Abstract
Background:
Mediastinum harbors a mixed bag of neoplastic lesions with varied therapeutic and prognostic implications. Distinguishing pulmonary squamous cell carcinoma from thymic carcinoma is a challenging task owing to rather nonspecific clinicoradiological features and a considerable overlap in histomorphological features. Thus, we evaluated the diagnostic utility of cluster of differentiation 5 (CD5) and CD117 immunohistochemical markers in distinguishing these tumors.
Materials and Methods:
The archives of department of histopathology were retrospectively reviewed from June 2012 to May 2016. Formalin-fixed paraffin-embedded blocks of 1800 cases diagnosed as nonsmall cell lung carcinoma were retrieved, and immunohistochemical expression of CD5 and CD117 was evaluated in these cases.
Results:
Adenocarcinoma (980; 54.44%) was the most common histological subtype of lung carcinoma observed in our study. CD117 was positive in 171 out of 1800 cases (9.5%) of which 120 cases (70.17%) were adenocarcinoma, followed by 40 cases (23.4%) of squamous cell carcinoma. Immunoreactivity for CD5 was observed in 209 cases (11.61%), 200 (95.7%) cases of which were diagnosed as adenocarcinoma. None of the cases diagnosed as squamous cell carcinoma on histomorphology showed CD5 immunoexpression.
Conclusion:
While thymic squamous cell carcinomas are well known for CD5 and CD117 coexpression, none of the cases of squamous cell carcinoma arising in lung express CD5. These markers are a diagnostic tool to distinguish a primary lung squamous cell carcinoma from thymic carcinoma, particularly in the setting of a central pulmonary lesion with mediastinal involvement.
Keywords: Cluster of differentiation 117, cluster of differentiation 5, nonsmall cell lung cancer, thymic carcinoma
Introduction
Lung carcinoma is one of the leading causes of cancer mortality worldwide with a rising incidence.[1] Adenocarcinoma being the most common histological subtype comprises 70% of all nonsmall cell lung cancers (NSCLCs).[2,3] With the advent of newer diagnostic modalities, the terminology of lung carcinomas had been revised with special emphasis on the terminology used for reporting on small biopsies and aspirate smears. The International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society (IASLC/ATS/ERS) classification system 2011 is the first society to have standardized this terminology.[4,5] However, when these aspirates/biopsies are from central lung tumors along with mediastinal involvement, it becomes difficult to differentiate these from thymic carcinomas.[6,7] Among the thymic carcinomas, frequency of thymic squamous cell carcinoma is higher in Asia reaching up to 90% than in West (30%) and is known to frequently invade the adjacent lung tissue.[8] The most common histologic variant of thymic carcinoma is squamous cell carcinoma which cannot be differentiated from primary squamous cell carcinoma of the lung on morphological examination alone. At the same time, this differentiation is essential owing to different treatment modalities employed for each of these tumors along with their individual prognostic indicators.[9,10] As compared to squamous cell carcinoma of the lung, thymic squamous cell carcinomas are known to have better prognosis.[11]
On literature search, the utility of different immunohistochemical markers to distinguish squamous cell carcinoma of the lung and thymic carcinoma has been studied by few authors of which cluster of differentiation 5 (CD5) and CD117 (c-kit) have emerged as promising diagnostic tools.[9] However, further studies with a large sample size are essential to validate the data published. Keeping in view these considerations, we retrospectively analyzed cases of NSCLCs diagnosed on histopathology over a period of 4 years in a tertiary care setting. Our study evaluates the expression of CD5 and CD117 in NSCLCs to establish their role as differential diagnostic markers for lung and thymic squamous cell carcinomas.
