Abstract
Apigenin is a natural flavonoid which possesses multiple anti-cancer properties such as anti-proliferation, anti-inflammation, and anti-metastasis in many types of cancers including colorectal cancer. Neural precursor cell expressed developmentally downregulated 9 (NEDD9) is a multi-domain scaffolding protein of the Cas family which has been shown to correlate with cancer metastasis and progression. The present study investigates the role of NEDD9 in apigenin-inhibited cell migration, invasion, and metastasis of colorectal adenocarcinoma DLD1 and SW480 cells. The results show that knockdown of NEDD9 inhibited cell migration, invasion, and metastasis and that overexpression of NEDD9 promoted cell migration and invasion of DLD1 cells and SW4890 cells. Apigenin treatment attenuated NEDD9 expression at protein level, resulting in reduced phosphorylations of FAK, Src, and Akt, leading to inhibition on cell migration, invasion, and metastasis of both DLD1 and SW480 cells. The present study has demonstrated that apigenin inhibits cell migration, invasion, and metastasis through NEDD9/Src/Akt cascade in colorectal cancer cells. NEDD9 may function as a biomarker for evaluation of cancer aggressiveness and for selection of therapeutic drugs against cancer progression.
Keywords: Apigenin, NEDD9, Colorectal cancer, Migration, Invasion, Metastasis
1. Introduction
NEDD9 (neural precursor cell expressed developmentally downregulated 9), also known as human enhancer of fllamentation 1 (HEF1), is a non-catalytic scaffolding protein of the Crk-associated substrate (Cas) family (O'Neill et al., 2000). NEDD9 localizes to focal adhesions to coordinate focal adhesion kinase (FAK) and non–receptor tyrosine kinases c-Src (Src) signaling cascades, controlling multiple processes that are crucial for tumorigenesis and metastasis, including apoptosis, migration, invasion, and survival (Natarajan et al., 2006; Izumchenko et al., 2009). In clinical specimens, elevated expression of NEDD9 was positively associated with the malignant progression in melanoma, glioblastoma, breast cancer, head and neck cancer, and lung cancer (Natarajan et al., 2006; Izumchenko et al., 2009; Kim et al., 2006; Speranza et al., 2012; Kong et al., 2011; Lucas et al., 2010; Li et al., 2011; Xia et al., 2010; Kondo et al., 2012; Feng et al., 2012; Chang et al., 2012; Miao et al., 2013). Several in vivo studies have demonstrated that deletion of NEDD9 attenuates tumor growth and metastasis in breast cancer, melanoma, and lung cancer (Izumchenko et al., 2009; Kim et al., 2006; Ji et al., 2007).
The mechanisms of NEDD9 in cancer cell migration, invasion, and metastasis have been extensively studied in recent years. It has been reported that NEDD9 acts through integrin beta 3 and Src to promote invasion in melanoma (Ahn et al., 2012). An in vivo study indicates that NEDD9 is involved in activation of oncogenic signaling pathways including Akt, Erk, and Src in breast cancer development (Izumchenko et al., 2009). In colon cancers, NEDD9 was identified as a mediator of the proliferative effects of prostaglandin E(2) (Xia et al., 2010). Hypoxia is able to induce NEDD9 expression in colorectal cancer cells. Inhibition of hypoxia by YC-1, a hypoxia inducible factor (HIF)-1α inhibitor, abolished NEDD9 expression, indicating that NEDD9 is a downstream target of HIF-1α (Kim et al., 2010). Another recent study indicates that NEDD9 is a potential target of Wnt signaling that promotes migration and cancer progression in colorectal cancer (Li et al., 2011). These studies indicate that NEDD9 could be as a potent biomarker for cancer resistance for therapeutics.
NEDD9 has been identified as a key regulator in cancer cell migration, invasion, and metastasis. Identification of drugs targeting NEDD9 to inhibit tumor progression is of great interest. Apigenin (4′,5,7-trihydroxyflavone, 5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one) is a natural flavonoid abundant in many vegetables and fruits, such as onions, oranges, parsley, tea, and wheat sprouts. Accumulating evidences suggest that apigenin is a promising molecule for cancer prevention in many types of cancers through different mechanisms. Apigenin inhibits hepatocyte growth factor (HGF)-promoted invasive growth and metastasis by blocking PI3K/Akt pathway and integrin β4 function in breast cancer cells (Lee et al., 2008). Apigenin inactivates Akt to trigger apoptosis in human prostate cancer in vitro and in vivo (Kaur et al., 2008). Apigenin also induces apoptosis in human leukemia cells and exhibits anti-leukemic activity in vivo (Budhraja et al., 2012). Apigenin inhibits expression of VEGF and angiogenesis in human lung cancer cells (Liu et al., 2005). The adenomatous polyposis coli (APC) dysfunction may be critical for apigenin to induce cell cycle arrest in human colon cancer cells. In addition, apigenin enhances APC expression and apoptosis in cells with wild-type APC (Chung et al., 2007). Moreover, apigenin promotes cell migration in nontumorigenic colon epithelial cells differing in APC genotype, implicating its matrix metalloproteinase activity (Fenton and Hord, 2004). Apigenin administration sensitizes colon cancer cells to TNFα-induced apoptosis (Farah et al., 2003). Consistent with the identification of NEDD9 as a therapeutic target and as an important protein involved in migration, invasion, and metastasis in cancer, we hypothesized that apigenin downregulates NEDD9, leading to inhibition of cancer progression. The present study investigates (a) whether apigenin is able to inhibit cell migration, invasion, and metastasis in colorectal ad-enocarcinoma DLD1 and SW480 cells in vitro and in vivo; (b) whether apigenin treatment downregulates NEDD9; (c) whether inhibition of apigenin on migration, invasion, and metastasis is through NEDD9; and (d) what is the underlying mechanism of NEDD9 downregulation by apigenin.
