Abstract
Purpose
Extensive follicle loss has been demonstrated in ovarian grafts post transplantation, reducing their productivity and lifespan. Several mechanisms for this loss have been proposed, and this study aims to clarify when and how the massive follicle loss associated with transplantation of ovarian tissue graft occurs. An understanding of the mechanisms of follicle loss will pinpoint potential new targets for optimization and improvement of this important fertility preservation technique.
Methods
Frozen-thawed marmoset (n = 15), bovine (n = 37), and human (n = 46) ovarian cortical tissue strips were transplanted subcutaneously into immunodeficient castrated male mice for 3 or 7 days. Histological (H&E, Masson’s trichrome) analysis and immunostaining (Ki-67, GDF9, cleaved caspase-3) were conducted to assess transplantation-associated follicle dynamics, with untransplanted frozen-thawed tissue serving as a negative control.
Results
Evidence of extensive primordial follicle (PMF) activation and loss was observed already 3 days post transplantation in marmoset, bovine, and human tissue grafts, compared to frozen-thawed untransplanted controls (p < 0.001). No significant additional PMF loss was observed 7 days post transplantation. Recovered grafts of all species showed markedly higher rates of proliferative activity and progression from dormant to growing follicles (Ki-67 and GDF9 staining) as well as higher growing/primordial (GF/PMF) ratio (p < 0.02) and higher collagen levels compared with untransplanted controls.
Conclusions
This multi-species study demonstrates that follicle activation plays an important role in transplantation-induced follicle loss, and that it occurs within a very short time frame after grafting. These results underline the need to prevent this activation at the time of transplantation in order to retain the maximal possible follicle reserve and extend graft lifespan.
Keywords: Ovarian tissue cryopreservation and transplantation, Follicle activation, Fertility preservation
Introduction
Ovarian tissue cryopreservation and transplantation (OTCP-TP) has proven to be a successful fertility preservation technique [1, 2]. This technique is gaining increasing traction as a result of accumulative success with more than 80 live births reported by various teams across the world and over 90% return of ovarian function in graft recipients [3–5]. Extended graft survival has been reported in animal models and humans [6, 7], as demonstrated by prolonged restoration of ovarian hormonal production, repeated pregnancies, and live births. However, in order to optimize graft potential, it is essential to diminish the significant follicle loss that accompanies the OTCP-TP process [8, 9].
OTCP-TP is a multi-step procedure wherein each step can contribute to the overall decline in follicle numbers. The initial disconnection of the cortical tissue from its physical environment and blood and oxygen supply begins a deleterious ischemic cascade of events [10–12]. This is accompanied by physical and chemical assaults in preparation for freezing and thawing [13, 14]. Additional factors such as graft size, transplantation site, and operative technique and environmental factors also affect OTCP-TP follicle loss and success rate [8, 15, 16]. Diminished ovarian graft follicle stockpile potentially reduces the long-term endocrine function and reproductive potential of the graft, necessitating repeat transplantations of additional tissue from a precious limited supply [12].
While all steps may contribute to graft follicle loss, studies have shown that the most significant loss occurs immediately post transplantation [17–19]. The immediate transplantation period is characterized by ischemia and graft signaling to generate new blood and oxygen supply, during which acute loss of both growing and primordial follicles occurs [12, 20, 21]. However, the significant primordial follicle loss that occurs shortly after transplantation has been shown to be simultaneously accompanied by an increase in growing follicle population and proliferation of granulosa cells [16]. These data suggest, at least in human and bovine tissue, an additional important mechanism for follicle loss following ovarian graft transplantation: activation and loss (“burnout”) of the primordial follicle pool [22].
In order to better understand the mechanism leading to acute primordial activation and loss related to ovarian tissue grafting with clinical relevancy, this study conducted a cross species (bovine, non-human primate, and human) investigation of follicle dynamics and primordial follicle activation at two different time points immediately post transplantation in frozen thawed ovarian tissue.
Materials and methods
Ovarian tissue preparation from different species
Marmoset monkeys
Five healthy adult female marmoset monkeys (Callithrix jacchus) at the age range of 13 to 23 months were made available for ovaries excision [23] from the breeding colony of the Centre of Reproductive Medicine and Andrology, Germany. The marmosets were maintained in families at a 12/12 photoperiod. Monkeys received a complex diet consisting of pellets, insects, fruits, and vegetables and unlimited access to water. Animals were sacrificed due to other indications. The animals were sedated with Ketaject and killed by exsanguination. All procedures were conducted according to the German Law on the Care and Use of Laboratory Animals under license 84-02.05.20.12.0.018. Bilateral oophorectomy was performed, and the ovaries were immediately rinsed briefly in sterile phosphate-buffered saline (PBS; Gibco, Paisley, UK).
