Abstract
Molecular biomarkers play little role in the current treatment of metastatic castration resistant prostate cancer (CRPC). The advent of next-generation sequencing (NGS) has enabled the comprehensive molecular characterization of the genomic and transcriptomic landscape of both untreated primary prostate cancer and CRPC. Recent studies demonstrating the feasibility of inter-institution studies obtaining and NGS profiling of metastatic biopsies, targeted NGS approaches applicable to routine formalin fixed paraffin embedded (FFPE) specimens, and NGS approaches applicable to circulating DNA and circulating CTCs portend near term adoption of NGS approaches in the management and treatment of CRPC. Important considerations in the clinical implementation of NGS include inter and intra-patient heterogeneity, disease progression to neuroendocrine/small cell prostate carcinoma and incorporation into clinical trial design to demonstrate clinical utility. Herein we review the recent progress in NGS based characterization of CRPC to understand disease biology and inform on barriers to widespread clinical adoption.
Keywords: exome sequencing, transcriptome sequencing, cell free DNA, circulating tumor cells, PARP inhibitor, molecular landscape, small cell carcinoma
Comprehensive next-generation sequencing (NGS) of primary prostate cancer and castration-resistant prostate cancer (CRPC) has provided a foundational understanding of the prostate cancer genomic and transcriptomic landscape, elucidating key biological and molecular components of progression and potential therapeutic opportunities1,2. NGS-based profiling of CRPC has identified the most frequent molecular alterations in advanced, treatment refractory disease, as well as demonstrated the unique therapeutic challenges in using molecular information to guide treatment1. At present, NGS-based profiling can enable relatively fast, accurate, and comprehensive assessment of driving genomic and transcriptomic alterations in advanced cancer. However, prostate cancer is a dynamic, inherently heterogeneous disease, and within this context, considerable challenges remain around how best to leverage NGS-based screening, prognostic, and disease monitoring strategies in the context of current standards of care 3. Here we review some of the key NGS-based approaches and findings that are enabling the tracking of the evolution of metastatic CRPC, including applications for informing treatment, and explore challenges for prospective implementation of NGS-based assays aimed at guiding precision medicine approaches for CRPC.
Genomic/Transcriptomic Landscape and NGS Profiling in CRPC
Multiple recent large-scale sequencing studies have helped to characterize the diverse genomic and transcriptomic landscape of both primary prostate cancer and CRPC, as well as small cell/neuroendocrine prostatic carcinoma (NePC)1,2,4–6. These studies have leveraged comprehensive DNA and RNA sequencing of fresh frozen tissue samples, describing a heterogeneous set of somatic alterations present in CRPC and/or NePC, including those enriched or unique in CRPC or NePC compared to primary disease 1,2. Alterations of particular relevance include frequent adaptive AR amplifications and mutations often conferring resistance to first and second generation anti-androgen therapies, TP53 and RB1 mutations and deletions particularly in NePC, and an increased prevalence of germline or somatic alterations in DNA repair pathway genes in CRPC1,2,4–6. Comprehensive RNA sequencing of advanced prostate cancers have also been recently reported, building on prior expression profiling studies of CRPC2,4–9. Sequencing based approaches for assessing the CRPC transcriptome may have particular relevance given that the presence of AR splice variants in both primary and advanced prostate cancer may lead to increased resistance to second generation anti-androgens10. Overall, these sequencing initiatives have helped to outline the feasibility and efficacy of comprehensive (whole genome, whole exome) sequencing-based profiling of CRPC patients in large-scale single- or multi-institutional collaborations1.
Technical challenges persist, however, for widespread prospective implementation of comprehensive NGS based profiling of patients with advanced prostate cancer. Access to fresh frozen tissue biopsy samples is often limited, leaving formalin-fixed, paraffin-embedded (FFPE) tissue samples as the primary source analyte for many sequencing-based assays11–13. Whole genome or transcriptome scale sequencing of routine FFPE clinical core biopsy samples has proven challenging14. Further, even when fresh frozen tissue is obtainable, it is still generally cost-prohibitive for many clinical centers to deploy comprehensive genomic and transcriptomic NGS-based profiling of CRPC samples in a prospective fashion 12,15. Additionally, routine biopsy sampling of metastases in patients with advanced disease is not always performed given the utility of serum PSA as a recurrence/response biomarker, limiting tissue availability for widespread understanding of molecular relationships between primary and metastatic lesions and hindering development of personalized treatment approaches for individuals with CRPC16.
