Abstract
Liver dysfunction is a major problem in patients with severe preeclampsia (PE), hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome, or in patients receiving anti-vascular endothelial growth factor (VEGF) therapy. Excessive soluble fms-like tyrosine kinase 1 (sFlt1) that antagonizes VEGF has been implicated in the pathogenesis of PE. VEGF increases the expression of endothelial nitric oxide synthase (eNOS) and activates it. eNOS polymorphisms that cause reduced NO production are associated with PE. The aim of this study was to clarify the role on hepatic function by excess sFlt1 in the absence of eNOS gene product. We first overexpressed sFlt1 using adenovirus in eNOS −/− and eNOS +/+ mice. Excessive sFlt1 and lack of eNOS synergistically increased plasma levels of liver transaminases, exacerbated infiltration of inflammatory cells, elevated expression levels of cytokines in the liver, and aggravated oxidative stress and coagulation abnormalities. Lack of eNOS in the presence of excess sFlt1 also induced thrombocytopenia, whereas eNOS +/+ mice with excess sFlt1 alone showed no or modest liver phenotype. Taken together, excessive sFlt1 and lack of eNOS synergistically induce hepatic dysfunction and thrombocytopenia, suggesting a novel role for VEGF and nitric oxide signaling in hepatocyte-endothelial cross-talk in health and in liver injury states.
Introduction
Vascular endothelial growth factor (VEGF) is indispensable in the maturation and maintenance of endothelial cells1. VEGF produced by hepatocytes acts on VEGF receptors expressed in sinusoidal endothelial cells, and is essential for maintaining liver homeostasis2,3. Although inhibitors of VEGF signaling are widely used as anti-cancer therapy, their hepatotoxicity is problematic4. Moreover, placental upregulation of endogenous sFlt1 that acts as an inhibitor of VEGF and placental growth factor (PlGF) signaling, has been linked to the pathogenesis of preeclampsia (PE)5,6 and possibly hemolysis, elevated liver enzyme levels, and low platelet levels (HELLP) syndrome that exhibits liver dysfunction7,8.
The effect of inhibiting VEGF on liver injury has not been well studied. Some investigators showed that knocking down hepatic VEGF or excessive sFlt1 causes hepatotoxicity2,9, whereas others have demonstrated VEGF inhibitors do not affect liver function10. These findings may indicate that additional factor(s) may be required for VEGF inhibitors to induce liver dysfunction.
VEGF activates eNOS through phosphorylation of Ser 117711. Typical eNOS gene polymorphisms, G894T and T-786C, are associated with the onset of PE or HELLP syndrome12,13. Consistent with these findings, it was recently reported that hypertension and placental ischemia induced by sFlt1 may be dependent on impaired NO signaling, and that sildenafil, a cyclic GMP agonist, can reverse sFlt1 mediated adverse pregnancy outcomes14. However, it is likely that sFlt1 also induces several NO independent pathways. In this regard, we have previously demonstrated that lack of eNOS exacerbates sFlt1-induced kidney injury through endothelin activation15. Based on these findings, we hypothesized that eNOS dysfunction is likely involved in the exacerbation of tissue injury caused by VEGF inhibition.
Here, we demonstrate that excessive sFlt1 combined with lack of eNOS in non-pregnant mice causes severe liver dysfunction accompanied by hepatic inflammation, oxidative stress, and dyslipidemia. Coagulation abnormalities and thrombocytopenia were also evident.
Results
Characteristics and liver dysfunction induced by excessive sFlt1 in mice lacking eNOS
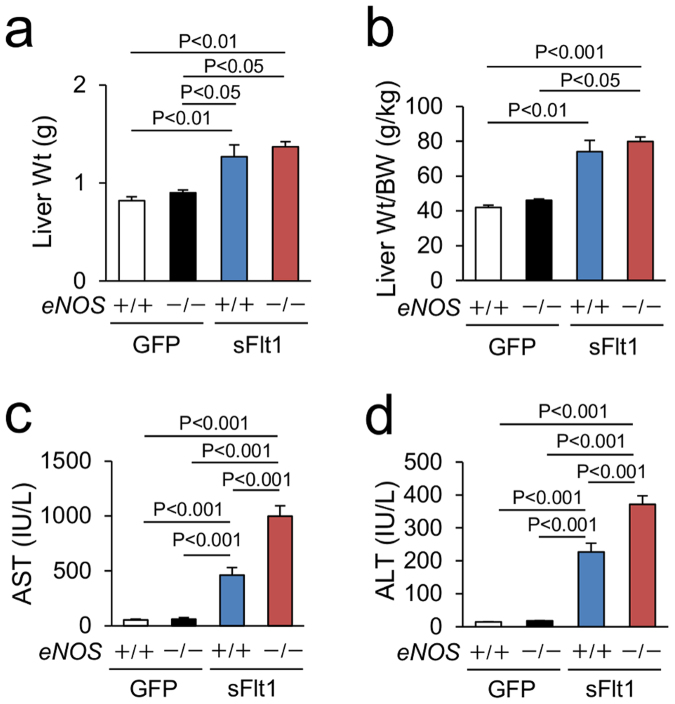
We used non-pregnant eNOS −/− mice overexpressing sFlt1. eNOS −/−; sFlt1 mice showed severe glomerular injury and massive albuminuria that is consistent with our previous observation (Supplementary Figure 1a,b)15. Liver weight and liver weight/body weight were larger in mice with excessive sFlt1 (Fig. 1a,b). Lack of eNOS did not affect them. In contrast, levels of plasma aspartate transaminase (AST) were significantly elevated in eNOS −/−; sFlt1 (998.5 ± 96.8 IU/L) compared to those of controls (55.1 ± 6.3 IU/L in eNOS +/+ and 63.8 ± 11.5 IU/L in eNOS −/−) and of eNOS +/+; sFlt1 mice (463.0 ± 68.5 IU/L) (Fig. 1c). The levels of alanine transaminase (ALT) were also elevated in eNOS −/−; sFlt1 (371.1 ± 26.4 IU/l) compared to those of controls (14.7 ± 0.6 IU/L in eNOS +/+ and 17.8 ± 0.5 IU/L in eNOS −/−) and eNOS +/+; sFlt1 mice (227.2 ± 26.4 IU/L) (Fig. 1d). These data were specific to sFlt1 overexpression as GFP overexpressing control mice did not demonstrate any hepatic phenotype (Fig. 1a–d).
