Abstract
Objective
This systematic review sought to evaluate the clinical outcomes of vitrification at the cleavage stage and blastocyst stage for embryo transfer in patients undergoing assisted reproductive technology (ART) treatment.
Methods
We searched for related comparative studies published in the PubMed, EMBASE, and Cochrane Library databases up to July 2017. The primary outcomes were clinical pregnancy rate (CPR) and embryo implantation rate (IR). Secondary outcomes were multiple pregnancy rate (MPR), miscarriage rate (MR), live birth rate (LBR), and ongoing pregnancy rate (OPR). The Mantel-Haenszel fixed effects model and random effects model were used to analyze the summary risks ratios (RRs) with 95% confidence intervals (CIs).
Results
Eight studies with more than 6590 cycles were included in our meta-analysis. Seven studies were observational retrospective comparative studies. One was a prospective study. Overall, the current study summarizes information from 6590 vitrification warming cycles (cleavage stage n = 4594; blastocysts n = 1996). There was no difference in the primary outcome clinical pregnancy rate (RR = 0.97, 95% CI = 0.90–1.04; fixed effects model; I 2 = 21%), whereas vitrified blastocyst transfer was significantly superior to vitrified cleavage-stage embryo transfer regarding the implantation rate (RR = 0.85, 95% CI = 0.74–0.97; random effects model; I 2 = 43). Regarding the secondary outcomes, no differences were found in the multiple pregnancy rate (RR = 1.20, 95% CI = 0.79–1.82; fixed effects model; I 2 = 22), live birth rate (RR = 1.07, 95% CI = 0.98–1.16; fixed effects model; I 2 = 0), and ongoing pregnancy rate (RR = 1.01, 95% CI = 0.92–1.120; fixed effects model; I 2 = 0), whereas a higher miscarriage rate was observed with vitrified blastocyst transfer (RR = 0.65, 95% CI = 0.45–0.93; random effects model; I 2 = 23).
Conclusion
In summary, this meta-analysis shows that vitrification at any stage has no detrimental effect on clinical outcome. Blastocyst transfer will still remain a favorable and promising option in ART. Due to the small sample evaluated in the pool of included studies, large-scale, prospective, and randomized controlled trials are required to determine if these small effects are clinically relevant.
Keywords: Blastocyst, Cleavage stage, Clinical outcome, Vitrification, Embryo transfer
Introduction
Cryopreservation has become a well-established adjuvant technique since the publication of the first reports on successful clinical pregnancies with frozen-thawed human embryos in the early 1980s [1]. Some studies have shown that improvements in cryopreservation technology have led to a higher embryo survival rate and implantation rate, without significantly increasing any adverse short-term health outcomes for the infants [2–4]. Slow freezing is the most widely used cryopreservation method for human embryos; however, vitrification is rapidly replacing slow freezing as the method of choice for embryo freezing in clinics worldwide. The decision to switch to the vitrification cryopreservation protocol was due to the reported high embryo survival rate, the lower risk of losing the opportunity for embryo transfer and higher blastulation rates [5–9]. The first report on the efficacy of the vitrification cryopreservation protocol was published in 1985 [10]. Vitrification, using different types of devices and methodologies, is an ultra-rapid freezing procedure that prevents ice crystal formation. It is the routine cryopreservation method used for surplus human cleavage-stage embryos and blastocyst embryo transfers [8, 9, 11–20]. The major obstacle of slow freezing is damage to the extra-cellular structure of large tissues and organs due to the crystallization of ice; this has to be avoided if structural and functional integrity are to be maintained [21–23]. The principle of vitrification aims to eliminate the formation of intra- and extra-cellular ice crystals and to prevent osmotic shock from ultra-rapid cooling, thereby reducing damage to living cells. Using this method, cells are vitrified in a glass-like amorphous solid state free of any crystallized structures [11].
The optimal stage for embryo vitrification remains to be determined. There are a number of studies where the pregnancy rates between transfers performed at the cleavage stage and blastocyst stage have been compared. Each stage has its own inherent advantages [13, 24–30]. Blastocyst transfer (BT) results in a higher implantation rate (IR) and a better clinical pregnancy rate than cleavage-stage transfer [31, 32], with a smaller number of transferred embryos [33–35]. Extended culture and transfer of blastocysts increase the synchronization of the endometrium and permits the selection of more advanced embryos considered best suited for transfer [33, 36]. BT decreases uterine contractility, thus reducing the chance of embryo expulsion [37]. In addition, a study found that chromosome abnormalities were lower with BT compared to cleavage-stage embryo transfer [38]. BT prevents premature contact with an altered uterine environment after controlled ovarian stimulation, as supraphysiological concentrations of estrogen (E2) and progesterone (P) may influence endometrial receptivity [39, 40]. However, it seems that the cumulative clinical pregnancy rate for cleavage-stage embryos is higher than that for blastocysts [41].