2. Materials and methods
2.1. Chemicals and reagents
Apigenin, dimethyl sulfoxide (DMSO), polybrene, and MG132 were from Sigma (St Louis, MO). RNeasy mini kit and plasmid prep kit were from Qiagen (Valencia, CA). CellTiter 96® AQueous non-radioactive cell proliferation assay kit and M-MLV reverse transcriptase were from Promega (Madison, WI). Dulbecco's Modified Eagle Medium (DMEM), fetal bovine serum (FBS), puromycin, and Oligo (dT)20 AccuPrime Taq DNA polymerase high fidelity were from Invitrogen (Carlsbad, CA). Leibovitz's L-15 medium was from American Type Culture Collection (ATCC) (Manassas, VA). Enhanced chemiluminescence (ECL) kit was from Thermo Scientific (Rockford, IL). Matrigel basement membrane matrix was from BD Biosciences (Sparks, MD). Antibodies against p-FAKTyr397, FAK, p-SrcTyr527, Src, p-CrkLTyr207, CrkL, p-AktSer473, and Akt were from Cell Signaling Technologies (Beverly, MA). Antibodies against NEDD9 and β-actin were from Santa Cruz Biotechnology (San Diego, CA). Antibody against CK20 was from Abcam (Cambridge, MA).
2.2. Cell culture
Human colorectal adenocarcinoma DLD1 cells, SW480 cells, and normal human colorectal epithelial CRL-1807 were from American Type Culture Collection (Manassas, VA). DLD1 cells and CRL-1807 cells were cultured in DMEM supplement with 10% FBS at 37 °C with 5% CO2. SW480 cells were cultured in Leibovitz's L-15 medium with 10% FBS at 37 °C without CO2.
2.3. Cell viability assay
DLD1 and SW480 cells were plated in 96-well plates (3 × 104 cells/well) and treated with indicated dose of apigenin or vehicle DMSO for 24 or 48 h. Cell viability was determined using a CellTiter 96® AQueous non-radioactive cell proliferation assay according to manufacturer's instruction. Formazan (proportional to the number of living cells) was quantitated by measuring absorbance at 570 nm using a microplate reader (Bio-Rad Laboratory, Hercules, CA).
2.4. Genetic alteration of NEDD9 expression
Plasmid LZRS/IresGFP, LZRS/IresGFP-hNEDD9, pLKO, pLKO/shC, and pLKO/shD were from Addgene (Cambridge, MA). DLD1 and SW480 cells were transfected with 4 μg plasmid using Lipofectamine. Cells that stably expressing or knockdown NEDD9 were selected using 10 μg/mL puromycin for 2 weeks followed by immunoblotting analysis to verify NEDD9 expression.
2.5. Scratch wound healing assay
DLD1 and SW480 cells were allowed to grow to full confiuency in 24-well plates. Scratch wound was made by scratching the confiuent monolayer with a 10 μL pipette tip. After washing with PBS, the cells were treated with 20 μM of apigenin or vehicle DMSO. Images were taken at 24 and 48 h after wounding. The percentage of wound closure was determined using Image J software.
2.6. Boyden chamber migration and invasion assay
Migration and invasion assay were carried out using transwell chambers according to the manufacture's protocol. 2.5 × 105 (invasion) or 1.25 × 105 (migration) cells were plated onto cell culture inserts pre-coated with (invasion) or without (migration) matrigel and incubated with 20 μM of apigenin or vehicle DMSO for 48 (invasion) or 24 (migration) hours. The migrated or invaded cells were stained with 0.05% crystal violet and counted in five random fields.
2.7. Immunoblotting
Immunoblotting was performed as described previously (Wang et al., 2012). Cell lysates were prepared in RIPA buffer. The protein concentration was measured using Bradford protein assay reagent. 20 μg of total protein from cell lysate was separated by SDS-PAGE, and incubated with primary antibodies. The blots were then re-probed with second antibodies conjugated to horseradish peroxidase. Proteins were visualized using an enhanced chemiluminescence kit.
2.8. Real time PCR
RNA was extracted and purified using the RNeasy mini kit. 0.5 μg of RNA was reverse transcribed using M-MLV reverse transcriptase. Primers of NEDD9 were designed using Primer-Blast yielding the following sense and anti-sense sequences: Forward-GAC CTG CAT AGA GCA GAA CAC and Reverse-TGCATGGGACCAATCAGAAGC. Values were normalized by β-actin: Forward-TCACCCACACTGTGCCCATCTACGA, reverse-CAGCGGAACCGCTCATTGCCAATGG. PCR was performed using Perfecta Sybr Green Fastmix (Quanta Biosciences) in MyiQ (Bio-Rad) and the data were analyzed with CFX Manager software (Bio-Rad).
2.9. In vivo metastasis assay
6–8 weeks old, female athymic nude mice were from The Jackson Laboratory (Bar Harbor, ME). The animals were housed in a specific pathogen-free room within the animal facilities at the University of Kentucky, Lexington, KY. Animals were allowed to acclimatize to their new environment for 1 week prior to use. All animals were handled according to the Institutional Animal Care and Use Committee (IACUC) guidelines, University of Kentucky. DLD1 cells (1 × 106) or DLD1 cells that NEDD9 was stably knockdown were re-suspended in 100 μL of PBS and injected into the mouse tail vein. The mice were intraperitoneally (i.p.) treated with PBS or apigenin (20 mg/kg) once a week. 14 weeks of post-injection of cancer cells, the mice were sacrificed using CO2. The lung tissues were isolated and fixed in 10% formalin for further histopathological examination.
2.10. Statistical analysis
Data were expressed as the mean ± standard deviation (SD). Statistical significance of differences among treatment groups were determined by ANOVA with Student's t-test. A p < 0.05 was considered as statistical significance.