Figure 1.
Liver dysfunction induced by excessive sFlt1 in mice lacking eNOS. (a) Liver weight (Wt). (b) Liver Wt/body weight (BW). Excessive sFlt1 causes hepatomegaly. The levels of plasma Aspartate transaminase (AST) and Alanine transaminase (ALT) are severely increased in eNOS −/−; sFlt1 mice (c,d) n = 7–9. Data are shown as mean ± s.e.m. ANOVA or Kruskal-Wallis test.
Histological damage and inflammation in the liver
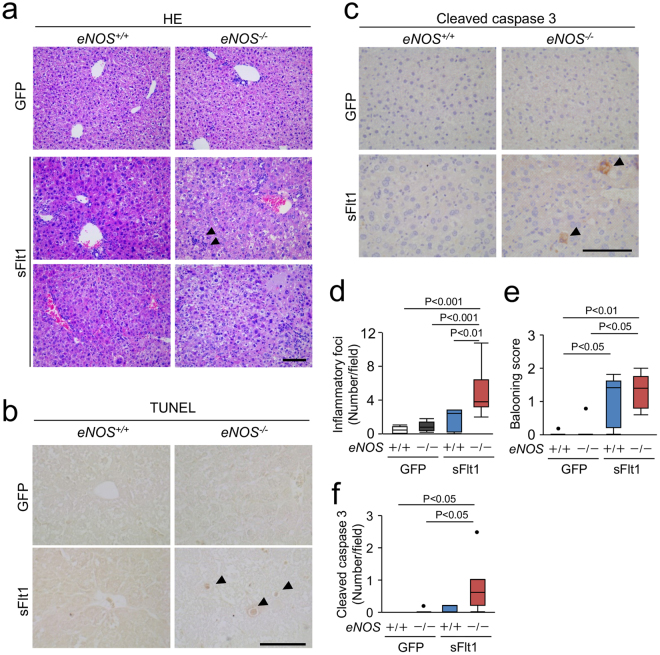
Since lack of eNOS in mice with excessive sFlt1 further increased the levels of ALT and AST, we next performed pathological analysis of the liver. Figure 2a–c shows the representative hepatic photomicrographs of Hematoxylin-Eosin stain, TdT-mediated dUTP nick end labeling (TUNEL) stain and immunohistochemistry against cleaved caspase 3. The liver from eNOS −/−; sFlt1 mice exhibited hepatocyte ballooning accompanied by vacuolar degeneration, necrotic lesion, and infiltration of inflammatory cells (Fig. 2a). The number of inflammatory foci was significantly increased in eNOS −/−; sFlt1 mice compared to that of control and eNOS +/+; sFlt1 mice (Fig. 2d). There was an increase in the hepatocyte ballooning score with excessive sFlt1 in both eNOS +/+ mice and eNOS −/− mice (Fig. 2e). The cleaved caspase 3 positive hepatocytes were frequently observed in the liver from eNOS −/−; sFlt1 mice (Fig. 2f).
Figure 2.
Histological damage in the liver. (a) Representative photomicrographs of Hematoxylin Eosin (HE). Inflammatory foci (arrowheads), vacuolar degeneration, and necrosis are shown in the liver from eNOS −/−; sFlt1 mice. TUNEL (b) and immunohistochemistry against cleaved caspase 3 (c) in the liver. Scale bar indicates 100 µm. (d) The number of inflammatory foci is significantly increased in the liver from eNOS −/−; sFlt1 mice. (e) The score of ballooning hepatocytes. (f) Increased cleaved caspase 3 positive cells in the liver from eNOS −/−; sFlt1 mice. n = 5–8. Data are shown as box plot. ANOVA or Kruskal-Wallis test.
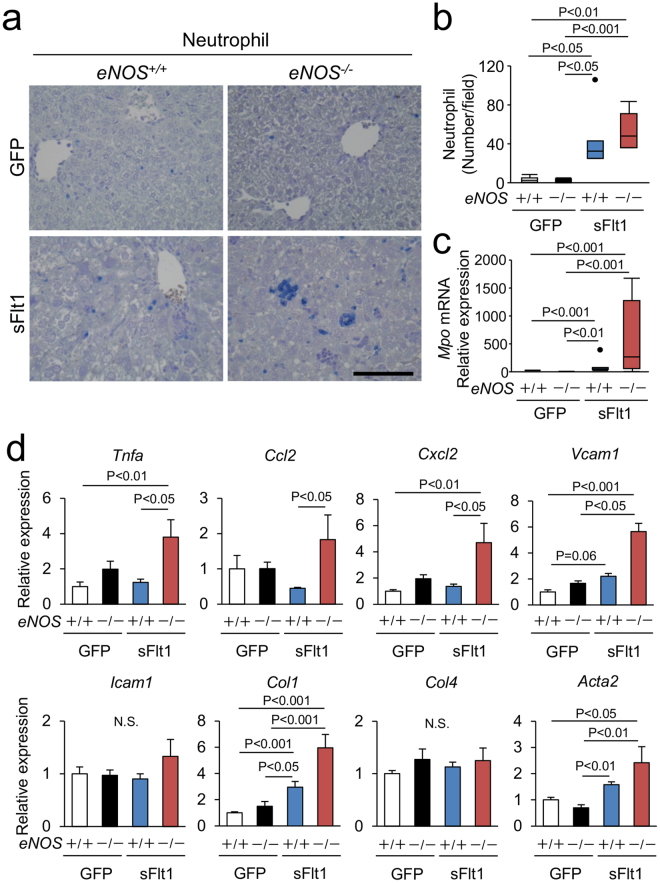
To further analyze the inflammation in the liver, we tested the changes in neutrophil infiltration and the expression of proinflammatory and profibrotic genes in the liver. Excessive sFlt1 in the eNOS −/− mice increased the number of infiltrating neutrophils (Fig. 3a,b). The levels of hepatic myeloperoxidase (Mpo) mRNA in the eNOS −/−; sFlt1 mice were more than 500 fold higher than that of the eNOS +/+ mice (Fig. 3c), whereas excessive sFlt1 per se did not affect macrophage infiltration (Supplementary Figure 2a,b). As shown in Fig. 3d, lack of eNOS elevated the expression levels of Tnfa, Ccl2, Cxcl2 and Vcam1 only in the setting of excessive sFlt1. Similarly, the levels of pro-fibrotic genes, Col1 and Acta2, were elevated in the eNOS −/−; sFlt1 mice (Fig. 3d). These findings indicate that in the setting of excessive sFlt1, lack of eNOS exacerbates histological damage and inflammation in the liver.