There are several potential limitations to the transfer of blastocysts. The cancelation rate in blastocyst stage is significantly higher than in the cleavage stage [42, 43]. Blastocysts have lower freezing rates compared with the cleavage stage [44]. BT is also associated with higher monozygotic twin rates [45]. BT also increases the risk of premature delivery and babies with lower birth weights [46], and may contribute to the generation of epigenetic mutations in the offspring [47] and altered sex ratios [48] compared with cleavage-stage embryo transfer. A meta-analysis found no evidence of any differences in pregnancy outcomes between day 2/3 and day 5/6 embryo transfer [49]. De Vos et al. debated the benefit of day 5 versus day 3 single embryo transfer [24]. Hreinsson et al. demonstrated that embryo transfer is equally effective at the cleavage stage and the blastocyst stage [50].
The reported optimized embryo transfer stage for vitrification cycles is variable and inconsistent. As these findings are inconsistent in the literature, we decided to perform a systematic review to investigate the optimal embryo transfer strategy for vitrification cycles.
Materials and methods
Search strategy
To identify relevant articles that compared the clinical outcomes of vitrified cleavage stage and blastocyst embryo transfers, electronic databases were searched and the reference lists of articles were scanned by two independent reviewers. The search was last performed from start dates to July 2017. The electronic searches were performed using the PubMed, EMBASE, and Cochrane Library databases with no language restrictions. The following terms were searched: “day 2” (day two), “day 3” (day three), “cleavage stage,” “day 5” (day five), “day 6” (day six), “blastocyst,” and “vitrification”.
Selection of studies
Only reports meeting the following inclusion criteria were included in the meta-analysis. Eligibility inclusion criteria were the following: (1) any female age; (2) human vitrified embryos; (3) embryo vitrification had been performed on day 2/day 3 (cleavage stage) or day 5/day 6 (blastocyst stage); (4) the study provided comparative data on clinical outcomes after embryo transfer. Exclusion criteria were the following: (1) assisted hatching cycles; (2) non-human vitrified embryos; (3) embryo transfer did not compare the cleavage stage and blastocyst stage; (4) preimplantation genetic diagnosis (PGD); (5) oocyte in vitro maturation (IVM) was performed.
Data extraction
Two researchers assessed the eligible studies and extracted the data independently. Any disagreement was resolved by discussion with each other. Study characteristics and outcome data were generated from eight eligible studies.
Quality assessment
We used the Newcastle-Ottawa Scale to assess the methodological quality. The Newcastle-Ottawa Scale star system (range 0 to 9 stars) was used to assess the eight eligible studies. In the present study, a score of > 5 points was considered to be an adequately qualification for the meta-analysis, because standard validated criteria for important end points have not been established. The mean value for the eight studies assessed was 7 stars. A table containing the rankings for each study is provided in the online data supplement (Table 1).
Table 1.
Characteristics of the included studies
| Study | Design | Outcome | ET stage | Vitrification | Quality scores |
|---|---|---|---|---|---|
| Cobo et al. [13] | R | CPR, IR, MR, LBR, OPR | day2/3+day5/6 | Cryotop | 7 |
| Lee et al. [27] | R | CPR | day3+day5 | No statement | 6 |
| Vos et al. [24] | R | LBR | day3+day5 | No statement | 8 |
| Sugiyama et al. [29] | R | CPR, IR, MR, LBR | day3+BL | Cryotop | 6 |
| Shaw et al. [28] | Prospective study | CPR, IR, MPR, MR | day3+day5 | No statement | 7 |
| Tong et al. [30] | R | CPR, IR, MPR | CL+BL | Cryotop | 7 |
| Desai et al. [25] | R | CPR, IR | day3+day 5/6 | Cryoloop | 6 |
| Desai et al. [26] | R | CPR, IR, MPR, MR, LBR, OPR | CL+BL | Rapid-i+cryoloop | 7 |
CL cleavage stage ET, BL blastocyst stage ET. R retrospective study
Outcome parameters
The primary outcome measures chosen for this meta-analysis were the clinical pregnancy rate and implantation rate. Secondary outcomes were the rate of multiple pregnancy, miscarriage, live birth, and ongoing pregnancy.
Statistical analysis
All statistical analysis was conducted using Rev. Man software [version 5.3]. For the included studies, the dichotomous data results for each of the studies eligible for the meta-analysis were expressed as a risk ratio (RR) with 95% confidence intervals (CI). The fixed effects model was to be adopted if there was no heterogeneity among studies; otherwise, the random effects model was used. Statistical heterogeneity between studies was evaluated with the chi-squared test and the I 2 statistic. An I 2 value greater than 50% is considered to represent a substantial heterogeneity. P < 0.05 was considered statistically significant. All statistical tests were two-sided. Sensitivity analysis was performed by omitting one study each time to detect extreme values.