3. Results
3.1. NEDD9 is a positive regulator of migration and invasion of DLD1 cell and SW480 cells
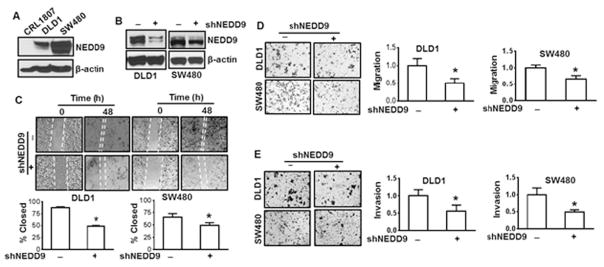
A high level of NEDD9 has been found in the colorectal cancer patients (Shagisultanova et al., 2015). To learn whether NEDD9 is also highly expressed in colorectal cancer cell lines, we have examined NEDD9 protein level in CRL1807 non-transformed human colonocytes and colorectal carcinoma DLD1 and SW480 cells using immunoblotting analysis. A high level of NEDD9 was observed in both colorectal carcinoma DLD1 and SW480 cells, but not in CR1807 non-transformed cells (Fig. 1A). To explore whether increased protein level of NEDD9 is critical for invasion and migration in colorectal cancer cells, NEDD9 expression was inhibited by its shRNA (Fig. 1B) and migration and invasion assays were performed. The results show that knockdown of NEDD9 reduced migration of DLD1 cells by 38% and 46% as shown in the scratching wound healing assay and Boyden chamber migration assay, respectively (Fig. 1C and D). Similar results were observed using SW480 cells (Fig. 1C and D). Knockdown of NEDD9 inhibited invasion of both DLD1 and SW480 cells by 45% and 51%, respectively (Fig. 1E). The results above indicate that NEDD9 positively regulates migration and invasion of DLD1 and SW480 cells.
Fig. 1.
Inhibition of NEDD9 suppresses cell migration and invasion of DLD1 and SW480 cells. (A) NEDD9 expression in colorectal cancer DLD1, SW480 cells, or CRL1807 non-transformed colonocytes was examined using immunoblotting analysis. (B) DLD1 cells and SW480 cells were transfected with NEDD9 shRNA followed by antibiotic selection for 1 month. The cells were harvested for examination of NEDD9 expression using immunoblotting analysis. The results are representative of three independent experiments (A and B). (C) Wound healing assay. Representative images of wounded DLD1 cells and SW480 cells with or without knockdown of NEDD9 at 0 and 48 h. Cell migration was determined by percentage of closure of wound gap at time 0. * p < 0.05 compared to scramble control. (D) and (E) Cell migration and invasion determined by Boyden chamber assay. Representative images of cell migration (D) and invasion (E) in DLD1 cells and SW480 cells with or without knockdown of NEDD9. All values were expressed as fold changes relative to scramble control. * p < 0.05 compared to scramble control.
3.2. Apigenin inhibits migration and invasion of DLD1 and SW480 cells
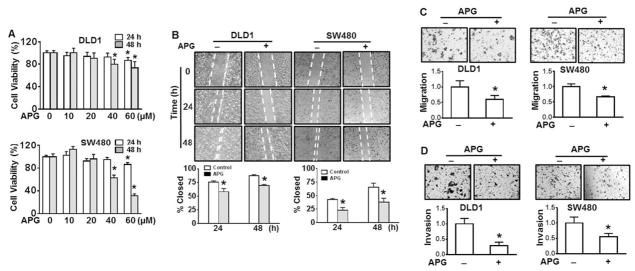
Apigenin, a polyphenol in many vegetables and fruits, exhibits various anti-cancer properties. The results from viability assay show that apigenin treatment up to 40 μM for 24 h did not exhibit cytotoxicity in DLD1 or SW480 cells (Fig. 2A). In contrast, the cell viability was markedly reduced when the cells were treated with 40 μM apigenin for 48 h (Fig. 2A). Taking together the results above and those from NEDD9 expression in apigenin-treated cells (Fig. 3A), we believe that the dosages up to 40 μM of apigenin are appropriate for the cell treatments. Both wound healing assay and Boyden chamber invasion assay were performed to evaluate the inhibition of apigenin on cell migration and invasion. The results show that the wound closure was significantly inhibited by 20 μM of apigenin (58%) compared to that of control group (76%) for 24 h in DLD1 cells (Fig. 2B, left). At 48 h of apigenin treatment, the percentage of closure was 70% as control without treatment being 87% (Fig. 2B, left). Similar results were observed using SW480 cells (Fig. 2B, right). The results from Boyden chamber assay show that DLD1 cell migration and invasion were significantly decreased by 40% at 24 h and 61% at 48 h upon apigenin treatment compared to control (Fig. 2C and D, left), respectively. The similar results were obtained using SW480 cells (Fig. 2C and D, right). These results suggest that apigenin is able to inhibit cell migration and invasion of DLD1 and SW480 cells.
Fig. 2.
Apigenin inhibits cell migration and invasion of DLD1 and SW480 cells. (A) DLD1 and SW480 cells were treated with various doses of apigenin (APG) or vehicle (DMSO) for 24 or 48 h. Cell viability was determined by CellTiter 96® cell proliferation assay. * p < 0.05 compared to control without treatment. (B) Representative images of wounded DLD1 cells and SW480 cells treated with or without 20 μM APG for 24 and 48 h. Cell migration was determined by percentage of closure of wound gap at time 0. * p < 0.05 compared to control without treatment at corresponding treatment time. (C) and (D) Cell migration and invasion determined by Boyden chamber assay. Representative images of cell migration (C) and invasion (D) in DLD1 cells and SW480 cells with or without 20 μM of APG treatment. All values were expressed as fold changes relative to none treatment control. * p < 0.05 compared to none treatment control.
