Abstract
Measles is a highly transmissible disease caused by measles virus and remains a major cause of child mortality in developing countries. Measles virus nucleoprotein (N) encapsidates the RNA genome of the virus for providing protection from host cell endonucleases and for specific recognition of viral RNA as template for transcription and replication. This protein is over-expressed at the time of viral replication. The C-terminal of N protein is intrinsically disordered, which enables this protein to interact with several host cell proteins. It was previously proved in our laboratory that N expressing human cancerous cells undergo programmed cell death because of reactive oxygen species (ROS) generation as well as Caspase 3 activation. The phosphoprotein (P) along with N protein enclosed viral genomic RNA forming a ribonucleoprotein complex (RNP). It also establishes interaction with the large protein (L) i.e. viral RNA dependent RNA polymerase to ensure viral replication within host cells. The host cell receptors of this virus are CD46, SLAM/CD150 and PVRL4. Measles virus is latently oncotropic in nature and possesses oncolytic property by syncytia formation. We try to highlight the application of this property in developing a virotherapeutic vehicle.
Keywords: Measles virus, Edmonston vaccine strain, CD46 (cluster of differentiation 46), Syncytia, Oncotropic and oncolytic viruses
Highlights
-
•
Measles, caused by Measles Virus, is one of the leading causes of child mortality.
-
•
Vaccination against measles has decreased the child mortality rate worldwide.
-
•
Its oncolytic property is used to develop a virotherapeutic vehicle against cancer.
-
•
Measles virus lyses the cancer cells by syncytia formation.
-
•
The recombinant virus can be generated using reverse genetic system.
1. Introduction
Measles virus belongs to the genus Morbillivirus under family Paramyxoviridae of the order Mononegavirales. The Paramyxoviridae family also includes mumps virus and other viruses causing infections in respiratory tract. Measles is transmitted either through air or by direct contact with body secretions. The primary site of viral infection is respiratory tract. Then it disperses throughout the body viz. lymphoid tissue, liver, lungs, conjunctiva, and skin. This virus infects humans only [1], [2]. Death is common among young children (below 5 years) and occurs due to measles related complications viz. infections in respiratory tracts like pneumonia, brain swelling or encephalitis, blindness and ear infections, dehydration and diarrhoea. Recovery from measles makes an individual immune for rest of one's life [3]. The worldwide death rate due to measles is decreased amazingly due to routine vaccination and mass campaigning. The measles vaccine is often injected along with rubella and/or mumps (MMRV: Measles Mumps Rubella Vaccine). Compared to 73% in 2000, 85% of world's children had received a vaccine dose on their first birthday 2013 [4].
2. Viral structure
Measles virus is generally of round shape but shows pleomorphism i.e. the appearance of two or more distinctly different forms in the life cycle of some organisms. It contains single-stranded RNA of negative polarity. It is an enveloped virus with non-segmented genome. The RNA genome is 15,894 nucleotides in length and forms complex with N protein. The arrangement is helical and follows the “rule of six” [5]. The genome contains a 52 nucleotide non-coding leader region at the beginning that acts as a transcriptional promoter and a 37 nucleotide non-coding trailer region at the end that acts as a transcriptional terminator. The MV genome organization bears essential similarity to other members of Paramyxoviridae. The genome contains six genes code for eight viral proteins. The anti-genome arrangement is 5′-N, P, M, F, H, L-3′. V and C are two accessory proteins produced by editing and use of an alternative ORF respectively from P gene sequence [6].
The N protein is coded by N gene. The N terminal region of this protein is conserved and is called NCORE. It is 400 amino acids long. The C-terminal- known as NTAIL is intrinsically disordered, which enables it to establish interaction with many viral and host cell proteins. The C-terminal domain of P protein and matrix protein (M) are its interacting partners [7]. The host cell partners that were reported to interact with N tail are Heat shock protein (Hsp72), eukaryotic translation initiation factor 3 (eIF3-p40) and Interferon Regulatory Factor (IRF3) [8]. Nucleocapsid protein provides protection to the viral RNA. The N enclosed RNA is a template for viral multiplication.
The P, V and C proteins are coded by P gene. V is the result of RNA editing and C is coded from an alternative reading frame [9]. These proteins hardly play any role in maintaining viral structure but act as virulence factors to suppress innate immune response in host by interfering with Interferon (IFN) signaling pathway [10]. The P protein establishes connection with both N and L proteins to ensure proper replication and transcription. It acts as a cofactor and is a part of ribonucleoprotein (RNP) complex [11].
The hydrophobic M protein is coded by M gene. Though it is not a membrane protein, its association with the membrane has been reported [12]. It fills the spaced between lipid envelope and nucleocapsid core. This protein interacts with RNP complex as well as cytoplasmic tails of H and F proteins. Interestingly, this is necessary for cell fusion. It has an inhibitory effect on viral polymerase and takes part in viral lifecycle and organizing viral structure [13].
