Abstract
This review summarizes the evidence derived from studies utilizing denervation procedures to demonstrate sympathetic control of white adipose tissue metabolism and body fat mass. A majority of the work demonstrating neural control of white fat was performed in the Bartness laboratory with Siberian hamsters as the predominant experimental model. These animals experience dramatic changes in body fat mass in response to changes in photoperiod, however, the mechanisms identified in hamsters have been reproduced or further elucidated by experiments with other animal models. Evidence for the role of sympathetic innervation contributing to the control of white adipocyte lipolysis and preadipocyte proliferation is summarized. In addition, evidence from denervation experiments for neural communication between different white fat depots as well as for a feedback control loop between sensory afferents from individual fat depots and sympathetic efferents to the same or distant white fat depots is discussed.
Keywords: sympathectomy, adipocyte lipolysis, preadipocyte proliferation, leptin
Introduction
The objective of this review is to provide a summary of the work contributed by Timothy J. Bartness to the understanding of central control of adipose mass through methods that involve direct manipulation of the innervation of individual fat depots. The animal model used in a large number of these studies is the hamster (Syrian or Siberian) which can be induced to gain or lose body fat simply by changing photoperiod. Thus the change in fat is mediated by endogenous control systems that respond to a normal biological signal rather than representing a response to an unusual insult such as dietary induced weight gain or surgical loss (lipectomy) or gain (transplant) of body fat.
Seasonal Changes in Body Fat of Hamsters
Tim initiated his work with photoperiod in hamsters in the 1980s during his time as a postdoctoral fellow with George N. Wade at the University of Massachusetts and solidified this interest during a subsequent postdoctoral fellowship with Bruce D. Goldman at the Worcester Foundation. It was already established that photoperiod influenced adiposity in hamsters. Syrian hamsters (Mesocricetus auratis) gain a large amount of fat in short photoperiod, winter-like, days [1] whereas Siberian hamsters (Phodopus sungorus sungorus) lose fat in similar conditions [2]. The changes in body composition are fully reversible and follow the seasons when the animals are living in the wild. It is important to note that Siberian hamsters exposed to short photoperiod lose weight and then this is followed by a reduction in food intake which means that the change in body fat is a primary response rather than a secondary outcome following changes in energy balance [3]. Thus, the change in body composition is caused by a photoperiod responsive system that modulates lipolysis and/or lipogenesis.
It had already been established that the photoperiodic changes in body weight and composition were driven by the hormone melatonin released from the pineal gland during the dark period [2]. Tim completed a work intensive study with pinealectomized hamsters housed in long photoperiod and treated with melatonin and found that the changes in body weight were driven by the duration of the nocturnal peak of melatonin, rather than the actual concentration of melatonin in the circulation. Hamsters infused with 10 ng melatonin/day during a short infusion of 6 hour duration gained body weight and fat comparable to that in Siberian hamsters housed in long day photoperiod. However, animals infused with the same amount of melatonin over a longer period of 10 hours did not show any change in body composition [4]. In a complementary study, long duration melatonin infusions into long day housed hamsters produced responses typical of an animal housed in a short day length [5]. In male Siberian hamsters housed in short photoperiod conditions intraperitoneal fat (epididymal and retroperitoneal) decreased in size more than subcutaneous fat depots (inguinal and dorsal), but were also restored more rapidly when the animals were transferred to long photoperiod conditions [6]. This regional response was not apparent in females.
The next step in the process of understanding the seasonal control of body fat mass was to identify the melatonin-responsive signal that changed fat metabolism. The seasonal changes in body weight and composition of hamsters are accompanied by other physiologic adaptations including testicular regression when fat is lost and recrudescence when fat is increased [3, 7, 8]. An initial study tested whether the changes in fat were secondary to the change in gonadal hormone levels, but neither castration of male Siberian hamsters nor ovariectomy of females had any effect on the loss of body fat in short-day conditions [3]. This was the first in a series of studies designed to identify a hormonal mediator of the melatonin-driven photoperiodic changes in body weight, body fat and reproductive status. Subsequent depletion, repletion experiments tested prolactin [9], insulin [10], thyroxine [11] and adrenal norepinephrine as potential mediators [12]. Although none of these hormones were found to be the exclusively driver of white adipose tissue lipolysis, it did appear that the combination of sympathetic norepinephrine and adrenal epinephrine accounted for the short day loss of fat in Siberian hamsters [12]. Subsequently the Bartness lab identified melatonin receptors on sympathetic neurons originating in the suprachiasmatic nucleus and terminating in white fat depots [13]. Thus, Tim’s interest in neural control of adipose tissue developed from a failure to find a hormonal explanation for the photoperiodic-dependent changes in hamsters, but the amount of work involved in the hormone studies and the frustration of obtaining only negative results remained a topic of conversation throughout his career.
The first evidence for sympathetic innervation of white fat from the Bartness lab [14] used anterograde and retrograde tract tracing to provide anatomic evidence of sympathetic and sensory innervation of different white fat depots (subcutaneous inguinal and intraperitoneal epididymal). This paper clearly showed that there was innervation of the fat cells and not just blood vessels within a depot. The specificity of the tracing was confirmed by surgical denervation of fat depots and by injecting the tracers directly into blood vessels. In this and future studies norepinephrine turnover (NETO) in individual fat pads was determined by measuring the decline in adipose norepinephrine (NE) content following inhibition of tyrosine hydroxylase with alpha-methyl-p-tyrosine (α-MPT). The results showed a correlation between NETO in discrete fat depots and the sequence of fat pad depletion of Siberian hamsters as they transitioned from fat to lean after transfer from long to short day photoperiod conditions [6]. These initial studies led to a body of work that developed during the next three decades to establish the anatomic and functional role of both sympathetic and sensory innervation of white [15, 16] and brown fat [17–19], the unique responses obtained from individual fat depots [20] and most recently how white and brown fat communicate to produce a coordinated metabolic and thermogenic response to physiologic challenges [19, 21, 22].
Methods of Denervation
The most direct ways to test the importance of nerves in controlling adipose tissue metabolism and function is to remove the neural supply to the tissue. There are several alternate current methodologies for denervating brown or white fat depots and each of these were described in detail by Vaughan et al in 2014 [23]. One option is surgical denervation in which nerve fibers supplying a particular fat depot are physically cut. This is the most difficult and least specific procedure. It is difficult because of the necessity to locate all of the nerves that enter a specific fat depot and to dissect the nerve without damaging closely associated tissue or blood vessels. Some of these nerves may also transition through the fat depot to supply other tissue, such as adjacent skin or muscle. It is non-specific because the sympathetic and sensory nerves innervating white or brown fat depots travel together and are both destroyed when the nerve bundle is transected. Benefits are that this technique can be close to 100% effective in removing neural input and output from a fat pad and, as with chemical denervation described below, it is possible to denervate one pad in a bilateral depot and leave the other pad intact as a within animal control.
An alternative to surgical denervation is selective chemical destruction of either sympathetic or sensory neurons within a specific fat pad. 6-hydroxydopamine (6OHDA) was the first chemical agent used to produce selective destruction of sympathetic nerves. The compound is taken up into noradrenergic storage vesicles [24] through the noradrenaline re-uptake pump [25]. Oxidative damage disrupts the membrane due to oxidative damage to produce a reversible loss of sympathetic innervation [26]. Sympathectomy of a specific fat pad is achieved by making 10 to 20 small volume injections (1 or 2 µl) of 8–10 mg/ml 6OHDA across the pad [27]. Control pads receive a similar number of injections of vehicle. This is a time consuming process because the needle is held in place for a minute at the end of each injection to prevent efflux of the solution out of the tissue. However, unlike surgical denervation, sensory nerves remain intact. In a mouse study sympathetic denervation achieved with 6OHDA, measured as tissue NE content, was 60% effective 24 hours after the injections. Four weeks after the injections the amount of NE per mg of tissue remained the same as at 24 hours after injection [28]. Others have reported that sympathetic nerves regenerate following systemic treatment with 6OHDA. Thureson-Klein et al [29] reported a partial recovery of brown fat NE content within 4 weeks of subcutaneous administration of 6OHDA in rats. Regeneration of the sympathetic nerves may be a disadvantage for long-term experiments designed to test the effects of denervation, but could be an advantage if the process of reinnervation is of interest. Although the sympathetic denervation of fat depots or the whole animal with 6OHDA is reversible in adults, whole animal sympathectomy of neonates is irreversible [30]. The impact of fat specific denervation in neonates has not been investigated, but may be more stable than the process in adults.
