Abstract
Cannabinoid hyperemesis syndrome (CHS) is a clinical entity in which marijuana users develop nausea, vomiting, and abdominal pain that improves with hot water bathing or cannabis cessation. Previous models suggest that CHS arises solely from the derangement of cannabinoid receptor type 1 signaling. However, involvement of transient receptor potential vanilloid subtype 1 (TRPV1) receptor, which is activated by marijuana, capsaicin, and heat, could fill gaps in existing models, including the enigmatic role of hot water bathing. We propose that chronic cannabis use decreases TRPV1 signaling and alters gastric motility, and we report the case of a CHS patient whose symptoms improved after topical capsaicin.
Introduction
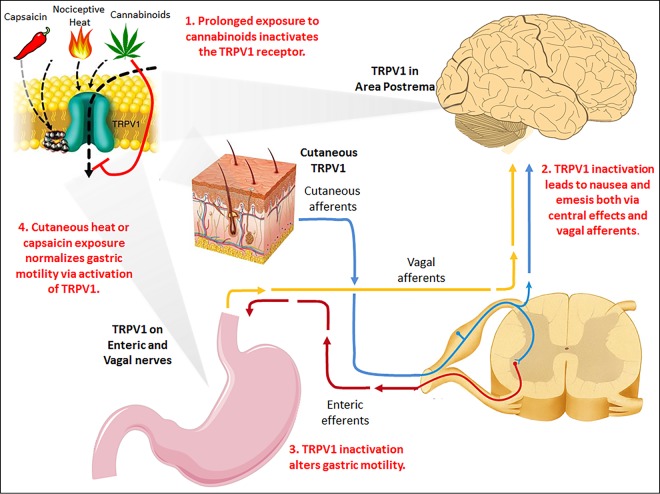
Cannabinoid hyperemesis syndrome (CHS) is a clinical entity characterized by chronic marijuana use, intractable vomiting, and relief of symptoms with hot-water bathing, exemplifying the enigmatic role of temperature in attenuating symptoms.1-3 A recent review proposed that transient receptor potential vanilloid subtype 1 (TRPV1) receptor, which is involved in gastric motility and is activated by cannabinoids, high temperatures, and capsaicin, is centrally involved in the pathogenesis of CHS (Figure 1).4
Figure 1.
Proposed pathophysiology of cannabinoid hyperemesis syndrome. TRPV1 is expressed in area postrema of the medulla, along gastric enteric and vagal nerves, and on cutaneous receptors in the dermis and epidermis. Prolonged exposure to cannabinoids inactivates TRPV1, potentially resulting in central nausea, altered gastric motility, and abdominal pain. Exposure to nociceptive heat, such as with compulsive hot-water bathing, may transiently augment cutaneous TRPV1 firing and restore gastric motility, temporarily mitigating symptoms. Use of another TRPV1 agonist, capsaicin, may also provide relief. Cessation of marijuana use gradually leads to normalization of TRPV1 function and fully ameliorates symptoms.
Case Report
A 47-year-old man with a decade-long history of marijuana use of up to several grams daily presented to the gastroenterology clinic with 8 years of abdominal pain, nausea, and vomiting relieved by up to 4 hours of hot-water bathing daily. Computed tomography (CT) of the abdomen and pelvis showed an unremarkable liver, no biliary duct dilatation, normal gallbladder, normal pancreatic body and head, normal spleen, and no bowel obstruction or inflammation. Abdominal ultrasound revealed a liver with normal size and echotexture, antegrade portal flow, non-dilated bile ducts, and a gallbladder without gallstones, wall thickening, or pericholecystic fluid. Upper endoscopy demonstrated a normal esophagus, normal gastric mucosa, and normal examined duodenum. Biopsies were negative for Helicobacter pylori. Colonoscopy showed normal colonic mucosa and terminal ileum. The patient had no improvement with dicyclomine, ranitidine, and twice-daily omeprazole. He was diagnosed with CHS and encouraged to discontinue marijuana.