Bovine
Fresh bovine ovaries (lactating milking cows, ages 1.5–3 years, n = 12) were collected from the local abattoir and transferred to the lab in a sterile phosphate-buffered saline (Beit Ha’emek, Israel). Handling of the ovaries and tissue preparation were performed as previously described [24].
Human
Ovarian tissues were harvested from eight patients (age range 13–26 years) by laparoscopic surgery prior to any chemotherapy treatments in order to cryopreserve cortical tissue for optional future use to restore fertility. These patients consent to donate 5% of the cortex for research (written informed consent, ethical approval no. 8065). Tissue was immediately transferred to the lab in a sterile Gamete buffer (Cook Medical, IN, USA).
Cortical tissue isolation, cryopreservation, and thawing
Cortical tissue in all experiments was obtained by removal of the underlying stromal tissue using a scalpel and forceps under a stereomicroscope in 37 °C L-15 medium (Beit Ha’emek, Israel). Slow freezing cryopreservation was performed according to previously published protocol [25]. Briefly, tissue fragments were equilibrated with 0.1 M sucrose and 1.5 M DMSO (both from Sigma-Aldrich, Israel) for 30 min following introduction into the cryopreservation device (Planer, UK). A multi-gradient slow-freezing protocol cooled the samples to − 150 °C followed by storage in liquid nitrogen.
Prior to transplantation, tissue was thawed rapidly by equilibrating the samples in decreasing DMSO gradient solutions in 0.1 M sucrose and cut into 2 × 2 × 1–2-mm3 pieces (marmoset, n = 15; bovine, n = 37; human, n = 46). Transplantation of ovarian cortex strips into castrated SCID mice in all experiments was performed as described below, under ethical approval no. 873/13/ANIM.
Transplantation
Seven-week-old castrated male immunodeficient mice (Athymic nude, Harlan Israel, n = 54) were used as recipient animals. Mice were castrated at 4 weeks using bilateral orchiectomy. The mice were maintained at 28 °C under controlled SPF conditions, with a 12-h light/dark cycle and free access to an autoclaved pelleted diet and water. Xenotransplantation experiments commenced at least 3 weeks post orchiectomy.
Ovarian tissue transplantation surgical procedures were performed as described previously [16]. Briefly, mice were anesthetized by intraperitoneal (i.p.) injection of 100 mg/kg Ketamine (Vitamed) and 10 mg/kg Xylazine (UDIM Pharmacy) solution. A longitudinal incision was made in the dorsum of the recipient mouse, the skin was lifted, and the ovarian cortical strips were inserted subcutaneously. Size of transplanted tissue from all species was 2 × 2 × 1–2 mm3. Each graft was attached in place with a 6–0 non-absorbable prolene suture. The incision was sutured using a 4–0 suture with emphasis on using the suture to press the underlying graft. Transplanted tissues were recovered after 3 or 7 days.
Histology and immunohistochemistry
Recovered grafts and control frozen-thawed non-transplanted samples were fixed in 4% paraformaldehyde and embedded in paraffin, and serial 5-μm sections were prepared from the whole grafts for follicle count and immunohistochemistry. Follicle classification as primordial or growing was performed on every sixth section of hematoxylin and eosin-stained sections by two independent observers under a light microscope (slides/graft: eight for marmoset, nine for bovine, six for human). Only non-atretic follicles with clearly visible oocytes circled by granulosa cell layers were referred to in the “Results” section since no significant difference was found in the number of atretic follicles among the groups. PMF was defined as an oocyte surrounded by a single layer of flattened pre-granulosa cells. GF includes follicles in all developing stages. In Sirius red collagen staining, collagen fibers stained purple-red, tissue counterstained green.
For immunohistochemistry, the sections were incubated for 1 h with primary antibodies Ki-67 (Rabbit Monoclonal, 275R, 1:200, Cell Marque) and GDF9 (Goat Polyclonal, sc-12244, 1:50, Santa Cruz, USA) at RT in a humidity chamber, washed, and incubated with secondary antibodies: for Ki-67 (HiDef Detection Polymer, 954D, Cell Marque, Rocklin, CA, USA) and GDF9 (IH-8063-15, ImmunoBioscience, Washington, USA). A peroxidase substrate kit (SK-4100, Vector Labs, USA) was used as a chromogen and hematoxylin as a counterstain.
