Abstract
Objectives:
The present study aimed to investigate the efficacy, toxicity, and impact of induction chemotherapy (IC) in technically unresectable T4a oral cavity squamous cell cancers (OSCCs).
Materials and Methods:
Patients diagnosed with technically unresectable locally advanced T4a OSCC from January 2013 and November 2016 at our center, who received 2–3 cycles of IC and then assessed for resectability, were reviewed retrospectively. Patients' profile, response rates and toxicity of IC, resectability status, and overall survival (OS) were evaluated. Statistical analyses were performed using SPSS version 17.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results:
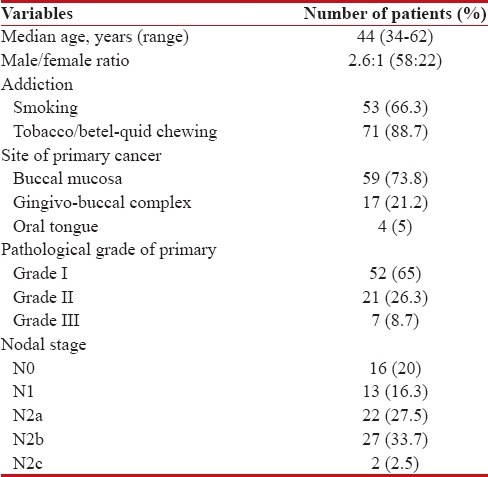
Totally 80 patients received IC, and of them 58 (72.5%) were males. Median age at diagnosis was 44 years (range, 34–62 years). All our patients received IC with doublet regimen. Majority of the patients had buccal mucosa cancers (73.8%), followed by gingivobuccal complex (21.2%) and oral tongue (5%) primaries. After IC, partial response was achieved in 17 (21.3%) patients, stable disease in 49 (61.3%) patients and disease progression was noted in 14 (17.4%) patients. Post-IC, resectability was achieved in 19 (23.8%) of 80 patients, but 4 of them did not undergo surgery due to logistic and personal reasons. The median OS of patients who underwent surgery followed by adjuvant local therapy (n = 15) was 16.9 months (95% CI: 15.2–19.8 months) and for those treated with nonsurgical local therapy (n = 65) was 8.8 months (95% CI: 6.8–10.6 months) (log-rank P = 0.000).
Conclusions:
IC had a manageable toxicity profile and achieved resectability in 23.8% of our patients with technically unresectable T4a OSCC. Patients underwent resection had a significantly better median OS than those who received nonsurgical local treatment.
Keywords: Induction chemotherapy, oral cavity squamous cell cancers, technically unresectable
Introduction
Squamous cell carcinoma is the most common tumor of the oral cavity.[1] The majority of oral cavity squamous cell cancers (OSCCs) present at locally advanced stage and have a relatively poor prognosis with a reported 5-year overall survival (OS) <50%–60%.[2,3,4] The current standard of care for resectable locally advanced OSCC is surgical resection followed by adjuvant radiation with or without concurrent chemotherapy, as indicated.[5]
As per the current American Joint Committee on Cancer (AJCC) staging system, locally advanced T4a OSCCs are considered as resectable tumors.[6] However, in routine clinical practice, certain T4a OSCCs are not technically resectable at baseline, due to extensive loco-regional disease extent which does not come under the definition of T4b,– for example, presence of skin induration/edema up to the zygomatic arch and/or involvement of the pterygoid muscles. For the purpose of achieving a negative pathological margin, extensive surgical procedures are required in these tumors, which are associated with unacceptable amount of cosmetic deformity and functional morbidity.[7] As a consequence, locally advanced T4a OSCCs with these above mentioned features, are still considered technically unresectable, despite recent advances in surgical and reconstructive techniques. As of current practice, these technically unrestable oral cavity tumors are treated with definitive chemoradiation, at most centers. However, the results of this nonsurgical local treatment modality in locally advanced OSCCs are disappointing with a reported 1-year disease-free survival ranging from 10% to 40%, in various studies.[8,9,10,11,12,13,14]
Two large landmark studies, the TAX323 and TAX 324, had highlighted the role of induction chemotherapy (IC), in unresectable and locally advanced head and neck cancers.[15,16] The use true-positive fraction (TPF) (docetaxel, cisplatin, and 5-fluorouracil) regimen in these trials led to an overall response rate (ORR) of about 68%. However, these two landmark studies were not exclusively designed for OSCC and <15% of the included patients had oral cavity cancers. In a phase III randomized controlled trial, Licitra et al. showed that the use of IC in resectable OSCCs was associated with 33% clinical complete response (CR) and 82% ORR.[17]
We believe that a proportion of patients with technically unresectable T4a OSCCs, might be made resectable by the use of IC and this may improve the overall outcome. To examine the validity of such a hypothesis, we performed a retrospective analysis of our patients with technically unresectable T4a OSCCs who were treated with IC.