Two glycoproteins that protrude out of viral envelope, are coded by fusion (F) and hemagglutinin (H) genes respectively. The H protein recognizes, hence attaches with the host cell receptors like SLAM/CD150 and CD46, thereby determining viral tropism. Only after successful attachment, fusion to the host cell membrane is mediated by F protein. This protein undergoes structural transformation only after a successful attachment [14], [15].
The viral polymerase (an RNA-dependent RNA polymerase, RdRp) or large protein is coded by the L gene. It catalyzes RNA synthesis, polymerization, capping, polymerization, methylation and polyadenylation. The negative sense RNA is transcribed directly into mRNA without forming any DNA intermediate [16] (Fig. 1).
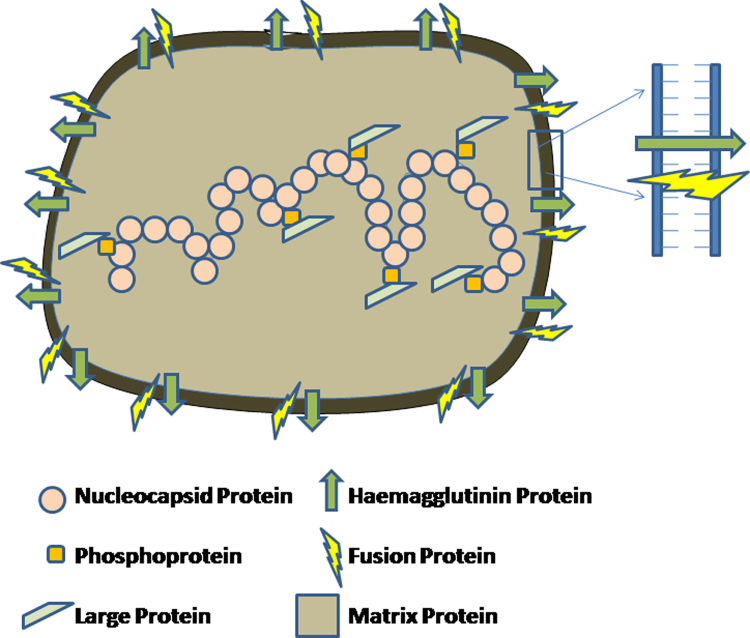
Fig. 1.
A diagrammatic representation of measles virus structure. MV contains RNA that is encapsidated by N protein. P and L protein maintain connection with the N protein encapsidated RNA. The M protein is dispersed all over. The H and F proteins remain attached to the lipid bilayer obtained from the host cell membrane.
3. Receptors
Measles virus interacts with three host cell receptors viz. CD46, SLAM/ CD150 and PVRL4.
The cluster differentiation marker CD46 is a complement regulatory protein. The CD46 gene is located on chromosome 1q32. It acts as a complement receptor as well as an inhibitor [17]. This type I membrane protein plays a key role in regulating the complement system. It acts as a cofactor of serum factor I, which mediates the cleavage of complement components viz. C3b and C4b, thereby protecting the host cells [18]. The action of this protein is necessary at the time of human fertilization process. It is a receptor molecule for Edmonston vaccine strain of MV, human herpesvirus-6 (HHV-6) and type IV pili of Neisseria [19]. The extracellular domain is composed of four short consensus repeats (SCR1-4), containing 60 amino acids. This domain is a closely packed beta-barrel bordered by flexible loops. The receptor contains a STP (Ser/Thr/Pro) domain, a helical transmembrane domain and an intracellular cytoplasmic domain, which takes part in signal transduction [20]. CD46 is expressed at basal level in all nucleated cells but is over expressed in all adenocarcinoma cells. This receptor has a predominant role in protecting the tumor cells from host immune response. The vaccine of measles Edmonston strain can be used for treatment of adenocarcinoma as CD46 is proved to be the receptor of entry of this strain. The interacting partners of CD46 are CD9, CD29 and CD151 [21].
Signaling lymphocytic activation molecule (SLAM) or CD150 is encoded by SLAMF1 gene located on chromosome 1q22 in humans. The interacting partners of SLAM are SH2D1A, SH2D1B and PTPN11 [22]. This glycoprotein is expressed on the surface of certain immune cells viz. dendritic cells, natural killer cells, B and T cells. The wild type strain of measles virus recognizes CD150 as its receptor. It also functions as a co-activator on B and T cells. It possesses two extracellular domains, which correspond to variable (V) and constant (C) regions of immunoglobulin super family and an endodomain containing an SH2 binding region. The successive duplications in common ancestral gene resulted in the formation of molecules of CD150 family. The wild type vaccine strain cannot kill cancer cells unless it is attenuated [24].
