Abstract
Dr. Basil A. Pruitt Jr., a consummate clinical and translational surgeon-scientist, has been spent over half a century at the forefront of an advancing standard of burn care. Commanding the US Army Institute for Surgical Research in San Antonio, he trained generations of leading burn clinicians and allied scientists. At his direction, there were forged discoveries in resuscitation from shock, treatment of inhalation injury, control of burn-related infections, prevention of iatrogenic complications, and understanding the sympathetic, endocrine, and immune responses to burn injury. Most consequentially, this team was among the first recognize and define alterations in the basal metabolic rate and thermoregulation consequent to burn injury. These investigations prompted groundbreaking insights into the coordinated nervous, autonomic, endocrine, immune, and metabolic outflows that a severely-burned patient uses to remain alive and restore homeostasis. Marking his scientific consequence, many of his reports continue bear fruit when viewed through a contemporary lens. This paper summarizes some of the major findings of his career thus far, and is intended to complement a Festschrift recently held in his honor.
Keywords: metabolism, infection, smoke inhalation
Background
Great minds of the past century sparked a Renaissance that revolutionized our understanding of trauma, and consequently improved the standard of care for these injuries: Claude Bernard defined the semi-permeable membrane and the internal milieu, giving birth to the theory that constancy of an organism’s internal environment is the necessary condition for life(1). Walter B. Cannon expanded on the constancy theory to further describe this homeostasis as the interaction of complex cyclic biological processes that generate and maintain the steady-state(2, 3). David P. Cuthberson investigated the effects of injury, rest, and diet on nitrogen balance. He determined that localized injury (extremity fracture) produced negative nitrogen balance, an organism-level systemic effect, while rest and dietary composition conditioned this response (4–6). Hans Seyle showed that adrenal products, whether released in stress states or supplemented, wrought similar effects. Francis D. Moore described human body composition—the total body water, fat mass, skeletal weight, and (intracellular) body cell mass(7–9). He perceived that respiration occurs in the latter compartment alone. Taken together, this pantheon of surgical scientists put together a conceptual model: that the stable flow of biochemical energy within and through this compartment was life, and the irreversible cessation thereof was death(10). By describing how burn injuries could alter the basal metabolic rate, Basil A. Pruitt was at work in a moment in time when “the lights came on,” and his work energized the conceptual model built by these predecessors. His work with a succession of brilliant clinicians and allied scientists has extended and improved life for the burned patient in countless ways. At the dawn of his scientific career, he published a mortality review of 1,100 consecutive burns, presaging both his work on behalf of burned patients and the larger impact of these findings to the common understanding of biology(11). His corpus of research provides comprehensive description and analysis of post-injury systemic alterations, and lays the groundwork for physiological interventions that continue to define a higher standard in burn care.
The concept of hypermetabolism in burns
Spanning 60 years of progressive research, Dr. Pruitt and colleagues have been deciphering the systemic changes that occur after burn injury. In describing post-injury hypermetabolism, they showed that the burned patient enters a state of systemic activation. Post-burn hyperpyrexia, tachycardia, cardiac dysfunction, elevated energy expenditure, increased protein catabolism, peripheral wasting of muscle and fat, as well as profound alterations of the immune status resemble conditions such as those seen in patients with severe disseminated infection, hyperthyroid crisis, and other physiological “storms”. Here, we summarize a few of the main findings as we understand them.
Temperature regulation and blood flow
Burned patients need to be protected from evaporative, radiative, and conductive heat loss, partially owing to the disruption and dysregulation of their integument. However, the current understanding of the complex concept of temperature regulation after thermal injury originated consequent to the observation that burned patients have markedly elevated core and skin temperatures soon after injury. To further investigate, these patients were placed in a specialized metabolic room (laminar airflow, controlled humidity and temperature) and asked to set the environmental temperature to maximize comfort, resulting in the consistent selection of a higher room temperature, 30 degrees Celsius (12). These patients also consistently demonstrated skin and core temperature increases of almost 2 degrees Celsius above controls. These findings persisted at rest and during sleep, indicating an elevated hypothalamic temperature set point (“internally warm, not externally cold”). Taken together, these findings answered contemporary challenges attempting to attribute the elevated metabolic rate solely to the environmental conditions, pain, and agitation.
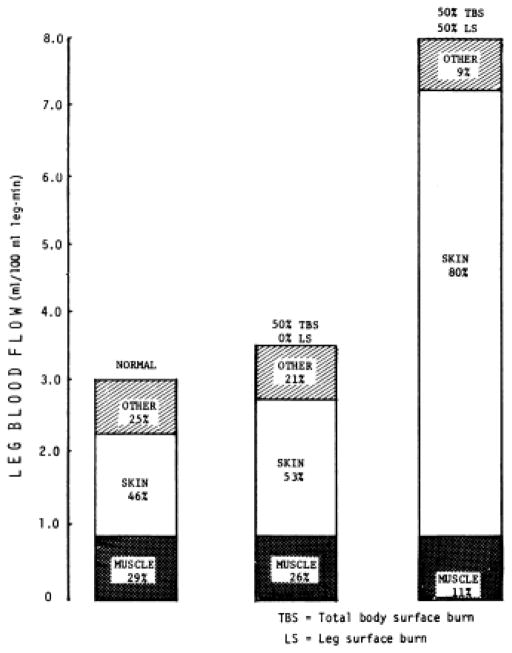
Along with elevated temperature and set-point, the course of recovery from burn injury is accompanied by increased cardiac index (whole-organism blood flow). In a group of burned patients undergoing visceral blood flow and metabolic measurements, average cardiac index was 8.2 +/− 0.5 liters/m^2 min (13). Aulick et al. demonstrated in 1977 that blood flow in the injured limbs of burn patients is increased in relation to the size of the injury, to a maximum around 8mL/min per 100mL leg volume seen with 50% surface area burn of the leg. Normal levels of blood flow were observed in unburned limbs. In both burned and unburned limbs, blood flow was modulated via variations in temperature, with increasing temperature resulting in increased blood flow and apparent intact responsiveness to sympathetic stimuli (14) These increases in blood flow correlated with increased glucose uptake and lactate production, though there were no alterations in limb oxygen consumption (15). Blood flow to the muscle groups in the burned legs was also investigated through the clearance of locally injected radioactive xenon gas clearance (Figure 1). It was determined that blood flow to the underlying muscles is not altered after burn injury (16).
Figure 1.
Leg blood flow. Taken with permission from Aulick et al. Muscle blood flow following thermal injury. Ann Surg. 1978;188(6):778. http://journals.lww.com/annalsofsurgery/pages/default.aspx
In the previously-referenced study by Wilmore and Goodwin et al., liver and kidney metabolic and blood flow measurements were performed. The patients were classified as non-infected, bacteremic, and bacteremic with organ failure complication. Hepatic blood flow was markedly elevated in non-infected burn patients and even further when bacteremia was present. However, in bacteremic cases with organ failure complications, hepatic blood flow was decreased below the level of non-infected burn patients, consistent with others’ observation of elevated hepatic resistance in shock and endotoxinemic states; this finding opened an early window into the pathophysiology of “shock liver,” more correctly termed hypoxic hepatitis(17). Oxygen uptake was elevated in the non-infected and bacteremic groups and elevated still further in the complicated bacteremic group.
