Abstract
Pediatric dentistry provides primary and comprehensive preventive and therapeutic oral health care for infants and children through adolescence, together with special health care needs. This specialty encompasses a variety of skills, disciplines, procedures and techniques that share a common origin with other dental specialties however these have been modified and reformed to the distinctive requirements of infants, children, adolescents and special health care needs. Disciplines comprise of behavior guidance, care of the medically and developmentally compromised and disabled patient, supervision of orofacial growth and development, caries prevention, sedation, pharmacological management, and hospital dentistry including other traditional fields of dentistry. The skills apply to the ever-changing stages of dental, physical, and psychosocial development for treating conditions and diseases distinctive to growing individuals. Hence with the changing scope of practice it is imperative that the clinician stays updated with the current evidence based trends in practice, collaborates with other disciplines and Imparts quality oral health care tailored to the specific needs of every child.
Keywords: Paediatric dentistry, Developmental anomaly, Pediatric dental management, Advances in dentistry, Lasers
Highlights
-
•
Dental care is medically-necessary because it contributed to the prevention and elimination of orofacial disease, infection, and pain, to restore the form and function of the dentition, and to correct facial disfiguration or dysfunction.
-
•
Collaboration between families, early care and educational professionals, and health care professionals is a prerequisite for effective oral health.
-
•
An understanding of early establishment of a dental home by 12 months ensures awareness of age-specific oral health issues with long term positive effects for the children.
-
•A Professional prophylaxis is indicated to Instruct the caregiver and child or adolescent in pro-per oral hygiene techniques, for removal of dental plaque, extrinsic stain, and calculus deposits from the teeth.
-
•Facilitate the examination of hard and soft tissues.
-
•Introduce dental procedures to the young child and apprehensive patient.
-
•
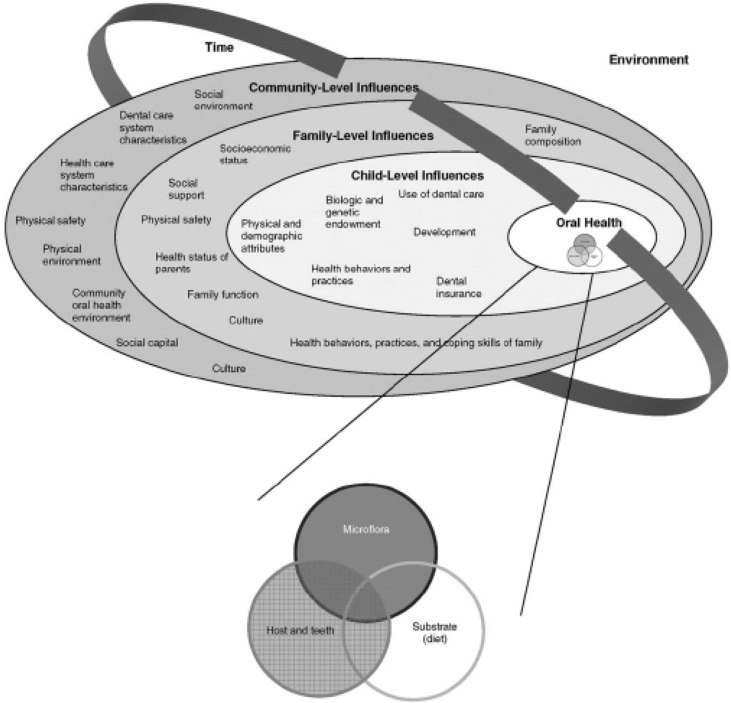
Pediatric dentistry provides primary and comprehensive preventive and therapeutic oral health care for infants and children through adolescence including special health care needs [1]. It assimilates a variety of skills, disciplines, procedures and techniques which have been modified and reformed to the unique requirements of infants, children, adolescents and special health care needs [2]. The disciplines comprise of behavior guidance, care for the medically and developmentally compromised and disabled patient, supervision of orofacial growth and development, caries prevention, sedation, pharmacological management and hospital dentistry including other traditional fields of dentistry. The skills for treating conditions and diseases distinctive to growing individuals apply to the altering stages of dental, physical, and psychosocial development [2] (see Fig. 1).
Fig. 1.
Influence on children's oral health status. From Fisher-Owen SA, Gansky SA, Platt LJ, Weintraub JA, Soobader M-J, Bramlett MD, Newacheck PW. Influences on children's oral health: a conceptual model. Pediatrics 2007; 120: e510-e520.
Oral health is a fundamental part of the overall health and welfare of every infant, child and adolescent because oral diseases affect function, development and quality of life. Child oral health care should be safe, continual, wide-ranging, easily accessible, cost effective, good in quality and reverent of every child and their family [3]. This care precludes and eradicates disease, pain and infection; restores function and form of dentitions and corrects apparent facial dysfunction or disfiguration [4].
The practice of dentistry experiences a new paradigm shift with the advent and use of new technology. New imaging devices, restorative procedures, internet and powerful electronic devices, laser dentistry and new materials are examples of advances impacting dentistry. Although pediatric dentistry may not have as many new tools of treatments their practices have nevertheless improved tremendously in the recent years.
This review is an overview of the evidence based current practices and advances in pediatric dentistry advances and guides the provision of primary prevention, early intervention and reparative care in the primary and permanent dentition.
1. Dental home
A dental home is a continuing relationship between a dentist and a patient encompassing all aspects of oral health care delivery [5]. It considers the patient's age, developmental and psychosocial status with the aim of health promotion, disease prevention and anticipatory guidance in a comprehensive, accessible and family-centered way [5]. This service may be attained via provider training, prevention emphasis and alliance among stakeholders [6]. It should be set up by 6 months of age and no later than 12 months of age [7].
Emergent evidence shows the effectiveness of early founding of a dental home in reducing early childhood caries [8]. A dentist in a dentist-directed care examines, diagnoses oral conditions and tailors a treatment plan including preventive services and all services are carried out under the dentist's supervision alongside allied dental personnel. Affiliating with other health providers like pediatricians, pediatric nurses, family physicians who most often see children during the first years of life improves the oral health of a child [9].
2. Prophylaxis
Dental plaque, stains and calculus are removed by the dentists and hygienists via several approaches. In a toothbrush prophylaxis; toothbrush and toothpaste remove plaque from all the surfaces [10]. In a rubber cup prophylaxis; a dental polishing paste is applied to tooth surfaces by a rotary rubber cup or rotary bristle brushes for eliminating dental plaque and stains [11]. In dental scaling an ultrasonic or hand instrument is used for removal of dental calculus and stains [12].
Professional prophylaxis is beneficial for introducing dental procedures to the young child and apprehensive patient, assessing patient cooperation, facilitating hard and soft tissue examination, instructing the caregiver and child or adolescent in pro-per oral hygiene techniques and removal of plaque, calculus and extrinsic stains. Caries/periodontal disease are the determinants of recall interval [12].
3. Fluoride
Fluoride favors caries risk reduction and reversal of enamel demineralization in a safe and effective manner [13]. Topical cariostatic effect [14] may be better when supplemented with good oral hygiene practices like complete tooth brushing with a fluoride toothpaste [15]. Adjusting fluoride level in community water supply to an optimal concentration reduces caries occurrence [16]. Currently recommended fluoride level in community water supply is 0.7 ppm [17].
The anti-caries effect of professional topical fluoride treatments is exhibited by various measures including 1.23% acidulated phosphate fluoride, 5% neutral sodium fluoride varnish, 0.09% fluoride mouthrinse and 0.5% fluoride gel/paste [18]. The content of fluoride in ready-to-use infant formulas in the U.S. and Canada ranges from 0.1 to 0.3 mg/L [19]. Fluoridated milk and fluoridated salt could be considered for high caries prevalence and low compliance for tooth brushing in areas without water fluoridation.
Fluoride tablets (0.25 mg NaF) and fluoride drops may be recommended for children at high risk of caries on an individual basis. Topical fluorides (gels, varnishes, and rinses) should be used in children at an increased caries risk and for children with special oral health care needs [20]. Gels for professional use contain 5000–12,500 ppm F [21]. Rinses for home use or at schools on a daily basis contain 0.05% NaF (225 ppm F); and weekly basis contain 0.2% NaF (900 ppm F) [21]. Varnishes for professional use contain 1000–56,300 ppm Fluoride [21].
The duration of tooth brushing should surpass one minute on each brushing, excess toothpaste spat out and water rinses avoided. Teeth may be brushed with either manual or powered toothbrushes with a soft small head. The recommended fluoride concentration for toothpaste in children 6 months- <2 years is 500 ppm, twice a day and as a pea sized amount. For children 2-<6 years of age use a1000 (+) ppm toothpaste twice a day as a pea-sized amount. For 6 years and over 1450 ppm use twice daily ranging between 1-2 cm [22].
4. Interim therapeutic restoration
ITR is performed when traditional cavity preparation and/or placement of dental restorations are not possible or when caries control is necessary prior to a definitive dental restoration [23]. The tooth is prepared by removing caries without pulpal exposure by hand or rotary instruments and restored with an adhesive restorative material like glass ionomer or resin-modified glass ionomer cement [24]. Highest success is achieved on applying to single surface or small two surface restorations [25]. Inadequate cavity preparation with subsequent lack of retention and insufficient bulk can lead to failure [26,27]. Leakage of a restoration can be minimized by maximum caries removal from the margin of the lesion.
Oral hygiene instructions and follow-up care with topical fluorides may improve the treatment outcome in high caries-risk populations, particularly when glass ionomer cements with fluoride releasing and recharging properties are used [28]. ITR reduces levels of cariogenic oral bacteria (e.g., Mutans Streptococci, lactobacilli) instantly after it is placed. This level however may return to pre-procedure counts six months after ITR placement if another treatment is not delivered [29].