Nectins, belonging to the family of cell adhesion molecules, play a role in adhesion in chemical synapse of neuronal tissues and adherens junction of epithelial tissue. The cellular adhesion mediated by nectins is Ca2+-independent [25]. Four nectin molecules have been identified so far, they are nectin-1, nectin-2, nectin-3 and nectin-4, in humans. Each molecule is composed of an intracellular domain, a single transmembrane domain and three extracellular immunoglobulin domains; the latter contain two constant regions and one variable region. The intracellular domain interacts with afadin, a scaffold protein, to form dimers. The interactions are homophilic or heterophilic and cadherin molecules are recruited for strengthening binding affinity. The H protein of measles virus interacts specifically with the V (variable) domain of Nectin 4/PVRL4 (primary vitreoretinal lymphoma). In many adenocarcinoma cell lines it is over expressed on the apical surface and can be a potential therapeutic target [23], [25] (Fig. 2).
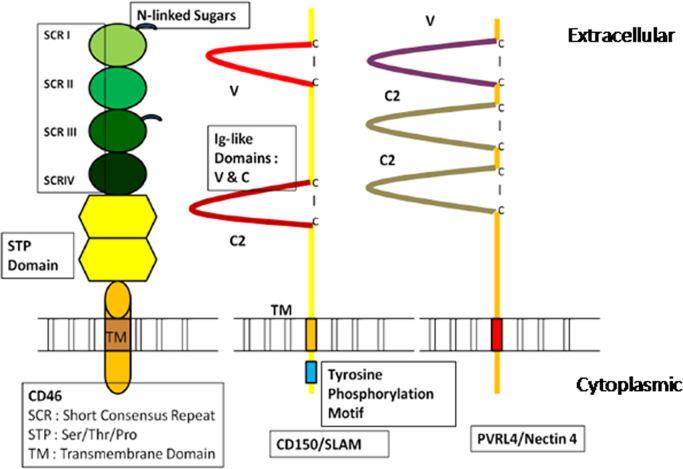
Fig. 2.
Organization of cell surface receptors for measles virus on host cell (after Noyce et al.[23]). The receptors for MV are CD46, SLAM/CD150 and PVRL4/ Nectin-4. The extracellular, transmembrane and cytoplasmic domains of these receptors are depicted in this figure.
4. Viral replication
The strategy of measles replication and transcription bears similarity with other Paramyxoviridae, which is placed in group V according to Baltimore classification. The viral polymerase recognizes RNP complex as its template to initiate viral replication and transcription [26]. The process of transcription starts at 3′ end and continues towards the 5′ end of the genomic RNA template. The synthesized mRNA is translated into proteins. The virus utilizes host translation machinery to ensure proper multiplication. The complementary positive strand is also synthesized from negative-sense genome by viral polymerase for production of more copies of the genome. The N protein accumulation is essential for switching from transcription to replication process [27].
Following the process of transcription and replication, the viral molecules assemble for generation of fully infectious particles. The C-terminal of N protein establishes interaction with M protein, this was confirmed by co-immunoprecipitation and yeast two-hybrid assay. The M protein plays an important role in incorporating the RNP into virions. It also assists in linking RNP with those parts of host cell membrane, where viral glycoproteins are introduced. An ESCRT-independent Virion release independent of endosomal sorting complex required for transport (ESCRT) has also been reported [28].
The phenomenon of polar attenuation occurs in measles virus- the gene expression at 3′ end of the genomic RNA is greater compared to those at 5′ end [29] (Fig. 3).
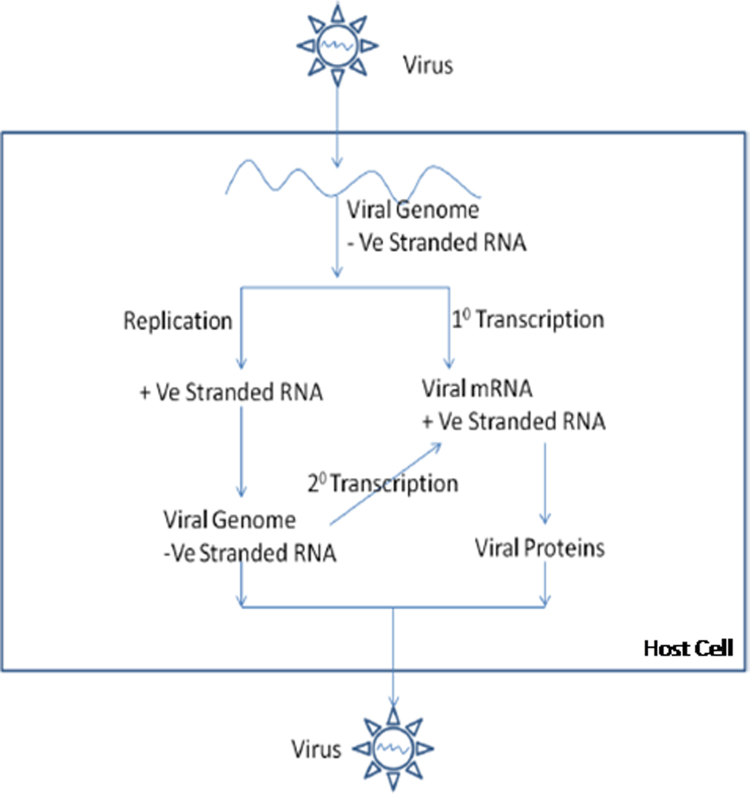
Fig. 3.