Figure 3.
Inflammation in the liver. (a,b) Infiltrating neutrophils are visualized by Naphthol AS-D chloroacetate Esterase stain (blue). Scale bar indicates 100 µm. Number of neutrophil is increased by excessive sFlt1, which is further up-regulated by eNOS deletion. (c) The level of Mpo (myeloperoxidase) mRNA drastically increased in the liver from eNOS −/−; sFlt1 mice. (d) Expression of inflammation and pro-fibrotic related genes in the liver. N.S., not significant. n = 7–8. Data are shown as mean ± s.e.m or box plot. ANOVA or Kruskal-Wallis test.
Oxidative stress and hypoxia
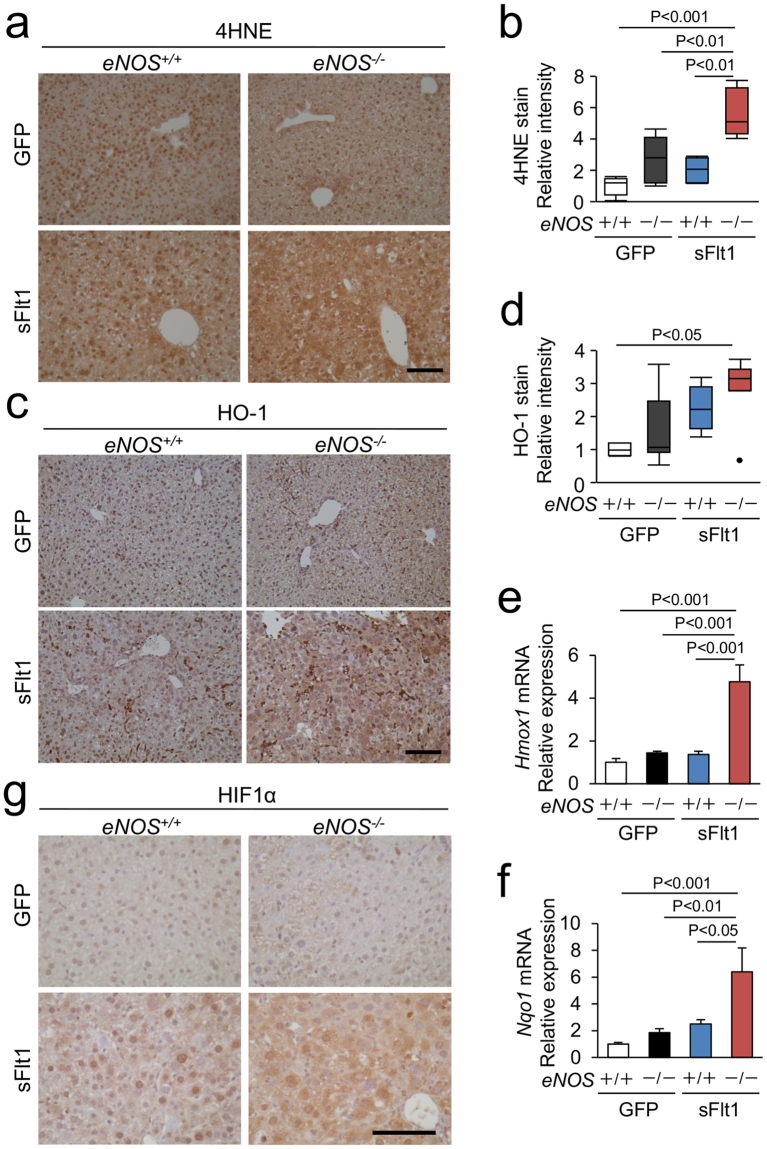
Inhibition of VEGF or of eNOS exacerbates hepatic hypoxia pathways2,16, which increases oxidative stress and promotes liver injury17. Accordingly, we examined oxidative stress and hypoxia in this model. Intensity of immunoreactive 4-hydroxy-2-nonenal (4HNE) in the liver from the eNOS −/−; sFlt1 mice was significantly higher than that from the eNOS +/+ mice with or without excessive sFlt1, suggesting that oxidative stress is increased in the liver from the eNOS −/−; sFlt1 mice (Fig. 4a,b). The protein and gene expression of HO-1 (Hmox1), an anti-oxidative enzyme, was increased in the liver from the eNOS −/−; sFlt1 mice (Fig. 4c–e). Similarly, gene expression of Nqo1 was up-regulated (Fig. 4f). Strong immunoreactive hypoxia inducible factor 1α (HIF1α) was observed in the liver from the eNOS −/−; sFlt1 mice (Fig. 4g). Moreover, the gene expression of Glut1 and Epo, other typical target genes of HIF, was significantly upregulated in the eNOS −/−; sFlt1 mice (Supplementary Figure 3a,b). We conclude that lack of eNOS in mice with excessive sFlt1 aggravates oxidative stress and hypoxia, which likely induces severe liver injury.