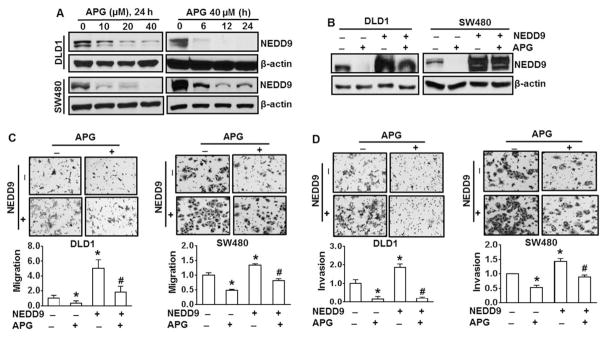
Fig. 3.
Apigenin inhibits migration and invasion of DLD1 and SW480 cells through downregulation of NEDD9. (A) Both DLD1 and SW480 cells were treated with various doses of apigenin (APG) for up to 24 h. NEDD9 expression was examined by immunoblotting analysis. (B) DLD1 and SW480 cells were transfected with NEDD9 overexpression vector or control vector followed by antibiotic selection for additional one month. DLD1 and SW480 cells with or without stable overexpression of NEDD9 were treated with 40 μM of APG for 24 h. NEDD9 expression was examined by immunoblotting analysis. The results are representative of three independent experiments (A and B). (C) and (D) Cell migration and invasion determined by Boyden chamber assay. Representative images of cell migration (C) and invasion (D) in DLD1 cells and SW480 cells with or without overexpression of NEDD9 in the combination of 20 μM of APG treatment for 24 (migration) or 48 h (invasion). All values were expressed as fold changes relative to scramble control without APG treatment. * and #, p < 0.05 compared to scramble control and NEDD9 expressing cells without APG, respectively.
3.3. Inhibition of apigenin on cell migration, invasion, and metastasis through downregulation of NEDD9 in vitro and in vivo
Giving the observations in Figs. 1 and 2, we investigated whether apigenin is able to inhibit NEDD9 in DLD1 and SW480 cells. The results show that apigenin treatment reduced NEDD9 protein level in a dose-and time-dependent manner in both DLD1 and SW480 of cells (Fig. 3A). To further confirm the ability of apigenin on downregulation of NEDD9, DLD1 or SW480 cells with stable NEDD9 expression were established. The results show that apigenin is able to reduce NEDD9 level in NEDD9-expressing DLD1 or SW480 cells (Fig. 3B), indicating that potential inhibition of apigenin on NEDD9. Forced expression of NEDD9 increased migration by 5-fold and 1.34-fold, respectively, compared to the wild-type DLD1 cells and SW480 cells (Fig. 3C). Similarly, forced expression of NEDD9 elevated invasion by 86% and 40%, respectively, compared to wild-type DLD1 and SW480 cells (Fig. 3D). Those results provide an additional evidence for the positive role of NEDD9 in migration and invasion of DLD1 and SW480 cells. Apigenin is able to reduce migration and invasion of NEDD9-expressing cells (Fig. 3C and D), indicating that suppression of apigenin on migration and invasion is mediated at least partially by NEDD9, if not fully.
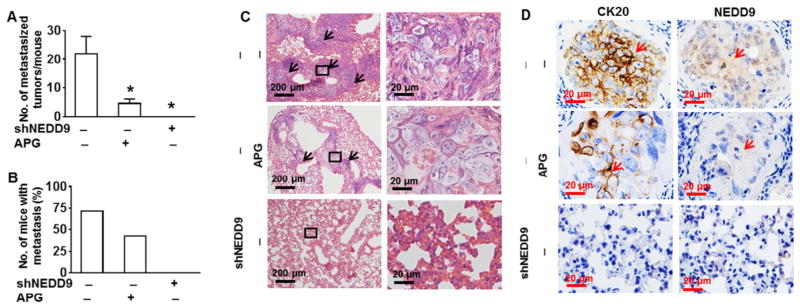
Our in vitro studies have demonstrated that apigenin is able to inhibit migration and invasion of DLD1 and SW480 cells through downregulation of NEDD9. When tumor cells invade through blood vessel walls into the bloodstream, intravasation is the key step of the carcinoma process leading to metastasis (Mignatti and Rifkin, 1993). Thus we investigated capability of apigenin to suppress metastasis in vivo. DLD1 cells with or without NEDD9 knockdown were injected into nude mice via tail vein. The mice were treated with apigenin through intraperitoneal injection once a week. After 14 weeks the animals were euthanized and lung tissues were isolated. The results show that injection with wild type DLD1 cells caused metastasis of these cells to the lung as evidenced by increased number of metastasized tumor per mouse and number of mice with metastasis (Fig. 4A and B). No tumor in lung was observed in the animals injected with DLD1 cells with knockdown of NEDD9 (Fig. 4A and B). Apigenin treatment significantly reduced the numbers of metastatic tumor in the lung per animal compared to that in wile-type DLD1 cells only without any treatment (Fig. 4A). In apigenin treatment group, 3 out of 7 animals (43%) were found with metastastic tumors (Fig. 4B). In the group without any treatment, 5 out of 7 (71%) were found with metastastic tumors (Fig. 4B). The results from histological HE staining show that lung from animal injected with wild-type DLD1 exhibited thicken and enlarged bronchus and irregular architecture with tumor cells compared to the lung from animal injected with apigenin-treated or NEDD9-knockdown (Fig. 4C). CK20 was highly expressed in these metastatic lung tumors, confirming the colorectal origin of those tumors (Fig. 4D).
Fig. 4.
