Abstract
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder among reproductive-aged women and the main cause of infertility due to anovulation. However, this syndrome spans the lives of women affecting them from in-utero life until death, leading to several health risks that can impair quality of life and increase morbidity and mortality rates. Fetal programming may represent the beginning of the condition characterized by hyperandrogenism and insulin resistance which leads to a series of medical consequences in adolescence, adulthood, and old age. Menstrual and fertility problems evolve into metabolic complications as age advances. An early and precise diagnosis is important for an adequate management of PCOS, especially at the extreme ends of the reproductive lifespan. However, many different phenotypes are included under the same condition, being important to look at these different phenotypes separately, as they may require different treatments and have different consequences. In this way, PCOS exhibits a great metabolic complexity and its diagnosis needs to be revised once again and adapted to recent data obtained by new technologies. According to the current medical literature, lifestyle therapy constitutes the first step in the management, especially when excess body weight is associated. Pharmacotherapy is frequently used to treat the most predominant manifestations in each age group, such as irregular menses and hirsutism in adolescence, fertility problems in adulthood, and metabolic problems and risk of cancer in old age. Close surveillance is mandatory in each stage of life to avoid health risks which may also affect the offspring, since fetal and post-natal complications seem to be increased in PCOS women.
Keywords: Polycystic ovary syndrome, Childhood, Adolescence, Perimenopause, Fertility, Pregnancy complications
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder among reproductive-aged women (5–10%) and the main cause of infertility due to anovulation [1]. PCOS affects women from in-utero life until death, leading to several health risks that can impair quality of life and increase morbidity and mortality rates. This condition really includes many different phenotypes which may require different treatments and may have different consequences and exhibits a great metabolic complexity, thus needing an urgent revision of its diagnosis. The aim of the present review is to describe the medical consequences of the syndrome from the beginning of the reproductive life to its end according to the current medical literature (Fig. 1). New research may change future knowledge about the syndrome once its different phenotypes are analyzed as complete separate entities.
Fig. 1.
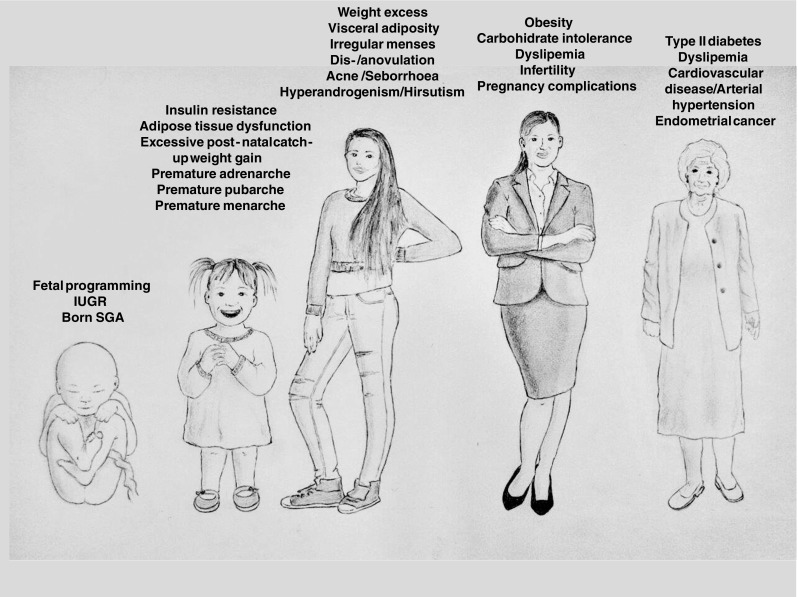
Main clinical and metabolic manifestations of polycystic ovary syndrome according to women’s stage of life. Clinical manifestations of PCOS appear in adolescence, but the disease seems to be originated in the intra-uterine environment through developmental programming. Menstrual, hyperandrogenic, and reproductive manifestations change to metabolic alterations as age advances, thus affecting this syndrome from the beginning of life to its end. IUGR: intrauterine growth retardation; SGA: small for gestational age
Definition and epidemiology
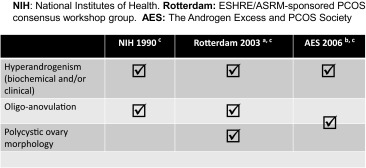
PCOS is a complex syndrome with diagnostic criteria that have been grouped in different, somewhat controversial, classifications [2–6] (Table 1). According to the features of the syndrome considered, up to 16 phenotypes may exist with different metabolic and reproductive consequences. Some of these phenotypes will be included in the criteria of the commented classifications (Table 2). The Rotterdam criteria are the most commonly used, although they are now over 10 years old and not accepted by all [7], with calls for them to be updated [8, 9]. The criteria used to define oligo-anovulation (OA) are insufficient, an adequate definition of biological hyperandrogenism (HA) is yet to be established, and the characterization of polycystic ovarian morphology (PCOM) proposed in 2003 has become obsolete in the face of the latest generations of ultrasound machines [10]. In addition, these diagnostic criteria are not valid for early and late ages (i.e., for teenagers and aged women). High anti-Müllerian hormone (AMH) serum concentrations have emerged as a useful marker of PCOS, although a universally agreed cut-off has not been established [11, 12]. In any case, PCOS must always be diagnosed after all other conditions that involve HA or OA have been excluded [13].
Table 1.
Different classifications of diagnostic criteria for PCOS
aTwo criteria required out of three
bHyperandrogenism and ovarian dysfunction (oligo-anovulation and/or polycystic ovarian morphology)
cExclusion of other androgen excess or related disorders: 21-hydroxylase-deficiency, non-classic adrenal hyperplasia, androgen-secreting neoplasm, androgenic/anabolic drug use or abuse, Cushing’s syndrome, severe insulin resistance, thyroid dysfunction and hyperprolactinemia
Table 2.
Potential phenotypes of polycystic ovary syndrome (adapted from Azziz et al., 2009; reference 5)
| Phenotype | Hyperandrogenemia | Hirsutism | Oligo-anovulation | Polycystic ovaries | NIH 1990 criteria | Rotterdam 2003 criteria | AE-PCOS 2006 criteria |
|---|---|---|---|---|---|---|---|
| A | + | + | + | + | X | X | X |
| B | + | + | + | X | X | X | |
| C | + | + | + | X | X | X | |
| D | + | + | X | X | X | ||
| E | + | + | + | X | X | X | |
| F | + | + | X | X | X | ||
| G | + | + | + | X | X | ||
| H | + | + | X | X | |||
| I | + | + | X | X | |||
| J | + | + | X | ||||
| K | + | + | |||||
| L | + | ||||||
| M | + | ||||||
| N | + | ||||||
| O | + | ||||||
| P |
The described prevalence of PCOS in women of reproductive age in the general population varies by geographic region, ranging from 1 to 19% according to population samples analyzed in the USA, Western Europe, the Middle East, East Asia, and Australia [14, 15]. The varying prevalence of PCOS may be due to genetic and environmental factors. A lower socio-economic development is also associated with poorer health which can lead to hormonal alterations and/or activate a genetic predisposition for the development of the syndrome. Lack of adequate healthcare provision also results in lower rates of correct diagnosis and appropriate treatments [14, 16].
Pathophysiology
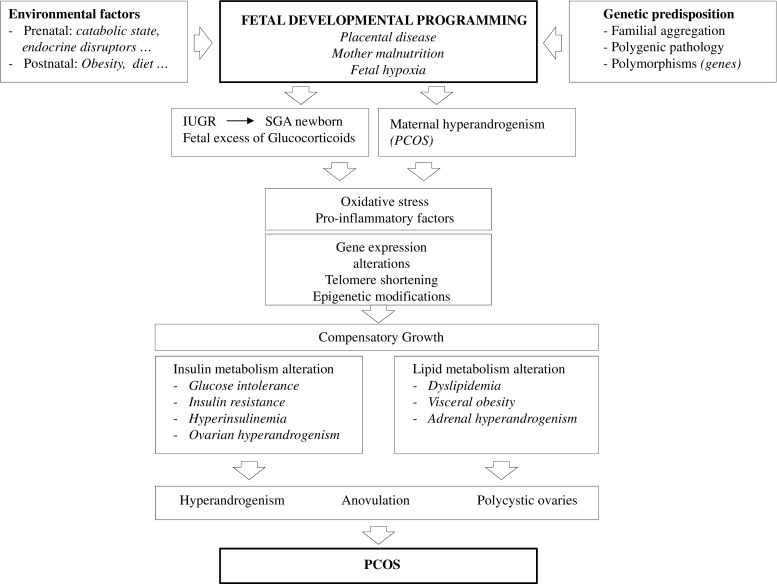
PCOS is characterized by diverse clinical presentations, both reproductive and metabolic, throughout a woman’s life. Its pathophysiology is not yet clear, but the heterogeneity of its characteristics suggests that genetic, metabolic, endocrine, environmental, and lifestyle factors are important in its clinical manifestations [17] (Fig. 2).
Fig. 2.
Pathophysiology of polycystic ovary syndrome. The interaction between a genetic predisposition and some prenatal and postnatal environmental factors seems to be responsible for the development of the syndrome. Oxidative stress, low-grade chronic inflammation, and insulin and lipid metabolism alterations are present in the background of the main clinical manifestations of the syndrome which constitute their diagnostic criteria during women’s reproductive age: hyperandrogenism, anovulation and polycystic ovary morphology. IUGR: intrauterine growth retardation; PCOS: polycystic ovary syndrome; SGA: small for gestational age
There is evidence of a pattern of inheritance of PCOS that is not yet well defined. Most authors define PCOS as a polygenic pathology. Key genes encoding factors involved in the synthesis, transport and regulation of androgens, the metabolism of insulin, and folliculogenesis have been described as the main candidate genes [18, 19]. Environmental conditions may mimic hormonal actions and activate pre-existing predisposing factors, triggering the characteristic endocrine alterations of PCOS. They can be classified as prenatal (epigenetic fetal programming) or postnatal (diet, obesity, sedentary lifestyle, and environmental toxins). It has also been proposed that PCOS may present a pattern of non-genetic inheritance in populations with an unhealthy lifestyle such as those with a diet high in saturated fat, sedentary lifestyle, and alcohol and tobacco consumption [20].