At least 20 images of five tissue sections from different mice per group were obtained for each analysis. A murine LLC tumor was used as positive control for Ki-67 and caspase-3 immunostaining, and a 6-week-old murine ovary was used as positive control for GDF9 immunostaining. Ovary tissue from each species exposed to secondary antibody only was used as negative control.
Statistical analysis
Results were expressed as mean follicle count per section ± SE or GF/PMF ratio ± SE. Data were subjected to one-way analysis of variance (ANOVA) and all-pairs, Tukey-Kramer HSD test. p values lower than 0.05 were considered statistically significant.
Results
Follicle loss post transplantation
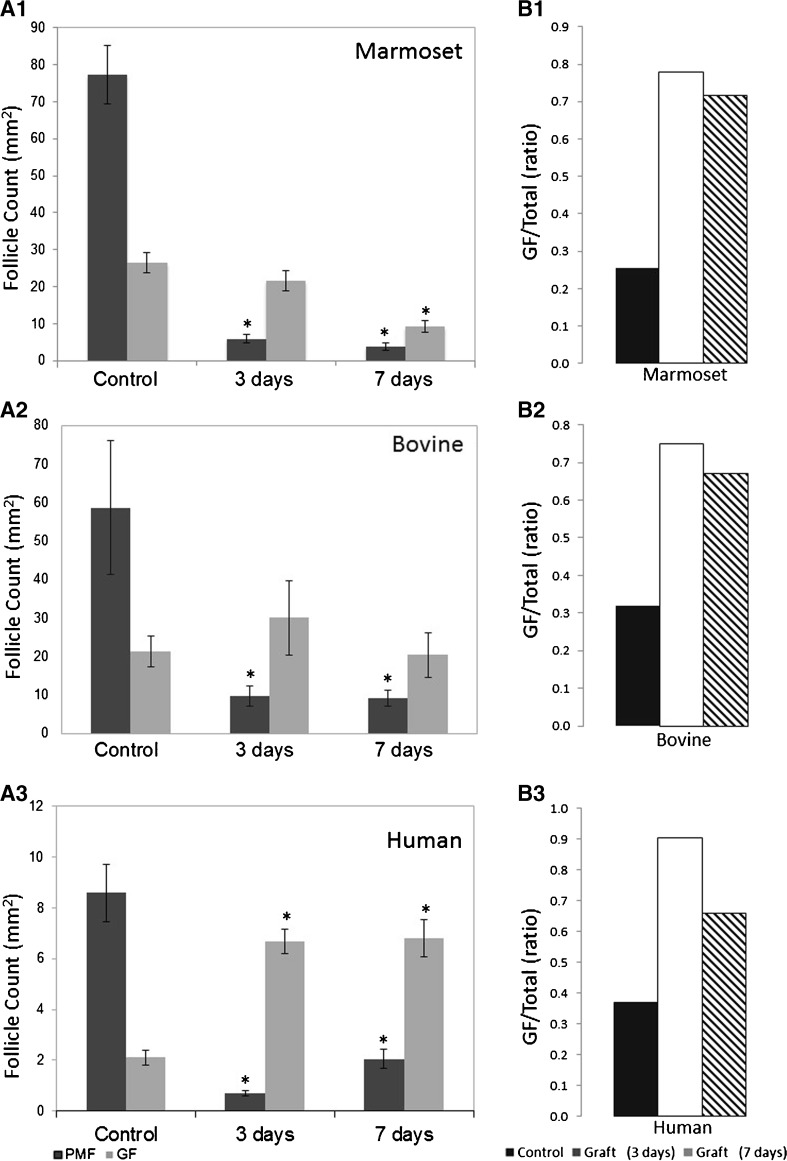
All grafts were recovered and evaluated. The mean dormant and growing follicle numbers (± SE) in ovarian grafts 3 and 7 days post transplantation compared with untransplanted controls are presented in Fig. 1. Grafts of all tissue species demonstrated significant and dramatic PMF loss already 3 days post transplantation compared to untransplanted controls, 92% (5.72 ± 0.97 vs 76.80 ± 6.91) in marmoset grafts, 83% (9.58 ± 1.01 vs 58.67 ± 6.70) in bovine grafts, and 91% (0.70 ± 0.12 vs 8.60 ± 1.44) in human grafts (p < 0.001), (Fig. 1 (A)). In all species, no additional significant PMF loss was observed in 7-day grafts compared to 3-day grafts. In human grafts, there were on average more PMFs in day 7 grafts than in day 3 grafts. This was not statistically significant and does not represent an actual increase in follicle number; rather, it is most likely the result of the low density of PMF in human tissue and thus higher variation in the final counts compared with marmoset or bovine (see Fig. 2a, b).
Fig. 1.