5. Bleaching
Internal or external dental whitening for individual teeth of primary and young permanent teeth are indicated for discoloration secondary to a traumatic injury (darkening with devitalization, calcific metamorphosis), anomalies in enamel color of a permanent tooth due to trauma or infection of the primary tooth or intrinsic discoloration/staining (tetracycline staining, fluorosis) [[30], [31], [32], [33], [34], [35]].
This may be accomplished by in-office professional whitening or at-home bleaching modalities (custom trays or over-the-counter products including bleaching gels, whitening strips, brush-on agents, toothpaste, mints, chewing gum, and mouth rinse). Whiteners or bleaching agents (professional-use products) containing peroxide range from 10% carbamide peroxide (3% hydrogen peroxide) to 38% (13 percent hydrogen peroxide). Carbamide peroxide is the most common active ingredient in dentist-dispensed tooth-bleaching products for home-use [36].
In-office bleaching products require rubber dam or a protective gel isolation to protect the gingival soft tissues. Home-use bleaching products contain lower concentrations of hydrogen peroxide or carbamide peroxide [37]. Whitening toothpastes contain polishing or chemical agents which remove extrinsic stains from the tooth surface through gentle polishing, chemically chelating or other non-bleaching action [38].
The side effects from vital tooth bleaching include sensitivity; tissue irritation and increased marginal leakage of an existing restoration [37] whereas internal bleaching of non-vital teeth exhibits external root resorption [39] and ankylosis. Degradation byproducts of hydrogen peroxide or carbamide peroxide result in a hydroxyl-free radicals associated with periodontal tissue damage and root resorption. Hence minimizing the exposure at the lowest effective concentration of hydrogen peroxide or carbamide peroxide is recommended beside sodium perborate mixed with water for bleaching nonvital teeth [40,41].
6. Lasers
Laser is an abbreviation for light amplification by stimulated emission of radiation. An active medium produces photons of energy on stimulation which is transported in a beam with a precise wavelength exclusive to that medium. Lasers are classified by the active medium used for creating the energy. They are a single wavelength and monochromatic since the energy radiated is only by one color [42]. Target or identified oral hard and soft tissues differ in their affinity for absorption of specific wavelengths of laser energy [43].
The main effect of a laser inside the target tissues is photothermal [44]. When the target tissue containing water is raised above 100 degrees centigrade, the water vaporizes leading to soft tissue ablation [45]. Hard dental tissues with minerals and hydroxyapatite crystals donot ablate at this temperature but the steam expands and disperses the material into small particles [43]. They provide dental restorative and soft tissue procedures for infants, children, and adolescents as well as those with special health care needs.
The CO2 is a soft tissue laser is effective in incising, excising, and coagulating soft tissue [45]. The diode laser encompasses a solid active medium and semiconductor crystals of aluminum or iridium, gallium, and arsenic is readily absorbed by pigmented tissues [46]. The Nd:YAG laser containing neodymium ions and crystal of yttrium, aluminum, and garnet [47] is effective in coagulation and hemostasis in soft tissue procedures since it is absorbed by pigmented tissues, minimally by hard tissue as well as by hemoglobin [48]. The Er:YAG comprises of erbium ions and a solid active medium of crystals of yttrium, aluminum, and garnet and the Er,Cr:YSGG encompasses erbium, chromium ions, and a crystal of yttrium, scandium, gallium, and garnet. It facilitates soft tissue procedures, removes caries and prepares enamel, dentin, cementum, and bone [49].
Lasers benefit pediatric dentistry on the basis of discerning and specific interface with diseased tissues [45]. They produce less thermal necrosis of adjacent tissues than electrosurgical instruments [48] obtain hemostasis during soft tissue procedures without sutures in most cases [50] exhibit rapid wound healing with less post-operative discomfort and reduce the need for analgesics [51]; less to no local anesthesia [51] and reduce operator chair time. The property of decontaminating and bactericidal action on tissues entails less antibiotic prescription post-operatively [45,51]. Relief from pain and inflammation concomitant with aphthous ulcers and herpetic lesions without pharmacological intervention is also apparent [50,51].
The erbium lasers performs minimal caries removal because affected carious tissue has a higher water content than the healthy dental tissue [52] and eliminate the vibratory effects of the conventional high-speed hand pieces rendering tooth preparations comfortable and less anxiety provoking for children and adolescents [53]. Analgesic effect of Nd:YAG and erbium lasers on hard tissues eliminate the need of local anesthesia during tooth preparations [54,55].
7. Pain management
Pain is difficult to measure in children due to its subjectivity and often reliant on parents [56].
Children experience pain and exhibit variability in the expression of pain due to the differences in their cognitive and emotional development, medical conditions. Pain scale indicators used with children include the FACES pain scale and the Wong-Baker FACES scale [57]. Pain assessment in special needs children needs observations such as vocalization, facial expressions and body movements [58].
Pain in a dental setup can be effectively managed and relieved by both non pharmacologic and pharmacological pain strategies pre-operatively. Non-pharmacologic behavior therapy includes guided imagery, distraction, play therapy and tell-show-do techniques. Pharmacologic therapy may consist of a hierarchy in terms of adequate local anesthesia, anxiolysis, moderate sedation, or deep sedation regimens [59].
Inadequate pain management may have significant physical and psychological consequences hence it is imperative to recognize and assess pain and document it in the patient's chart to determine a clinical diagnosis, plan a treatment and determine the analgesic requirement for the patient. Familiarity with the medical history aids to avoid prescription of a contraindicated drug and comprehend the consequences, morbidities and toxicities associated with the use of specific therapeutics. Selection of an analgesic depends on the individual patient, the extent of treatment, the duration of the procedure, psychological factors, patient's medical history and physiologic factors such as bleeding disorders, liver problems, or kidney problems [60].
Analgesics should initially be administered on a regular time schedule if moderate to severe pain is considered likely during the first 36 to 48 h and not as needed so as to create stable plasma levels of analgesics and decrease the chance of breakthrough pain [61,62]. Consider non-opioid analgesics [eg, nonsteroidal anti-inflammatory agents (NSAIDs), acetaminophen] as first line agents for acute mild to post-operative pain management. Combining NSAIDs with acetaminophen provides a greater analgesic effect than a single agent alone. Opioid analgesics (Codeine) combined with NSAIDs for treatment of moderate to severe post-operative pain in children and adolescents [63].
8. Antibiotic prophylaxis
Bacteremia, bacteria in the bloodstream, is anticipated following invasive dental procedures. Infective endocarditis is an uncommon but life-threatening complication resulting from bacteremia. Viridans group streptococci, Staphylococcus aureus, enterococcus, pseudomonas, serratia, and candida are some of the microorganisms implicated with IE. Many cases of IE caused by oral microflora can result from bacteremia associated with routine daily activities such as toothbrushing, flossing and chewing. However, antibiotic prophylaxis is recommended with certain dental procedures. An effective antibiotic regimen should be directed against the most likely infecting organism, with antibiotics administered shortly before the procedure. Antibiotics are recommended for cardiac patients at high risk (cardiac valve repair, congenital heart disease, cardiac transplantation with cardiac valvulopathy and previous endocarditis, immunocompromised patients (immunosuppression secondary to HIV, Severe Combined Immunodeficiency syndrome, cancer chemotherapy, hematopoietic stem cell therapy or organ transplant), head and neck cancer, Autoimmune disease, Sickle cell anemia, diabetes, chronic steroid and bisphosphonate therapy or asplenism. It is also prescribed for patients with prosthetic total joint arthroplasty where hematogenous infections may lead to loss of prosthetic joint [[64], [65], [66], [67], [68], [69], [70]].
9. Analgesis/anxiolysis
Analgesia/anxiolysis is the diminution or elimination of pain and anxiety in a conscious patient [72]. The patient is responsive to verbal commands, every vital sign is stable with no significant risk of loss of protective reflexes and with a safe return to pre-procedural mobility. Analgesia/anxiolysis may accelerate the delivery of procedures not particularly uncomfortable but which require the patient to minimize movement [71,73].
Nitrous oxide is a colorless and virtually odorless gas with a faint, sweet smell. This effective analgesic/anxiolytic agent causes central nervous system (CNS) depression and euphoria with a very slight effect on the respiratory system [73,74]. Nitrous oxide has several mechanisms of action. The analgesic effect is initiated by neuronal release of endogeneous opioid peptides with subsequent activation of opioid receptors and descending Gamma-aminobutyric acid type A (GABAA) receptors and noradrenergic pathways modulating nociceptive processing at the spinal level. The anxiolytic effect involves activation of the GABAA receptor either directly or indirectly through the benzodiazepine binding site [75,76]. Since nitrous oxide is 34 times more soluble than nitrogen in blood, diffusion hypoxia may occur. Nitrous oxide causes minor depression in cardiac output while peripheral resistance is slightly increased, hence maintaining the blood pressure [73].
Nitrous oxide inhalation/sedation is a predictable pharmacological approach for reducing or alleviating anxiety, discomfort or pain, enhances patient communication and cooperation, raises the pain threshold, increases tolerance for longer appointments, reduces gagging, potentiates sedative effect and aids the treatment of special needs children. The disadvantages include lack of potency, dependence on psychological reassurance and interference of the nasal hood to anterior maxilla, nitrous oxide pollution and occupational hazard. Contraindications include chronic obstructive pulmonary disease, emotional disturbance, coblamin deficiency, treatment with bleomycin sulphate, methylenetetrahydrofolate reductase deficiency and the first trimester of pregnancy [[74], [75], [76]].