The patient continued marijuana use and presented several weeks later to the emergency department with severe, periumbilical, stabbing pain associated with nausea and vomiting. He had a temperature of 37.1°C, heart rate 86 beats/min, blood pressure 146/74 mm Hg, and oxygen saturation 98% on ambient air. Labs showed a white blood cell count 14,000 cells/µL, potassium 3.1 mEq/L, and normal hemoglobin, creatinine, blood urea nitrogen, aspartate aminotransferase, alanine aminotransferase, total bilirubin, albumin, lipase, and urinalysis. Abdominal CT scan was unchanged from the previous scan. He was treated with intravenous fluids, potassium, ondansetron, metoclopramide, prochlorperazine, fentanyl, viscous lidocaine, aluminum hydroxide/magnesium hydroxide/simethicone, and pantoprazole without improvement of symptoms. We applied capsaicin cream (0.075%) to a 15 × 25 cm area in the periumbilical region, with reapplications every 4 hours. The patient reported burning of the skin and improvement in the intensity of his stabbing abdominal pain and nausea a few hours after the first application of capsaicin. After the second dose, he noted complete resolution of his nausea. He received 2 more doses, which resulted in complete improvement of his abdominal pain. He was discharged the following day with a prescription for topical capsaicin. Over the following 3 months, the patient had no visits to our hospital system or any other providers included in Epic Care Everywhere within Washington State.
Discussion
The efficacy of capsaicin in CHS in our case lends credence to the role of TRPV1 in this syndrome. Previous models suggesting that cannabinoid receptor type 1 (CB1) is solely responsible for CHS are unsatisfying for several reasons. First, peripheral activation of CB1 slows gastric transit, but a case series of CHS patients described normal or increased transit time and no gastroparesis symptoms.5,6 Second, previous models suggested that hot-water bathing is an adaptive response to the hypothermic effects of cannabinoids. However, activation of CB1 should lead to hot-water bathing in all marijuana users, not just CHS patients who seem to engage in hot-water bathing as a learned behavior to treat symptoms. Finally, this model fails to explain why only some patients are susceptible to CHS, why there is latency from onset of marijuana use to symptoms, and why only chronic users are affected.
TRPV1 is a nonselective cation channel activated by noxious heat and capsaicin. This receptor is expressed throughout the gastrointestinal tract, including vagal sensory neurons, intrinsic enteric neurons in the myenteric plexus, and gastric epithelial cells.7,8 Within the central nervous system, there is a high density of TRPV1 in the area postrema, known as the chemoreceptor trigger zone. Activation of TRPV1 has potent anti-emetic effects, which may be mediated by depletion of substance P from neural circuits traveling to the nucleus tractus solitarius.9,10
Exogenous cannabinoids, including delta-9-tetrahydrocannabinoil, activate both CB1 and TRPV1.11 In vitro studies demonstrate that exogenous cannabinoids lead to dephosphorylation of TRPV1 and subsequent receptor desensitization.10 Therefore, chronic exposure to cannabinoids could downregulate or desensitize TRPV1 signaling, explaining how prolonged exposure to cannabinoids might lead to decreased TRPV1 signaling, altered gastric motility, and emesis. The learned behavior of compulsive hot-water bathing may be an attempt to normalize diminished TRPV1 activity by deliberate exposure to another TRPV1 agonist, nociceptive heat. Therefore, TRPV1 agonists such as topical capsaicin, which has a longer half-life than oral capsaicin, might augment TRPV1 activity and provide a less burdensome approach to treating CHS.12
One published case report and 2 case series have described successful treatment of 15 CHS patients with topical capsaicin.13-15 In all cases, patients with heavy marijuana use presented to the emergency department with nausea, vomiting, and abdominal pain improved by application of topical capsaicin preparation (0.075%). Some have questioned the lack of advanced diagnostics, such as endoscopic studies and CT imaging in these cases. In addition, these patients had a short period of observation in the emergency department with unclear clinical courses after discharge.16 These weaknesses do not apply to our case, given the extensive workup and prolonged inpatient observation.
Marijuana is the most frequently used illicit drug in the United States. Its recent legalization in many states has raised concerns of increased use and a resulting rise in CHS incidence.17,18 For these reasons, a better understanding of CHS pathogenesis and novel treatment strategies are increasingly important. Our case lends further credence to the role of TRPV1 in CHS, although only limited conclusions can be drawn from this single case with incomplete confirmed follow-up. We believe a prospective study, such as the planned randomized controlled trial examining capsaicin in cyclic vomiting syndrome, could adequately assess the efficacy of capsaicin for CHS.19
Disclosures
Author contributions: All authors contributed equally to the manuscript. A. Moon is the article guarantor.