A Primordial follicle loss and activation following transplantation. Primordial (dark gray) and growing (light gray) follicle counts in frozen-thawed marmoset (A1), bovine (A2), and human (A3) ovarian tissue before (control) or after transplantation for 3 or 7 days s.c. in immunodeficient mice (counts per whole graft, mean ± SE, *p < 0.05 compared with untransplanted control). B Ratios of growing (GF) to primordial follicle (PMF) counts post transplantation. Ratios of GF/PMF in frozen-thawed marmoset (B1), bovine (B2), and human (B3) ovarian tissue before (untransplanted control, black) or 3 and 7 days after transplantation (white and stripes, respectively). Ratios in each time point significantly different from those of untransplanted control are marked by different letters (p < 0.05)
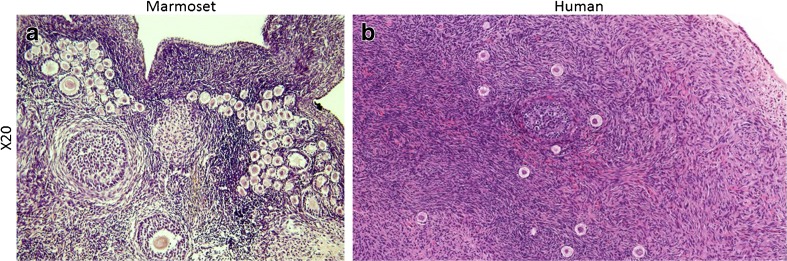
Fig. 2.
Comparison of follicle density in human vs marmoset ovarian tissue grafts. PMF density in human ovarian tissue grafts (b) is substantially lower than in the equivalent size marmoset tissue (a). The result of this lack of density is increased variability between pieces of tissue
In contrast to the massive drop in PMF population, growing follicle numbers were higher post transplantation in bovine and human grafts, reaching maximal numbers at 3 days compared with untransplanted control and those at 7 days post transplantation. While not higher than in untransplanted controls, marmoset tissue grafts also exhibited high numbers of growing follicles—higher than the number of PMFs—at both timepoints.
The relative distribution in follicle subclass was expressed by calculating the ratio of growing to primordial follicles (GF/PMF), where changes in this ratio post transplantation provide a measure of follicle activation [16, 26]. Untransplanted control tissues from all species exhibited a similar GF/PMF ratio of approximately 0.3, indicating that PMF comprised the substantial majority of the follicle population. However, 3 and 7 days post transplantation, the GF/PMF ratios in all species increased significantly showing a maximal ratio at 3 days post transplantation (p < 0.02) (Fig. 1 (B)). The decrease in the GF/PMF ratio observed in human grafts 7 days compared to 3 days post transplantation reflects the higher numbers of PFs (non-significant) at that timepoint, a factor of the low density and high variability in PMF numbers in human tissue.
Increased proliferation and fibrosis observed in transplanted grafts
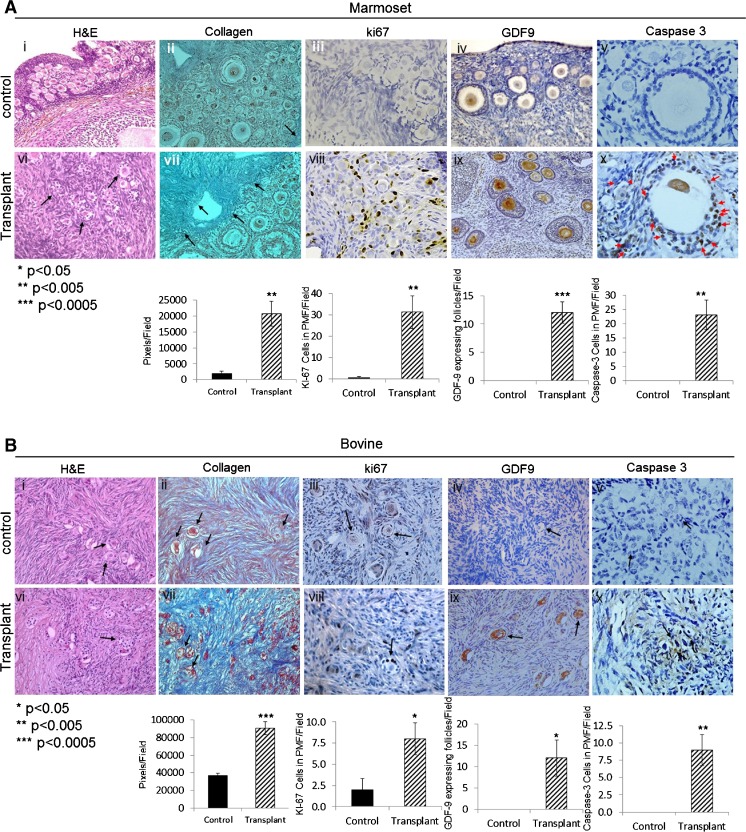
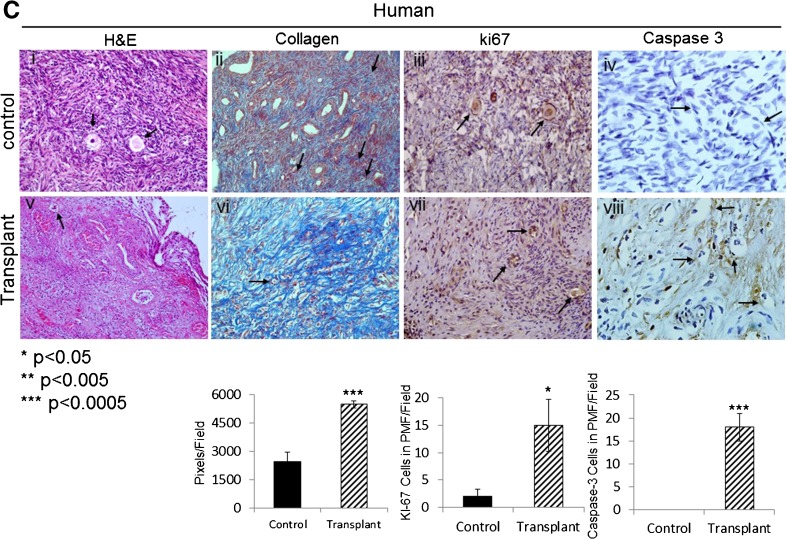
Seven days after transplantation, all transplanted grafts demonstrated a significant increase in staining for Ki-67 in granulosa cells, indicating proliferation in activated PMF and in GF (Fig. 3a (iii) vs (viii), b (iii) vs (viii), c (iii) vs (vii)). Extensive staining for GDF9 (representing progression from primordial follicle [27]) was observed in follicles in grafts of marmoset and bovine (Fig. 3a (iv) vs (ix), b (iv) vs (ix)). Human grafts did not show immunoreactivity for GDF9. Caspase-3 staining showed a significant increased apoptosis in granulosa cells of growing follicles in all grafted samples (Fig. 3a (x), b (x), c (viii)). In addition, stromal changes, specifically increased fibrosis, were observed in all grafted samples compared to untransplanted control (Fig. 3a (vii), b (vii), c (vi)).
