Abstract
Purpose
The purpose of this study was to evaluate telomere homeostasis in sub-fertile compared to fertile human sperm.
Methods
This observational, comparative study included 16 sub-fertile men who required intracytoplasmic sperm injection and 10 fertile men. At least 100 sperm cells from each participant were assessed. Main outcome measures were telomere length and telomere aggregates. Telomerase RNA component (TERC) copy number and telomere capture were assessed using fluorescence in situ hybridization technique and human telomerase reverse transcriptase (hTERT) using immunohistochemistry.
Results
Clinical backgrounds were similar. The percentage of sperm cells with shorter telomeres was higher among the sub-fertile compared to the fertile participants (3.3 ± 3.1 vs. 0.6 ± 1.2%, respectively; P < 0.005). The percentage of cells with telomere aggregates was significantly higher in the sub-fertile group (15.12 ± 3.73 vs. 4.73 ± 3.73%; P < 0.005). TERC gene copy number was similar between groups. The percentage of cells that were positive for hTERT was lower in the sub-fertile group (3.81 ± 1.27 vs. 8.42 ± 1.80%; P < 0.005). Telomere capture rates were higher among the sub-fertile sperm cells (P < 0.005).
Conclusions
Sub-fertile sperm cells have short telomeres that are elongated by the alternative pathway of telomere capture. Dysfunctional telomeres may affect sperm fertilizability.
Keywords: Male sub-fertility, Telomeres, Sperm
Introduction
It is well established that female fertility declines with age and with DNA aberrations and genomic instability. The pathophysiology leading to abnormal sperm and male infertility is not fully understood [1]. DNA variations and abnormalities can affect sperm quality and fertilizability [2–4].
Telomeres are repeating DNA sequences located at the ends of chromosomes. They are responsible for chromosomal stability [5, 6]. Telomeres of healthy somatic cells become shorter as cells divide, when cells age, or are exposed to stressors such as hypoxia or uncontrolled glucose levels. Short telomeres reflect genomic instability [7], and they tend to aggregate and become dysfunctional [8].
Telomerase is an RNA-dependent DNA polymerase. It has a functional RNA component: hTERC, which is the template for telomeric DNA synthesis, and a catalytic protein, hTERT (human telomerase reverse transcriptase). hTERT expression is associated with telomerase activity and determines enzyme activity [9, 10]. Telomerase is crucial for maintaining spermatogenesis in germline cells [11, 12].
When telomeres become short, a compensational mechanism of “telomere capture” might occur, with telomeres moving between chromosomes to compensate for the loss of telomere length [13, 14]. Telomere capture maintains telomere length and chromosomal function. This mechanism is associated with genomic abnormality of cancerous cells. It was also described in cells exposed to metabolic stressors, such as abnormal glucose or hyperlipidemia, as well as hypoxia and infections [15, 16].
Telomeres are well-known markers of cellular aging [17]. They are shorter among individuals with age-related diseases, such as metabolic syndrome and cardiovascular diseases. Long telomeres are associated with increased survival, especially among men [18, 19]. Given the association between reproductive and biological aging, including that shortened telomeres are associated with premature ovarian failure [20] and the correlation between ovarian telomerase activity and the number of primordial follicles [21], we hypothesized a potential effect of telomere length on sperm quality.
Previous studies regarding telomeres and sperm quality demonstrated shortened telomeres in sperm from men with idiopathic infertility [22] and oligozoospermia [23]. However, telomere homeostasis and the mechanisms of telomere shortening in sub-fertile sperm are not yet completely understood. It is also not clear whether shortened telomeres lead to dysfunctional sperm or only exemplify sperm abnormalities. The current study investigated telomere homeostasis in sub-fertile compared to normal sperm.
Materials and methods
Study groups and sample collection
Residual sperm from men receiving Intra Cytoplasmatic Sperm Injection (ICSI) was collected and analyzed. The study group consisted of 16 men with sub-fertility, ranging in age from 29 to 42 years. The men in the sub-fertile group and their partners underwent a fertility evaluation after 1 year of unprotected intercourse. All female partners were evaluated and diagnosed as normo-ovulatory, with at least one patent tube. The men were categorized as sub-fertile after sperm analysis, following the 2010 WHO guidelines. Indications for ICSI were total motile count (TMC) less than 1.5 million, less than 20% fertilization in previous IVF cycle, or less than 2% sperm with normal morphology.
The control group included 10 healthy males with proven fertility in the last year. Analysis of their donated sperm was normal. Clinical characteristics, sperm analysis and telomere features were compared between the two groups.
The study was approved by the institutional Ethics Review Board. All patients provided signed informed consent.