Changes in gene expression produced by exposure to steroids (mainly glucocorticoids and/or androgens) during critical periods of fetal development have been related to the different phenotypes of PCOS described [20]. In the case of fetal hypoxia due to maternal dietary restriction or placental insufficiency, catabolic phenomena lead to intrauterine growth restriction (IUGR) and low birth weight (LBW). As a survival mechanism, energy expenditure is reduced by redistributing fetal blood flow to essential organs (heart, brain, and adrenal glands). This leads to an increased production of glucocorticoids as a consequence of the hyperactivity of the hypothalamic-pituitary-adrenal axis, which may induce epigenetic modifications [21]. Studies with experimental animals have found that fetuses with IUGR caused by placental insufficiency or maternal malnutrition were born small for gestational age (SGA) and that these animals showed a predisposition to develop pathologies in postnatal life after compensatory growth during the first 2 years of life. Clinical data show that compensatory growth might also be associated with the development of comorbidities in humans, including PCOS. Thus, children born with SGA would exhibit a clinical marker of developmental programming by glucocorticoids associated with the development of PCOS and its associated comorbidities [20].
PCOS has been described as an ovarian disease characterized by excessive androgen production [18, 22]. Studies in rhesus monkeys and sheep have shown that exposure of fetuses to high levels of androgens during the intrauterine period can alter folliculogenesis and induce the onset of clinical manifestations of PCOS in adolescence [19]. HA in fetal life may also lead to epigenetic reprogramming of reproductive fetal tissues, resulting in a PCOS phenotype in adulthood. A significant increase in peripheral serum androgen concentrations has been demonstrated in singleton 22–28-week pregnancies of PCOS women versus non-PCOS pregnant women of similar gestational age [23], thus being a possible source of fetal androgenization. However, other potential sources should be considered, since placental aromatase would protect the fetus from high maternal androgen concentrations. It has been suggested that maternal obesity, diabetes mellitus, insulin resistance, and excessive weight gain during pregnancy are predictors of large for gestational age (LGA) offspring. Because these conditions may also be associated with HA, LGA babies might exhibit a higher risk for PCOS via developmental programming by androgen excess, but this hypothesis remains to be confirmed through clinical studies [20].
The manifestation of PCOS may be complete in adolescence, with the activation of the hypothalamic-pituitary-ovarian axis [20], although many women show regular cycles in the beginning which continue on to become oligomenorrheic. In puberty, there is a physiological increase in insulin levels, resulting in a reduction of SHBG levels and an increase in free androgen concentrations, with the subsequent stimulation of ovarian steroidogenesis. In women with PCOS, physiological hyperinsulinemia in adolescence may trigger HA and ovulatory dysfunction. Girls predisposed to insulin resistance and weight excess are at a higher risk of early adrenarche and subsequent PCOS.
A normal folliculogenesis depends on intra- and extra-ovarian factors. An imbalance of these factors can alter follicular development and the production of mature oocytes, compromising fertility in PCOS women. Extra-ovarian factors include FSH deficit, hypersecretion of LH, HA of ovarian or adrenal origin, and hyperinsulinemia with insulin resistance (IR). Intra-ovarian factors correspond to the family of growth factors, cytokines, and inhibins present in the follicular fluid and whose concentrations correlate with plasma levels [24]. The relationship between vitamin D deficiency and PCOS is inconclusive. Existing data on vitamin D supplementation do not demonstrate a clear effect on metabolic and reproductive parameters of PCOS [25].
Regardless of weight or body mass index (BMI), 50–70% of PCOS patients present with IR, which means that greater amounts of insulin are required for normal function. This is reflected in an increase in insulin secretion by pancreatic β cells, leading to compensatory hyperinsulinemia and normal glycaemia. When the pancreatic response is inadequate, glucose intolerance and/or type 2 diabetes can develop [22]. The mechanisms leading to IR consist of a defective binding of insulin to its receptor or to changes in insulin signal transduction. It is postulated that hyperinsulinemia contributes to HA by stimulating ovarian androgen production and inhibiting hepatic synthesis of SHBG, thus increasing levels of free testosterone. Insulin also increases adrenal androgens and stimulates ovarian steroidogenesis mediated by LH through an action on thecal and granulosa cells [26].
In addition, an imbalance between oxygen free radicals or reactive oxygen species (ROS) and antioxidant factors can lead to cell damage, a situation that can occur in the follicular fluid of women with PCOS which undermines oocyte maturation and embryo quality. The inflammatory environment caused by oxidative stress also promotes IR and contributes to HA [24]. Alteration in cortisol metabolism can also cause HA. In PCOS women, there is an elevated activity of 5α-reductase leading to increased inactivation of cortisol or impaired 11β-hydroxysteroid dehydrogenase and thus impaired regeneration of cortisol. Impaired activity of these enzymes causes increased ACTH secretion with decreased negative feedback signaling thereby maintaining normal serum cortisol with increased adrenal androgen. Thus, adrenal androgen excess in PCOS is associated with increased inactivation of cortisol by 5β-reductase that may lower cortisol blood levels and stimulate ACTH-dependent steroidogenesis [27].
A metabolic disorder has been widely documented in PCOS women. Vascular alterations such as endothelial dysfunction, increased arterial stiffness, and intima-media thickness are more prevalent in these patients. Most women with PCOS also exhibit some or most components of the metabolic syndrome, including obesity, hypertension, dyslipidemia, and IR. Up to 30–40% of women with PCOS have impaired glucose tolerance and as many as 10% develop type 2 diabetes mellitus by the age of 40 [28]. The phenotype with HA and IR seems to carry a higher metabolic risk.
Childhood and adolescence
LBW followed by excessive postnatal catch-up weight gain may initiate a cascade of metabolic changes including increased adipose body composition, IR, and a less favorable adipokine profile as early as pre-school age. These events can lead to an increased risk of premature adrenarche, pubertal development and menarche (by nearly a year, compared to non-LBW counterparts), and PCOS [29–31].
Premature pubarche (PP) is defined as the appearance of pubic hair before the age of 8 in girls and 9 in boys. PP in girls may be a precedent of metabolic syndrome and clinical ovarian androgen excess in adolescence, particularly when it occurs after LBW and excessive postnatal catch-up, and can moderately reduce final height. In girls with PP, early metformin therapy is initiated in pre-puberty, at a mean age of 8 years, and maintained throughout puberty (for 4 years). It normalizes body composition and excessive visceral fat, delays menarche by about 1 year, and increases adult stature [32, 33]. The benefits of early metformin therapy on body composition, lipids, circulating insulin, and testosterone are maintained 2 years after the treatment has terminated. Other independent prepubertal risk factors for the development of PCOS are obesity and metabolic syndrome [34].
While the diagnosis of PCOS in adulthood is based on the presence of clinical or biochemical HA in combination with ovarian dysfunction or PCOM—once other possible causes have been excluded—diagnosis during childhood and adolescence is more challenging, mainly because some features related to PCOS are common in the transition from puberty to adulthood (Tables 3 and 4). First, menstrual irregularities and anovulatory cycles are very common in the first 2 years after menarche (25% in the first year, 35–40% up to the third and fourth year). Complete maturation of the hypothalamic-pituitary-ovarian axis is usually achieved in the first 5 years after menarche. In the context of variations in the adolescent menstrual cycle, the persistence of oligomenorrhea (menstrual cycles > 35 days), secondary amenorrhea (absence of cycles for more than 3 months), or primary amenorrhea (absence of menarche by age 15 years [35]) in girls with complete pubertal development and adult stature suggests an excess of androgens [36]. Prolonged adolescent oligomenorrhea at 14–19 years is reported to be predictive of persistent ovarian dysfunction later in life [37].
Table 3.
Clinical features of polycystic ovary syndrome in adolescent (14–21 years old) and advanced age (> 50 years old) women
| Adolescence | Advanced age | |
|---|---|---|
| Hyperandrogenism | -Hirsutism -Moderate to severe or persistent acne and poor response to treatments. |
- Hirsutism - Less pronounced phenotype |
| Oligo-anovulation (OA) | -Persistence of oligomenorrhea -Secondary amenorrhea -Primary amenorrhea in girls with complete pubertal development |
- More regular menstrual cycles after age of 40 - 2-year delay of menopause |
| Metabolic and endocrine disorders | -Fewer vasomotor symptoms -Overweight or obese -Insulin resistance -Metabolic syndrome |
Table 4.
Diagnostic criteria of polycystic ovary syndrome in adolescent (14–21 years old) and advanced age (> 50 years old) women
| Adolescence | Advanced age | |
|---|---|---|
| Hyperandrogenism | -Persistent elevation of total testosterone serum levels -Hirsutism -Moderate to severe acne |
-Well-documented long-term history of hyperandrogenism |
| Oligo-anovulation (OA) | -Persistent oligomenorrhea for at least 2 years after menarche -Primary amenorrhea by age 16 and complete pubertal development |
-Well-documented long-term history of oligomenorrhea |
| Polycystic ovarian morphology | Ovarian volume > 12 cm3 |
Secondly, mild acne, hirsutism, and physiologic IR, as well as obesity, are frequently observed during puberty. A diagnosis of HA should be considered in adolescents with hirsutism (male-like hair growth), moderate to severe inflammatory acne, and/or menstrual irregularities. Acne that is persistent and poorly responsive to topical dermatologic treatment also suggests HA. Due to variability in the results of testosterone assays and limited data regarding normal developmental fluctuations in testosterone levels during puberty, there is no clear testosterone cut-off level. In general, for an assay using an extraction step, total testosterone concentrations > 55 ng/dl are consistent with HA [38].
Third, PCOM occurs in up to 25% of healthy adolescents, and ovarian volume is typically greater during puberty than in adulthood [39]. PCOM is highly prevalent in healthy non-hyperandrogenic girls since multifollicular ovaries can be a normal stage of development in adolescence and early adulthood [40].
There is a consensus that an ovarian volume > 12.0 cm3 (calculated by the formula for a prolate ellipsoid) should be considered enlarged. Follicle counts should not be used to define PCOM in adolescents. Available data regarding the usefulness of AMH for diagnosing PCOS among adolescent girls are inconsistent [41].
There is no consensus about clinical criteria to define PCOS in the adolescent population. The ESHRE/ASRM working group [42] and the Endocrine Society [43] have suggested that, when adolescent PCOS is not clearly evident by adult standards, the disorder should be considered if there have been increased serum androgen levels and/or progressive hirsutism in association with persistent oligomenorrhea for at least 2 years after menarche and/or primary amenorrhea by age 16 years and/or ovarian volume > 10 cm3, after exclusion of secondary causes. More recently, international professional societies of pediatric and adolescent medicine have defined that persistent elevation of total and/or free testosterone serum levels is the most determinant biochemical test for confirming HA in the adolescent population. Importantly, treatment may be indicated even in the absence of a definitive diagnosis [36].