Figure 4.
Markers of oxidative stress and hypoxia in the liver. (a) Representative photomicrographs of immunohistochemistry against 4-hydroxy-2-nonenal (4HNE). (b) Strong immunoreactive 4HNE is shown in the liver from eNOS −/−; sFlt1 mice. (c) Representative photomicrographs of immunohistochemistry against HO-1. (d) Strong immunoreactive HO-1 is shown in the liver from eNOS −/−; sFlt1 mice. (e,f) The levels of Hmox1 and Nqo1 mRNA in the liver. (g) Representative photomicrographs of immunohistochemistry against Hypoxia inducible factor 1α (HIF1α) in the liver. n = 5–8. Data are shown as mean ± s.e.m or box plot. ANOVA or Kruskal-Wallis test.
Lipid metabolism in the liver
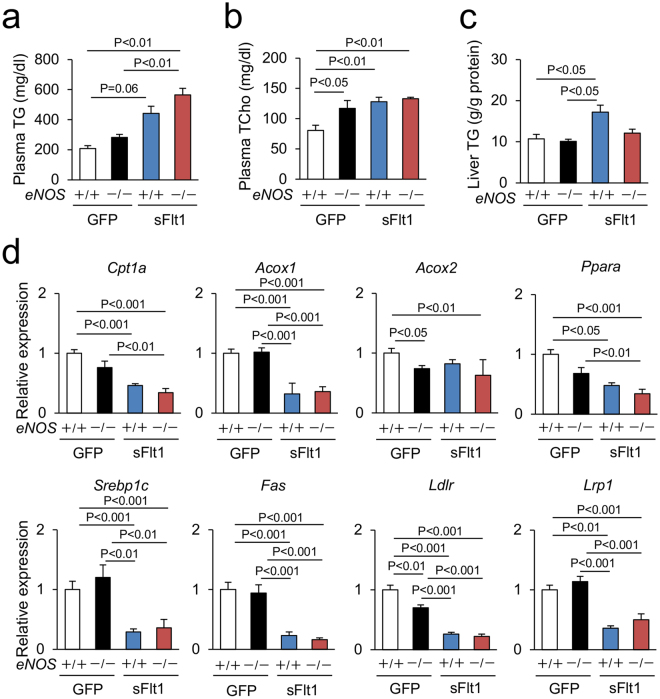
Liver plays a central role in lipid metabolism, and dyslipidemia is commonly observed in preeclamptic women18,19. Accordingly, we quantified lipid parameters in the four groups of mice. Excessive sFlt1 increased the levels of plasma triglyceride, plasma total cholesterol, and liver triglyceride content in the wild type eNOS mice, but lack of eNOS did not affect these parameters in mice with excessive sFlt1 (Fig. 5a–c). The expression levels of key regulators of hepatic lipid metabolism, fatty acid oxidation (Cpt1a, Acox1, and Ppara), lipogenesis (Srebp1c and Fas), and lipoprotein clearance receptors (Ldlr and Lrp1) were reduced by excessive sFlt1, but lack of eNOS did not affect them (Fig. 5d).
Figure 5.
Lipid metabolism in the liver. (a–c) Plasma triglyceride (TG), total cholesterol (TCho), and hepatic TG. (d) Expression of Fatty acid oxidation, lipogenesis, and lipoprotein clearance receptor related genes in the liver, which are down-regulated by excessive sFlt1. n = 7–8. Data are shown as mean ± s.e.m. ANOVA or Kruskal-Wallis test.
Thrombocytopenia induced by excessive sFlt1 in mice lacking eNOS
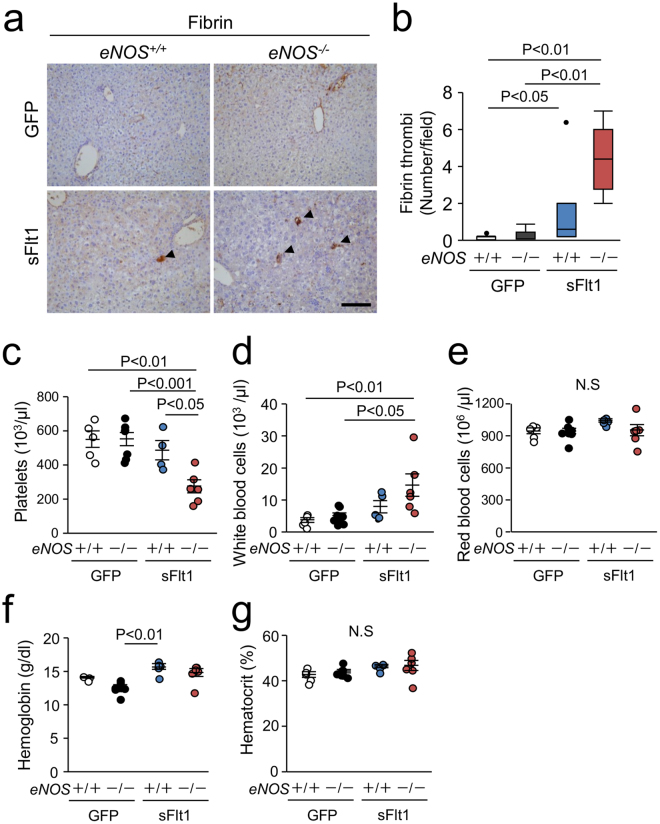
Because VEGF inhibitor therapies and severe preeclampsia is characterized by hematological abnormalities20,21, we next examined hematological parameters in this model. The number of fibrin thrombi was significantly higher in the liver from the eNOS −/−; sFlt1 mice than that from other three groups of mice (Fig. 6a,b). Complete blood count showed reduced platelet number and increased white blood cell number in the eNOS −/−; sFlt1 mice (Fig. 6c,d). The red blood cell count and hematocrit were similar among the groups (Fig. 6e–g). These findings indicate that lack of eNOS in mice with excessive sFlt1 caused hypercoagulability and thrombocytopenia. Although inhibiting VEGF causes thrombotic microangiopathy and hemolytic anemia22,23, excessive sFlt1 and lack of eNOS did not cause anemia. Consistent with this observation, the level of plasma haptoglobin, a marker of hemolysis, was not statistically different between the groups (Supplementary Figure 4). Moreover, schistocytes were not observed in their smears (data not shown). We conclude that lack of eNOS in the context of excessive sFlt1 exacerbates hypercoagulability and thrombocytopenia without obvious hemolysis.
