Abstract
JAK2V617F mutation is found in about 60% of cases of essential thrombocytemia (ET) and represents a driving mutation. Immune thrombocytopenia (ITP) is an autoimmune disease characterized by a low platelet (PLT) count. So far, only 2 reports described ET following ITP. For the first time we analyzed in a patient the JAK2V617F allele burden at ITP onset occurred 13 years before the ET diagnosis and found the presence of a small clone JAK2V617F positive clone (3%) raised to 27% in the following years. The association of ET and ITP could suggest similar pathogenetic mechanisms that should be further investigated.
Keywords: Essential thrombocytemia, Immune thrombocytopenia, JAK2V617F mutation
1. Introduction
The cytokine receptor-JAK-STAT pathway is a key signaling pathway during myelopoiesis. JAK2V617F mutation is found in about 60% of cases of essential thrombocytemia (ET) and represents a driving mutation that allows a cytokine-independent growth of megakaryocytes [1]. Although it is accepted that JAK2V617F drives clonal proliferation in ET, less is known about what factors influence the onset and development of the disease [2]. JAK2 V617F mutation may also be present in individuals without signs of myeloprolifrative neoplasm (MPNs), but its the significance is still not clear [3], [4]. In general population JAK2 V617F mutation is associated with increased morbidity and mortality, although only present in 18 of 10 507 (0,2%) persons [5]. Immune thrombocytopenia (ITP) is an acquired autoimmune disorder of increased platelet (PLT) destruction and decreased platelet production, that may be associated to bleeding, especially in the skin and the mucosa [6]. ITP can be primary (80%) whereas other 20% occurs in the setting of lymphoproliferative disorders; systemic lupus erithematosus, or other autoimmune disorders; infections such as hepatitis C, HIV, and Helicobacter pylori. ET and ITP are different blood diseases with PLT counts at opposite extremes. So far, only 2 previous reports described ET following ITP [7], [8]. Hereby we describe a third case of ET diagnosed 13 years after ITP diagnosis. To our best knowledge, for the first time, we retrospectively showed the presence of a JAK2V617F clone since the onset of ITP.
2. Case report
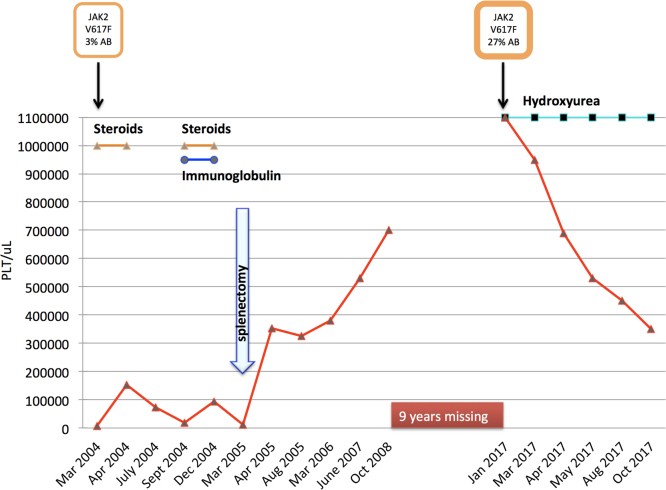
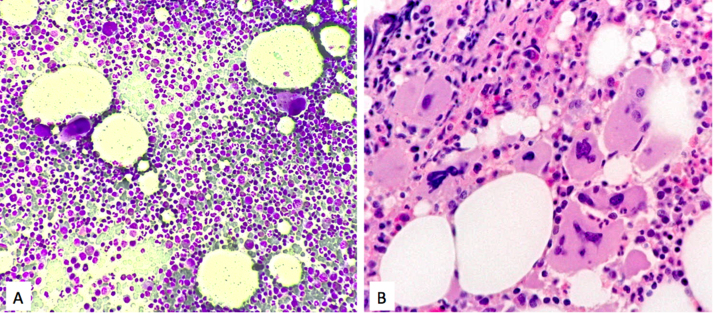
In March 2004, a 72 year old woman suffering from rheumatoid arthritis, presented with petechiae, ecchymoses, epistaxis and a platelet (PLT) count of 13,000/μL was diagnosed with ITP (Fig. 1). White blood cell count (WBC) and hemoglobin (Hb) were within the normal ranges. Coagulation tests were normal. Tests for viral B and C hepatitis, CMV, and HIV were all negative. Bone marrow aspirate showed increased megakaryocytes and normal myeloid/erythroid ratio (Fig. 2A). Treatment with high dose prednisone (1 mg/Kg) was started with good PLT response (154,000/μL), but the drug was not well tolerated due to arterial hypertension and high blood sugar value. After 1 month prednisone was tapered and stopped in July. However, the platelet count fell and bleeding symptoms recurred. Prednisone was reinitiated in combination with immunoglobulin (400 mg/Kg for 5 days), obtaining only a transient response. Due to poor efficacy and tolerance, in March 2005, the patient underwent splenectomy, achieving a rapid normalization