The main objective of the treatment of PCOS in adolescent girls should be to restore normal ovulation and menstrual cycle, to reduce/eliminate hirsutism and acne, to achieve weight loss in overweight or obese individuals, and to treat hyperlipidemia and hyperglycaemia. A healthy diet combined with exercise should be the priority in overweight/obese adolescents. Losing weight will help to prevent clinical manifestations of PCOS, improve self-esteem, and normalize menstrual periods [44].
Hirsutism treatments may need to integrate pharmacological agents, cosmetic procedures, and psychological support [45]. The cornerstone of treatment of hirsutism is the application of cosmetic measures. The most commonly used are shaving and waxing techniques. They are quite efficacious, inexpensive, and safe. Other additional but more expensive measures are topical agents that inhibit local hair growth (eflornithine hydrochloride cream) and thermal destruction of dermal papilla (laser therapy or electrolysis). When these methods are not effective enough for the patient, the combination of both physical and hormonal therapies constitutes an option [46]. Oral contraceptives with anti-androgenic gestagen preparations (cyproterone and drospirenone) increase SHBG and reduce free testosterone serum index levels, hirsutism, and acne [47]. Other pharmacological therapies that can be used in combination with androgen suppression are flutamide 250 mg twice daily, spironolactone 100 mg daily, and finasteride 5 mg daily [45].
Adolescents with classical PCOS have alterations in some surrogate markers of cardiovascular risk which can be ameliorated by metformin. Hyperandrogenic girls such as those with adolescent PCOS exhibit abnormal insulin responses to glucose loading, higher low-density lipoprotein cholesterol (LDLC) to high-density lipoprotein cholesterol (HDLC) ratios, and lowered SHBG levels. In addition, elevated levels of circulating insulin coincide with a reciprocal decline of SHBG, thereby allowing for increased availability of free testosterone [48]. A combination of low-dose flutamide and metformin normalizes HA, IR, dyslipidemia, body adiposity, low-grade cardiovascular markers of inflammation, and hirsutism/acne in addition to regularizing menstrual cycles [49]. Low-dose polytherapy [metformin (850 mg/day), flutamide (62.5 mg/day), pioglitazone (7.5 mg/day), ethinylestradiol (20 μg/day) plus drospirenone (3 mg/day) 21/28 days] for 24 months leads to improvements in endocrine-metabolic profile, total and visceral adiposity, and markers of cardiovascular health [50].
Adolescent women with PCOS are also at an increased risk for depression and anxiety disorders which can be prominent in those faced with issues of self-presentation, and in women of all ages with respect to eating, overweight, and clinical manifestations of androgen excess [51]. The overemphasizing for weight loss in some psychological predisposed adolescents with PCOS could contribute to the development of eating disorders, most often anorexia and bulimia nervosa. Moreover, it has been hypothesized that PCOS may promote bulimic behavior since androgens have appetite-stimulating effects and could impair impulse control. Nevertheless, dieting and overall eating disorder symptoms in PCOS adolescents have not been described significantly higher than in women with normal ovaries [52].
Adulthood: fertility (Fig. 3)
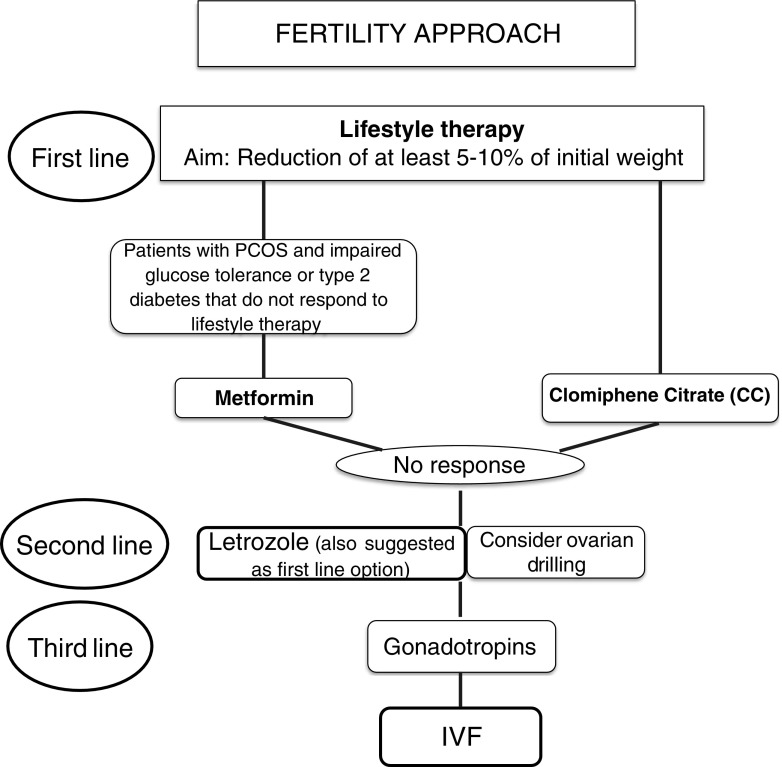
Fig. 3.
Fertility approach of polycystic ovary syndrome. The first-line therapeutic approach in obese infertile women with PCOS consists of lifestyle therapy. When it fails or weight excess is not present, pharmacotherapy is advised. In patients with impaired glucose tolerance or type 2 diabetes, metformin can be administered. In the remaining cases, clomiphene citrate is the first choice for ovulation induction and letrozole the second option, although some recent studies suggest letrozole as the first pharmacological option. Laparoscopic ovarian drilling can be considered in selected cases with caution. Gonadotropins are recommended when no success is achieved with the drugs previously commented for ovulation induction or when there is an indication for IVF. BMI: body mass index; CC: clomiphene citrate; PCOS: polycystic ovary syndrome
Infertility and hirsutism are the two main clinical problems that bring a woman with PCOS to the clinic. Regarding infertility, the main cause is androgen and LH hypersecretion, which lead to OA, irregular menses, and reduced fecundability. Irregular periods—oligo/amenorrhea—will be a first sign of ovarian dysfunction that can be easily detected in the first visit. Usually, these women will have a very high antral follicle count and AMH concentrations [53].
Lifestyle therapy: bariatric surgery
The first line of treatment for ovulation induction in PCOS is lifestyle intervention, especially in obese patients, which seeks to improve body composition (BMI, body weight, and waist-to-hip ratio), HA, and IR [54]. The objective should be a reduction of at least 5 to 10% of the initial weight [55]. Although controversial, better body-weight control has been reported when metformin has been added to lifestyle modification [56]. Lifestyle therapy consists of a hypocaloric diet (1200–1400 kcal/day for 3 months [57] or a 500–1000 Kcal/day deficit [58]) in combination with physical exercise (120 min of exercise per day, 3 to 5 days/week for 6 months) [59]. The patient’s capacity to adhere to diet and exercise programs and to maintain an appropriate weight over time is paramount [60]. In this context, psychological support is essential. Pharmacotherapy for weight reduction has not demonstrated its effectiveness for fertility purposes. Bariatric surgery can be considered in women with BMI ≥ 40 kg/m2 or ≥ 35 kg/m2 with associated comorbidities and who have failed to lose enough weight with other treatments in ≥ 6 months. It shows several benefits, including the improvement or resolution of type 2 diabetes mellitus [61], IR, and fertility problems [62]. However, surgery is a risky option with important associated complications before and during pregnancy [63]. Moreover, it is not a quick approach to achieve pregnancy, since it should be avoided for at least 12 to 18 months after surgery to reduce fetal complications [64]. Therefore, bariatric surgery should be considered the last therapeutic option in obese women with fertility problems.
Anti-androgen therapy
When considering anti-androgen therapy in adult women, there are several pragmatic approaches to be discussed with the patient: (a) minimizing circulating androgens with either oral contraceptive pill and/or insulin sensitizing agents, (b) peripheral blockage of androgen receptor with spironolactone, flutamide, ciproterone acetate, or finasteride, or (c) inhibiting facial or other body parts hair growth by eflornithine hydrochloride [65].
Metformin
Metformin is an insulin-sensitizing agent which acts by reducing gluconeogenesis and lipogenesis and by enhancing glucose uptake in the liver, skeletal muscle, adipose tissue, and ovaries. In PCOS women, metformin inhibits ovarian androgen production by approximately 20 to 25%, decreases serum LH, and increases SHBG [66]. Metformin may also improve ovulatory function. A meta-analysis of PCOS patients revealed an increase in pregnancy rates but not in live birth rates among those receiving metformin [67]. A systematic review and meta-analysis of patients with PCOS undergoing IVF or ICSI treatment did not show significant differences in terms of pregnancy and live birth outcomes between patients receiving metformin and those given a placebo [68]. Therefore, metformin is not recommended as a first-line agent for ovulatory infertility, but it is advised in women with PCOS and impaired glucose tolerance or type 2 diabetes that do not respond to lifestyle modifications [69].
Clomiphene citrate
Clomiphene citrate (CC) is considered the first-line treatment for ovulation induction in women with PCOS [43]. This drug is an estrogen receptor modulator with a controversial mechanism of action. When administered in the first days of the follicular phase, it competes with estrogens for their receptors in the hypothalamus and pituitary, blocking the negative feedback mechanism. Subsequently, endogenous gonadotropins are released and the dominant follicle is selected [70]. Endometrial proliferation may be inappropriate due to the anti-estrogenic effect of CC, which can decrease the chance of embryo implantation. In addition, the cervical mucus characteristics can also change making sperm penetration more difficult [71]. The starting dose of CC is 50 mg/day for 5 days from the second to fifth day of the cycle. This dose should be increased if there is no response after two cycles, as only two-thirds of patients respond to 50 mg/day in the first cycle [64]. With this 5-day course of treatment, the ovulation rate can reach 46%, rising to 85% if the dose is increased to more than 150 mg/day [72]. According to ESHRE/ASRM consensus, the maximal dose should not exceed 150 mg/day, as there is no clear evidence of efficacy at higher doses [73]. The multiple pregnancy rate per conception cycle is around 8% after CC [74]. This rate is lower than the obtained with gonadotropin but higher than with letrozole (0.46, 0.23 to 0.92) and metformin (0.22, 0.05 to 0.92) [75]. The advantages of CC are its low cost and its oral administration. Approximately 15% of women with PCOS do not respond to the maximum dose of CC and are considered resistant to this medication [76].