Glucose output, lactate uptake, and alanine uptake were also measured. The kidney was not a net producer of glucose. Liver glucose production was elevated 1.5× in the non-infected group, 2× in the bacteremic group, and dropped to baseline values in the complicated bacteremic group. Lactate uptake was elevated >2× in the non-infected group, and still further in the bacteremic group, and was decreased to 1.5× baseline in the complicated bacteremic group. Liver alanine uptake was elevated 3× and 4× in the non-infected and bacteremic groups, respectively; but dropped to unburned values in the complicated group(13). Together with the observation that all 4 patients in the complicated bacteremic group did not survive, these findings provide a previously unseen window into the flux of biochemical energy during critical illness. They also suggest elevated substrate flux through the Cori (lactate) and Cahill (alanine) cycles, as well as associated obligatory hepatic gluconeogenesis (Table 1). After injury, concurrent with an elevated hypothalamic temperature set-point and cardiac index, occur qualitative and quantitative changes in the flow of biological energy and mass (substrate) through the organism.
Table 1. Visceral blood flow and metabolic fluxes.
Taken with permission from Wilmore et al. Effect of injury and infection on visceral metabolism and circulation. Ann Surg. 1980;192(4):491. http://journals.lww.com/annalsofsurgery/pages/default.aspx
| Normal | Noninfected Burn Patients | Bacteremic Burn Patients | Bacteremic Burn Patients with Complications | |
|---|---|---|---|---|
| ICG and ESBF | ||||
| k/min | 0.2–0.3 | 0.328 ± 0.027 | 0.273 ± 0.030 | 0.141 ± 0.021† |
| Indocyanine green dye per cent extraction | 75–90 | 67 ± 4 | 50 ± 5 | 46 ± 8 |
| Blood volume, ml/kg | 70–80 | 82.4 ± 4.8 | 81.9 ± 6.3 | 104.8 ± 14.1 |
| Estimated splanchnic blood flow, L/min·m2 | 0.63–0.85 | 1.54 ± 0.12 | 1.74 ± 0.17 | 1.19 ± 0.18 |
| Splanchnic blood flow as a per cent of cardiac index | 22–28 | 19.1 ± 1.8 | 20.1 ± 2.1 | 16.1 ± 2.5 |
| Hematocrit per cent | 39–46 | 34 ± 1 | 33 ± 1 | 33 ± 1 |
| Splanchnic Exchange | ||||
| Splanchnic VO2, ml/min·m2 | 34–40 | 68 ± 4 | 66 ± 5 | 73 ± 3 |
| Splanchnic VO2 as a per cent of total VO2 | 20–25 | 29.8 ± 1.5 | 27.8 ± 2.2 | 30.3 ± 1.5 |
| Glucose production, mM/min·m2 | 0.35–0.45 | 0.635 ± 0.035 | 0.835 ± 0.054† | 0.362 ± 0.060†‡ |
| Lactate uptake, mM/min·m2 | 0.13–0.16 | 0.377 ± 0.077 | 0.431 ± 0.107 | 0.268 ± 0.108 |
| Per cent of glucose from lactate | 20–24 | 30.5 ± 6.7 | 28.8 ± 7.4 | 45.5 ± 21.9 |
| Pyruvate uptake, mM/min·m2 | 0.005–0.010 | 0.019 ± 0.008 | 0.018 ± 0.007 | 0.011 ± 0.004 |
| Per cent glucose from pyruvate | 1–3 | 1.52 ± 0.66 | 1.20 ± 0.44 | 1.32 ± 0.40 |
| Alanine uptake, mM/min·m2 | 0.030–0.045 | 0.124 ± 0.031 | 0.213 ± 0.040 | 0.042 ± 11‡ |
| Per cent of glucose from alanine | 5–9 | 9.2 ± 2.3 | 13.2 ± 2.0 | 6.3 ± 1.5 |
Data reported as range or mean ± SEM.
Noninfected burn patients versus bacteremic burn patients, p < 0.05.
Noninfected burn patients versus bacteremic burn patients with complications, p < 0.05.
Bacteremic burn patients versus bacteremic burn patients with complications, p < 0.05.
Metabolic rate and substrate utilization
The hypermetabolic state in burned patients can be observed through measurements of resting energy expenditure. Using multiple methods of indirect calorimetry, Pruitt et al. showed that the resting metabolic rate was elevated with increasing burn size, with a maximal resting metabolic rate twice that of unburned controls (18). Subsequent studies showed that these elevations in energy requirements could be partially modulated through adjustments in environmental temperature. Burn patients treated in warm environments of 32°C exhibited reduced metabolic rates compared to those treated at 25°C, although both groups remained hypermetabolic (12). Improvements in burn care can be appreciated through the lens of decreasing energy requirements of burned patients. Comparing populations of patients burned 15 years apart (in both populations increased resting energy expenditure correlated positively with burn size) the more recent patients showed reductions in their post-burn resting energy expenditures when compared to prior cohorts (19).
The increased energy requirements due to burns lead to severe weight loss and negative nitrogen balance (20). These alterations in nutritional needs prompted research into optimal caloric and protein replacement strategies. In the setting of an adequate, constant nitrogen intake, increasing carbohydrate feeding spared protein catabolism and mitigated negative nitrogen balance, while intravenous fat emulsion did not affect nitrogen balance independently (21), (22). These studies extended the findings of Cuthbertson to burned patients, and indicated that the primary determinants of nitrogen excretion were the carbohydrate intake and the metabolic rate. Nitrogen excretion was minimized when the carbohydrate intake approximated the metabolic rate. Exogenous insulin was additive to carbohydrate in further decreasing nitrogen excretion when patients were provided constant, adequate protein intake(23).
On a cellular level, Curreri et al. deftly illustrated an increased intracellular accumulation of sodium in burned patients. Convalescent burn patients showed a slight increase from the unburned comparison group; however, in acutely burned patients fed only maximal oral intake, red blood cell (RBC) intracellular sodium increased drastically from normal <12 to >16mEq, while average caloric intake was 1,600 kcal per day. Patients receiving supplemental feedings, at 3,000–6,000 kcal per day showed normalization of this RBC intracellular sodium disturbance to levels seen in the convalescent group (24). In this under-appreciated report, the authors demonstrated a globally-important indicator of cellular energy homeostasis (intracellular sodium concentration; reflecting the activity of the Na/K ATPase) to be deranged in burned patients receiving then-standard feeding, and suggested an improved treatment that normalizes this—adequate feeding of burned patients. This study exemplifies one perspective of the tip of an iceberg that is the biochemical alterations underlying post-burn hypermetabolism.
Applying the methods of Hlad et al. (25) to burned patients, Wilmore et al. demonstrated large increases in both glucose flux, and the rate constant for glucose disappearance after thermal injury(26). However with superimposed sepsis, burned patients exhibited glucose flux and a glucose disappearance rate constant that were both below unburned controls(27). Together, these findings imply that increased metabolic flux may be necessary to rebuild injured tissues, and demonstrate the derangement wrought by superimposed systemic infection.
