To the Editor
IgE antibodies (Abs) can mediate allergic reactions, including systemic anaphylaxis by activating the high affinity FcεRI on mast cells and basophils, leading to release of inflammatory mediators1,2,E1–3 (see References E1–45 in this article´s Online Repository). In contrast, allergen-specific IgG Abs, which are also induced by allergen-specific immunotherapies (AIT), can inhibit IgE-mediated anaphylaxis caused by low levels of allergen through allergen-masking and crosslinking of the FcεRI with the classical IgG inhibitory receptor FcγRIIb2,3,E1,4,5. However, when allergen levels are high, IgG Abs induced in untreated and SIT-treated allergic patients as well as to medical drugs also have the potential to mediate anaphylaxis by activating classical activating FcγRs on different immune cell types2,3,4,E6–12.
The effector functions of IgG Abs depend on their subclassE11,13 and the type of Fc N-glycosylation (Fig 1, A, and Fig E1). Agalactosylated IgG Abs generally promote inflammationE14,15, whereas galactosylation and terminal sialylation of IgG Abs generally suppress inflammation5–8,E15–19. However, the effects of IgG subclass and Fc glycosylation pattern in allergy remain unclear.
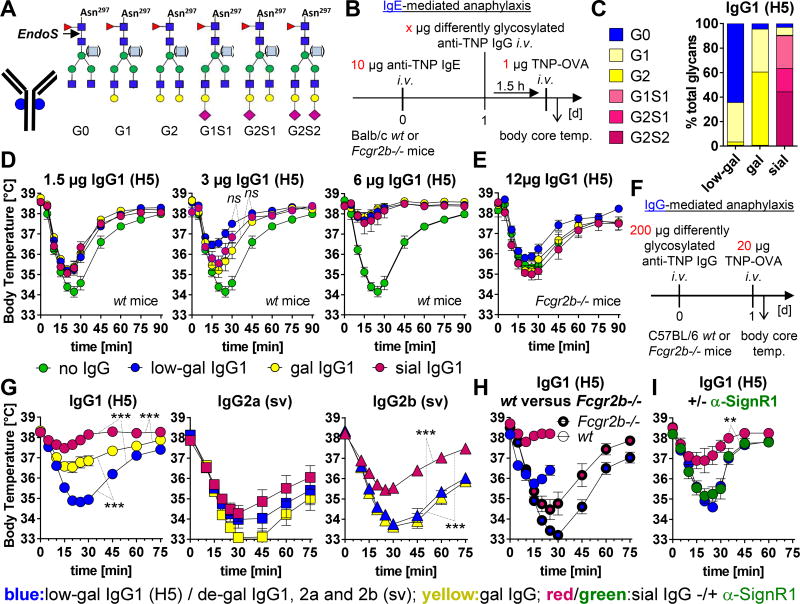
FIG 1. Effect of differently glycosylated IgG subclass Abs on IgE- and IgG-mediated murine anaphylaxis.
A, The conserved biantennary N-glycan (four N-acetylglucosamines (dark blue) and three mannoses (green)) at Asn 297 in the IgG Fc part can be modified by fucose (red), bisecting GlcNAc (light blue), galactose (G; yellow) and sialic acid (S; magenta). The cleavage site of EndoS used for IgG glycan analysis is depicted. B, Experimental design of IgE-mediated anaphylaxis as done in D and E. C, Fc glycosylation profiles of the differently glycosylated murine IgG1 anti-TNP mAbs (clone H5) that were used in the murine experiments; native=low-galactosylated (low-gal), in vitro galactosylated (gal) or galactosylated plus sialylated (sial). D and E, Inhibition of IgE-mediated temperature drop by differently glycosylated IgG1 anti-TNP mAbs (H5) in (D) wt and (E) FcγRIIb-deficient mice (in each graph: no IgG, n=8–10; low-gal, n=8–10; gal, n=5; sial, n=5); symbols represent means. One of two independent experiments is shown. F, Experimental design of IgG-mediated anaphylaxis as done in G–I. G–I, Decrease in body temperature induced with differently glycosylated IgG subclass anti-TNP mAbs in (G–I) wt or (H) FcγRIIb-deficient mice (G–I) without or (I) with α-SignR1 treatment; de-gal: in vitro de-sialylated plus de-galactosylated. (G) Pooled data (n=10–15) from independent experiments with n=5/group/experiment or (H, I) one of two independent experiments is shown.