Guanethidine has also been used to sympathectomize fat depots and the procedure has been described in detail by Demas and Bartness [31]. Technically the process is similar to that for 6OHDA in that multiple small volume injections of 10 ug/ul guanethidine are made into the tissue. The guanethidine is transported into sympathetic terminals by the noradrenaline re-uptake transporter and accumulates in the vesicles containing NE [32]. Subsequently the neurons are destroyed [33] possibly due to an autoimmune response [34]. Guanethidine depletes tissue NE content by 30–80% 2 weeks after surgery [31]. As with 6OHDA this procedure leaves sensory nerves intact, but also has the added advantage of being considered permanent [35]. In 2005 we tested guanethidine, but did not find a substantial reduction in NE content of treated fat pads [36]. Others, however, continue to report effective global [37] and tissue specific [38] sympathectomy with guanethidine
It also is possible to selectively destroy sensory nerves in adipose tissue. Capsaicin activates vanilloid receptors [39] expressed by unmyelinated and some small diameter myelinated sensory neurons. The resulting influx of calcium and sodium has an excitotoxic effect. Sensory denervation is achieved by making multiple small volume injections of 20 µg/µl capsaicin across the extent of a fat pad [23]. Destruction of the unmyelinated sensory neurons is demonstrated by a reduction in tissue calcitonin gene related protein (CGRP) and substance P content. Capsaicin does not affect unmyelinated efferent fibers [40]. Shi et al [41] reported that tissue CGRP was reduced by approximately 40% twelve weeks after capsaicin treatment, compared with 80% in surgically denervated pads, but the advantage of chemical sensory denervation is that sympathetic fibers remain intact.
Sympathetic Denervation and Adipocyte Lipolysis
The role of the sympathetic nervous system in controlling lipolysis was reviewed in depth by Bartness et al in 2014 [42], therefore, this section will summarize the evidence with an emphasis on contributions made by experiments that involved denervation of adipose tissue. Indirect evidence for sympathetic control of adipose tissue lipolysis came from early studies with cats in which splanchnic nerves were cut only on the left side. Approximately 2 weeks after the surgery denervated fat pads from overfed or food deprived cats were larger than the intact innervated fat pads on the right side [43]. The results of these experiments were subsequently confirmed in fasted rats with surgically denervated retroperitoneal fat [44]. Additional indirect evidence for sympathetic control of lipolysis came from experiments showing an increased rate of NETO in both white and brown fat of cold exposed [45] or fasted rats [46] and in white fat of Siberian hamsters losing fat as they transitioned from long- to short-day photoperiod [14]. Surgical denervation was also used to demonstrate that an estradiol-induced reduction of body fat in ovariectomized female Siberian hamsters was dependent on an increase in NETO [47]. More direct evidence came from in vitro studies in which electrical stimulation of nerves to an excised epididymal fat pad increased the concentration of free fatty acids in the bathing media [48].
Central regulation of lipolysis in white fat was demonstrated by the Migliorini laboratory [49]. Hypothalamic deafferentation, achieved by making selective knife cuts between the anterior and medial hypothalamus, completely blocked free fatty acid mobilization caused by peripheral administration of 2-deoxyglucose (2DG). By contrast adrenal demedullation did not affect the response, excluding a role for adrenal catecholamines. Subsequent experiments confirmed a role for central control of lipolysis in response to cold exposure, forced exercise and fasting [50]. The potential for central control was confirmed when Youngstrom and Bartness [14] provided anatomical evidence for sympathetic innervation of white adipose tissue and subsequent studies identified the areas of the brain that controlled white fat sympathetic outflow [15]. Testing for sympathetic stimulation of lipolysis has depended upon detecting increases in circulating concentrations of free fatty acids and glycerol and/or measuring activation of lipolytic enzymes in fat pads of interest. Adipose tissue lipase (AGTL), perilipin A and abhydrolase domain containing 5 (Abdh5) hydrolyze triglycerides to free fatty acids and diacyglycerides which are the substrate for hormone sensitive lipase (HSL). Monoacylglyceride derived from hydrolysis by HSL is processed to glycerol and free fatty acids by monoglyceride lipase (MGL). In basal conditions AGTL, perilipin A and Abdh5 form a complex on the surface of the lipid droplet, but Abdh5 is inactive and HSL is in the cytoplasm so lipolysis is limited. Upon activation and phosphorylation of HSL and perilipinA the complex is reorganized, Abdh5 is activated and HSL migrates to the lipid droplet which facilitates full hydrolysis of triglycerides [51].
The melanocortin system has been shown to play an important role in the control of energy balance and preventing weight gain [52] in part by sympathetic stimulation of thermogenesis in BAT [53]. It has been reported that melanocortin receptors 3 and 4 (MCR3 and MCR4) mediate the changes energy balance [54], but MCR4, which is widely distributed in the brain [55], has the dominant role [56]. Endogenous ligands for the receptors are derived from the preprotein proopiomelanocortin (POMC) and α-melanocyte stimulating hormone (αMSH) is the primary ligand for MCR4 whereas α- β- and γ-MSH and ACTH have equal affinity for MCR3, but γ-MSH is thought to be the primary ligand for this receptor [52]. In 1999 Haynes et al [57] reported that third ventricle administration of an MCR3, MCR4 agonist, melanotan II (MTII), increased sympathetic nerve activity in rats. Subsequently the Bartness lab identified many areas of the brain in which sympathetic nerves from both white and brown fat expressed MCR4 [58, 59]. Injecting MTII into the 3rd ventricle of rats significantly increased NETO and phosphorylation, but not expression, of HSL and perilipin A in subcutaneous, but not intraperitoneal, fat of rats [60, 61]. These data showed that the measured increases in circulating concentrations of glycerol and free fatty acids in MTII-treated animals were due to depot specific stimulation of lipolysis [61], consistent with previous observations that other conditions that stimulate lipolysis, such as food deprivation and cold exposure, also influenced NETO in a depot specific pattern [20]. In the latter experiment each of the white fat pads tested (retroperitoneal, inguinal, dorsosubcutaneous and epididymal) responded to some, but not all of the stimuli tested, except inguinal fat which showed a reliable increase in NETO to each of the lipolytic stimuli tested as well as to central administration of MTII [20, 61].
Sympathetic stimulation of lipolysis is mediated by Gs-coupled β-adrenergic receptors [62]. In rodents the β3-receptor is the primary driver of lipolysis [63], but in humans β1- and β2- receptors are the mediators of sympathetic-driven lipolysis [64]. Different fat depots have different levels of expression of β-adrenergic receptors, but the ratio of expression of β-receptors to the anti-lipolytic Gi-coupled α2-adrenoreceptor also determines the lipolytic response to catecholamines. Sustained stimulation with catecholamines results in down-regulation of β1- and β2-, but not β3-receptors [65] which also have the lowest affinity for catecholamines [65], suggesting that this receptor is most active in conditions of extreme, sustained activation of the sympathetic nervous system and adrenal medulla. Chemical sympathectomy of inguinal and epididymal fat in hamsters has also demonstrated that sympathetic tone can influence the ratio of β- to α2-receptros in a depot specific manner [66]. Thus, sympathetic nervous system stimulated lipolysis in any specific fat depot is determined by the relative level of expression of different adrenergic receptors, the degree of sympathetic innervation, expression of specific receptors on the sympathetic efferents and the strength and duration of sympathetic activation.
The sympathetic nervous system is a primary determinant of adipocyte lipolysis, but its effect is modulated by interactions with other lipolytic and anti-lipolytic hormones. For example, Demas and Bartness [12] demonstrated that the reduction in fat pad size of Siberian hamsters moved from long- to short-day length photoperiod required a combination of both sympathetic and adrenal catecholamines. An increase in sympathetic activation often occurs at the same time as glucagon release is stimulated. Lefebvre et al [67] used denervation to demonstrate that in the absence of sympathetic drive glucagon promoted glycerol, but decreased free fatty acid release from white fat and that glucose uptake by adipose tissue increased, suggesting that activation of the sympathetic nervous system may prevent reesterification of fatty acids released in response to other lipolytic agents. It is well established that insulin inhibits lipolysis and suppresses catecholaminergic stimulation of lipolysis [68]. This may be due to insulin promoting internalization of β-adrenergic receptors and changing the ratio of β- to α2-adrenergic receptors on the fat cell [69] and/or insulin increasing glucose conversion to lactate which activates an anti-lipolytic orphan Gi-coupled receptor that is expressed in adipocytes [70].