Figure 6.
Fibrin deposition in the liver and thrombocytopenia. (a) Representative photomicrographs of immunohistochemistry against Fibrin. Scale bar indicates 100 µm. (b) Number of Fibrin thrombi is significantly increased in the liver from eNOS −/−; sFlt1 mice. (c–g) Data of blood count; platelets (c), white blood cells (d), red blood cells (e), hemoglobin (f), and hematocrit (g). Excessive sFlt1 combined eNOS deletion causes thrombocytopenia. N.S., not significant. n = 4–8. Data are shown as mean ± s.e.m or box plot. ANOVA or Kruskal-Wallis test.
Discussion
We have demonstrated that the lack of eNOS in the presence of excessive sFlt1 exacerbates hepatic injury and causes hypercoagulability and thrombocytopenia. Our data show that the livers from the eNOS −/−; sFlt1 mice have enhanced hepatic inflammation, prominent neutrophil infiltration, and increased oxidative stress and the expression of genes induced by hypoxia. Literature shows that nitric oxide (NO) derived from eNOS is anti-inflammatory in vitro and in vivo 16,24–26. NO-donor directly reduces the expression levels of hypoxia-induced cytokines and chemokines in HepG2 cells24. Lack or inhibition of eNOS exacerbates hepatic inflammation in obesity and ischemic models16,25,26. Moreover, hepatocyte specific deletion of VEGF causes hypoxia2,27, which causes tissue injury mediated by HIF28. Up-regulated HIF1α and HIF2α increase hepatic inflammation, and contribute to alcoholic or non-alcoholic liver disease and acetaminophen induced liver injury29–31. Consistent with these findings, our data suggest that inflammation, hypoxia, and oxidative stress could be an important pathogenic factor in the exacerbation of liver injury in setting of reduced VEGF signaling and impaired NO production.
In our experimental condition, adenovirus increased plasma sFlt1 concentration to ~1.0 × 104 ng/ml (Supplementary Figure 5). Previous report demonstrated that such a high level of sFlt1 almost completely inhibits VEGF signaling10. Furthermore, the lack of any phenotype in the control adenoviral group suggests that the phenotype induced by sFlt1 is specific to VEGF inhibition. Moreover, eNOS dysfunction is likely crucial to the onset or exacerbation of VEGF inhibitor-induced liver injury, because wild type eNOS mice with extremely excessive sFlt1 did not show hepatic damage.
The patients with PE and HELLP syndrome have elevated levels of serum triglyceride and fatty acid compared to those of normal pregnancy18,19,32, suggesting that inhibiting VEGF is associated with abnormal lipid metabolism in the liver. In accordance with this finding, our data indicate that sFlt1 overexpression increases the levels of plasma triglyceride and total cholesterol. However, lack of eNOS did not further exacerbate these parameters (Fig. 5). Literature shows that hepatocyte specific inhibition of triglyceride-rich lipoprotein clearance receptors Ldlr or Lrp, elevated plasma lipoprotein33, and that skeletal muscle and adipose tissue actively regulates lipoprotein clearance34. Consistent with these findings, excessive sFlt1 reduced the expression levels of Ldlr and Lrp1 in the liver (Fig. 5d). Despite liver damage and abnormal lipid profile in the plasma, the liver did not show increased triglyceride content in the eNOS −/−; sFlt1 mice. It is likely that reduced fatty acid oxidation in the liver suppresses lipogenesis and lipid uptake in the liver, leading to increased plasma triglyceride levels. There was no remarkable effect of lack of eNOS on lipid metabolism in the liver.
Thrombocytopenia is a characteristic feature of VEGF inhibitor-induced thrombotic microangiopathy22, but its pathogenesis remains unclear. Previous report demonstrates that excessive sFlt1 together with lack of Adamts13 develops hemolysis and thrombocytopenia in mice35. Pregnant mice with excessive sFlt1 and soluble endoglin mimic features of human HELLP syndrome8. Our data and these findings suggest that excessive sFlt1 alone is not sufficient to cause thrombocytopenia. Because NO derived from eNOS inhibits platelet activation24,36, we suggest that reduced NO from eNOS causes thrombocytopenia when VEGF is inhibited.
Stringent VEGF inhibition increases hepatocyte erythropoiesis and polycythemia, which is mediated by increased erythropoietin production due to HIF2 activation10,37. But this was not evident in our model, although the levels of Epo mRNA in the liver were elevated with excessive sFlt1 (Supplementary Figure 4b). Overexpression of both sFlt1 and soluble endoglin displays the phenotype of HELLP syndrome including hemolysis8. Excessive sFlt1 together with lack of eNOS is not sufficient to cause hemolysis, and overexpression of both sFlt1 and soluble endoglin is likely necessary for hemolysis to cause HELLP syndrome. However, some preeclamptic patients have liver injury and thrombocytopenia without hemolysis38, and our model could explain the pathogenesis of these patients.
sFlt1 is known to inhibit PlGF signaling. However, PlGF is largely made during pregnancy and at least 7–8 folds lower in non-pregnant states39. Moreover, lack of Plgf does not affect normal angiogenesis, and PlGF blockade rather ameliorates liver fibrosis and inflammation in cirrhotic mice40–42. Hepatic expression of PlGF is undetectably low in our preliminary observation and in prior reports40,42. Accordingly, we believe inhibition of PlGF does not contribute to exacerbation of liver toxicity by excessive sFlt1 in our model. However, whether this is true during pregnancy where PlGF is abundantly made needs additional studies.