It was shown in 1978 that burned patients exhibit markedly increased phenylalanine flux; this being further augmented with the development of clinical sepsis. They demonstrated that conversion to tyrosine occurred normally, thus the elevated circulating levels of phenylalanine were due to massively increased release from muscle breakdown (28). Dong et al. replicated the observation of elevated circulating aromatic amino acids post-burn and even more so with superimposed sepsis/multi-organ failure(29). They correlated elevated aromatic amino acids with the serum acetoacetate/beta-hydroxybutyrate ratio (a marker for hepatocyte mitochondrial energy status) and showed a significant decrease in this ratio after post-burn day 7 for the patients who developed multi-organ failure. This decrease was not seen in those who did not develop multi-organ failure. Taken together, these works demonstrated the pervasive and vitally-important post-injury perturbations in micro- and macro-biochemistry which we may now increasingly appreciate because of Dr. Pruitt’s work. An expanding vista is now available for study whereby we may discern how an organism responds to injury, supporting explosive, regulated growth and cellular proliferation, via increased glycolytic metabolism, increased intracellular NADPH, and increased carbon skeleton precursor molecules for proliferating/growing cells. This is a different, but related, post-injury correlate of Otto Warburg’s observation of aerobic glycolysis in neoplasia(30–33). The study of genomic and post-translational mediators that orchestrate this metabolic reprogramming is an active frontier. Early data suggesting a shift in splicesome composition presage a potential major advance in our understanding of how the stress response implements metabolomic change (34–36).
Hormones and Hypothalamic Response
Manifold changes in circulating hormone levels occur in burned patients. Multiple studies demonstrated generally normal total T3 and T4, Free T3 and T4, and TSH levels in uncomplicated burn patients. However, in those patients with infection or sepsis, decreased free T4 and T3 were noted (37). T3 replacement did not grossly alter metabolic rate or mortality post-burn (38) but may have decreased the circulating levels of norepinephrine and epinephrine. These studies suggest that the hypermetabolic response to burns is independent of thyroid hormones(39), but that hypothyroidism in critically ill patients may permit, or contribute to, elevated catecholamines.
In a study of nine burn patients, it was found that there was diminished growth hormone release following both insulin hypoglycemia and arginine infusion as compared to control patients, as well as a significant increase in levels of growth hormone in fasting, hyperglycemic burn patients (40). Burned men exhibit hypogonadism and Leydig cells failure following burn injury, resulting in depressed testosterone levels(41). Burn injury results in increased estradiol levels and sex hormone-binding globulin, along with a concomitant decrease in the secretion of bioactive LH, resulting in suppression of testosterone. Together, these studies indicated a pervasive shift from normal hypothalamic function to a post-injury mobilization, a prolonged fight-or-flight response, which remains partially understood. Wilmore, Long, Mason and Pruitt wrote a review of the coordinated hypothalamic response to injury in 1976 called: “Stress in Surgical Patients as a Neurophysiological Reflex Response;” it remains as relevant today as it was on the day it was published.
Glucocorticoids are elevated following burn injury (whether measured by serum or urinary excretion markers). Regulation of glucocorticoid secretion is complex, with multiple determinants of adrenal cortex secretion of cortisol, including pituitary adrenocorticotrophic hormone (ACTH), but also afferent neural control and hyperthermia itself (which blunts adrenal response to ACTH). Furthermore, the usual circadian rhythm of cortisol secretion is dampened after burn injury. In 1982, Vaughn et al. reviewed the former and demonstrated the latter. They showed elevation in circulating and urinary cortisol, but found a weak correlation with the level of ACTH. Better correlations were observed between cortisol and total body surface area burned, metabolic rate, or average temperature. These observations indicate adrenal hypersecretion of cortisol in response to increased body temperature, circulating mediators of hypermetabolism, and direct adrenal innervation, with a decrease in adrenal cortex responsiveness to ACTH. On this basis, the authors’ concluded that cortisol may play a secondary role in permitting and promoting the changes seen in post-injury hypermetabolism (42). They attributed post-injury metabolic and thermal changes primarily to sympathetic tone and circulating catecholamines (norepinephrine and epinephrine).
Abnormal leukocyte function and cytokine production
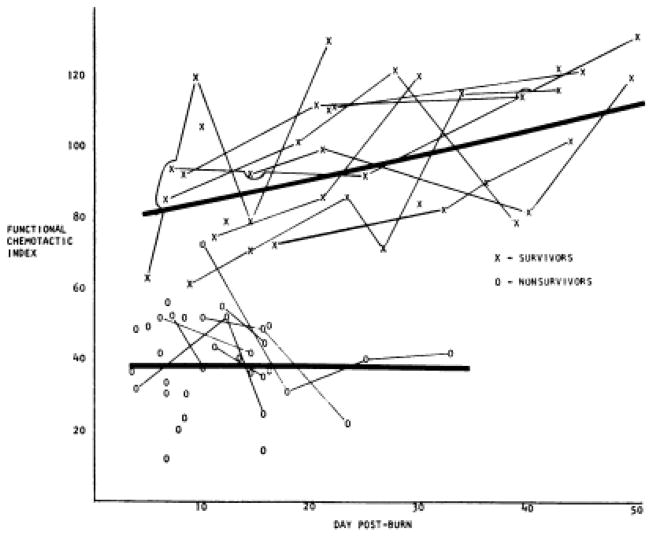
Warden et al. in 1976 demonstrated powerful prediction of post-burn mortality by evaluating neutrophil chemotactic function as a percentage of normal volunteers’ neutrophil chemotaxis (Figure 2). Less than 50% of normal neutrophil chemotaxis was consistently observed in those patients who did not survive (43).
Figure 2.
Impaired neutrophil chemotactic index predicts early burn mortality. Taken with permission from Warden et al. Evaluation of leukocyte chemotaxis in vitro in thermally injured patients. J Clin Inves. 1974;54(4):1001.
Depressed levels of circulating immunoglobulins (Ig) have been frequently reported after severe burn injury. Given the increased susceptibility to infection observed in burn patients, Burleson et al. administered hyper immune IgG (2× per week, 500mg/kg, for up to 3 weeks) to 35 patients with ~45% TBSA burn. In comparison with 34 controls equivalent in age and TBSA burn, IgG levels were increased (44). B cell population was decreased during therapy and subtle differences in cultured B cell response to mitogens were observed, while T cell populations appeared unaltered in number or response to mitogen by IgG therapy.
Post-burn leukopenia is a common occurrence after severe burns, and Cioffi et al. studied the effects of granulocyte-macrophage colony stimulating factor (GM-CSF). They found a 50% increase in mean leukocyte counts after the first week post burn in treated patients. Treatment with GM-CSF increased myeloperoxidase activity (cytosolic oxidative function) week 1 post burn, but these levels then returned to unburned control levels during week 2 and 3 of treatment, while remaining elevated in the untreated patients. Extracellular oxidative function, measured by superoxide production, was initially depressed in both burned groups compared to non-burned controls, although GM-CSF treated patients demonstrated a return to non-burned control levels after week 1, while these levels remained below control levels in the untreated burn patients (45).
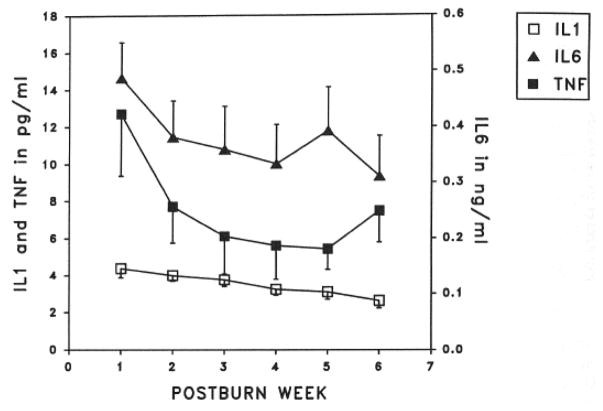
Burn injury and subsequent infections increase circulating levels of endogenous and exogenous pyrogens. As reported by Drost et al. in two 1993 papers, marked and prolonged cytokine elevations are seen after burn injury (Figure 3) (46, 47).