Fig. 3.
Immunostaining demonstrates actively growing follicles in transplanted grafts. Histological analysis of marmoset (a), bovine (b), and human (c) frozen-thawed ovarian tissues before and 7 days post transplantation. Samples were stained with H&E (a i, vi; b i, vi; c i, v). Arrows pointing at PMF, with Masson’s trichrome for collagen (a ii, vii; b ii, vii; c ii, vi). Arrows pointing at collagen (blue), for Ki-67 (a iii, viii, b iii, viii; c iii, vii). Arrows pointing at Ki-67 expressing PMF, cleaved caspase-3 (a v, x; b v, x; c iv, viii). Arrows pointing at cleaved caspase-3 expressing PMF (a v; b v, x; c iv, viii), growing follicles (a v), and GDF9 (a iv, ix; b iv, ix). Arrows pointing at GDF9 expressing growing follicles. Magnification: ×40. Image analysis and quantification were performed for each antibody on four slides per group, and the mean counts per field ± SE are shown below the histological images (*p < 0.05, **p < 0.005, ***p < 0.0005 compared with untransplanted control average). Quantification is expressed as follows: for Ki-67 and caspase-3 expressed as cells in PMF/field, for GDF9 as GDF9 expressing follicles/field, and for collagen as pixels/field (using ImageJ software)
Discussion
This study looked at the phenomenon and time frame of follicle activation post ovarian tissue transplantation across multiple species (marmoset, bovine, and human). Results demonstrated that massive PMF loss occurs in grafts of all species already 3 days of transplantation, with no significant additional loss thereafter. This is new information which narrows the time frame reported in the only previously published studies to examine this topic, which documented significant PMF loss in cryopreserved thawed human ovarian cortex grafts 7 days after transplantation [16, 18]. The mechanism of this dramatic loss of PMFs was evident in the significant increase in the number of growing follicles observed, together with the increase in granulosa cell proliferation (as demonstrated by Ki-67 staining) as well as in staining for GDF9 (representing the progression of PMF to primary stage follicles) all of which point to the activation and growth of PMFs as a significant mechanism behind the follicle loss that occurs post transplantation. This conclusion is supported by previous data which showed an increase in the number of growing follicles in newborn mouse ovarian tissue grafts [17]. The ratio of GF to PMF, which provides a measure of follicle activation, was shown to be significantly higher in grafts of all three species compared with untransplanted (frozen-thawed) control tissue, both 3 and 7 days post transplantation. In addition, all transplanted grafts showed a significant increase in apoptosis in granulosa cells of growing follicles as well as increased fibrosis compared with untransplanted tissue, confirming previous studies (Israely, 2006; Damasio, 2016; Dath, et al., 2010; Nisolle, et al., 2000; Gavish, 2014).