Sympathetic Denervation and Cell Proliferation
Before Youngstrom and Bartness [14] provided anatomic evidence of sympathetic innervation of white fat, Jones et al [71] used primary adipocyte culture to show that norepinephrine had a direct inhibitory effect on preadipocyte proliferation. The following year Cousin et al [72] reported that surgical denervation of retroperitoneal fat increased fat pad size by increasing fat cell number. As early as one week after surgery there was evidence of an increase in preadipocyte number and by one month there was an increase in adipocyte number. Interestingly they did not find any change in adipocyte rate limiting enzymes for fatty acid synthesis or lipolysis or in vitro measures of basal or norepinephrine stimulated lipolysis. In a subsequent study with hamsters Youngstrom and Bartness [73] examined the effect of surgical denervation of inguinal fat in long- and short-day housed Siberian hamsters. Eleven weeks after surgery denervated fat pads contained less norepinephrine, but were larger and had a significantly greater number of adipocytes than the undisturbed contralateral pad. There was no significant change in adipocyte size between the denervated and contralateral pads. Photoperiod did not influence the effect of denervation on proliferation, thus there was an increase in fat cell number even in the depots of short-day housed animals in which fat mass and food intake were down-regulated compared with long-day housed animals.
It is well documented that different fat depots have different degrees of cellularity [74] and metabolic response to hormones [75] in addition to conferring different levels of risk for metabolic syndrome [76]. Therefore, it is not surprising to find that the origin and density of innervation also varies between depots. Bowers et al [77] tested the effect of surgical denervation on cellularity of retroperitoneal and inguinal fat in long-day housed hamsters. The denervated pads showed similar reductions in tissue norepinephrine content and a significant increase in cell number compared with the contralateral pad, but the effect was exaggerated in inguinal compared with retroperitoneal fat (180% versus 155%) five weeks after surgery. The difference in proliferation was attributed to differences in central origins of innervation of the fat depot, but may be attributable to the more recent observation that subcutaneous fat has a much higher population of adipose progenitor cells than visceral fat [78]. Although a role for sensory output was not excluded in the study by Bowers et al [77], this issue was addressed in a separate study that compared surgical denervation (sympathetic plus sensory) to sensory-specific denervation [41]. Surgical denervation led to an increase in fat cell number in inguinal and epididymal fat whereas sensory denervation did not change cell number, but did produce a small increase in inguinal, but not epididymal, fat cell size. As in the previous study [77] the increase in cell number was exaggerated in inguinal fat and particularly apparent in the population of small diameter cells (25–100 µm diameter). Because measurements were made on fully differentiated fat cells, neither of these studies [41, 77] distinguished between lipid filling of existing preadipocytes and promotion of both proliferation and differentiation of adipocytes. In a study reported by Foster and Bartness [79] surgical, chemical sympathetic and chemical sensory denervation of inguinal fat were compared. In order to test for development of new preadipocytes hamsters were injected with BrDU for 6 days after denervation as a marker of de novo cell proliferation. Ten days after denervation there was a small, insignificant increase in fat pad weight, but a 4-fold increase in BrDu labeled adipocytes compared with the contralateral intact fat in rats that had one surgically or sympathetically denervated fat pad. In rats that had undergone sensory denervation there was a 60% decrease in fat pad CGRP content, but no change in the number of BrdU labeled adipocytes. The specificity of BrdU to adipocytes was confirmed by double staining with the adipocyte specific membrane marker AD-3 [80]. These results confirmed that sympathetic innervation of white fat inhibits cell proliferation and that the increase in measureable white adipocytes following denervation cannot simply be attributed to differentiation of existing cells.
If sympathetic denervation is permissive for adipocyte proliferation, then proliferation and differentiation of white fat cells would be expected to be inhibited in conditions of chronic sympathetic activation. Consistent with this Cousin et al [81] reported no change in DNA content or expression of A2COL6, a marker for preadipocytes, in ovarian or inguinal fat of female rats housed at 4°C for 9 days. This study also showed a steady increase in DNA and a spike in A2COL6 in IBAT, consistent with well-established evidence that chronic activation of adrenergic receptors in brown fat leads to a rapid and substantial increase in cell number [82]. By contrast, others reported that cold exposure of young male rats caused a substantial increase in white fat preadipocyte proliferation, but that new cells had the characteristics of brown or beige adipocytes [83] and that newly formed adipocytes in epididymal fat from rats treated with a β3-adrenergic agonist for 6 days were multilocular [84]. Similarly, Wang et al. [85] found increased preadipocyte proliferation in epididymal and inguinal depots of male AdipoChaser mice exposed to the cold or treated with a β3 adrenergic agonist. In these animals beige fat cells accounted for the proliferation in inguinal fat, whereas the new cells in epididymal fat were reported to be white adipocytes.
The reason for the discrepant results for the effects of sympathetic activation on white adipocyte proliferation has not been investigated, but may be explained by the difference in sensitivity of techniques used to identify new preadipocytes. The development of inducible progenitor markers, such as AdipoChaser [85], that can subsequently be detected in mature adipocytes allows a for reliable identification of cells that develop during a specific treatment period. An alternate explanation for the discrepant results could be based on differences in the relative levels of expression of different adrenergic receptors across species and fat depots [86, 87].
As noted above, white fat expresses the α2-adrenoreceptor, which inhibits adenylate cyclase, counteracts the effects of β-adrenergic receptors in fat cells and inhibits lipolysis [62], but promotes adipocyte proliferation [88]. The number of α2-receptors present in white fat increases following denervation [72], suggesting that down regulation of this receptor in intact fat depots may contribute to the stability of cell number. Receptors remain functional in a denervated fat depot because α2-receptors have a high affinity for low concentrations of catecholamines and bind both norepinephrine released from surviving sympathetic terminals and epinephrine from the adrenal gland [87]. An increased rate of proliferation in response to α2-adrenergic receptor activation was first demonstrated in vitro [89] and then confirmed in vivo using β-adrenergic receptor deficient mice overexpressing human α2-adrenergic receptors in adipose tissue [88]. When these mice became obese on a high-fat diet the gain in body fat was entirely due to an increase in fat cell number, with no change in fat cell size [88]. If these receptors are confirmed as essential to the sympathetic control of adipocyte proliferation, then species differences in response to sympathetic denervation or chronic activation would be expected due to large inter-species differences in the level of expression in white fat [86] and differences in the ratio of α2- to β–adrenergic receptors. Hamsters have one of the highest ratios [87] and potentially the greatest potential for proliferation following denervation.
Sympathetic Denervation and Leptin Responsiveness
The sympathetic innervation of white adipose tissue demonstrates the expected responses to physiologic and environmental stimuli that are known to influence sympathetic activity [20]. Leptin, a hormone produced by white adipose tissue, is hypothesized to function as a negative feedback signal in the control of energy balance [90] and body fat mass is decreased in animals treated with exogenous leptin [91]. Loss of body fat has been attributed to both a suppression of food intake [91] and an increase of brown fat thermogenesis [92], driven by increased sympathetic activation of the tissue. Studies with knockout mice suggested that the increase in thermogenesis may require activation of MCR4 [93] and the Bartness lab established that sympathetic nerves to both white and brown fat express MCR4 [58]. Therefore, in a collaborative study with the Bartness laboratory we investigated whether increased sympathetic drive also contributed to the leptin-induced loss of white fat [27]. In these experiments we used 6OHDA to selectively destroy sympathetic nerve terminals in one epididymal fat pad of C56BL mice or Sprague Dawley rats. There also were some rats in which one retroperitoneal fat pad was denervated. The rats were fitted with an intraperitoneal miniosmotic pump delivering leptin (10 µg/day for mice and 50 µg/day for rats) at the same time as the denervation surgery was performed. Fat depot weight and NE content were measured 13 days later. We chose to denervate epididymal fat because previous experiments had shown this depot to be very responsive to peripheral infusions of leptin [94].