FISH assay
Telomere length and aggregates
Sperm was fixed on slides and dried at room temperature for 1 h. The samples were prepared for fluorescence in situ hybridization (FISH) using a Tissue Pretreatment Kit (Biomedicals, North America, Solon, OH, USA). Slides were first placed in a plastic jar containing 40 ml of xylene for 10 min twice at room temperature, followed by fixation in an acetic acid–ethanol solution l at a ratio of 1:3 for 15 min. The slides were then placed in fresh 100% ethanol for 5 min twice, allowed to air dry and then placed on a plate warmed to 80 °C for 60 min. Slides were then incubated for 25 min in a glass jar, containing the pretreatment solution, preheated to 45 °C (12 g of pretreatment powder in 40 cm3 of 2× sodium saline solution (SCC)), rinsed twice in 2× SSC buffer at room temperature, and then incubated in a glass jar containing a protein digesting enzyme that was preheated to 45 °C for 45 min (400 ml in 40 cm3 of 2× SCC). The slides were rinsed twice in 2× SCC buffer at room temperature, dehydrated for 1 min in 70, 80, and 100% ethanol at room temperature, and left to air dry for 5 min. The slides were then placed on a plate warmed to 94 °C for 4 min.
The telomere length and the number of aggregates were assessed using fluorescence CY3-labeled, telomere-specific, peptide nucleic acid probe (vial 2 in K 532 DAKO, Glostrup, Denmark) following manufacturer’s instructions. Internal control was performed by using slides exposed to the entire procedure except for the CY3-labeled, telomere-specific peptide nucleic acid probe and referred to as “negative telomere” control slides. The slides were counterstained with 40-6-diamidino-w-phenylindole (DAPI) (1000 ng/ml, VYS-32-804830, Vysis, Abott, IL, USA) and overlaid with glass cover slips for observation. Internal controls for that step of the study were performed using the CY3-labeled, telomere-specific, peptide nucleic acid probe without counterstaining for DAPI. These slides were referred to as “DAPI negative controls.” Each specimen was placed under a fluorescent microscope. We used one filter for three colors (DAPI, TRIS, FITC), automatic exposure for both images, and × 100 magnification on an AX70 Olympus Provis microscope (Olympus Corp., Tokyo, Japan). For each specimen, 100 cells were counted. The slides were blindly scored twice (three times whenever there was a difference of more than 10% between the counts). Negative control, study, and control samples were scored simultaneously to avoid potential differences related to the experiment itself.
Telomere length was quantified by signal intensity and telomere number. The cells were categorized as having high (strong) or low (weak) fluorescence and by the number of telomere signals (dots per cell) < 10 or > 10. Those with low signal intensity and < 10 dots per cell were defined as having short telomeres. Strong signal and > 10 dots per cell represent long telomeres [24].
The manual FISH counts were followed by computer-assisted analysis of digital images to ensure the accuracy of the counts. The software was developed in our laboratory using Matlab. It analyzes the intensity of telomere staining in DAPI-stained nuclei by comparing the difference between the intensity of the brightest and the dimmest red stained pixels in each nucleus that stained blue, using a derivative-based algorithm. The total intensity of each telomere is calculated as the sum of the intensity of all the pixels that belong to the telomere.
The telomere length in the study samples was determined relative to the controls. The percentage of cells with short telomeres and the percentage of cells with telomere aggregates were calculated for each slide.
TERC gene copy number
We used the TERC–Kreatech probe (Cat. No. KBI hTERC, (3 of 26) (red)/3q11 (green)). The hTERC (3q26)-specific DNA probe is optimized to detect copy numbers of the hTERC gene region 3q26. It appears as a red signal. The 3q11 region probe is included to facilitate chromosome identification. It appears as a green signal and serves as a control. We compared the number of signals of the probe targeted to the specific loci of TERC gene (red) and the number of signals from the control probe (green). The number of TERC copies was obtained by counting the signals in 100 nuclei. Cells containing more than two copies of TERC gene (red signal) were considered amplified.
Telomere capture
Telomere capture was analyzed using the FISH technique. In the nuclei, the number of the specific locus of the specific chromosome (SNRPN or 13q 14.3: orange, the “normal disomic loci”) and its telomere (15qter or 13qter: green) was compared. The number of signals of the SNRPN or 13q 14.3 locus was compared to the number of signals in the sub-telomeric region of the specific chromosome (green signals). For example, normal appearance is two orange signals and two green signals, while abnormal appearance, which represents telomere capture, is two orange compared with one, three, or more green signals (the rearranged captured sub-telomeric regions). Approximately 100 nuclei from each sample were analyzed. Only cells in which two orange signals (normal “disomic loci”) appeared were analyzed. Positive and negative controls were used to ensure the reliability of the assay.
