J Chron Obstruct Pulmon Dis. 2010;5:327–34. doi: 10.2147/COPD.S11343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