Here, we first compared the capacity of differently glycosylated forms of murine IgG1, a subclass that resembles AIT-induced human IgG4 in its limited ability to activate complement and classical activating FcγRs3,E1,5,11,13, to inhibit IgE-mediated systemic anaphylaxis (Fig 1, B–E, and Fig E1 (see Figs E1–4 and Methods in this article´s Online Repository)). IgE-mediated anaphylaxis (assessed as decreased rectal temperature) was induced intravenously (i.v.) with 10 µg of IgE anti-2,4,6-trinitrophenyl (TNP) monoclonal Ab (mAb), followed by an i.v. challenge 24 h later with 1 µg of TNP-coupled ovalbumin (TNP-OVA) (Fig 1, B). Increasing doses of differently glycosylated murine IgG1 anti-TNP mAbs (clone H5; native=low-galactosylated (low-gal), in vitro galactosylated (gal) or in vitro galactosylated plus sialylated (sialylated; sial)) decreased IgE-mediated hypothermia in a FcγRIIb-dependent manner (Fig 1, B–E, and Fig E1).
Even though low-galactosylated IgG1 showed a tendency for more efficient inhibition (Fig 1, C; 3 µg of IgG1; not significant), possibly due to its higher affinity than sialylated IgG1 for FcγRIIb16, the IgG glycosylation pattern (Fig 1, D and E) and IgG subclass (studied by comparing IgG1, IgG2a and IgG2b anti-TNP class switch variant (sv) mAbs with identical V(D)J sequences)E20 (Fig E1, G and data not shown) had only a slight effect on extent of inhibition.
In contrast, the severity of IgG-mediated systemic anaphylaxis, which required challenge with a higher antigen dose (20 µg)2,3, was IgG subclass- and glycosylation-dependent (Fig 1, G and H, and Fig E1). De-sialylated plus de-galactosylated (de-gal) IgG2a and IgG2b subclass anti-TNP (sv) mAbs induced more severe anaphylaxis than de-galactosylated (sv) and low-galactosylated (H5) IgG1 mAbs (IgG2a=IgG2b>IgG1) (Fig E1)4.
IgG1-mediated anaphylaxis was inhibited by galactosylation and especially by additional sialylation (Fig 1, G); sialylation also significantly reduced the anaphylaxis potential of IgG2b and tended to reduce that of IgG2a (Fig 1, G). Sialylation even reduced the increased anaphylaxis potential of IgG1 in FcγRIIb-deficient mice4 (Fig 1, H), suggesting the importance of additional/other inhibitory mechanisms of IgG1 sialylation, such as one dependent on the C-type lectin receptor, SignR1 (Fig 1, I)7,8,E17,18.
These observations suggest that AIT protocols that promote sialylation of human IgG4 might optimally limit the possibility of IgG-mediated systemic anaphylaxis in the presence of higher allergen doses.
To evaluate this assumption, we analyzed how conventional AIT with birch pollen extract and the adjuvant aluminium hydroxide (alum) (ALK-depot SQ from ALK-Abelló) affects the IgG subclass and glycosylation of anti-Bet v 1 (Betula verrucosa 1; the major birch pollen allergen) Abs (Fig E4)9,E21–23.
In untreated patients, Bet v 1-specific IgG4 titers were constantly low, while IgE but also IgG1 titers increased uring the pollen season (Fig 2, A, and Fig E2). In contrast, during AIT, levels of Bet v 1-specific IgG1 increased in the first 12 months but decreased afterwards, while Bet v 1-specific IgG4 titers persistantly increased (Fig 2, A, and Fig E2)1,9,E21,22,24.
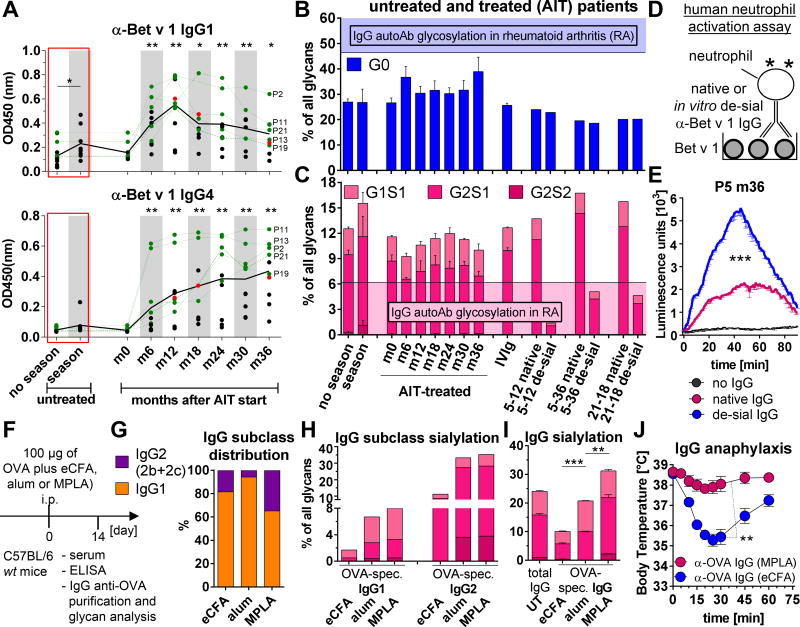
FIG 2. Bet v 1-specific serum IgG Fc glycosylation of untreated and treated allergic patients and influence of different adjuvants on IgG Fc glycosylation.