We used non-pregnant mice with excessive sFlt1 because increased sFlt-1 recapitulates the phenotype of maternal syndrome of preeclampsia regardless of pregnancy in rodent models6,15. However, sFlt1 explains only some aspects of the pathogenesis of preeclampsia. Various factors including endoglin, endothelin, catechol-O-methyltransferase, or angiotensin-II are likely involved in endothelial dysfunction in pregnant or preeclampsia condition8,15,43,44. Their interaction with eNOS and the role in hepatic injury should be clarified in the future.
In conclusion, we have demonstrated that hepatotoxicity of sFlt1 is exacerbated by lack of eNOS. Further studies should evaluate the nitric oxide independent pathways induced by VEGF inhibition. These findings might open a novel role of hepatocyte-endothelial communication in the liver homeostasis and underling mechanism of liver injury induced by impaired VEGF signaling.
Methods
Animals
All experiments were conducted in compliance with the guidelines of Tohoku University. Experimental protocol was approved by the Institutional Animal Care and Use Committee at Tohoku University. Ten to fourteen-week-old non-pregnant female eNOS −/− mice with C57BL/6 J genetic background were injected with 1 × 109 PFU adenovirus to overexpress sFlt1 (Adeno sFlt1) or adenovirus encoding GFP protein (Adeno GFP) at equivalent doses as we previously described15,45. These mice were maintained for 7 days. Previous studies have shown that increased sFlt-1 recapitulates the phenotype of preeclampsia regardless of whether the animal is pregnant6,15,45. Mice cannot maintain pregnancy if excessive sFlt-1 and lack of eNOS are combined (our unpublished observation)15. Accordingly, we used non-pregnant female eNOS −/− mice for these studies.
Biochemical measurement
ELISA kits were used to measure urinary albumin (Exocell Inc., Philadelphia, PA), plasma sFlt1 (R&D Systems Inc, Minneapolis, MN) and plasma haptoglobin (Life Diagnostics, Inc. West Chester, PA). Colorimetric detection kits were used to measure AST, ALT, triglyceride and total cholesterol (Wako chemicals, Osaka, Japan) in plasma and liver homogenate. Urinary creatinine was determined by the method we developed using LC-MS/MS46.
Blood count
Blood was collected with EDTA and analyzed using Microsemi LC-662 (Horiba, Japan).
Quantitative RT-PCR
Total RNA from the liver was extracted using TRI Reagent (Molecular Research Center, Inc., Cincinnati, OH). Hypoxanthine-guanine phosphoribosyltransferase (Hprt) was used as a reference gene as we previously reported47,48. The primers used in this study have been previously described elsewhere. Their sequences are available on request.
Morphological study
Livers were fixed in 2% PFA and embedded in paraffin. The sections 2 μm in thickness were stained with Hematoxylin-Eosin stain to evaluate histological damage. The degree of lobular inflammation was evaluated by counting of inflammatory foci. The degree of Injured hepatocytes was examined using ballooning score as previously described49. Ballooning score was determined according to number of ballooned hepatocytes: 0 (none), 1 (few), and 2 (many). 5 consecutive fields were examined in each slide at 100-fold magnification. All examination was performed under blinded manner.
Immunohistochemistry
For immunohistochemistry, rabbit anti-human cleaved caspase 3 antibody (1:300, Cell Signaling Technology, Danvers, MA), rabbit anti-human hypoxia-inducible factor 1a antibody (1:1000, Novus Biologicals, Littleton, CO), anti-human 4-hydroxy-2-nonenal antibody (10 µg/ml, Japan Institute for the Control of Aging, Japan), rabbit anti-human HO-1 antibody (1:500, Enzo Life Sciences, Farmingdale, NY), rabbit anti-human fibrin/fibrinogen antibody (1:4000, Dako, Denmark), and rat anti-mouse MOMA2 antibody (1:400, AbD Serotec, Raleigh, NC) were used. TUNEL stain kit was from Wako chemicals (Osaka, Japan). Neutrophils were visualized using Naphthol AS-D chloroacetate Esterase stain (Muto Pure Chemicals, Tokyo, Japan). About 5 consecutive fields were examined in each slide at 100 or 200-fold magnification. All assessments were performed with ImageJ (National Institutes of Health, Bethesda, MD).
Statistical Analyses
Multiple groups were compared using two-way ANOVA with the Tukey-Kramer test for parametric values, if necessary logarithm transition was performed. Otherwise, Kruskal-Wallis test with Dunn’s test was used for non-parametric values. All analyses were performed using JMP 11.0.0 (SAS Institute Inc., Cary, NC). Values are presented as mean ± s.e.m or box plot. Differences were considered statistically significant with P < 0.05.
Electronic supplementary material
Acknowledgements
We thank members of Tohoku University, Faculty of Pharmaceutical Sciences, for their assistance and Drs Oliver Smithies and Nobuyo Maeda (The University of North Carolina at Chapel Hill) for their helpful comments. Our work was supported by Grants-In-Aid from the Japan Society for Promotion of Science (JSPS 16J03192), The Kidney Foundation, Japan (JKFB15-14), and Kanzawa Medical Research Foundation, Japan.
Author Contributions
Y.O., K.M., T.F. and E.S. performed experiments. Y.O. and N.T. analyzed data and co-wrote manuscript. H.S., J.S., S.I., and S.A.K. interpreted data and edited manuscript. N.T. contributed to conception of research.