Results
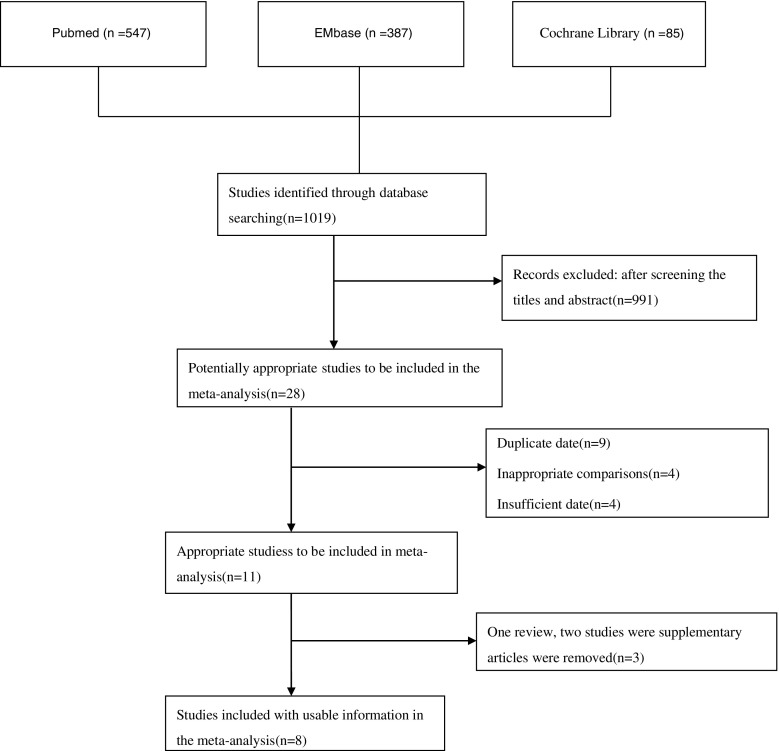
A total of 1019 available publications were identified after the primary comprehensive literature research using aforementioned strategy (Fig. 7). After the titles and abstracts were retrieved, 991 irrelevant studies were excluded and the resulting in 28 potentially eligible studies was reviewed in detail. Of these 28 articles, 17 were subsequently excluded, the reasons for which were nine duplicates, four inappropriate comparisons, and four because of insufficient data. We subsequently excluded three articles (one review and two studies published by the same group with overlapping recruitment periods), resulting in eight studies finally being included in the current meta-analysis. The main characteristics and quality features of the eight included trials are presented in Table 1.
Fig. 7.
Published related comparative studies in the PubMed, EMBASE, and Cochrane Library databasesᅟ
Clinical pregnancy
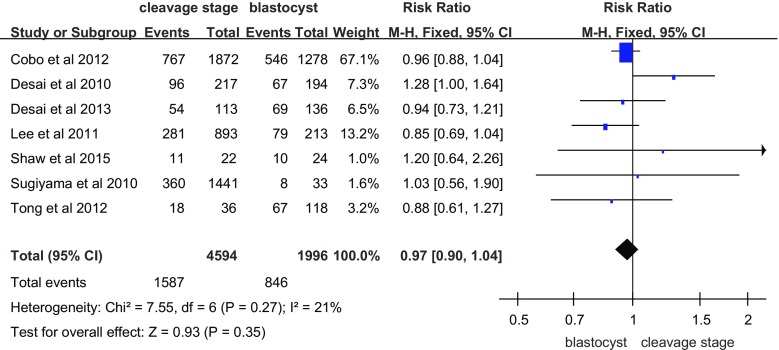
Seven suitable studies investigated the clinical pregnancy rate after vitrification at the cleavage and blastocyst stages (Fig. 1). The clinical pregnancy rate of cleavage-stage embryos after vitrification was not different compared to vitrified blastocysts (RR = 0.97, 95% CI = 0.90–1.04; fixed effects model). No heterogeneity was detected (I 2 = 21%).
Fig. 1.
Clinical pregnancy rate per transfer cycle
Implantation rate
The embryo implantation rate, a limiting factor for success, represents the capacity of each embryo transferred during this period to implant in the uterus and to result in pregnancy. There were six trials in eight studies that reported the embryo implantation rate (Fig. 2). There was a statistically significant difference in the implantation rate between cleavage-stage embryo transfer and blastocyst transfer after vitrification (RR = 0.85; 95% CI = 0.74–0.97; random effects model). Blastocyst transfer had a higher implantation rate than cleavage-stage embryo transfers. Heterogeneity was observed (I 2 = 43%).
Fig. 2.
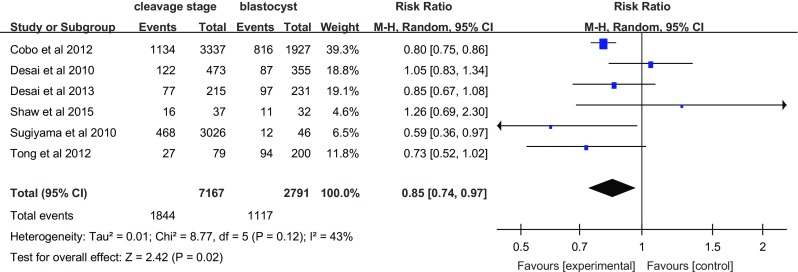
Implantation rate per embryo transfer
Multiple pregnancies
Three studies investigated the effect of vitrification on multiple pregnancies (Fig. 3). Compared with those women in the blastocyst transfer group, women who underwent cleavage-stage embryo transfer did not show and increased rate of multiple pregnancies (RR = 1.20, 95% CI = 0.79–1.82; fixed effects model), without heterogeneity (I 2 = 22%).
Fig. 3.