Several groups have shown that targeted DNA and RNA sequencing of FFPE tissue samples may be a feasible strategy for profiling clinically relevant somatic alterations in both primary and advanced prostate cancer 11–13. Targeted strategies have shown promise in assaying recurrently altered oncogenes and tumor suppressors, genes with frequent copy number alterations, and driver gene fusions such as TMPRSS2-ERG in order to identify the salient driving molecular alterations present in an advanced prostate cancer. Both targeted and more comprehensive approaches have also proven effective at identifying alterations that define well-established prostate cancer subtypes, including samples with ETS family gene fusions as well as those with SPOP mutations, SPINK1 overexpression, CHD1 mutations and deletions, and IDH1 mutations 1,2,6,17–19. Given the initial success in tissue-based targeted sequencing of CRPC, some have even proposed strategies for monitoring disease via rebiopsy of lesions profiled pre- and post-treatment paired with NGS profiling8. However, these targeted and comprehensive approaches all require repeat invasive procedures for individual patient tracking, presenting limited feasibility for widespread clinical implementation, particularly in an era where biopsy of metastatic lesions to obtain material for molecular testing is not routinely reimbursed.
For this reason, recent efforts reporting efficacy of non-invasive NGS-based approaches to identify and track clinically relevant somatic alterations over time within patients with CRPC may be particularly relevant in the near term17,20–24. These approaches have shown that by targeted and more comprehensive sequencing of cell-free DNA (cfDNA), somatic point mutations, insertions/deletions, and copy-number alterations can be detected across a broad spectrum of tumor-derived cfDNA fractions. Alterations detected have highlighted or confirmed a number of important resistance mechanisms that emerge over the course of anti-androgen treatment (including both AR amplifications and point mutations), as well as suggesting that AR amplification alone may be a strong predictor of resistance to second generation anti-androgens abiraterone and enzalutamide17,21–23. Perhaps most importantly, this work has demonstrated dynamic temporal changes in circulating tumor DNA fractions in cfDNA representing different tumor subclones over the course of anti-androgen treatment, hinting at myriad molecular changes in primary and metastatic lesions occurring in response to substantial therapeutic and fitness-related selective pressures21.
Further work suggesting utility of whole exome and RNA sequencing from circulating tumor cells (CTCs) in patients with advanced prostate cancer has also been reported 25,26, however the clinical utility of these approaches have not been fully investigated. Overall, these non-invasive approaches represent an important first step in understanding the dynamic nature of tumor clone and subclone representation detectable in the blood, as well as identifying technical hurdles that must be overcome for widespread clinical use of non-invasive NGS-based monitoring of molecular alterations in patients with advanced disease. Substantive work is required to enhance the sensitivity of these non-invasive approaches and validate the prognostic utility of these tools in personalizing patient care for individuals with advanced prostate cancer. Of note, very focused assays (including single gene assays) may be the final clinical assays used after more discovery based NGS approaches have defined critical alterations, such as RT-PCR based assays for ARv7 expression in CTCs10.
Intertumoral heterogeneity
Despite the broad characterization of the genomic and transcriptomic landscape of castrate-resistant prostate cancer (CRPC) and efforts to non-invasively characterize molecular alterations during treatment, a complete understanding of the intra-patient progression from localized primary prostate cancer to metastatic castrate-resistant disease remains elusive, limited primarily by the long timeline of typical prostate cancer progression that complicates longitudinal sample collection. Complicating the long arc of disease progression is the relatively recent discovery of substantial intra- and inter-individual heterogeneity for patients with metastatic disease, which may complicate development of personalized approaches to CRPC treatment, particularly for AR based therapies3,5,27. Prostate cancer is an inherently multifocal disease28, with recent reports describing multiple clonal expansions even within a single morphologic tumor focus29,30. While multiple reports support the monoclonal origin of lethal metastatic CRPC3,5,6,31, recent evidence supports the potential of lethal metastases arising from one or several clones or subclones in the primary tumor 5,27,31. Likewise recent work in heavily treated patients suggests there may be a more complex series of metastasis-to-metastasis or metastasis-to-surgical bed seeding events that enable widespread metastatic spread as well as elimination and recurrence of individual clones during treatment 3,21,32. While truncal mutations are typically shared across most lesions, these reports suggest substantial inter-tumoral heterogeneity may be present in patients with CRPC, although the degree of “relevant” heterogeneity is less well established3,5. Nevertheless, this heterogeneity presents significant challenges for using NGS to inform clinical decision-making in patients with CRPC, primarily owing to the limited molecular resolution available from a single core biopsy sample of a particular lesion in an inherently multi-focal, heterogeneous disease. Likewise, use and interpretation of non-invasive NGS-based disease monitoring approaches must account for the heterogeneous mix of physical locations from which tumor derived cfDNA or CTCs being assayed were originally shed.