Selection of an appropriately sized nasal hood is important. A flow rate of five to six L/min generally is acceptable to most patients and can be adjusted after observation of the reservoir bag. The bag should pulsate gently with each breath and should not be either over- or underinflated. Introduction of 100 percent oxygen for one to two minutes followed by titration of nitrous oxide in 10 percent intervals is recommended. During nitrous oxide/oxygen analgesia/anxiolysis the concentration of nitrous oxide should not routinely exceed 50 percent. Studies have demonstrated that gas concentrations dispensed by the flow meter vary significantly from the end expired alveolar gas concentrations; it is the latter that is responsible for the clinical effects [75,76]. To achieve sedation, the scavenging vacuum should not be so strong as to prevent adequate ventilation of the lungs with nitrous oxide [77]. A review of records of patients undergoing nitrous oxide-oxygen inhalation sedation demonstrate that the typical patient requires from 30 to 40 percent nitrous oxide to achieve ideal sedation [31]. Nitrous oxide concentration may be decreased during easier procedures (eg, restorations) and increased during more stimulating ones (eg, extraction, injection of local anesthetic).
Side effects such as nausea and vomiting are more likely to be observed when titration is not employed [31]. During treatment, it is important to continue the visual monitoring of the patient's respiratory rate and level of consciousness. The effects of nitrous oxide largely are dependent on psychological reassurance. It is therefore important to continue traditional behavior guidance techniques during treatment. Once the nitrous oxide flow is terminated, 100 percent oxygen should be delivered for five minutes. The patient must return to pretreatment responsiveness before discharge.
Facilities for delivering nitrous oxide/oxygen must be checked for proper gas delivery and fail-safe function checked and calibrated prior to use. Inhalation equipment must have the capacity for delivering 100 percent and never less than 30 percent, oxygen concentration at a flow rate appropriate to the child's size, appropriate scavenging and delivery system to minimize room air contamination and occupational risk should have BLS training and an emergency cart should be available. A positive pressure oxygen delivery system capable of administering greater than 90 percent oxygen at a 10 L/min flow for at least 60 min (650 L, “E” cylinder) must be available. When a self-inflating bag valve mask device is used for delivering positive pressure oxygen, a 15 L/min flow is recommended [[78], [79], [80], [81], [82], [83], [84], [85]].
10. Restorative techniques
A multitude of restorative treatment decisions are available for primary and permanent dentition. These include dental amalgam, glass ionomers, resin modified glass ionomers, conventional glass ionomers, atraumatic/alternative restorative technique (ART), interim therapeutic restoration (ITR), resin infiltration, dental composites, pit and fissure sealants, resin-based sealants, glass ionomer sealants, resin based composite, dental composites, compomers, stainless steel crowns, primary molar, preformed metal crown, strip crowns, pre-veneered crowns and esthetic restorations [86].
The contemporary management of dental caries encompasses identification of an individual risk for caries progression, understanding of the disease process and active surveillance to assess disease progression and manage with appropriate preventive services, supplemented by the most adequate restorative therapy when indicated [87]. Decisions for when to restore carious lesions include clinical criteria of visual detection of enamel cavitation, visual identification of shadowing of the enamel, and/or radiographic recognition of enlargement of lesions over time [88].
The benefits of restorative therapy include removal of cavitation or defects to eliminate areas that are susceptible to caries; stopping the progression of tooth demineralization; restoring the integrity of tooth structure; preventing the spread of infection into the dental pulp; and preventing the shifting of teeth and space loss due to loss of tooth structure. The risks of restorative therapy include lessening the longevity of teeth by making them more susceptible to fracture, recurrent lesions, restoration failure, pulp exposure during caries excavation, future pulpal complications, and iatrogenic damage to adjacent teeth [[89], [90], [91]]. Primary teeth may be more susceptible to restoration failures than permanent teeth [92]. In addition before restoring primary teeth, one needs to consider the time of primary tooth exfoliation.
Stepwise excavation is a two-step caries removal process in which carious dentin is partially removed at the first appointment, leaving caries over the pulp, with placement of a temporary filling. At the second appointment, all remaining carious dentin is removed and a final restoration placed [93]. Partial, or one-step, caries excavation removes part of the carious dentin, but leaves caries over the pulp and subsequently places a base and final restoration [94,95]. No removal of caries before restoration of primary molars in children aged three to 10 years also has been reported [96]. This preserves the tooth structure and pulp vitality.
Pit and fissure sealant are a material placed into the pits and fissures of caries-susceptible teeth that micromechanically bonds to the tooth preventing access by cariogenic bacteria to their source of nutrients [97] thus reduce caries risk in susceptible sites especially primary molars by lowering the number of viable bacteria including streptococcus mutans and lactobacilli [98].
Resin infiltration is an innovative approach primarily to arrest the progression of non-cavitated interproximal caries lesions. The aim of the resin infiltration technique is to allow penetration of a low viscosity resin into the porous lesion body of enamel caries [98]. An additional use of resin infiltration has been suggested to restore white spot lesions formed during orthodontic treatment [99].
Dental amalgam has been the most commonly used restorative material in posterior teeth for over 150 years and is still widely used throughout the world today [100]. Amalgam contains a mixture of metals such as silver, copper, and tin, in addition to approximately 50 percent mercury [101]. Dental amalgam has declined in use over the past decade, perhaps due to the controversy surrounding perceived health effects of mercury vapor, environmental concerns from its mercury content, and increased demand for esthetic alternatives. There is strong evidence that dental amalgam is efficacious in the restoration of Class I and Class II cavity restorations in primary and permanent teeth [100,101].
Composites consist of a resin matrix and chemically bonded fillers [102]. They are classified according to their filler size, because filler size affects polishability/esthetics, polymerization depth, polymerization shrinkage and physical properties. Hybrid resins combine a mixture of particle sizes for improved strength while retaining esthetics [103]. The smaller filler particle size allows greater polishability and esthetics, while larger size provides strength. Flowable resins have a lower volumetric filler percentage than hybrid resins [104]. Enamel and dentin bonding agents decrease marginal staining and detectable margins for the different types of composites. In primary molars, there is strong evidence from randomized controlled trials that composite resins are successful when used in Class I restorations and Class II lesions in primary teeth.
Advancements in conventional glass ionomer formulation led to better properties, including the formation of resin-modified glass ionomers. These products showed improvement in handling characteristics, decreased setting time, increased strength and improved wear resistance. The properties that make GIC favorable for use in children include: chemical bond to both enamel and dentin; thermal expansion similar to that of tooth structure; biocompatibility; uptake and release of fluoride and decreased moisture sensitivity as compared to resins. Resin modified glass ionomer cements (RMGIC), with the acid-base polymerization supplemented by a second resin light cure polymerization is efficacious in primary teeth [105,106].
GIC is recommended in class 1 restorations and class II restorations in primary teeth. Other applications of glass ionomers where fluoride release has advantages are for interim therapeutic restorations (ITR) and the atraumatic/alternative restorative technique (ART). These procedures have similar techniques but different therapeutic goals. ITR may be used in a very young [107] uncooperative or special health care needs [108] for whom traditional cavity preparation and/or placement of traditional dental restorations are not feasible or need to be postponed. Additionally, ITR may be used for caries control in children with multiple open carious lesions, prior to definitive restoration of the teeth [[109], [110], [111]].
Compomers Polyacid-modified resin-based composites or compomers; contain 72 percent (by weight) strontium fluorosilicate glass and the average particle size is 2.5 micrometers. Moisture is attracted to both acid functional monomer and basic ionomer-type in the material. This moisture triggers a reaction that releases fluoride and buffers acidic environments. Considering the ability to release fluoride, esthetic value and simple handling properties of compomer, it can be useful in pediatric dentistry. Compomers can be an alternative to other restorative materials in the primary dentition in Class I and Class II restorations [112,113].
Preformed metal crowns (also known as stainless steel crowns) are prefabricated metal crown forms which adapt to individual teeth and cement with a biocompatible luting agent. They are indicated for the restoring primary and permanent teeth with extensive caries, cervical decalcification, and/or developmental defects (eg, hypoplasia, hypocalcification), when other available restorative materials are likely to fail (eg, interproximal caries extending beyond line angles, patients with bruxism), following pulpotomy or pulpectomy, as fixing abutments for space maintainers, the intermediate restoration of fractured teeth, definitive restorative treatment for high caries-risk children and in patients whose treatment is performed under sedation or general anesthesia [113].
Anterior esthetic restorations are a challenge due to: the small size of the teeth; close proximity of the pulp to the tooth surface; relatively thin enamel; lack of surface area for bonding and child behavior. Treatment options for primary anterior teeth include composites for Class III and Class V restorations and permanent dentition as well as resin modified GIC, strip crowns, pre-veneered stainless steel crowns, preformed stainless steel crowns and open-faced stainless steel crowns for full coverage restorations [114].
11. Pulp therapy
In pulp therapy the primary objective is to maintain the integrity and health of the tooth and supporting tissues whereas the treatment objective is to maintain the vitality of the pulp of a tooth affected by caries, traumatic injury or other causes. Pulp preservation in young primary teeth allows apexogenesis for long term retention of a tooth with a favorable crown root ratio and thick dentinal walls [115].
The indications, objectives, and type of pulpal therapy depend on the pulpal status subject to whether the pulp is vital or non-vital. This is based on the clinical and radiographic diagnosis of normal pulp (symptom free and normally responsive to vitality testing), reversible pulpitis (pulp is capable of healing), symptomatic or asymptomatic irreversible pulpitis (vital inflamed pulp is incapable of healing) or pulp necrosis [116]. Clinical tests which help include palpation, percussion, and mobility and for permanent teeth, electric pulp tests and thermal test are recommended [117,118].