Materials And Methods
Patient selection, evaluation, and treatment
All consecutive cases diagnosed with locally advanced technically unresectable T4a OSCC and having Eastern Cooperative Oncology Group performance score ≤2, between January 2013 and October 2016 at the Department of Medical Oncology, Kidwai Memorial Institute of Oncology, Bengaluru, Karnataka, India, were investigated retrospectively. All the patients underwent a detailed history and clinical examination. The locoregional disease extent was investigated by contrast-enhanced computed tomography scans, and the diagnosis was confirmed by microscopic examination of the biopsy specimens. These patients were considered technically unresectable upfront at a multidisciplinary clinic due to advanced local disease (e.g., the presence of skin induration/edema up to the zygomatic arch and/or involvement of pterygoid muscles) and offered IC with an aim to achieve resectability. All our patients received IC with a 2-drug regimen of Paclitaxel 175 mg/m2 on day 1 and carboplatin area under the curve 5 on day 1 (plus carboplatin [PC] regimen), with secondary G-CSF prophylaxis. A 25% dose reduction in subsequent cycles was done in patients developing any Grade 4 or life-threatening toxicity. Responses to IC were reported according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1). The adverse events were classified based on the Common Terminology Criteria for Adverse Events version 4.0. After the completion of 2–3 cycles of IC, all patients were evaluated clinico-radiologically for response assessment. In patients who had adequate down-staging of the tumor, surgery had been offered as an option. Following surgery, all patients received adjuvant radiation with or without concurrent chemotherapy (Cisplatin 40 mg/m2/weekly), depending on the histopathological report of the operated specimen. Patients with the unresectable disease even after IC, were treated with either definitive chemo-radiation or radical radiation or palliative radiation or best supportive care (BSC), depending on the performance status, locoregional disease extent after IC, patients choice and logistics.
Statistical analysis
Duration of progression-free survival (PFS) was defined by the time from the treatment initiation until the documented recurrence or disease progression, and overall survival (OS) was calculated from the date of diagnosis until death. Kaplan Meier survival analysis was used for estimation of median OS in the whole cohort. Impact of therapeutic strategies on survival was tested using the log rank test. OS was taken as the primary end-point for this retrospective analysis, and the secondary end-points were PFS, response, and toxicity profile of IC. All statistical analyses were performed using SPSS version 17.0 for Windows (IBM, SPSS Inc., Chicago, IL, USA).
Results
Totally 80 cases of locally advanced technically unresectable T4a OSCCs treated with IC over the mentioned time period, were retrospectively reviewed. The baseline patients' characteristics are shown in Table 1. All our patients received doublet IC with PC regimen. Majority of patients (83.8%, n = 67) received 3 cycles of IC and 8 patients (10%) received 2 cycles. Five patients (6.2%) received only 1 cycle of IC due to rapid disease progression.
Table 1.