Denervation of one epididymal fat pad did not change the leptin responsiveness of that fat pad or of any other fat depot in the mice. Epididymal levels of NE were very low in rats and were not significantly changed by 6OHDA treatment, however, this denervation did increase the size of the mesenteric fat depot and reduced leptin responsiveness of retroperitoneal and inguinal fat of the same animal, measured by the degree of change in fat pad weight during leptin infusion. Denervation of one retroperitoneal fat pad gave much clearer results and confirmed that peripherally administered leptin does not require sympathetic innervation of white fat to reduce fat depot size. Unlike epididymal denervation, loss of sympathetic innervation to retroperitoneal fat had no effect on the size or leptin responsiveness of other fat depots. These results were consistent with a previous report that hyperleptinemia caused by a leptin-secreting adenovirus reduced the size of transplanted fat that had no established neural connections [95]. They also demonstrated the potential for communication between different white fat depots on a depot specific basis. To follow-up on these observations with peripheral infusions of leptin [27] we measured NETO in different fat depots of rats receiving central injections or peripheral infusions of leptin to test whether there was a direct association between leptin-induced activation of sympathetic outflow and weight loss of specific fat depots [96]. This study also included rats pair-fed to the leptin treated animals to account for any change in sympathetic drive caused by hypophagia. We found that neither peripheral infusions nor central injections of leptin caused a uniform change in NETO across fat depots, although all of the fat pads measured were reduced in size in leptin treated animals. Consistent with the results from hamsters, NETO was higher in inguinal fat than any other white fat depot and we found that pair-feeding had greater effects than leptin on NETO, increasing NE degradation in mesenteric fat, but decreasing it in retroperitoneal fat. The results of this study combined with those from the denervation experiment [27] provided convincing evidence that loss of fat in leptin-treated animals was independent of a change in sympathetic drive to individual white fat depots, but also provided new information on the differences in basal levels of sympathetic drive to different depots.
To our knowledge the importance of the sympathetic nervous system in mediating white fat responses to other hormones has not been tested in detail. By contrast to the lack of association between leptin and sympathetic activity in white fat, the Bartness lab found that sensory nerves in white fat respond to leptin. Leptin receptors have been identified on the dorsal root ganglia pseudopolar neurons innervating white fat and injection of leptin directly into the inguinal fat of hamsters stimulated afferent nerve activity [97]. These data can be interpreted as leptin functioning as a paracrine signal of the size of fat stores, allowing this information to be integrated with other signals related to adipocyte size or metabolism and contribute to the central control of white fat mass.
Sensory Denervation and Total Body Fat mass
Although there are fewer studies examining the impact of sensory denervation on white fat morphology, they provide critical evidence for a neural feedback loop between fat depots and areas of the brain that send sympathetic afferents to the anatomically distributed fat depots. The presence of sensory innervation of white fat was first demonstrated by Fishman and Dark [98] using anterograde tract tracing and confirmed by the presence in rat white adipose tissue of neuropeptides typically associated with sensory fibers [99]. Subsequent tract tracing studies from the Bartness lab identified the central distribution of sites that receive input from sensory neurons originating in white and brown fat and demonstrated significant overlap between these areas and those that represent the origins of sympathetic efferents to white fat [100]. Most recently there is anatomic [19] and functional [21, 22] evidence of central integration of neural control of white and brown fat.
It was assumed that sensory nerves informed the brain of the size of fat depots, and this was tested by Shi et al [41] who used capsaicin injections to sensory denervate inguinal or epididymal fat in long-day housed Siberian hamsters. Sensory denervation did not change the weight of either the denervated fat pad or of distant, untreated fat pads, but did increase adipocyte size in the denervated pad. This was interpreted as representing a decrease in lipolysis caused by loss of a hypothesized communication between sensory and sympathetic innervation of the fat depot. It was assumed that the sensory nerves provided central control systems with information on the size of body fat stores and to test this a second sensory denervation study directly compared the response to bilateral epididymal sensory denervation with that produced by removal of the epididymal fat (lipectomy) [101]. It is well established that removal of fat pads from hamsters, rats or mice results in a compensatory increase in the size of remaining fat depots in an attempt to correct total body fat [102]. Selective sensory denervation of white fat resulted in an identical pattern of compensation as was found for lipectomy, even though sensory denervation reduced epididymal fat CGRP by only 50%. Removal or sensory denervation of epididymal fat increased the size of retroperitoneal and inguinal fat and sensory denervation also caused an increase in the size of the denervated pad. Thus, these experiments confirmed not only an essential role for a sympathetic-sensory feedback loop for specific fat pads, but also a contribution of information derived from individual white fat pads to the integrated control of total body fat mass.
Denervation and communication between fat depots
As described above, in 2005 we reported a study that was a collaboration with the Bartness laboratory, testing the effect of selectively sympathectomizing one fat pad on its response to leptin [27]. An unexpected result was that sympathetic denervation of one inguinal or one retroperitoneal fat pad changed the size and leptin responsiveness of other intact, distant fat depots. These data suggested that there was neural communication between the fat depots which led us to test the impact of sympathetic denervation of one fat depot on the NE content and turnover (NETO) in other intact fat pads [28]. The study included mice in which one or both inguinal or epididymal fat pads were subjected to chemical sympathectomy with 6OHDA. Denervation of one inguinal pad resulted in a decrease in NE content of both the treated pad and the contralateral, intact inguinal pad and in IBAT measured 4 weeks after the surgery. It also led to an increase in weight of the contralateral inguinal pad, both epididymal pads, perirenal and mesenteric fat. None of these effects were apparent when both inguinal pads were denervated, but there was a small increase in the weight of IBAT. The increase in weight of denervated pads was associated with an increase in the number of small fat cells (20–60 um diameter), consistent with loss of sympathetic drive and cell proliferation, whereas the increased weight of epididymal fat and mesenteric fat of mice with one denervated inguinal pad was associated with an increase in the number of larger fat cells (>70um diameter) consistent with increased lipid filling, an effect that was previously observed following sensory denervation [101]. By contrast, denervation of epididymal fat had little effect on the NE content or size of distant fat depots. A reduction in NE content was limited to the injected pad, ipsilateral retroperitoneal pads and IBAT with no change in the weight or fat cell size in any fat depot. When norepinephrine turnover (NETO) was measured in different fat pads of mice that had both inguinal or both epididymal fat pads denervated, there was a suppression of NETO in inguinal, retroperitoneal and IBAT irrespective of which depot had been denervated. NETO was too low to measure in epididymal fat, even before it was denervated, but denervation still inhibited NETO in the distant fat depots.
The results of this study implied that the sympathetic outflow to different fat pads is a coordinated event. It seemed likely that sensory neurons in a white fat pad detected some aspect of the metabolic response to sympathetic activation and that central integration of information from distributed fat depots determined the outflow to other depots. Specifically, the ability of a change in sympathetic activity in one fat pad to decrease NE content or NETO of other pads is presumed to result from a sensory signal from the denervated pad. Recently, electrophysiology has shown that increased sympathetic activity in inguinal fat causes an increase in activity of the afferent nerves from the same fat pad [21]. The identity of the signal monitored by the sensory nerves has not been defined, but a product of lipolysis has been implicated [21]. In the mouse study, described above, sympathetic denervation of one inguinal fat pad was enough to change central control of sympathetic drive to multiple fat depots resulting in a decrease in lipolysis and an increase in fat cell size. Interpretation of a low rate of lipolysis as a stimulant for fat storage may imply either that the low rate of lipolysis is indicative of exhaustion of lipid stores in fat depots and inhibition of further lipolysis is a survival mechanism, or that there are adequate alternate substrates available to support metabolism and that there is no additional need for release of lipid from fat depots. The difference in response to denervation of one inguinal versus one epididymal fat depot [28] implies that different fat depots play different roles in homeostasis. This notion is supported by evidence from rodents that loss of epididymal fat (a depot that has no direct equivalent in humans [103]) may result in infertility [104], whereas loss of inguinal fat increases risk for insulin resistance [105].