Vital pulp therapy for primary teeth with a diagnosis of a normal pulp or reversible pulpitis entails a protective liner. A protective liner is a liquid applied thinly on the pulpal surface of a deep cavity preparation for providing coverage to the exposed dentin tubules and to serve as a protective barrier between the restorative material or cement and the pulp. Calcium hydroxide, dentin bonding agent and glass ionomer cements liners are usually placed [119,120].
Indirect pulp treatment is instituted in a tooth with a deep carious lesion approximating the pulp but without signs or symptoms of pulp degeneration [115]. Caries encircling the pulp is left behind to avoid pulpal exposure and is subsequently covered with a biocompatible material [121]. A radiopaque liner such as a dentin bonding agent [122], resin modified glass ionomer [123,124], calcium hydroxide [125,126], zinc oxide/eugenol [127], or glass ionomer cement [128,129] are applied over the remaining carious dentin for stimulation of healing and repair.
Direct pulp capping is indicated for a permanent tooth that has a small carious or mechanical exposure in a tooth with a normal pulp [130]. Calcium hydroxide or MTA is placed prior to the definitive restoration [131,132].
Partial pulpotomy for carious exposures involves the removal of inflamed pulp tissue beneath an exposure 1–3 millimeters or deeper to reach healthy pulp tissue and the surrounding dentine. Pulpal bleeding is controlled by irrigation with a bacteriocidal agent such as sodium hypochlorite or chlorhexidineb [133,134] within a few minutes after which the site is covered with calcium hydroxide [[135], [136], [137]] or MTA. Refs. [138,139] followed by resin modified glass ionomer cement [140].
Partial pulpotomy for traumatic exposures (Cvek pulpotomy). Is indicated for a vital, traumatically exposed, young permanent tooth ln which the inflamed pulp tissue beneath an exposure is removed to a depth of one to three millimeters or more to reach the deeper healthy tissue. Pulpal bleeding is controlled via bacteriocidal irrigants such as sodium hypochlorite or chlorhexidine [141], and the site then is covered with calcium hydroxide [142,143], or MTA [144].White, rather than gray, MTA is recommended in anterior teeth to decrease the chance of discoloration. It is independent of the time between treatment and the accident and the size of exposure.
Nonvital pulp treatment Pulpectomy (conventional root canal treatment). In apexified permanent teeth is conventional root canal (endodontic) treatment for exposed, infected, and/or necrotic teeth to eliminate pulpal and periradicular infection. The roof of the pulp chamber is cleared to gain access to the canals and remove all coronal pulp tissue. Following debridement, the canals are disinfected, shaped and obturated in entirety with a biologically-acceptable, nonresorbable filling as close to the cementoenamel junction as possible [145].
Apexification (root end closure) is indicated for inducing root end closure in an incompletely formed permanent non-vital tooth by removal of the coronal andl radicular tissue and placing a biocompatible agent such as calcium hydroxide in the canals for two to four weeks to disinfect. Root end closure is accomplished with an apical barrier such as MTA [146] and conformed by clinical and radiographic diagnosis. If complete closure cannot be accomplished by MTA, an absorbable collagen wound dressing (eg, Colla-Cote®) [147]can be placed at the root end and MTA packed subsequently in the canal space. Gutta percha fill the remaining canal space. If the walls of the canal are thin, fill with MTA or composite resin instead of gutta percha to protect from tooth fracture [148].
11.1. Stem cells
Pulpalal tissue of exfoliating primary teeth and surgically removed third molars may be a source of mesenchymal stem cells [149]. While sources of dental stem cells are readily accessible, those cells must be attained with consent, secured and stored properly to maintain the potential to proliferate and differentiate [150] for autologous regenerative therapies.
12. Radiographs
They aid diagnosis of disease and dentofacial development in oral health of children across all age groups. Good radiological practices (eg, use of lead apron, thyroid collars, and high-speed film; beam collimation) are important. The benefits of obtaining radiographs against the patient's risk of radiation exposure must be weighed. New imaging technologies [ie, cone beam computed tomography (CBCT)] have added three-dimensional capabilities to the practice. Posterior bitewings, periapical xrays, panoramic exam, Occlusal x rays are tailored to the caries risk and type of clinical presentation [151].
13. Pulpotomy and pulpal medicaments
The pulpotomy procedure is performed in a primary tooth with extensive caries when removal results in pulpal exposure but without evidence of radicular pathology or pulp exposure due to mechanical trauma. The coronal pulp tissue is amputated, and the remaining vital radicular pulp tissue surface judged to be vital without suppuration, purulence, necrosis or excessive haemorrhage uncontrolled after few minutes is treated with a long-term clinically-successful medicament such as Buckley's Solution of formocresol or ferric sulfate [[152], [153], [154], [155]]. Calcium hydroxide has less long term success [156]. MTA is a recent pulpotomy medicament with a high rate of success. Clinical trials show that MTA performs equal to or better than formocresol or ferric sulfate [157,158] and may be the preferred pulpotomy agent in the future.
Electrosurgery is another method which demonstrates success [159]. After filling the coronal pulp chamber with zinc/oxide eugenol or other suitable base, the tooth is restored with a restoration that seals the tooth and prevents microleakage. Stainless steel crown is the most long term restoration but if sufficient supporting enamel remains, amalgam or composite resin may offer functional alternative given that the primary tooth has a life span of two years or less [[160], [161], [162]].
Hence with the changing scope of practice it is imperative that the clinician stays updated with the current evidence based trends in practice, collaborates with other disciplines and Imparts quality oral health care tailored to the specific needs of every child.
Ethical approval
N/A.
Funding
N/A.
Author contribution
Self.
Conflicts of interest
The authors whose names are listed immediately below certify that they have NO affi liations with or involvement in any organization or entity with any fi nancial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-fi nancial interest (such as personal or professional relationships, affi liations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Guarantor
Self.
N/A.
Registration of research studies
researchregistry3071.
Consent
N/A.
References
- 1.American Dental Association Commission on Dental Accreditation . 2013. Accreditation Standards for Advanced Specialty Education Programs in Pediatric Dentistry. Chicago, Ill. [Google Scholar]
- 2.American Academy of Paediatric Dentistry Reference Manual V 38/N O 6 1 6/17. 2015. [Google Scholar]
- 3.American Academy of Pediatric Dentistry Core Values. 2009. [PubMed] [Google Scholar]
- 4.American Academy of Paediatric Dentistry Definition of Medically Necessary Care. 2015. [Google Scholar]
- 5.American Academy of Pediatric Dentistry Definition of dental home. Pediatr. Dent. 2014;36(special issue):12. [Google Scholar]
- 6.American Academy of Pediatric Dentistry Reference Manual Definition of dental home. Pediatr. Dent. 2007;29(7):10. [Google Scholar]
- 7.Academy of General Dentistry. When Should My Child First See a Dentist? Available at: “http://www.knowyourteeth.com/infobites/abc/article/?abc=c&iid=296&a id=1186”. Accessed August 22, 2014.