Apigenin suppresses lung metastasis through downregulation of NEDD9. Athymic nude mice were injected with DLD1 cells with or without stable knockdown of NEDD9 via tail vein. The mice were i.p. treated with apigenin (APG) (20 mg/kg) once per week. The lungs were isolated at week 14. The total number of metastatic lesions was counted (n = 7–8 mice per group). (A) The number of metastatic foci in the lungs of each mouse. * p < 0.05 compared to scramble control cells without APG treatment. (B) The proportion of mice with metastasis in each group. (C) Representative H&E stained lung section. Arrows indicate metastatic foci in the lungs. (D) Representative immunohistological staining for expressions of NEDD9 and CK20 in the metastatic foci. Arrows indicate metastatic DLD1 cells in the lung section.
3.4. Apigenin inhibits NEDD9-Src-Akt signaling cascade
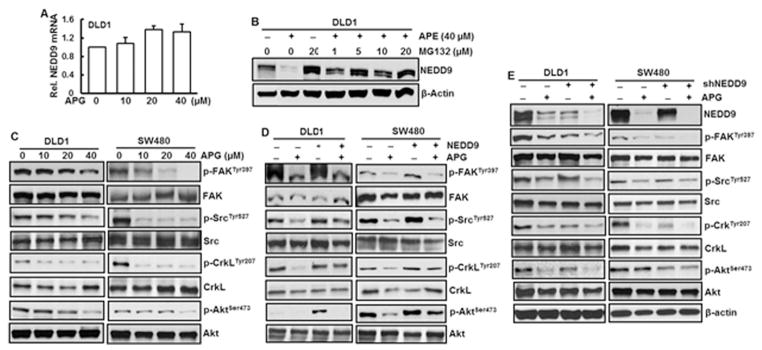
Our results indicate that NEDD9 is important in apigenin-inhibited cell migration, invasion, and metastasis. Identification of mechanism of NEDD9 inhibition by apigenin becomes important and necessary. To learn whether decreased NEDD9 protein level by apigenin is due to reduction at transcriptional level, NEDD9 mRNA level was quantitated. The results show that treatment of the DLD1 cells with apigenin did not alter NEDD9 at mRNA level (Fig. 5A), indicating that decreased NEDD9 protein level by apigenin is through post-translation modification of this protein. For additional study, MG132, a proteinase inhibitor, was treated with or without apigenin. The results show that NEDD9 protein level was dramatically elevated in the cells treated with MG132 together with apigenin compared to those treated with apigenin alone (Fig. 5B). Increase in MG132 dose did not elevate NEDD9 protein level (Fig. 5B), further showing that reduced NEDD9 protein level by apigenin was through its post-translation modification. It has been reported that Akt together with other two scaffold proteins p130Cas and NEDD9 forms complex with FAK, Src, and Crk-L proteins (Guerrero et al., 2012). To investigate whether apigenin is able to inactivate FAK, Src, Crk-L, and Akt, phosphorylations of those proteins were examined. The results show that apigenin caused a dose-dependent reduction of phosphorylations of FAK at Tyr397, Src at Tyr527, CrkL at Tyr207, and Akt at Ser473 in DLD1 and SW480 cells (Fig. 5C). As expected, forced expression of NEDD9 increased phosphorylations of FAK, Src, CrkL, and Akt (Fig. 5D), providing an additional support that those proteins are downstream targets of NEDD9. In DLD1 and SW480 cells with NEDD9 overexpression, apigenin is able to overcome forced expression of NEDD9 to reduce NEDD9 expression, resulting in decreased phosphorylations of FAK, Src, CrkL, and Akt (Fig. 5D). Knockdown of NEDD9 in DLD1 cells or SW480 cells decreased phosphorylations of FAK, Src, CrkL, and Akt (Fig. 5E). Treatment with apigenin in NEDD9 knockdown cells further reduced NEDD9 protein level and phosphorylations of FAK, Src, CrkL, and Akt (Fig. 5E), indicating the essential role of NEDD9 in regulation of those proteins.
Fig. 5.
Apigenin inhibits NEDD9-Src-Akt signaling cascade. (A) DLD1 cells were treated with various doses of apigenin (APG) for 24 h. mRNA level of NEDD9 was measured by RT-PCR. (B) DLD1 cells were pretreated with MG132 for 4 h prior to APG treatment. After 24 h, the cells were collected for examination of NEDD9 expression by immunoblotting analysis. (C) DLD1 and SW480 cells were treated with APG at indicated doses for 24 h. Whole cell lysate were isolated and protein expression was analyzed by immunoblotting analysis. (D) DLD1 cells and SW480 cells with or without knockdown of NEDD9 were treated with 40 μM of APG for 24 h. Whole cell lysates were isolated for immunoblotting analysis. (E) DLD1 and SW480 cells with or without stable overexpression of NEDD9 were treated with 40 μM of APG for 24 h. Whole cell lysates were isolated for immunoblotting analysis. The results from immunoblotting analyses are representative of three independent experiments.
4. Discussion
Flavonoids are a ubiquitous dietary phenolics and have been extensively investigated for their anticancer properties including in vitro anti-invasive and in vivo anti-metastatic activities (Weng and Yen, 2012). Apigenin has been shown to have a potential to inhibit migration and invasion of ovarian (Hu et al., 2008), breast (Lee et al., 2008), prostate (Plaisant et al., 2011), colorectal (Chunhua et al., 2013), and melanoma cells (Agullo et al., 1997). The present study has found that downregulation of NEDD9 by apigenin suppresses cell migration and invasion of colorectal cancer DLD1 and SW480 cells and lung metastasis in vivo.