A schematic representation of replication cycle of measles virus in host cell. MV is a negative stranded RNA virus. The RNA dependent RNA polymerase (RdRp) produces positive strand RNA from the negative strand by transcription. The viral proteins are coded by the respective genes. The negative stranded RNA is also generated by replication process. New progeny viruses are generated. The entire process occurs in the host cell cytoplasm.
5. Immune suppression due to viral infection
Measles virus infection causes transient immune suppression that renders the affected individual more susceptible to other pathogenic infections. It causes lymphopenia, where the count of both B and T cells decreases extensively. The cytotoxic T-cells play a role in viral clearance and the affected individuals acquire a lifelong immunity against measles virus infection [30]. Within a week the lymphocyte number becomes normal after viral clearance but immune suppression may last for more than two years. The viral infection also suppresses delayed-type hypersensitivity responses. The T cells become insensitive to proliferation signals and IL-2 signaling pathway becomes impaired in T cells. IL-10 is over expressed and IL-12 is down regulated upon infection. The Fas expression on surface of lymphocytes causes apoptosis of the latter [31], [32].
The innate immune response after viral infection stimulates some signaling pathways, which act through transcription factors like NF-κB and IRF3 [33]. The adaptive immune response functions in viral clearance. The appearance of CD4+ and CD8+ T lymphocytes is a unique feature of this response [34].
6. Oncolytic measles virus and virotherapy
Cancer, a group of diseases, is characterized by abnormal and uncontrolled cell growth as well as cell migration and invasion. Cancer cells are capable of escaping apoptosis. The tumors are viewed as either benign or malignant. The benign tumors are localized at the point of origin and never invade other parts of body but the malignant ones invade tissues away from the point of origin via blood or lymph- a phenomenon called metastasis [35]. Cancers can be classified based on the tissue of origin into the following types- 1) carcinoma (cancer of epithelial tissue), 2) sarcoma (cancer of connective tissue), 3) lymphoma and leukemia (cancer of hematopoietic cells), 4) germ cell tumor (cancer of pluripotent cells) and 5) blastoma (cancer of embryonic tissue or precursor cells [36].
In 1954 Enders and Peebles isolated measles virus from a patient, named Edmonston [37]. The vaccine against measles was developed after multiple passages. Further passages generated Schwarz and Moraten strains, which are used today [38]. These vaccines are used for protection of millions all over the world successfully. These live-attenuated vaccine strains possess oncolytic activity and are effectively used in clinical trials [39]. As CD46 is over expressed in many tumor cells, it becomes the preferred target of the virus. Recently it has been reported that nectin-4 also acts as a receptor for both wild type and vaccine strains. The unmodified attenuated viral vaccine strain (Edmonston Vaccine Strain) has been used to treat several cancers viz. leukemia, myeloma, ovarian and breast cancer. Genetically engineered measles viruses are utilized for oncolytic virotherapy after “blinding” it to its natural receptors. Table 1 presents the genetically modified viruses used against cancer [38], [39]. MV-H protein is solely associated with viral tropism, so the latter is modified by inserting at the C-terminal of H protein the ligands of the receptors displayed by the cancer cells. The ligand is a single chain antibody for the receptors e.g. CD20, CD38, CD133, epidermal growth factor receptor (EGFR) and carcinoembryogenic antigen (CEA) [40], [41], [42]. When the cancer cells are infected with MV-CEA, the soluble peptide CEA is released into the blood stream. Thus CEA level can be detected in serum of patients, treated with MV-CEA vaccine [43]. Purine nucleoside phosphorylase (PNP), found in E. Coli, act as a prodrug convertase to assist conversion of chemotherapeutic prodrugs such as fludarabine into 2-fluoroadenine, a highly toxic metabolite, which intercalates in DNA to promote apoptosis. Recombinant measles virus, expressing prodrug convertase, was generated [44]. To ensure invasion and metastasis, the cancer cells secrete matrix metalloproteinases (MMP) to carry out degradation of extracellular matrix. Recombinant measles virus with MMP-cleavable sequences in place of furin-cleavable site of F protein induces the virus to fuse selectively with MMP-expressing cancer cells [45]. For quantifying tumor development