A, Serum titers of Bet v 1-specific IgG1 and IgG4 from untreated (season, n=8; no season, n=6 + 11 (AIT-treated, month (m) 0)) and AIT-treated (n=11) birch pollen allergic patients; black line: mean; gray: pollen season. The green data points depict the 5 AIT-treated patients who were selected for the glycan analysis in B and C and Fig E2, while the red data points depict the 3 samples (patient 5 at m12 (5–12) and 5–36 and 21-18) that were chosen for in vitro de-sialylation and neutrophil activation in B–E and Fig E2. One of two independent ELISAs is shown. B and C, Percentage of (B) agalactosylated (G0) and (C) sialylated glycans from purified Bet v 1-specific IgG Abs of untreated (season, n=5; no season, n=5 + 5 (AIT-treated, m0) and the 5 selected AIT-treated patients and from IVIg and purified native and in vitro de-sialylated total serum IgG from patient samples 5–12, 5–36 and 21-18. The filled areas indicate the levels of agalactosylated (G0) or sialylated IgG autoAbs in RA patients for comparisonE14. D and E, Human neutrophil activation assay. (D) Experimental setup and (E) ROS production after activation with native or in vitro de-sialylated Bet v 1-specific IgG Abs of patient 5 (P5 m36); no IgG (black). One of two independent ROS assays is shown. F–J, The effect of distinct adjuvants (eCFA, alum or MPLA) on the induction of OVA-specific serum IgG Abs. F, Experimental design. G, IgG1 and IgG2 (IgG2b+IgG2c; both cannot be distinguished by glycopeptide analysis because of the comparable peptide sequence) frequencies in purified OVA-specific IgG Abs as determined by glycopeptide analysis. H and I, (H) IgG1 and IgG2 or (I) total IgG Fc sialylation profiles of pooled and purified OVA-specific IgG Abs as determined by (H) glycopeptide or (I) total IgG glycan analysis. J, IgG-mediated anaphylaxis as described in Fig 1, F with 100 µg of pooled and purified OVA-specific serum IgG Abs; n=4–5 per group. Symbols represent means.
However, the Fc glycosylation profile of Bet v 1-specific serum IgG Abs from untreated and AIT-treated patients remained stable and were more highly galactosylated and sialylated than IgG autoAbs from rheumatoid arthritis (RA) patientsE14 (Fig 2, B and C, and Fig E2). The glycosylation profiles of the AIT-treated patients resembled those of two recently described AIT patients who had received similar therapy with alum (Allergovit from Allergopharma)5 and of those in therapeutic IVIg, which have Fc sialylation-dependent anti-inflammatory properties (Fig 2, B and C, and Fig E2)E16–18.
Consistent with an inverse relationship between IgG sialylation and inflammatory potential, we found that de-sialylation of native Bet v 1-specific IgG from the serum of AIT-treated patients strongly increased its ability to activate neutrophils in vitro (Fig 2, B–E, and Fig E2).
These observations suggest that conventional AIT with alum induces sialylated IgG(4) Abs that probably have low potential to induce IgG-mediated allergic reactions.
However, studies remain required to assess how Fc glycosylation modulates the effector functions of human IgG1 and IgG4 and how new AIT protocols with distinct adjuvants1,E25–29, will influence the human IgG subclass distribution and Fc glycosylation pattern and consequently, the risk of IgG-mediated allergic reactions.
To initiate such studies, we compared the effects of enriched complete Freund’s adjuvant (eCFA; highly inflammatory), alum and Monophosphoryl Lipid A (MPLA; recently approved for AIT)1,6,E26,27 on IgG subclass and Fc glycosylation profiles in OVA-immunized mice (Fig 2, F, and Fig E3). eCFA induced the highest IgG titer (eCFA>MPLA=alum) (Fig E3, C), but all three immunizations induced predominantly IgG1 (alum/94%>eCFA/81%>MPLA/65%) followed by IgG2b and hardly IgG2c (IgG2 (IgG2b+IgG2c): MPLA/35%>eCFA/19%>alum/6%) (Fig 2, G, and Fig. E3, C), whose functions depend on galactosylation (only IgG1) and sialylation (IgG1 and at least in part IgG2b) (Fig 1, G).