Patients’ profile at baseline
According to the assessment of response to IC, stable disease was documented in 49 patients (61.3%), followed by a partial response (PR) in 17 patients (21.3%) and disease progression in 14 patients (17.4%). None of our patients achieved a CR after IC. 19 patients (including the 17 partial responders) achieved resectability after IC. On univariate analysis, achievement of PR was found to be a significant factor for achievement of resectability (P = 0.000).
The rate of febrile neutropenia was 18.8% (n = 15) with IC. The rate of other Grade 3-4 toxicities were as follows: hematological toxicity– 21.3% (n = 17); mucositis–2.5% (n = 2), and diarrhea– 2.5% (n = 2). Two of our patients required 25% dose reduction in the next cycle, due to severe mucositis and diarrhea.
After IC, 19 patients (23.8%) achieved resectability, but 4 of them did not undergo surgery due to logistic and personal reasons. One of these 4 patients opted for definitive chemo-radiation, and the rest of 3 patients defaulted after IC and received palliative radiation latter. All the 15 patients, who underwent surgery post IC, had a R0 resection and received adjuvant chemo-radiation at standard doses. Among the 61 patients who had not achieved resectability after IC, 27 received definitive chemo-radiation, 8 received definitive radiation, 14 received palliative radiation, and 12 patients received BSC alone.
At the time of last follow-up, 49 patients (61.3%) had disease failure, either in the form of loco-regional recurrence/progression (n = 44) or as distant recurrence (n = 2) or as both (n = 3). The median PFS and OS for the whole cohort were 7.6 months and 11.2 months, respectively. The median OS of patients who underwent surgery followed by adjuvant local therapy (n = 15) was 16.9 months (95% confidence interval [CI]: 15.2-19.8 months) and for those treated with nonsurgical local therapy (n = 65) was 8.8 months (95% CI: 6.8–10.6 months) (log-rank P = 0.000) [Figure 1]. Among the 65 patients, who received nonsurgical local treatment post IC, the median OS for patients receiving definitive chemo-radiation, definitive radiation, palliative radiation and BSC were 11.8 months, 8.5 months, 6.8 months, and 5.3 months, respectively [Figure 2].
Figure 1.
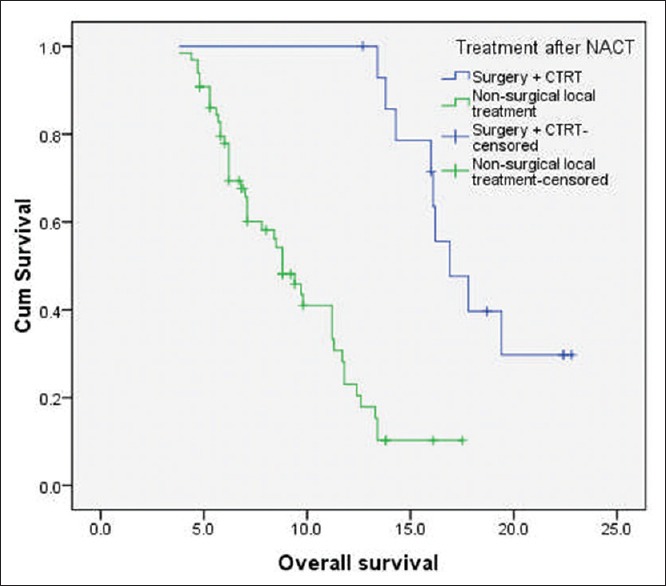
Kaplan–Meier curve of overall survival (in months) of patients with technically unresectable T4a oral cavity squamous cell cancers. The median overall survival of patients who underwent surgery followed by adjuvant local therapy (n = 15) was 16.9 months and for those treated with nonsurgical local therapy (n = 65) was 8.8 months (log-rank P = 0.000)
Figure 2.