and metastasis, the tumor cells are transduced with recombinant MV containing LacZ reporter gene to quantify beta-galactosidase enzymatic activity that is reported to be higher in invading cancer cells [46], [47]. Recombinant measles virus expressing neutrophil activating protein (NAP), a H. Pylori protein, stimutates the release of proimflammatory cytokines to boost anti-cancer immune response [48]. Iodine uptake in thyroid gland is mediated by a membrane ion channel called thyroidal sodium iodide symporter (NIS). Genetically modified measles expressing this ion channel are capable of inducing iodine symport, hence increased iodine concentration within cancer cells [49], [50], [51], [52]. The expression of programmed death ligand 1 or CD274 is up regulated in cancer cells to equip the latter to evade anti-tumor immune response. When oncolytic virotherapy is combined with immune checkpoint modulators, the tumor burden has been reported to be reduced by direct cell lysis and anti-tumor immunity is stimulated. Genetically modified measles vaccine strain encoding PDL1 antibody is found to be an efficient oncolytic agent against melanoma [53]. Recombinant measles vaccine strain encoding human lambda light immunoglobulin chain (MV-lambda) is used effectively against multiple myeloma. The myeloma cell, which over-expresses human kappa light immunoglobulin chain, secretes a chimeric immunoglobulin (one kappa and one lambda chain) upon infection. This converted marker is quantified easily by immunological techniques [54]. Granulocyte macrophage colony stimulating factor (GM-CSF) by genetically engineered measles vaccine strain has been reported to cause an up regulation of anti-tumor response by neutrophil [55]. The outcomes of clinical trials are encouraging [39]. The cancer cells are destroyed by syncytia formation mediated by the viral fusion (F) protein.
Table 1.
Genetically engineered measles virus used for oncolytic virotherapy.
| Cancer Type | Genetically Modified Virus |
|---|---|
| Blood Cancer | MV-CD20, MV-PNP, MV-LacZ |
| Breast Cancer | MV-NAP, MV-CEA |
| Ovarian Cancer | MV-CD38, MV-her/neu, MV-CEA, MV-NIS |
| Liver Cancer | MV-CD133, MV-MMP, MV-CEA, NV-NIS |
| Pancreatic Cancer | MV-PNP, MV-NIS |
| Colorectal Cancer | MV-CD133, MV-NIS, MV-PNP |
| Prostate Cancer | MV-NIS, MV-CEA |
| Brain Cancer | MV-CD38, MV-CD133 |
| Skin Cancer | MV-alphaPDL1 |
| Thyroid Cancer | MV-NIS |
| Myeloma | MV-NIS, MV-lambda, MV-CD38 |
| Fibrosarcoma | MV-MMP, MV-CD20 |
| Burkitt's Lymphoma | MV-GMCSF |
| Lung Cancer | MV-CEA, rMV-EGFP-SLAMblind |
| Mesothelioma | MV-NIS, MV-GMCSF |
7. Measles virus reverse genetics and rescue
The 293-3-46 helper cells, constitutively expressing T7 RNA polymerase and measles proteins (MV-N and MV-P) and derived from 293 cell line, are co-transfected with constructs containing cDNA of virus antigenome and viral polymerase kept under T7 promoter. Measles virus multiplication occurs within the helper cells leading to syncytia formation. A modified rescue technique has been adopted for improvement in viral rescue efficiency. Briefly 14–16 h after co-transfection of 293-3-46 cell, the transfection media is replaced. Following that at heat shock at 440C was given to the cells for 3 h. The transfected cells are transferred from each well of six-well dish to a Vero cell monolayer in a 10 cm dish. 3–5 days after transfer the plaques are counted and the recombinant virus is harvested [56].
The recombinant viruses are designed specifically so that progeny viruses will contain a tag- a six histidine peptide at the C-terminal of MV-H. This is recognized by Vero-a –His cells, Vero cells displaying a single chain membrane bound antibody, which recognizes the viral tag. This is termed as a ‘pseudoreceptor system’ and is utilized for viral rescue and propagation [49].
Recombinant viruses rescued employing reverse genetics system can be designed to (a) directly infect and lyse cancer cells, (b) deliver toxins to kill cancer cells, and (c) deliver therapeutic molecules to cancer cells targeting crucial cancer-specific molecules.
8. Limitations
N protein is reported to be apoptotic in nature [57] and interacts with protein tyrosine kinase 2 to suppress the expression of GTPases like RhoA and cdc42 to check cancer proliferation. But the P protein has an antagonistic role- it interacts with phosphoinositide 3-kinase and increases the expression of anti-apoptotic markers viz. Bcl-2 and Bcl-xL. So it will be a cause of concern during therapy [58]. The limitations do exist in oncolytic virotherapy- they are innate and adaptive immune response of the host, range of host and safety risks in using recombinant viruses [59].