In contrast to only small differences in Fc glycosylation pattern between human IgG subclasses in the same sample30,31, glyopeptide analysis confirmed that murine IgG2 (IgG2b and IgG2c) was, on average, much more highly galactosylated and sialylated than IgG1 (Fig 2, H, and Fig E3)32. Because alum and MPLA induced higher galactosylation and sialylation levels of both OVA-specific IgG1 and IgG2(b) than OVA-eCFA (Fig 2, H), MPLA, with the highest ratio of IgG2(b), induced the highest levels of total IgG galactosylation and sialylation as determined by HPLC glycan analysis (Fig 2, I, and Fig E3). Consistent, only 100 µg of purified OVA-specific IgG Abs from the OVA-eCFA group, but not from the OVA-MPLA group, induced IgG-mediated anaphylaxis (Fig 2, J).
Taken together, our data suggest that although IgG subclass and glycosylation pattern have relatively little effect on IgG Ab blocking of IgE-mediated anaphylaxis, increased sialylation of IgG(4) Abs should decrease the risk of IgG-induced anaphylaxis in the presence of high allergen doses. Accordingly, it seems advisable to select adjuvants for new AIT protocols1,E25,26 for their ability to promote sialylated IgG(4) Ab responses.
Supplementary Material
Acknowledgments
The murine IgG1, IgG2a and IgG2b anti-TNP hybridoma switch variants were a gift from Lucien Aarden (Amsterdam, Netherlands) and the murine IgG1 anti-TNP (clone H5) hybridoma cell line from Birgitta Heyman (Uppsala, Sweden).
This study was supported by the German Research foundation (grants nos. EH 221/8-1, International Research Training Group (iGRK) 1911, GRK 1727, CRU 303 and Excellence cluster 306 to M.E., iGRK1911 to F.D.F., as well as MO 2076/3-1, HE 1602/10-1, PF 344/3-1 and SFB/TR22 to C.M, M.H. and W.P.), the Else-Kröner-Fresenius Foundation (2014_A91 to M.E.), the U.S. Department of Veterans Affairs Merit Award to F.D.F., the NIH (R01 AI072040 to F.D.F., as well as GM103390 and GM107012 to K.W.M.), and the Food Allergy Research and Education (FARE) to F.D.F.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of potential conflict of interest:
Hansa Medical AB (HMAB) (www.hansamedical.com) holds patents for using EndoS as a treatment for antibody-mediated diseases. M.C. is listed as one of the inventors on these applications and has a royalty agreement with HMAB. Genovis AB (GAB) (www.genovis.com) holds patents for the biotechnological use of EndoS where M.C. is listed as an inventor. HMAB and GAB were not involved in any way in the design of the study, writing of the manuscript or the decision to publish.
The rest of the authors declare that they have no relevant conflicts of interest and no financial interests.
References
- 1.Akdis CA, Akdis M. Advances in allergen immunotherapy: aiming for complete tolerance to allergens. Sci Transl Med. 2015;7:280ps6. doi: 10.1126/scitranslmed.aaa7390. [DOI] [PubMed] [Google Scholar]
- 2.Finkelman FD, Khodoun MV, Strait R. Human IgE-independent systemic anaphylaxis. J Allergy Clin Immunol. 2016;137:1674–80. doi: 10.1016/j.jaci.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strait RT, Morris SC, Finkelman FD. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and Fc gamma RIIb cross-linking. J Clin Invest. 2006;116:833–41. doi: 10.1172/JCI25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beutier H, Gillis CM, Iannascoli B, Godon O, England P, Sibilano R, et al. IgG subclasses determine pathways of anaphylaxis in mice. J Allergy Clin Immunol. 2017;139:269–80. e7. doi: 10.1016/j.jaci.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oefner CM, Winkler A, Hess C, Lorenz AK, Holecska V, Huxdorf M, et al. Tolerance induction with T cell-dependent protein antigens induces regulatory sialylated IgGs. Journal of Allergy and Clinical Immunology. 2012;129:1647–55. doi: 10.1016/j.jaci.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 6.Hess C, Winkler A, Lorenz AK, Holecska V, Blanchard V, Eiglmeier S, et al. T cell-independent B cell activation induces immunosuppressive sialylated IgG antibodies. J Clin Invest. 2013;123:3788–96. doi: 10.1172/JCI65938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collin M, Ehlers M. The carbohydrate switch between pathogenic and immunosuppressive antigen-specific antibodies. Experimental dermatology. 2013;22:511–4. doi: 10.1111/exd.12171. [DOI] [PubMed] [Google Scholar]
- 8.Pincetic A, Bournazos S, DiLillo DJ, Maamary J, Wang TT, Dahan R, et al. Type I and type II Fc receptors regulate innate and adaptive immunity. Nat Immunol. 2014;15:707–16. doi: 10.1038/ni.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Möbs C, Ipsen H, Mayer L, Slotosch C, Petersen A, Würtzen PA, et al. Birch pollen immunotherapy results in long-term loss of Bet v 1-specific TH2 responses, transient TR1 activation, and synthesis of IgE-blocking antibodies. J Allergy Clin Immunol. 2012;130:1108–16. e6. doi: 10.1016/j.jaci.2012.07.056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.