Letrozole
This aromatase inhibitor is administered to women with PCOS as an alternative in order to avoid the anti-estrogenic effect of CC on the endometrium. Furthermore, it preserves ovarian pituitary feedback, has a lower risk of multiple follicle development than CC [77], and reduces the peripheral conversion of androgens to estrogens in ovarian granulosa cells by blocking aromatase [78]. The recommended dose is 5 or 7.5 mg/day for 5 to 10 days starting 3–7 days after menses [79]. A potential risk of teratogenic effects of letrozole was suggested in a preliminary report [80], but subsequent studies have discarded them [78]. Letrozole has been suggested as a second-line pharmacological therapy in women resistant to CC, and as a first-line therapy for ovulation induction in women with PCOS and a BMI greater than 30 kg/m2 given the higher live birth rate in comparison to that achieved with CC []. However, the PPCOS trial (pregnancy in PCOS patients) [74] evaluated in a double-blind, multicenter trial the efficacy of letrozole vs CC not only in determining ovulation but also pregnancy in a very large sample size (1500 patients). This trial showed that PCOS women who received letrozole had more cumulative live births than those who received CC (27.5 vs 19.1%, p = 0.007, RR 1.44, 95% CI 1.10–1.87) for all BMIs, with no differences among miscarriage or twinning rates. This data is encouraging enough to consider letrozole a first-line drug in PCOS women when considering ovulation induction.
Gonadotropin therapy
Gonadotropins (FSH or HMG) constitute the third line of pharmacological treatment in PCOS women in whom CC and letrozole have not been effective. Low doses are recommended to achieve monofollicular growth (37.5 to 75 IU/day or every other day). In the low-dose step-up regimen, the dose is increased after 14 days if there is no response. Currently, an ultra-low increment of FSH (8.3–12.5 IU) is possible with the newly available pen devices [82]. As the required starting dose of gonadotropin is not easy to determine, a careful monitoring of follicular development is essential, and patients should be made aware of cancelation rates of hCG administration in the first attempts of ovarian stimulation. A cohort study of 343 PCOS patients receiving a starting dose of 50 IU FSHr/day presented a cancelation rate of 13.5% owing to hyperresponse, defined as the presence of more than three follicles ≥ 16 mm [83]. A Cochrane review about the use of gonadotropins for ovulation induction in women with PCOS highlighted similar live birth rates and OHSS incidence with the use of urinary-derived gonadotropins, recombinant FSH, or HMG/HP-HMG [84]. Prescription of gonadotropins requires consideration regarding the cost, the intensive monitoring required, and the potential risks of multiple pregnancy and OHSS.
In IVF, gonadotropins should be administered in a GnRH antagonist protocol, since different meta-analyses and randomized controlled trials have shown similar outcome parameters but lower OHSS rates than in classic long agonist protocols in PCOS women [85].
Laparoscopic ovarian drilling
A review about the role of laparoscopic ovarian drilling (LOD) in PCOS women drew attention to its benefits in patients with CC resistance, BMI < 30 kg/m2 and preoperative LH above 10 IU/L. In addition, the authors described that the performance of 5 to 10 perforations on the surface of each ovary using monopolar energy was the preferred surgical technique [86]. LOD may be considered in infertile PCOS women resistant to CC or letrozole, especially if laparoscopy is indicated by another reason such as tubal patency assessment or salpingectomy. A prospective RCT analyzed the effect of unilateral and bilateral LOD in PCOS women; 87 PCOS patients with ovulation induction failure were randomly allocated to either unilateral or bilateral LOD by electrocautery. Ovulation, pregnancy, and miscarriage rates were similar in both groups, but unilateral LOD was less time-consuming and probably associated with fewer complications than bilateral drilling [87]. In the long term, not only adhesions may be generated by the healing process after LOD, but also a compromised ovarian function and even induced iatrogenic premature ovarian failure if the procedure is too aggressive [88]. Therefore, it is a treatment option that should be selected with caution.
Ovulation induction and intrauterine insemination (IUI) outcome
Few studies have analyzed the outcome of ovulation induction or IUI in PCOS patients in terms of pregnancy or live birth rates. A recent review showed that gonadotropins are the most effective treatment in patients resistant to CC [89]. In a study of 945 treatment cycles in which a low-dose regimen of 50 IU of rFSH was applied, 61.3% monofollicular development was achieved with 14.4% PR per cycle and 53.1% cumulative PR after six treatment cycles [83]. Compared to timed intercourse (TI), IUI does not increase PRs in couples with PCOS and normal semen undergoing ovulation induction. In a recent study, PR was similar in the TI group (17.5%) vs IUI group (16.6%) [90]. In the absence of other fertility problems that require IVF, ovulation induction for TI or IUI seems to be the indicated first step of fertility treatment in PCOS women.
IVF outcome
Whether oocyte quality is affected in PCOS patients remains an open question. Some studies have shown lower fertilization rates in conventional IVF in PCOS patients compared to controls, but similar pregnancy, miscarriage, and live birth rates [91]. Other authors have described a poor oocyte quality, leading to lower fertilization, embryo cleavage and implantation rates, and higher miscarriage rates [92]. Although an association between poor oocyte and embryo quality and increased aneuploidy and miscarriage rates has been described [93, 94], recent data show that PCOS patients present similar percentages of euploid embryos than controls, and even a higher absolute numbers of euploid embryos due to the higher oocyte yield after controlled ovarian hyperstimulation [95]. Alterations in the intrafollicular microenviroment or in intra-ovarian factors belonging to growth factor families have been also suggested to affect the oocyte competence and maturation [96, 97].
According to the current evidence, despite a higher cancelation rate is observed in PCOS women [91], biochemical pregnancy, implantation, clinical pregnancy, ongoing pregnancy, live birth, and cumulative live birth rates are comparable to those of non-PCOS women [98, 99]. However, reproductive outcome in PCOS may be influenced by BMI [100]; in fact, a different endometrial gene expression during the window of implantation has been described in obese patients, particularly in those with PCOS [101].
Ovarian stimulation risks
Multiple pregnancy and OHSS are serious risks in patients undergoing ART. Multiple pregnancy can be prevented by careful monitoring of the number of follicles larger than 12 mm during ovulation induction. Recent data suggests that cancelation of cycles should be considered when estradiol levels are > 1200 pg/mL or there is an excessive number (≥ 5) of developing follicles [102]. When more than 3 follicles > 14 mm are observed, cancelation should be seriously considered. In IVF, the multiple pregnancy rate can be decreased by a strict policy of elective single embryo transfer [103].
OHSS is one of the most serious, and potentially lethal, complications of ovarian stimulation. Induction of final oocyte maturation with a bolus of gonadotropin-releasing hormone (GnRH) agonist, rather than the standard criterion hCG, in patients undergoing ovarian stimulation with a GnRH antagonist protocol significantly reduces the risk of OHSS by inducing a quick and reversible luteolysis [104], although luteal phase becomes defective. This is not a problem in cases of freeze-all policy, but in a fresh embryo transfer luteal support needs to be much more intensive than the conventional support. Main approaches have been either low dose hCG added to oral estradiol and vaginal progesterone or high doses of estradiol combined with i.m. progesterone [105]. Antagonist protocols are strongly recommended in these cases, since they reduce OHSS incidence by 50% and allow GnRH agonist triggering [106].
Adulthood: pregnancy
Women with PCOS exhibit a significantly increased risk of obstetric and neonatal complications compared to controls, regardless of the definition of PCOS applied [107], and even when BMI and other confounders, such as age, parity, time to conception or number of fetuses, have been taken into account [108–113]. HA, obesity, low-grade chronic inflammation, dyslipemia, IR and other metabolic disturbances may play a role in this increased risk and probably also in the reported higher risk of metabolic and reproductive abnormalities in the offspring [108, 112–114]. Moreover, factors related to infertility, including prolonged time to pregnancy, fertility drugs and assisted conception treatments, and a higher rate of multiple pregnancies, also constitute risk factors for complications during pregnancy [108]. All these elements may act directly and/or through a defective trophoblast invasion and placentation to induce fetal-maternal complications, although an alteration in placental gene expression has been also suggested as a mechanism [115].
Maternal complications
Pregnancy-induced hypertension (PIH) and pre-eclampsia
The risk of PIH and pre-eclampsia has been reported to be three to four times higher in PCOS women, but most of the reports in question have been retrospective studies or meta-analyses without an adequate sub-analysis of confounders [109–111]. A population-cohort study by Roos et al. [116] found a significantly increased incidence of pre-eclampsia in 3787 women with PCOS compared to 1,191,336 women without PCOS after adjusting for BMI and use of ART (OR 1.45, 95%CI 1.24–1.69). Another two more recent studies have shown similar results [114, 117], but in one the increased risk of pre-eclampsia was detected only in a subgroup of hyperandrogenic PCOS women [117]. A more recent retrospective cohort analysis detected a fourfold increase in the risk of hypertensive disorders during pregnancy in women with PCOS after adjusting for age, parity, BMI, and time to conception in singleton pregnancies [113].
Gestational diabetes mellitus
Gestational diabetes is the most frequent pregnancy complication described in PCOS women, with a two- to threefold risk in comparison to non-PCOS women, even after adjusting data for confounders [109–111, 113, 116, 118]. Metformin therapy does not seem to reduce its incidence, according to a recent randomized controlled trial in which this drug was compared with a placebo [119]. A recent meta-analysis of five studies including 502 PCOS patients administered metformin throughout pregnancy and 427 controls administered the drug only to achieve conception did not highlight differences in the risk of gestational diabetes mellitus or pre-eclampsia between the two groups [120].
Fetal/neonatal complications
Miscarriage
Currently, there is no clear evidence regarding an increased risk of miscarriage in PCOS women compared with fertile controls or non-PCOS infertile women undergoing IVF [91, 112]. Some authors suggested that the hypersecretion of LH, HA, and hyperinsulinemia present in PCOS women were interlinked and together might result in disordered ovarian and endometrial function related to an increased risk of early pregnancy loss [121] However, miscarriage seems to be strongly influenced by BMI, but not by PCOS itself [122]. In addition, in early miscarriages, a lower aneuploidy rate has been described in PCOS women than in non-PCOS women, even when adjusting for maternal age [123].