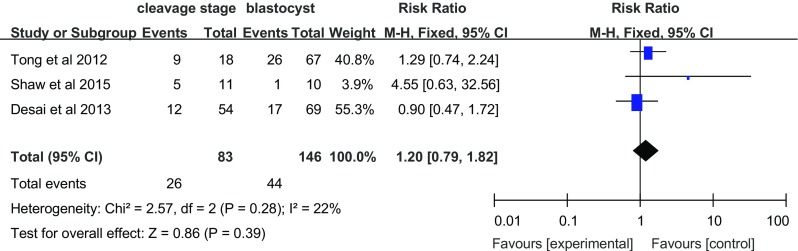
Multiple pregnancy rate per transfer cycle
Miscarriage rate
The most common cause of spontaneous miscarriage during the first trimester is chromosomal abnormalities of the embryo or fetus. Four articles investigated the effect of vitrification on the miscarriage rate (Fig. 4). The miscarriage rate with blastocyst transfer was higher than that with cleavage-stage embryo transfer (RR = 0.65, 95% CI = 0.45–0.93; random effects model), without heterogeneity (I 2 = 23%).
Fig. 4.
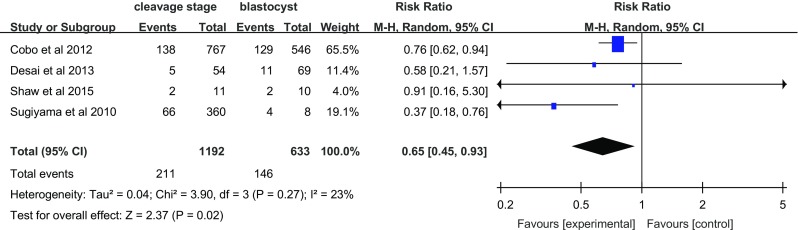
Miscarriage rate per transfer cycle
Ongoing pregnancy
Two studies investigated the effect of vitrification on ongoing pregnancy (Fig. 5). Compared with women in the blastocyst transfer group, women who underwent cleavage-stage embryo transfer did not show an increase in ongoing pregnancy (RR = 1.01, 95% CI 0.92–1.12; fixed effects model), without heterogeneity (I 2 = 0%).
Fig. 5.
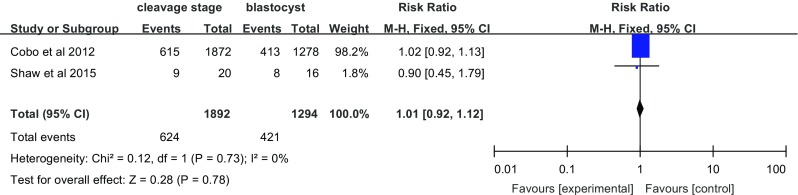
Ongoing pregnancy per transfer cycle
Live birth
Four studies investigated the effect of vitrification on live birth (Fig. 6). Compared with women in the blastocyst transfer group, women who underwent cleavage-stage embryo transfer showed no difference in the live birth rate (RR = 1.07, 95% CI = 0.98–1.16; fixed effects model), without heterogeneity (I 2 = 0%).
Fig. 6.
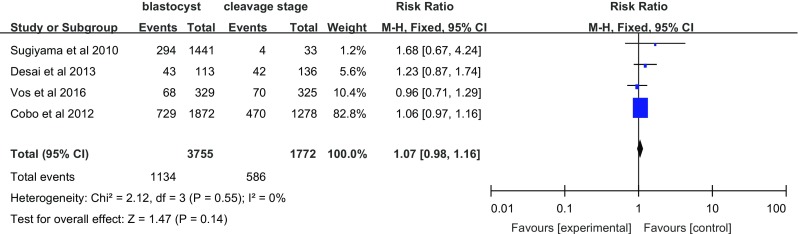
Live birth rate per transfer cycle
The main characteristics and quality features of the eight included studies are presented in Table 1.
Discussion
Eight studies were included, comparing the clinical outcomes of vitrified cleavage-stage and blastocysts embryo transfers in patients undergoing ART treatment. Our meta-analysis demonstrated that BT is associated with a higher implantation rate than cleavage-stage embryo transfers in patients undergoing vitrification cycles. However, BT also increased the miscarriage rate compared with cleavage-stage embryo transfer. This systematic review showed that there was no significant difference in the clinical pregnancy rate. So, the BT group and the cleavage-stage embryo transfer group had the same rates of multiple pregnancy, live birth, and ongoing pregnancy. The results of this study therefore demonstrate that blastocyst culture and transfer is not suited for all ART patients.
In order to explain the clinical outcomes between the cleavage stage and blastocyst stage in vitrification cycles, a variety of factors to be considered. This meta-analysis showed a higher implantation rate with blastocyst transfer than cleavage-stage transfer, as previous studies have reported. This may be due to a number of reasons. First, vitrification BT has an extremely high survival rate, so the great majority of warming cycles result in embryo placement, which is why the rate of transfer cancelation is quite low [13]. Shaw et al. found a similar survival rate after vitrification and warming for embryos in the cleavage and blastocyst stages [28]. Second, vitrification BT has a very high cooling rate and, most importantly, an extremely high warming rate, which impedes the formation of ice during warming [13, 51]. Blastocyst collapse is integral to the successful vitrification of expanded blastocysts. Desai et al. showed that cell death and DNA damage are reduced if the size of the blastocoel cavity is small prior to vitrification [52]. Third, BT increases the synchronization of the endometrium and permits the selection of more advanced embryos, which are better suited for transfer compared to cleavage-stage transfer [33, 36]. Tong et al. found that blastocyst culture and transfer should be offered primarily to younger patients (less than 35 years of age) with a better prognosis, in tandem with blastocyst vitrification [30]. Assisted hatching improves the chances of implantation rate during assisted reproduction [53]. Zhu et al. showed that BT with assisted hatching has a higher implantation rate compared to cleavage-stage embryos in vitrification cycles [54]. However, we excluded this article in the detailed analysis, considering that this article was a source of heterogeneity. The reason is that this study used assisted hatching while the other studies did not (Fig. 7).