Figure 3.
Cytokine elevations after burn injury. Taken with permission from Drost et al. Plasma cytokines following thermal injury and their relationship with patient mortality, burn size, and time postburn. J Trauma. 1993;35(3):335–9.
Ishihara et al. (2009) showed preservation of cardiac contractility with veno-venous hemofiltration in a swine model of endotoxemia that otherwise caused loss of end-systolic elastance and cardiac index. The authors avoided changes in temperature or preload between the groups(48). Notably, all groups demonstrated >100× elevation in circulating epinephrine and norepinephrine following endotoxin infusion; these catecholamine levels were not significantly altered by hemofiltration.
Catecholamines
Despite myriad factors reported to promote or inhibit the development of post-burn hypermetabolic state, the work of Pruitt et al. clearly demonstrates sympathetic catecholamines (norepinephrine more so than epinephrine) to be the effector limb of the transition to, and maintenance of this hypermetabolic state. Wilmore et al. demonstrated catecholamines to be the mediator of the hypermetabolic response to thermal injury via a seminal paper (49). Several key findings were produced by this study: beta- adrenergic blockade (but not alpha blockade) reduced metabolic rate, pulse, blood pressure, and free fatty acids. Also, the investigators documented the “non-living” response to thermal injury— poikilothermia. They noted that when burned patients were placed in cooler environments (21°C), the metabolic rate generally increased, and urinary catecholamine excretion increased in parallel, except in four non-survivors who also showed less catecholamine elaboration. The reason for the failure of these patients to develop a sufficient hypermetabolic response to allow survival remains only partially understood.
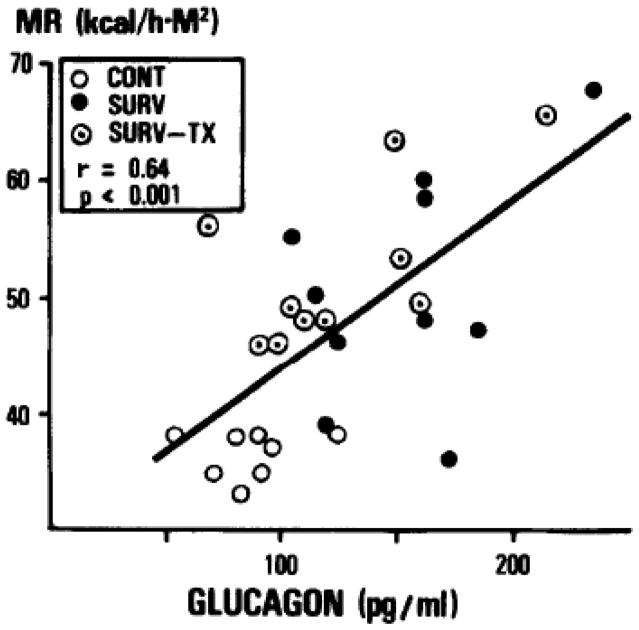
A further analysis of data collected as part of the triiodothyronine replacement trial described above showed that metabolic rate increased proportionally to TBSA burn, plasma norepinephrine, and plasma glucagon (Figures 4 and 5); and it varied inversely with total cholesterol (50).
Figure 4.
Correlation between serum glucagon and metabolic rate. Taken with permission from Vaughan et al. Nonthyroidal control of metabolism after burn injury possible role of glucagon. Metabolism. 1985;34(7):637–41.
Figure 5.
Correlation between urinary catecholamines and metabolic rate. Taken with permission from Wilmore et al. Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg. 1974;180(4):653. http://journals.lww.com/annalsofsurgery/pages/default.aspx
Inhalation Injury
Inhalation injury increases the risk of mortality and the risk of developing pneumonia, which increases mortality further(51). Early investigations focused on diagnostic methods. Moylan et al. described the clinical presentation of inhalation injury in 1972 in a report describing the utility of pulmonary clearance of intravenous xenon-133 (52, 53). Fiberoptic bronchoscopy was reported in 1975, providing primary evidence of the airway pathophysiology: hyperemia, increased secretions, mucosal sloughing, airway occlusion, and carbonaceous sputum(54). Using an ovine model, the dose-response effect of smoke inhalation injury was investigated. These studies suggested that smoke inhalation exhibits a threshold effect, and also recapitulated many of the physiological and histological features of smoke inhalation(55–57). This model also demonstrated that the airway pathophysiology is absent with carbon monoxide inhalation alone(58). The addition of a 40% TBSA scald burn exaggerated the hemodynamic changes seen after smoke inhalation injury, as well as lung lipid peroxidation and hypoproteinemia, although pulmonary function parameters were unchanged(59). In 1989, high-frequency percussive ventilation was shown to be safe, and associated with lower incidence of pneumonia and increased survival in patients with severe inhalation injuries(60–64).
Burn Infections
Pseudomonas burn wound histopathological invasion was described by Foley (65), and the correlation between histological invasion and quantitative tissue cultures was investigated by McManus et al. They observed that negative cultures generally (>95%) implied absence of tissue invasion on histological preparations. Half of the rare false negatives in this group were due to fungal infections. When 105 or greater colony counts were observed on quantitative tissue culture, only 36% of specimens demonstrated invasion of viable tissue (Table 2). Although quantitative tissue culture informs organism identification and antimicrobial therapy, these authors suggested it should not be used as a surrogate for the presence or severity of wound infection (66, 67).
Table 2. Local signs and histological staging of wound bacterial growth.
Taken with permission from Becker et al. Fungal burn wound infection: A 10-year experience. Arch Surg-Chicago. 1991;126:44–8.
| Local Signs of Burn Wound Infection | Classification of Microbial Status of Wounds |
|---|---|
| Conversion of partial-thickness injury to full-thickness necrosis | Stage 1: Wound colonization |
| Focal or generalized brown, black, or violaceous discoloration of the burn wound | 1a: Superficial colonization |
| Rapid separation of eschar layer | 1b: Microorganisms in nonviable tissue |
| Hemorrhagic discoloration of the subeschar tissue | 1c: Microorganisms at the interface of viable tissue |
| Green pigment in subcutaneous fat | Stage 2: Invasive infection |
| Red or black nodular lesions in unburned skin (ecthyma gangrenosum) | 2a: Microinvasion of viable tissue |
| Edema and/or red or black discoloration of unburned skin at the wound margin | 2b: Deep or generalized invasion of viable tissue |
| Centrifugal spread of subcutaneous edema with central wound necrosis | 2c: Microvascular invasion |
Levine et al. reported a simple, rapid, technique of wound swab determination of wound microbiology. Obtaining wound swabs from the surface of healing (non-necrotic) tissues, with enough pressure to cause punctate bleeding, they reported that positive Gram’s stain from this swab consistently predicted >106 colony counts, and that swab cultures performed in broth and in serial dilutions, correlated strongly with flamed tissue biopsy quantitative bacterial counts. This report highlights the utility of Gram’s stain in detecting clinically-significant bacterial colonization, and suggests that properly set-up (quantitative, via serial dilutions) bacterial culture is able to serve as a surrogate for quantitative tissue biopsy and culture (68).