Immunohistochemistry for hTERT
Immunohistochemistry for hTERT was performed using Histostain-SP Detection Kit (Zymed Laboratories, San Francisco, CA). For peroxidase quenching, the sections were incubated at room temperature, for 15 min, in 3% hydrogen peroxide and methanol, and then blocked with blocking solution (reagent A from the above commercial kit) for 15 min and washed twice with PBS. The slides were incubated at room temperature for 1 h with rabbit monoclonal antibody against hTERT (Novus Biologicals, Littleton, CO) at a dilution of 1:50 and then for 10 min with secondary antibody (Reagent B, biotinylated secondary antibody). The slides were then incubated at room temperature for 10 min with streptavidin and washed twice with PBS. The nuclei were stained with DAB for 3 min and washed with distilled water. The slides were then stained with hematoxylin and eosin, washed with water, covered, and viewed with a light microscope. hTERT expression was evaluated based on presence of the signal. We counted 100 cells per slide and grouped cells according to presence or absence of staining for hTERT. The slides were scored blindly, twice.
Statistical analysis
A two-tailed sample t test and the nonparametric Kruskal–Wallis and Mann–Whitney U tests were used to test the differences between the study groups. P < 0.05 was considered statistically significant. SPSS software (SPSS, Inc., Chicago, IL) was used for statistical analysis.
Results
Background characteristics of the participants were consistent with the inclusion criteria and the definitions of the study and the control groups. Sub-fertile patients tended to be older, but the differences did not reach statistical significance. There were no other differences (Table 1).
Table 1.
Clinical characteristics of the participants
| Parameter | Sub-fertile sperm | Fertile sperm | P value |
|---|---|---|---|
| Age (years ± SD) | 37.4 ± 5 | 36.5 ± 7 | 0.65 |
| BMI ± SD | 26.2 ± 6 | 24.8 ± 4 | 0.53 |
| Smokers | 0 | 0 | NS |
| Patients with diabetes mellitus | 0 | 0 | NS |
| Sperm concentration × 106 (± SD) | 7 ± 4.6 | 87 ± 12 | < 0.001 |
| Total motile count × 106 (± SD) | 1.2 ± 0.3 | 48 ± 16 | < 0.001 |
| Normal morphology (% ± SD) | 1.3 ± 0.7 | 38 ± 13 | < 0.001 |
NS not significant
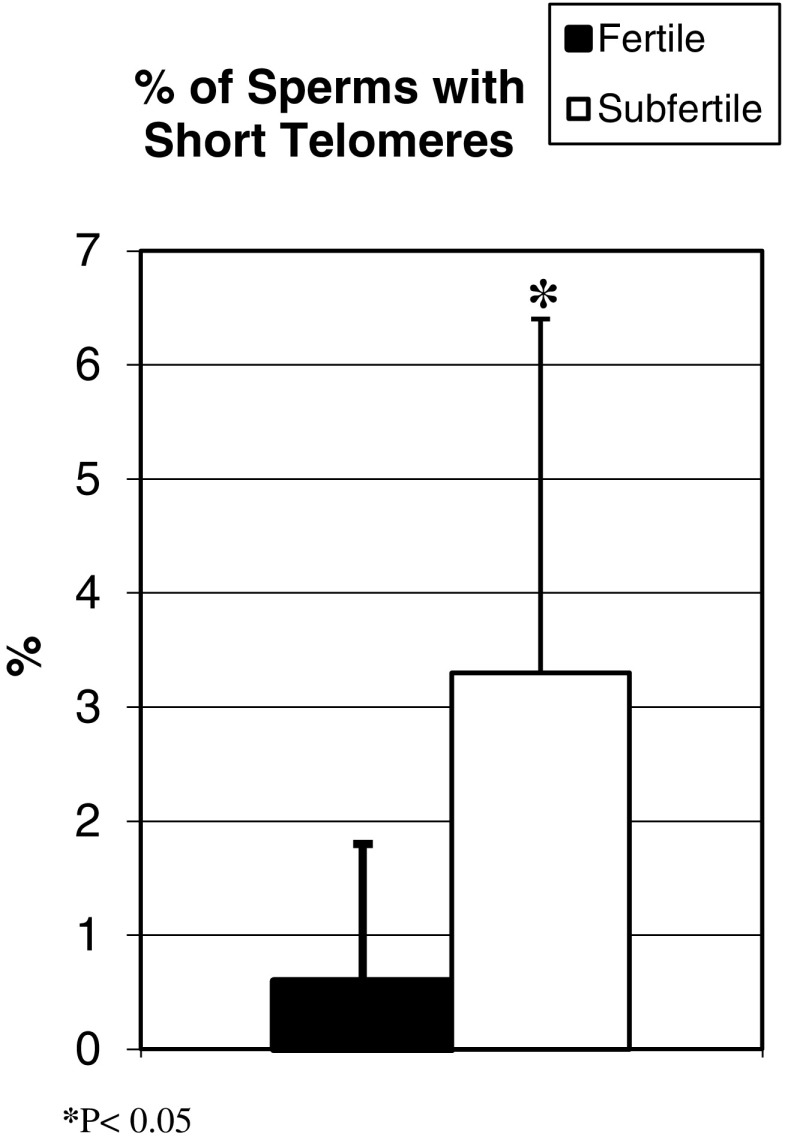
Telomeres were shorter among the participants with sub-fertile sperm (0.6 ± 1.2%) compared to fertile sperm (3.3 ± 3.1%; P < 0.005). More cells had parameters consistent with short telomeres (Fig. 1).