Preterm delivery
Although a recent meta-analysis did not show a higher risk of preterm delivery in PCOS women [111], two previous meta-analyses [109, 110] and two cohort studies [116, 117] described a twofold increase in the said risk. In the cohort study published by Naver et al. [117], the risk was confined to hyperandrogenic women with PCOS. In a recent retrospective cohort study, the increased risk of preterm delivery up until 37 weeks was eliminated after adjusting for PIH [113]. In twin pregnancies, the presence of maternal PCOS has been associated with an increased risk of preterm delivery (RR 1.18 CI 1.03–1.37) and very premature delivery (< 32 weeks) [124].
Small-for-gestational-age (SGA) neonates
One meta-analysis did not detect a higher risk of SGA infants in women with PCOS [109], but a more recent one found a twofold increased risk [110]. Similarly, two recent case-control studies have reported opposite results; a similar risk of SGA in PCOS women and controls was observed in one [117], while a twofold increased risk was found in non-obese infertile women with PCOS who underwent ART compared with control patients with tubal infertility factor in the other [125]. Palomba et al. [114] also detected a significantly higher incidence of SGA in a recent prospective controlled clinical study in which 150 pregnant PCOS women were compared with 150 age- and BMI-matched healthy pregnant controls.
Large-for-gestational-age (LGA) neonates and macrosomia
One population-based study revealed a higher risk of LGA neonates in women with PCOS compared to controls with tubal factor (OR 1.39, 95% CI 1.19–1.62) [133]. However, two meta-analyses failed to detect differences regarding LGA infants [110] or macrosomia [109] in women with PCOS versus those without. Obesity is an evident confounder, since a difference in the incidence of LGA has not been described between non-obese controls and non-obese women with PCOS [125]. However, a prospective controlled clinical study by Palomba et al. [114] detected a significantly higher incidence of LGA in 150 pregnant PCOS women compared to 150 age- and BMI-matched healthy pregnant controls, and a recent retrospective cohort study reported a significantly increased risk of LGA infants after adjusting for BMI and gestational diabetes mellitus status in singleton pregnancies [113]. In the Palomba et al.’s paper [114], both LGA and SGA babies were more prevalent in PCOS women. The increased risk of gestational diabetes in PCOS pregnant women may be related to the higher incidence of LGA babies, and the poorer trophoblastic invasion/ placentation described in some PCOS women to the increased risk of SGA babies.
Other complications
In women with PCOS, a higher risk of admission to the NICU and a low Apgar score at 5 min have been observed in neonates, as well as higher perinatal mortality [109, 111, 116], although not all the literature supports a risk of adverse fetal or neonatal outcomes, or a risk of cesarean section or operative vaginal delivery [113, 124, 126].
Perimenopause and elderly women
Diagnosis of PCOS in the perimenopause can be difficult, since the clinical and biochemical manifestations change with age (Tables 3 and 4). As women grow older, there is a significant decrease in androgenic production, but not as much as in non-PCOS women [127]. This is why the classical phenotype is tempered, with more regular cycles after the age of 40. There is also an attenuated decrease in AMH and ovarian volume, with a normal postponement of the menopause of 2 years [128]. Postmenopausal women with PCOS report hirsutism more frequently, but exhibit fewer climacteric symptoms than controls [129].
During this period, metabolic and endocrine disorders take preference over the gynecological problems. Around 40 to 85% of women with PCOS are overweight or obese compared with age-matched controls. IR is more prevalent among both lean and obese women with PCOS (30 and 70%, respectively) compared with age- and weight-matched controls [130], with PCOS subjects running an increased risk of developing type 2 diabetes. Metabolic syndrome is also more predominant in PCOS women in this age group. In fact, these women show elevated total cholesterol, triglycerides, insulin secretion, lipid accumulation product, and blood pressure. At perimenopausal period (41–55 years old), these parameters are not different between controls and PCOS women. HOMA-β is lower at late reproductive and perimenopausal periods compared to the early reproductive age within the PCOS group. Therefore, no further deterioration of metabolic parameters seems to occur during perimenopause [131, 132].
The higher rate of obesity, IR, impaired glucose intolerance (or type 2 diabetes), and dyslipidemia may lead to the development of coronary heart disease (CHD) [133]. However, uncertainty exists as to whether PCOS status per se increases cardiovascular mortality [42]. Non-alcoholic fatty liver disease (NAFLD) is also more prevalent in older PCOS patients [134], as well as mood (depression and anxiety) [135] and eating (binge eating and bulimia nervosa) disorders, even with respect to women with the same BMI. Sleeping apnea is also frequent due to obesity, androgen excess, and IR. Women with PCOS also present risk factors for endometrial cancer (oligo-ovulation, excess weight, and hyperinsulinemia). In fact, those under 54 years of age present an increased risk of endometrial cancer, but not of breast or ovarian cancer [136].
Once a diagnosis of PCOS is confirmed, a cardio-metabolic risk assessment including estimation of blood pressure and BMI, fasting lipid profile and 2-h glucose tolerance test (OGTT) is recommended. Patients with normal glucose tolerance should be re-screened at least once every 2 years, or more frequently if additional risk factors are detected. In the event of impaired glucose tolerance, an assessment of type 2 diabetes should be performed [137]. Women with PCOS should be informed about the signs and side effects of sleeping apnea and excessive daytime sleepiness. Therapy in these women should generally aim to manage hidden metabolic abnormalities, reduce risk factors for developing type 2 diabetes and cardiovascular disease, and prevent endometrial hyperplasia and carcinoma. Available data suggest that lifestyle (diet, exercise, and behavioral interventions) can improve metabolic risk factors and should constitute the first line of intervention for most women [54].
Conclusions
PCOS is a condition that spans the lives of women. Fetal programming may represent the beginning of a syndrome characterized by HA and IR which can have a series of medical consequences in adolescence, adulthood, and old age. Menstrual and fertility problems evolve into metabolic complications as age advances, with early diagnosis being crucial if the aforementioned, sometimes fatal, complications are to be avoided. A precise diagnosis is important, especially at the extreme ends of the reproductive lifespan. However, many different phenotypes are included under the same condition, being important to look at these different phenotypes separately, as they may require different treatments and have different consequences. In this way, PCOS exhibits a great metabolic complexity and its diagnosis should be revised once again according to recent data obtained by new technologies. Lifestyle therapy tends to be the first step in PCOS management, especially when excess body weight is associated. Pharmacotherapy is frequently used to manage the most predominant manifestations in each age group, such as irregular menses and hirsutism in adolescence, fertility problems in adulthood, and metabolic problems and risk of cancer in old age. New research may change future information once different phenotypes of the syndrome are analyzed as complete separate entities.
Acknowledgments
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Compliance with ethical standards
Conflict of interests
The authors declare that they have no conflict of interest.
References
- 1.Solomon CG. Polycystic ovary syndrome. N Engl J Med. 2016;375:54–64. doi: 10.1056/NEJMcp1514916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stein IF, Levethal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181–191. doi: 10.1016/S0002-9378(15)30642-6. [DOI] [Google Scholar]
- 3.Zawadzki JK, Dunaif A, et al. Diagnosis criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, et al., editors. Polycystic ovary syndrome. Boston: Blackwell Scientific Publications; 1992. pp. 377–384. [Google Scholar]
- 4.Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 5.Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456–488. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 6.National Institutes of Health. Evidence-based methodology workshop on polycystic ovary syndrome. December 3–5, 2012. Executive summary. Available at https/prevention.nih.gov/docs/programs/pcos/FinalReport.pdf. Accesed March 1, 2017.
- 7.Ning N, Balen A, Brezina PR, et al. How to recognize PCOS: results of a web-based survey at IVF-worldwide.com. Reprod BioMed Online. 2013;26:500–505. doi: 10.1016/j.rbmo.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Dewailly D. Diagnostic criteria for PCOS: is there a need for a rethink? Best Pract Res Clin Obstet Gynaecol. 2016;37:5–11. doi: 10.1016/j.bpobgyn.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E. American Androgen Excess and PCOS Society Disease State Clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome- part 1. Endocr Pract. 2015;21:1291–1300. doi: 10.4158/EP15748.DSC. [DOI] [PubMed] [Google Scholar]
- 10.Zhu RY, Wong YC, Yong EL. Sonographic evaluation of polycystic ovaries. Best Pract Res Clin Obstet Gynaecol. 2016;35:25–37. doi: 10.1016/j.bpobgyn.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Dewailly D, Pigny P, Soudan B, et al. Reconciling the definitions of polycystic ovary syndrome: the ovarian follicle number and serum anti-Müllerian hormone concentrations aggregate with the markers of hyperandrogenism. J Clin Endocrinol Metab. 2010;95:4399–4405. doi: 10.1210/jc.2010-0334. [DOI] [PubMed] [Google Scholar]