Prognostic and screening considerations
While NGS-based profiling has played a critical role in elucidating key components of prostate cancer biology and some aspects of disease progression, NGS-based prognostic assays are still limited. Existing tissue-based prognostic assays, including Oncotype DX, Prolaris, Promark and Decipher, use RT-PCR, protein expression, or genomewide expression arrays to determine gene/protein expression for their component markers33. Ultimately, orthogonal NGS-based validation of these assays, incorporation of DNA based alterations, and their robustness to multifocality and intratumoral heterogeneity will likely be necessary to further improve prostate cancer prognosis and prediction.
Meanwhile, prospective sequencing of select genes may be an important consideration for germline screening and advanced disease monitoring in patients at risk for primary or advanced prostate cancer. Germline alterations in BRCA2 and BRCA1 have been shown to increase the lifetime risk for prostate cancer 34–37 and germline BRCA2 carriers show worse prognosis than non-BRCA2 carriers 38. DNA damage repair genes are also an important consideration, particularly in advanced prostate cancer, as approximately 20% of CRPC patients have been shown to harbor germline and/or somatic alterations in DNA damage repair genes such as BRCA1, BRCA2, or ATM 1,6,11,12. With only ~3% of primary prostate cancer reporting germline or somatic alterations in BRCA1 or BRCA2, there may need to be a particular focus in screening for or monitoring BRCA1/BRCA2 alterations in men with previous primary prostate cancer diagnosis or at higher baseline risk for primary disease, particularly in light of the potential predictive nature of these alterations (see below). Sequencing of additional genes that predispose men to higher risk of prostate cancer (e.g., HOXB13) may also be warranted 39.
Neuroendocrine/small cell prostate cancer
NGS profiling has also informed on the subset of patients who develop AR-independent small cell/neuroendocrine prostate cancer (NePC)4,40. The increasing relevance of NePC (whether due to selection by more potent AR signaling therapies or increased survival of patients with CRPC beyond AR driven disease) has led to investigation on both the morphologic and molecular characterization of this disease subtype4,12,40,41. Importantly, both single gene and comprehensive NGS approaches support transdifferentiation as the typical mechanism of NePC development, where NePC is clonally related to preceding AR driven disease4,42,43. NePC, particularly small cell carcinoma, shows a unique transcriptional profile (typically AR signaling low, neuroendocrine gene expression high and proliferation high) as well as characteristic genomic alterations including RB1 and TP53 loss and MYCN (or MYCL) amplification40,44–46. Of particular relevance, comprehensive NGS interrogation has demonstrated that typical adenocarcinoma and small cell carcinoma represent a spectrum, with the opportunity for molecular assessment to complement clinicopathologic assessment in determining treatment strategies4,12,41,46,47.
Clinical trial design
NGS-based molecular stratification strategies have emerged as a way to more intelligently enroll patients most likely to benefit in targeted therapy clinical oncology trials. However, recent reports indicate only 2% of all clinical trials enrolling patients with prostate cancer from September 2011 to September 2014 used biomarkers or molecular alterations to select patients for trial enrollment 48. Conversely, the 20% of CRPC tumors showing germline or somatic alterations in DNA damage repair genes (most frequently BRCA2, BRCA1, or ATM) carry clear implications for ongoing and prospective clinical trial design, given the success of poly ADP ribose polymerase (PARP) inhibitors in BRCA-deficient advanced breast and ovarian cancers49. Of particular note, Mateo et al. recently reported a phase II study of PARP inhibition with olaparib in metastatic CRPC, with response rates >80% in cases with germline or somatic alterations in DNA damage repair genes (BRCA2, BRCA1, ATM, CHEK2, FANCA, and PALB2) compared to 6% in patients without DNA damage repair gene alterations50, leading to breakthrough status. This study underscores the benefit for employing NGS assay guided patient selection for clinical trial design, where even rare potentially targetable alterations (e.g. those in RAF family members and IDH1) can be assessed enabling umbrella or basket trials, similar to the approach taken by the NCI-MATCH trial (NCT02465060), where NGS from metastatic FFPE samples guides enrollment on patient-specific molecular alterations.