In our meta-analysis, in the vitrification BT group, the first trimester miscarriage rate was higher compared with that in the vitrification cleavage-stage embryo transfer group. It is thought that cleavage-stage embryo transfer cycles may produce a higher quality of embryo compared with vitrified-warmed BT cycles. Cleavage-stage embryo transfer cycles are usually performed on young patients with a good prognosis, while vitrified-warmed BT cycles are performed women who did not became pregnant after a cleavage-stage embryo transfer cycle [55].
In vitrification embryo transfer, BT did not show any benefit regarding the clinical pregnancy rate, multiple pregnancy rate, live birth rate, or ongoing pregnancy compared with cleavage-stage embryo transfer. There may be some factors that affect these pregnancy outcomes. Although the vitrification process avoids intra-cellular ice crystal formation during cooling and prevents injury to the cryopreserved cells, it also protects cells from damage due to ice crystals during thawing [56]. However, the vitrification procedure may also induce hardening of the zona pellucida, resulting in hatching difficulties, as the zona thickness has been correlated negatively with embryo implantation rates [57]. Vitrification uses high concentrations of cryoprotectants which may be toxic to oocytes and embryos. If the cooling rate applied is insufficient, intra-cellular ice formation and freezing may occur. These are two major shortcomings affecting the development of embryos [6, 29, 58]. Barsky et al. found that vitrified-warmed embryo transfers are 3.1-fold more likely to result in pregnancies complicated by preeclampsia as compared to pregnancies resulting from fresh embryo transfers [59]. Although we collected a relatively large number of cleavage-stage frozen embryo transfer cases, there are limitations due to the small number of blastocyst frozen embryo transfer cases. The vitrification protocol, using different types of devices and methodologies, may also have an effect on clinical outcomes. Desai et al. found that the lower cooling rate with the Rapid-i method was not a deterrent to the successful vitrification of Day 3 cleavage embryos despite the larger size of the individual blastomeres compared to the cryoloop device [26].
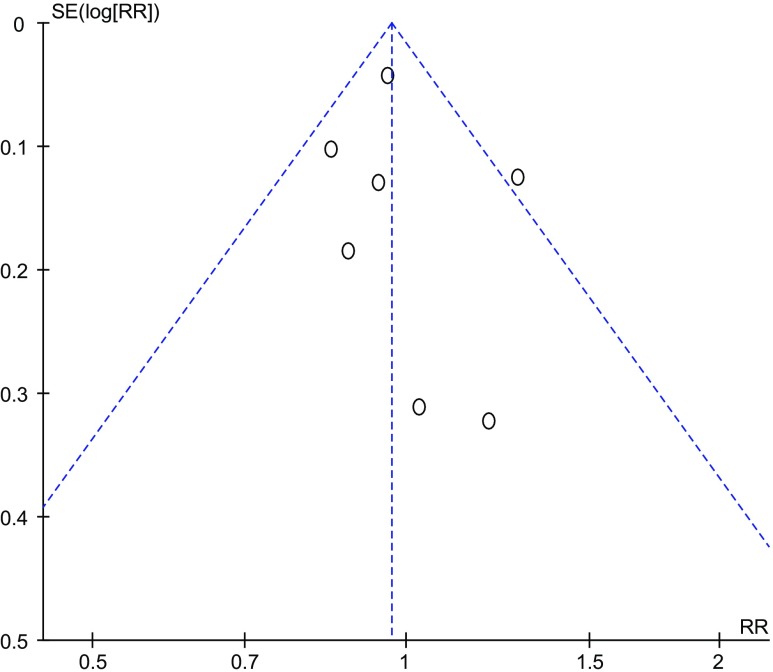
Our study has several strengths. First, it is a comprehensive meta-analysis comparing the optimal stage for embryo vitrification. We conducted sensitivity analyses and used a random effects model throughout to incorporate heterogeneity into our analysis; no apparent heterogeneity was detected, making the results reliable. Moreover, the pooled RR was not affected by any individual study. In particular, when we omitted the study of Cobo et al., the RRs were not significantly different from those obtained in the overall analyses. For the meta-analysis of a large sample size, there was no evidence of significant publication bias by inspection of the funnel plot (Fig. 8).
Fig. 8.