Human ovarian tissue is problematic for examining follicle dynamics in transplanted grafts due to the relative scarcity of PMFs compared to other species. The low density of follicles, together with natural variability, makes it harder to reach conclusions regarding changes in follicle numbers. As such, the marmoset model used in this study serves a particularly important purpose. As a primate model with greater density of follicles (Fig. 3), we were able to use marmoset ovarian grafts to statistically demonstrate the activation of follicles post transplantation. So while the human tissue allowed us to observe the same trend, the marmoset graft confirmed that follicle activation is a significant mechanism behind transplantation-induced follicle loss also in primates. Together, these results establish the universality of follicle activation and “burnout” as a mechanism of post-transplantation follicle loss in ovarian tissue grafts across species.
The process of transplantation is linked to other causes of tissue damage such as ischemia, oxidative stress, and fibrosis, all of which have been proposed to induce the follicle loss observed post transplantation. However, while ischemia has been shown to contribute to graft follicle loss [28, 29], the extensive primordial follicle loss coupled with the increase in growing follicles observed in our present and previous study [16] cannot be solely attributed to these causes. In particular, primordial and primary follicles have been shown to be less susceptible to the damaging effects of ischemia and oxidative stress than more metabolically active growing follicles [17, 20, 30], and given growing follicles’ greater susceptibility to ischemic damage, there is certainly no mechanism by which ischemia would cause an increase in growing follicles. As such, while other mechanisms of tissue damage may contribute to the follicle loss associated with frozen-thawed ovarian tissue transplantation, our results support the hypothesis that this loss is, in a significant measure, due to activation and burnout of the PMF population [22].
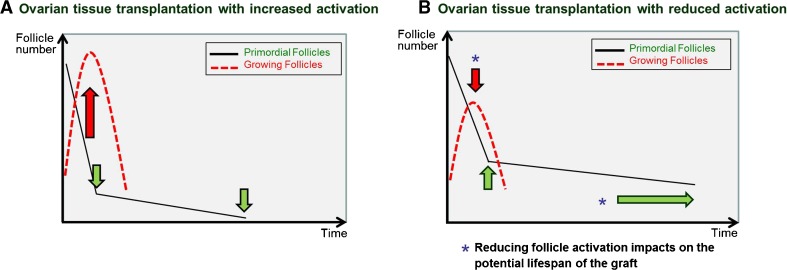
Some studies have looked at manipulating follicle growth in transplanted tissue grafts via inhibition of the PI3K and the Hippo signaling pathways [31, 32], both of which are involved in the promotion of follicle growth [33]. Manipulation of these pathways was used to promote follicle activation and growth and the generation of mature oocytes immediately after transplantation. This was done in order to rapidly produce mature follicles that they were able to use for in vitro fertilization. However, it is worth noting that the promotion of follicle activation in grafts via exposure to Akt inhibitors is actually entirely unnecessary since the act of transplantation alone induces follicle activation. Moreover, promoting follicle activation further reduces the reserve of PMFs, a process which we ultimately aim to prevent as it reduces the potential fertility and lifespan of the graft [34] (Fig. 4).
Fig. 4.
The magnitude of follicle activation directly impacts PMF reserve in ovarian grafts. a Inducing activation in ovarian follicle grafts increases the number of growing follicles in the immediate short term at the expense of the PMF population, resulting in decreased graft lifespan. This may be suitable for immediate IVF retrieval but comes at the expense of the overall fertility potential and long-term functioning of the graft. b Limiting activation at the time of transplantation reduces PMF loss thereby extending graft lifespan. It is preferable to reduce the activation that occurs with transplantation in order to maximize the fertility outcomes of the procedure
The importance of reducing follicle loss and thus retaining the largest possible number of dormant follicles in the graft cannot be overstated. Ovarian tissue cryopreservation is a labor-intensive technique involving surgery, expert preparation of the tissue, and long-term storage. The resulting grafts are by necessity a very limited resource and often an end-of-the-line option for the patient involved. The functional lifespan of an ovarian graft is entirely dependent on the number of dormant follicles in that graft. It is for this reason that protecting the dormant follicle population in the graft by reducing activation, and thereby maximizing the follicle reserve and the lifespan of the graft, is so crucial.