- 8.Lee J.Y., Bouwens T.J., Savage M.F., Vann W.F., Jr. Examining the cost-effectiveness of early dental visits. Pediatr. Dent. 2006;28(2):102–105. discussion 192–198. [PubMed] [Google Scholar]
- 9.American Academy of Paediatric Dentistry Policy on Workforce Issues and Delivery of Oral Health Care Services in a Dental Home. 2014. [PubMed] [Google Scholar]
- 10.Ramos-Gomez F., Crystal Y.O., Ng M.W., Tinanoff N., Featherstone J.D. Caries risk assessment, prevention, and management in pediatric dental care. Gen. Dent. 2010;58(6) 505-17; quiz 518–519. [PubMed] [Google Scholar]
- 11.Wilkins E.M. tenth ed. Lippincot Williams and Wilkins; Baltimore, Md: 2009. Clinical Practice of Dental Hygiene; pp. 728–735. [Google Scholar]
- 12.American Academy of Paediatric Dentistry Policy on the Role of Dental Prophylaxis in Pediatric Dentistry. 2012. [Google Scholar]
- 13.American Academy of Paediatric Dentistry Policy on Use of Fluoride. 2014. [Google Scholar]
- 14.Featherstone J.D. Prevention and reversal of dental caries: role of low level fluoride. Community Dent. Oral Epidemiol. 1999;27:31–40. doi: 10.1111/j.1600-0528.1999.tb01989.x. [DOI] [PubMed] [Google Scholar]
- 15.Rolla G., Ogaard, Cruz R.D.A. Clinical effect and mechanism of cariostatic action of fluoride- containing toothpastes: a review. Int. Dent. J. 1991;41:171–174. [PubMed] [Google Scholar]
- 16.CDC Recommendations for using fluoride to prevent and control dental caries in the United States. MMWR Recomm. Rep. 2001;50(RR14):1–42. [PubMed] [Google Scholar]
- 17.Department of Health and Human Services . January 7, 2011. News Re-lease: HHS and EPA Announce New Scientific Assessments and Actions on Fluoride.http://yosemite.epa.gov/opa/admpress.nsf/3881d73f4d4a a a 0 b 8 5 2 5 7 3 5 9 0 0 3 f 5 3 48/8 6 9 6 4 a f 577c37ab28525781100 5a8417!OpenDocument Available at: [Google Scholar]
- 18.Weyant R.J., Tracy S.L., Anselmo T., Beltrán-Aguilar E.J., Donly K.J., Frese W.A. Topical fluoride for caries prevention: executive summary of the updated clinical recommendations and supporting systematic review. J. Am. Dent. Assoc. 2013;144(11):1279–1291. doi: 10.14219/jada.archive.2013.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foman S.J., Ekstrand J. Fluoride intake. In: Fejerskov O., Ekstrand J., Burt B.A., editors. Fluoride in Dentistry. second ed. Munksgaard; Copenhagen: 1996. pp. 40–52. [Google Scholar]
- 20.European Archives of Paediatric Dentistry Guidelines on the use of fluoride in children: an EAPD policy document. 2009;10(3) doi: 10.1007/BF03262673. [DOI] [PubMed] [Google Scholar]
- 21.Poulsen S., Gadegaard E., Mortensen B. Cariostatic effect of daily use of a fluoride-containing lozenge compared to fortnightly rinses with 0.2% sodium fluoride. Caries Res. 1981;15:236–242. doi: 10.1159/000260519. [DOI] [PubMed] [Google Scholar]
- 22.Marinho V.C., Higgins J.P., Logan S., Sheiham A. Fluoride toothpastes for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2003;(1) doi: 10.1002/14651858.CD002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Academy of Pediatric Dentistry Clinical guide-line on pediatric restorative dentistry. Pediatr. Dent. 2012;34(special issue):173–180. [Google Scholar]
- 24.Yip H.K., Smales R.J., Ngo H.C., Tay F.R., Chu F. Selection of restorative materials for the atraumatic restorative treat-ment (ART) approach: a review. Spec. Care Dent. 2001;21(6):216–221. doi: 10.1111/j.1754-4505.2001.tb00257.x. [DOI] [PubMed] [Google Scholar]
- 25.Mandari G.J., Frencken J.E., van't Hof M.A. Six-year success rates of occlusal amalgam and glass-ionomer restorations placed using three minimal intervention approaches. Caries Res. 2003;37(4):246–253. doi: 10.1159/000070866. [DOI] [PubMed] [Google Scholar]
- 26.da Franca C., Colares V., Van Amerongen E. Two-year evaluation of the atraumatic restorative treatment approach in primary molars class I and II restorations. Int. J. Paediatr. Dent. 2011;21(4):249–253. doi: 10.1111/j.1365-263X.2011.01125.x. [DOI] [PubMed] [Google Scholar]
- 27.van Gemert-Schriks M.C.M., van Amerongen W.E., ten Cate J.M., Aartman I.H.A. Three-year survival of single- and two-surface ART restorations in a high-caries child population. Clin. Oral Investig. 2007;11(4):337–343. doi: 10.1007/s00784-007-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tam L.E., Chan G.P., Yim D. In vitro caries inhibition effects by conventional and resin modified glass ionomer restorations. Oper. Dent. 1997;22(1):4–14. [PubMed] [Google Scholar]
- 29.Roshan N.M., Shigli A.L., Deshpande S.D. Microbiological evaluation of salivary Streptococcus mutans from children of age 5-7 years, pre- and post-atraumatic restorative treat-ment. Contemp. Clin. Dent. 2010;1(2):94–97. doi: 10.4103/0976-237X.68602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zekonis R., Matis B.A., Cochran M.A., Al Shetri S.E., Eckert G.J., Carlson T.J. Clinical evaluation of in-office and at-home bleaching treatments. Oper. Dent. 2003;28(2):114–121. [PubMed] [Google Scholar]
- 31.Abbott P., Heah S.Y. Internal bleaching of teeth: an analysis of 255 teeth. Aust. Dent. J. 2009;54(4):326–333. doi: 10.1111/j.1834-7819.2009.01158.x. [DOI] [PubMed] [Google Scholar]
- 32.Matis B.A., Wang Y., Jiang T., Eckert G.J. Extended at-home bleaching of tetracycline-stained teeth with differ-ent concentrations of carbamide peroxide. Quintessence Int. 2002;33(9):645–655. [PubMed] [Google Scholar]
- 33.Kugel G., Gerlach R.W., Aboushala A., Ferreira S., Magnuson B. Long-term use of 6.5% hydrogen peroxide bleaching strips on tetracycline stain: a clinical study. Comp. Cont. Educ. Dent. 2011;32(8):50–56. [PubMed] [Google Scholar]
- 34.Bizhang M., Muller M., Phark J.H., Barker M.L., Gerlach R.W. Clinical trial of long-term color stability of hydrogen peroxide strips and sodium percarbonate film. Am. J. Dent. 2007;20(Spec No A):23A–27A. [PubMed] [Google Scholar]
- 35.Donly K.J., Gerlach R.W. Clinical trials on the use of whitening strips in children and adolescents. Gen. Dent. 2002;50(3):242–245. [PubMed] [Google Scholar]
- 36.Metz M.J., Cochran M.A., Batis B.A., Gonzalez C., Platt J.A., Pund M.R. Clinical evaluation of 15% carbamide peroxide on the surface microhardness and shear bond strength of human enamel. Oper. Dent. 2007;32(5):427–436. doi: 10.2341/06-142. [DOI] [PubMed] [Google Scholar]
- 37.Dawson P.F., Sarif Mo, Smith A.B., Brunton P.A. A clinical study comparing the efficacy and sensitivity of home vs combined whitening. Oper. Dent. 2011;36(5):460–466. doi: 10.2341/10-159-C. [DOI] [PubMed] [Google Scholar]
- 38.American Dental Association Council on Scientific Affairs . April 2012. Statement on the Safety and Effectiveness of Tooth Whitening Products.http://www.ada.org/en/about-the-ada/ada-positions-policies-and-statements/tooth-whitening-safety-and-effectiveness Available at: [Google Scholar]
- 39.Heithersay G.S. Treatment of invasive cervical resorption: an analysis of results using topical application of trichlor-acetic acid, curettage, and restoration. Quintessence Int. 1999;30(2):96–110. [PubMed] [Google Scholar]
- 40.Palo R.M., Valera M.C., Camargo S.E., Carmago C.H., Cardoso P.E. Peroxide penetration from the pulp chamber to the external root surface after internal bleaching. Am. J. Dent. 2010;23(3):171–174. [PubMed] [Google Scholar]
- 41.Firat E., Ercan E., Gurgan S., Yucel O.O., Cakir F.Y., Berker E. The effect of bleaching systems on the gingiva and the levels of IL-1B and iL-10 in gingival crevicular fluid. Oper. Dent. 2011;36(6):572–580. doi: 10.2341/10-058-C. [DOI] [PubMed] [Google Scholar]
- 42.Fasbinder D.J. Dental laser technology. Comp. Cont. Educ. Dent. 2008;29(8):452–459. [PubMed] [Google Scholar]
- 43.Martens L.C. Laser physics and review of laser applications in dentistry for children. Eur. Arch. Paediatr. Dent. 2011;12(2):61–67. doi: 10.1007/BF03262781. [DOI] [PubMed] [Google Scholar]
- 44.White JM, Goodis HE, Kudler JJ, Tran KT. Thermal laser effects on intraoral soft tissue, teeth and bone in vitro. Third International Congress on Lasers in Dentistry. Salt Lake City, UT: University of Utah Printing Services;1 992:189–190.