NEDD9 is one of Cas family of scaffold and adaptor proteins. Cas family proteins have no enzymatic activity but instead function as a platform for the assembly of multiprotein complexes, most notably Src and FAK, to influence cell behavior (Guerrero et al., 2012). Among Cas family members, NEDD9 and p130Cas are extensively studied and implicated in cell motility such as adhesion, cell migration, and invasion. While p130Cas is a consistently and ubiquitously expressed protein, the expression of NEDD9 is temporally regulated and is tissue- and cell type-specific (Lin et al., 1997). This protein has been identified as a metastatic gene in multiple types of cancer such as breast (Kandel and Hay, 1999), colon (Li et al., 2011), and melanoma (Kim et al., 2006). The present study has observed that NEDD9 is highly expressed in colorectal DLD1 and SW480 cancer cells but not in CRL1807 normal colonocytes. Inhibition of NEDD9 by its shRNA reduced migration, invasion, and lung metastasis of those colorectal cancer cells. In contrast, forced expression of NEDD9 in DLD1 and SW480 cells promoted cell migration and invasion. The results from our preliminary study have also shown that NEDD9 expression in HCT116 cells, another colorectal cancer cell line, is much lower than that in DLD1 or SW480 cells (data not shown). The low expression of NEDD9 is correlated to the moderate migration and invasion of HCT116 cells (data not shown). These results indicate the essential role of NEDD9 in aggressiveness of colorectal cancer cells.
The mechanisms of apigenin against cell migration and invasion and metastasis remain to be investigated. Flavonoids are known to regulate protein kinase C (PKC) and protein tyrosine kinase (PTK), important proteins in cancer biology (Agullo et al., 1997; Lin et al., 1997; Ravindranath et al., 2004). It has been reported that apigenin inhibits HGF-induced migration and invasion of human breast cancer cells (Lee et al., 2008). Apigenin upregulates transgelin and inhibits invasion and migration of colorectal cancer through inactivation of Akt (Chunhua et al., 2013). Akt or protein kinase B (PKB) is a serine/threonine-specific protein kinase which plays crucial roles in various cellular processes such as cell migration, cell proliferation, and apoptosis (Kandel and Hay, 1999). It has also reported that apigenin inhibits cell migration and invasion by downregulation of FAK in human ovarian cancer A2780 cells (Hu et al., 2008). Apigenin inhibits prostate cancer cell motility through FAK/Src signaling (Franzen et al., 2009). An in vivo metastasis study has demonstrated that apigenin suppresses HGF-promoted metastasis of MDA-MB-231 human breast cancer cells through PI3K/Akt pathway (Lee et al., 2008). Despite various mechanisms of apigenin protection against migration and invasion of cancer cells have been reported, no studies was found to investigate the relationship between inhibition of cancer aggressiveness by apigenin and NEDD9. The present study has found that apigenin downregulates NEDD9, leading to suppression of migration and invasion of DLD1 and SW480 cells. Our animal study has demonstrated that apigenin treatment markedly reduced the lung metastasis of DLD1 cells. The decreased expression of NEDD9 in metastatic lung tumors from animals treated with apigenin further supports the essential role of NEDD9 in apigenin-inhibited metastasis of DLD1 cells.
NEDD9 is subject to tight control of expression levels through post-translational protein degradation mechanisms (Zheng and McKeown-Longo, 2006). The degree of phosphorylation of NEDD9 influences its scaffolding activity and localization and proteasomal degradation (Shagisultanova et al., 2015). Currently the role of individual phosphor-ylation site of NEDD9 is poorly understood. It has been reported that NEDD9 migrates as a doublet of 105-kDa and 115-kDa which are associated with distinct phosphorylation state (Law et al., 1998). 105-kDa NEDD9 is both tyrosine and serine phosphorylated and undergoes further phosphorylation modifications to produce a 115-kDa hyper-phosphorylated protein. 115-kDa NEDD9 is the primary target for proteasomal degradation (Zheng and McKeown-Longo, 2006). It is lost following treatments that disrupt the actin cytoskeleton (Zheng and McKeown-Longo, 2006). In the present study, both 105-kDa and 115-kDa isoforms were detected in DLD1 and SW480 cells. Interestingly, apigenin suppressed both 105-kDa and 115-kDa NEDD9 protein through post-translation modification. It has been reported that inhibitors of protein phosphatase 2A (PP2A) stabilized 115-kDa isoform, causing degradation of NEDD9 (Zheng and McKeown-Longo, 2006). The present study has not yet examined the mechanism of apigenin-inhibited NEDD9 protein level, one possibility is that apigenin may target PP2A, resulting in increased degradation of NEDD9. Apigenin failed to alter p130Cas expression in DLD1 or SW480 cells (data not shown), indicating that apigenin selectively inhibits inducible but not essential/baseline function of Cas family proteins.
Among numerous binding partners of Cas and NEDD9, three proteins have been most consistently implicated in Cas/NEDD9 activity and signaling: Src, FAK, and the SH2 domain-containing adaptor molecule CrkL (Guerrero et al., 2012). The NEDD9/Src/FAK signaling complex plays an essential role in mediating movement toward growth factors and in translating mechanical forces into biochemical signals. These signals initiate motility, promoting cancer cell metastasis processes including migration, invasion, intravasation, survival in circulation (anoikis resistance), and extravasation. NEDD9 activation includes two steps: (a) NEDD9 is phosphorylated by FAK at C-terminal and (b) the C-terminal phosphorylated NEDD9 is further phosphorylated by Src kinase at substrate domain, creating more binding sites for effector proteins such as CrkL. Cas-CrkL complex has been described as a “master switch” for cell migration. Through CrkL and DOCK180, NEDD9 activates Rac and other components of the cell migration machinery (O'Neill et al., 2007). The present study has found that apigenin caused a dose-dependent reduction of phosphorylations of Src, FAK, CrkL, and Akt. To confirm Src-Akt cascade is the downstream target of NEDD9, DLD1 and SW480 cells with either stable overexpression or knockdown of NEDD9 were established. The reduction in phosphorylations of Src, FAK, CrkL, and Akt by apigenin in the cells with forced NEDD9 expression is similar to that in wildtype cells. Apigenin further reduced both NEDD9 level and phosphorylation of those downstream proteins in the cells with NEDD9 knockdown compared to that in wildtype cells treated with apigenin, indicating the essential role of NEDD9 in apigenin's function. Our preliminary study has also observed that overexpression of activated Akt does not activate NEDD9 or Src in DLD1 cells (data not shown). These observations indicate that Akt is a downstream effector of NEDD9/Src complex. Akt is a central node of a signaling pathway consisting of many components that have been implicated in cancer development and progression (Altomare and Testa, 2005). For example, Akt has been shown to contribute to tumor invasion and metastasis by promoting the secretion of matrix metalloproteinases (MMPs) and the induction of epithelial–mesenchymal transition (EMT) (Larue and Bellacosa, 2005). In the present study, apigenin treatment or NEDD9 knockdown inhibited Akt phosphorylation, resulting in suppression of the downstream effectors of Akt, such as MMP9, contributing to overall mechanism of apigenin against cell migration, invasion, and metastasis.
