Abstract
Background
Activation of cannabinoid CB1 receptors suppresses pathological pain but also produces unwanted central side effects. We hypothesized that a positive allosteric modulator (PAM) of CB1 signaling would suppress inflammatory and neuropathic pain without producing cannabimimetic effects or physical dependence. We also asked whether a CB1-PAM would synergize with inhibitors of endocannabinoid deactivation and/or an orthosteric cannabinoid agonist.
Methods
GAT211, a novel CB1-PAM, was evaluated for antinociceptive efficacy and tolerance in models of neuropathic and/or inflammatory pain. Cardinal signs of direct CB1-receptor activation were evaluated together with propensity to induce reward/aversion and physical dependence. Comparisons were made with inhibitors of endocannabinoid deactivation (JZL184, URB597) or an orthosteric cannabinoid agonist (WIN55,212-2). All studies used 5-11 subjects per group.
Results
GAT211 suppressed allodynia induced by complete Freund’s adjuvant (CFA) and the chemotherapeutic agent paclitaxel in wildtype but not CB1 knockout mice. GAT211 did not impede paclitaxel-induced tumor cell line toxicity. GAT211 did not produce cardinal signs of direct CB1-receptor activation in the presence or absence of pathological pain. GAT211 produced synergistic anti-allodynic effects with fatty-acid amide hydrolase (FAAH) and monoacylglycerol lipase (MGL) inhibitors in paclitaxel-treated mice. Therapeutic efficacy was preserved over 19 days of chronic dosing with GAT211 but not the MGL inhibitor JZL184. The CB1 antagonist rimonabant precipitated withdrawal in mice treated chronically with WIN55,212-2 but not GAT211. GAT211 did not induce condition place preference or aversion.
Conclusions
Positive allosteric modulation of CB1 receptor signaling shows promise as a safe and effective analgesic strategy that lacks tolerance, dependence and abuse liability.
Keywords: neuropathic pain, reward, withdrawal, physical dependence, allosteric modulator, endocannabinoid
Introduction
The endocannabinoid system, the body’s own cannabis-like system, consists of cannabinoid receptors (i.e. CB1, CB2), endocannabinoids (i.e. anandamide, 2-arachidonoylglycerol (2-AG)) and enzymes that catalyze endocannabinoid synthesis and degradation(1). Cannabinoid agonists such as Δ9-tetrahydrocannabinol (Δ9-THC) suppress pathological pain in preclinical studies(2-4) but also produce unwanted psychotropic effects that limit therapeutic use(5-7). Inhibitors of fatty-acid amide hydrolase (FAAH) and monoacylglycerol lipase (MGL), the major enzymes degrading anandamide and 2-AG, respectively, suppress pain in rodent models of traumatic nerve injury, toxic neuropathy(8, 9) and hyperglycemia(10) with efficacy comparable to or surpassing conventional treatments(9). However, FAAH and MGL degrade other lipids that do not bind to cannabinoid receptors(11, 12). Moreover, MGL inhibition can produce tolerance and CB1-dependent withdrawal (13, 14). Thus, alternate strategies that maximize and selectively amplify the therapeutic potential of CB1 receptor signaling without deleterious consequences are needed.
Allosteric binding site(s) distinct from the classical (orthosteric) CB1 binding site have been identified(15-20). Binding of a pure positive allosteric modulator (PAM) to an allosteric site increases the affinity of the endogenous ligand for the orthosteric binding site and/or enhances the efficiency of signaling by the endogenous ligand(21, 22). By amplifying ongoing signaling by endogenous agonists, allosteric modulators may produce a more optimal and circumscribed spectrum of physiological effects compared to indiscriminate activation of cannabinoid receptors. Both negative (Org27596, Org29647(20), pregnenolone(23), PSNCBAM-1(24, 25), PEPCAN12(25), cannabidiol (26)) and positive (ZCZ011(18), lipoxin A4(27), GAT211(17)) allosteric modulators of CB1 receptor signaling have been described. Lipoxin A4 is an endogenous CB1 positive allosteric modulator (PAM) that produces cardinal signs of CB1 activation following intracerebroventricular administration(27, 28). ZCZ011 exhibits antinociceptive efficacy that is preserved following six days of repeated dosing while avoiding cardinal signs of CB1 receptor activation(18). Thus, CB1 PAMs represent a promising therapeutic strategy.
We synthesized GAT211 (Supplemental Figure 1), a novel PAM of CB1 receptor signaling(17). Unlike orthosteric CB1 ligands, GAT211 does not displace [3H]CP55,940 bound to human CB1 receptors or CB1 in mouse brain membranes(17). However, GAT211 slows dissociation kinetics of [3H]CP55,940 binding, consistent with allosteric modulation of CB1 signaling(17). The in vivo profile of GAT211 has not previously been described. We compared antinociceptive efficacy of GAT211 with an orthosteric cannabinoid agonist (WIN55,212-2) and inhibitors of endocannabinoid deactivation targeting FAAH (URB597) or MGL (JZL184) using inflammatory and neuropathic pain models. We asked whether GAT211 would synergize with the CB1 orthosteric agonist and also FAAH and MGL inhibitors. We evaluated propensity of GAT211 to induce CB1-mediated cannabimimetic effects and physical dependence in the presence and absence of pathological pain. We also examined the impact of GAT211 on tetrad behaviors in FAAH and MGL KO mice exhibiting elevated endocannabinoid tone. We evaluated whether chronic dosing with GAT211 would induce physical dependence by precipitating a withdrawal syndrome with a CB1 antagonist. Finally, we evaluated whether GAT211 would exhibit rewarding or aversive properties.
Methods
Subjects
Adult male mice (~7-8 weeks of age) on C57BL/6J, and CD1 strains were used. CB1KO mice on a CD1 background and FAAH knockout (FAAH KO) and MGL knockout (MGL KO) mice, both on a C57 background, were also used. Mice were single housed prior to experimental manipulations. Mice were maintained on a 12 h light/12 h dark cycle (lights from 7am to 7pm) in a temperature-controlled facility with food and water ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) and followed the guidelines for the treatment of animals of the International Association for the Study of Pain(29).
Materials
GAT211 was synthesized by the authors (by PMK and GT(30)). Paclitaxel (Tecoland Corporation, Edison, NJ, USA), complete Freund’s adjuvant (CFA; Sigma Aldrich, St. Louis, MO), WIN55,212-2, AM251, AM630 and URB597 (all from Cayman Chemical Company, Ann Arbor, MI), rimonabant (National Institute on Drug Abuse; Bethesda, Maryland) and JZL184 (MedChem101, Plymouth Meeting, PA) were used. Drugs were dissolved in 20% DMSO with 80% ethanol: emulphor: saline in a 1:1:8 ratio and administered via intraperitoneal (i.p.) injection in a volume of 5mL/kg. For in vitro studies, L-glutamine, fetal bovine serum, penicillin streptomycin and Dulbecco’s Modified Eagle Medium (DMEM) were from GIBCO (Grand Island, NY, USA). Dimethyl sulfoxide (DMSO) was from Sigma-Aldrich. 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl-2H-tetrazolium bromide (MTT) was from Promega (Madison, WI). HeyA8 cells were a gift from Dr. Kenneth Nephew.
General Behavioral Experimental Protocol
All behavioral experiments were conducted by an experimenter (RAS) blinded to genotype and treatment condition. Mice were randomly assigned to experimental conditions. See Supplement 1 for detailed methods.
Allodynia
Paw withdrawal thresholds (g) to mechanical stimulation were measured in duplicate in each paw using an electronic von Frey anesthesiometer (IITC model Alemo 2390–5, Woodland Hills, CA) as described previously(31). Duration of hypersensitivity to cold stimulation was measured using the acetone method in the same animals used to assess mechanical hypersensitivity (32).
CFA-induced inflammatory nociception
CFA (diluted 1:1 in saline) was administered (20 μl) via unilateral intraplantar (i.pl.) injection in the hindpaw with a 28 ½ gauge needle(33). Approximately 48h later, escalating doses of GAT211, WIN55,212-2 or a combination based upon the ED50 ratios of each drug (GAT211+WIN55,212-2) was administered (i.p.) when allodynia was stable.
Paclitaxel-induced neuropathic pain
Paclitaxel (4mg/kg i.p.) was dissolved in vehicle containing 5% cremophor-EL (Sigma-Aldrich), 5% ethanol, 90% saline. Paclitaxel or cremophor-based vehicle was injected (i.p.) on day 0, 2, 4 and 6(6, 33).
Tolerance in comparison to orthosteric cannabinoid agonist
GAT211 (5, 10, 20mg/kg/day i.p. × 9 days) or vehicle was administered once daily to naïve and paclitaxel-treated mice. Separate naive groups received the orthosteric cannabinoid agonist WIN55,212-2 (3mg/kg/day i.p. × 9 days). Responses to mechanical and cold stimulation were recorded before and 30 min following pharmacological manipulations. On day 9, mice were challenged with rimonabant (10mg/kg i.p.) to precipitate CB1 receptor-dependent withdrawal.
Pharmacological specificity
GAT211 (20mg/kg i.p. × 8 days) was administered once daily alone or co-administered with either the CB1 antagonist AM251 (5mg/kg i.p. × 8 days) or the CB2 antagonist AM630 (5mg/kg i.p. × 8 days) (See supplemental material). To confirm mediation by CB1, GAT211 (20mg/kg i.p. × 8 days) or vehicle was administered to paclitaxel-treated CB1 KO mice.