Materials and Methods
The archives of department of histopathology were retrospectively reviewed from June 2012 to May 2016. Of the 56,706 pathology case records reviewed, 1800 cases of NSCLCs were diagnosed over a period of 4 years. The clinical details along with formalin-fixed paraffin-embedded blocks of these cases were retrieved. All the cases were further classified according to the new international multidisciplinary classification system (IASLC/ATS/ERS). An immunohistochemical panel of CD5 (Thermo Scientific, Clone: SP 19) and CD117 (Biocare Medical, Clone: EP10) was put in all the cases. In brief, sections measuring 3–4 μm thick were cut, deparaffinized with xylene and brought to water through graded levels of alcohol. Endogenous peroxidase activity was blocked by treating the slides with hydrogen peroxide for 30 min at room temperature. Antigen retrieval was done by immersing the slides in citrate buffer using the pressure cooker method. Then, the slides were incubated overnight with the primary antibody (rabbit polyclonal) at 4°C in a humidified chamber. The following day secondary antibody was added. The sections were then incubated with diaminobenzidine for visualization of the peroxidase reaction. After being washed in water, the sections were counter stained with hematoxylin, dehydrated in alcohol, cleared in xylene, and mounted. Immunohistochemistry (IHC) was evaluated in a blinded fashion, i.e., without knowing the histopathological diagnosis. The cases were considered positive for CD5 and CD117 if 1% of the tumor cells showed diffuse cytoplasmic and/or membranous brown staining. Statistical analysis was performed using IBM SPSS software version 21.0 (Armonk, NY: IBM Corp) and P < 0.05 was considered statistically significant.
Results
Clinicopathological features of 1800 cases of NSCLC were analyzed. The mean patient age at the time of diagnosis was 55 years (range: 28–84 years). Males (69.44%) were more commonly affected than females (30.55%) with a male: female ratio of 2.2:1. Adenocarcinoma (980; 54.44%) was the most common histological subtype of lung carcinoma diagnosed. Squamous cell carcinoma was the next common tumor diagnosed in 720 patients (40%). This was followed by large cell carcinoma (60; 3.33%) and adenosquamous carcinoma (40; 2.22%).
On IHC, both CD117 and CD5 showed diffuse cytoplasmic and membranous positivity [Figures 1 and 2]. Two hundred and nine (11.61%) cases showed immunoreactivity for CD5, and 171 cases (9.5%) were positive for CD117. Coexpression of both CD117 and CD5 immunomarkers was seen in 34 (1.88%) cases [Table 1].
Figure 1.
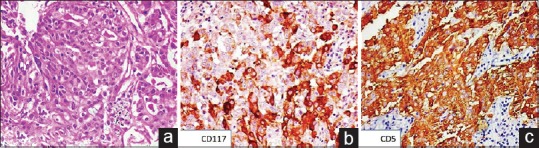
(a) Photomicrograph showing glandular arrangement of cells with moderate amount of eosinophilic cytoplasm and central vesicular nuclei (H and E, ×200). On immunohistochemistry, the tumor cells showed cytoplasmic and membranous positivity for (b) cluster of differentiation 117 (immunostain, ×200) and (c) cluster of differentiation 5 (immunostain, ×200)
Figure 2.
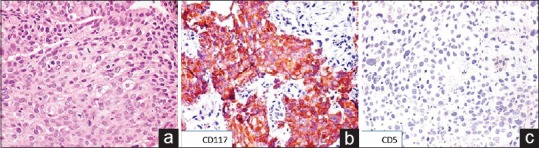
(a) Photomicrograph showing polygonal cells arranged in diffuse sheets with dense eosinophilic cytoplasm and central hyperchromatic nucleus (H and E, ×200). (b) On immunohistochemistry, the tumor cells showed cytoplasmic and membranous positivity for cluster of differentiation 117 (immunostain, ×200). (c) The tumor cells were negative for cluster of differentiation 5 (immunostain, ×200)
Table 1.
Clinicopathological features and immunohistochemical expression in 1800 cases of nonsmall cell lung cancers
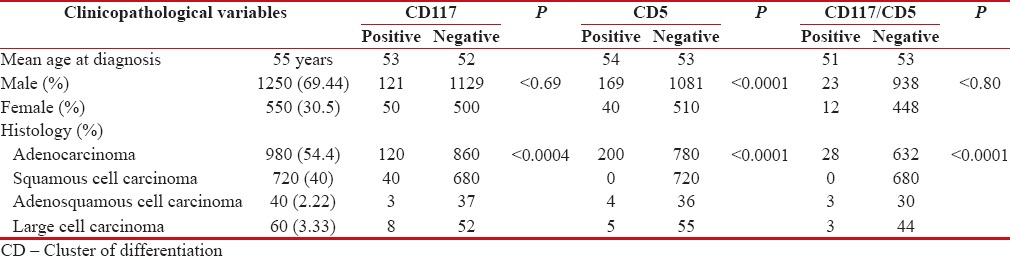
The CD117-positive group (171 cases) comprised of 120 (70.17%) cases of adenocarcinoma, 40 (23.4%) cases of squamous cell carcinoma, 8 (4.67%) cases of large cell carcinoma, and 3 (1.75%) cases of adenosquamous carcinoma. The frequency of CD117 positivity was higher in adenocarcinoma as compared to other histological subtypes. This was found to be statistically significant (P < 0.0004).