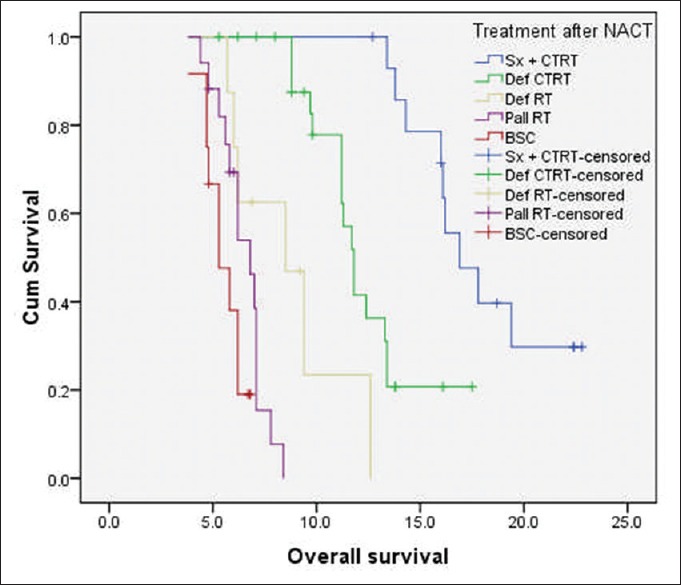
Kaplan–Meier curve of overall survival (in months) of patients with technically unresectable T4a oral cavity squamous cell cancers, who received nonsurgical local treatment postinduction chemotherapy (n = 65). The median overall survival for patients receiving definitive chemo-radiation, definitive radiation, palliative radiation, and best supportive care were 11.8, 8.5, 6.8, and 5.3 months, respectively
Discussion
AJCC staging 2010 defines moderately advanced local disease, or T4a OSCC as tumor invading any one of the following-the cortical bone, inferior alveolar nerve, floor of mouth, skin of face, extrinsic muscles of tongue, and maxillary sinus.[6] Conventionally, T4a OSCCs are considered as resectable tumors. However, “resectability” remains a controversial term with limited consensus among the surgical teams. The balance between the extent of surgery required to achieve negative pathological margins and acceptable cosmetic and functional deformity defines the resectability of a tumor.[18]
The role of IC in head and neck SCC, is being explored with the basic premise of reducing extent of surgical resection, improving loco-regional disease control and decreasing distant metastasis, thereby improving treatment outcomes by decreasing mortality and morbidity. However, till date the indications of IC in oral cavity cancers are not clearly defined. Majority of studies have failed to demonstrate a significant benefit of IC in terms of loco-regional control (LRC) and OS in resectable OSCC.[17,19,20,21,22,23] However, almost all the published reports have shown encouraging results in terms of achieving high ORR with IC.
The results of definitive chemo-radiation or definitive radiation alone in locally advanced OSCCs are not satisfactory.[9] A few small studies showed encouraging results in terms of achieving good LRC using up front compartmental resections, in OSCC patients with the advanced locoregional disease.[24,25] However, these are single institutional reports and are not generally reproducible in daily practice. A rationale of proposing IC in these advanced local tumors is to improve the overall outcome by facilitating possible surgical resection following tumor down-staging. Interestingly, there are a few published retrospective reports also, from our country to support this concept.[26,27]
In a study reported by Patil et al. the role of IC followed by resection was retrospectively tested in 123 patients with technically unresectable locally advanced oral cavity cancers.[26]
Unresectibility in these cases was defined as disease reaching up to the zygoma and/or soft tissue swelling up to the zygoma, extensive soft tissue involvement reaching up to the hyoid cartilage, extensive skin infiltration, and the involvement of the infratemporal fossa. The patients were given IC with TPF or TP and assessed for resectibility. The response rate with the three drug and two drug regimens was 32% and 27.37%, respectively. Resectability was achieved in 17 patients with triplet regimen (68%) and 36 patients with doublet regimen (37.89%). The estimated median OS was 12.7 months, for the whole cohort. The estimated median OS was not reached for patients undergoing postchemotherapy resection. This was statistically significant compared to patients treated with nonsurgical modalities postchemotherapy. The estimated median OS in these patients was 8 months (P = 0.0001). They demonstrated the effectiveness of IC in down-staging tumors and enabling radical surgery with comparable 2 years survival to primary radical surgery.