9. Conclusion
Once the scientists were engrossed in designing an effective vaccine against measles but now measles Edmonston vaccine strain is being utilized as an effective therapeutic vaccine against cancer. Clinical trials are ongoing and the response is optimistic. Besides chemotherapy and radiotherapy, virotherapy has become an effective tool to treat malignancy. The genetically engineered virus that can boost the cancer suppressed immune system has been developed. The anti-tumor activity of CD8+ NKG2D+ cells is enhanced up on infection of hepatocellular carcinoma cells with Edmonston vaccine strain of measles virus and CD8+ NKG2D+ cells induce apoptosis via extrinsic pathway [60]. Other viruses of Paramyxoviridae family also possess this character and their oncolytic property has been studied vividly. However it should be kept in mind that long term vaccine against wild type measles has not yet been developed. The virus, which possesses a property to fight against cancer, is still one of the major causes of child mortality.
Acknowledgements
Our study was supported by grants received from UGC [No.2(1036)/SLS/2011-12] and DST, [EML PAGE NO. DST PURSE (090)] Government of India. SB is recipient of a Senior Research Fellowship of UGC.
Footnotes
Transparency document associated with this article can be found in the online version at doi:10.1016/j.bbrep.2017.12.004.
Appendix A. Transparency document
Transparency document
References
- 1.Biesbroeck L. Viral exanthems: an update. Dermatol. Ther. 2013;26(6):433–438. doi: 10.1111/dth.12107. [DOI] [PubMed] [Google Scholar]
- 2.Ludlow M. Pathological consequences of systemic measles virus infection. J. Pathol. 2015;235(2):253–265. doi: 10.1002/path.4457. [DOI] [PubMed] [Google Scholar]
- 3.Huiming Y. Vitamin A for treating measles in children. Cochrane Database Syst. Rev. 2005;4 doi: 10.1002/14651858.CD001479.pub3. (CD001479) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leuridan E. Measles outbreak in Europe: susceptibility of infants too young to be immunized. Vaccine. 2012;30(41):5905–5913. doi: 10.1016/j.vaccine.2012.07.035. [DOI] [PubMed] [Google Scholar]
- 5.Bourhis J.M. The intrinsically disordered C-terminal domain of the measles virus nucleoprotein interacts with the C-terminal domain of the phosphoprotein via two distinct sites and remains predominantly unfolded. Protein Sci. 2005;14(8):1975–1992. doi: 10.1110/ps.051411805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horikami S.M. Structure, transcription, and replication of measles virus. Curr. Top. Microbiol. Immunol. 1995;191:35–50. doi: 10.1007/978-3-642-78621-1_3. [DOI] [PubMed] [Google Scholar]
- 7.Dutch R.E. Paramyxoviruses. In Encyclopedia of Virology. 3rd ed. Academic Press; Cambridge, MA, USA: 2008. pp. 52–57. [Google Scholar]
- 8.Zhang X. Hsp72 recognizes a P binding motif in the measles virus N protein C-terminus. Virology. 2005;337(1):162–174. doi: 10.1016/j.virol.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 9.Bellini W.J. Measles virus P gene codes for two proteins. J. Virol. 1985;53(3):908–919. doi: 10.1128/jvi.53.3.908-919.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devaux P. Measles virus P protein gene products: conformational flexibility of the P/V protein amino-terminal domain and C protein infectivity factor function. J. Virol. 2004;78(21):11632–11640. doi: 10.1128/JVI.78.21.11632-11640.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krumm S.A. The measles virus nucleocapsid protein tail domain is dispensable for viral polymerase recruitment and activity. J. Biol. Chem. 2013;288(41):29943–29953. doi: 10.1074/jbc.M113.503862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pohl C. Measles virus M and F proteins associate with detergent-resistant membrane fractions and promote formation of virus-like particles. J. Gen. Virol. 2007;88(Pt 4):1243–1250. doi: 10.1099/vir.0.82578-0. [DOI] [PubMed] [Google Scholar]
- 13.Iwasaki M. The matrix protein of measles virus regulates viral RNA synthesis and assembly by interacting with the nucleocapsid protein. J. Virol. 2009;83(20):10374–10383. doi: 10.1128/JVI.01056-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muhlebach M.D. The measles virus fusion protein transmembrane region modulates availability of an active glycoprotein complex and fusion efficiency. J. Virol. 2008;82(22):11437–11445. doi: 10.1128/JVI.00779-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navaratnarajah C.K. The heads of the measles virus attachment protein move to transmit the fusion-triggering signal. Nat. Struct. Mol. Biol. 2011;18(2):128–134. doi: 10.1038/nsmb.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blumberg B.M. Measles virus L protein evidences elements of ancestral RNA polymerase. Virology. 1988;164(2):487–497. doi: 10.1016/0042-6822(88)90563-6. [DOI] [PubMed] [Google Scholar]