There were 200 cases (95.7%) of adenocarcinoma in the CD5-positive group (209 cases), 5 cases (2.4%) of large cell carcinoma, and 4 cases (1.91%) of adenosquamous carcinoma. There was no case of squamous cell carcinoma of the lung showing immunoreactivity for CD5. The frequency of CD5-positive tumors was statistically higher in adenocarcinoma than in other histological subtypes (P < 0.0001).
Coexpression of CD117 and CD5 was seen in 28 cases (82.35%) of adenocarcinoma and 3 cases (8.82%) each of adenosquamous and large cell carcinoma. None of the squamous cell carcinoma cases showed immunohistochemical coexpression of CD117 and CD5. In this group also, coexpression was seen more frequently in adenocarcinoma which was statistically significant (P < 0.0001) [Table 1].
Discussion
Mediastinum is the central portion of the thoracic cavity, housing numerous organs, and harboring a mixed bag of nonneoplastic and neoplastic lesions. Accurate diagnosis is essential owing to the widely variable therapeutic and prognostic implications. Preoperative diagnosis of mediastinal masses is a challenging task due to rather nonspecific clinicoradiological features and technical difficulty in performing fine-needle aspiration cytology. Imaging is the initial preoperative investigation of choice for a mediastinal mass as it helps in compartmentalizing the lesion and narrowing the differential diagnosis. However, histopathological examination remains the gold standard for categorization and an accurate diagnosis of these lesions.[9] Morphological diagnosis of squamous cell carcinoma needs to be correlated with the exact site of the biopsy. The sites of involvement within this anatomical compartment include the more common sites such as lung, esophagus, metastasis, and the less commonly thymus.[12] Morphology alone fails to categorize the tumor as metastatic or primary and cannot identify the site of origin in case of latter. Thus, immunohistochemical analysis is imperative before a decisive diagnosis.[9]
CD117 is a transmembrane tyrosine kinase receptor protein which is encoded by the proto-oncogene c-kit that maps to chromosome 4.[13] It is normally expressed at high levels in tissues including germ cells, hematopoietic stem cells, mast cells, melanocytes, and the interstitial cells of Cajal.[14] Overexpression of CD117 is noted in a number of human malignancies, mainly gastrointestinal stromal tumors, seminoma, and myeloproliferative disorders. CD117 expression has been reported in adenoid cystic carcinoma of the salivary glands and occasionally in breast carcinomas, small cell lung carcinomas, and NSCLCs.[15,16,17,18,19]
CD5 belongs to ancient scavenger receptor superfamily present at 11q13. It is a monomeric glycoprotein which is expressed by T-cells during the various stages of T-cell differentiation in the thymus.[20,21] It is a type of receptor molecule which interacts with one of its ligands CD72 present on the surface of B-cells. This interaction results in the increased proliferation of both the cells.[22]
Borrowing from literature, thymic carcinomas express CD117 and CD5 in approximately 85% and 70% cases, respectively. Coexpression of both the markers is seen in about 60% of these cases.[13,23,24] Therefore, positive expression of CD117 and CD5 supports the histological diagnosis of thymic carcinoma.[9] Various authors have evaluated the diagnostic utility of CD117 and CD5 in thymic carcinomas; however, data regarding the expression of these immunomarkers in NSCLC are limited. To the best of our knowledge, ours is the first study to evaluate the expression of CD117 and CD5 in a large sample size of 1800 cases of NSCLC. Most studies have reported CD117 positivity in 20% of NSCLC with a range of 7%–64%.[23,25] This wide range may be attributed to the different cutoff levels applied in different studies. In the current study, we found CD117 positivity in 171 out of 1800 cases (9.5%). Adenocarcinoma was the most common tumor to express CD117 (70.1% of all CD117 positive cases), followed by squamous cell carcinoma, large cell carcinoma, and adenosquamous carcinoma. These findings are in concordance with the previously published data.[9,25]
Similar to CD117, CD5 expression is also seen in a wide range of NSCLC cases (0%–85%).[23,25] None of the studies had a substantial number of sample size. Therefore, the prevalence of CD5 in NSCLC still remains unclear.[23,25,26] In the present study, 209 out of 1800 NSCLC cases (11.61%) showed CD5 positivity. It was maximally expressed by adenocarcinoma (95.7% of all CD5-positive cases) followed by large cell carcinoma and adenosquamous carcinoma. Coexpression of CD117 and CD5 was seen in 34 (1.88%) cases of NSCLC. Interestingly, in our study, none of the squamous cell carcinoma (720) cases expressed CD5 immunoreactivity. Thus, expression of CD5 or coexpression of CD117 and CD5 in a mediastinal mass rules out squamous cell carcinoma lung.