The observation that cell number increased only in inguinal pads that were denervated, even though there was an equivalent drop in NETO of inguinal fat from mice in which with epididymal fat was denervated [28] suggests that fat cell number and size are controlled by factors other than NE, but that are associated with the sympathetic innervation of adipose tissue. Cousin et al [72] reported an increase in the ratio of α2- to β-adrenergic receptors in retroperitoneal fat that had been denervated and, as discussed above, it has since been shown that α2-receptors support preadipocyte proliferation in preference to adipocyte hypertrophy [88]. These changes in receptor expression could potentially explain the proliferation in the denervated fat pad, but do not explain why a decline in NETO with an intact sympathetic nerve supports enlargement of existing adipocytes. One possible explanation is that sympathetic nerve terminals release NPY in addition to NE and NPY has been shown to promote lipid filling of adipocytes [106]. In the fat depots that were denervated the sympathetic terminals were destroyed and incapable of releasing any neuropeptide, whereas the intact contralateral pads had a low level of NE release but would have remained capable of releasing other neuropeptides, including NPY. As the sensory nerves must act indirectly to modify metabolism in a way that increases fat cell size an alternate interpretation is that the decline in sympathetic activity in intact fat depots was small compared with that produced by sympathetic denervation. It is possible that there is a graded response to sympathetic activity in white fat such that a small or moderate drop in activity inhibits lipolysis and results in hypertrophy, whereas denervation causes a major decline in sympathetic activity which results in a drop in both lipolysis and an increase in proliferation. This would be consistent with the observation that although denervation of one inguinal pad reduced the NE content of multiple fat depots, adipocyte proliferation was apparent only in the denervated pad [28].
Conclusions
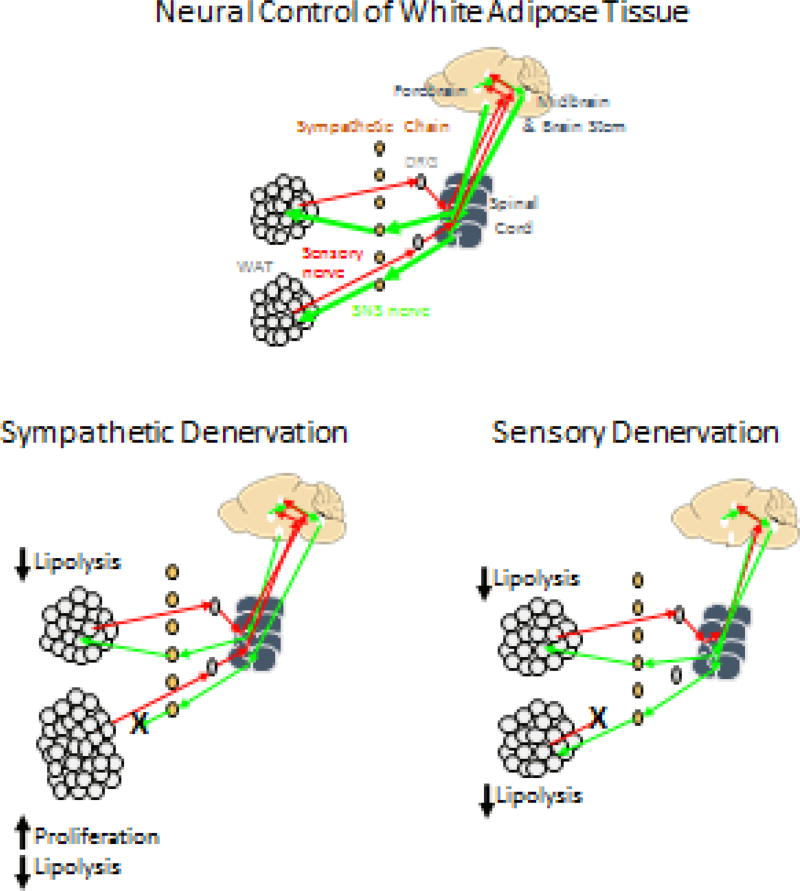
A large body of work from the Bartness lab over the last two decades has demonstrated an anatomically diverse, but coordinated, control of white and brown adipose tissue mass by the sympathetic nervous system (see [42, 107] for reviews). Denervation of specific fat depots played a crucial role in demonstrating that sympathetic nerves control lipolysis and thus fat cell size in addition to inhibiting proliferation, whereas sensory feedback determines sympathetic outflow which ultimately controls lipolysis and fat cell size (see Figure 1). The use of chemical denervation to selectively destroy sympathetic or sensory nerves in specific fat depots has proven invaluable in testing the impact of environmental and physiological conditions on the control of fat cell size [41], number [79] and metabolism [46] and also in testing for interactions between hormones and the sympathetic nervous system [27]. Selective denervation of individual pads has also proven essential to the demonstration of a feedback loop between sympathetic and sensory systems [101] and for the communication between different fat depots [28] (see Figure 1). This communication between individual fat depots allows for a coordinated control of total body fat stores, but also has recently been shown to coordinate metabolic and thermogenic responses to physiological challenges [21, 22].
Figure 1.
Schematic representation of sympathetic and sensory innervation of white adipose tissue and the response to selective sympathetic or sensory denervation. Sympathetic denervation of one pad results in an enlargement of the denervated pad due to a decrease in lipolysis, which allows enlargement of existing cells, and an increase in fat cell number. Distant fat depots enlarge due to a decrease in lipolysis, but no change in fat cell number. Sensory denervation of one fat pad results in a reduction in sympathetic drive to multiple fat pads which increases lipolysis and increases fat cell size.
Highlights.
Denervation has demonstrated control of lipolysis by sympathetic nerves
Denervation has demonstrated that preadipocyte proliferation by sympathetic nerves
Denervation has demonstrated feedback between sympathetic and sensory nerves in white fat
Denervation has provided evidence of communication between individual fat depots
Acknowledgments
This work was supported in part by National Institutes of Health grants R01DK109997 and R01DK053903 awarded to RBSH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Campbell CS, Tabor J, Davis JD. Small effect of brown adipose tissue and major effect of photoperiod on body weight in hamsters (Mesocricetus auratus) Physiol Behav. 1983;30:349–352. doi: 10.1016/0031-9384(83)90137-3. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman K. The influence of photoperiod and melatonin on testis size, body weight and pelage color in the Djungarian hamster (Phodopus sungorus) Journal of Comparative Physiology. 1973;85:267–282. [Google Scholar]
- 3.Wade GN, Bartness TJ. Effects of photoperiod and gonadectomy on food intake, body weight, and body composition in Siberian hamsters. Am J Physiol. 1984;246:R26–30. doi: 10.1152/ajpregu.1984.246.1.R26. [DOI] [PubMed] [Google Scholar]
- 4.Bartness TJ, Goldman BD. Effects of melatonin on long-day responses in short-day housed adult Siberian hamsters. Am J Physiol. 1988;255:R823–830. doi: 10.1152/ajpregu.1988.255.5.R823. [DOI] [PubMed] [Google Scholar]
- 5.Bartness TJ, Goldman BD. Peak duration of serum melatonin and short-day responses in adult Siberian hamsters. Am J Physiol. 1988;255:R812–822. doi: 10.1152/ajpregu.1988.255.5.R812. [DOI] [PubMed] [Google Scholar]
- 6.Bartness TJ, Hamilton JM, Wade GN, Goldman BD. Regional differences in fat pad responses to short days in Siberian hamsters. Am J Physiol. 1989;257:R1533–1540. doi: 10.1152/ajpregu.1989.257.6.R1533. [DOI] [PubMed] [Google Scholar]
- 7.Carter DS, Goldman BD. Progonadal role of the pineal in the Djungarian hamster (Phodopus sungorus sungorus): mediation by melatonin. Endocrinology. 1983;113:1268–1273. doi: 10.1210/endo-113-4-1268. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann K. Photoperiodic effects in the Djungarian hamster: one minute of light during dark time mimics influence of long photoperiods on testicular recrudescence, body weight and pelage colour. Experientia. 1979;35:1529–1530. doi: 10.1007/BF01962828. [DOI] [PubMed] [Google Scholar]
- 9.Bartness TJ, Wade GN, Goldman BD. Are the short-photoperiod-induced decreases in serum prolactin responsible for the seasonal changes in energy balance in Syrian and Siberian hamsters? J Exp Zool. 1987;244:437–454. doi: 10.1002/jez.1402440310. [DOI] [PubMed] [Google Scholar]
- 10.Bartness TJ, McGriff WR, Maharaj MP. Effects of diabetes and insulin on photoperiodic responses in Siberian hamsters. Physiol Behav. 1991;49:613–620. doi: 10.1016/0031-9384(91)90287-x. [DOI] [PubMed] [Google Scholar]
- 11.O'Jile JR, Bartness TJ. Effects of thyroxine on the photoperiodic control of energy balance and reproductive status in Siberian hamsters. Physiol Behav. 1992;52:267–270. doi: 10.1016/0031-9384(92)90269-8. [DOI] [PubMed] [Google Scholar]