Two papers studied the utility of subeschar antibiotic administration. A rodent burn wound Pseudomonas infection model indicated the carbenicillin, an antibiotic known for area-under-the-curve dependent bactericidal effect, was effective either under the wound or injected subcutaneously distant from the wound. Although colistin, gentamycin, and neomycin were studied, these were not effective. The authors concluded that the gradual absorption (or depot effect) and perhaps prolonged antibiotic level was responsible for the improved survival observed in the carbenicillin group, rather than a specific local effect of antibiotic in proximity to the wound/viable tissue interface (69). Subsechar antibiotics were used in 19 histopathologically-proven Pseudomonas burn wound infections. There were only five survivors, indicating the severity of Pseudomonas burn wound infection, but five others died with no evidence of wound infection at autopsy. Overall, bacterial clearance was seen in >50% of patients, and the authors indicate that subeschar infusion of semisynthetic penicillins was partially effective in their series (70). Evolving antimicrobial susceptibility, particularly of Acinetobacter and Pseudomonas, may influence the future generalizability of this work.
In a 10-year (1979–1989) review of fungal burn unit infections, Becker et al. showed that the combination of topical, subeschar, and systemic chemotherapeutic agents along with the evolving standard of burn care had significantly reduced the incidence of bacterial burn wound infections as histopathologically defined and staged.
However, the incidence of fungal burn wound infections remained relatively constant over this period, around 7% of admissions. These patients had large burns, half had inhalation injuries, and high associated mortality was experienced (74%). Notably, Candida was responsible for only 20% of these infections; the remainder were molds, predominantly Aspergillus and Fusarium. The authors emphasize the need for histopathological diagnosis and rapid, wide debridement (71).
Warden described in 1973 the dangers of central venous cannulation, including a 35% incidence of thrombosis and nearly 20% incidence of superimposed suppuration, attributing these to “a hazard of medical progress(72).” Munster reported on 64 cases of cardiac infection in burned patients, most occurring in the setting of septicemia from gram positive, gram negative, or yeast organisms(73). Other infections, including acalculous cholecystitis (74) and herpesviridae(75) were described in burned patients with customary rigor and insight.
In a 25-year, nearly 6,000 patient review, examining the association with bacteremia and mortality, Mason et al. observed that gram negative bacteremia is associated with a significant excess mortality (40–50%) than would be predicted based on injury characteristics alone. Surprisingly, gram positive bacteremia was not associated with increased mortality across any of the subgroups. Yeast in the bloodstream was marginally associated with increased mortality. The highest mortality group (100% excess mortality, or twice the risk of death) had gram negative (enteric) + gram negative (Pseudomonas) + yeast in the bloodstream during their burn convalescence (76).
Past and Future Impact of Dr. Pruitt’s Research
The following areas of research indicate the breadth of Dr. Pruitt’s contribution to science and burn care. Suppurative chondritis is a disfiguring complication of ear burns, and leads to one of the most challenging problems in burn reconstruction. It is painful for the patient, and this secondary infection represents an additional scourge of the burned patient. Mills II et al. demonstrated a 10-fold decrease in suppurative chondritis using scrupulous avoidance of pressure to burned ears topical 10% mafenide acetate cream applied twice daily, daily washing, and early recognition with limited debridement of necrotic material. This study demonstrates the concern of Pruitt and colleagues, beyond the preservation of the body cell mass, that the burned patient may be restored as fully as possible to function and happiness—through the avoidance of all suffering and tissue loss that can possibly be avoided (77).
Special populations also received attention from Dr. Pruitt: Amy et al. provided a 33-year review (1950–1982) of thermal injuries in pregnancy. 30 patients were cared for, and there were 20 maternal and 17 fetal survivors. 50% of survivors successfully carried to term, indicating the need for obstetrical consultation and follow-up in the care of these patients. Maternal complications negatively influenced fetal outcome, with fetal loss in all patients who became septic in this series. The authors point out the need for continuous fetal monitoring during resuscitation and during acute volume status changes; 13% of the fetuses (2 nonviable and 1 viable) were spontaneously aborted in the first 96 hours post injury (78). Graves et al. examined the burn resuscitation of almost 50 children under 25kg. Defining adequate end-organ perfusion by urine output of 0.5–1mL/kg/hr, they identified a group they felt to be over-resuscitated. Noting that maintenance requirements, on a per-kilogram basis, are higher in children, they cautioned that typical ml/kg/% burn formulae may dangerously under resuscitate children. As such, they separated the predicted maintenance requirement for each child from the resuscitation volume. On the basis of this report, the authors recommend, in addition to maintenance, burn resuscitation initiation at 3mL/kg/%TBSA burn. In their review, they note that half of children will require adjustment to a higher rate, and the importance of monitoring hourly urine output(79).
Conversely, in 2000, when alarmed by increased occurrence of complications due to too much resuscitation fluid, Dr. Pruitt warned the burn community about excessive resuscitation as a cause of fluid overload. He decried “fluid creep” and recommended monitoring of intrabdominal and peak inspiratory pressure to detect fluid overload when the volume of infused resuscitation fluid approached 25% of body weight(80). To reduce the risk of excessive resuscitation, he recommended use of the modified Brooke formula to predict initial resuscitation fluid needs of burn patients (81).
Finally, via the subjects of a 50% full-thickness scald burn rat model, Herndon et al. examined the interaction between many of the above-discussed factors. They showed that thyroidectomy did not influence the post-burn metabolic rate. Adrenalectomy blunted over half the increase in metabolic rate observed in burn-control rats. A higher unburned control metabolic rate was observed in adrenalectomized, dexamethasone-replaced rats, but post-burn the metabolic rate was similar to the non-replaced adrenalectomized rats, indicating a smaller incremental increase in metabolic rate after burn. Catecholamine depletion with reserpine similarly reduced the post-burn metabolic rate increase by over half of control values; unburned, depleted rats had metabolic rates equivalent to untreated, unburned controls. Inhibition of prostaglandin synthesis or effect with indomethacin, meclofenamate, or RO2-5270 (Hoffman-LaRoche) also blunted by half the post-burn metabolic rate compared with controls, although the latter slightly increased the unburned, basal, metabolic rate(82). This last study provided the initial fuel for a much greater increase in the common understanding of post-burn metabolism; writ large, how life adapts and grows past burn injury.
Acknowledgments
Conflicts of Interest and Sources of Funding: None declared. This work was supported by grants from NIH (P50GM060338, R01GM056687, R01HD049471, H133A120091, R01GM112936, T32GM008256) and Shriners of North America (84080, 79141, 79135, 71009, 80100, 71008, 84291). It was also supported by UTMB’s Institute for Translational Sciences, supported in part by a Clinical and Translational Science Award (UL1TR000071) from the National Center for Advancing Translational Sciences.
Footnotes
This manuscript is submitted as a postscript to the Festschrift honoring Dr. Basil A. Pruitt Jr. at the American Association for the Surgery of Trauma Board of Manager’s Meeting, April-08-2017 in San Antonio, Texas.