Conclusion
This study demonstrates that though NSCLC expresses CD117 and CD5, however, squamous cell carcinomas of the lung are always negative for CD5. Therefore, an immunohistochemical panel including CD5 and CD117 is helpful in the differential diagnosis of primary pulmonary squamous cell carcinomas and primary thymic squamous cell carcinomas with coexpression as a strong argument for a thymic primary.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Okada M. Subtyping lung adenocarcinoma according to the novel 2011 IASLC/ATS/ERS classification: Correlation with patient prognosis. Thorac Surg Clin. 2013;23:179–86. doi: 10.1016/j.thorsurg.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura H, Saji H. Worldwide trend of increasing primary adenocarcinoma of the lung. Surg Today. 2014;44:1004–12. doi: 10.1007/s00595-013-0636-z. [DOI] [PubMed] [Google Scholar]
- 4.Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: Relevance for clinical practice and clinical trials. J Clin Oncol. 2013;31:992–1001. doi: 10.1200/JCO.2012.46.9270. [DOI] [PubMed] [Google Scholar]
- 5.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger K, Yatabe Y, et al. Diagnosis of lung adenocarcinoma in resected specimens: Implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification. Arch Pathol Lab Med. 2013;137:685–705. doi: 10.5858/arpa.2012-0264-RA. [DOI] [PubMed] [Google Scholar]
- 6.Castle SM, Salas N, Leveillee RJ. Radio-frequency ablation helps preserve nephrons in salvage of failed microwave ablation for a renal cancer in a solitary kidney. Urol Ann. 2013;5:42–4. doi: 10.4103/0974-7796.106966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang ER, Schreiner AM, Pua BB. Advances in lung adenocarcinoma classification: A summary of the new international multidisciplinary classification system (IASLC/ATS/ERS) J Thorac Dis. 2014;6(Suppl 5):S489–501. doi: 10.3978/j.issn.2072-1439.2014.09.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG, editors. WHO Classification of Tumours of Lung, Pleura, Thymus and Heart. France: IARC Press; 2015. [DOI] [PubMed] [Google Scholar]
- 9.Kriegsmann M, Muley T, Harms A, Tavernar L, Goldmann T, Dienemann H, et al. Differential diagnostic value of CD5 and CD117 expression in thoracic tumors: A large scale study of 1465 non-small cell lung cancer cases. Diagn Pathol. 2015;10:210. doi: 10.1186/s13000-015-0441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Syrios J, Diamantis N, Fergadis E, Katsaros L, Logothetis M, Iakovidou I, et al. Advances in thymic carcinoma diagnosis and treatment: A review of literature. Med Oncol. 2014;31:44. doi: 10.1007/s12032-014-0044-2. [DOI] [PubMed] [Google Scholar]
- 11.Marx A, Rieker R, Toker A, Länger F, Ströbel P. Thymic carcinoma: Is it a separate entity? From molecular to clinical evidence. Thorac Surg Clin. 2011;21:25–31. doi: 10.1016/j.thorsurg.2010.08.010. v-vi. [DOI] [PubMed] [Google Scholar]
- 12.Yoneda KY, Louie S, Shelton DK. Mediastinal tumors. Curr Opin Pulm Med. 2001;7:226–33. doi: 10.1097/00063198-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Heinrich MC, Rubin BP, Longley BJ, Fletcher JA. Biology and genetic aspects of gastrointestinal stromal tumors: KIT activation and cytogenetic alterations. Hum Pathol. 2002;33:484–95. doi: 10.1053/hupa.2002.124124. [DOI] [PubMed] [Google Scholar]