- 12.Demas GE, Bartness TJ. Direct innervation of white fat and adrenal medullary catecholamines mediate photoperiodic changes in body fat. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1499–1505. doi: 10.1152/ajpregu.2001.281.5.R1499. [DOI] [PubMed] [Google Scholar]
- 13.Song CK, Bartness TJ. CNS sympathetic outflow neurons to white fat that express MEL receptors may mediate seasonal adiposity. Am J Physiol Regul Integr Comp Physiol. 2001;281:R666–672. doi: 10.1152/ajpregu.2001.281.2.R666. [DOI] [PubMed] [Google Scholar]
- 14.Youngstrom TG, Bartness TJ. Catecholaminergic innervation of white adipose tissue in Siberian hamsters. Am J Physiol. 1995;268:R744–751. doi: 10.1152/ajpregu.1995.268.3.R744. [DOI] [PubMed] [Google Scholar]
- 15.Bamshad M, Aoki VT, Adkison MG, Warren WS, Bartness TJ. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am J Physiol. 1998;275:R291–299. doi: 10.1152/ajpregu.1998.275.1.R291. [DOI] [PubMed] [Google Scholar]
- 16.Bartness TJ, Song CK. Thematic review series: adipocyte biology. Sympathetic and sensory innervation of white adipose tissue. J Lipid Res. 2007;48:1655–1672. doi: 10.1194/jlr.R700006-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol. 1999;276:R1569–1578. doi: 10.1152/ajpregu.1999.276.6.R1569. [DOI] [PubMed] [Google Scholar]
- 18.Vaughan CH, Bartness TJ. Anterograde transneuronal viral tract tracing reveals central sensory circuits from brown fat and sensory denervation alters its thermogenic responses. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1049–1058. doi: 10.1152/ajpregu.00640.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryu V, Watts AG, Xue B, Bartness TJ. Bidirectional crosstalk between the sensory and sympathetic motor systems innervating brown and white adipose tissue in male Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2017;312:R324–R337. doi: 10.1152/ajpregu.00456.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brito NA, Brito MN, Bartness TJ. Differential sympathetic drive to adipose tissues after food deprivation, cold exposure or glucoprivation. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1445–1452. doi: 10.1152/ajpregu.00068.2008. [DOI] [PubMed] [Google Scholar]
- 21.Garretson JT, Szymanski LA, Schwartz GJ, Xue B, Ryu V, Bartness TJ. Lipolysis sensation by white fat afferent nerves triggers brown fat thermogenesis. Mol Metab. 2016;5:626–634. doi: 10.1016/j.molmet.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen NL, Barr CL, Ryu V, Cao Q, Xue B, Bartness TJ. Separate and shared sympathetic outflow to white and brown fat coordinately regulates thermoregulation and beige adipocyte recruitment. Am J Physiol Regul Integr Comp Physiol. 2017;312:R132–R145. doi: 10.1152/ajpregu.00344.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaughan CH, Zarebidaki E, Ehlen JC, Bartness TJ. Analysis and measurement of the sympathetic and sensory innervation of white and brown adipose tissue. Methods Enzymol. 2014;537:199–225. doi: 10.1016/B978-0-12-411619-1.00011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennett T, Burnstock G, Cobb JL, Malmfors T. An ultrastructural and histochemical study of the short -term effects of 6-hydroxydopamine on adrenergic nerves in the domestic fowl. Br J Pharmacol. 1970;38:802–809. doi: 10.1111/j.1476-5381.1970.tb09889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jonsson G, Sachs C. Effects of 6-hydroxydopamine on the uptake and storage of noradrenaline in sympathetic adrenergic neurons. Eur J Pharmacol. 1970;9:141–155. doi: 10.1016/0014-2999(70)90293-1. [DOI] [PubMed] [Google Scholar]
- 26.Kostrzewa RM, Jacobowitz DM. Pharmacological actions of 6-hydroxydopamine. Pharmacol Rev. 1974;26:199–288. [PubMed] [Google Scholar]
- 27.Rooks CR, Penn DM, Kelso E, Bowers RR, Bartness TJ, Harris RB. Sympathetic denervation does not prevent a reduction in fat pad size of rats or mice treated with peripherally administered leptin. Am J Physiol Regul Integr Comp Physiol. 2005;289:R92–102. doi: 10.1152/ajpregu.00858.2004. [DOI] [PubMed] [Google Scholar]
- 28.Harris RB. Sympathetic denervation of one white fat depot changes norepinephrine content and turnover in intact white and brown fat depots. Obesity (Silver Spring) 2012;20:1355–1364. doi: 10.1038/oby.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thureson-Klein A, Lagercrantz H, Barnard T. Chemical sympathectomy of interscapular brown adipose tissue. Acta Physiol Scand. 1976;98:8–18. doi: 10.1111/j.1748-1716.1976.tb10296.x. [DOI] [PubMed] [Google Scholar]
- 30.Finch L, Haeusler G, Thoenen H. A comparison of the effects of chemical sympathectomy by 6-hydroxydopamine in newborn and adult rats. Br J Pharmacol. 1973;47:249–260. doi: 10.1111/j.1476-5381.1973.tb08322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demas GE, Bartness TJ. Novel method for localized, functional sympathetic nervous system denervation of peripheral tissue using guanethidine. J Neurosci Methods. 2001;112:21–28. doi: 10.1016/s0165-0270(01)00452-6. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell JR, Oates JA. Guanethidine and related agents. I. Mechanism of the selective blockade of adrenergic neurons and its antagonism by drugs. J Pharmacol Exp Ther. 1970;172:100–107. [PubMed] [Google Scholar]
- 33.Angeletti PU, Levi-Montalcini R, Caramia F. Structural and ultrastructural changes in developing sympathetic ganglia induced by guanethidine. Brain Res. 1972;43:515–525. doi: 10.1016/0006-8993(72)90405-2. [DOI] [PubMed] [Google Scholar]
- 34.Picklo MJ. Methods of sympathetic degeneration and alteration. J Auton Nerv Syst. 1997;62:111–125. doi: 10.1016/s0165-1838(96)00121-x. [DOI] [PubMed] [Google Scholar]
- 35.Evans BK, Heath JW, Burnstock G. Reinnervation following guanethidine-induced sympathectomy of adult rats. J Neurocytol. 1979;8:381–400. doi: 10.1007/BF01236127. [DOI] [PubMed] [Google Scholar]
- 36.Rooks C, Bennet T, Bartness TJ, Harris RB. Compensation for an increase in body fat caused by donor transplants into mice. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1149–1155. doi: 10.1152/ajpregu.00634.2003. [DOI] [PubMed] [Google Scholar]
- 37.Nascimento FP, Magnussen C, Yousefpour N, Ribeiro-da-Silva A. Sympathetic fibre sprouting in the skin contributes to pain-related behaviour in spared nerve injury and cuff models of neuropathic pain. Mol Pain. 2015;11:59. doi: 10.1186/s12990-015-0062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Consigny PM, Davalian D, Donn R, Hu J, Rieser M, Stolarik D. Chemical renal denervation in the rat. Cardiovasc Intervent Radiol. 2014;37:218–223. doi: 10.1007/s00270-013-0796-7. [DOI] [PubMed] [Google Scholar]
- 39.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 40.Cervero F, McRitchie HA. Neonatal capsaicin does not affect unmyelinated efferent fibers of the autonomic nervous system: functional evidence. Brain Res. 1982;239:283–288. doi: 10.1016/0006-8993(82)90853-8. [DOI] [PubMed] [Google Scholar]
- 41.Shi H, Song CK, Giordano A, Cinti S, Bartness TJ. Sensory or sympathetic white adipose tissue denervation differentially affects depot growth and cellularity. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1028–1037. doi: 10.1152/ajpregu.00648.2004. [DOI] [PubMed] [Google Scholar]
- 42.Bartness TJ, Liu Y, Shrestha YB, Ryu V. Neural innervation of white adipose tissue and the control of lipolysis. Front Neuroendocrinol. 2014;35:473–493. doi: 10.1016/j.yfrne.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beznak ABL, Hasch Z. The effect of sympathectomy on the fatty deposit in connective tissue. Experimental Physiology. 1937;27:1–15. [Google Scholar]