Additional NGS-based Applications in Treatment of CRPC
Given the reported inter-tumoral heterogeneity and temporal changes in circulating DNA from tumor subclones in response to therapeutic pressures, utilizing molecular sequencing to improve prostate cancer prognostication and therapeutic prediction may be particularly challenging 51. Challenges including technical limitations, tissue availability, the inherent biological variability in prostate cancer and the established utility (and known limitations) of serum PSA mean that serial monitoring and disease tracking in patients at risk or with CRPC is still a fledgling enterprise. For patients on anti-androgen therapy, PSA monitoring and imaging are typically used as a primary metric for response to treatment, but we expect prospective NGS-based tracking strategies may improve sensitivity in screening for and detecting genomic & transcriptomic alterations – including AR mutations, splice variants, and amplifications – signaling the start of or susceptibility to treatment resistance at earlier time points than existing strategies 52. It must be stressed however, that the clinical adoption of NGS to detect recurrence or resistance based on ultrasensitive detection of molecular alterations will require proven benefit of initiating/changing therapy at that time vs. waiting for clinical progression.
Although comprehensive NGS is critical to characterize the molecular landscape of CRPC, we anticipate that small, customized targeted sequencing panels compatible with DNA or RNA isolated from tissue, blood, or urine will prove invaluable for the eventual treatment guidance and monitoring of disease- or progression-associated alterations in patients with CRPC, much like those employed in recent reports 17,21. Alternatively, some groups have reported utility in using low coverage whole-genome sequencing to screen cfDNA in patients with CRPC for clinically informative copy number alterations (including AR amplifications), a strategy which could help complement a more targeted NGS approach given the high prevalence of driving copy-number alterations in CRPC 1,24.
Recent discoveries have also characterized a series of long non-coding RNAs (lncRNAs) associated with aggressive prostate cancer, most notably SChLAP1, which is prognostic in localized prostate cancer7,9,53, and the landscape of lncRNAs in CRPC remains poorly described. Together with work summarizing the expression of myriad AR splice variants (several of which may confer resistance to second-generation anti-androgens) in both primary and advanced prostate cancer10,54, these reports highlight a potential key role for serial RNA-based NGS profiling in guiding treatment of patients with CRPC. However, established clinical benefit associated with these newly discovered mechanisms and biomarkers is still being explored in ongoing trials, and systematic validation of the clinical and prognostic utility is warranted prior to widespread implementation.
Epigenomic analyses in localized and advanced prostate cancer have also reported preliminary evidence supporting the role of epigenetic alterations as potential biomarkers for both aggressive and castrate-resistant prostate cancer, however limited work has been carried out to determine whether these markers can be reliably detected non-invasively55. These analyses have, however, helped to identify the role that epigenetic AR co-activators such as TIF2, p300, CBP, and EZH2 play in CRPC, nominating important candidates for NGS-based gene expression profiling over the course of disease 55,56. Ultimately, the prognostic ability for proposed epigenetic biomarkers will require more systematic evaluation before being considered for use in guiding treatment decisions in CRPC.
Conclusions
In the near term future, tissue-based NGS profiling coupled with non-invasive (cfDNA- or CTC-based) NGS profiling will likely present a powerful approach for capturing a relatively complete assessment of the intra- and inter-tumoral molecular heterogeneity present in patients with advanced prostate cancer and identifying the most promising treatment hypotheses. Non-invasive tracking of clinically relevant somatic alterations in patients with advanced prostate cancer paired with standard-of-care PSA screening should enable more comprehensive feedback for clinicians both prior to and in response to treatment, enabling a nimbler, more pro-active treatment approach if such approaches improve outcome beyond waiting for clinical progression. Comprehensive whole genome and transcriptome sequencing in patients with untreated and treated metastatic disease will continue to refine our understanding of the relationship between metastatic spread and both fundamental and treatment-induced molecular heterogeneity. Intelligently designed germline screening approaches could help to identify men at higher risk for development of aggressive or metastatic prostate cancer, while improved non-invasive approaches hold significant promise for better understanding CRPC disease progression through identification of novel biomarkers and early detection of treatment resistance mechanism. Ultimately, significant contributions from pathology, oncology, bioinformatics, basic science, and cancer genomics will be required to demonstrate clinical utility of NGS enabling widespread adoption in the treatment of men with CRPC.