- 45.Coluzzi D. Fundamentals of dental lasers: science and instruments. Dent. Clin. North Am. 2004;48(4):751–770. doi: 10.1016/j.cden.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 46.Convissar R.A., Goldstein E.E. An overview of lasers in dentistry. Gen. Dent. 2003;51(5):436–440. [PubMed] [Google Scholar]
- 47.Green J., Weiss A., Stern A. Lasers and radiofrequency devices in dentistry. Dent. Clin. North Am. 2011;55(3):585–597. doi: 10.1016/j.cden.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 48.Kotlow L.A. Lasers in pediatric dentistry. Dent. Clin. North Am. 2004;48(4):889–922. doi: 10.1016/j.cden.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 49.van As G. Erbium lasers in dentistry. Dent. Clin. North Am. 2004;48(4):1017–1059. doi: 10.1016/j.cden.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 50.Boj J.R., Poirer C., Hernandez M., Espassa E., Espanya A. Review: laser soft tissue treatments for paediatric dental patients. Eur. Arch. Paediatr. Dent. 2011;12(2):100–105. doi: 10.1007/BF03262788. [DOI] [PubMed] [Google Scholar]
- 51.Olivi G., Genovese M.D., Caprioglio C. Evidence-based dentistry on laser paediatric dentistry: review and out-look. Eur. J. Paediatr. Dent. 2009;10(1):29–40. [PubMed] [Google Scholar]
- 52.Coluzzi D.J. An overview of laser wavelengths used in dentistry. Dent. Clin. North Am. 2000;44(4):753–766. [PubMed] [Google Scholar]
- 53.Tanboga I., Eren F., Altinok B., Peker S., Ertugal F. The effect of low level laser therapy on pain during cavity preparation with laser in children. Eur. Arch. Paediatr. Dent. 2011;12(2):93–95. doi: 10.1007/BF03262786. [DOI] [PubMed] [Google Scholar]
- 54.Whitters C.J., Hall A., Creanor S.L. A clinical study of pulsed Nd:YAG laser induced pulpal analgesia. J. Dent. 1995;23(3):145–150. doi: 10.1016/0300-5712(95)93571-i. [DOI] [PubMed] [Google Scholar]
- 55.Matsumoto K., Hossain M., Hossain M.M., Kawano H., Kimura Y. Clinical assessment of Er,Cr:YSGG laser applications for caries removal and cavity preparation in children. Med. Laser Appl. 2002;20(1):17–21. doi: 10.1089/104454702753474968. [DOI] [PubMed] [Google Scholar]
- 56.Barrêtto E.P.R., Ferreira E.F., Pordeus I.A. Evaluation of toothache severity in children using a visual analog scale of faces. Pediatr. Dent. 2004;26(6):485–491. [PubMed] [Google Scholar]
- 57.Hicks C.L., von Baeyer C.L., Spafford P., van Korlaar I., Goodenough B. The Faces Pain Scale-Revised: toward a common metric in pediatric pain measurement. Pain. 2001;93(2):173–183. doi: 10.1016/S0304-3959(01)00314-1. [DOI] [PubMed] [Google Scholar]
- 58.NIH Pain Consortium . National Institutes of Health; Bethesda, Md: 2007. http://painconsortium.nih.gov/pain_scales/index.html Available at: [Google Scholar]
- 59.American Academy of Pediatric Dentistry Use of local anesthesia for pediatric dental patients. Pediatr. Dent. 2011;33(special issue):174–180. [Google Scholar]
- 60.American Academy of Pediatrics American Pain Society. The assessment and management of acute pain in infants, children and adolescents. Pediatrics. 2001;108(3):793–797. doi: 10.1542/peds.108.3.793. [DOI] [PubMed] [Google Scholar]
- 61.Becker D.E. Pain management: Part 1: managing acute and postoperative dental pain. Anesth. Prog. 2010;57(2):67–79. doi: 10.2344/0003-3006-57.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sutters K.A., Miaskowsk C., Holdridge-Zeuner D. A randomized clinical trial of the efficacy of scheduled dosing of acetaminophen and hydrocodone for the management of postoperative pain in children after tonsillectomy. Clin. J. Pain. 2010;26(2):95–103. doi: 10.1097/AJP.0b013e3181b85f98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yaksh T.L., Wallace M.S. Opioids, analgesia and pain managment. In: Brunton L.L., Chabner B.A., Knollmann B.S., editors. Goodman and Gilman's the Pharmacological Basis of Therapeutics. twelfth ed. McGraw-Hill; New York, NY: 2010. pp. 481–526. [Google Scholar]
- 64.American Academy of Paediatric Dentistry Guidelines on Antibiotic Prophylaxis for Dental Patients at Risk. 2014. [Google Scholar]
- 65.Wilson W., Taubert K.A., Gevitz M. Prevention of infective endocarditis: guidelines from the American heart association—a guideline from the American heart association rheumatic fever, endocarditis and kawasaki disease committee, council on cardiovascular disease in the young, and the council on clinical cardiology, council on cardiovascular surgery and anesthesia anesthesia, and the quality of care and outcomes research interdisciplinary working group. J. Am. Dent. Assoc. 2007;138(6):739–745. doi: 10.14219/jada.archive.2007.0262. 747-60. Erratum in: J Am Dent Assoc 2008;139(3):253. [DOI] [PubMed] [Google Scholar]
- 66.Wilson W., Taubert K.A., Gevitz M. Prevention of infective endocarditis: guidelines from the American heart association—a guideline from the American heart association rheumatic fever, endocarditis and kawasaki disease committee, council on cardiovascular disease in the young, and the council on clinical cardiology, council on cardiovascular surgery and anesthesia anesthesia, and the quality of care and outcomes research interdisciplinary working group. Circulation. 2007;116(15):1736–1754. doi: 10.1161/CIRCULATIONAHA.106.183095. E-published April 19, 2007. Erratum in: Circulation 2007;116(15):e376-e7. [DOI] [PubMed] [Google Scholar]
- 67.Roberts G.J., Jaffrey E.C., Spract D.A., Petrie A., Greville C., Wilson M. Duration, prevalence and intensity of bacteremia after dental extractions in children. Heart. 2006;92(9):1274–1277. doi: 10.1136/hrt.2004.046581. de Sa DD, Tleyieh IM, Anavekar NS, et al. Epidemiological trends of infective endocarditis: A populationbased study in Olmsted County, Minnesota. Mayo Clin Proc 2010;85(5):422-6. Erratum in: Mayo Clin Proc 2010;85(8):722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lockhart P.B., Loven B., Brennan M.T., Baddour L.M., Levinson M. The evidence base for the efficiency of antibiotic prophylaxis in dental practice. J. Am. Dent. Assoc. 2007;138(4):458–474. doi: 10.14219/jada.archive.2007.0198. [DOI] [PubMed] [Google Scholar]
- 69.Nishimura R.A., Carabello B.A., Faxon D.P. ACC/AHA 2008 guideline update on valvular heart disease: focused update on infective endocarditis: a report of the American college of cardiology/American heart association task force on practice guidelines: endorsed by the society of cardiovascular anesthesiologists, society for cardiovascular angiography and interventions, and society of thoracic surgeons. Circulation. 2008;118:887–896. doi: 10.1161/CIRCULATIONAHA.108.190377. [DOI] [PubMed] [Google Scholar]
- 70.American Academy of Pediatric Dentistry Guideline on dental management of pediatric patients receiving chemotherapy, hematopoietic cell transplantation, and/or radiation. Pediatr. Dent. 2014;36(special issue):293–301. [Google Scholar]
- 71.American Dental Association . 2007. Guideline for the Use of Sedation and General Anesthesia by Dentists.http://www.ada.org/sections/about/pdfs/anesthesia_guidelines.pdf Available at: [Google Scholar]
- 72.American Society of Anesthesiologists Practice guidelines for sedation and analgesia by nonanesthesiologists: an updated report by the American Society of Anesthesiologists task force on sedation and analgesia by nonanesthesiologists. Anesthesiology. 2002;96:1004–1017. [Google Scholar]
- 73.Paterson S.A., Tahmassebi J.F. Pediatric dentistry in the new millennium: use of inhalation sedation in pediatric dentistry. Dent. Update. 2003;30(7):350–356. doi: 10.12968/denu.2003.30.7.350. 358. [DOI] [PubMed] [Google Scholar]
- 74.Dock M., Creedon R.L. Pharmacologic management of patient behavior. In: Dean J.A., Avery D.R., McDonald R.E., editors. McDonald and Avery's Dentistry for the Child and Adolescent. ninth ed. Mosby; Maryland Heights, Mo: 2011. pp. 261–264. [Google Scholar]
- 75.Emmanouil D.E., Quock R.M. Advances in understanding the actions of nitrous oxide. Anesth. Prog. 2007;54(1):9–18. doi: 10.2344/0003-3006(2007)54[9:AIUTAO]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sanders R.D.B., Weimann J., Maze M. Biologic effects of nitrous oxide: a mechanistic and toxicologic review. Anesthesiology. 2008;109(4):707–722. doi: 10.1097/ALN.0b013e3181870a17. [DOI] [PubMed] [Google Scholar]
- 77.Patel R., Lenczyk M., Hannallah R.S., McGill W.A. Age and onset of desaturation in apnoeic children. Can. J. Anesth. 1994;41(9):771–774. doi: 10.1007/BF03011582. [DOI] [PubMed] [Google Scholar]
- 78.Foley J. A prospective study of the use of nitrous oxide inhalation sedation for dental treatment in anxious children. Eur. J. Paediatr. Dent. 2005;6(3):21–27. [PubMed] [Google Scholar]
- 79.Clark M.S. Contemporary issues surrounding nitrous oxide. In: Malamed S.F., editor. Sedation: a Guide to Patient Management. fifth ed. Mosby Elsevier; St. Louis, Mo: 2010. p. 256. [Google Scholar]
- 80.Klein U., Robinson T.J., Allshouse A. End-expired nitrous oxide concentrations compared to flowmeter settings during operative dental treatment in children. Pediatr. Dent. 2011;33(1):56–62. [PubMed] [Google Scholar]
- 81.Klein U., Bucklin B.A., Poulton T.J., Bozinov D. Nitrous oxide concentrations in the posterior nasopharynx during administration by nasal mask. Pediatr. Dent. 2004;26(5):410–416. [PubMed] [Google Scholar]
- 82.Malamed S.F. fifth ed. Mosby Elsevier; St. Louis, MO: 2010. Sedation: a Guide to Patient Management; pp. 248–259. [Google Scholar]
- 83.Malamed S.F., Clark M.S. Nitrous oxide-oxygen: a new look at a very old technique. J. Calif. Dent. Assoc. 2003;31(5):397–403. [PubMed] [Google Scholar]
- 84.American Academy of Pediatrics, American Academy of Pediatric Dentistry Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: an update. Pediatr. Dent. 2006;28(suppl):115–132. [Google Scholar]
- 85.Donaldson D., Meechan J.G. The hazards of chronic exposure to nitrous oxide: an update. Br. Dent. J. 1995;178(3):95–100. doi: 10.1038/sj.bdj.4808673. [DOI] [PubMed] [Google Scholar]
- 86.American Academy of Paediatric dentistry Guidelines on restorative dentistry Refernce Manual v. 37/n06 15/16.