Antinociceptive synergism with endocannabinoid deactivation inhibitors
Dose-response curves for GAT211, URB597 and JZL184 in suppressing paclitaxel-induced allodynia were generated. ED50 values derived for each drug were used to administer 1:1 combination ratios of GAT211 with either URB597 or JZL184. Combination ED50 values were determined in a separate set of otherwise drug naïve paclitaxel-treated mice and analyzed as described below.
Tolerance in comparison to MGL inhibitor
GAT211 (20mg/kg/day i.p.), JZL184 (16mg/kg/day i.p) or vehicle was administered once daily over 20 consecutive days to paclitaxel-treated mice. Responses to mechanical and cold stimulation were recorded over 19 days. On day 20, mice were challenged with vehicle or rimonabant to evaluate possible CB1-dependent withdrawal behaviors.
Cardinal signs of CB1 activation
Naïve mice receiving GAT211 (20mg/kg i.p. daily × 8 days), WIN55,212-2 (3mg/kg i.p. × 8 days) or vehicle were evaluated for mechanical and cold responsiveness on days 2, 4, and 7 of chronic dosing. On day 9, mice were challenged with rimonabant (10mg/kg i.p.) to precipitate CB1-dependent withdrawal (6).
Tetrad behaviors
Impact of GAT211(20mg/kg i.p.) was evaluated in a modified tetrad as previously described(6) using WT, FAAH and MGL KO mice (34):
Rotarod test
An accelerating rotarod was used to measure impact of GAT211 on motor coordination in WT, FAAH and MGL KO mice(6, 32, 35).
Rectal temperature
Rectal temperature (°C) was measured using a thermometer (Physitemp Instruments, Inc, Clifton, NJ) and rectal probe (Braintree Laboratories, Inc, Braintree, MA).
Tail-Flick antinociception
The latency for the mouse to withdraw its tail from a hot water bath (52°C) was used to assess tail-flick antinociception.
Ring test
The amount of time the mouse spent immobile (minus respiratory movements) on an elevated wire ring (6.35cm diameter wire ring suspended 16cm above a flat platform) was recorded over 5 minutes to assess catalepsy(36).
CB1-dependent withdrawal symptoms
Mice were challenged with vehicle or rimonabant (10mg/kg i.p.) to determine whether allosteric modulation of CB1 receptor signaling with GAT211 would elicit a CB1-dependent withdrawal syndrome.
Conditioned place testing
We evaluated whether GAT211 produced rewarding/aversive properties following repeated pairings using a three chamber conditioned place preference apparatus(37-39).
Cell culture
Human ovarian cancer HeyA8 cells were grown in T75 flasks containing DMEM medium containing penicillin-streptomycin (100units/mL-100μg/mL), L-glutamine (4mM) and 10% fetal bovine serum (v/v) at 37°C under 5% CO2 in humidified incubator conditions. Every third day cells were passaged and confluent cells were obtained for use in the cytotoxicity assay.
MTT cytotoxicity assay
Cultured HeyA8 ovarian tumor cells were used to assess impact of GAT211 on paclitaxel-induced reductions in tumor cell viability (by ZX). Viable cells are capable of reducing 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to insoluble formazan, which can be detected in a colorimetric assay for assessing cell metabolic activity(32, 40, 41).
Statistical analyses
Data were analyzed by repeated measures analysis of variance (ANOVA), one-way ANOVA followed by Bonferroni post hoc tests and planned comparison or paired t-tests, as appropriate. All statistical analyses were performed using IBM-SPSS Statistics version 19.0 (SPSS Inc., an IBM company, Chicago, IL, USA) or Graphpad Prism version 5.02 for Windows (GraphPad Software, San Diego CA, USA, www.graphpad.com). p<0.05 was considered significant.
Isobolographic analysis(42, 43) was performed to evaluate the impact of dose-combinations of GAT211 with WIN55,212-2, JZL184, or URB597 on either CFA or paclitaxel-induced allodynia.
Results
GAT211 suppresses CFA-induced mechanical hypersensitivity in a CB1-dependent manner that is comparable to the orthosteric agonist WIN55,212-2
Intraplantar CFA produced mechanical hypersensitivity in the injected (ipsilateral) paw (p<0.001) relative to baseline (pre-CFA) responding. Both GAT211 and WIN55,212-2 dose-dependently reduced CFA-induced hypersensitivity with ED50s (95% confidence intervals) of 9.75 (5.78–13.72) mg/kg and 1.07 (0.09-1.42) mg/kg i.p. (Figure 1A). CB1 KO mice exhibited CFA-induced hypersensitivity comparable to WT animals (p>0.786) (Figure 1A). In CB1 KO mice, the highest efficacious dose of GAT211 (30mg/kg i.p) failed to alter CFA-induced mechanical allodynia (p>0.05) (Figure 1A).
Figure 1. GAT211 produces dose-dependent anti-allodynic effects in CFA-treated mice.
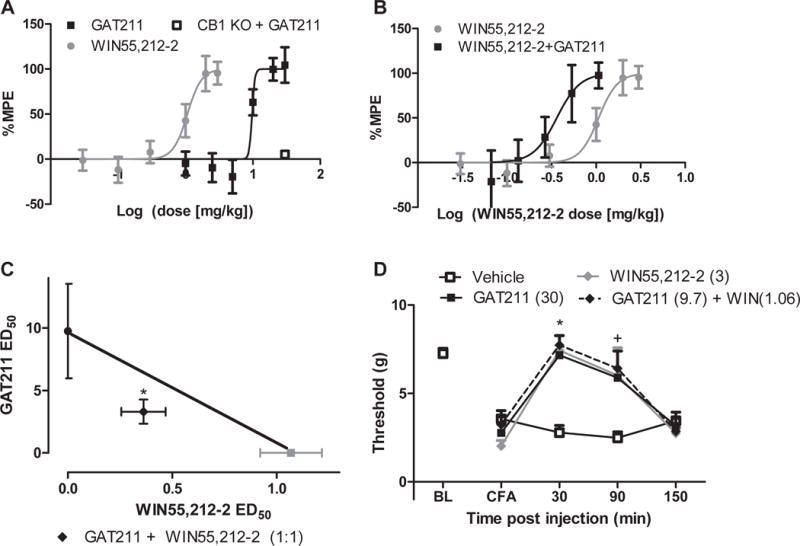
(A) GAT211 (1, 2.5, 5, 10, 20, 30 mg/kg i.p.), WIN55,212-2 (0.01, 0.03, 0.1, 0.3, 1, 2, 3 mg/kg i.p.) suppressed CFA-induced mechanical allodynia. GAT211 (30 mg/kg i.p.) was devoid of anti-allodynic efficacy in CFA-treated CB1 KO mice. (B) When administered in a 1:1 combination based on ED50 values, GAT211 shifted the dose-response curve of WIN55,212-2 leftward. (C) GAT211 synergized with the orthosteric cannabinoid agonist WIN55,212-2. (D) Timecourse of anti-allodynic efficacy of active GAT211 (30 mg/kg i.p.), WIN55,212-2 (3 mg/kg i.p.) and combination (GAT211 (9.7 mg/kg i.p.) + WIN55,212-2 (1.06 mg/kg i.p.) relative to vehicle. Figure legend shows dose administered for each compound (mg/kg i.p.). Data are expressed as mean ±SEM (n = 6 per group). #P<0.05 vs. pre-paclitaxel baseline, repeated measures ANOVA. *P<0.05 all groups vs. vehicle, +P<0.05 GAT211 20 mg/kg i.p. and 30 mg/kg i.p. vs. vehicle two-way ANOVA followed by Bonferroni post hoc test.
GAT211 synergized with WIN55,212-2 to reduce CFA-induced mechanical allodynia. The observed combination ED50 (95% confidence intervals) of 3.67 (3.25–4.08)mg/kg i.p.) was lower (p<0.02, two-tailed t-test) than the theoretical additive ED50 (5.41 (4.38–6.44) mg/kg i.p.) (Figure 1C). GAT211 shifted the ED50 of WIN55,212-2 leftward (0.31 (0.2–0.66) mg/kg i.p.) (Figure 1B). Only the highest combination dose (GAT211 (9.75mg/kg i.p.)+WIN55,212-2 (1.07mg/kg i.p.) decreased body temperature (p<0.05 vs. vehicle; Supplemental Figure 2B). WIN55,212-2 (2 and 3mg/kg i.p.) lowered body temperature (p<0.05 vs. vehicle; Supplemental Figure 2A) whereas GAT211 failed to do so following acute or chronic dosing (Supplemental Figure 3).
GAT211, WIN55,212-2, and GAT211+WIN55,212-2 in combination produced a time-dependent [F3,20 = 22.46, p<0.0001] reduction in CFA-induced mechanical hypersensitivity. All treatments reduced CFA-induced mechanical hypersensitivity at 30 (p<0.01 vs. vehicle) and 90 (p<0.05 vs. vehicle) minutes post-injection (Figure 1D). None of the treatments showed antinociceptive efficacy 150 minutes post-injection (p>0.05 vs. vehicle).
Effects of GAT211 in naïve and paclitaxel-treated WT mice
Paclitaxel produced behavioral hypersensitivities to mechanical [F3, 8=146.9, p<0.001] and cold [F3, 8=41.41, p<0.001] (Figure 2A,B) stimulation. In paclitaxel-treated WT mice, GAT211 (10 and 20mg/kg/day i.p.) suppressed mechanical [F3, 17=10.336, p<0.02] and cold [F3, 17=7.267, p<0.01] allodynia relative to vehicle (Figure 2C,D). GAT211 (5mg/kg/day i.p.) did not alter paclitaxel-induced mechanical (p=1.00) or cold (p=1.00) allodynia.