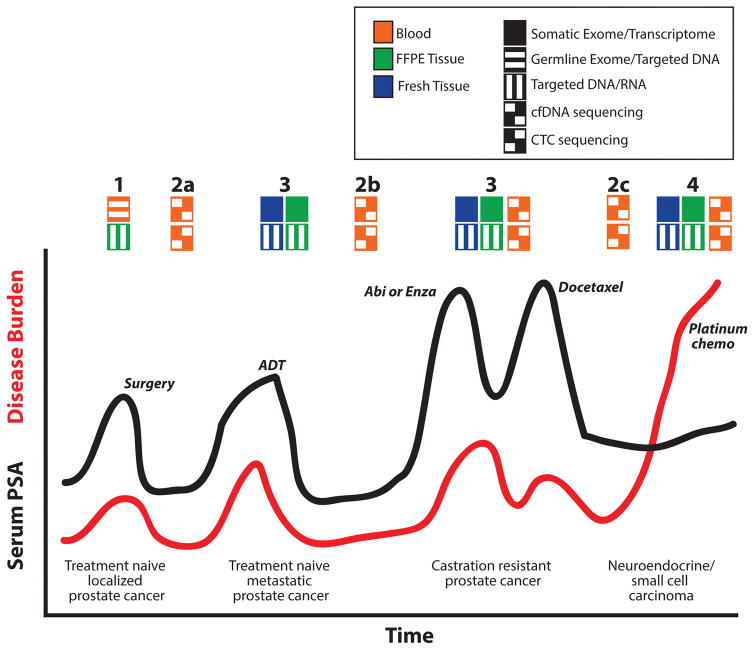
Figure 1.
Potential clinical utility of next generation sequencing (NGS) during prostate cancer progression. A timeline of serum PSA (black line) and disease burden (red line) along with treatments (italics) are shown for a hypothetical patient who progresses from localized untreated prostate cancer diagnosed and treated by radical prostatectomy to untreated treatment naïve metastatic prostate cancer to castration resistant prostate cancer (CRPC) and eventually neuroendocrine/small cell carcinoma. Opportunities for NGS to guide clinical management are shown above the graph according to the biocompartment assessed (color of the box) and NGS approach (pattern of the box) as indicated in the legend. At diagnosis (1), germline NGS assessment may be utilized to identify predisposing germline variants that may inform on later therapy and identify hereditary predisposition. Likewise, targeted DNA and RNA based assessment of FFPE biopsy and/or prostatectomy tissues may be used for prognosis and assessment of presumed clonal alterations that can be tracked and/or targeted during progression. NGS of cfDNA and/or CTCs isolated from blood may be used for non-invasive assessment of disease recurrence (2a) and assessment of clonal dynamics upon treatment. Diagnosis of metastatic disease by biopsy enables targeted DNA and RNA assessment of FFPE tissue (or comprehensive assessment if fresh tissue is obtained [most likely in the translational research setting]), and may have utility in predicting response to ADT or enrollment on clinical trials in the castration sensitive space (3). In addition to monitoring for development of CRPC after ADT (2a), NGS of cfDNA and/or CTCs may have particular utility for predicting response to second generation anti-androgens (such as abiraterone [abi] or enzalutamide [enza]) based on assessment of AR amplifications, mutations, or splice variant expression. Likewise, targeted or comprehensive NGS of CRPC biopsy tissue may have utility for identifying resistance mechanisms, novel targetable alterations, and identification of alterations enabling enrollment on umbrella and/or basket studies (3). NGS assessment of cfDNA and/or CTCs may be useful as a non-invasive complement to serum PSA to identify the development of AR independent clones (2c) and neuroendocrine/small cell prostate carcinoma when serum PSA may not be an accurate measurement of disease burden. Lastly, NGS of neuroendocrine/small cell prostate carcinoma (4) tissue may identify potential novel targetable alterations that developed during progression.