- 12.Dewailly D, Gronier H, Poncelet E, et al. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26:3123–3129. doi: 10.1093/humrep/der297. [DOI] [PubMed] [Google Scholar]
- 13.Dewailly D, Catteau-Jonard S, Reyss AC, et al. Oligoanovulation with polycystic ovaries but not overt hyperandrogenism. J Clin Endocrinol Metab. 2006;91:3922–3927. doi: 10.1210/jc.2006-1054. [DOI] [PubMed] [Google Scholar]
- 14.Stein S, Jennifer L, Sites CK, Yang D. Environmental determinants of polycystic ovary syndrome. Fertil Steril. 2016;106:16–24. doi: 10.1016/j.fertnstert.2016.07.339. [DOI] [PubMed] [Google Scholar]
- 15.Yildiz BO, Bozdag G, Yapici Z, Esinler I, Yarali H. Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum Reprod. 2012;27:3067–3073. doi: 10.1093/humrep/des232. [DOI] [PubMed] [Google Scholar]
- 16.Sirmans SM, Parish RC, Blake S, Wang X. Epidemiology and comorbidities of polycystic ovary syndrome in an indigent population. J Investig Med. 2014;62:868–874. doi: 10.1097/01.JIM.0000446834.90599.5d. [DOI] [PubMed] [Google Scholar]
- 17.Rutkowska AZ, Diamanti-Kandarakis E. Polycystic ovary syndrome and environmental toxins. Fertil Steril. 2016;106:948–958. doi: 10.1016/j.fertnstert.2016.08.031. [DOI] [PubMed] [Google Scholar]
- 18.Franks S, Mc Carthy M, Hardy K. Development of polycystic ovary syndrome: involvement of genetic and environmental factors. Int J Androl. 2006;29:278–285. doi: 10.1111/j.1365-2605.2005.00623.x. [DOI] [PubMed] [Google Scholar]
- 19.De Leo V, Musacchio MC, Cappelli V, Massaro MG, Morgante G, Petraglia F. Genetic, hormonal and metabolic aspects of PCOS: an update. Reprod Biol Endocrinol. 2016;14:38. doi: 10.1186/s12958-016-0173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Melo AS, Dias SV, De Carvalho R, et al. Pathogenesis of polycystic ovary syndrome: multifactorial assessment from the foetal stage to menopause. Reproduction. 2015;150:11–24. doi: 10.1530/REP-14-0499. [DOI] [PubMed] [Google Scholar]
- 21.Longo S, Bollani L, Decembrino L, Di Comite A, Angelini M, Stronati M. Short-term and long-term sequelae in intrauterine growth retardation (IUGR) J Matern Fetal Neonatal Med. 2013;26:222–225. doi: 10.3109/14767058.2012.715006. [DOI] [PubMed] [Google Scholar]
- 22.Delitala AP, Capobianco G, Delitala G, Cherchi PL, Dessole S. Polycystic ovary syndrome, adipose tissue and metabolic syndrome. Arch Gynecol Obstet 2017. [DOI] [PubMed]
- 23.Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Pérez-Bravo F, Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod. 2002;17:2573–2579. doi: 10.1093/humrep/17.10.2573. [DOI] [PubMed] [Google Scholar]
- 24.Qiao J, Feng HL. Extra- and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Hum Reprod Update. 2011;17:17–33. doi: 10.1093/humupd/dmq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voulgaris N, Papanastasiou L, Piaditis G, et al. Vitamin D and aspects of female fertility. Hormones (Athens) 2017;16:5–21. doi: 10.14310/horm.2002.1715. [DOI] [PubMed] [Google Scholar]
- 26.Bremer AA, Miller WL. The serine phosphorylation hypothesis of polycystic ovary syndrome: a unifying mechanism of hyperandrogenemia and IR. Fertil Steril. 2008;89:1039–1048. doi: 10.1016/j.fertnstert.2008.02.091. [DOI] [PubMed] [Google Scholar]
- 27.Krishnan A, Muthusami S. Hormonal alterations in PCOS and its influence on bone metabolism. J Endocrinol. 2017;232:R99–R113. doi: 10.1530/JOE-16-0405. [DOI] [PubMed] [Google Scholar]
- 28.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33:981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ibáñez L, Jaramillo A, Enríquez G, et al. Polycystic ovaries after precocious pubarche: relation to prenatal growth. Hum Reprod. 2007;22:395–400. doi: 10.1093/humrep/del395. [DOI] [PubMed] [Google Scholar]
- 30.Ibáñez L, Díaz R, López-Bermejo A, Marcos MV. Clinical spectrum of premature pubarche: links to metabolic syndrome and ovarian hyperandrogenism. Rev Endocr MetabDisord. 2009;10:63–76. doi: 10.1007/s11154-008-9096-y. [DOI] [PubMed] [Google Scholar]
- 31.Idkowiak J, Lavery GG, Dhir V, et al. Premature adrenarche: novel lessons from early onset androgen excess. Eur J Endocrinol. 2011;165:189–207. doi: 10.1530/EJE-11-0223. [DOI] [PubMed] [Google Scholar]
- 32.Ibáñez L, Lopez-Bermejo A, Diaz M, Marcos MV, de Zegher F. Early metformin therapy to delay menarche and augment height in girls with precocious pubarche. Fertil Steril. 2011;95:727–730. doi: 10.1016/j.fertnstert.2010.08.052. [DOI] [PubMed] [Google Scholar]
- 33.Ibáñez L, López-Bermejo A, Díaz M, Marcos MV, de Zegher F. Early metformin therapy (age 8-12 years) in girls with precocious pubarche to reduce hirsutism, androgen excess, and oligomenorrhea in adolescence. J Clin Endocrinol Metab. 2011;96:E1262–E1267. doi: 10.1210/jc.2011-0555. [DOI] [PubMed] [Google Scholar]
- 34.Rosenfield RL. Identifying children at risk of polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:787–796. doi: 10.1210/jc.2006-2012. [DOI] [PubMed] [Google Scholar]
- 35.Marsh CA, Grimstad FW. Primary amenorrhea: diagnosis and management. Obstet Gynecol Surv. 2014;69:603–612. doi: 10.1097/OGX.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 36.Witchel SF, Oberfield S, Rosenfield RL, et al. The diagnosis of polycystic ovary syndrome during adolescence. Horm Res Paediatr. 2015;87:376–389. doi: 10.1159/000375530. [DOI] [PubMed] [Google Scholar]
- 37.Glueck CJ, Woo JG, Khoury PR, Morrison JA, Daniels SR, Wang P. Adolescent oligomenorrhea (age 14–19) tracks into the third decade of life (age 20–28) and predicts increased cardiovascular risk factors and metabolic syndrome. Metabolism. 2015;64:539–553. doi: 10.1016/j.metabol.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Carmina E, Oberfield SE, Lobo RA. The diagnosis of polycystic ovary syndrome in adolescents. Am J Obstet Gynecol. 2010;203:201.e1–201.e5. doi: 10.1016/j.ajog.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Fruzzetti F, Campagna AM, Perini D, Carmina E. Ovarian volume in normal and hyperandrogenic adolescent women. FertilSteril. 2015;104:196–199. doi: 10.1016/j.fertnstert.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 40.Johnstone EB, Rosen MP, Neril R, et al. The polycystic ovary post-Rotterdam: a common, age-dependent finding in ovulatory women without metabolic significance. J Clin Endocrinol Metab. 2010;95:4965–4972. doi: 10.1210/jc.2010-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Welt CK, Carmina E. Clinical review: lifecycle of polycystic ovary syndrome (PCOS): from in utero to menopause. J Clin Endocrinol Metab. 2013;98:4629–4638. doi: 10.1210/jc.2013-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fauser BC, Tarlatzis RW, Rebar RS, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97:28–38.e25. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 43.Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565–4592. doi: 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsikouras P, Spyros L, Manav B, et al. Features of polycystic ovary syndrome in adolescence. J Med Life. 2015;8:291–296. [PMC free article] [PubMed] [Google Scholar]
- 45.van Zuuren EJ, Fedorowicz Z, Carter B, Pandis N. Interventions for hirsutism (excluding laser and photoepilation therapy alone) Cochrane Database Syst Rev. 2015;4:CD010334. doi: 10.1002/14651858.CD010334.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buggs C, Rosenfield RL. Polycystic ovary syndrome in adolescence. Endocrinol MetabClin North Am. 2005;34:677. doi: 10.1016/j.ecl.2005.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhattacharya SM, Jha A, DasMukhopadhyay L. Comparison of two contraceptive pills containing drospirenone and 20 μg or 30 μg ethinyl estradiol for polycystic ovary syndrome. Int J Gynaecol Obstet. 2016;132:210–213. doi: 10.1016/j.ijgo.2015.06.065. [DOI] [PubMed] [Google Scholar]
- 48.Fruzzetti F, Perini D, Lazzarini V, Parrini D, Genazzani AR. Adolescent girls with polycystic ovary syndrome showing different phenotypes have a different metabolic profile associated with increasing androgen levels. Fertil Steril. 2009;92:626–634. doi: 10.1016/j.fertnstert.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 49.de Zegher F, Ibáñez L. Therapy: low-dose flutamide for hirsutism: into the limelight, at last. Nat Rev Endocrinol. 2010;6:421–422. doi: 10.1038/nrendo.2010.119. [DOI] [PubMed] [Google Scholar]
- 50.Ibáñez L, López-Bermejo A, Díaz M, Enríquez G, del Río L, de Zegher F. Low-dose pioglitazone and low-dose flutamide added to metformin and oestro-progestagens for hyperinsulinaemic women with androgen excess: add-on benefits disclosed by a randomized double-placebo study over 24 months. Clin Endocrinol. 2009;71:351–357. doi: 10.1111/j.1365-2265.2008.03472.x. [DOI] [PubMed] [Google Scholar]
- 51.Cooney LG, Lee I, Sammel MD, Dokras A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2017;32:1075–1091. doi: 10.1093/humrep/dex044. [DOI] [PubMed] [Google Scholar]
- 52.Michelmore KF, Balen AH, Dunger DB, et al. Polycystic ovaries and eating disorders: are they related? Hum Reprod. 2001;16:765–769. doi: 10.1093/humrep/16.4.765. [DOI] [PubMed] [Google Scholar]
- 53.Bachelot A. Polycystic ovarian syndrome: clinical and biological diagnosis. Ann Biol Clin (Paris) 2016;74:661–667. doi: 10.1684/abc.2016.1184. [DOI] [PubMed] [Google Scholar]
- 54.Moran LJ, Hutchison SK, Norman RJ, Teede HJ. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2011;7:CD007506. doi: 10.1002/14651858.CD007506.pub3. [DOI] [PubMed] [Google Scholar]
- 55.Radon PA, McMahon MJ, Meyer WR. Impaired glucose tolerance in pregnant women with polycystic ovary syndrome. Obstet Gynecol. 1999;94:194–197. doi: 10.1016/s0029-7844(99)00252-5. [DOI] [PubMed] [Google Scholar]
- 56.Naderpoor N, Shorakae S, de Courten B, Misso ML, Moran LJ, Teede HJ. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update. 2015;21:560–574. doi: 10.1093/humupd/dmv025. [DOI] [PubMed] [Google Scholar]