Figure 2. Chronic dosing with GAT211 reverses hypersensitivity to mechanical and cold stimulation induced by paclitaxel.
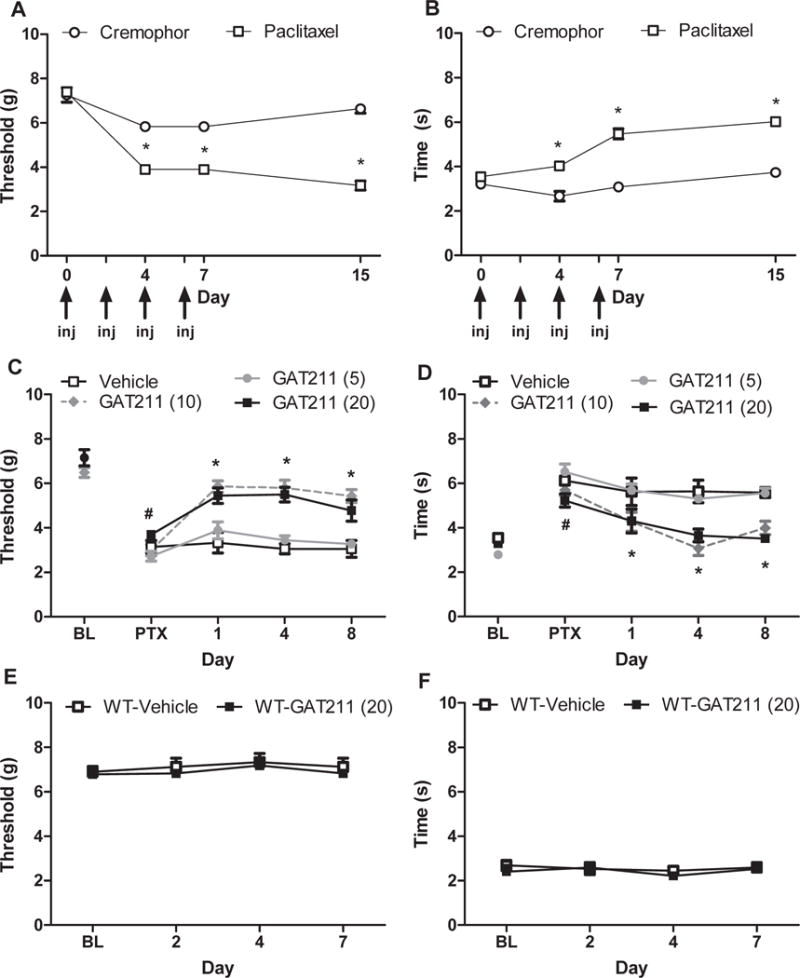
Paclitaxel, but not its cremophor-based vehicle, induces hypersensitivities to (A) mechanical and (B) cold stimulation. GAT211 (10 and 20 mg/kg i.p.) suppressed paclitaxel-induced (B) mechanical and (C) cold allodynia relative to vehicle over an 8 day once daily dosing interval whereas GAT211 (5 mg/kg i.p.) was ineffective. (E,F) GAT211 (20 mg/kg i.p. × 8 days) did not alter baseline thresholds to either (E) mechanical or (F) cold stimulation over the same time course. Arrows show timing of paclitaxel or cremophor vehicle injection (inj). Figure legend shows dose administered for each compound (mg/kg i.p.). Data are expressed as mean ± SEM (n = 5-6 per group). *P<0.05 vs. vehicle or cremophor, one-way ANOVA followed by Bonferroni post hoc test. #P<0.05 vs. pre-paclitaxel baseline, repeated measures ANOVA.
GAT211 (20mg/kg/day i.p. × 8 days) did not alter baseline responses to mechanical (p>0.51) (Figure 2E) or cold (p>0.22) stimulation (Figure 2F) compared to vehicle on any test day in naïve C57 or CD1 WT mice.
Anti-allodynic effects of GAT211 are mediated by CB1 receptors
In paclitaxel-treated WT mice, GAT211 (20mg/kg/day i.p. × 8 days)-induced suppressions of mechanical [F3, 17=10.336, p<0.02] and cold [F3, 17=7.267, p<0.01] allodynia (Figure 3A,B) were blocked by AM251 (5mg/kg/day i.p.) at all-time points (p<0.0001 vs. GAT211). AM630 (5mg/kg/day i.p.) did not alter the anti-allodynic efficacy of GAT211 for either stimulus modality (p=1.00 mechanical, p=1.00 cold vs. GAT211 20mg/kg i.p.) (Figure 3A,B). The same dose of AM630 blocked anti-allodynic effects of a CB2 agonist in paclitaxel-treated mice in our previous work(6).
Figure 3. GAT211-induced a CB1-mediated suppression of paclitaxel-induced neuropathic pain.
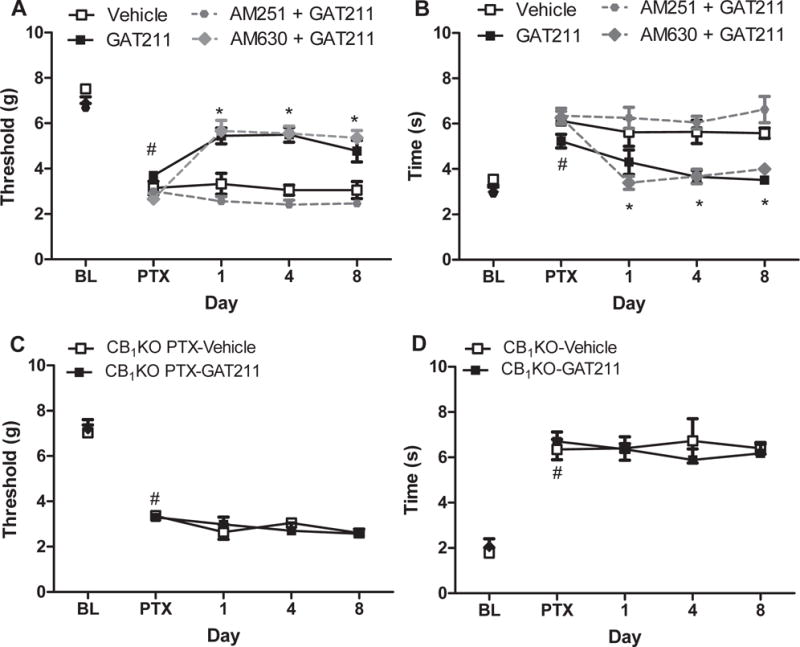
The GAT211-induced suppression of paclitaxel-induced (A) mechanical and (B) cold hypersensitivity was blocked by the CB1 receptor antagonist AM251 (5 mg/kg i.p.) but not by the CB2 antagonist AM630 (5 mg/kg i.p.). (C,D) GAT211 (20 mg/kg i.p.) did not suppress paclitaxel-induced (C) mechanical or (D) cold hypersensitivities in mice lacking CB1 receptors (CB1 KO). Data are expressed as mean ± SEM (n=5–6 per group). *P<0.05 vs. vehicle, one-way ANOVA followed by Bonferroni post hoc test. #P<0.05 vs. pre-paclitaxel baseline, repeated measures ANOVA.
GAT211 did not suppress paclitaxel-induced mechanical (p>0.42) or cold (p>0.16) allodynia in CB1 KO mice (Figure 3C,D).
GAT211 synergizes with inhibitors of endocannabinoid deactivation
GAT211 dose-dependently reduced paclitaxel-induced hypersensitivity to mechanical and cold stimulation (Figure 4A,B). GAT211 suppressed chemotherapy-induced mechanical and cold allodynia with ED50s (95% confidence intervals) of 11.35 (8.66–14.88) and 9.90 (9.47–10.33) mg/kg i.p., respectively (Figure 4A,B). GAT211 synergized with JZL184 to suppress mechanical and cold allodynia (Figure 5A,B). The combination of GAT211+ JZL184 suppressed paclitaxel-induced mechanical allodynia with an observed ED50 of 6.09 (3.94–9.42) mg/kg i.p. that was lower (p<0.05, two-tailed t-test) than the theoretical additive value of the combination [ED50 (95% confidence intervals) of GAT211+JZL184: 10.27 (7.52–13.02) mg/kg i.p. (Figure 5A and Table 1)]. GAT211 + JZL184 similarly suppressed cold allodynia with an observed ED50 (8.17 (6.97–9.58) mg/kg) that was lower than the theoretical additive value (12.04 (10.73–13.35) mg/kg i.p.) of the combination (p<0.05, two-tailed t-test), consistent with antinociceptive synergism (Figure 5B and Table 1).
Figure 4. Dose response of GAT211 and inhibitors of endocannabinoid deactivation URB597 and JZL184 in suppressing paclitaxel-induced neuropathic pain.