of PLT count (354,000/μL). Over the following 3 years PLT count increased to 700,000/μL but this was interpreted as a consequence of splenectomy; only treatment with aspirin was prescribed. Thereafter, the patient was lost to follow-up. After 9 years, in January 2017, the patient (age 85) was again referred to our department by her general practitioner owing to a PLT count of 1100,000/μL. A bone marrow trephine was performed, showing increased cellularity and several clusters of large to giant mature megacaryocytes with folded nuclei (Fig. 2B). A qualitative polymerase chain reaction (ASO-PCR) analysis demonstrated the presence of JAK2V617F mutation in her peripheral blood. An allele burden (AB) of 27% was obtained by quantitative real-time polymerase chain reaction (qRT-PCR) using JAK2MutaQuant™ (Ipsogen Inc.,Qiagen Germany). We retrospectively analyzed the presence of JAK2V617F mutation in the bone marrow smear of 2004: genomic DNA was recovered from a stained smear by scraping off the cells into a tube using a scalpel blade for manual extraction using the DNA blood mini kit (Qiagen, Germany) according to the manufacturer's instructions. The AB in 2004 was 3%. Based on WHO 2008 criteria [9] the patient was diagnosed with ET and treated with Hydroxyurea (HU) 500 mg/die. PLT count decreased progressively until reaching normal range in August 2017. Currently the patient is doing well on the same dosage of HU.
Fig. 1.
: Clinical outcome and platelet (PLT) count in a ITP patient associated with 3% of a JAK2V617F allele burden (AB) followed ET with 27% of AB after 13 years.
Fig. 2.
A: Bone marrow film of ITP diagnosed in 2004 (magnification 20×). Megakaryocytes are diffuse and slightly increased in number, with normal features.
B. Bone marrow trephine of ET diagnosed in 2017 (magnification 20×). Clusters of large to giant mature megacaryocytes with folded nuclei.
3. Discussion
JAK2V617F represents a driving mutation, and almost all healthy individuals carrying it develop a MPN within 5–9 years [10]. Nevertheless, the factors that influence the development of disease are poorly understood. An association between previous autoimmune disease and increased risk of MPN has been suggested [11]. Huang et al. suggested the possibility of the coexistence of a clonal MPN with low JAK2V617F allele burden and autoimmune-associated cytopenia, where thrombocytosis was probably masked by immune-mediated cellular destruction in the spleen pre-operatively, but data on JAK2V617F at ITP onset nor bone marrow finding at ET transformation were not available [7]. Notably, in our experience as well, progressive thrombocytosis developed after splenectomy. Sobas et al. reported data on bone marrow trephine in a case of JAK2V617F positive ET 11 years after ITP, but mutation status at ITP diagnosis was not available. An inverted case of ITP following JAK2V617F ET was also reported [12]. For the first time we retrospectively analyzed the JAK2V617F AB at ITP onset occurred 13 years before the ET diagnosis and found the presence of a small clone JAK2V617F positive clone (3%) raised to 27% in the following years. All described cases of ET following ITP are associated with JAK2V617F mutation and no reports are known with Calreticuline or MPL. The association of ET and ITP could suggest similar pathogenetic mechanisms that should be further investigated.