- 87.American Academy of Pediatric Dentistry Guideline on caries risk assessment and management for infants, children, and adolescents. Pediatr. Dent. 2014;36(special issue):127–134. [PubMed] [Google Scholar]
- 88.Ismail A.I., Sohn W., Tellez M. The international caries detection and assessment system (ICDAS): an integrated system for measuring dental caries. Community Dent. Oral Epidemiol. 2007;35(3):170–178. doi: 10.1111/j.1600-0528.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 89.Downer M.C., Azli N.A., Bedi R., Moles D.R., Setchell D.J. How long do routine dental restorations last? A systematic review. Br. Dent. J. 1999;187(8):432–439. doi: 10.1038/sj.bdj.4800298a1. [DOI] [PubMed] [Google Scholar]
- 90.Lenters M., van Amerongen W.E., Mandari G.J. Iatrogenic damage to the adjacent surface of primary molars in three different ways of cavity preparation. Eur. Arch. Paed. Dent. 2006;1(1):6–10. doi: 10.1007/BF03320808. [DOI] [PubMed] [Google Scholar]
- 91.Ricketts D., Lamont T., Innes N.P.T., Kidd E., Clarkson J.E. March 28, 2013. Techniques for Managing Decay in Teeth.http://summaries.cochrane.org/CD003808/techniques-formanaging-decay-in-teeth The Cochrane Summaries. Available at: [Google Scholar]
- 92.Hickel R., Kaaden C., Paschos E., Buerkle V., García-Godoy F., Manhart J. Longevity of occlusally-stressed restorations in posterior primary teeth. Am. J. Dent. 2005;18:198–211. [PubMed] [Google Scholar]
- 93.Bjørndal L., Reit C., Bruun G. Treatment of deep caries lesions in adults: randomized clinical trials comparing stepwise vs. direct complete excavation, and direct pulp capping vs. partial pulpotomy. Eur. J. Oral Sci. 2010;118(3):290–297. doi: 10.1111/j.1600-0722.2010.00731.x. [DOI] [PubMed] [Google Scholar]
- 94.Maltz M., Garcia R., Jardim J.J. Randomized trial of partial vs. stepwise caries removal: 3-year follow-up. J. Dent. Res. 2012;91(11):1026–1031. doi: 10.1177/0022034512460403. [DOI] [PubMed] [Google Scholar]
- 95.Maltz M., Jardim J.J., Mestrinho H.D. Partial removal of carious dentine: a multicenter randomized controlled trial and 18-month follow-up results. Caries Res. 2013;47(2):103–109. doi: 10.1159/000344013. [DOI] [PubMed] [Google Scholar]
- 96.Simonsen R.J. Clinical Applications of the Acid Etch Technique. Quintessence Publishing Co, Inc; Chicago, Ill: 1978. Pit and fissure sealants; pp. 19–42. [Google Scholar]
- 97.Griffin S.O., Gray S.K., Malvitz D.M., Gooch B.F. Caries risk in formerly sealed teeth. J. Am. Dent. Assoc. 2009;140(4):415–423. doi: 10.14219/jada.archive.2009.0190. [DOI] [PubMed] [Google Scholar]
- 98.Paris S., Hopfenmuller W., Meyer-Lueckel H. Resin infiltration of caries lesions: an efficacy randomized trial. J. Dent. Res. 2010;89(8):823–826. doi: 10.1177/0022034510369289. [DOI] [PubMed] [Google Scholar]
- 99.Meyer-Lueckel H., Bitter K., Paris S. Randomized controlled clinical trial on proximal caries infiltration: three-year follow-up. Caries Res. 2012;46(6):544–548. doi: 10.1159/000341807. [DOI] [PubMed] [Google Scholar]
- 100.Beazoglou T., Eklund S., Heffley D., Meiers J., Brown L.J., Bailit H. Economic impact of regulating the use of amalgam restorations. Publ. Health Rep. 2007;122(5):657–663. doi: 10.1177/003335490712200513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Department of Health and Human Services. Final Rule. Federal Register 75: Issue 112. Friday, June 11, 2010. http://www.fda.gov/downloads/medicaldevices/productsandmedicalprocedures/dentalproducts/dental amalgam/ucm174024.pdf Availble at: [Google Scholar]
- 102.Heintze S.D., Rousson V. Clinical effectiveness of direct Class II restorations - a meta-analysis. J. Adhes. Dent. 2012;14(5):407–431. doi: 10.3290/j.jad.a28390. [DOI] [PubMed] [Google Scholar]
- 103.Burgess J.O., Walker R., Davidson J.M. Posterior resin based composite: review of the literature. Pediatr. Dent. 2002;24(5):465–479. [PubMed] [Google Scholar]
- 104.Pallav P., De Gee A.J., Davidson C.L., Erickson R.L., Glasspoole E.A. The influence of admixing microfiller to small-particle composite resins on wear, tensile strength, hardness and surface roughness. J. Dent. Res. 1989;68(3):489–490. doi: 10.1177/00220345890680031101. [DOI] [PubMed] [Google Scholar]
- 105.Mitra S.B., Kedrowski B.L. Long-term mechanical properties of glass ionomers. Dent. Mater. 1994;10(2):78–82. doi: 10.1016/0109-5641(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 106.Douglas W.H., Lin C.P. Strength of the new systems. In: Hunt P.R., editor. Glass Ionomers: the Next Generation. International Symposia in Dentistry, PC; Philadelphia, PA: 1994. pp. 209–216. [Google Scholar]
- 107.Wambier D.S., dos Santos F.A., Guedes-Pinto A.C., Jaeger R.G., Simionato M.R. Ultrastructural and microbiological analysis of the dentin layers affected by caries lesions in primary molars treated by minimal intervention. Pediatr. Dent. 2007;29(3):228–234. [PubMed] [Google Scholar]
- 108.Mandari G.J., Frencken J.E., van't Hof M.A. Six years success rates of occlusal amalgam and glass ionomer restorations placed using minimal intervention approaches. Caries Res. 2003;37(4):246–253. doi: 10.1159/000070866. [DOI] [PubMed] [Google Scholar]
- 109.Dulgergil D.T., Soyman M., Civelek A. Atraumatic restorative treatment with resin-modified glass ionomer material: short-term results of a pilot study. Med. Princ. Pract. 2005;14(3):277–280. doi: 10.1159/000085750. [DOI] [PubMed] [Google Scholar]
- 110.Nicholson J.W. Polyacid-modified composite resins (‘compomers’) and their use in clinical dentistry. Dent. Mater. 2007;23(5):615–622. doi: 10.1016/j.dental.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 111.Cildir S.K., Sandalli N. Fluoride release/uptake of glassionomer cements and polyacid-modified composite resins. Dent. Mater. J. 2005;24(1):92–97. doi: 10.4012/dmj.24.92. [DOI] [PubMed] [Google Scholar]
- 112.Peng D., Smales R.J., Yip H.K., Shu M. In vitro fluoride release from aesthetic restorative materials following recharging with APF gel. Aust. Dent. J. 2000;45(3):198–203. doi: 10.1111/j.1834-7819.2000.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 113.American Academy of Pediatric Dentistry Guideline on pediatric restorative dentistry. Pediatr. Dent. 2013;35(special issue):226–234. [Google Scholar]
- 114.Shah P.V., Lee J.Y., Wright J.T. Clinical success and parental satisfaction with anterior preveneered primary stainless steel crowns. Pediatr. Dent. 2004;26(5):391–395. [PubMed] [Google Scholar]
- 115.Fuks A.B. Pulp therapy for the primary dentition. In: Pinkham J.R., Casamassimo P.S., Fields H.W. Jr., McTigue D.J., Nowak A., editors. Pediatric Dentistry: Infancy through Adolescence. fifth ed. Elsevier Saunders Co; St. Louis, Mo: 2013. pp. 331–351. [Google Scholar]
- 116.American Association of Endodontists . seventh ed. American Association of Endodontists; Chicago, Ill: 2003. Glossary of Endodontic Terms. [Google Scholar]
- 117.American Association of Endodontists . 1996. Systematic Endodontic Diagnosis. Insert to the Fall/Winter edition of Endodontics: Colleagues for Excellence. [Google Scholar]
- 118.McDonald R.E., Avery D.R., Dean J.A., Jones J.E. Management of trauma to the teeth and supporting tissues. In: Dean J.A., Avery D.R., McDonald R.E., editors. McDonald and Avery's Dentistry for the Child and Adolescent. ninth ed. Mosby Elsevier Inc; St Louis, Mo: 2011. pp. 403–442. [Google Scholar]
- 119.Itota T., Nakabo S., Torii Y., Narukami T., Doi J., Yoshiyama M. Effect of fluoride-releasing liner on demineralized dentin. Quintessence Int. 2006;37(4):297–303. [PubMed] [Google Scholar]
- 120.Weiner R.S., Weiner L.K., Kugel G. Teaching the use of bases and liners: a survey of North American dental schools. J. Am. Dent. Assoc. 1996;127(11):1640–1645. doi: 10.14219/jada.archive.1996.0100. [DOI] [PubMed] [Google Scholar]
- 121.Büyükgüral B., Cehreli Z.C. Effect of different adhesive protocols vs calcium hydroxide on primary tooth pulp with different remaining dentin thicknesses: 24 month results. Clin. Oral Investig. 2008;12(1):91–96. doi: 10.1007/s00784-007-0152-x. [DOI] [PubMed] [Google Scholar]
- 122.Falster C.A., Araújo F.B., Straffon L.H., Nör J.E. Indirect pulp treatment: in vivo outcomes of an adhesive resin system vs calcium hydroxide for protection of the dentin pulp complex. Pediatr. Dent. 2002;24(3):241–248. [PubMed] [Google Scholar]
- 123.Lo E.C., Holmgren C.J., Hu D., Van Palenstein Helderman W. Six-year follow up of atraumatic restorative treatment restorations placed in Chinese school children. Community Dent. Oral Epidemiol. 2007;35(5):387–392. doi: 10.1111/j.1600-0528.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 124.de Souza E.M., Cefaly D.F., Terada R.S., Rodrigues C.C., de Lima Navarro M.F. Clinical evaluation of the ART technique using high density and resin-modified glass ionomer cements. Oral Health Prev. Dent. 2003;1(3):201–207. [PubMed] [Google Scholar]