Competing Interests
S.A.K. is a coinventor of several patents related to angiogenic biomarkers that are held by BIDMC. S.A.K. reports serving as a consultant to Thermofisher Scientific, and has financial interest in Aggamin LLC.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-18260-7.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 2.Walter TJ, Cast AE, Huppert KA, Huppert SS. Epithelial VEGF signaling is required in the mouse liver for proper sinusoid endothelial cell identity and hepatocyte zonation in vivo. Am J Physiol Gastrointest Liver Physiol. 2014;306:G849–862. doi: 10.1152/ajpgi.00426.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimizu H, et al. Vascular endothelial growth factor secreted by replicating hepatocytes induces sinusoidal endothelial cell proliferation during regeneration after partial hepatectomy in rats. J Hepatol. 2001;34:683–689. doi: 10.1016/S0168-8278(00)00055-6. [DOI] [PubMed] [Google Scholar]
- 4.Iacovelli R, et al. Incidence and relative risk of hepatic toxicity in patients treated with anti-angiogenic tyrosine kinase inhibitors for malignancy. Br J Clin Pharmacol. 2014;77:929–938. doi: 10.1111/bcp.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hod, T., Cerdeira, A. S. & Karumanchi, S. A. Molecular Mechanisms of Preeclampsia. Cold Spring Harb Perspect Med5, 10.1101/cshperspect.a023473 (2015). [DOI] [PMC free article] [PubMed]
- 6.Maynard SE, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Westbrook RH, Dusheiko G, Williamson C. Pregnancy and liver disease. J Hepatol. 2016;64:933–945. doi: 10.1016/j.jhep.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 8.Venkatesha S, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 9.Mahasreshti PJ, et al. Intravenous delivery of adenovirus-mediated soluble FLT-1 results in liver toxicity. Clin Cancer Res. 2003;9:2701–2710. [PubMed] [Google Scholar]
- 10.Tam BY, et al. VEGF modulates erythropoiesis through regulation of adult hepatic erythropoietin synthesis. Nat Med. 2006;12:793–800. doi: 10.1038/nm1428. [DOI] [PubMed] [Google Scholar]
- 11.Gélinas DS, Bernatchez PN, Rollin S, Bazan NG, Sirois MG. Immediate and delayed VEGF-mediated NO synthesis in endothelial cells: role of PI3K, PKC and PLC pathways. Br J Pharmacol. 2002;137:1021–1030. doi: 10.1038/sj.bjp.0704956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leonardo DP, et al. Association of Nitric Oxide Synthase and Matrix Metalloprotease Single Nucleotide Polymorphisms with Preeclampsia and Its Complications. PLoS One. 2015;10:e0136693. doi: 10.1371/journal.pone.0136693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng F, et al. Associations between nitric oxide synthase 3 gene polymorphisms and preeclampsia risk: a meta-analysis. Sci Rep. 2016;6:23407. doi: 10.1038/srep23407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burke SD, et al. Soluble fms-like tyrosine kinase 1 promotes angiotensin II sensitivity in preeclampsia. J Clin Invest. 2016;126:2561–2574. doi: 10.1172/JCI83918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li F, et al. eNOS deficiency acts through endothelin to aggravate sFlt-1-induced pre-eclampsia-like phenotype. J Am Soc Nephrol. 2012;23:652–660. doi: 10.1681/ASN.2011040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taniai H, et al. Susceptibility of murine periportal hepatocytes to hypoxia-reoxygenation: role for NO and Kupffer cell-derived oxidants. Hepatology. 2004;39:1544–1552. doi: 10.1002/hep.20217. [DOI] [PubMed] [Google Scholar]
- 17.Elias-Miró M, Jiménez-Castro MB, Rodés J, Peralta C. Current knowledge on oxidative stress in hepatic ischemia/reperfusion. Free Radic Res. 2013;47:555–568. doi: 10.3109/10715762.2013.811721. [DOI] [PubMed] [Google Scholar]
- 18.Gallos ID, et al. Pre-eclampsia is associated with, and preceded by, hypertriglyceridaemia: a meta-analysis. BJOG. 2013;120:1321–1332. doi: 10.1111/1471-0528.12375. [DOI] [PubMed] [Google Scholar]
- 19.Enquobahrie DA, et al. Maternal plasma lipid concentrations in early pregnancy and risk of preeclampsia. Am J Hypertens. 2004;17:574–581. doi: 10.1016/j.amjhyper.2004.03.666. [DOI] [PubMed] [Google Scholar]
- 20.Dusse LM, Rios DR, Pinheiro MB, Cooper AJ, Lwaleed BA. Pre-eclampsia: relationship between coagulation, fibrinolysis and inflammation. Clin Chim Acta. 2011;412:17–21. doi: 10.1016/j.cca.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 21.Ma L, et al. In vitro procoagulant activity induced in endothelial cells by chemotherapy and antiangiogenic drug combinations: modulation by lower-dose chemotherapy. Cancer Res. 2005;65:5365–5373. doi: 10.1158/0008-5472.CAN-04-3156. [DOI] [PubMed] [Google Scholar]
- 22.Usui J, et al. Clinicopathological spectrum of kidney diseases in cancer patients treated with vascular endothelial growth factor inhibitors: a report of 5 cases and review of literature. Hum Pathol. 2014;45:1918–1927. doi: 10.1016/j.humpath.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Eremina V, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuo KK, et al. NO donor KMUP-1 improves hepatic ischemia-reperfusion and hypoxic cell injury by inhibiting oxidative stress and pro-inflammatory signaling. Int J Immunopathol Pharmacol. 2013;26:93–106. doi: 10.1177/039463201302600109. [DOI] [PubMed] [Google Scholar]
- 25.Tateya S, et al. Endothelial NO/cGMP/VASP signaling attenuates Kupffer cell activation and hepatic insulin resistance induced by high-fat feeding. Diabetes. 2011;60:2792–2801. doi: 10.2337/db11-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawachi S, et al. Nitric oxide synthase and postischemic liver injury. Biochem Biophys Res Commun. 2000;276:851–854. doi: 10.1006/bbrc.2000.3559. [DOI] [PubMed] [Google Scholar]