Fig. 1.
Telomere length: The graph represents the percentage of sperm with short telomeres in each study group. The cells with short telomeres have few telomeric signals and low intensity using specific FISH probes
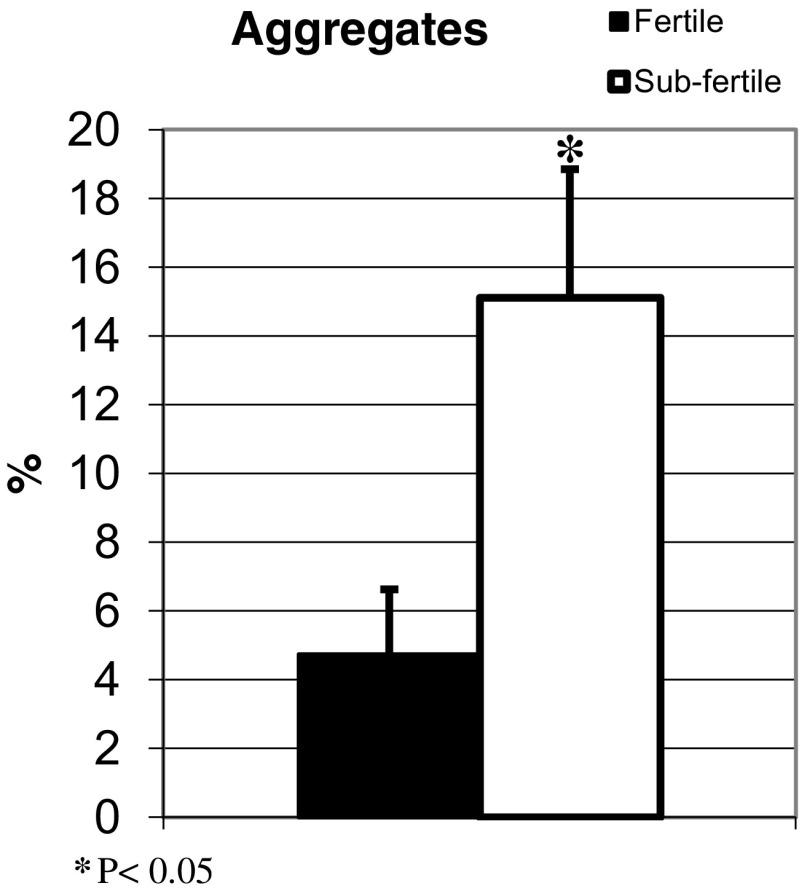
The sub-fertile sperm expressed a higher percentage of telomere aggregates (15.12 ± 3.73%) compared to the fertile sperm (4.73 ± 3.73%; P < 0.05; Fig. 2).
Fig. 2.
Telomere aggregates: Using FISH signals, the percentage of telomeric aggregates in each sample was calculated
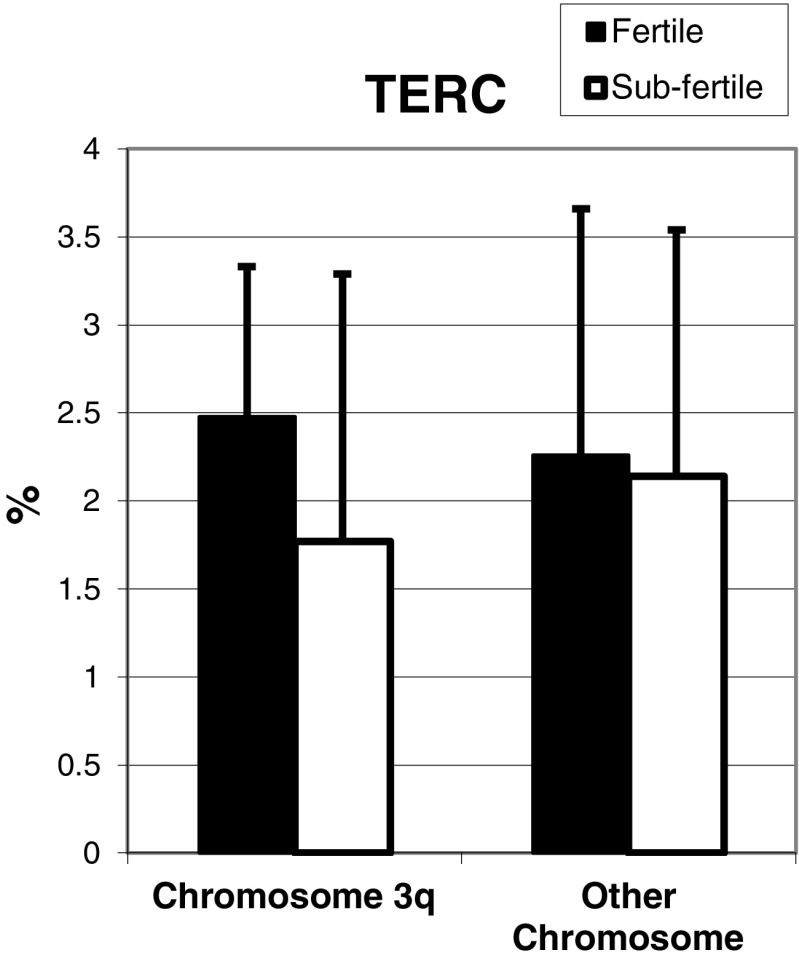
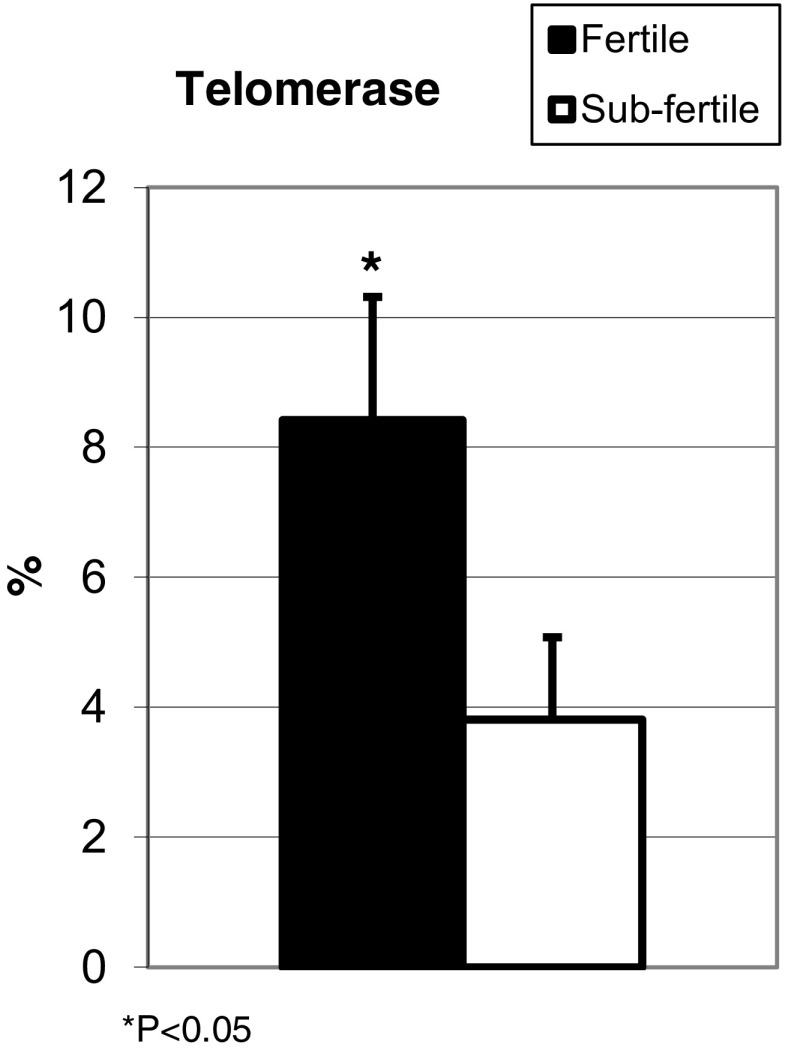
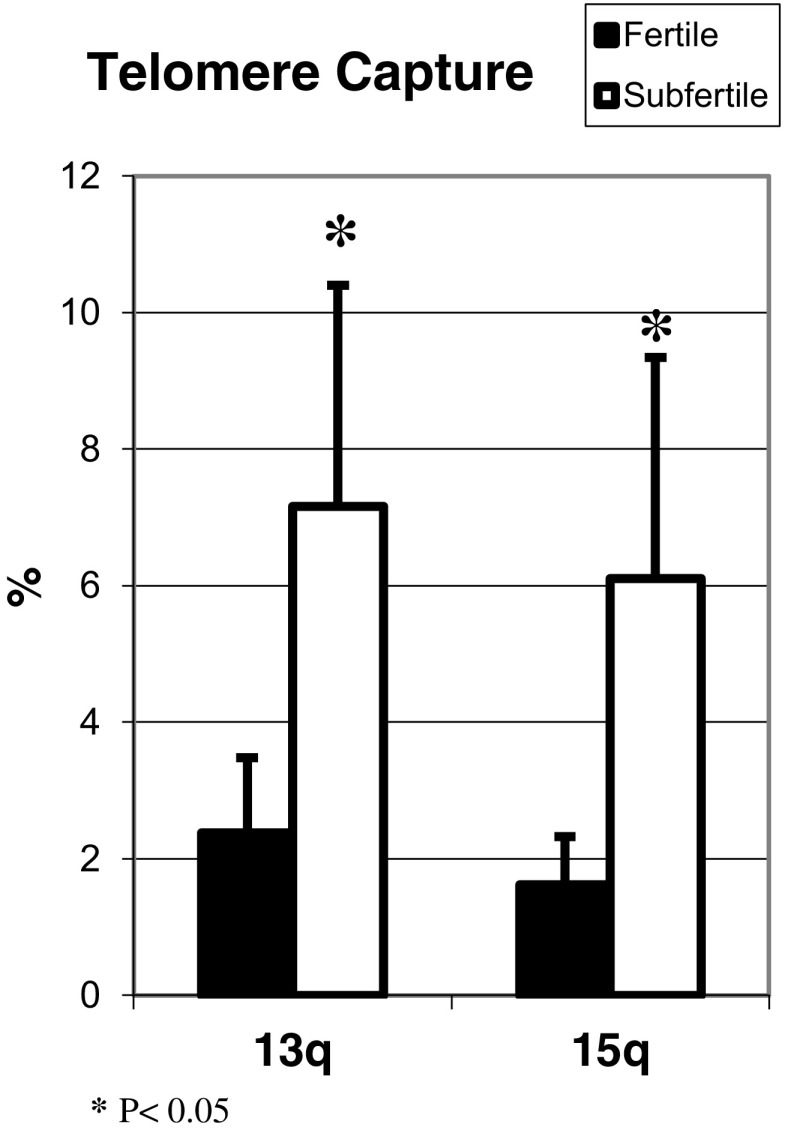
Although TERC gene copy number was lower among the sub-fertile compared to fertile sperm, the differences did not reach statistical significance (Fig. 3). However, telomerase was lower in sperm from sub-fertile (3.81 ± 1.27%) compared to fertile (8.42 ± 1.89%; P < 0.05) men (Fig. 4). Compensation for shortened telomeres by telomere capture was higher in sub-fertile compared to fertile sperm (Fig. 5).
Fig. 3.
TERC gene copy number: FISH with the specific DNA probe was used to detect copy numbers of the hTERC gene region. The number of TERC copies was obtained by counting the signals in 100 nuclei. The graph represents the percentage of cells containing more than two copies of the TERC gene, which is considered amplified
Fig. 4.
Telomerase: The percentage of sperm in each group that stained positive for telomerase using immunohistochemistry
Fig. 5.
Telomere capture: Telomere capture was analyzed using the FISH technique. Approximately 100 nuclei from each sample were analyzed. The graph represents the percentage of cells with a signal of telomere capture in each study group