In summary, the present study has demonstrated that NEDD9 is a key regulator in the migration, invasion, and metastasis of colorectal cancer cells. Apigenin attenuates NEDD9 expression, resulting in inhibition on cell migration, invasion, and metastasis of those cells. NEDD9 may function as a biomarker for evaluation of cancer aggressiveness and for selection of drug target for therapeutic potential against cancer progression.
Acknowledgments
This work was support by University of Kentucky for start-up to Zhang Z.
Footnotes
Conflict of interest statement
The authors declare no conflict of interest to report.
Transparency document
The Transparency document associated with this article can be found, in the online version.
References
- Agullo G, et al. Relationship between flavonoid structure and inhibition of phosphatidylinositol 3-kinase: a comparison with tyrosine kinase and protein kinase C inhibition. Biochem Pharmacol. 1997;53(11):1649–1657. doi: 10.1016/s0006-2952(97)82453-7. [DOI] [PubMed] [Google Scholar]
- Ahn J, Sanz-Moreno V, Marshall CJ. The metastasis gene NEDD9 product acts through integrin beta3 and Src to promote mesenchymal motility and inhibit amoeboid motility. J Cell Sci. 2012;125(Pt 7):1814–1826. doi: 10.1242/jcs.101444. [DOI] [PubMed] [Google Scholar]
- Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24(50):7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- Budhraja A, et al. Apigenin induces apoptosis in human leukemia cells and exhibits anti-leukemic activity in vivo. Mol Cancer Ther. 2012;11(1):132–142. doi: 10.1158/1535-7163.MCT-11-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JX, et al. Role of NEDD9 in invasion and metastasis of lung adenocarcinoma. Exp Ther Med. 2012;4(5):795–800. doi: 10.3892/etm.2012.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CS, et al. Impact of adenomatous polyposis coli (APC) tumor supressor gene in human colon cancer cell lines on cell cycle arrest by apigenin. Mol Carcinog. 2007;46(9):773–782. doi: 10.1002/mc.20306. [DOI] [PubMed] [Google Scholar]
- Chunhua L, et al. Apigenin up-regulates transgelin and inhibits invasion and migration of colorectal cancer through decreased phosphorylation of AKT. J Nutr Biochem. 2013;24(10):1766–1775. doi: 10.1016/j.jnutbio.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Farah M, et al. 5,6-Dichloro-ribifuranosylbenzimidazole- and apigenin-induced sensitization of colon cancer cells to TNF-alpha-mediated apoptosis. Am J Physiol Gastrointest Liver Physiol. 2003;285(5):G919–G928. doi: 10.1152/ajpgi.00205.2003. [DOI] [PubMed] [Google Scholar]
- Feng Y, et al. The CRTC1-NEDD9 signaling axis mediates lung cancer progression caused by LKB1 loss. Cancer Res. 2012;72(24):6502–6511. doi: 10.1158/0008-5472.CAN-12-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton JI, Hord NG. Flavonoids promote cell migration in nontumorigenic colon epithelial cells differing in Apc genotype: implications of matrix metalloproteinase activity. Nutr Cancer. 2004;48(2):182–188. doi: 10.1207/s15327914nc4802_8. [DOI] [PubMed] [Google Scholar]
- Franzen CA, et al. The chemopreventive bioflavonoid apigenin inhibits prostate cancer cell motility through the focal adhesion kinase/Src signaling mechanism. Cancer Prev Res (Phila) 2009;2(9):830–841. doi: 10.1158/1940-6207.CAPR-09-0066. [DOI] [PubMed] [Google Scholar]
- Guerrero MS, Parsons JT, Bouton AH. Cas and NEDD9 contribute to tumor progression through dynamic regulation of the cytoskeleton. Genes Cancer. 2012;3(5–6):371–381. doi: 10.1177/1947601912458585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XW, Meng D, Fang J. Apigenin inhibited migration and invasion of human ovarian cancer A2780 cells through focal adhesion kinase. Carcinogenesis. 2008;29(12):2369–2376. doi: 10.1093/carcin/bgn244. [DOI] [PubMed] [Google Scholar]
- Izumchenko E, et al. NEDD9 promotes oncogenic signaling in mammary tumor development. Cancer Res. 2009;69(18):7198–7206. doi: 10.1158/0008-5472.CAN-09-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448(7155):807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- Kandel ES, Hay N. The regulation and activities of the multifunctional serine/thre-onine kinase Akt/PKB. Exp Cell Res. 1999;253(1):210–229. doi: 10.1006/excr.1999.4690. [DOI] [PubMed] [Google Scholar]
- Kaur P, Shukla S, Gupta S. Plant flavonoid apigenin inactivates Akt to trigger apoptosis in human prostate cancer: an in vitro and in vivo study. Carcinogenesis. 2008;29(11):2210–2217. doi: 10.1093/carcin/bgn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, et al. Comparative oncogenomics identifies NEDD9 as a melanoma metastasis gene. Cell. 2006;125(7):1269–1281. doi: 10.1016/j.cell.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Kim SH, et al. Human enhancer of fllamentation 1 is a mediator of hypoxia-inducible factor-1alpha-mediated migration in colorectal carcinoma cells. Cancer Res. 2010;70(10):4054–4063. doi: 10.1158/0008-5472.CAN-09-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, et al. Impact of the integrin signaling adaptor protein NEDD9 on prognosis and metastatic behavior of human lung cancer. Clin Cancer Res. 2012;18(22):6326–6338. doi: 10.1158/1078-0432.CCR-11-2162. [DOI] [PubMed] [Google Scholar]