Level of Evidence: Not applicable
Authorship statement
All authors were actively involved in drafting and critical revision of the manuscript. All have given their final approval for submission.
Contributor Information
Karel D. Capek, Email: kdcapek@utmb.edu.
Guillermo Foncerrada, Email: gffoncer@utmb.edu.
R. Patrick Clayton, Email: rpclayto@utmb.edu.
Michaela Sljivich, Email: misljivi@utmb.edu.
Charles D. Voigt, Email: chvoigt@utmb.edu.
Gabriel Hundeshagen, Email: gahundes@utmb.edu.
Janos Cambiaso-Daniel, Email: jacambia@utmb.edu.
Craig Porter, Email: cr2porte@utmb.edu.
Ashley Guillory, Email: asguillo@utmb.edu.
David N. Herndon, Email: dherndon@utmb.edu.
References
- 1.Bernard C. Lectures on the phenomena of life common to animals and plants. Springfield, Ill: Thomas; 1974. p. v. [Google Scholar]
- 2.Cannon WB. Traumatic shock. New York, London: D. Appleton and company; 1923. p. xvii.p. 201. [Google Scholar]
- 3.Cannon WB. A Consideration of Possible Toxic and Nervous Factors in the Production of Traumatic Shock. Ann Surg. 1934;100(4):704–13. doi: 10.1097/00000658-193410000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuthbertson DP. The significance of proteins in nutrition; their particular importance during convalescence. Br Med J. 1948;2(4581):731–7. doi: 10.1136/bmj.2.4581.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuthbertson DP. The disturbance of metabolism produced by bony and non-bony injury, with notes on certain abnormal conditions of bone. Biochem J. 1930;24(4):1244–63. doi: 10.1042/bj0241244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuthbertson DP. The influence of prolonged muscular rest on metabolism. Biochem J. 1929;23(6):1328–45. doi: 10.1042/bj0231328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore FD. Metabolic care of the surgical patient. Philadelphia: Saunders; 1959. p. 1011. [Google Scholar]
- 8.Moore FD. The body cell mass and its supporting environment; body composition in health and disease. Philadelphia: W.B. Saunders Co; 1963. p. 535. [Google Scholar]
- 9.Moore FD, Ball MR. The metabolic response to surgery. Springfield, Ill: Thomas; 1952. p. xv.p. 156. [Google Scholar]
- 10.Moore FD. A miracle and a privilege : recounting a half century of surgical advance. Washington, D.C: Joseph Henry Press; 1995. p. xii.p. 450. [Google Scholar]
- 11.Pruitt BA, Jr, Tumbusch WT, Mason AD, Jr, Pearson E. Mortality in 1,100 consecutive burns treated at a burns unit. Ann Surg. 1964;159(3):396. doi: 10.1097/00000658-196403000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilmore DW, Mason AD, Johnson DW, Pruitt BA. Effect of ambient temperature on heat production and heat loss in burn patients. J Appl Physiol. 1975;38(4):593–7. doi: 10.1152/jappl.1975.38.4.593. [DOI] [PubMed] [Google Scholar]
- 13.Wilmore DW, Goodwin CW, Aulick LH, Powanda MC, Mason AD, Jr, Pruitt BA., Jr Effect of injury and infection on visceral metabolism and circulation. Ann Surg. 1980;192(4):491. doi: 10.1097/00000658-198010000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aulick LH, Wilmore DW, Mason AD, Pruitt BA. Influence of the burn wound on peripheral circulation in thermally injured patients. Am J Physiol Heart Circ. 1977;233(4):H520–H6. doi: 10.1152/ajpheart.1977.233.4.H520. [DOI] [PubMed] [Google Scholar]
- 15.Wilmore DW, Aulick LH, Mason AD, Jr, Pruitt BA., Jr Influence of the burn wound on local and systemic responses to injury. Ann Surg. 1977;186(4):444. doi: 10.1097/00000658-197710000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aulick LH, Wilmore DW, Mason AD, Jr, Pruitt BA., Jr Muscle blood flow following thermal injury. Ann Surg. 1978;188(6):778. doi: 10.1097/00000658-197812000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henrion J. Hypoxic hepatitis. Liver Int. 2012;32(7):1039–52. doi: 10.1111/j.1478-3231.2011.02655.x. [DOI] [PubMed] [Google Scholar]
- 18.Pruitt BA, Jr, Goodwin CW, Vaughan GM, Aulick LH, Newman JJ, Strome DR, Mason AD., Jr The metabolic problems of the the burn patient. Acta Chir Scand Supplementum. 1985;522:119–39. [PubMed] [Google Scholar]
- 19.Carlson DE, Cioffi WG, Jr, Mason AD, Jr, McManus WF, Pruitt BA., Jr Resting energy expenditure in patients with thermal injuries. Surg Gynecol Obstet. 1992;174(4):270–6. [PubMed] [Google Scholar]
- 20.Newsome TW, Mason AD, Jr, Pruitt BA., Jr Weight loss following thermal injury. Ann Surg. 1973;178(2):215–7. doi: 10.1097/00000658-197308000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long JM, Wilmore DW, Mason AD, Jr, Pruitt BA., Jr Fat-carbohydrate interaction: effects on nitrogen-sparing in total intravenous feeding. Surg Forum. 1974;25:61–3. [PubMed] [Google Scholar]
- 22.Long JM, III, Wilmore DW, Mason AD, Jr, Pruitt BA., Jr Comparison of carbohydrate and fat as caloric sources. Surg Forum. 1975;26:108–10. [PubMed] [Google Scholar]
- 23.Long JM, 3rd, Wilmore DW, Mason AD, Jr, Pruitt BA., Jr Effect of carbohydrate and fat intake on nitrogen excretion during total intravenous feeding. Ann Surg. 1977;185(4):417. doi: 10.1097/00000658-197704000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curreri PW, Wilmore DW, Mason AD, Jr, Newsome TW, Asch MJ, Pruitt BA., Jr Intracellular cation changes following major trauma: effect of supranormal caloric intake. J Trauma. 1971;11(5):390–6. doi: 10.1097/00005373-197105000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Elrick H, Hlad CJ, Jr, Witten TA. Studies on the kinetics of glucose utilization. J Clin Invest. 1956;35(10):1139–49. doi: 10.1172/JCI103368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilmore DW, Mason AD, Pruitt BA., Jr Alterations in glucose kinetics following thermal injury. Surg Forum. 1975;26:81–3. [PubMed] [Google Scholar]
- 27.Wilmore DW, Mason AD, Jr, pruitt BA., Jr Impaired glucose flow in burned patients with gram-negative sepsis. Surg Gynecol Obstet. 1976;143(5):720–4. [PubMed] [Google Scholar]
- 28.Herndon DN, Wilmore DW, Mason AD, Pruitt BA. Abnormalities of phenylalanine and tyrosine kinetics: Significance in septic and nonseptic burned patients. Arch Surg. 1978;113(2):133–5. doi: 10.1001/archsurg.1978.01370140023004. [DOI] [PubMed] [Google Scholar]