4. Conclusion
In conclusion, splenectomy and autoimmunity might be factors influencing the development of MPN and JAK2V617F mutation status analysis could be suggested to those ITP patients candidate to splenectomy or treatment with new thrombopoietin receptor agonists.
Conflict of interest statement
The authors declare that there are no conflicts of interest to disclose.
References
- 1.Cahu X., Constantinescu S.N. Oncogenic drivers in myeloproliferative neoplasms: from JAK2 to calreticulin mutations. Curr. Hematol. Malig. Rep. 2015;10:335–343. doi: 10.1007/s11899-015-0278-x. [DOI] [PubMed] [Google Scholar]
- 2.Tapper W., Jones A.V., Kralovics R. Genetic variation at MECOM, TERT, JAK2 and HBS1L-MYB predisposes to myeloproliferative neoplasms. Nat. Commun. 2015;6:6691. doi: 10.1038/ncomms7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Stefano V., Fiorini A., Rossi E. Incidence of the JAK2 V617F mutation among patients with splanchnic or cerebral venous thrombosis and without overt chronic myeloproliferative disorders. J. Thromb. Haemost. 2007;5:708–714. doi: 10.1111/j.1538-7836.2007.02424.x. [DOI] [PubMed] [Google Scholar]
- 4.Sidon P., El Housni H., Dessars B., Heimann P. The JAK2V617F mutation is detectable at very low level in peripheral blood of healthy donors. Leukemia. 2006;20:1622. doi: 10.1038/sj.leu.2404292. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen C., Birgens H.S., Nordestgaard B.G. The JAK2 V617F somatic mutation, mortality and cancer risk in the general population. Haematologica. 2011;96:450–453. doi: 10.3324/haematol.2010.033191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Audia S., Mahévas M., Samson M. Pathogenesis of immune thrombocytopenia. Autoimmun. Rev. 2017;16:620–632. doi: 10.1016/j.autrev.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Huang C.E., Chen Y.Y., Liu J.L. JAK2V617F mutation in immune thrombocytopenia. Thromb. Res. 2016;144:149–151. doi: 10.1016/j.thromres.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 8.Sobas M.A., Wróbel T., Zduniak K. Immune thrombocytopenia and JAK2V617F positive essential thrombocythemia: literature review and case report. Case Rep. Hematol. 2017;2017:3725089. doi: 10.1155/2017/3725089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thiele J., Kvasnicka H.M. The 2008 WHO diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis. Curr. Hematol. Malig. Rep. 2009;4:33–40. doi: 10.1007/s11899-009-0005-6. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen C., Bojesen S.E., Nordestgaard B.G. JAK2V617F somatic mutation in the general population: myeloproliferative neoplasm development and progression rate. Haematologica. 2014;99:1448–1455. doi: 10.3324/haematol.2014.107631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kristinsson S.Y., Landgren O., Samuelsson J. Autoimmunity and the risk of myeloproliferative neoplasms. Haematologica. 2010;95:1216–1220. doi: 10.3324/haematol.2009.020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farhat M.H., Kuriakose P., Jawad M. Sequential occurrence of thrombotic thrombocytopenic purpura, essential thrombocythemia, and idiopathic thrombocytopenic purpura in a 42-year-old African-American woman: a case report and review of the literature. J. Med Case Rep. 2012;6:93. doi: 10.1186/1752-1947-6-93. [DOI] [PMC free article] [PubMed] [Google Scholar]