Discussion
The current study identified significantly impaired telomere homeostasis among samples of sub-fertile sperm. Sub-fertile sperm have shorter telomeres and increased aggregate formation, which imply dysfunction. We also observed less telomerase, which might explain the shortened telomeres, with increased compensation by telomere capture. These observations might be a key finding for interventional studies to improve sperm quality.
Among women, telomere dysfunction has been established as a factor in lower fertility and increased miscarriage rates, related to aging and premature ovarian failure. Shortened telomeres with decreased telomerase activity were found in granulosa cells of women with ovarian failure and infertility. Aberrant telomere homeostasis was suggested as a molecular marker for occult ovarian insufficiency [25, 26].
Among men, it is well documented that chromosomal abnormalities due to meiotic nondisjunction, as well as DNA damage, are more common in sub-fertile sperm compared to that of the general population [3, 4, 27]. Telomere dysfunction as a component of male infertility is less well established in the literature and requires further investigation.
Our findings of disrupted telomere function in sub-fertile sperm samples agree with previously published data. PCR assessment of DNA from spermatozoa revealed fewer repeats of telomere sequences among infertile men [2, 4, 22, 23]. The findings of these studies were inconsistent regarding the correlation between telomere length and other parameters of sperm analysis. Although, a recent study found a positive correlation between short telomeres and decreased sperm motility and vitality [28]. Moreover, the current study adds information about mechanisms of telomere shortening.
Telomere length determines the number of mitosis cycles the cell undergoes; the longer the telomeres, the higher the number of potential mitosis. A positive correlation was found between reproductive life span and telomere length [29].
Telomere length is maintained by telomerase activity throughout spermatogenesis in the germ cells of human testes. We found decreased telomerase expression in sub-fertile sperm with shortened telomeres. A previous study found similar telomerase activity among patients with oligozoospermia secondary to maturation arrest or obstruction, and no correlation between telomerase activity and sperm concentration, but almost no telomerase activity in patients with Sertoli cell-only syndrome and azoospermia [12]. Azoospermia is a different type of sperm pathology, and telomere homeostasis might be different.
If shortened telomeres in germ cells are not lengthened by telomerase, chromosomal abnormalities of fusion, unbalanced translocations, and terminal micro-deletions might occur. Short telomeres in germ cells potentially limit the replicative capacity of the fertilized zygote and increase its genomic instability due to chromosomal fusion or aberrant meiosis. Consequently, shortened telomeres might increase the incidence of early spontaneous abortions and genetic syndromes [11]. Decreased telomerase in sub-fertile sperm might contribute to nuclear damage and infertility, as well as to spontaneous miscarriages in these patients.