- Kong C, et al. NEDD9 is a positive regulator of epithelial-mesenchymal transition and promotes invasion in aggressive breast cancer. PLoS One. 2011;6(7):e22666. doi: 10.1371/journal.pone.0022666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene. 2005;24(50):7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- Law SF, et al. Cell cycle-regulated processing of HEF1 to multiple protein forms differentially targeted to multiple subcellular compartments. Mol Cell Biol. 1998;18(6):3540–3551. doi: 10.1128/mcb.18.6.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WJ, et al. Apigenin inhibits HGF-promoted invasive growth and metastasis involving blocking PI3K/Akt pathway and beta 4 integrin function in MDA-MB-231 breast cancer cells. Toxicol Appl Pharmacol. 2008;226(2):178–191. doi: 10.1016/j.taap.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Li Y, et al. HEF1, a novel target of Wnt signaling, promotes colonic cell migration and cancer progression. Oncogene. 2011;30(23):2633–2643. doi: 10.1038/onc.2010.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JK, et al. Suppression of protein kinase C and nuclear oncogene expression as possible molecular mechanisms of cancer chemoprevention by apigenin and curcumin. J Cell Biochem Suppl. 1997;28–29:39–48. [PubMed] [Google Scholar]
- Liu LZ, et al. Apigenin inhibits expression of vascular endothelial growth factor and angiogenesis in human lung cancer cells: implication of chemoprevention of lung cancer. Mol Pharmacol. 2005;68(3):635–643. doi: 10.1124/mol.105.011254. [DOI] [PubMed] [Google Scholar]
- Lucas JT, Jr, et al. Regulation of invasive behavior by vascular endothelial growth factor is HEF1-dependent. Oncogene. 2010;29(31):4449–4459. doi: 10.1038/onc.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y, et al. Overexpression of NEDD9 is associated with altered expression of E-cadherin, beta-catenin and N-cadherin and predictive of poor prognosis in non-small cell lung cancer. Pathol Oncol Res. 2013;19(2):281–286. doi: 10.1007/s12253-012-9580-2. [DOI] [PubMed] [Google Scholar]
- Mignatti P, Rifkin DB. Biology and biochemistry of proteinases in tumor invasion. Physiol Rev. 1993;73(1):161–195. doi: 10.1152/physrev.1993.73.1.161. [DOI] [PubMed] [Google Scholar]
- Natarajan M, et al. HEF1 is a necessary and specific downstream effector of FAK that promotes the migration of glioblastoma cells. Oncogene. 2006;25(12):1721–1732. doi: 10.1038/sj.onc.1209199. [DOI] [PubMed] [Google Scholar]
- O'Neill GM, Fashena SJ, Golemis EA. Integrin signalling: a new Cas(t) of characters enters the stage. Trends Cell Biol. 2000;10(3):111–119. doi: 10.1016/s0962-8924(99)01714-6. [DOI] [PubMed] [Google Scholar]
- O'Neill GM, et al. A new central scaffold for metastasis: parsing HEF1/Cas-L/NEDD9. Cancer Res. 2007;67(19):8975–8979. doi: 10.1158/0008-5472.CAN-07-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaisant M, et al. Inhibition of hedgehog signaling decreases proliferation and clonogenicity of human mesenchymal stem cells. PLoS One. 2011;6(2):e16798. doi: 10.1371/journal.pone.0016798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindranath MH, et al. Anticancer therapeutic potential of soy isoflavone, genistein. Adv Exp Med Biol. 2004;546:121–165. doi: 10.1007/978-1-4757-4820-8_11. [DOI] [PubMed] [Google Scholar]
- Shagisultanova E, et al. Preclinical and clinical studies of the NEDD9 scaffold protein in cancer and other diseases. Gene. 2015;567(1):1–11. doi: 10.1016/j.gene.2015.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speranza MC, et al. NEDD9, a novel target of miR-145, increases the invasiveness of glioblastoma. Oncotarget. 2012;3(7):723–734. doi: 10.18632/oncotarget.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, et al. Arsenic and chromium in drinking water promote tumorigenesis in a mouse colitis-associated colorectal cancer model and the potential mechanism is ROS-mediated Wnt/beta-catenin signaling pathway. Toxicol Appl Pharmacol. 2012;262(1):11–21. doi: 10.1016/j.taap.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng CJ, Yen GC. Flavonoids, a ubiquitous dietary phenolic subclass, exert extensive in vitro anti-invasive and in vivo anti-metastatic activities. Cancer Metastasis Rev. 2012;31(1–2):323–351. doi: 10.1007/s10555-012-9347-y. [DOI] [PubMed] [Google Scholar]
- Xia D, et al. HEF1 is a crucial mediator of the proliferative effects of prostaglandin E(2) on colon cancer cells. Cancer Res. 2010;70(2):824–831. doi: 10.1158/0008-5472.CAN-09-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, McKeown-Longo PJ. Cell adhesion regulates Ser/Thr phosphorylation and proteasomal degradation of HEF1. J Cell Sci. 2006;119(Pt 1):96–103. doi: 10.1242/jcs.02712. [DOI] [PubMed] [Google Scholar]