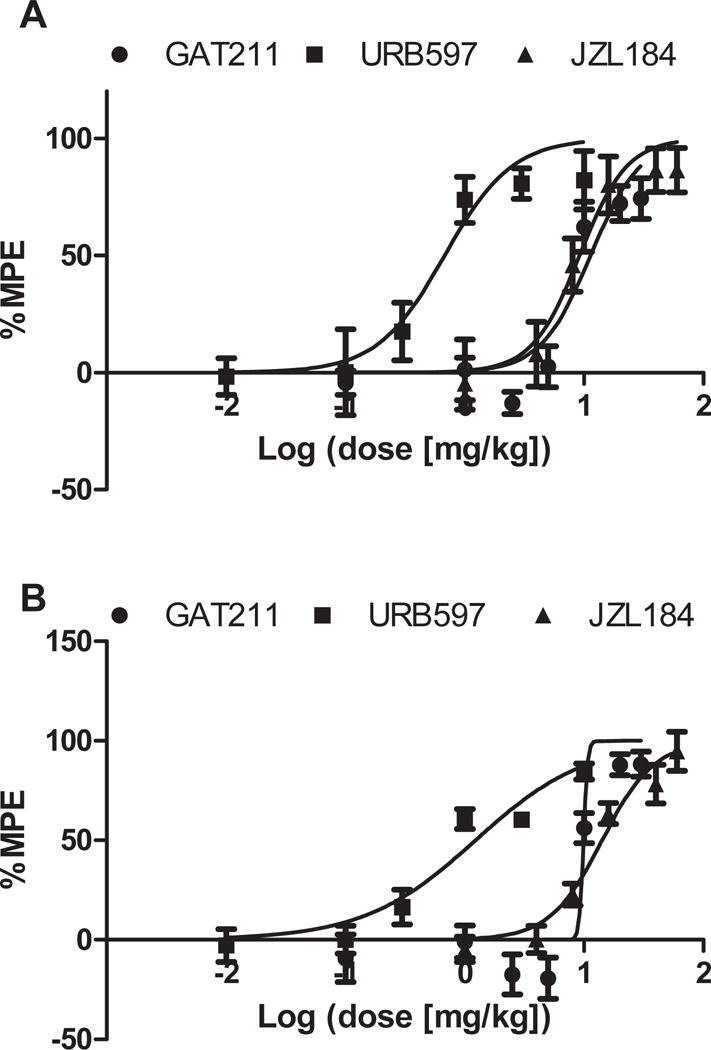
GAT211 (0.1, 1, 2.5, 5, 10, 20, 30 mg/kg i.p.), the FAAH inhibitor URB597 (0.01, 0.1, 0.3, 1, 3, 10 mg/kg i.p.) and the MGL inhibitor JZL184 (1, 4, 8, 16, 40, 60 mg/kg i.p.) attenuated paclitaxel-induced (A) mechanical and (B) cold allodynia in a dose-dependent manner. Data are expressed as mean± SEM (n = 5-6 per group)
Figure 5. The CB1 positive allosteric modulator GAT211 produces synergistic anti-allodynic effects with inhibitors of MGL or FAAH.
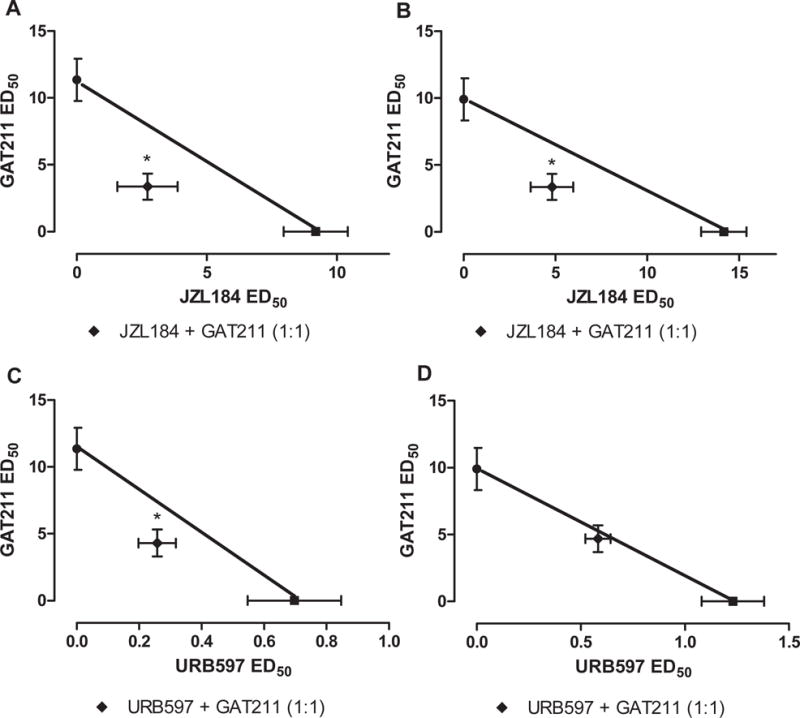
GAT211 synergized with (A) the MGL inhibitor JZL184 in suppressing paclitaxel-induced mechanical and (B) cold allodynia. GAT211 produced (C) synergistic antinociceptive effects with the FAAH inhibitor URB597 in suppressing paclitaxel-induced mechanical allodynia but was (D) only additive in suppressing paclitaxel-induced cold allodynia. Data are expressed as mean ED50 ± SEM (n = 6 per group) *P<0.05 vs. theoretical additive values, two-tailed t-test using Welch’s correction.
Table 1.
Isobolographic analysis reveals antinociceptive synergism of combinations of GAT211 with FAAH and MGL inhibitors in a paclitaxel model of neuropathic pain
| Combination with GAT211 | |||
|---|---|---|---|
|
| |||
| Compound | Modality | Theoretical | Experimental |
| JZL184 | Mechanical | 10.27 (7.52 – 13.02) | 6.09 (3.94 – 9.42)* |
| Cold | 12.04 (10.73 – 13.35) | 8.17 (6.96 – 9.58)* | |
|
| |||
| URB597 | Mechanical | 6.01 (5.08 – 7.74) | 4.56 (3.38 – 6.16)* |
| Cold | 5.57 (5.39 – 5.74) | 5.27 (4.50 – 6.17) | |
Mean ED50 values (with 95 % confidence intervals) mg/kg i.p.
p < 0.05 vs. theoretical ED50
URB597 dose-dependently suppressed paclitaxel-induced mechanical (ED50 (95% confidence interval): 0.68 (0.4049–1.141) and cold (ED50 (95% confidence interval): 1.23 (0.8109–1.867)mg/kg) allodynia (Figure 5A,B). Isobolographic analysis revealed that GAT211 synergized with URB597 in suppressing mechanical allodynia (Figure 5C). GAT211 + URB597 suppressed mechanical allodynia with an observed ED50 (ED50: 4.56 (3.38–6.16) mg/kg) that was lower (p<0.05 for each comparison, two-tailed t-test) than the theoretical additive value of the combination (ED50: 6.01 (5.08–7.74) mg/kg) (Figure 5C and Table 1). By contrast, GAT211 + URB597 produced an additive anti-allodynic effect in suppressing cold allodynia (Figure 5D). The observed ED50 (5.27 (4.50–6.17) mg/kg i.p.) of the combination of GAT211+URB597 did not differ from the theoretical additive value for the combination (5.57 (5.393–5.742) mg/kg i.p.) in suppressing cold allodynia (p>0.05 two-tailed t-test; Figure 5D). Theoretical and observed ED50s for combination treatments are summarized in Table 1.
Antinociceptive tolerance develops following chronic MGL inhibition in paclitaxel-treated mice
Both GAT211 and JZL184 reduced paclitaxel-induced hypersensitivities to mechanical [F14,105=8.019, p<0.0001] and cold [F14,105 =29.028, p<0.0001] stimulation. JZL184 displayed initial efficacy in reducing mechanical (p<0.001; day 1 vs. vehicle) and cold (p<0.001; day 1 vs. vehicle) allodynia (Figure 6A,B). However, complete tolerance developed to the anti-allodynic efficacy of JZL184 (mechanical: p=0.118, day 8 vs. vehicle; cold: p=1.00, day 8 vs. vehicle) by day 8 of repeated dosing and JZL184 remained ineffective throughout the remainder of the chronic dosing period. By contrast, GAT211 exhibited initial anti-allodynic efficacy upon acute administration (mechanical: p<0.001; vs. cold: p<0.001; day 1 vs. vehicle) and remained fully effective with no loss of antinociceptive efficacy throughout the 19 day chronic dosing period (mechanical: p<0.01; cold: p<0.001; vs. vehicle for all days) (p>0.573 GAT211 (20mg/kg); day 1 mechanical vs. day 19, p>0.374 GAT211 (20mg/kg); day 1 cold vs. day 19) (Figure 6A,B).
Figure 6. The CB1 positive allosteric modulator GAT211 does not produce tolerance typical of MGL inhibitor JZL184 in paclitaxel-treated mice or induce physical dependence.
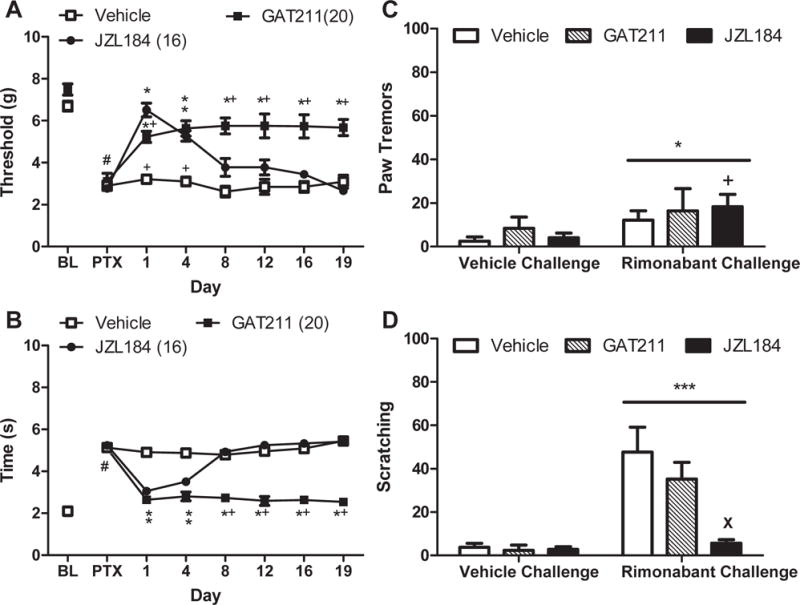
GAT211 (20 mg/kg i.p. × 19 days) suppressed paclitaxel-induced (A) mechanical and (B) cold allodynia throughout the entire 19 day dosing period relative to vehicle. JZL184 (16 mg/kg × 19 days i.p.) suppressed paclitaxel-induced (A) mechanical and (B) cold allodynia but tolerance developed by day 8 of chronic dosing. When challenged with vehicle, mice treated chronically with GAT211 (20 mg/kg/day i.p. × 20 days) or JZL184 (16 mg/kg/day i.p. × 20 days) did not exhibit (C) paw tremors or (D) scratching behaviors. (C,D) Rimonabant challenge did not induce withdrawal behaviors in either GAT211-treated or vehicle-treated mice but reduced the numbers of scratching bouts in mice treated with JZL184. Data are expressed as mean ± SEM (n=4-6 per group). (A),(B)*P<0.05 vs. vehicle, +P<0.05 vs. JZL184 one-way ANOVA followed by Bonferroni post hoc test. #P<0.05 vs. pre-paclitaxel baseline, repeated measures ANOVA. (C),(D) *P<0.05 vs. vehicle challenge, two-way ANOVA. +P<0.05 JZL184 Rimonabant Challenge vs. JZL184 vehicle challenge, paired t-test. XP<0.05 vs vehicle, two-way ANOVA followed by Bonferroni post-hoc test.