The likely explanation behind transplantation-induced activation as a mechanism of loss lies in the process of cortical tissue preparation for freezing and/or transplantation, which by necessity isolates the PMFs from the growing (antral, pre-antral, and secondary) follicles which maintain the quiescence of the dormant follicles via production of inhibitory factors such as AMH. The absence of the growing follicles disrupts the balance between stimulatory and inhibitory follicle activation factors in the graft, thereby instigating follicular activation [35].
Conclusion
This study provides new evidence that primordial follicle activation and burnout are clinically relevant mechanisms of acute follicle depletion after transplantation in ovarian tissue grafts, and that this loss occurs within the short time frame of 3 days post transplantation. This advance in our understanding of the timing and mechanisms behind the follicle loss that limits the lifespan of ovarian tissue grafts opens up potential new targets for optimization and improvement of this important fertility preservation technique.
Acknowledgements
The authors wish to thank Mrs. Sanaz Dereh-Haim, Mrs. Reinhild Sandhowe-Klawerkamp, and Mr. Martin Heuermann for excellent technical assistance and animal care. The following foundations contributed to the funding of this study: Israel Science Foundation and Kahn Foundation.
Compliance with ethical standards
All procedures were conducted according to the German Law on the Care and Use of Laboratory Animals under license 84-02.05.20.12.0.018.
References
- 1.Donnez J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364(9443):1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 2.Meirow D, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353(3):318–321. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- 3.Meirow D, Ra’anani H, Biderman H. Ovarian tissue cryopreservation and transplantation: a realistic, effective technology for fertility preservation. Methods Mol Biol. 2014;1154:455–473. doi: 10.1007/978-1-4939-0659-8_21. [DOI] [PubMed] [Google Scholar]
- 4.Stoop D, Cobo A, Silber S. Fertility preservation for age-related fertility decline. Lancet. 2014;384(9950):1311–1319. doi: 10.1016/S0140-6736(14)61261-7. [DOI] [PubMed] [Google Scholar]
- 5.Donnez J, Dolmans MM. Transplantation of ovarian tissue. Best Pract Res Clin Obstet Gynaecol. 2014;28(8):1188–1197. doi: 10.1016/j.bpobgyn.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Andersen CY, et al. Long-term duration of function of ovarian tissue transplants: case reports. Reprod BioMed Online. 2012;25(2):128–132. doi: 10.1016/j.rbmo.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Meirow D, et al. Transplantations of frozen-thawed ovarian tissue demonstrate high reproductive performance and the need to revise restrictive criteria. Fertil Steril. 2016;106(2):467–474. doi: 10.1016/j.fertnstert.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 8.Kawamura K, et al. Ovary transplantation: to activate or not to activate. Hum Reprod. 2015;30(11):2457–2460. doi: 10.1093/humrep/dev211. [DOI] [PubMed] [Google Scholar]
- 9.Silber S. Ovarian tissue cryopreservation and transplantation: scientific implications. J Assist Reprod Genet. 2016;33(12):1595–1603. doi: 10.1007/s10815-016-0814-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 11.Van Eyck AS, et al. Both host and graft vessels contribute to revascularization of xenografted human ovarian tissue in a murine model. Fertil Steril. 2010;93(5):1676–1685. doi: 10.1016/j.fertnstert.2009.04.048. [DOI] [PubMed] [Google Scholar]
- 12.Donnez J, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99(6):1503–1513. doi: 10.1016/j.fertnstert.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 13.Donnez J, Dolmans MM. Cryopreservation and transplantation of ovarian tissue. Clin Obstet Gynecol. 2010;53(4):787–796. doi: 10.1097/GRF.0b013e3181f97a55. [DOI] [PubMed] [Google Scholar]
- 14.Lee J, et al. Ovarian injury during cryopreservation and transplantation in mice: a comparative study between cryoinjury and ischemic injury. Hum Reprod. 2016;31(8):1827–1837. doi: 10.1093/humrep/dew144. [DOI] [PubMed] [Google Scholar]
- 15.Donnez J, et al. Ovarian tissue cryopreservation and transplantation: a review. Hum Reprod Update. 2006;12(5):519–535. doi: 10.1093/humupd/dml032. [DOI] [PubMed] [Google Scholar]