- 57.Qublan HS, Yannakoula EK, Al-Qudah MA, El-Uri FI. Dietary intervention versus metformin to improve the reproductive outcome in women with polycystic ovary syndrome. A prospective comparative study. Saudi Med J. 2007;28:1694–1699. [PubMed] [Google Scholar]
- 58.Tang T, Glanville J, Hayden CJ, White D, Barth JH, Balen AH. Combined lifestyle modification and metformin in obese patients with polycystic ovary syndrome. A randomized, placebo-controlled, double-blind multicentre study. Hum Reprod. 2006;21:80–89. doi: 10.1093/humrep/dei311. [DOI] [PubMed] [Google Scholar]
- 59.Karimzadeh MA, Javedani M. An assessment of lifestyle modification versus medical treatment with clomiphene citrate, metformin, and clomiphene citrate-metformin in patients with polycystic ovary syndrome. Fertil Steril. 2010;94:216–220. doi: 10.1016/j.fertnstert.2009.02.078. [DOI] [PubMed] [Google Scholar]
- 60.Domecq JP, Prutsky G, Mullan RJ, et al. Lifestyle modification programs in polycystic ovary syndrome: systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98:4655–4663. doi: 10.1210/jc.2013-2385. [DOI] [PubMed] [Google Scholar]
- 61.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes-3-year outcomes. N Engl J Med. 2014;370:2002–2013. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malik SM, Traub ML. Defining the role of bariatric surgery in polycystic ovarian syndrome patients. World J Diabetes. 2012;3:71–79. doi: 10.4239/wjd.v3.i4.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johansson K, Cnattingius S, Näslund I, et al. Outcomes of pregnancy after bariatric surgery. N Engl J Med. 2015;372:814–824. doi: 10.1056/NEJMoa1405789. [DOI] [PubMed] [Google Scholar]
- 64.Balen AH, Morley LC, Misso M, et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update. 2016;22:687–708. doi: 10.1093/humupd/dmw025. [DOI] [PubMed] [Google Scholar]
- 65.Mihailidis J, Dermesropian R, Taxel P, Luthra P, Grant-Kels JM. Endocrine evaluation of hirsutism. Int J Womens Dermatol. 2017;3(1 Suppl):S6–S10. doi: 10.1016/j.ijwd.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Homburg R, Insler V. Ovulation induction in perspective. Hum Reprod Update. 2002;8:449–462. doi: 10.1093/humupd/8.5.449. [DOI] [PubMed] [Google Scholar]
- 67.Tang T, Lord JM, Norman RJ, Yasmin E, Balen AH. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev. 2012;16(5):CD003053. doi: 10.1002/14651858.CD003053.pub5. [DOI] [PubMed] [Google Scholar]
- 68.Huang X, Wang P, Tal R, Lv F, Li Y, Zhang X. A systematic review and meta-analysis of metformin among patients with polycystic ovary syndrome undergoing assisted reproductive technology procedures. Int J Gynaecol Obstet. 2015;131:111–116. doi: 10.1016/j.ijgo.2015.04.046. [DOI] [PubMed] [Google Scholar]
- 69.Goodman NF, Cobin RH, Futterweit W, et al. American association of clinical endocrinologists, American college of endocrinology, and androgen excess and PCOS society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome - part 2. Endocr Pract. 2015;21:1415–1426. doi: 10.4158/EP15748.DSCPT2. [DOI] [PubMed] [Google Scholar]
- 70.Imani B, Eijkemans MJ, te Velde ER, Habbema JD, Fauser BC. A nomogram to predict the probability of live birth after clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. Fertil Steril. 2002;77:91–97. doi: 10.1016/S0015-0282(01)02929-6. [DOI] [PubMed] [Google Scholar]
- 71.Brown J, Farquhar C, Beck J, Boothroyd C, Hughes E. Clomiphene and anti-estrogens for ovulation induction in PCOS. Cochrane Database Syst Rev. 2009;4:CD002249. doi: 10.1002/14651858.CD002249.pub4. [DOI] [PubMed] [Google Scholar]
- 72.Rostami-Hodjegan A, Lennard MS, Tucker GT, Ledger WL. Monitoring plasma concentrations to individualize treatment with clomiphene citrate. Fertil Steril. 2004;81:1187–1193. doi: 10.1016/j.fertnstert.2003.07.044. [DOI] [PubMed] [Google Scholar]
- 73.Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Consensus on infertility treatment related to polycystic ovary syndrome. Hum Reprod. 2008;23:462–477. doi: 10.1093/humrep/dem426. [DOI] [PubMed] [Google Scholar]
- 74.Legro RS, Brzysli RG, Diamond MP, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371:119–129. doi: 10.1056/NEJMoa1313517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang R, Kim B, van Wely M, et al. Treatment strategies for women with WHO group II anovulation: systematic review and network meta-analysis. BMJ. 2017;356:j138. doi: 10.1136/bmj.j138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Melo AS, Ferriani RA, Navarro PA. Treatment of infertility in women with polycystic ovary syndrome: approach to clinical practice. Clinics (Sao Paulo) 2015;70:765–769. doi: 10.6061/clinics/2015(11)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Homburg R. Choices in the treatment of anovulatory PCOS. Current management of polycystic ovary syndrome. London: RCOG Press; 2010. pp. 143–152. [Google Scholar]
- 78.Palomba S. Aromatase inhibitors for ovulation induction. J Clin Endocrinol Metab. 2015;100:1742–1747. doi: 10.1210/jc.2014-4235. [DOI] [PubMed] [Google Scholar]
- 79.Mitwally MF, Casper RF. Use of an aromatase inhibitor for induction of ovulation in patients with an inadequate response to clomiphene citrate. Fertil Steril. 2001;75:305–309. doi: 10.1016/S0015-0282(00)01705-2. [DOI] [PubMed] [Google Scholar]
- 80.Biljan MM, Hemmings R, Brassard N. The outcome of 150 babies following the treatment with Letrozole or Letrozole and gonadotropins. Fertil Steril. 2005;84:S95. doi: 10.1016/j.fertnstert.2005.07.230. [DOI] [Google Scholar]
- 81.Committee Opinion No. 663 Summary: Aromatase Inhibitors in Gynecologic Practice. Obstet Gynecol. 2016. 10.1097/AOG.0000000000001478. [DOI] [PubMed]
- 82.Orvieto R, Homburg R. Chronic ultra-low dose follicle-stimulating hormone regimen for patients with polycystic ovary syndrome: one click, one follicle, one pregnancy. Fertil Steril. 2009;91:1533–1535. doi: 10.1016/j.fertnstert.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 83.Calaf Alsina J, Ruiz Balda JA, Romeu Sarrió A, et al. Ovulation induction with a starting dose of 50 IU of recombinant follicle stimulating hormone in WHO group II anovulatory women: the IO-50 study, a prospective, observational, multicentre, open trial. BJOG. 2003;110:1072–1077. doi: 10.1111/j.1471-0528.2003.02290.x. [DOI] [PubMed] [Google Scholar]
- 84.Weiss NS, Nahuis M, Bayram N, Mol BW, Van der Veen F, van Wely M. Gonadotropins for ovulation induction in women with polycystic ovarian syndrome. Cochrane Database Syst Rev. 2015;9:CD010290. doi: 10.1002/14651858.CD010290.pub2. [DOI] [PubMed] [Google Scholar]
- 85.Lin H, Li Y, Li L, Wang W, Yang D, Zhang Q. Is a GnRH antagonist protocol better in PCOS patients? A meta-analysis of RCTs PLoS One. 2014;9:e91796. doi: 10.1371/journal.pone.0091796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hueb CK, Dias Junior JA, Abrão MS, Filho EK. Drilling: medical indications and surgical technique. Rev Assoc Med Bras. 2015;61:530–535. doi: 10.1590/1806-9282.61.06.530. [DOI] [PubMed] [Google Scholar]
- 87.Youssef H, Atallah MM. Unilateral ovarian drilling in polycystic ovarian syndrome: a prospective randomized study. Reprod BioMed Online. 2007;15:457–462. doi: 10.1016/S1472-6483(10)60373-2. [DOI] [PubMed] [Google Scholar]
- 88.Lebbi I, Ben Temime R, Fadhlaoui A, Feki A. Ovarian drilling in PCOS: is it really useful? Front Surg. 2015;2:30. doi: 10.3389/fsurg.2015.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Birch Petersen K, Pedersen NG, Pedersen AT, Lauritsen MP, la CourFreiesleben N. Mono-ovulation in women with polycystic ovary syndrome: a clinical review on ovulation induction. Reprod BioMed Online. 2016;32:563–583. doi: 10.1016/j.rbmo.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 90.Wiser A, Shalom-Paz E, Reinblatt SL, Holzer H, Tulandi T. Controlled ovarian hyperstimulation in women with polycystic ovarian syndrome with or without intrauterine insemination. Gynecol Endocrinol. 2012;28:502–504. doi: 10.3109/09513590.2011.634938. [DOI] [PubMed] [Google Scholar]
- 91.Heijnen EM, Eijkemans MJ, Hughes EG, et al. A meta-analysis of outcomes of conventional IVF in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12:13–21. doi: 10.1093/humupd/dmi036. [DOI] [PubMed] [Google Scholar]
- 92.Sahu B, Ozturk O, Ranierri M, Serhal P. Comparison of oocyte quality and intracytoplasmic sperm injection outcome in women with isolated polycystic ovaries or polycystic ovarian syndrome. Arch Gynecol Obstet. 2008;277:239–244. doi: 10.1007/s00404-007-0462-x. [DOI] [PubMed] [Google Scholar]
- 93.Munné S, Dailey T, Sultan KM, Grifo J, Cohen J. The use of first polar bodies for preimplantation diagnosis of aneuploidy. Hum Reprod. 1995;10:1014–1020. doi: 10.1093/oxfordjournals.humrep.a136027. [DOI] [PubMed] [Google Scholar]
- 94.Gianaroli L, Magli MC, Ferraretti AP, Lappi M, Borghi E, Ermini B. Oocyte euploidy, pronuclear zygote morphology and embryo chromosomal complement. Hum Reprod. 2007;22:241–249. doi: 10.1093/humrep/del334. [DOI] [PubMed] [Google Scholar]
- 95.Weghofer A, Munne S, Chen S, Barad D, Gleicher N. Lack of association between polycystic ovary syndrome and embryonic aneuploidy. Fertil Steril. 2007;88:900–905. doi: 10.1016/j.fertnstert.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 96.Wood JR, Dumesic DA, Abbott DH, Strauss JF., III Molecular abnormalities inoocytes from women with polycystic ovary syndrome revealed by microarray analysis. J Clin Endocrinol Metab. 2007;92:705–713. doi: 10.1210/jc.2006-2123. [DOI] [PubMed] [Google Scholar]
- 97.Diamanti-Kandarakis E. Polycystic ovarian syndrome: pathophysiology, molecular aspects and clinical implications. Expert Rev Mol Med. 2008;10:e31–e21. doi: 10.1017/S1462399408000598. [DOI] [PubMed] [Google Scholar]
- 98.Nejad ES, Saedi T, Saedi S, Rashidi BH, Nekoo ZA, Jahangiri N. Comparison of in vitro fertilisation success in patients with polycystic ovary syndrome and tubal factor. Gynecol Endocrinol. 2011;27:117–120. doi: 10.3109/09513590.2010.501872. [DOI] [PubMed] [Google Scholar]