- 44.Cantu RC, Goodman HM. Effects of denervation and fasting on white adipose tissue. Am J Physiol. 1967;212:207–212. doi: 10.1152/ajplegacy.1967.212.1.207. [DOI] [PubMed] [Google Scholar]
- 45.Garofalo MA, Kettelhut IC, Roselino JE, Migliorini RH. Effect of acute cold exposure on norepinephrine turnover rates in rat white adipose tissue. J Auton Nerv Syst. 1996;60:206–208. doi: 10.1016/0165-1838(96)00037-9. [DOI] [PubMed] [Google Scholar]
- 46.Migliorini RH, Garofalo MA, Kettelhut IC. Increased sympathetic activity in rat white adipose tissue during prolonged fasting. Am J Physiol. 1997;272:R656–661. doi: 10.1152/ajpregu.1997.272.2.R656. [DOI] [PubMed] [Google Scholar]
- 47.Lazzarini SJ, Wade GN. Role of sympathetic nerves in effects of estradiol on rat white adipose tissue. Am J Physiol. 1991;260:R47–51. doi: 10.1152/ajpregu.1991.260.1.R47. [DOI] [PubMed] [Google Scholar]
- 48.Correll JW. Adipose tissue: ability to respond to nerve stimulation in vitro. Science. 1963;140:387–388. doi: 10.1126/science.140.3565.387. [DOI] [PubMed] [Google Scholar]
- 49.Teixeira VL, Antunes-Rodrigues J, Migliorini RH. Evidence for centers in the central nervous system that selectively regulate fat mobilization in the rat. J Lipid Res. 1973;14:672–677. [PubMed] [Google Scholar]
- 50.Gross JL, Migliorini RH. Further evidence for a central regulation of free fatty acid mobilization in the rat. Am J Physiol. 1977;232:E165–171. doi: 10.1152/ajpendo.1977.232.2.E165. [DOI] [PubMed] [Google Scholar]
- 51.Girousse A, Langin D. Adipocyte lipases and lipid droplet-associated proteins: insight from transgenic mouse models. Int J Obes (Lond) 2012;36:581–594. doi: 10.1038/ijo.2011.113. [DOI] [PubMed] [Google Scholar]
- 52.Cone RD, Mountjoy KG, Robbins LS, Nadeau JH, Johnson KR, Roselli-Rehfuss L, Mortrud MT. Cloning and functional characterization of a family of receptors for the melanotropic peptides. Annals of the New York Academy of Sciences. 1993;680:342–363. doi: 10.1111/j.1749-6632.1993.tb19694.x. [DOI] [PubMed] [Google Scholar]
- 53.Voss-Andreae A, Murphy JG, Ellacott KL, Stuart RC, Nillni EA, Cone RD, Fan W. Role of the central melanocortin circuitry in adaptive thermogenesis of brown adipose tissue. Endocrinology. 2007;148:1550–1560. doi: 10.1210/en.2006-1389. [DOI] [PubMed] [Google Scholar]
- 54.Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 55.Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Molecular Endocrinology. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 56.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 57.Haynes WG, Morgan DA, Djalali A, Sivitz WI, Mark AL. Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension. 1999;33:542–547. doi: 10.1161/01.hyp.33.1.542. [DOI] [PubMed] [Google Scholar]
- 58.Song CK, Jackson RM, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA is expressed in sympathetic nervous system outflow neurons to white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1467–1476. doi: 10.1152/ajpregu.00348.2005. [DOI] [PubMed] [Google Scholar]
- 59.Song CK, Vaughan CH, Keen-Rhinehart E, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA expressed in sympathetic outflow neurons to brown adipose tissue: neuroanatomical and functional evidence. Am J Physiol Regul Integr Comp Physiol. 2008;295:R417–428. doi: 10.1152/ajpregu.00174.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shrestha YB, Vaughan CH, Smith BJ, Jr, Song CK, Baro DJ, Bartness TJ. Central melanocortin stimulation increases phosphorylated perilipin A and hormone-sensitive lipase in adipose tissues. Am J Physiol Regul Integr Comp Physiol. 2010;299:R140–149. doi: 10.1152/ajpregu.00535.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology. 2007;148:5339–5347. doi: 10.1210/en.2007-0621. [DOI] [PubMed] [Google Scholar]
- 62.Lafontan M, Berlan M. Fat cell alpha 2-adrenoceptors: the regulation of fat cell function and lipolysis. Endocr Rev. 1995;16:716–738. doi: 10.1210/edrv-16-6-716. [DOI] [PubMed] [Google Scholar]
- 63.Carpene C, Bousquet-Melou A, Galitzky J, Berlan M, Lafontan M. Lipolytic effects of beta 1-, beta 2-, and beta 3-adrenergic agonists in white adipose tissue of mammals. Ann N Y Acad Sci. 1998;839:186–189. doi: 10.1111/j.1749-6632.1998.tb10756.x. [DOI] [PubMed] [Google Scholar]
- 64.Lafontan M, Berlan M. Do regional differences in adipocyte biology provide new pathophysiological insights? Trends Pharmacol Sci. 2003;24:276–283. doi: 10.1016/S0165-6147(03)00132-9. [DOI] [PubMed] [Google Scholar]
- 65.Langin D, Tavernier G, Lafontan M. Regulation of beta 3-adrenoceptor expression in white fat cells. Fundam Clin Pharmacol. 1995;9:97–106. doi: 10.1111/j.1472-8206.1995.tb00268.x. [DOI] [PubMed] [Google Scholar]
- 66.Robidoux J, Pirouzi P, Lafond J, Savard R. Site-specific effects of sympathectomy on the adrenergic control of lipolysis in hamster fat cells. Can J Physiol Pharmacol. 1995;73:450–458. doi: 10.1139/y95-057. [DOI] [PubMed] [Google Scholar]
- 67.Lefebvre P, Luyckx A, Bacq ZM. Effects of denervation on the metabolism and the response to glucagon of white adipose tissue of rats. Horm Metab Res. 1973;5:245–250. doi: 10.1055/s-0028-1093959. [DOI] [PubMed] [Google Scholar]
- 68.Stich V, Pelikanova T, Wohl P, Sengenes C, Zakaroff-Girard A, Lafontan M, Berlan M. Activation of alpha2-adrenergic receptors blunts epinephrine-induced lipolysis in subcutaneous adipose tissue during a hyperinsulinemic euglycemic clamp in men. Am J Physiol Endocrinol Metab. 2003;285:E599–607. doi: 10.1152/ajpendo.00502.2002. [DOI] [PubMed] [Google Scholar]
- 69.Engfeldt P, Hellmer J, Wahrenberg H, Arner P. Effects of insulin on adrenoceptor binding and the rate of catecholamine-induced lipolysis in isolated human fat cells. J Biol Chem. 1988;263:15553–15560. [PubMed] [Google Scholar]
- 70.Ahmed K, Tunaru S, Tang C, Muller M, Gille A, Sassmann A, Hanson J, Offermanns S. An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell Metab. 2010;11:311–319. doi: 10.1016/j.cmet.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 71.Jones DD, Ramsay TG, Hausman GJ, Martin RJ. Norepinephrine inhibits rat pre-adipocyte proliferation. Int J Obes Relat Metab Disord. 1992;16:349–354. [PubMed] [Google Scholar]
- 72.Cousin B, Casteilla L, Lafontan M, Ambid L, Langin D, Berthault MF, Penicaud L. Local sympathetic denervation of white adipose tissue in rats induces preadipocyte proliferation without noticeable changes in metabolism. Endocrinology. 1993;133:2255–2262. doi: 10.1210/endo.133.5.8404678. [DOI] [PubMed] [Google Scholar]
- 73.Youngstrom TG, Bartness TJ. White adipose tissue sympathetic nervous system denervation increases fat pad mass and fat cell number. Am J Physiol. 1998;275:R1488–1493. doi: 10.1152/ajpregu.1998.275.5.R1488. [DOI] [PubMed] [Google Scholar]
- 74.DiGirolamo M, Fine JB, Tagra K, Rossmanith R. Qualitative regional differences in adipose tissue growth and cellularity in male Wistar rats fed ad libitum. Am J Physiol. 1998;274:R1460–1467. doi: 10.1152/ajpregu.1998.274.5.R1460. [DOI] [PubMed] [Google Scholar]