Funnel plot of publication bias
The shortcomings of this meta-analysis are as follows. First, the studies included in our meta-analysis were mostly retrospective studies, and only one was a prospective study. A prospective randomized controlled trial is needed in the future. Second, 67% of the total transfer cycles of the meta-analysis was provided by one study [13], which included women with embryo transfers in which vitrification occurred between the cleavage stage and blastocyst stage. However, it should be highlighted that the assessment and measurement of most outcomes included in our review are considered objective in nature. It is noteworthy that the estimates of pooled RRs obtained after excluding this study were not significantly different from those obtained in the overall analyses. Selection bias is an inevitable problem and it is difficult to overcome this problem through statistical methods. There are many factors that can influence the pregnancy rate, such as the physician or the embryologist performing the transfer, difficulty in inserting the transfer catheter, and endometrial thickness. Another limitation of this review is that it only included articles that were written in English and some reports not written in English might have been missed. Moreover, the lack of standardization of vitrification equipment restricts a direct comparison of the studies included in this meta-analysis. However, it is not possible to account for differences between centers in terms of surgical techniques or vitrification protocols. We were unable to adjust for confounders such as age, controlled ovarian stimulation, duration of infertility, and pre-existing medical illness due to the varied design of the studies. Without individual patient data, we were unable to adjust for confounders and determine whether the risks were different for embryos frozen by vitrification at the cleavage and blastocyst stages. When it comes to thawing embryos, there is uncertainty as to whether the method of thawing and protocol used (a natural or hormonally mediated cycle) for replacement has any bearing on different clinical outcomes.
Undoubtedly, blastocyst transfer will still remain a favorable and promising option for ART. In the future, the efficacy of embryo transfer at the blastocyst stage could be fully optimized through further refinement and improvement of current blastocyst cryopreservation protocols.
References
- 1.Trounson A, Mohr L. Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature. 1998;3(305):707–709. doi: 10.1038/305707a0. [DOI] [PubMed] [Google Scholar]
- 2.Ozgur K, et al. Perinatal outcomes after fresh versus vitrified-warmed blastocyst transfer: retrospective analysis. Fertil Steril. 2015;104(4):899–907. doi: 10.1016/j.fertnstert.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 3.Li Z, et al. Clinical outcomes following cryopreservation of blastocysts by vitrification or slow freezing: a population-based cohort study. Hum Reprod. 2014;29:2794–2801. doi: 10.1093/humrep/deu246. [DOI] [PubMed] [Google Scholar]
- 4.Wikland M, et al. Obstetric outcomes after transfer of vitrified blastocysts. Hum Reprod. 2010;25:1699–1707. doi: 10.1093/humrep/deq117. [DOI] [PubMed] [Google Scholar]
- 5.Wong KM, Mastenbroek S, Repping S. Cryopreservation of human embryos and its contribution to in vitro fertilization success rates. Fertil Steril. 2014;102:19–26. doi: 10.1016/j.fertnstert.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 6.Zhu HY, et al. Slow freezing should not be totally substituted by vitrification when applied to day 3 embryo cryopreservation: an analysis of 5613 frozen cycles. J Assist Reprod Genet. 2015;32:1371–1377. doi: 10.1007/s10815-015-0545-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolibianakis EM, Venetis CA, Tarlatzis BC. Cryopreservation of human embryos by vitrification or slow freezing: which one is better? Curr Opin Obstet Gynecol. 2009;21:270–274. doi: 10.1097/GCO.0b013e3283297dd6. [DOI] [PubMed] [Google Scholar]
- 8.Rezazadeh Valojerdi M, et al. Vitrification versus slow freezing gives excellent survival, post warming embryo morphology and pregnancy outcomes for human cleaved embryos. J Assist Reprod Genet. 2009;26(6):347–354. doi: 10.1007/s10815-009-9318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balaban B, et al. A randomized controlled study of human day 3 embryo cryopreservation by slow freezing or vitrification: vitrification is associated with higher survival, metabolism and blastocyst formation. Hum Reprod. 2008;23:1976–1982. doi: 10.1093/humrep/den222. [DOI] [PubMed] [Google Scholar]
- 10.Rall WF, Fahy GM. Ice-free cryopreservation of mouse embryos at -196 C by vitrification. Nature. 1985;313:573–575. doi: 10.1038/313573a0. [DOI] [PubMed] [Google Scholar]
- 11.Wong YY, Wong YK. Phasing-in of vitrification into routine practice: why, how, and what. Hong Kong Med J. 2011;17:119–126. [PubMed] [Google Scholar]
- 12.Belva F, et al. Neonatal health including congenital malformation risk of 1072 children born after vitrified embryo transfer. Hum Reprod. 2016;10:1093. doi: 10.1093/humrep/dew103. [DOI] [PubMed] [Google Scholar]
- 13.Cobo A, et al. Outcomes of vitrified early cleavage-stage and blastocyst-stage embryos in a cryopreservation program: evaluation of 3,150 warming cycles. Fertil Steril. 2012;98(5):1138–1146.e1. doi: 10.1016/j.fertnstert.2012.07.1107. [DOI] [PubMed] [Google Scholar]