The correlation between short telomeres in sperm and sub-fertility could reflect a natural mechanism of preventing transmission of cells with dysfunctional telomeres and chromosomal abnormalities. The shortened telomeres damage chromosomal meiosis and germ cell development, and these cells undergo apoptosis at the first stage of meiosis [30, 31].
A strength of the current study is that it is based on a relatively novel approach to infertility secondary to abnormal sperm. Little has been studied about telomere homeostasis among sub-fertile men. The information presented here presents a new aspect of the problem and opens a window for additional research in this field.
This study is not without limitations, as it is observational without interventional experiments. The sample size is small. Also, using qualitative methods such as FISH and immunohistochemistry is not as accurate as quantitative methodologies. We tried to overcome those limitations by conducting repeated evaluations, as well as computer analyses. Therefore, conclusions based on the data should be drawn carefully and further research with interventional experiments is justified. Also, the clinical implications of the findings should be studied further.
To conclude, interrupted telomere homeostasis in sub-fertile sperm might be one explanation for male infertility. This is a preliminary proposition, as it is not yet completely understood. Additional research based on these data might improve our understanding of male sub-fertility and serve as a basis for therapeutic research.
Funding information
This study was not funded.
Compliance with ethical standards
The study was approved by the institutional Ethics Review Board. All patients provided signed informed consent.
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Tal Biron-Shental and Amir Wiser have equal contribution.
References
- 1.Desai N, Sabanegh E, Kim T, Agarwal A. Free radical theory of aging: implications in male infertility. Urology. 2010;75:14–19. doi: 10.1016/j.urology.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 2.San Gabriel M, Zhang X, Zini A. Estimation of human sperm gene specific deoxyribonucleic acid damage by real time polymerase chain reaction analysis. Fertil Steril. 2006;85:797–799. doi: 10.1016/j.fertnstert.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 3.Huges CM, Lewis SE, McKelvey-Martin VJ, Thompson W. A comparison of baseline and induced DNA damage in human spermatozoa from fertile and infertile men, using a modified comet assay. Mol Hum Reprod. 1996;2:613–619. doi: 10.1093/molehr/2.8.613. [DOI] [PubMed] [Google Scholar]
- 4.Zini A, Bielecki R, Phang D, Zenzes MT. Correlations between two markers of sperm DNA integrity, DNA denaturation and DNA fragmentation, in fertile and infertile men. Fertil Steril. 2001;75:674–677. doi: 10.1016/S0015-0282(00)01796-9. [DOI] [PubMed] [Google Scholar]
- 5.Zalenskaya IA, Zalensky AO. Telomeres in mammalian male germline cells. Int Rev Cytol. 2002;218:37–67. doi: 10.1016/S0074-7696(02)18011-9. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Bohr VA, Lansdorp P. Telomere, telomerase and aging. Mech Ageing Dev. 2008;129:1–2. doi: 10.1016/j.mad.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Harley CB. Telomeres shorten during aging of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 8.Biron-Shental T, Sukenik-Halevy R, Sharon Y, Goldberg-Bittman L, Kidron D, Fejgin MD, et al. Short telomeres may play a role in placental dysfunction in preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol. 2010;202:381.e17. doi: 10.1016/j.ajog.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 9.Hug N, Lingner J. Telomere length homeostasis. Chromosoma. 2006;115:413–425. doi: 10.1007/s00412-006-0067-3. [DOI] [PubMed] [Google Scholar]
- 10.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 11.Baird DM, Britt-Compton B, Rowson J, Amso NN, Gregory L, Kipling D. Telomere instability in males germline. Hum Mol Genet. 2006;15:45–51. doi: 10.1093/hmg/ddi424. [DOI] [PubMed] [Google Scholar]
- 12.Fujisawa M, Tanka H, Tatsumi N, Okada H, Arakawa S, Kamidono S. Telomeres activity in the testis of infertile patients with selected causes. Hum Rep. 1998;13:1476–1479. doi: 10.1093/humrep/13.6.1476. [DOI] [PubMed] [Google Scholar]