GAT211 does not elicit cardinal signs of CB1 receptor activation across 19 days of chronic dosing
Neither GAT211 (20mg/kg/day i.p. ×19 days) nor JZL184 (16mg/kg/day i.p. ×19 days) altered rotarod performance (p>0.7; JZL184:p>0.1, GAT211:p>0.41) or body temperature (p>0.5; JZL184:p>0.08; GAT211:p>0.6 for all days) relative to vehicle (Supplemental Figure 3) in the same mice evaluated for anti-allodynic efficacy. However, pharmacological manipulations altered immobility time in the ring test [F2,15=4.330, p<0.034], although post hoc comparisons did not reach statistical significance (p>0.1 for each comparison). Planned comparisons confirmed that JZL184 produced a modest but reliable increase in immobility time vs. vehicle across the observation interval [F1,10=5.508, p<0.05] whereas GAT211 (p>0.4) failed to do so (Supplemental Figure 3).
Challenge with a CB1 antagonist does not elicit a prototypic cannabinoid withdrawal syndrome in mice treated chronically with GAT211: comparison with JZL184
Rimonabant challenge increased the number of paw tremors relative to vehicle challenge (F1,14=6.773, p<0.05), but there was no main effect of treatment (p>0.60) or interaction (p>0.80). Planned comparisons nonetheless revealed that JZL184 (p<0.05) but not GAT211 (p>0.50) or vehicle (p>0.05) increased paw tremors following rimonabant challenge relative to vehicle challenge (Figure 6C). Rimonabant challenge increased scratching relative to vehicle challenge (F1,14 =32.50, p<0.0001; Figure 6D) in a treatment-dependent manner (F2,14 =6.800, p<0.01), and the interaction was significant (F2,14 =7.373, p<0.01). Post-hoc analysis revealed that chronic JZL184 treatment reduced rimonabant-induced scratching relative to chronic vehicle or GAT211 treatment (p<0.01 for all comparisons). GAT211 did not differ from either group (p>0.05 for all comparisons)(Figure 6D).
GAT211 does not reduce tumor cell viability in vitro
Paclitaxel reduced HeyA8 tumor cell viability in concentrations ranging from 0.1 nM to 100 nM [F6,105=42.17; p<0.0001] (p<0.05 vs. basal for all comparisons) (Figure 7A). GAT211 (20 μM) (p>0.05 vs. paclitaxel) alone did not alter tumor cell viability and did not impede the ability of paclitaxel (20 nM) to reduce tumor cell viability (p<0.001 vs. basal) in HeyA8 cells (Figure 7B).
Figure 7. Effects of GAT211 and paclitaxel on ovarian tumor cell viability.
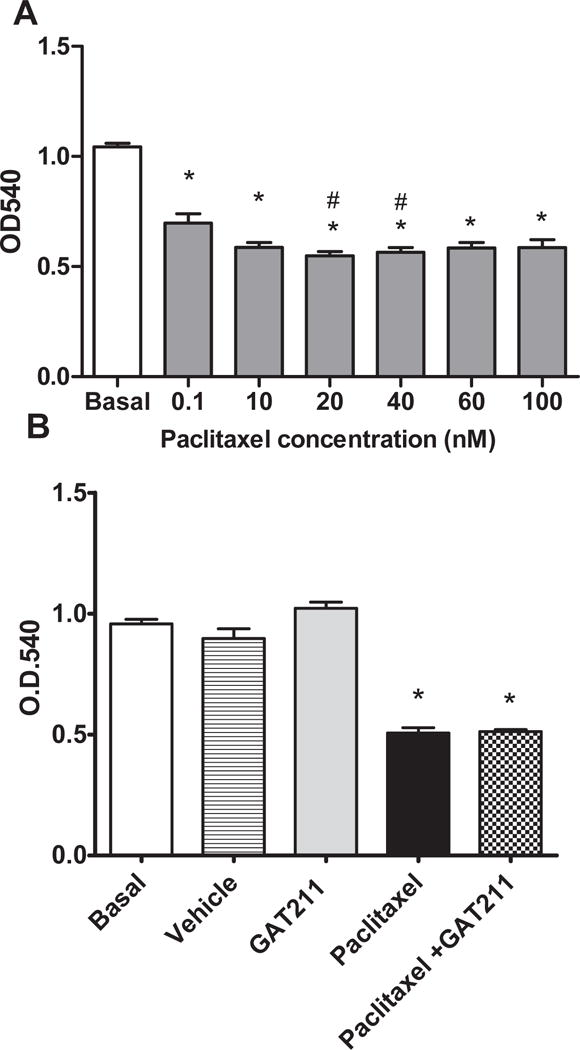
(A) Paclitaxel reduces HeyA8 tumor cell viability in MTT assay. (B) GAT211 (20 μM) alone did not alter tumor cell viability and did not impede ability of paclitaxel (20 nM) to reduce tumor cell viability in HeyA8 cells. Values are means ± SEM of 8-16 replicates per determination. Effects of GAT211shown are representative of three independent assays. *P<0.05 vs. basal, #P<0.05 vs. 0.01nM paclitaxel, one-way ANOVA followed by Bonferroni post hoc test.
GAT211 increases immobility time in MGL and FAAH KO mice
In MGL KO mice, GAT211 (20mg/kg/i.p.) increased immobility time (p<0.05) (Figure 8A,B) but did not affect motor performance (p>0.36), tail flick latencies (p>0.82), or body temperature (p>0.12) compared to vehicle (Figure 8D-F).
Figure 8. GAT211 increases immobility time in MGL and FAAH KO mice but not in WT mice.
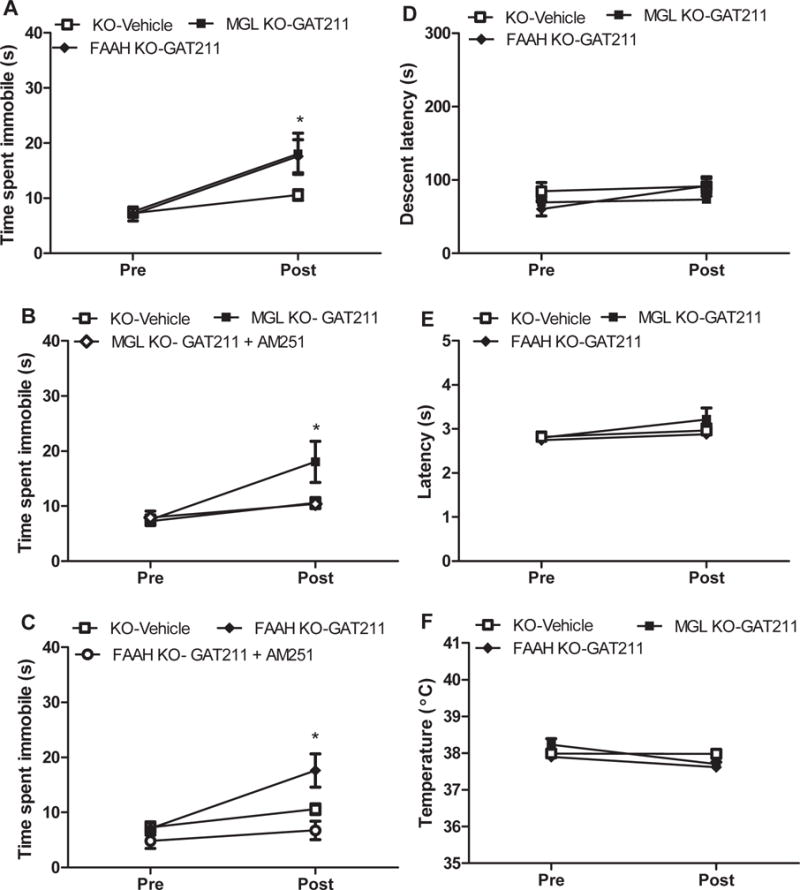
GAT211 (20 mg/kg i.p.) increased (A) time spent immobile in the ring test in both (B) MGL KO and (C) FAAH KO mice that was reversed following co-administration with AM251 (5 mg/kg i.p.). When administered alone, GAT211 did not produce (D) motor impairment in the rotarod test, (E) antinociception in the tail-flick test, or (F) hypothermia relative to vehicle in either genotype. Responses in FAAH KO mice and MGL KO mice receiving vehicle did not differ and were pooled for statistical analysis. *P<0.05 vs. vehicle, one-tailed t-test. Mean ±SEM (n = 5-12 per group)
In FAAH KO animals, GAT211 (20mg/kg i.p.) increased immobility time (p<0.03) (Figure 8A,C) but did not alter rotarod performance (p>0.82), tail flick responses (p>0.78) or body temperature (p>0.43) compared to vehicle (Figure 8A-D). GAT211-induced increases in catalepsy observed in each KO were blocked by concomitant AM251 (5mg/kg i.p.) administration (p>0.05) (Figure 8B,C).