In a larger follow-up study, the same group retrospectively analyzed 721 patients with T4a and T4b OSCC deemed as technically unresectable who received IC.[27] Nearly 43% of these patients had sufficient reduction in tumor size that made them resectable. Three drug regimen achieved resectability in 66.21% and two drug regimen in 40.34% of patients. The LRC rate was 20.6% for the overall cohort. For patients undergoing surgery, the LRC was 32% and 15% for the nonsurgical group. The median estimated survival was 19.6 and 8.16 months, respectively.
In the present study, the response rates and resectability after IC are lower than previously reported,[26,27] which could be explained by differences in the patient profile and IC regimens. Moreover, the assessment of resectability is dependent on the surgical skills available in any oncology center. Hence, our results need to be validated by other Indian centers.
Conclusions
In this study, the use of IC in technically unresectable locally advanced T4a oral cavity cancers seems to be feasible with a manageable toxicity profile. In a subset of patients with these advanced tumors, IC has been shown to cause significant tumor shrinkage and improve resectability. Clearly, these hypothesis generating findings of improved resectability and overall outcomes with IC in patients with technically unresectable T4a OSCC, need further validation via large prospective randomized trials.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Scully C, Bagan J. Oral squamous cell carcinoma overview. Oral Oncol. 2009;45:301–8. doi: 10.1016/j.oraloncology.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Neville BW, Day TA. Oral cancer and precancerous lesions. CA Cancer J Clin. 2002;52:195–215. doi: 10.3322/canjclin.52.4.195. [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Head and Neck Cancers, Version 2. 2016. [Last accessed on 2016 Nov 17]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/headandneck.pdf .
- 6.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th ed. Bangalore: Springer; 2009. [Google Scholar]
- 7.Kekatpure VD, Manjula BV, Mathias S, Trivedi NP, Selvam S, Kuriakose MA, et al. Reconstruction of large composite buccal defects using single soft tissue flap – Analysis of functional outcome. Microsurgery. 2013;33:184–90. doi: 10.1002/micr.22063. [DOI] [PubMed] [Google Scholar]
- 8.Mohanti BK, Umapathy H, Bahadur S, Thakar A, Pathy S. Short course palliative radiotherapy of 20 gy in 5 fractions for advanced and incurable head and neck cancer: AIIMS study. Radiother Oncol. 2004;71:275–80. doi: 10.1016/j.radonc.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Corry J, Peters LJ, Costa ID, Milner AD, Fawns H, Rischin D, et al. The 'QUAD SHOT' – A phase II study of palliative radiotherapy for incurable head and neck cancer. Radiother Oncol. 2005;77:137–42. doi: 10.1016/j.radonc.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Pathak KA, Gupta S, Talole S, Khanna V, Chaturvedi P, Deshpande MS, et al. Advanced squamous cell carcinoma of lower gingivobuccal complex: Patterns of spread and failure. Head Neck. 2005;27:597–602. doi: 10.1002/hed.20195. [DOI] [PubMed] [Google Scholar]
- 11.Ghoshal S, Mallick I, Panda N, Sharma SC. Carcinoma of the buccal mucosa: Analysis of clinical presentation, outcome and prognostic factors. Oral Oncol. 2006;42:533–9. doi: 10.1016/j.oraloncology.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal JP, Nemade B, Murthy V, Ghosh-Laskar S, Budrukkar A, Gupta T, et al. Hypofractionated, palliative radiotherapy for advanced head and neck cancer. Radiother Oncol. 2008;89:51–6. doi: 10.1016/j.radonc.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Al-mamgani A, Tans L, Van rooij PH, Noever I, Baatenburg de jong RJ, Levendag PC, et al. Hypofractionated radiotherapy denoted as the “Christie scheme”: An effective means of palliating patients with head and neck cancers not suitable for curative treatment. Acta Oncol. 2009;48:562–70. doi: 10.1080/02841860902740899. [DOI] [PubMed] [Google Scholar]