Acknowledgments
Source of Funding: S.A.T. has received research support from Astellas and had a sponsored research agreement with, and has received travel support from ThermoFisher Scientific/Life Technologies. S.A.T. is a co-founder and equity holder in Strata Oncology. D.H.H. has received travel support from ThermoFisher Scientific. This work supported in part by the National Institutes of Health (R01 CA 183857 to S.A.T). S.A.T. is supported by the A. Alfred Taubman Medical Research Institute and the Prostate Cancer Foundation.
Footnotes
Conflicts of Interest: The University of Michigan has been issued a patent on the use of ETS gene fusions in patients with prostate cancer, on which S.A.T. is listed as a co-inventor; similarly, a patent application on the use of SPINK1 in patients with prostate cancer has also been filed. The diagnostic field of use has been licensed to Gen-Probe (California, USA), which has sublicensed rights to Ventana Medical Systems (Arizona, USA). S.A.T. has consulted for, and received honoraria from AbbVie, Astellas, Jannsen and Ventana Medical Systems.
References
- 1.Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215–1228. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Research N. The Molecular Taxonomy of Primary Prostate Cancer. Cell. 2015;163(4):1011–1025. doi: 10.1016/j.cell.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gundem G, Van Loo P, Kremeyer B, et al. The evolutionary history of lethal metastatic prostate cancer. Nature. 2015;520(7547):353–357. doi: 10.1038/nature14347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beltran H, Prandi D, Mosquera JM, et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med. 2016;22(3):298–305. doi: 10.1038/nm.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar A, Coleman I, Morrissey C, et al. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat Med. 2016;22(4):369–378. doi: 10.1038/nm.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grasso CS, Wu YM, Robinson DR, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487(7406):239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prensner JR, Zhao S, Erho N, et al. RNA biomarkers associated with metastatic progression in prostate cancer: a multi-institutional high-throughput analysis of SChLAP1. Lancet Oncol. 2014;15(13):1469–1480. doi: 10.1016/S1470-2045(14)71113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajan P, Stockley J, Sudbery IM, et al. Identification of a candidate prognostic gene signature by transcriptome analysis of matched pre- and post-treatment prostatic biopsies from patients with advanced prostate cancer. BMC Cancer. 2014;14:977. doi: 10.1186/1471-2407-14-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ylipaa A, Kivinummi K, Kohvakka A, et al. Transcriptome Sequencing Reveals PCAT5 as a Novel ERG-Regulated Long Noncoding RNA in Prostate Cancer. Cancer Res. 2015;75(19):4026–4031. doi: 10.1158/0008-5472.CAN-15-0217. [DOI] [PubMed] [Google Scholar]
- 10.Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371(11):1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beltran H, Yelensky R, Frampton GM, et al. Targeted next-generation sequencing of advanced prostate cancer identifies potential therapeutic targets and disease heterogeneity. Eur Urol. 2013;63(5):920–926. doi: 10.1016/j.eururo.2012.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hovelson DH, McDaniel AS, Cani AK, et al. Development and validation of a scalable next-generation sequencing system for assessing relevant somatic variants in solid tumors. Neoplasia. 2015;17(4):385–399. doi: 10.1016/j.neo.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng HH, Klemfuss N, Montgomery B, et al. A pilot study of clinical targeted next generation sequencing for prostate cancer: Consequences for treatment and genetic counseling. Prostate. 2016 doi: 10.1002/pros.23219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cieslik M, Chugh R, Wu YM, et al. The use of exome capture RNA-seq for highly degraded RNA with application to clinical cancer sequencing. Genome Res. 2015;25(9):1372–1381. doi: 10.1101/gr.189621.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Allen EM, Wagle N, Stojanov P, et al. Whole-exome sequencing and clinical interpretation of formalin-fixed, paraffin-embedded tumor samples to guide precision cancer medicine. Nat Med. 2014;20(6):682–688. doi: 10.1038/nm.