Chronic administration of GAT211 does not induce the cardinal signs of CB1 receptor activation associated with orthosteric cannabinoid agonists
As expected, WIN55,212-2 (3mg/kg i.p.) produced motor ataxia (p<0.05), catalepsy (p<0.001), tail-flick antinociception (p<0.01) and reductions in body temperature (p<0.004) relative to vehicle (Figure 9A-D) on the first day of administration. Tolerance developed to the CB1-mediated pharmacological effects of WIN55,212-2 by day 8 of repeated dosing (p>0.402). By contrast, GAT211 (20mg/kg/day × 8 days i.p.) did not alter motor performance (p>0.44), produce immobility (p>0.59), or induce tail flick antinociception (p>0.18) relative to vehicle treatment on either day 1 or 8 of chronic dosing (Figure 9A-C). GAT211 did not induce hypothermia (p>0.24) on any day (Figure 9D).
Figure 9. The CB1 positive allosteric modulator GAT211 lacks cardinal signs of CB1 activation typical of the orthosteric cannabinoid agonist WIN55,212-2.
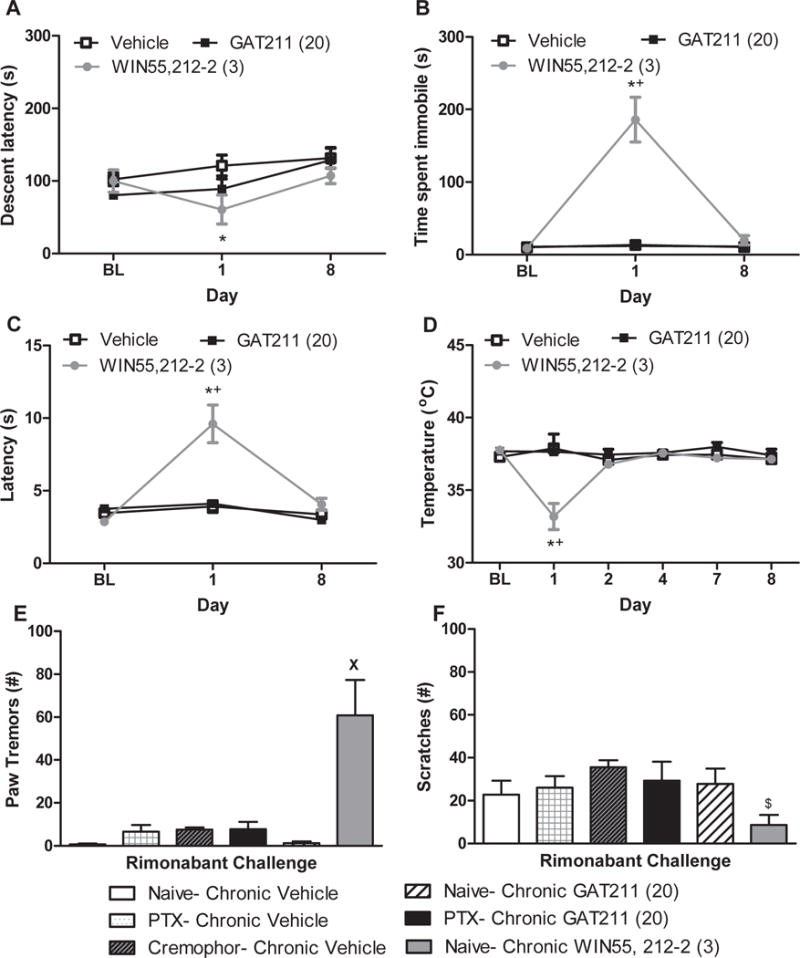
(A) The orthosteric cannabinoid agonist WIN55,212-2 (3 mg/kg i.p.) decreased rotarod descent latency relative to vehicle on day 1 but not day 8 of chronic dosing whereas GAT211 was ineffective. WIN55,212-2 increased (B) immobility time in the ring test and (C) tail flick latency in the hot water tail immersion test relative to either vehicle or GAT211 (20 mg/kg i.p.) on day 1 but not day 8 of chronic dosing. (D) WIN55,212-2 decreased body temperature relative to either vehicle or GAT211. Figure legend shows dose administered for each compound (mg/kg i.p.). (E) Challenge with the CB1 antagonist rimonabant (10 mg/kg i.p.) increased paw tremors in mice treated chronically with WIN55,212-2 (3 mg/kg/day i.p. × 9 days) but not GAT211 (20 mg/kg/day i.p. × 9 days). (F) WIN55,212-2 decreased rimonabant-induced scratching bouts relative to cremophor-treated animals. Figure legend shows dose administered for each compound (mg/kg i.p.).
*P<0.05 vs. vehicle, +P<0.05 vs. GAT211, XP<0.05 vs. all other groups, $P<0.05 vs. Cremophor one-way analysis of variance followed by Bonferroni post hoc test. Data are expressed as mean ± SEM (n = 5-12 per group)
Challenge with a CB1 antagonist elicits a prototypic cannabinoid CB1-dependent withdrawal syndrome in mice treated chronically with an orthosteric CB1 agonist but not a CB1 PAM
Rimonabant challenge markedly increased paw tremors in mice treated chronically with WIN55,212-2 (3mg/kg/day × 8 days i.p.; (F5,22=5.17 p<0.01); p<0.05 for all comparisons; Figure 9E) but not GAT211 (p>0.05; Figure 9E). Rimonabant-induced scratching (F5,22=2.812, p<0.05) was altered by WIN55,212-2 (p<0.05 vs. cremophor vehicle) but not by GAT211 (Figure 9F). Paclitaxel did not alter any of the observed behaviors relative to cremophor vehicle treatment (p=1.00) (Figure 9E,F).
Repeated administration of GAT211 does not cause reward or aversion
In WT mice, GAT211 (20mg/kg i.p./session) did not induce conditioned place preference or aversion compared to vehicle pairings (p>0.36) (Figure 10A) whereas morphine (8mg/kg i.p./session) produced robust conditioned place preference (p<0.02) (Figure 10B).
Figure 10. GAT211 does not produce conditioned place preference or aversion.
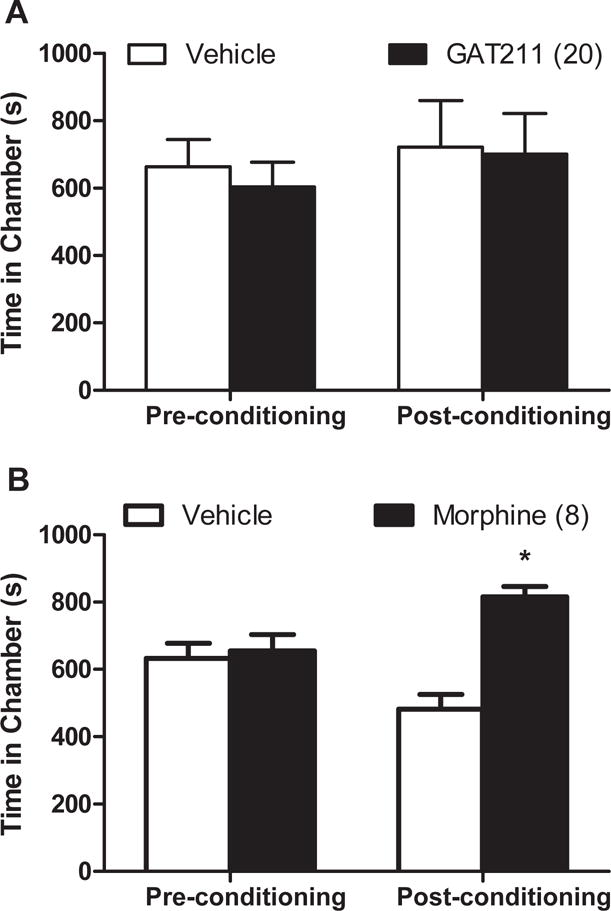
(A) GAT211 did not alter the time spent in either chamber. (B) Mice exhibited preference for the morphine paired chamber. Figure legend shows dose administered for each compound (mg/kg i.p.). *P<0.05 vs. vehicle-paired chamber, two-tailed t-test using Welch’s correction.