- 27.Wei K, et al. A liver Hif-2α-Irs2 pathway sensitizes hepatic insulin signaling and is modulated by Vegf inhibition. Nat Med. 2013;19:1331–1337. doi: 10.1038/nm.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosmorduc O, Housset C. Hypoxia: a link between fibrogenesis, angiogenesis, and carcinogenesis in liver disease. Semin Liver Dis. 2010;30:258–270. doi: 10.1055/s-0030-1255355. [DOI] [PubMed] [Google Scholar]
- 29.Nath B, et al. Hepatocyte-specific hypoxia-inducible factor-1α is a determinant of lipid accumulation and liver injury in alcohol-induced steatosis in mice. Hepatology. 2011;53:1526–1537. doi: 10.1002/hep.24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qu A, et al. Hypoxia-inducible transcription factor 2α promotes steatohepatitis through augmenting lipid accumulation, inflammation, and fibrosis. Hepatology. 2011;54:472–483. doi: 10.1002/hep.24400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sparkenbaugh EM, et al. The role of hypoxia-inducible factor-1α in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2011;338:492–502. doi: 10.1124/jpet.111.180521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wetzka B, et al. Altered lipid metabolism in preeclampsia and HELLP syndrome: links to enhanced platelet reactivity and fetal growth. Semin Thromb Hemost. 1999;25:455–462. doi: 10.1055/s-2007-994950. [DOI] [PubMed] [Google Scholar]
- 33.Foley EM, et al. Hepatic remnant lipoprotein clearance by heparan sulfate proteoglycans and low-density lipoprotein receptors depend on dietary conditions in mice. Arterioscler Thromb Vasc Biol. 2013;33:2065–2074. doi: 10.1161/ATVBAHA.113.301637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams KJ. Molecular processes that handle–and mishandle–dietary lipids. J Clin Invest. 2008;118:3247–3259. doi: 10.1172/JCI35206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erpenbeck L, et al. ADAMTS13 Endopeptidase Protects against Vascular Endothelial Growth Factor Inhibitor-Induced Thrombotic Microangiopathy. J Am Soc Nephrol. 2016;27:120–131. doi: 10.1681/ASN.2014121165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iafrati MD, et al. Compensatory mechanisms influence hemostasis in setting of eNOS deficiency. Am J Physiol Heart Circ Physiol. 2005;288:H1627–1632. doi: 10.1152/ajpheart.00819.2004. [DOI] [PubMed] [Google Scholar]
- 37.Tojo Y, et al. Hypoxia Signaling Cascade for Erythropoietin Production in Hepatocytes. Mol Cell Biol. 2015;35:2658–2672. doi: 10.1128/MCB.00161-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parnas M, et al. Moderate to severe thrombocytopenia during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2006;128:163–168. doi: 10.1016/j.ejogrb.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 39.Kumasawa K, et al. Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc Natl Acad Sci USA. 2011;108:1451–1455. doi: 10.1073/pnas.1011293108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Steenkiste C, et al. Inhibition of placental growth factor activity reduces the severity of fibrosis, inflammation, and portal hypertension in cirrhotic mice. Hepatology. 2011;53:1629–1640. doi: 10.1002/hep.24238. [DOI] [PubMed] [Google Scholar]
- 41.Li, X. et al. Placental growth factor silencing ameliorates liver fibrosis and angiogenesis and inhibits activation of hepatic stellate cells in a murine model of chronic liver disease. J Cell Mol Med, 10.1111/jcmm.13158 (2017). [DOI] [PMC free article] [PubMed]
- 42.Autiero M, Luttun A, Tjwa M, Carmeliet P. Placental growth factor and its receptor, vascular endothelial growth factor receptor-1: novel targets for stimulation of ischemic tissue revascularization and inhibition of angiogenic and inflammatory disorders. J Thromb Haemost. 2003;1:1356–1370. doi: 10.1046/j.1538-7836.2003.00263.x. [DOI] [PubMed] [Google Scholar]
- 43.Zhou CC, et al. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med. 2008;14:855–862. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanasaki K, et al. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature. 2008;453:1117–1121. doi: 10.1038/nature06951. [DOI] [PubMed] [Google Scholar]
- 45.Li F, et al. Nicotinamide benefits both mothers and pups in two contrasting mouse models of preeclampsia. Proc Natl Acad Sci USA. 2016;113:13450–13455. doi: 10.1073/pnas.1614947113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi N, Boysen G, Li F, Li Y, Swenberg JA. Tandem mass spectrometry measurements of creatinine in mouse plasma and urine for determining glomerular filtration rate. Kidney Int. 2007;71:266–271. doi: 10.1038/sj.ki.5002033. [DOI] [PubMed] [Google Scholar]
- 47.Oe Y, et al. Coagulation Factor Xa and Protease-Activated Receptor 2 as Novel Therapeutic Targets for Diabetic Nephropathy. Arterioscler Thromb Vasc Biol. 2016;36:1525–1533. doi: 10.1161/ATVBAHA.116.307883. [DOI] [PubMed] [Google Scholar]
- 48.Hayashi S, et al. Protease-activated receptor 2 exacerbates adenine-induced renal tubulointerstitial injury in mice. Biochem Biophys Res Commun. 2017;483:547–552. doi: 10.1016/j.bbrc.2016.12.108. [DOI] [PubMed] [Google Scholar]
- 49.Brunt EM, Tiniakos DG. Histopathology of nonalcoholic fatty liver disease. World J Gastroenterol. 2010;16:5286–5296. doi: 10.3748/wjg.v16.i42.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.