- 29.Dong YL, Sheng CY, Herndon DN, Waymack JP. Metabolic abnormalities of mitochondrial redox potential in postburn multiple system organ failure. Burns. 1992;18(4):283–6. doi: 10.1016/0305-4179(92)90148-n. [DOI] [PubMed] [Google Scholar]
- 30.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21(3):297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie M, Yu Y, Kang R, Zhu S, Yang L, Zeng L, Sun X, Yang M, Billiar TR, Wang H, et al. PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation. Nat Commun. 2016;7:13280. doi: 10.1038/ncomms13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, Zhu M, Brasier AR, Kudlicki AS. Inferring genome-wide functional modulatory network: a case study on NF-kappaB/RelA transcription factor. J Comput Biol. 2015;22(4):300–12. doi: 10.1089/cmb.2014.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang J, Chen X, Liu H, Shao J, Xie R, Gu P, Duan C. Polypyrimidine Tract-Binding Protein 1 promotes proliferation, migration and invasion in clear-cell renal cell carcinoma by regulating alternative splicing of PKM. Am J Cancer Res. 2017;7(2):245–59. [PMC free article] [PubMed] [Google Scholar]
- 35.Su CH, Hung KY, Hung SC, Tarn WY. RBM4 Regulates Neuronal Differentiation of Mesenchymal Stem Cells by Modulating Alternative.,12Splicing of Pyruvate Kinase M. Mol Cell Biol. 2017;37(3) doi: 10.1128/MCB.00466-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen M, Zhang J, Manley JL. Turning on a fuel switch of cancer: hnRNP proteins regulate alternative splicing of pyruvate kinase mRNA. Cancer Res. 2010;70(22):8977–80. doi: 10.1158/0008-5472.CAN-10-2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Becker RA, Wilmore DW, Goodwin CW, Zitzka CA, Wartofsky L, Burman KD, Mason AD, Pruitt BA. Free T4, free T3, and reverse T3 in critically ill, thermally injured patients. J Trauma. 1980;20(9):713–21. doi: 10.1097/00005373-198009000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Becker RA, Vaughan GM, Ziegler MG, Seraile LG, Goldfarb IW, Mansour EH, McManus WF, Pruitt BA, Jr, Mason AD., Jr Hypermetabolic low thriidothyronine syndrome of burn injury. Crit Care Med. 1982;10(12):870–5. doi: 10.1097/00003246-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 39.Becker RA, Vaughan GM, Goodwin CW, Ziegler MG, Harrison TS, Mason AD, Pruitt BA. Plasma norepinephrine, epinephrine, and thyroid hormone interactions in severely burned patients. Arch Surg. 1980;115(4):439–43. doi: 10.1001/archsurg.1980.01380040067012. [DOI] [PubMed] [Google Scholar]
- 40.Wilmore DW, Orcutt TW, Mason AD, Jr, Pruitt BA., Jr Alterations in hypothalmic function following thermal injury. J Trauma. 1975;15(8):697–703. doi: 10.1097/00005373-197508000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Plymate SR, Vaughan GM, Mason AD, Pruitt BA. Central hypogonadism in burned men. Horm Res. 1987;27(3):152–8. doi: 10.1159/000180803. [DOI] [PubMed] [Google Scholar]
- 42.Vaughan GM, Becker RA, Allen JP, Goodwin CW, Pruitt BA, Jr, Mason AD., Jr Cortisol and Corticotrophin in Burned Patients. J Trauma. 1982;22(4):263–73. doi: 10.1097/00005373-198204000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Warden GD, Mason AD, Jr, Pruitt BA., Jr Evaluation of leukocyte chemotaxis in vitro in thermally injured patients. J Clin Invest. 1974;54(4):1001. doi: 10.1172/JCI107815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burleson DG, Mason AD, McManus AT, Pruitt BA. Lymphocyte phenotype and function changes in burn patients after intravenous IgG therapy. Arch Surg. 1988;123(11):1379–82. doi: 10.1001/archsurg.1988.01400350093014. [DOI] [PubMed] [Google Scholar]
- 45.Cioffi WG, Jr, Burleson DG, Jordan BS, Becker WK, McManus WF, Mason AD, Jr, Pruitt BA., Jr Effects of granulocyte-macrophage colony-stimulating factor in burn patients. Arch Surg. 1991;126(1):74–9. doi: 10.1001/archsurg.1991.01410250080013. [DOI] [PubMed] [Google Scholar]
- 46.Drost AC, Burleson DG, Cioffi WG, Jr, Mason AD, Jr, Pruitt BA., Jr Plasma cytokines after thermal injury and their relationship to infection. Ann Surg. 1993;218(1):74. doi: 10.1097/00000658-199307000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drost AC, Burleson DG, Cioffi WG, Jr, Jordan BS, Mason AD, Jr, Pruitt BA., Jr Plasma cytokines following thermal injury and their relationship with patient mortality, burn size, and time postburn. J Trauma. 1993;35(3):335–9. doi: 10.1097/00005373-199309000-00001. [DOI] [PubMed] [Google Scholar]
- 48.Ishihara S, Ward JA, Tasaki O, Pruitt BA, Jr, Mozingo DW. Cardiac contractility during hemofiltration in an awake model of hyperdynamic endotoxemia. J Trauma. 2009;67(5):1055–61. doi: 10.1097/TA.0b013e3181a5f405. [DOI] [PubMed] [Google Scholar]
- 49.Wilmore DW, Long JM, Mason AD, Jr, Skreen RW, Pruitt BA., Jr Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg. 1974;180(4):653. doi: 10.1097/00000658-197410000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaughan GM, Becker RA, Unger RH, Ziegler MG, Siler-Khodr TM, Pruitt BA, Jr, Mason AD., Jr Nonthyroidal control of metabolism after burn injury possible role of glucagon. Metabolism. 1985;34(7):637–41. doi: 10.1016/0026-0495(85)90091-5. [DOI] [PubMed] [Google Scholar]
- 51.Shirani KZ, Pruitt BA, Jr, Mason AD., Jr The influence of inhalation injury and pneumonia on burn mortality. Ann Surg. 1987;205(1):82–7. doi: 10.1097/00000658-198701000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moylan JA, Jr, Wilmore DW, Mouton DE, Pruitt BA., Jr Early diagnosis of inhalation injury using 133 xenon lung scan. Ann Surg. 1972;176(4):477–84. doi: 10.1097/00000658-197210000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agee RN, Long JM, 3rd, Hunt JL, Petroff PA, Lull RJ, Mason AD, Jr, Pruitt BA., Jr Use of 133xenon in early diagnosis of inhalation injury. J Trauma. 1976;16(3):218–24. doi: 10.1097/00005373-197603000-00007. [DOI] [PubMed] [Google Scholar]
- 54.Hunt JL, Agee RN, Pruitt BA., Jr Fiberoptic bronchoscopy in acute inhalation injury. J Trauma. 1975;15(8):641–9. doi: 10.1097/00005373-197508000-00004. [DOI] [PubMed] [Google Scholar]