Acknowledgements: We thank Dr. Jasmine Zia and Dr. Anne Peery for reviewing this manuscript and providing expert assistance.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
References
- 1.Sontineni SP, Chaudhary S, Sontineni V, Lanspa SJ. Cannabinoid hyperemesis syndrome: Clinical diagnosis of an underrecognised manifestation of chronic cannabis abuse. World J Gastroenterol. 2009;15(10):1264–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen JH, de Moore GM, Heddle R, Twartz JC. Cannabinoid hyperemesis: Cyclical hyperemesis in association with chronic cannabis abuse. Gut. 2004;53(11):1566–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallace D, Martin AL, Park B. Cannabinoid hyperemesis: Marijuana puts patients in hot water. Australas Psychiatry. 2007;15(2):156–8. [DOI] [PubMed] [Google Scholar]
- 4.Richards JR, Lapoint JM, Burillo-Putze G. Cannabinoid hyperemesis syndrome: potential mechanisms for the benefit of capsaicin and hot water hydrotherapy in treatment. Clin Toxicol (Phila). 2017;1:1–10. [DOI] [PubMed] [Google Scholar]
- 5.Izzo AA, Sharkey KA. Cannabinoids and the gut: New developments and emerging concepts. Pharmacol Ther. 2010;126(1):21–38. [DOI] [PubMed] [Google Scholar]
- 6.Simonetto DA, Oxentenko AS, Herman ML, Szostek JH. Cannabinoid hyperemesis: A case series of 98 patients. Mayo Clin Proc. 2012;87(2):114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–24. [DOI] [PubMed] [Google Scholar]
- 8.Ward SM, Bayguinov J, Won KJ, Grundy D, Berthoud HR. Distribution of the vanilloid receptor (VR1) in the gastrointestinal tract. J Comp Neurol. 2003;465(1):121–35. [DOI] [PubMed] [Google Scholar]
- 9.Darmani NA, Ray AP. Evidence for a re-evaluation of the neurochemical and anatomical bases of chemotherapy-induced vomiting. Chem Rev. 2009;109(7):3158–99. [DOI] [PubMed] [Google Scholar]
- 10.Rudd JA, Nalivaiko E, Matsuki N, Wan C, Andrews PL. The involvement of TRPV1 in emesis and anti-emesis. Temperature (Austin). 2015;2(2):258–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Petrocellis L, Ligresti A, Moriello AS, et al. Effects of cannabinoids and cannabinoid-enriched cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol. 2011;163(7):1479–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chanda S, Bashir M, Babbar S, Koganti A, Bley K. In vitro hepatic and skin metabolism of capsaicin. Drug Metab Dispos. 2008;36(4):670–5. [DOI] [PubMed] [Google Scholar]
- 13.Roman F, Llorens P, Burillo-Putze G. [Topical capsaicin cream in the treatment for cannabinoid hyperemesis syndrome]. Med Clin (Barc). 2016; [DOI] [PubMed] [Google Scholar]
- 14.Lapoint J. Capsaicin cream for treatment of cannabinoid hyperemesis syndrome . Presented at: ACMT Annual Scientific Meeting 2014; Phoenix, AZ. [Google Scholar]
- 15.Dezieck L, Hafez Z, Conicella A, et al. Resolution of cannabis hyperemesis syndrome with topical capsaicin in the emergency department: A case series. Clin Toxicol (Phila). 2017;1–6. [DOI] [PubMed] [Google Scholar]
- 16.Wang JJ, Villeneuve E, Amiel JA, Gosselin S. Cannabinoid hyperemesis syndrome and topical capsaicin: Treating smoke with fire? Clin Toxicol (Phila). 2017;1:1235–42. [DOI] [PubMed] [Google Scholar]
- 17.Hasin DS, Saha TD, Kerridge BT, et al. Prevalence of marijuana use disorders in the United States between 2001–2002 and 2012–2013. JAMA Psychiatry. 2015;72(12):1235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kilmer B. Altered state? Assessing how marijuana legalization in California could influence marijuana consumption and public budgets. Santa Monica, Calif: RAND Corporation; 2010. [Google Scholar]
- 19.Topical Capsaicin for Cyclical Vomiting. Retrieved from https://ClinicalTrials.gov/show/NCT03223350 (Identification No. NCT03223350).