Discussion
The present studies validate positive allosteric modulation of cannabinoid CB1 signaling as a potential therapeutic strategy for suppressing pathological pain without producing tolerance, physical dependence, reward/aversion or CB1-mediated cannabimimetic effects. Our findings support an emerging literature validating the therapeutic potential of CB1 PAMs. Only two recent reports(18, 28) have evaluated in vivo efficacy of CB1 PAMs. In our study, GAT211 exhibited dose-dependent CB1-mediated antinociceptive efficacy in models of inflammatory and neuropathic pain and synergized with both an orthosteric cannabinoid agonist and endocannabinoid deactivation inhibitors. Moreover, GAT211 did not elicit cardinal signs of CB1 activation or produce physical dependence. Therapeutic efficacy was maintained over 19 days of repeated dosing, suggesting possible superiority of CB1 PAMs as an analgesic strategy over either orthosteric CB1 agonists (see also(6, 7)) or MGL inhibitors. By contrast, tolerance develops rapidly to CB1 agonists(6) and high dose MGL inhibitors(13, 14). The lack of tolerance to GAT211’s therapeutic efficacy may be due to enhancement of endocannabinoid signaling following chemotherapeutic treatment(9) and/or failure to desensitize or downregulate CB1. No tolerance was observed following chronic dosing with GAT21, consistent with observations using a ZCZ011 (twice daily × 6 days), which exhibits a longer duration of action relative to GAT211(18). Moreover, antinociceptive efficacy of GAT211 was dependent upon the presence of a pathological pain state. Finally, GAT211 did not impede paclitaxel-induced tumor cell toxicity in vitro.
The antinociceptive effects of GAT211 were mediated by CB1 as they were blocked by a CB1 (but not CB2) antagonist and absent in CB1 KO mice. GAT211 also produced synergistic antinociceptive effects with WIN55,212-2 without competing directly for the orthosteric CB1 binding site(17) and did not produce cannabimimetic effects in the tetrad. These observations suggest that GAT211 is likely to exhibit a favorable therapeutic ratio in vivo.
GAT211 enhanced the impact of constitutive loss of endocannabinoid hydrolytic enzymes. GAT211 produced equivalent enhancements of CB1-dependent catalepsy in FAAH KO and MGL KO mice. Thus, GAT211 did not potentiate endocannabinoid signaling in a probe-specific manner (e.g. heightening effects of anandamide over 2-AG or vice versa), consistent with in vitro findings(17). By contrast, lipoxin A4 preferentially enhances anandamide but not 2-AG signaling(28). However, FAAH and MGL also degrade lipid mediators that do not bind to cannabinoid receptors(8, 12, 44). Sustained inhibition of MGL does not desensitize CB1 receptors in brain regions relevant to catalepsy (i.e. caudate putamen and globus pallidus)(13) which may explain the selective sensitivity to catalepsy observed in FAAH and MGL KO mice. However, FAAH inhibitors and genetic deletion of FAAH has not been reported to alter tetrad behaviors(45).
Efficacy of GAT211 was specifically amplified by pharmacological manipulations that enhanced endocannabinoid tone. Our study is the first to document that CB1 PAMs synergize with orthosteric CB1 agonists and inhibitors of endocannabinoid deactivation. GAT211 exhibited antinociceptive synergism with either a FAAH or an MGL inhibitor. GAT211 synergized with the MGL inhibitor JZL184 to suppress paclitaxel-induced allodynia, irrespective of stimulus modality. However, GAT211 was synergistic with URB597 in suppressing mechanical allodynia but only additive in suppressing cold allodynia. This difference could be due to technical factors (i.e. duration of testing differs for mechanical and cold responsiveness), the steeper slope of the dose response curve for each stimulus modality, and/or higher levels of 2-AG compared to anandamide in the CNS. Our study is the first to demonstrate that a CB1 PAM does not interfere with paclitaxel’s ability to reduce tumor cell viability in vitro. Because the MTT assay is a probe of mitochondrial function and is unable to differentiate between cellular proliferation and cell death(46), future studies employing tumor bearing animals and assays of cellular death are needed to better understand how a CB1 PAM could impact chemotherapy treatment on cancer cells.
Because PAMs increase the signaling impact of the endogenous ligand at a receptor, repeated administration of a CB1 PAM could potentially produce CB1-mediated physical dependence. Challenge with a CB1 antagonist increases withdrawal-like behaviors (i.e. paw tremors/flutters and/or headshakes) in mice treated chronically with orthosteric CB1 agonists (present study and see (6, 7, 47)) and the MGL inhibitor JZL184 (13, 14) but not in mice treated chronically with the CB1 PAM GAT211. In our study, rimonabant challenge increased somatic withdrawal behaviors (i.e. paw tremors) in paclitaxel-treated mice receiving chronic WIN55,212-2 but not GAT211 treatment, suggesting that orthosteric CB1 agonism but not positive allosteric modulation of CB1 was associated with physical dependence. By contrast, JZL184, decreased rimonabant-induced scratching behavior, consistent with previous reports(14), but did not elicit increases in paw tremors (but see(13)). The latter study(13) evaluated otherwise naïve mice (i.e. pain free) and employed a different dosing paradigm (10mg/kg/day i.p. × 6 days) and observation window(1h).
Unlike orthosteric CB1 agonists (39, 48-50), our CB1 PAM did not produce reward or aversion. ZCZ011 suppressed pain behavior over a 6 day dosing interval without eliciting cannabimimetic effects or producing conditioned place preference or aversion(18). Thus, CB1 PAMs may lack significant abuse liability, unlike orthosteric CB1 agonists.
In summary, positive allosteric modulation of CB1 receptor signaling suppressed neuropathic and inflammatory pain without producing tolerance, physical dependence, reward/aversion or cardinal signs of indiscriminate CB1 receptor activation. GAT211 produced antinociceptive synergism with an orthosteric CB1 agonist and FAAH and MGL inhibitors. In conclusion, the allosteric modulatory site(s) residing on the CB1 receptor represent a promising target for development of analgesics that are safe, effective, nontoxic, lack tolerance and possess limited abuse liability.
Supplementary Material
Acknowledgments
The authors are grateful to Ben Cornett for assistance with genotyping and to Ben Cravatt for providing FAAH KO and MGL KO mice used to establish our breeding colony.
Financial Disclosures
Supported by DA041229 (to AGH and KM), DA009158 (to KM and AGH), CA200417 (to AGH), DA021696 (to KM) and EY024717 (to GAT). RAS is supported by T32DA024628 and by the 2017 Harlan Research Scholars program.
Abbreviation
- ANOVA
analysis of variance
- CB1
cannabinoid receptor 1
- CB2
cannabinoid receptor 2
- CR
cremophor
- CFA
complete Freund’s adjuvant
- DMSO
dimethyl sulfoxide
- FAAH
fatty-acid amide hydrolase
- i.p
intraperitoneal injection
- KO
knockout
- MGL
monoacylglycerol lipase
- PAM
positive allosteric modulator
- PTX
paclitaxel
- Δ9-THC
Δ9-tetrahydrocannabinol
- WT
wildtype
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Lu HC, Mackie K. An Introduction to the Endogenous Cannabinoid System. Biological psychiatry. 2016;79:516–525. doi: 10.1016/j.biopsych.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guindon J, Hohmann AG. The endocannabinoid system and pain. CNS & neurological disorders drug targets. 2009;8:403–421. doi: 10.2174/187152709789824660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moulin D, Boulanger A, Clark AJ, Clarke H, Dao T, Finley GA, et al. Pharmacological management of chronic neuropathic pain: Revised consensus statement from the Canadian Pain Society. Pain research & management: the journal of the Canadian Pain Society = journal de la societe canadienne pour le traitement de la douleur. 2014;19:328–335. doi: 10.1155/2014/754693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahn EJ, Hohmann AG. Cannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedside. Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics. 2009;6:713–737. doi: 10.1016/j.nurt.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis MP. Cannabinoids in pain management: CB1, CB2 and non-classic receptor ligands. Expert opinion on investigational drugs. 2014;23:1123–1140. doi: 10.1517/13543784.2014.918603. [DOI] [PubMed] [Google Scholar]
- 6.Deng L, Guindon J, Cornett BL, Makriyannis A, Mackie K, Hohmann AG. Chronic Cannabinoid Receptor 2 Activation Reverses Paclitaxel Neuropathy Without Tolerance or Cannabinoid Receptor 1-Dependent Withdrawal. Biological psychiatry. 2014 doi: 10.1016/j.biopsych.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng L, Cornett BL, Mackie K, Hohmann AG. CB1 Knockout Mice Unveil Sustained CB2-Mediated Antiallodynic Effects of the Mixed CB1/CB2 Agonist CP55,940 in a Mouse Model of Paclitaxel-Induced Neuropathic Pain. Molecular pharmacology. 2015;88:64–74. doi: 10.1124/mol.115.098483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crowe MS, Leishman E, Banks ML, Gujjar R, Mahadevan A, Bradshaw HB, et al. Dual inhibition of monoacylglycerol lipase and cyclooxygenases synergistically reduces neuropathic pain in mice. British journal of pharmacology. 2014 doi: 10.1111/bph.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guindon J, Lai Y, Takacs SM, Bradshaw HB, Hohmann AG. Alterations in endocannabinoid tone following chemotherapy-induced peripheral neuropathy: effects of endocannabinoid deactivation inhibitors targeting fatty-acid amide hydrolase and monoacylglycerol lipase in comparison to reference analgesics following cisplatin treatment. Pharmacological research: the official journal of the Italian Pharmacological Society. 2013;67:94–109. doi: 10.1016/j.phrs.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caprioli A, Coccurello R, Rapino C, Di Serio S, Di Tommaso M, Vertechy M, et al. The novel reversible fatty acid amide hydrolase inhibitor ST4070 increases endocannabinoid brain levels and counteracts neuropathic pain in different animal models. The Journal of pharmacology and experimental therapeutics. 2012;342:188–195. doi: 10.1124/jpet.111.191403. [DOI] [PubMed] [Google Scholar]