- 75.Fried SK, Russell CD, Grauso NL, Brolin RE. Lipoprotein lipase regulation by insulin and glucocorticoid in subcutaneous and omental adipose tissues of obese women and men. J Clin Invest. 1993;92:2191–2198. doi: 10.1172/JCI116821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93:S57–63. doi: 10.1210/jc.2008-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bowers RR, Festuccia WT, Song CK, Shi H, Migliorini RH, Bartness TJ. Sympathetic innervation of white adipose tissue and its regulation of fat cell number. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1167–1175. doi: 10.1152/ajpregu.00558.2003. [DOI] [PubMed] [Google Scholar]
- 78.Joe AW, Yi L, Even Y, Vogl AW, Rossi FM. Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells. 2009;27:2563–2570. doi: 10.1002/stem.190. [DOI] [PubMed] [Google Scholar]
- 79.Foster MT, Bartness TJ. Sympathetic but not sensory denervation stimulates white adipocyte proliferation. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1630–1637. doi: 10.1152/ajpregu.00197.2006. [DOI] [PubMed] [Google Scholar]
- 80.Wright JT, Hausman GJ. Monoclonal antibodies against cell surface antigens expressed during porcine adipocyte differentiation. Int J Obes. 1990;14:395–409. [PubMed] [Google Scholar]
- 81.Cousin B, Bascands-Viguerie N, Kassis N, Nibbelink M, Ambid L, Casteilla L, Penicaud L. Cellular changes during cold acclimatation in adipose tissues. J Cell Physiol. 1996;167:285–289. doi: 10.1002/(SICI)1097-4652(199605)167:2<285::AID-JCP12>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 82.Bukowiecki L, Collet AJ, Follea N, Guay G, Jahjah L. Brown adipose tissue hyperplasia: a fundamental mechanism of adaptation to cold and hyperphagia. Am J Physiol. 1982;242:E353–359. doi: 10.1152/ajpendo.1982.242.6.E353. [DOI] [PubMed] [Google Scholar]
- 83.Loncar D, Afzelius BA, Cannon B. Epididymal white adipose tissue after cold stress in rats. I. Nonmitochondrial changes. J Ultrastruct Mol Struct Res. 1988;101:109–122. doi: 10.1016/0889-1605(88)90001-8. [DOI] [PubMed] [Google Scholar]
- 84.Lafontan M, Langin D. Cellular aspects of fuel mobilization and selection in white adipocytes. Proc Nutr Soc. 1995;54:49–63. doi: 10.1079/pns19950037. [DOI] [PubMed] [Google Scholar]
- 85.Wang QA, Scherer PE. The AdipoChaser mouse: A model tracking adipogenesis in vivo. Adipocyte. 2014;3:146–150. doi: 10.4161/adip.27656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Castan I, Valet P, Quideau N, Voisin T, Ambid L, Laburthe M, Lafontan M, Carpene C. Antilipolytic effects of alpha 2-adrenergic agonists, neuropeptide Y, adenosine, and PGE1 in mammal adipocytes. Am J Physiol. 1994;266:R1141–1147. doi: 10.1152/ajpregu.1994.266.4.R1141. [DOI] [PubMed] [Google Scholar]
- 87.Lafontan M, Bousquet-Melou A, Galitzky J, Barbe P, Carpene C, Langin D, Berlan M, Valet P, Castan I, Bouloumie A, et al. Adrenergic receptors and fat cells: differential recruitment by physiological amines and homologous regulation. Obes Res. 1995;3(Suppl 4):507S–514S. doi: 10.1002/j.1550-8528.1995.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 88.Valet P, Grujic D, Wade J, Ito M, Zingaretti MC, Soloveva V, Ross SR, Graves RA, Cinti S, Lafontan M, Lowell BB. Expression of human alpha 2-adrenergic receptors in adipose tissue of beta 3-adrenergic receptor-deficient mice promotes diet-induced obesity. J Biol Chem. 2000;275:34797–34802. doi: 10.1074/jbc.M005210200. [DOI] [PubMed] [Google Scholar]
- 89.Bouloumie A, Planat V, Devedjian JC, Valet P, Saulnier-Blache JS, Record M, Lafontan M. Alpha 2-adrenergic stimulation promotes preadipocyte proliferation. Involvement of mitogen-activated protein kinases. J Biol Chem. 1994;269:30254–30259. [PubMed] [Google Scholar]
- 90.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 91.Harris RB, Zhou J, Redmann SM, Jr, Smagin GN, Smith SR, Rodgers E, Zachwieja JJ. A leptin dose-response study in obese (ob/ob) and lean (+/?) mice. Endocrinology. 1998;139:8–19. doi: 10.1210/endo.139.1.5675. [DOI] [PubMed] [Google Scholar]
- 92.Commins SP, Watson PM, Frampton IC, Gettys TW. Leptin selectively reduces white adipose tissue in mice via a UCP1-dependent mechanism in brown adipose tissue. Am J Physiol Endocrinol Metab. 2001;280:E372–377. doi: 10.1152/ajpendo.2001.280.2.E372. [DOI] [PubMed] [Google Scholar]
- 93.Ste Marie L, Miura GI, Marsh DJ, Yagaloff K, Palmiter RD. A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc Natl Acad Sci U S A. 2000;97:12339–12344. doi: 10.1073/pnas.220409497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bowen H, Mitchell TD, Harris RB. Method of leptin dosing, strain, and group housing influence leptin sensitivity in high-fat-fed weanling mice. Am J Physiol Regul Integr Comp Physiol. 2003;284:R87–100. doi: 10.1152/ajpregu.00431.2002. [DOI] [PubMed] [Google Scholar]
- 95.Wang ZW, Zhou YT, Lee Y, Higa M, Kalra SP, Unger RH. Hyperleptinemia depletes fat from denervated fat tissue. Biochem Biophys Res Commun. 1999;260:653–657. doi: 10.1006/bbrc.1999.0918. [DOI] [PubMed] [Google Scholar]
- 96.Penn DM, Jordan LC, Kelso EW, Davenport JE, Harris RB. Effects of central or peripheral leptin administration on norepinephrine turnover in defined fat depots. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1613–1621. doi: 10.1152/ajpregu.00368.2006. [DOI] [PubMed] [Google Scholar]
- 97.Murphy KT, Schwartz GJ, Nguyen NL, Mendez JM, Ryu V, Bartness TJ. Leptin Sensitive Sensory Nerves Innervate White Fat. Am J Physiol Endocrinol Metab. 2013 doi: 10.1152/ajpendo.00021.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fishman RB, Dark J. Sensory innervation of white adipose tissue. Am J Physiol. 1987;253:R942–944. doi: 10.1152/ajpregu.1987.253.6.R942. [DOI] [PubMed] [Google Scholar]
- 99.Giordano A, Morroni M, Santone G, Marchesi GF, Cinti S. Tyrosine hydroxylase, neuropeptide Y, substance P, calcitonin gene-related peptide and vasoactive intestinal peptide in nerves of rat periovarian adipose tissue: an immunohistochemical and ultrastructural investigation. J Neurocytol. 1996;25:125–136. doi: 10.1007/BF02284791. [DOI] [PubMed] [Google Scholar]
- 100.Ryu V, Bartness TJ. Short and long sympathetic-sensory feedback loops in white fat. Am J Physiol Regul Integr Comp Physiol. 2014;306:R886–900. doi: 10.1152/ajpregu.00060.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shi H, Bartness TJ. White adipose tissue sensory nerve denervation mimics lipectomy-induced compensatory increases in adiposity. Am J Physiol Regul Integr Comp Physiol. 2005;289:R514–R520. doi: 10.1152/ajpregu.00036.2005. [DOI] [PubMed] [Google Scholar]
- 102.Mauer MM, Harris RB, Bartness TJ. The regulation of total body fat: lessons learned from lipectomy studies. Neurosci Biobehav Rev. 2001;25:15–28. doi: 10.1016/s0149-7634(00)00047-6. [DOI] [PubMed] [Google Scholar]
- 103.Chusyd DE, Wang D, Huffman DM, Nagy TR. Relationships between Rodent White Adipose Fat Pads and Human White Adipose Fat Depots. Front Nutr. 2016;3:10. doi: 10.3389/fnut.2016.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chu Y, Huddleston GG, Clancy AN, Harris RB, Bartness TJ. Epididymal fat is necessary for spermatogenesis, but not testosterone production or copulatory behavior. Endocrinology. 2010;151:5669–5679. doi: 10.1210/en.2010-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang W, Cline MA, Gilbert ER. Hypothalamus-adipose tissue crosstalk: neuropeptide Y and the regulation of energy metabolism. Nutr Metab (Lond) 2014;11:27. doi: 10.1186/1743-7075-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bartness TJ, Ryu V. Neural control of white, beige and brown adipocytes. Int J Obes Suppl. 2015;5:S35–39. doi: 10.1038/ijosup.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]