- 99.Li HW, Lee VC, Lau EY, Yeung WS, Ho PC, Ng EH. Cumulative live-birth rate in women with polycystic ovary syndrome or isolated polycystic ovaries undergoing in-vitro fertilisation treatment. J Assist Reprod Genet. 2014;31:205–211. doi: 10.1007/s10815-013-0151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McCormick B, Thomas M, Maxwell R, Williams D, Aubuchon M. Effects of polycystic ovarian syndrome on in vitro fertilization-embryo transfer outcomes are influenced by body mass index. Fertil Steril. 2008;90:2304–2309. doi: 10.1016/j.fertnstert.2007.10.077. [DOI] [PubMed] [Google Scholar]
- 101.Bellver J, Martinez-Conejero JA, Labarta E, et al. Endometrial gene expression in the window of implantation is altered in obese women especially in association with polycystic ovary syndrome. Fertil Steril. 2011;95:2335–2341. doi: 10.1016/j.fertnstert.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 102.Fong SA, Palta V, Oh C, Cho MM, Loughlin JS, McGovern PG. Multiple pregnancy after gonadotropin-intrauterine insemination: an unavoidable event? Obstet Gynecol. 2011;2011:465483. doi: 10.5402/2011/465483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Harbottle S, Hughes C, Cutting R, Roberts S, Brison D. Association of Clinical Embryologists & the (ACE) British Fertility Society (BFS). Elective single embryo transfer: an update to UK best practice guidelines. Hum Fertil (Camb) 2015;18:165–183. doi: 10.3109/14647273.2015.1083144. [DOI] [PubMed] [Google Scholar]
- 104.Fatemi HM, Garcia-Velasco J. Avoiding ovarian hyperstimulation syndrome with the use of gonadotropin-releasing hormone agonist trigger. Fertil Steril. 2015;103:870–873. doi: 10.1016/j.fertnstert.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 105.Garcia-Velasco JA. Agonist trigger: what is the best approach? Agonist trigger with vitrification of oocytes or embryos. Fertil Steril. 2012;97:527–528. doi: 10.1016/j.fertnstert.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 106.Al-Inany HG, Youssef MA, Ayeleke RO, Brown J, Lam WS, Broekmans FJ. Gonadotropin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev. 2016;4:CD001750. doi: 10.1002/14651858.CD001750.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kollmann M, Klaritsch P, Martins WP, et al. Maternal and neonatal outcomes in pregnant women with PCOS: comparison of different diagnostic definitions. Hum Reprod. 2015;30:2396–2403. doi: 10.1093/humrep/dev187. [DOI] [PubMed] [Google Scholar]
- 108.Palomba S, de Wilde MA, Falbo A, Koster MP, La Sala GB, Fauser BC. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update. 2015;21:575–592. doi: 10.1093/humupd/dmv029. [DOI] [PubMed] [Google Scholar]
- 109.Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12:673–683. doi: 10.1093/humupd/dml036. [DOI] [PubMed] [Google Scholar]
- 110.Kjerulff LE, Sanchez-Ramos L, Duffy D. Pregnancy outcomes in women with polycystic ovary syndrome: a metaanalysis. Am J Obstet Gynecol. 2011;204:558.e1–558.e6. doi: 10.1016/j.ajog.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 111.Qin JZ, Pang LH, Li MJ, Fan XJ, Huang RD, Chen HY. Obstetric complications in women with polycystic ovary syndrome: a systematic review and meta-analysis. Reprod Biol Endocrinol. 2013;11:56. [DOI] [PMC free article] [PubMed]
- 112.Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group Consensus on women’s health aspects of polycystic ovary syndrome (PCOS) HumReprod. 2012;27:14–24. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 113.Sterling L, Liu J, Okun N, Sakhuja A, Sierra S, Greenblatt E. Pregnancy outcomes in women with polycystic ovary syndrome undergoing in vitro fertilization. Fertil Steril. 2016;105:791–7.e2. doi: 10.1016/j.fertnstert.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 114.Palomba S, Falbo A, Chiossi G, et al. Low-grade chronic inflammation in pregnant women with polycystic ovary syndrome: a prospective controlled clinical study. J Clin Endocrinol Metab. 2014;99:2942–2951. doi: 10.1210/jc.2014-1214. [DOI] [PubMed] [Google Scholar]
- 115.Maliqueo M, SundströmPoromaa I, Vanky E, et al. Placental STAT3 signaling is activated in women with polycystic ovary syndrome. Hum Reprod. 2015;30:692–700. doi: 10.1093/humrep/deu351. [DOI] [PubMed] [Google Scholar]
- 116.Roos N, Kieler H, Sahlin L, Ekman-Ordeberg G, Falconer H, Stephansson O. Risk of adverse pregnancy outcomes in women with polycystic ovary syndrome: population based cohort study. BMJ. 2011;343:d6309. doi: 10.1136/bmj.d6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Naver KV, Grinsted J, Larsen SO, et al. Increased risk of preterm delivery and pre-eclampsia in women with polycystic ovary syndrome and hyperandrogenaemia. BJOG. 2014;121:575–581. doi: 10.1111/1471-0528.12558. [DOI] [PubMed] [Google Scholar]
- 118.Joham AE, Ranasinha S, Zoungas S, Moran L, Teede HJ. Gestational diabetes and type 2 diabetes in reproductive-aged women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2014;99:447–452. doi: 10.1210/jc.2013-2007. [DOI] [PubMed] [Google Scholar]
- 119.Vanky E, Stridsklev S, Heimstad R, et al. Metformin versus placebo from first trimester to delivery in polycystic ovary syndrome: a randomized, controlled multicenter study. J Clin Endocrinol Metab. 2010;95:448–455. doi: 10.1210/jc.2010-0853. [DOI] [PubMed] [Google Scholar]
- 120.Feng L, Lin XF, Wan ZH, Hu D, Du YK. Efficacy of metformin on pregnancy complications in women with polycystic ovary syndrome: a meta-analysis. Gynecol Endocrinol. 2015;31:833–839. doi: 10.3109/09513590.2015.1041906. [DOI] [PubMed] [Google Scholar]
- 121.van der Spuy Z, Dyer S. The pathogenesis of infertility and early pregnancy loss in polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2004;18:755–771. doi: 10.1016/j.bpobgyn.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 122.Joham AE, Boyle JA, Ranasinha S, Zoungas S, Teede HJ. Contraception use and pregnancy outcomes in women with polycystic ovary syndrome: data from the Australian Longitudinal Study on Women's Health. Hum Reprod. 2014;29:802–808. doi: 10.1093/humrep/deu020. [DOI] [PubMed] [Google Scholar]
- 123.Wang Q, Luo L, Lei Q, et al. Low aneuploidy rate in early pregnancy loss abortuses from patients with polycystic ovary syndrome. Reprod BioMed Online. 2016;33:85–92. doi: 10.1016/j.rbmo.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 124.Løvvik TS, Wikström AK, Neovius M, Stephansson O, Roos N, Vanky E. Pregnancy and perinatal outcomes in women with polycystic ovary syndrome and twin births: a population-based cohort study. BJOG. 2015;122:1295–1302. doi: 10.1111/1471-0528.13339. [DOI] [PubMed] [Google Scholar]
- 125.Han AR, Kim HO, Cha SW, et al. Adverse pregnancy outcomes with assisted reproductive technology in non-obese women with polycystic ovary syndrome: a case-control study. Clin Exp Reprod Med. 2011;38:103–108. doi: 10.5653/cerm.2011.38.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Palomba S, Santagni S, Falbo A, La Sala GB. Complications and challenges associated with polycystic ovary syndrome: current perspectives. Int J Womens Health. 2015;7:745–763. doi: 10.2147/IJWH.S70314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Winter S, Talbott E, Guzick D, Zborowski J, McHugh K. Serum testosterone levels decrease in middle age in women with the polycystic ovary syndrome. Fertil Steril. 2000;73:724–7229. doi: 10.1016/S0015-0282(99)00641-X. [DOI] [PubMed] [Google Scholar]
- 128.Brown ZA, Louwers YV, Fong SL. The phenotype of polycystic ovary syndrome ameliorates with aging. Fertil Steril. 2011;96:1259–1265. doi: 10.1016/j.fertnstert.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 129.Schmidt J, Brannstrom M, Landin-Wihelmsen K, Dahigren E. Reproductive hormone levels and anthropometry in postmenopausal women with polycystic ovary syndrome (PCOS): a 21-year-follow-up study of women diagnosed with PCOS around 50 years ago and their age-matched controls. J Clin Endocrinol Metab. 2011;96:2178–2185. doi: 10.1210/jc.2010-2959. [DOI] [PubMed] [Google Scholar]
- 130.Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in woman withpolycystic ovary syndrome: a systematicreview and meta-analysis. Hum Reprod Update. 2012;18:618–637. doi: 10.1093/humupd/dms030. [DOI] [PubMed] [Google Scholar]
- 131.Randeva HS, Tan BK, Weickert MO, et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev. 2012;33:812–841. doi: 10.1210/er.2012-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Echiburú B, Crisosto N, Maliqueo M, et al. Metabolic profile in women with polycystic ovary syndrome across adult life. Metabolism. 2016;65:776–782. doi: 10.1016/j.metabol.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 133.Lo JC, Feigenbaun SL, Yang J, Pressman AR, Selby JV, Go AS. Epidemiology and adverse cardiovascular riskprofile of diagnosedpolycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:1357–1363. doi: 10.1210/jc.2005-2430. [DOI] [PubMed] [Google Scholar]
- 134.Gutierrez-Grobe Y, Ponciano-Rodríguez G, Ramos MH, Uribe M, Méndez-Sánchez N. Prevalence of nonalcoholic fatty liver disease in premenopausal, posmenopausal and polycystic ovary syndrome women. The role of estrogens. Am Hepatol. 2010;9:402–409. [PubMed] [Google Scholar]
- 135.Veltman-Verhulst SM, Boivin J, Eijkemans MJ, Fauser BJ. Emotional distress is a common risk in women with polycystic ovary syndrome: a systematic review and meta-analysis of 28 studies. Hum Reprod Update 2012; 18:638–651. [DOI] [PubMed]
- 136.Barry JA, Mallika MA, Hardiman PJ. Risk of endometrial, ovarian and breast cancer with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:748–758. doi: 10.1093/humupd/dmu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Salley KE, Wickham EP, Cheang KI, Essah PA, Karjane NW, Nestler JE. Glucose intolerance in polycystic ovary syndrome- a position statement of the Androgen Excess Society. J Clin Endocrinol Metab. 2007;92:4546–4556. doi: 10.1210/jc.2007-1549. [DOI] [PubMed] [Google Scholar]