- 16.Gavish Z, et al. Follicle activation and ‘burn-out’ contribute to post-transplantation follicle loss in ovarian tissue grafts: the effect of graft thickness. Hum Reprod. 2014;29(5):989–996. doi: 10.1093/humrep/deu015. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, et al. Early massive follicle loss and apoptosis in heterotopically grafted newborn mouse ovaries. Hum Reprod. 2002;17(3):605–611. doi: 10.1093/humrep/17.3.605. [DOI] [PubMed] [Google Scholar]
- 18.Dolmans MM, et al. Short-term transplantation of isolated human ovarian follicles and cortical tissue into nude mice. Reproduction. 2007;134(2):253–262. doi: 10.1530/REP-07-0131. [DOI] [PubMed] [Google Scholar]
- 19.Hancke K, et al. Ovarian transplantation for fertility preservation in a sheep model: can follicle loss be prevented by antiapoptotic sphingosine-1-phosphate administration? Gynecol Endocrinol. 2009;25(12):839–843. doi: 10.3109/09513590903159524. [DOI] [PubMed] [Google Scholar]
- 20.Soleimani R, Heytens E, Oktay K. Enhancement of neoangiogenesis and follicle survival by sphingosine-1-phosphate in human ovarian tissue xenotransplants. PLoS One. 2011;6(4):e19475. doi: 10.1371/journal.pone.0019475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newton H, et al. Low temperature storage and grafting of human ovarian tissue. Hum Reprod. 1996;11(7):1487–1491. doi: 10.1093/oxfordjournals.humrep.a019423. [DOI] [PubMed] [Google Scholar]
- 22.Roness H, et al. Ovarian follicle burnout: a universal phenomenon? Cell Cycle. 2013;12(20):3245–3246. doi: 10.4161/cc.26358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hearn JP, et al. Use of the common marmoset, Callithrix jacchus, in reproductive research. Primates Med. 1978;10:40–49. [PubMed] [Google Scholar]
- 24.Telfer EE, et al. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23(5):1151–1158. doi: 10.1093/humrep/den070. [DOI] [PubMed] [Google Scholar]
- 25.Meirow D, et al. Monitoring the ovaries after autotransplantation of cryopreserved ovarian tissue: endocrine studies, in vitro fertilization cycles, and live birth. Fertil Steril. 2007;87(2):418 e7–418 e15. doi: 10.1016/j.fertnstert.2006.05.086. [DOI] [PubMed] [Google Scholar]
- 26.Kalich-Philosoph L, et al. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci Transl Med. 2013;5(185):185ra62. doi: 10.1126/scitranslmed.3005402. [DOI] [PubMed] [Google Scholar]
- 27.Vitt UA, et al. In vivo treatment with GDF-9 stimulates primordial and primary follicle progression and theca cell marker CYP17 in ovaries of immature rats. Endocrinology. 2000;141(10):3814–3820. doi: 10.1210/endo.141.10.7732. [DOI] [PubMed] [Google Scholar]
- 28.Aubard Y, et al. Orthotopic and heterotopic autografts of frozen-thawed ovarian cortex in sheep. Hum Reprod. 1999;14(8):2149–2154. doi: 10.1093/humrep/14.8.2149. [DOI] [PubMed] [Google Scholar]
- 29.Nottola SA, et al. Cryopreservation and xenotransplantation of human ovarian tissue: an ultrastructural study. Fertil Steril. 2008;90(1):23–32. doi: 10.1016/j.fertnstert.2007.05.069. [DOI] [PubMed] [Google Scholar]
- 30.Oktay K, Oktem O. Ovarian cryopreservation and transplantation for fertility preservation for medical indications: report of an ongoing experience. Fertil Steril. 2010;93(3):762–768. doi: 10.1016/j.fertnstert.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Kawamura K, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci U S A. 2013;110(43):17474–17479. doi: 10.1073/pnas.1312830110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki N, et al. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30(3):608–615. doi: 10.1093/humrep/deu353. [DOI] [PubMed] [Google Scholar]
- 33.Hsueh AJ, et al. Intraovarian control of early folliculogenesis. Endocr Rev. 2015;36(1):1–24. doi: 10.1210/er.2014-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meirow D, et al. Optimizing outcomes from ovarian tissue cryopreservation and transplantation; activation versus preservation. Hum Reprod. 2015;30(11):2453–2456. doi: 10.1093/humrep/dev210. [DOI] [PubMed] [Google Scholar]
- 35.Visser JA, et al. Anti-Müllerian hormone: a new marker for ovarian function. Reproduction. 2006;131(1):1–9. doi: 10.1530/rep.1.00529. [DOI] [PubMed] [Google Scholar]