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beltran H, Rubin MA. New strategies in prostate cancer: translating genomics into the clinic. Clin Cancer Res. 2013;19(3):517–523. doi: 10.1158/1078-0432.CCR-12-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romanel A, Gasi Tandefelt D, Conteduca V, et al. Plasma AR and abiraterone-resistant prostate cancer. Sci Transl Med. 2015;7(312):312re310. doi: 10.1126/scitranslmed.aac9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310(5748):644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 19.Tomlins SA, Rhodes DR, Yu J, et al. The role of SPINK1 in ETS rearrangement-negative prostate cancers. Cancer Cell. 2008;13(6):519–528. doi: 10.1016/j.ccr.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azad AA, Volik SV, Wyatt AW, et al. Androgen Receptor Gene Aberrations in Circulating Cell-Free DNA: Biomarkers of Therapeutic Resistance in Castration-Resistant Prostate Cancer. Clin Cancer Res. 2015;21(10):2315–2324. doi: 10.1158/1078-0432.CCR-14-2666. [DOI] [PubMed] [Google Scholar]
- 21.Carreira S, Romanel A, Goodall J, et al. Tumor clone dynamics in lethal prostate cancer. Sci Transl Med. 2014;6(254):254ra125. doi: 10.1126/scitranslmed.3009448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wyatt AW, Azad AA, Volik SV, et al. Genomic Alterations in Cell-Free DNA and Enzalutamide Resistance in Castration-Resistant Prostate Cancer. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lallous N, Volik SV, Awrey S, et al. Functional analysis of androgen receptor mutations that confer anti-androgen resistance identified in circulating cell-free DNA from prostate cancer patients. Genome Biol. 2016;17:10. doi: 10.1186/s13059-015-0864-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ulz P, Belic J, Graf R, et al. Whole-genome plasma sequencing reveals focal amplifications as a driving force in metastatic prostate cancer. Nat Commun. 2016;7:12008. doi: 10.1038/ncomms12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lohr JG, Adalsteinsson VA, Cibulskis K, et al. Whole-exome sequencing of circulating tumor cells provides a window into metastatic prostate cancer. Nat Biotechnol. 2014;32(5):479–484. doi: 10.1038/nbt.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyamoto DT, Zheng Y, Wittner BS, et al. RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. Science. 2015;349(6254):1351–1356. doi: 10.1126/science.aab0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haffner MC, Mosbruger T, Esopi DM, et al. Tracking the clonal origin of lethal prostate cancer. J Clin Invest. 2013;123(11):4918–4922. doi: 10.1172/JCI70354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wise AM, Stamey TA, McNeal JE, Clayton JL. Morphologic and clinical significance of multifocal prostate cancers in radical prostatectomy specimens. Urology. 2002;60(2):264–269. doi: 10.1016/s0090-4295(02)01728-4. [DOI] [PubMed] [Google Scholar]
- 29.Boutros PC, Fraser M, Harding NJ, et al. Spatial genomic heterogeneity within localized, multifocal prostate cancer. Nat Genet. 2015;47(7):736–745. doi: 10.1038/ng.3315. [DOI] [PubMed] [Google Scholar]
- 30.Cooper CS, Eeles R, Wedge DC, et al. Analysis of the genetic phylogeny of multifocal prostate cancer identifies multiple independent clonal expansions in neoplastic and morphologically normal prostate tissue. Nat Genet. 2015;47(4):367–372. doi: 10.1038/ng.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu W, Laitinen S, Khan S, et al. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat Med. 2009;15(5):559–565. doi: 10.1038/nm.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong MK, Macintyre G, Wedge DC, et al. Tracking the origins and drivers of subclonal metastatic expansion in prostate cancer. Nat Commun. 2015;6:6605. doi: 10.1038/ncomms7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ross AE, D’Amico AV, Freedland SJ. Which, when and why? Rational use of tissue-based molecular testing in localized prostate cancer. Prostate Cancer Prostatic Dis. 2016;19(1):1–6. doi: 10.1038/pcan.2015.31. [DOI] [PubMed] [Google Scholar]
- 34.Thompson D, Easton DF Breast Cancer Linkage C. Cancer Incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94(18):1358–1365. doi: 10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- 35.Liede A, Karlan BY, Narod SA. Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J Clin Oncol. 2004;22(4):735–742. doi: 10.1200/JCO.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 36.Tischkowitz M, Eeles R Cancer IsIoMwgptP, its Clinical Treatment c. Mutations in BRCA1 and BRCA2 and predisposition to prostate cancer. Lancet. 2003;362(9377):80. doi: 10.1016/S0140-6736(03)13823-8. author reply 80. [DOI] [PubMed] [Google Scholar]