- 14.Wilding MG, et al. Human cleavage-stage embryo vitrification is comparable to slowrate cryopreservation in cycles of assisted reproduction. J Assist Reprod Genet. 2010;27:549–554. doi: 10.1007/s10815-010-9452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakashima A, et al. Optimization of a novel nylon mesh container for human embryo ultrarapid vitrification. Fertil Steril. 2010;93:24052410. doi: 10.1016/j.fertnstert.2009.01.063. [DOI] [PubMed] [Google Scholar]
- 16.Schoolcraft WB, et al. Clinical application of comprehensive chromosomal screening at the blastocyst stage. Fertil Steril. 2010;94:1700–1706. doi: 10.1016/j.fertnstert.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Raju GA, et al. Vitrification of human early cavitating and deflated expanded blastocysts: clinical outcome of 474 cycles. J Assist Reprod Genet. 2009;26:523–529. doi: 10.1007/s10815-009-9356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanderzwalmen P, et al. Aseptic vitrification of blastocysts from infertile patients, egg donors and after IVM. Reprod BioMed Online. 2009;19:700–707. doi: 10.1016/j.rbmo.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 19.van Landuyt L, et al. Outcome of closed blastocyst vitrification in relation to blastocyst quality: evaluation of 759 warming cycles in a single-embryo transfer policy. Hum Reprod. 2011;26:527–534. doi: 10.1093/humrep/deq374. [DOI] [PubMed] [Google Scholar]
- 20.Liebermann J. Vitrification of human blastocysts: an update. 2009. Reprod BioMed Online. 2009;19 Suppl 4:4328. [PubMed] [Google Scholar]
- 21.Jacobsen IA, Pegg DE, et al. Effect of cooling and warming rate on glycerolized rabbit kidneys. Cryobiology. 1984;21:637–653. doi: 10.1016/0011-2240(84)90223-2. [DOI] [PubMed] [Google Scholar]
- 22.Pegg DE. Ice crystals in tissues and organs. In: Pegg DE, Karow AM Jr, editors. The biophysics of organ preservation. New York: Plenum; 1987. pp. 117–140. [Google Scholar]
- 23.Rubinsky B, Pegg ED. A mathematical model for the freezing process in biological tissue. Proc R Soc Lond. 1988;234:343–358. doi: 10.1098/rspb.1988.0053. [DOI] [PubMed] [Google Scholar]
- 24.De Vos A, et al. Cumulative live birth rates after fresh and vitrified cleavage-stage versus blastocyst-stage embryo transfer in the first treatment cycle. Hum Reprod. 2016;31(11):2442–2449. doi: 10.1093/humrep/dew219. [DOI] [PubMed] [Google Scholar]
- 25.Desai N, et al. What is the optimal stage for embryo vitrification-a comparison of embryo survival and clinical outcomes with day 3 cleavage versus blastocyst stage vitrification. Fertil Steril. 2010;94(4):S110. doi: 10.1016/j.fertnstert.2010.07.454. [DOI] [Google Scholar]
- 26.Desai N, et al. The new Rapid-i carrier is an effective system for human embryo vitrification at both the blastocyst and cleavage stage. Reprod Biol Endocrinol. 2013;11(44):2–9. doi: 10.1186/1477-7827-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JH, et al. Effect of day-3 embryo and day-5 blastocyst stage at vitrification on clinical outcome of cryopreserved-embryo transfer cycles. Stockholm: Annual Meeting of ESHRE; 2011. [Google Scholar]
- 28.Shaw SF. Ongoing and cumulative pregnancy rate after cleavage-stage versus blastocyst-stage embryo transfer using vitrification for cryopreservation: impact of age on the results. J Assist Reprod Genet. 2015;32:177–184. doi: 10.1007/s10815-014-0387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugiyama R, et al. Clinical outcomes resulting from the transfer of vitrified human embryos using a new device for cryopreservation (plastic blade) J Assist Reprod Genet. 2010;27(4):161–167. doi: 10.1007/s10815-010-9390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tong GQ, et al. Clinical outcome of fresh and vitrified-warmed blastocyst and cleavage-stage embryo transfers in ethnic Chinese ART patients. J Ovarian Res. 2012;5:27. doi: 10.1186/1757-2215-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamath M, et al. Comparison of clinical outcomes following vitrified warmed day 5/6 blastocyst transfers using solid surface methodology with fresh blastocyst transfers. J Hum Reprod Sci. 2013;6(1):59. doi: 10.4103/0974-1208.112384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu D, et al. Vitrified-warmed blastocyst transfer cycles yield higher pregnancy and implantation rates compared with fresh blastocyst transfer cycles—time for a new embryo transfer strategy? Fertil Steril. 2011;95(5):1691–1695. doi: 10.1016/j.fertnstert.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 33.Han AR, et al. Blastocyst transfer in frozen-thawed cycles. Clin Exp Reprod Med. 2012;39(3):114–117. doi: 10.5653/cerm.2012.39.3.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blake D, et al. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology (Review). Cochrane Libr. 2011;10
- 35.Papanikolaou EG, et al. In vitro fertilization with single blastocyst-stage versus single cleavage-stage embryos. N Engl J Med. 2006;354:1139–1146. doi: 10.1056/NEJMoa053524. [DOI] [PubMed] [Google Scholar]
- 36.SILLS ES, Palermo DG. Human blastocyst culture in IVF: current laboratory applications in reproductive medicine practice. Morphol Embryol. 2010;51(3):441–445. [PubMed] [Google Scholar]