- 14.Holst VA, Marshall CE, Moskaluk CA, Frierson HF., Jr KIT protein expression and analysis of c-kit gene mutation in adenoid cystic carcinoma. Mod Pathol. 1999;12:956–60. [PubMed] [Google Scholar]
- 15.Palmu S, Söderström KO, Quazi K, Isola J, Salminen E. Expression of C-KIT and HER-2 tyrosine kinase receptors in poor-prognosis breast cancer. Anticancer Res. 2002;22:411–4. [PubMed] [Google Scholar]
- 16.Araki K, Ishii G, Yokose T, Nagai K, Funai K, Kodama K, et al. Frequent overexpression of the c-kit protein in large cell neuroendocrine carcinoma of the lung. Lung Cancer. 2003;40:173–80. doi: 10.1016/s0169-5002(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 17.Micke P, Basrai M, Faldum A, Bittinger F, Rönnstrand L, Blaukat A, et al. Characterization of c-kit expression in small cell lung cancer: Prognostic and therapeutic implications. Clin Cancer Res. 2003;9:188–94. [PubMed] [Google Scholar]
- 18.Naeem M, Dahiya M, Clark JI, Creech SD, Alkan S. Analysis of c-kit protein expression in small-cell lung carcinoma and its implication for prognosis. Hum Pathol. 2002;33:1182–7. doi: 10.1053/hupa.2002.129199. [DOI] [PubMed] [Google Scholar]
- 19.Van de Velde H, von Hoegen I, Luo W, Parnes JR, Thielemans K. The B-cell surface protein CD72/Lyb-2 is the ligand for CD5. Nature. 1991;351:662–5. doi: 10.1038/351662a0. [DOI] [PubMed] [Google Scholar]
- 20.Luo W, Van de Velde H, von Hoegen I, Parnes JR, Thielemans K. Ly-1 (CD5), a membrane glycoprotein of mouse T lymphocytes and a subset of B cells, is a natural ligand of the B cell surface protein Lyb-2 (CD72) J Immunol. 1992;148:1630–4. [PubMed] [Google Scholar]
- 21.Hishima T, Fukayama M, Fujisawa M, Hayashi Y, Arai K, Funata N, et al. CD5 expression in thymic carcinoma. Am J Pathol. 1994;145:268–75. [PMC free article] [PubMed] [Google Scholar]
- 22.Pan CC, Chen PC, Chiang H. KIT (CD117) is frequently overexpressed in thymic carcinomas but is absent in thymomas. J Pathol. 2004;202:375–81. doi: 10.1002/path.1514. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa K, Matsuno Y, Kunitoh H, Maeshima A, Asamura H, Tsuchiya R. Immunohistochemical KIT (CD117) expression in thymic epithelial tumors. Chest. 2005;128:140–4. doi: 10.1378/chest.128.1.140. [DOI] [PubMed] [Google Scholar]
- 24.Asirvatham JR, Esposito MJ, Bhuiya TA. Role of PAX-8, CD5, and CD117 in distinguishing thymic carcinoma from poorly differentiated lung carcinoma. Appl Immunohistochem Mol Morphol. 2014;22:372–6. doi: 10.1097/PAI.0b013e318297cdb5. [DOI] [PubMed] [Google Scholar]
- 25.Butnor KJ, Burchette JL, Sporn TA, Hammar SP, Roggli VL. The spectrum of Kit (CD117) immunoreactivity in lung and pleural tumors: A study of 96 cases using a single-source antibody with a review of the literature. Arch Pathol Lab Med. 2004;128:538–43. doi: 10.5858/2004-128-538-TSOKCI. [DOI] [PubMed] [Google Scholar]
- 26.Thomas de Montpréville V, Ghigna MR, Lacroix L, Besse B, Broet P, Dartevelle P, et al. Thymic carcinomas: Clinicopathologic study of 37 cases from a single institution. Virchows Arch. 2013;462:307–13. doi: 10.1007/s00428-013-1371-y. [DOI] [PubMed] [Google Scholar]