- 14.Pearson RA, Bannister-Young RH, Ivison D, Kelly CG, Chatterjee S. Split-course hypofractionated palliative radiotherapy for patients with head and neck squamous cell carcinoma – A worthwhile treatment schedule in the UK? Clin Oncol (R Coll Radiol) 2010;22:890–1. doi: 10.1016/j.clon.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–704. doi: 10.1056/NEJMoa071028. [DOI] [PubMed] [Google Scholar]
- 16.Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–15. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 17.Licitra L, Grandi C, Guzzo M, Mariani L, Lo Vullo S, Valvo F, et al. Primary chemotherapy in resectable oral cavity squamous cell cancer: A randomized controlled trial. J Clin Oncol. 2003;21:327–33. doi: 10.1200/JCO.2003.06.146. [DOI] [PubMed] [Google Scholar]
- 18.Pradhan SA. Surgery for cancer of the buccal mucosa. Semin Surg Oncol. 1989;5:318–21. doi: 10.1002/ssu.2980050505. [DOI] [PubMed] [Google Scholar]
- 19.Okura M, Hiranuma T, Adachi T, Ogura T, Aikawa T, Yoshioka H, et al. Induction chemotherapy is associated with an increase in the incidence of locoregional recurrence in patients with carcinoma of the oral cavity: Results from a single institution. Cancer. 1998;82:804–15. [PubMed] [Google Scholar]
- 20.Bossi P, Lo Vullo S, Guzzo M, Mariani L, Granata R, Orlandi E, et al. Preoperative chemotherapy in advanced resectable OCSCC: Long-term results of a randomized phase III trial. Ann Oncol. 2014;25:462–6. doi: 10.1093/annonc/mdt555. [DOI] [PubMed] [Google Scholar]
- 21.Zhong LP, Zhang CP, Ren GX, Guo W, William WN, Jr, Sun J, et al. Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up-front surgery in locally advanced resectable oral squamous cell carcinoma. J Clin Oncol. 2013;31:744–51. doi: 10.1200/JCO.2012.43.8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong LP, Zhang CP, Ren GX, Guo W, William WN, Jr, Hong CS, et al. Long-term results of a randomized phase III trial of TPF induction chemotherapy followed by surgery and radiation in locally advanced oral squamous cell carcinoma. Oncotarget. 2015;6:18707–14. doi: 10.18632/oncotarget.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma J, Liu Y, Yang X, Zhang CP, Zhang ZY, Zhong LP. Induction chemotherapy in patients with resectable head and neck squamous cell carcinoma: A meta-analysis. World J Surg Oncol. 2013;11:67. doi: 10.1186/1477-7819-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trivedi NP, Kekatpure V, Kuriakose MA. Radical (compartment) resection for advanced buccal cancer involving masticator space (T4b): Our experience in thirty patients. Clin Otolaryngol. 2012;37:477–83. doi: 10.1111/j.1749-4486.2012.02529.x. [DOI] [PubMed] [Google Scholar]
- 25.Liao CT, Ng SH, Chang JT, Wang HM, Hsueh C, Lee LY, et al. T4b oral cavity cancer below the mandibular notch is resectable with a favorable outcome. Oral Oncol. 2007;43:570–9. doi: 10.1016/j.oraloncology.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Patil VM, Noronha V, Joshi A, Muddu VK, Gulia S, Bhosale B, et al. Induction chemotherapy in technically unresectable locally advanced oral cavity cancers: Does it make a difference? Indian J Cancer. 2013;50:1–8. doi: 10.4103/0019-509X.112263. [DOI] [PubMed] [Google Scholar]
- 27.Patil VM, Prabhash K, Noronha V, Joshi A, Muddu V, Dhumal S, et al. Neoadjuvant chemotherapy followed by surgery in very locally advanced technically unresectable oral cavity cancers. Oral Oncol. 2014;50:1000–4. doi: 10.1016/j.oraloncology.2014.07.015. [DOI] [PubMed] [Google Scholar]