- 55.Shimazu T, Yukioka T, Hubbard GB, Langlinais PC, Mason AD, Jr, Pruitt BA., Jr A dose-responsive model of smoke inhalation injury. Severity-related alteration in cardiopulmonary function. Ann Surg. 1987;206(1):89–98. doi: 10.1097/00000658-198707000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hubbard GB, Shimazu T, Yukioka T, Langlinais PC, Mason AD, Jr, Pruitt BA., Jr Smoke inhalation injury in sheep. Am J Pathol. 1988;133(3):660–3. [PMC free article] [PubMed] [Google Scholar]
- 57.Hubbard GB, Langlinais PC, Shimazu T, Okerberg CV, Mason AD, Jr, Pruitt BA., Jr The morphology of smoke inhalation injury in sheep. J Trauma. 1991;31(11):1477–86. doi: 10.1097/00005373-199111000-00003. [DOI] [PubMed] [Google Scholar]
- 58.Shimazu T, Ikeuchi H, Hubbard GB, Langlinais PC, Mason AD, Jr, Pruitt BA., Jr Smoke inhalation injury and the effect of carbon monoxide in the sheep model. J Trauma. 1990;30(2):170–5. doi: 10.1097/00005373-199002000-00006. [DOI] [PubMed] [Google Scholar]
- 59.Tasaki O, Goodwin CW, Saitoh D, Mozingo DW, Ishihara S, Brinkley WW, Cioffi WG, Jr, Pruitt BA., Jr Effects of burns on inhalation injury. J Trauma. 1997;43(4):603–7. doi: 10.1097/00005373-199710000-00007. [DOI] [PubMed] [Google Scholar]
- 60.Pruitt BA, Jr, Cioffi WG, Shimazu T, Ikeuchi H, Mason AD., Jr Evaluation and management of patients with inhalation injury. J Trauma. 1990;30(12 Suppl):S63–8. doi: 10.1097/00005373-199012001-00015. [DOI] [PubMed] [Google Scholar]
- 61.Cioffi WG, Graves TA, McManus WF, Pruitt BA., Jr High-frequency percussive ventilation in patients with inhalation injury. J Trauma. 1989;29(3):350–4. doi: 10.1097/00005373-198903000-00012. [DOI] [PubMed] [Google Scholar]
- 62.Cioffi WG, Jr, Rue LW, 3rd, Graves TA, McManus WF, Mason AD, Jr, Pruitt BA., Jr Prophylactic use of high-frequency percussive ventilation in patients with inhalation injury. Ann Surg. 1991;213(6):575–80. doi: 10.1097/00000658-199106000-00007. discussion 80–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rue LW, 3rd, Cioffi WG, Mason AD, McManus WF, Pruitt BA., Jr Improved survival of burned patients with inhalation injury. Arch Surg. 1993;128(7):772–8. doi: 10.1001/archsurg.1993.01420190066009. discussion 8–80. [DOI] [PubMed] [Google Scholar]
- 64.Fitzpatrick JC, Cioffi WG., Jr Ventilatory support following burns and smoke-inhalation injury. Respir Care Clin N Am. 1997;3(1):21–49. [PubMed] [Google Scholar]
- 65.Foley FD, Greenawald KA, Nash G, Pruitt BA., Jr Pathology of Pseudomonas infection. Tex Med. 1969;65(10):36–9. [PubMed] [Google Scholar]
- 66.McManus AT, Kim SH, McManus WF, Mason AD, Jr, Pruitt BA., Jr Comparison of quantitative microbiology and histopathology in divided burn-wound biopsy specimens. Arch Surg. 1987;122(1):74–6. doi: 10.1001/archsurg.1987.01400130080012. [DOI] [PubMed] [Google Scholar]
- 67.Pruitt BA., Jr Biopsy diagnosis of surgical infections. N Engl J Med. 1984;310(26):1737–8. doi: 10.1056/NEJM198406283102609. [DOI] [PubMed] [Google Scholar]
- 68.Levine NS, Lindberg RB, Mason AD, Jr, Pruitt BA., Jr The quantative swab culture and smear: a quick, simple method for determining the number of viable aerobic bacteria on open wounds. J Trauma. 1976;16(2):89–94. [PubMed] [Google Scholar]
- 69.McManus WF, Mason AD, Jr, Pruitt BA., Jr Subeschar antibiotic infusion in the treatment of burn wound infection. J Trauma. 1980;20(12):1021–3. doi: 10.1097/00005373-198012000-00002. [DOI] [PubMed] [Google Scholar]
- 70.McManus WF, Goodwin CW, Jr, Pruitt BA., Jr Subeschar treatment of burn-wound infection. Arch Surg. 1983;118(3):291–4. doi: 10.1001/archsurg.1983.01390030023004. [DOI] [PubMed] [Google Scholar]
- 71.Becker WK, Cioffi WG, Jr, McManus AT, Kim SH, McManus WF, Mason AD, Pruitt BA., Jr Fungal burn wound infection: A 10-year experience. Arch Surg. 1991;126:44–8. doi: 10.1001/archsurg.1991.01410250048008. [DOI] [PubMed] [Google Scholar]
- 72.Warden GD, Wilmore DW, Pruitt BA., Jr Central venous thrombosis: a hazard of medical progress. J Trauma. 1973;13(7):620–6. [PubMed] [Google Scholar]
- 73.Munster AM, DiVincenti FC, Foley FD, Pruitt BA., Jr Cardiac infections in burns. Am J Surg. 1971;122(4):524–7. doi: 10.1016/0002-9610(71)90480-6. [DOI] [PubMed] [Google Scholar]
- 74.Munster AM, Goodwin MN, Pruitt BA., Jr Acalculous cholecystitis in burned patients. Am J Surg. 1971;122(5):591–3. doi: 10.1016/0002-9610(71)90284-4. [DOI] [PubMed] [Google Scholar]
- 75.Foley FD, Greenawald KA, Nash G, Pruitt BA., Jr Herpesvirus infection in burned patients. N Engl J Med. 1970;282(12):652–6. doi: 10.1056/NEJM197003192821205. [DOI] [PubMed] [Google Scholar]
- 76.Mason AD, McManus AT, Pruitt BA. Association of burn mortality and bacteremia: a 25-year review. Arch Surg. 1986;121(9):1027–31. doi: 10.1001/archsurg.1986.01400090057009. [DOI] [PubMed] [Google Scholar]
- 77.Mills DC, 2nd, Roberts LW, Mason AD, Jr, McManus WF, Pruitt BA., Jr Suppurative chondritis: its incidence, prevention, and treatment in burn patients. Plast Reconstr Surg. 1988;82(2):267–76. [PubMed] [Google Scholar]
- 78.Amy BW, McManus WF, Goodwin CW, Mason AD, Jr, Pruitt BA., Jr Thermal injury in the pregnant patient. Surg Gynecol Obstet. 1985;161(3):209–12. [PubMed] [Google Scholar]
- 79.Graves TA, Cioffi WG, McManus WF, Mason AD, Jr, Pruitt BA., Jr Fluid resuscitation of infants and children with massive thermal injury. J Trauma. 1988;28(12):1656–9. doi: 10.1097/00005373-198812000-00007. [DOI] [PubMed] [Google Scholar]
- 80.Pruitt BA., Jr Protection from excessive resuscitation: “pushing the pendulum back”. J Trauma. 2000;49(3):567–8. doi: 10.1097/00005373-200009000-00030. [DOI] [PubMed] [Google Scholar]
- 81.Pruitt BA., Jr Advances in fluid therapy and the early care of the burn patient. World J Surg. 1978;2(2):139–50. doi: 10.1007/BF01553536. [DOI] [PubMed] [Google Scholar]
- 82.Herndon DN, Wilmore DW, Mason AD, Jr, Pruitt BA., Jr Humoral mediators of nontemperature-dependent hypermetabolism in 50% burned adult rats. Surg Forum. 1977;28:37–9. [PubMed] [Google Scholar]