- 13.Amiel A, Goldzak G, Gaber E, Yosef G, Fejgin MD, Yukla M, Lishner M. Random aneuploidy and telomere capture in chronic lymphocytic leukemia and chronic myeloid leukemia patients. Cancer Genet Cytogenet. 2005;163:12–16. doi: 10.1016/j.cancergencyto.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Zuffardi O, Bonaglia M, Ciccone R, Giorda R. Inverted duplications deletions: underdiagnosed rearrangements? Clin Genet. 2009;75:505–513. doi: 10.1111/j.1399-0004.2009.01187.x. [DOI] [PubMed] [Google Scholar]
- 15.Laish I, Mannasse-Green B, Hadary R, Biron-Shental T, Konikoff FM, Amiel A, Kitay-Cohen Y. Telomere dysfunction in nonalcoholic fatty liver disease and cryptogenic cirrhosis. Cytogenet Genome Res. 2016;150:93–99. doi: 10.1159/000454654. [DOI] [PubMed] [Google Scholar]
- 16.Biron-Shental T, Sadeh-Mestechkin D, Amiel A. Telomere homeostasis in IUGR placentas—a review. Placenta. 2016;39:21–23. doi: 10.1016/j.placenta.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Bernadotte A, Mikhelson VM, Spivak IM. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging (Albany NY) 2016;8:3–11. doi: 10.18632/aging.100871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng YY, Kao TW, Chang YW, Wu CJ, Peng TC, Wu LW, Yang HF, Liaw FY, Chen WL. Examining the gender difference in the association between metabolic syndrome and the mean leukocyte telomere length. PLoS One. 2017;12(7):e0180687. 10.1371/journal.pone.0180687.eCollection2017 [DOI] [PMC free article] [PubMed]
- 19.Révész D, Milaneschi Y, Verhoeven JE, Lin J, Penninx BW. Longitudinal associations between metabolic syndrome components and telomere shortening. J Clin Endocrinol Metab. 2015;100:3050–3059. doi: 10.1210/JC.2015-1995. [DOI] [PubMed] [Google Scholar]
- 20.Hanna CW, Bretherick KL, Gair JL, Fluker MR, Stephenson MD, Robinson WP. Telomere length and reproductive aging. Hum Rep. 2009;24:1206–1211. doi: 10.1093/humrep/dep007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinugawa C, Murakomi T, Okamura K, Yajima A. Telomerase activity in normal ovaries and premature ovarian failure. Tokahy J Exp Med. 2000;190:231–238. doi: 10.1620/tjem.190.231. [DOI] [PubMed] [Google Scholar]
- 22.Thilagavathi J, Kumar M, Mishra SS, Venkatesh S, Kumar R, Dada R. Analysis of sperm telomere length in men with idiopathic infertility. Arch Gynecol Obstet. 2013;287:803–807. doi: 10.1007/s00404-012-2632-8. [DOI] [PubMed] [Google Scholar]
- 23.Ferlin A, Rampazzo E, Rocca MS, Keppel S, Frigo AC, De Rossi A, Foresta C. In young men sperm telomere length is related to sperm number and parental age. Hum Reprod. 2013;28:3370–3376. doi: 10.1093/humrep/det392. [DOI] [PubMed] [Google Scholar]
- 24.O'Sullivan JN, Finley JC, Risques RA, Shen WT, Gollahon KA, Moslovitz AH, Gryaznov S, Harley CB, Rabinovitch PS. Telomere length assessment in tissue sections by quantitative FISH: image analysis algorithms. Cytometry. 2004;58:120–131. doi: 10.1002/cyto.a.20006. [DOI] [PubMed] [Google Scholar]
- 25.Keefe DL, Liu L. Telomeres and reproductive aging. Reprod Fertil Dev. 2009;21:10–14. doi: 10.1071/RD08229. [DOI] [PubMed] [Google Scholar]
- 26.Butts S, Riethman H, Ratcliffe S, Shaunik A, Coutifaris C, Barnhart K. Correlation of telomere length and telomerase activity with occult ovarian insufficiency. J Clin Endocrinol Metab. 2009;94:4835–4843. doi: 10.1210/jc.2008-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen H, Ong C. Detection of oxidative DNA damage in human sperm and its association with sperm function and male infertility. Free Radic Biol Med. 2000;28:529–536. doi: 10.1016/S0891-5849(99)00234-8. [DOI] [PubMed] [Google Scholar]
- 28.Rocca MS, Speltra E, Menegazzo M, Garolla A, Foresta C, Ferlin A. Sperm telomere length as a parameter of sperm quality in normozoospermic men. Hum Reprod. 2016;31:1158–1163. doi: 10.1093/humrep/dew061. [DOI] [PubMed] [Google Scholar]
- 29.Aydos SE, Elhan AH, Tukun A. Is telomere length one of the determinants of reproductive life span? Arch Gynecol Obstet. 2005;272:113–116. doi: 10.1007/s00404-004-0690-2. [DOI] [PubMed] [Google Scholar]
- 30.Hemann MT, Rudolph KL, Strong MA, DePinho RA, Chin L, Greider CW. Telomere dysfunction triggers developmentally regulated germ cell apoptosis. Mol Biol Cell. 2001;12:2023–2030. doi: 10.1091/mbc.12.7.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujisawa M, Tanaka H, Tatsumi N, Okada H, Arakawa S, Kamidono S. Telomerase activity in the testis of infertile patients with selected causes. Hum Reprod. 1998;13:1476–1479. doi: 10.1093/humrep/13.6.1476. [DOI] [PubMed] [Google Scholar]