- 37.Ostrander EA, Udler MS. The role of the BRCA2 gene in susceptibility to prostate cancer revisited. Cancer Epidemiol Biomarkers Prev. 2008;17(8):1843–1848. doi: 10.1158/1055-9965.EPI-08-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Attard G, Parker C, Eeles RA, et al. Prostate cancer. Lancet. 2016;387(10013):70–82. doi: 10.1016/S0140-6736(14)61947-4. [DOI] [PubMed] [Google Scholar]
- 39.Ewing CM, Ray AM, Lange EM, et al. Germline mutations in HOXB13 and prostate-cancer risk. N Engl J Med. 2012;366(2):141–149. doi: 10.1056/NEJMoa1110000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beltran H, Rickman DS, Park K, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 2011;1(6):487–495. doi: 10.1158/2159-8290.CD-11-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Epstein JI, Amin MB, Beltran H, et al. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am J Surg Pathol. 2014;38(6):756–767. doi: 10.1097/PAS.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nadal R, Schweizer M, Kryvenko ON, Epstein JI, Eisenberger MA. Small cell carcinoma of the prostate. Nat Rev Urol. 2014;11(4):213–219. doi: 10.1038/nrurol.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kadakia KC, Tomlins SA, Sanghvi SK, et al. Comprehensive serial molecular profiling of an “N of 1” exceptional non-responder with metastatic prostate cancer progressing to small cell carcinoma on treatment. J Hematol Oncol. 2015;8(1):109. doi: 10.1186/s13045-015-0204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beltran H, Rickman DS, Park K, et al. Molecular Characterization of Neuroendocrine Prostate Cancer and Identification of New Drug Targets. Cancer Discov. 2011;1(6):487–495. doi: 10.1158/2159-8290.CD-11-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grasso CS, Cani AK, Hovelson DH, et al. Integrative molecular profiling of routine clinical prostate cancer specimens. Ann Oncol. 2015;26(6):1110–1118. doi: 10.1093/annonc/mdv134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aggarwal R, Zhang T, Small EJ, Armstrong AJ. Neuroendocrine prostate cancer: subtypes, biology, and clinical outcomes. J Natl Compr Canc Netw. 2014;12(5):719–726. doi: 10.6004/jnccn.2014.0073. [DOI] [PubMed] [Google Scholar]
- 47.Aparicio AM, Harzstark A, Corn PG, et al. Platinum-Based Chemotherapy for Variant Castrate-Resistant Prostate Cancer. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-12-3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khemlina G, Ikeda S, Kurzrock R. Molecular landscape of prostate cancer: implications for current clinical trials. Cancer Treat Rev. 2015;41(9):761–766. doi: 10.1016/j.ctrv.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Lord CJ, Tutt AN, Ashworth A. Synthetic lethality and cancer therapy: lessons learned from the development of PARP inhibitors. Annu Rev Med. 2015;66:455–470. doi: 10.1146/annurev-med-050913-022545. [DOI] [PubMed] [Google Scholar]
- 50.Mateo J, Carreira S, Sandhu S, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N Engl J Med. 2015;373(18):1697–1708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spratt DE, Zumsteg ZS, Feng FY, Tomlins SA. Translational and clinical implications of the genetic landscape of prostate cancer. Nat Rev Clin Oncol. 2016 doi: 10.1038/nrclinonc.2016.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prensner JR, Rubin MA, Wei JT, Chinnaiyan AM. Beyond PSA: the next generation of prostate cancer biomarkers. Sci Transl Med. 2012;4(127):127rv123. doi: 10.1126/scitranslmed.3003180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prensner JR, Iyer MK, Sahu A, et al. The long noncoding RNA SChLAP1 promotes aggressive prostate cancer and antagonizes the SWI/SNF complex. Nat Genet. 2013;45(11):1392–1398. doi: 10.1038/ng.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sprenger CC, Plymate SR. The link between androgen receptor splice variants and castration-resistant prostate cancer. Horm Cancer. 2014;5(4):207–217. doi: 10.1007/s12672-014-0177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valdes-Mora F, Clark SJ. Prostate cancer epigenetic biomarkers: next-generation technologies. Oncogene. 2015;34(13):1609–1618. doi: 10.1038/onc.2014.111. [DOI] [PubMed] [Google Scholar]
- 56.Xu K, Wu ZJ, Groner AC, et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent. Science. 2012;338(6113):1465–1469. doi: 10.1126/science.1227604. [DOI] [PMC free article] [PubMed] [Google Scholar]