- 11.Piomelli D, Hohmann AG, Seybold V, Hammock BD. A lipid gate for the peripheral control of pain. J Neurosci. 2014;34:15184–15191. doi: 10.1523/JNEUROSCI.3475-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leishman E, Cornett B, Spork K, Straiker A, Mackie K, Bradshaw HB. Broad impact of deleting endogenous cannabinoid hydrolyzing enzymes and the CB1 cannabinoid receptor on the endogenous cannabinoid-related lipidome in eight regions of the mouse brain. Pharmacological research: the official journal of the Italian Pharmacological Society. 2016;110:159–172. doi: 10.1016/j.phrs.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nature neuroscience. 2010;13:1113–1119. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinsey SG, Wise LE, Ramesh D, Abdullah R, Selley DE, Cravatt BF, et al. Repeated low-dose administration of the monoacylglycerol lipase inhibitor JZL184 retains cannabinoid receptor type 1-mediated antinociceptive and gastroprotective effects. The Journal of pharmacology and experimental therapeutics. 2013;345:492–501. doi: 10.1124/jpet.112.201426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shore DM, Baillie GL, Hurst DH, Navas F, 3rd, Seltzman HH, Marcu JP, et al. Allosteric modulation of a cannabinoid G protein-coupled receptor: binding site elucidation and relationship to G protein signaling. The Journal of biological chemistry. 2014;289:5828–5845. doi: 10.1074/jbc.M113.478495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Picone RP, Kendall DA. Minireview: From the bench, toward the clinic: therapeutic opportunities for cannabinoid receptor modulation. Molecular endocrinology. 2015;29:801–813. doi: 10.1210/me.2015-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laprairie RB, Kulkarni PM, Deschamps JR, Kelly ME, Janero DR, Cascio MG, et al. Enantio-specific Allosteric Modulation of Cannabinoid 1 Receptor. ACS chemical neuroscience. 2017 doi: 10.1021/acschemneuro.6b00310. [DOI] [PubMed] [Google Scholar]
- 18.Ignatowska-Jankowska BM, Baillie GL, Kinsey S, Crowe M, Ghosh S, Owens RA, et al. A Cannabinoid CB1 Receptor-Positive Allosteric Modulator Reduces Neuropathic Pain in the Mouse with No Psychoactive Effects. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2015;40:2948–2959. doi: 10.1038/npp.2015.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross RA. Tuning the endocannabinoid system: allosteric modulators of the CB1 receptor. British journal of pharmacology. 2007;152:565–566. doi: 10.1038/sj.bjp.0707349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price MR, Baillie GL, Thomas A, Stevenson LA, Easson M, Goodwin R, et al. Allosteric modulation of the cannabinoid CB1 receptor. Molecular pharmacology. 2005;68:1484–1495. doi: 10.1124/mol.105.016162. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen T, Li JX, Thomas BF, Wiley JL, Kenakin TP, Zhang Y. Allosteric Modulation: An Alternate Approach Targeting the Cannabinoid CB1 Receptor. Medicinal research reviews. 2016 doi: 10.1002/med.21418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laprairie RB, Bagher AM, Denovan-Wright EM. Cannabinoid receptor ligand bias: implications in the central nervous system. Current opinion in pharmacology. 2016;32:32–43. doi: 10.1016/j.coph.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Vallee M, Vitiello S, Bellocchio L, Hebert-Chatelain E, Monlezun S, Martin-Garcia E, et al. Pregnenolone can protect the brain from cannabis intoxication. Science. 2014;343:94–98. doi: 10.1126/science.1243985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horswill JG, Bali U, Shaaban S, Keily JF, Jeevaratnam P, Babbs AJ, et al. PSNCBAM-1, a novel allosteric antagonist at cannabinoid CB1 receptors with hypophagic effects in rats. British journal of pharmacology. 2007;152:805–814. doi: 10.1038/sj.bjp.0707347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Straiker A, Mitjavila J, Yin D, Gibson A, Mackie K. Aiming for allosterism: Evaluation of allosteric modulators of CB1 in a neuronal model. Pharmacological research: the official journal of the Italian Pharmacological Society. 2015;99:370–376. doi: 10.1016/j.phrs.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laprairie RB, Bagher AM, Kelly ME, Denovan-Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. British journal of pharmacology. 2015;172:4790–4805. doi: 10.1111/bph.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pertwee RG. Lipoxin A4 is an allosteric endocannabinoid that strengthens anandamide-induced CB1 receptor activation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:20781–20782. doi: 10.1073/pnas.1218529110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pamplona FA, Ferreira J, Menezes de Lima O, Jr, Duarte FS, Bento AF, Forner S, et al. Anti-inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:21134–21139. doi: 10.1073/pnas.1202906109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 30.Kulkarni PM, Ranade A, Garai S, Thakur GA. Microwave-accelerated Conjugate Addition of 2-Arylindoles to Substituted β-Nitrostyrenes in the Presence of Ammonium Trifluoroacetate: An Efficient Approach for the Synthesis of a Novel Class of CB1 Cannabinoid Receptor Allosteric Modulators. Journal of Heterocyclic Chemistry. 2017:n/a–n/a. [Google Scholar]
- 31.Vachon P, Millecamps M, Low L, Thompsosn SJ, Pailleux F, Beaudry F, et al. Alleviation of chronic neuropathic pain by environmental enrichment in mice well after the establishment of chronic pain. Behavioral and brain functions: BBF. 2013;9:22. doi: 10.1186/1744-9081-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slivicki RA, Ali YO, Lu HC, Hohmann AG. Impact of Genetic Reduction of NMNAT2 on Chemotherapy-Induced Losses in Cell Viability In Vitro and Peripheral Neuropathy In Vivo. PloS one. 2016;11:e0147620. doi: 10.1371/journal.pone.0147620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee WH, Xu Z, Ashpole NM, Hudmon A, Kulkarni PM, Thakur GA, et al. Small molecule inhibitors of PSD95-nNOS protein-protein interactions as novel analgesics. Neuropharmacology. 2015;97:464–475. doi: 10.1016/j.neuropharm.2015.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacological reviews. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 35.Rahn EJ, Thakur GA, Wood JA, Zvonok AM, Makriyannis A, Hohmann AG. Pharmacological characterization of AM1710, a putative cannabinoid CB2 agonist from the cannabilactone class: antinociception without central nervous system side-effects. Pharmacol Biochem Behav. 2011;98:493–502. doi: 10.1016/j.pbb.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pertwee RG. The ring test: a quantitative method for assessing the ‘cataleptic’ effect of cannabis in mice. British journal of pharmacology. 1972;46:753–763. doi: 10.1111/j.1476-5381.1972.tb06900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, et al. Unmasking the tonic-aversive state in neuropathic pain. Nature neuroscience. 2009;12:1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song R, Zhang HY, Peng XQ, Su RB, Yang RF, Li J, et al. Dopamine D(3) receptor deletion or blockade attenuates cocaine-induced conditioned place preference in mice. Neuropharmacology. 2013;72:82–87. doi: 10.1016/j.neuropharm.2013.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lepore M, Vorel SR, Lowinson J, Gardner EL. Conditioned place preference induced by delta 9-tetrahydrocannabinol: comparison with cocaine, morphine, and food reward. Life sciences. 1995;56:2073–2080. doi: 10.1016/0024-3205(95)00191-8. [DOI] [PubMed] [Google Scholar]
- 40.Deng L, Lee WH, Xu Z, Makriyannis A, Hohmann AG. Prophylactic treatment with the tricyclic antidepressant desipramine prevents development of paclitaxel-induced neuropathic pain through activation of endogenous analgesic systems. Pharmacological research: the official journal of the Italian Pharmacological Society. 2016;114:75–89. doi: 10.1016/j.phrs.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of immunological methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 42.Tallarida RJ. An overview of drug combination analysis with isobolograms. The Journal of pharmacology and experimental therapeutics. 2006;319:1–7. doi: 10.1124/jpet.106.104117. [DOI] [PubMed] [Google Scholar]
- 43.Tham SM, Angus JA, Tudor EM, Wright CE. Synergistic and additive interactions of the cannabinoid agonist CP55,940 with mu opioid receptor and alpha2-adrenoceptor agonists in acute pain models in mice. British journal of pharmacology. 2005;144:875–884. doi: 10.1038/sj.bjp.0706045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carey LM, Slivicki RA, Leishman E, Cornett B, Mackie K, Bradshaw H, et al. A pro-nociceptive phenotype unmasked in mice lacking fatty-acid amide hydrolase. Molecular pain. 2016;12 doi: 10.1177/1744806916649192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bellamy WT. Prediction of response to drug therapy of cancer. A review of in vitro assays. Drugs. 1992;44:690–708. doi: 10.2165/00003495-199244050-00002. [DOI] [PubMed] [Google Scholar]
- 47.Deng L, Guindon J, Vemuri VK, Thakur GA, White FA, Makriyannis A, et al. The maintenance of cisplatin- and paclitaxel-induced mechanical and cold allodynia is suppressed by cannabinoid CB(2) receptor activation and independent of CXCR4 signaling in models of chemotherapy-induced peripheral neuropathy. Molecular pain. 2012;8:71. doi: 10.1186/1744-8069-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheer JF, Kendall DA, Marsden CA. Cannabinoid receptors and reward in the rat: a conditioned place preference study. Psychopharmacology. 2000;151:25–30. doi: 10.1007/s002130000481. [DOI] [PubMed] [Google Scholar]
- 49.Robinson L, Hinder L, Pertwee RG, Riedel G. Effects of delta9-THC and WIN-55,212-2 on place preference in the water maze in rats. Psychopharmacology. 2003;166:40–50. doi: 10.1007/s00213-002-1302-0. [DOI] [PubMed] [Google Scholar]
- 50.Maldonado R. Study of cannabinoid dependence in animals. Pharmacology & therapeutics. 2002;95:153–164. doi: 10.1016/s0163-7258(02)00254-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