- 125.Pinto A.S., de Araújo F.B., Franzon R. Clinical and microbiological effect of calcium hydroxide protection in indirect pulp capping in primary teeth. Am. J. Dent. 2006;19(6):382–386. [PubMed] [Google Scholar]
- 126.Al-Zayer M.A., Straffon L.H., Feigal R.J., Welch K.B. Indirect pulp treatment of primary posterior teeth: a retrospective study. Pediatr. Dent. 2003;25(1):29–36. [PubMed] [Google Scholar]
- 127.Davidovich E., Weiss E., Fuks A.B., Beyth N. Surface antibacterial properties of glass ionomer cements used in a traumatic restorative treatment. J. Am. Dent. Assoc. 2007;138(10):1347–1352. doi: 10.14219/jada.archive.2007.0051. [DOI] [PubMed] [Google Scholar]
- 128.Marchi J.J., de Araújo F.B., Froner A.M., Straffon L.H., Nör J.E. Indirect pulp capping in the primary dentition: a 4 year follow-up study. J. Clin. Pediatr. Dent. 2006;31(2):68–71. doi: 10.17796/jcpd.31.2.y4um5076341226m5. [DOI] [PubMed] [Google Scholar]
- 129.Vij R., Coll J.A., Shelton P., Farooq N.S. Caries control and other variables associated with success of primary molar vital pulp therapy. Pediatr. Dent. 2004;26(3):214–220. [PubMed] [Google Scholar]
- 130.Farooq N.S., Coll J.A., Kuwabara A., Shelton P. Success rates of formocresol pulpotomy and indirect pulp therapy in the treatment of deep dentinal caries in primary teeth. Pediatr. Dent. 2000;22(4):278–286. [PubMed] [Google Scholar]
- 131.Horsted P., Sondergaard B., Thylstrup A., El Attar K., Fejerskov O. A retrospective study of direct pulp capping with calcium hydroxide compounds. Endod. Dent. Traumatol. 1985;1(1):29–34. doi: 10.1111/j.1600-9657.1985.tb00555.x. [DOI] [PubMed] [Google Scholar]
- 132.Baume L.J., Holz J. Long term clinical assessment of direct pulp capping. Int. Dent. J. 1981;31(4):251–260. [PubMed] [Google Scholar]
- 133.Siqueira J.F., Jr., Rôças I.N., Paiva S.S., Guimarães-Pinto T., Magalhaes K.M., Lima K.C. Bacteriologic investigation of the effects of sodium hypochlorite and chlorhexidine during the endodontic treatment of teeth with apical periodontitis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007;104(1):122–130. doi: 10.1016/j.tripleo.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 134.Ercan E., Ozekinci T., Atakul F., Gül K. Antibacterial activity of 2% chlorhexidine gluconate and 5.25% sodium hypochlorite in infected root canal: in vivo study. J. Endod. 2004;30(2):84–87. doi: 10.1097/00004770-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 135.Camp J.H., Fuks A.B. Pediatric endodontics: endodontic treatment for the primary and young permanent dentition. In: Cohen S., Hargreaves K.M., editors. Pathways of the Pulp. tenth ed. Mosby Elsevier; St. Louis, Mo: 2011. pp. 808–857. [Google Scholar]
- 136.Mejàre I., Cvek M. Partial pulpotomy in young permanent teeth with deep carious lesions. Endod. Dent. Traumatol. 1993;9(6):238–242. doi: 10.1111/j.1600-9657.1993.tb00279.x. [DOI] [PubMed] [Google Scholar]
- 137.Nosrat I.V., Nosrat C.A. Reparative hard tissue formation following calcium hydroxide application after partial pulpotomy in cariously exposed pulps of permanent teeth. Int. Endod. J. 1998;31(3):221–226. doi: 10.1046/j.1365-2591.1998.00147.x. [DOI] [PubMed] [Google Scholar]
- 138.El-Meligy O.A.S., Avery D.R. Comparison of mineral trioxide aggregate and calcium hydroxide as pulpotomy agents in young permanent teeth (apexogenesis) Pediatr. Dent. 2006;28(5):399–404. [PubMed] [Google Scholar]
- 139.Ercan E., Ozekinci T., Atakul F., Gül K. Antibacterial activity of 2% chlorhexidine gluconate and 5.25% sodium hypochlorite in infected root canal: in vivo study. J. Endod. 2004;30(2):84–87. doi: 10.1097/00004770-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 140.Fuks A.B., Gavra S., Chosack A. Long-term follow-up of traumatized incisors treated by partial pulpotomy. Pediatr. Dent. 1993;15(5):334–336. [PubMed] [Google Scholar]
- 141.de Blanco L.P. Treatment of crown fractures with pulp exposure. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1996;82(5):564–568. doi: 10.1016/s1079-2104(96)80204-6. [DOI] [PubMed] [Google Scholar]
- 142.Blanco L., Cohen S. Treatment of crown fractures with exposed pulps. J. Calif. Dent. Assoc. 2002;30(6):419–425. [PubMed] [Google Scholar]
- 143.Cvek M. Endodontic management and the use of calcium hydroxide in traumatized permanent teeth. In: Andreasen J.O., Andreasen F.M., Andersson L., editors. Textbook and Color Atlas of Traumatic Injuries to the Teeth. fourth ed. Blackwell Munksgaard; Ames, Iowa: 2007. pp. 598–657. [Google Scholar]
- 144.Bakland L.K. New endodontic procedures using mineral trioxide aggregate (MTA) for teeth with traumatic injuries. In: Andreasen J.O., Andreasen F.M., Andersson L., editors. Textbook and Color Atlas of Traumatic Injuries to the Teeth. fourth ed. Blackwell Munksgaard; Ames, Iowa: 2007. pp. 658–668. [Google Scholar]
- 145.Weiner R.S., Weiner L.K., Kugel G. Teaching the use of bases and liners: a survey of North American dental schools. J. Am. Dent. Assoc. 1996;127(11):1640–1645. doi: 10.14219/jada.archive.1996.0100. [DOI] [PubMed] [Google Scholar]
- 146.Torabinejad M., Chivian N. Clinical applications of mineral trioxide aggregate. J. Endod. 1999;25(3):197–205. doi: 10.1016/S0099-2399(99)80142-3. [DOI] [PubMed] [Google Scholar]
- 147.Patino M.G., Neiders M.E., Andreana S., Noble B., Cohen R.E. Collagen as an implantable material in medicine and dentistry. J. Oral Implantol. 2002;28(5):220–225. doi: 10.1563/1548-1336(2002)028<0220:CAAIMI>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 148.Katebzadeh N., Dalton B.C., Trope M. Strengthening immature teeth during and after apexification. J. Endod. 1998;24(4):256–259. doi: 10.1016/s0099-2399(98)80108-8. [DOI] [PubMed] [Google Scholar]
- 149.Eslaminejad M.B., Vahabi S., Shariati M., Nazarian H. In vitro growth and characterization of stem cells from human dental pulp of deciduous versus permanent teeth. J Dent. Tehran. 2010;7(4):185–195. [PMC free article] [PubMed] [Google Scholar]
- 150.Perry B.C., Zhou D., Wu X. Collection, cryopreservation, and characterization of human dental pulp-derived mesenchymal stem cells for banking and clinical use. Tissue Eng. Part C Meth. 2008;14(2):149–156. doi: 10.1089/ten.tec.2008.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Carter L., Farman A.G., Geist J. American Academy of Oral and Maxillofacial Radiology executive opinion statement on performing and interpreting diagnostic cone beam computed tomography. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008;106(4):561–562. doi: 10.1016/j.tripleo.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 152.Smith N.L., Seale N.S., Nunn M.E. Ferric sulfate pulpotomy in primary molars: a retrospective study. Pediatr. Dent. 2000;22(3):192–199. [PubMed] [Google Scholar]
- 153.Burnett S., Walker J. Comparison of ferric sulfate, formocresol, and a combination of ferric sulfate/formocresol in primary tooth vital pulpotomies: a retrospective radiographic survey. ASDC J. Dent. Child. 2002;69(1):44–48. [PubMed] [Google Scholar]
- 154.Ibricevic H., Al-Jame Q. Ferric sulphate and formocresol in pulpotomy of primary molars: long term follow-up study. Eur. J. Paediatr. Dent. 2003;4(1):28–32. [PubMed] [Google Scholar]
- 155.Loh A., O'Hoy P., Tran X. Evidence-based assessment: evaluation of the formocresol versus ferric sulfate primary molar pulpotomy. Pediatr. Dent. 2004;26(5):401–409. [PubMed] [Google Scholar]
- 156.Markovic D., Zivojinovic V., Vucetic M. Evaluation of three pulpotomy medicaments in primary teeth. Eur. J. Paediatr. Dent. 2005;6(3):133–138. [PubMed] [Google Scholar]
- 157.Fuks A.B., Papagiannoulis L. Pulpotomy in primary teeth: review of the literature according to standardized criteria. Eur. Arch. Paediatr. Dent. 2006;7(2):64–71. doi: 10.1007/BF03320817. [DOI] [PubMed] [Google Scholar]
- 158.Seale N.S., Glickman G.N. Contemporary perspectives on vital pulp therapy: views from the endodontists and pediatric dentists. Pediatr. Dent. 2008;30(3):261–267. [PubMed] [Google Scholar]
- 159.Dean J.A., Mack R.B., Fulkerson B.T., Sanders B.J. Comparison of electrical and formocresol pulpotomy procedures in children. Int. J. Paediatr. Dent. 2002;12(3):177–182. doi: 10.1046/j.1365-263x.2002.00355.x. [DOI] [PubMed] [Google Scholar]
- 160.Guelmann M., Fair J., Bimstein E. Permanent versus temporary restorations after emergency pulpotomies in primary molars. Pediatr. Dent. 2005;27(6):478–481. [PubMed] [Google Scholar]
- 161.Holan G., Fuks A.B., Keltz N. Success rate of formocresol pulpotomy in primary molars restored with stainless steel crown vs amalgam. Pediatr. Dent. 2002;24(3):212–216. [PubMed] [Google Scholar]
- 162.Guelmann M., McIlwain M.F., Primosch R.E. Radiographic assessment of primary molar pulpotomies restored with resin-based materials. Pediatr. Dent. 2005;27(1):24–27. [PubMed] [Google Scholar]