- 37.Fanchin R, et al. Uterine contractility decreases at the time of blastocyst transfers. Hum Reprod. 2001;16:1115–1119. doi: 10.1093/humrep/16.6.1115. [DOI] [PubMed] [Google Scholar]
- 38.Ata B, et al. Array CGH analysis shows that aneuploidy is not related with the number of embryos generated. Reprod BioMed Online. 2012;24:614–620. doi: 10.1016/j.rbmo.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Carvalho BR, et al. Embryo stage of development is not decisive for reproductive outcomes in frozen-thawed embryo transfer cycles. JBRA Assist Reprod. 2017;21(1):23–26. doi: 10.5935/1518-0557.20170007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fatemi HM, Popovic-Todorovic B. Implantation in assisted reproduction: a look at endometrial receptivity. Reprod BioMed Online. 2013;27:530–538. doi: 10.1016/j.rbmo.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 41.Glujovsky D, et al. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. 2012;7:CD002118. doi: 10.1002/14651858.CD002118.pub4. [DOI] [PubMed] [Google Scholar]
- 42.Berkkanoglu M, et al. Optimal embryo transfer strategy in poor response may include freeze-all. J Assist Reprod Genet. 2016;34(1):79–87. doi: 10.1007/s10815-016-0825-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marek D, et al. Introduction of blastocyst culture and transfer for all patients in an in vitro fertilization program. Fertil Steril. 1999;72:1035e40. doi: 10.1016/S0015-0282(99)00409-4. [DOI] [PubMed] [Google Scholar]
- 44.Papanikolaou EG, et al. Live birth rates after transfer of equal number of blastocysts or cleavage-stage embryos in IVF. A systematic review and meta-analysis. Hum Reprod. 2008;23:91e9. doi: 10.1093/humrep/dem339. [DOI] [PubMed] [Google Scholar]
- 45.Practice Committee of the American Society for Reproductive Medicine.Practice Committee of the Society for Assisted Reproductive Technology Blastocyst culture and transfer in clinical-assisted reproduction. Fertil Steril. 2006;86:S89e92. doi: 10.1016/j.fertnstert.2006.07.1479. [DOI] [PubMed] [Google Scholar]
- 46.Dar S, et al. Increased risk of preterm birth in singleton pregnancies after blastocyst versus day 3 embryo transfer: Canadian ART Register (CARTR) analysis. Hum Reprod. 2013;28:924e8. doi: 10.1093/humrep/des448. [DOI] [PubMed] [Google Scholar]
- 47.Niemitz EL, Feinberg AP. Epigenetics and assisted reproductive technology: a call for investigation. Am J Hum Genet. 2004;74:599e609. doi: 10.1086/382897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang HJ, et al. Impact of blastocyst transfer on offspring sex ratio and the monozygotic twinning rate: a systematic review and meta-analysis. Fertil Steril. 2009;91:2381e90. doi: 10.1016/j.fertnstert.2008.03.066. [DOI] [PubMed] [Google Scholar]
- 49.Blake D, et al. Cleavage stage versus blastocyst stage embryo transfer in assisted conception. Cochrane Collab. 2005;4:CD002118. doi: 10.1002/14651858.CD002118.pub2. [DOI] [PubMed] [Google Scholar]
- 50.Hreinsson J, et al. Embryo transfer is equally effective at cleavage stage and blastocyst stage: a randomized prospective study. Eur J Obstet Gynecol Reprod Biol. 2004;117(2):194–200. doi: 10.1016/j.ejogrb.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 51.Seki S, Mazur P. The dominance of warming rate over cooling rate in the survival of mouse oocytes subjected to a vitrification procedure. Cryobiology. 2009;59:75–82. doi: 10.1016/j.cryobiol.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Desai N, et al. Artificial collapse of blastocysts before vitrification: mechanical vs. laser technique and effect on survival, cell number, and cell death in early and expanded blastocysts. Biopreserv Biobank. 2008;6:181–190. [Google Scholar]
- 53.Martins WP, et al. Assisted hatching of human embryos: a systematic review and meta-analysis of randomized controlled trials. Hum Reprod Update. 2011;17(4):438–453. doi: 10.1093/humupd/dmr012. [DOI] [PubMed] [Google Scholar]
- 54.Zhu L, et al. Blastocyst culture and cryopreservation to optimize clinical outcomes of warming cycles. Reprod BioMed Online. 2013;27(2):154–160. doi: 10.1016/j.rbmo.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 55.Feng GX, et al. Comparable clinical outcomes and live births after single vitrified–warmed and fresh blastocyst transfer. Reprod BioMed Online. 2012;25:466–473. doi: 10.1016/j.rbmo.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 56.Ku P-Y, et al. Comparison of the clinical outcomes between fresh blastocyst and vitrified-thawed blastocyst transfer. J Assist Reprod Genet. 2012;29(12):1353–1356. doi: 10.1007/s10815-012-9874-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Edi-Osagie E, Hooper L, Seif MW. The impact of assisted hatching on live birth rates and outcomes of assisted conception: a systematic review. Hum Reprod. 2003;18(9):1828–1835. doi: 10.1093/humrep/deg334. [DOI] [PubMed] [Google Scholar]
- 58.Maheshwari A, Bhattacharya S. Elective frozen replacement cycles for all: ready for prime time? Hum Reprod. 2013;28:6–9. doi: 10.1093/humrep/des386. [DOI] [PubMed] [Google Scholar]
- 59.Barsky M, et al. Are perinatal outcomes affected by blastocyst vitrification and warming? Am J Obstet Gynecol. 2016;215:603.e1–603.e5. doi: 10.1016/j.ajog.2016.06.002. [DOI] [PubMed] [Google Scholar]