- 17.Liszewski M.K. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu. Rev. Immunol. 1991;9:431–455. doi: 10.1146/annurev.iy.09.040191.002243. [DOI] [PubMed] [Google Scholar]
- 18.Riley-Vargas R.C. CD46: expanding beyond complement regulation. Trends Immunol. 2004;25(9):496–503. doi: 10.1016/j.it.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Cattaneo R. Four viruses, two bacteria, and one receptor: membrane cofactor protein (CD46) as pathogens' magnet. J. Virol. 2004;78(9):4385–4388. doi: 10.1128/JVI.78.9.4385-4388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arbuckle J.H. The molecular biology of human herpesvirus-6 latency and telomere integration. Microbes Infect. 2011;13(8–9):731–741. doi: 10.1016/j.micinf.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Studebaker A.W. Treatment of medulloblastoma with a modified measles virus, Neuro. Oncol. 2010;12(10):1034–1042. doi: 10.1093/neuonc/noq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morra M. Characterization of SH2D1A missense mutations identified in X-linked lymphoproliferative disease patients. J. Biol. Chem. 2001;276(39):36809–36816. doi: 10.1074/jbc.M101305200. [DOI] [PubMed] [Google Scholar]
- 23.Noyce R.S. Nectin 4 is the epithelial cell receptor for measles virus. Trends Microbiol. 2012;20(9):429–439. doi: 10.1016/j.tim.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Wang N. CD150 is a member of family of gene that encode glycoproteins on the surface of hematopoietic cells. Immunogenetics. 2001;53(5):382–394. doi: 10.1007/s002510100337. [DOI] [PubMed] [Google Scholar]
- 25.Miyoshi J. Nectin and nectin-like molecules: biology and pathology. Am. J. Nephrol. 2007;27(6):590–604. doi: 10.1159/000108103. [DOI] [PubMed] [Google Scholar]
- 26.Whelan S.P. Transcription and replication of nonsegmented negative-strand RNA viruses. Curr. Top. Microbiol. Immunol. 2004;283:61–119. doi: 10.1007/978-3-662-06099-5_3. [DOI] [PubMed] [Google Scholar]
- 27.Gutsche I. Near-atomic cryo-EM structure of the helical measles virus nucleocapsid. Science. 2015;348(6235):704–707. doi: 10.1126/science.aaa5137. [DOI] [PubMed] [Google Scholar]
- 28.Rima B.K. The measles virus replication cycle. Curr. Top. Microbiol. Immunol. 2009;329:77–102. doi: 10.1007/978-3-540-70523-9_5. [DOI] [PubMed] [Google Scholar]
- 29.Iverson L.E. Localized attenuation and discontinuous synthesis during vesicular stomatitis virus transcription. Cell. 1981;23(2):477–484. doi: 10.1016/0092-8674(81)90143-4. [DOI] [PubMed] [Google Scholar]
- 30.Griffin D.E. Immune activation in measles. N. Engl. J. Med. 1989;320(25):1667–1672. doi: 10.1056/NEJM198906223202506. [DOI] [PubMed] [Google Scholar]
- 31.Avota E. Disruption of Akt kinase activation is important for immunosuppression induced by measles virus. Nat. Med. 2001;7(6):725–731. doi: 10.1038/89106. [DOI] [PubMed] [Google Scholar]
- 32.Karp C.L. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 1996;273(5272):228–231. doi: 10.1126/science.273.5272.228. [DOI] [PubMed] [Google Scholar]
- 33.TenOever B.R. Recognition of the measles virus nucleocapsid as a mechanism of IRF-3 activation. J. Virol. 2002;76(8):3659–3669. doi: 10.1128/JVI.76.8.3659-3669.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Polack F.P. Production of atypical measles in rhesus macaques: evidence for disease mediated by immune complex formation and eosinophils in the presence of fusion-inhibiting antibody. Nat. Med. 1999;5(6):629–634. doi: 10.1038/9473. [DOI] [PubMed] [Google Scholar]
- 35.Hanahan D. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 36.Varricchio C.G. Jones and Bartlett Publishers; Boston: 2004. A cancer source book for nurses; p. 229. [Google Scholar]
- 37.Enders J.F. Studies on an attenuated measles-virus vaccine. I. Development and preparations of the vaccine: technics for assay of effects of vaccination. N. Engl. J. Med. 1960;263:153–159. doi: 10.1056/NEJM196007282630401. [DOI] [PubMed] [Google Scholar]
- 38.Bellini W.J. Virology of measles virus. J. Infect. Dis. 1994;170(Suppl 1):S15–S23. doi: 10.1093/infdis/170.supplement_1.s15. [DOI] [PubMed] [Google Scholar]
- 39.Aref S. Measles to the Rescue: a Review of Oncolytic Measles Virus. Viruses. 2016;8(10) doi: 10.3390/v8100294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bucheit A.D. An oncolytic measles virus engineered to enter cells through the CD20 antigen. Mol. Ther. 2003;7(1):62–72. doi: 10.1016/s1525-0016(02)00033-3. [DOI] [PubMed] [Google Scholar]
- 41.Hammond A.L. Single-chain antibody displayed on a recombinant measles virus confers entry through the tumor-associated carcinoembryonic antigen. J. Virol. 2001;75(5):2087–2096. doi: 10.1128/JVI.75.5.2087-2096.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng K.W. Oncolytic measles viruses displaying a single-chain antibody against CD38, myeloma cell marker. Blood. 2003;101(7):2557–2562. doi: 10.1182/blood-2002-07-2195. [DOI] [PubMed] [Google Scholar]
- 43.Peng K.W. Non-invasive in vivo monitoring of track able viruses expressing soluble marker peptides. Nat. Med. 2002;8(5):527–531. doi: 10.1038/nm0502-527. [DOI] [PubMed] [Google Scholar]
- 44.Ungerechts G. Lymphoma chemovirotherapy: CD20-targeted and convertase-armed measles virus can synergize with fludarabine. Cancer Res. 2007;67(22):10939–10947. doi: 10.1158/0008-5472.CAN-07-1252. [DOI] [PubMed] [Google Scholar]
- 45.Springfeld C. Oncolytic efficacy and enhanced safety of measles virus activated by tumor-secreted matrix metalloproteinases. Cancer Res. 2006;66(15):7694–7700. doi: 10.1158/0008-5472.CAN-06-0538. [DOI] [PubMed] [Google Scholar]
- 46.Waszkiewicz N. The activity of serum beta-galactosidase in colon cancer patients with a history of alcohol and nicotine dependence: preliminary data. Postepy. Hig. Med. Dosw. 2013;67:896–900. doi: 10.5604/17322693.1064081. [DOI] [PubMed] [Google Scholar]
- 47.Buller C.J. Measurement of beta-galactosidase tissue levels in a tumor cell xenograft model. Methods Find. Exp. Clin. Pharmacol. 2003;25(9):713–716. doi: 10.1358/mf.2003.25.9.793338. [DOI] [PubMed] [Google Scholar]
- 48.Iankov I.D. Expression of immunomodulatory neutrophil-activating protein of helicobacter pylori enhances the antitumor activity of oncolytic measles virus. Mol. Ther. 2012;20(6):1139–1147. doi: 10.1038/mt.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng K.W. Targeting virus entry and membrane fusion through specific peptide/MHC complexes using a high-affinity T-cell receptor. Gene Ther. 2004;11(15):1234–1239. doi: 10.1038/sj.gt.3302286. [DOI] [PubMed] [Google Scholar]
- 50.Hadac M.E. Reengineering paramyxovirus tropism. Virology. 2004;329(2):217–225. doi: 10.1016/j.virol.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 51.Nakamura T. Rescue and propagation of fully retargeted oncolytic measles viruses. Nat. Biotechnol. 2005;23(2):209–214. doi: 10.1038/nbt1060. [DOI] [PubMed] [Google Scholar]
- 52.Watanabe M. Engineered serine protease inhibitor prevents furin-catalyzed activation of the fusion glycoprotein and production of infectious measles virus. J. Virol. 1995;69(5):3206–3210. doi: 10.1128/jvi.69.5.3206-3210.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Engeland C.E. CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Mol Ther. 2014;22(11):1949–1959. doi: 10.1038/mt.2014.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allen C. Retargeted oncolytic measles strains entering via the EGFRvIII receptor maintain significant antitumor activity against gliomas with increased tumor specificity. Cancer Res. 2006;66(24):11840–11850. doi: 10.1158/0008-5472.CAN-06-1200. [DOI] [PubMed] [Google Scholar]
- 55.Grossardt C. Granulocyte-macrophage colony-stimulating factor-armed oncolytic measles virus is an effective therapeutic cancer vaccine. Hum. Gene Ther. 2013;24(7):644–654. doi: 10.1089/hum.2012.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Radecke F. Rescue of measles viruses from cloned DNA. EMBO J. 1995;14(23):5773–5784. doi: 10.1002/j.1460-2075.1995.tb00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhaskar A. Expression of measles virus nucleoprotein induces apoptosis and modulates diverse functional proteins in cultured mammalian cells. PLoS One. 2011;6(4):e18765. doi: 10.1371/journal.pone.0018765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bhattacharjee S. Measles virus N and P proteins find interacting partners in HeLa cells. Int. J. Adv. Res. 2017;5(7):2691–2698. [Google Scholar]
- 59.Davis J.J. Oncolytic virotherapy for cancer treatment: challenges and solutions. J. Gene Med. 2005;7(11):1380–1389. doi: 10.1002/jgm.800. [DOI] [PubMed] [Google Scholar]
- 60.Chen A. Oncolytic measles virus enhances antitumour responses of adoptive CD8+NKG2D+ cells in hepatocellular carcinoma treatment. Sci. Rep. 2017;7(1):5170. doi: 10.1038/s41598-017-05500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document