Abstract
Decision-making regarding the use and selection of adjuvant chemotherapy for breast cancer in elderly patients is challenging due to the presence of age-related comorbidities, frailty, and competing causes of mortality. One area, relatively neglected in most guidelines, is the effect of competing causes of mortality on presumed benefit of adjuvant chemotherapy for breast cancer in these patients. This article utilizes a clinical case to illustrate the principles of risk-benefit assessment of adjuvant chemotherapy in elderly patients. We suggest an approach that incorporates validated tools for estimating survival benefits of adjuvant chemotherapy, geriatric assessment, predicting toxicity, and estimating remaining life expectancy without cancer. Integration of all these variables provides a better picture of the possible benefits and harms of adjuvant chemotherapy in this population compared to conventional approaches that incorporate tumor-related variables and nonstandard measures of geriatric assessment.
Keywords: Adjuvant chemotherapy, comorbidities, competing causes of mortality, early breast cancer, elderly
Clinical Case
A 65-year-old postmenopausal woman presented with the complaints of progressive painless right breast lump for 2 months and occasional low backache for 4 months which started after a fall in her bathroom. She had hypertension for 15 years controlled on amlodipine, and cervical and lumbar spondylosis for the preceding 5 years for which she self-medicated with nonsteroidal analgesics. There was no history of diabetes, chronic obstructive pulmonary disease, tobacco or alcohol use, and her family history was not significant. She reported her overall health to be good, could perform all daily activities without help, walk a few blocks, and climb one flight of stairs but had stopped regular exercise for a few months.
On examination, her weight was 65 kg, body mass index (BMI) was 26 kg/m2, and Eastern Cooperative Oncology Group (ECOG) performance status (PS) was 1. She had a 4-cm right breast lump with normal overlying skin and a single 1.5-cm mobile palpable node in the right axilla. Systemic examination was unremarkable except mild tenderness over L1 spine. Core biopsy revealed a high-grade invasive ductal carcinoma (IDC) negative for estrogen receptor (ER), progesterone receptor, and human epidermal growth factor 2 receptor. Chest radiograph and ultrasound of abdomen did not reveal any metastases, and MRI evaluation of dorso-lumbar spine showed compression fracture of L1 vertebral body without any mass lesion. Routine blood investigations including complete blood count, renal and liver functions were normal, and there was evidence of Vitamin D deficiency for which she was started on calcium and Vitamin D supplementation. A final diagnosis of clinically T2, N1, and M0 triple-negative breast cancer (TNBC) was made.
She was further evaluated for cardiac function as part of routine workup. Electrocardiogram did not reveal any significant abnormality, but two-dimensional echocardiogram showed a left ventricular ejection fraction (LVEF) of 45%. Left and right ventricular systolic functions were reported to be fair, there was Grade II diastolic dysfunction, and regional wall motion abnormality was reported in the left ventricle. There was a jerky motion of interventricular septum with moderate tricuspid regurgitation and moderate pulmonary hypertension with pulmonary arterial systolic pressure of 50 mmHg. On further questioning, she denied ever having experienced symptoms related to cardiac illnesses or restriction in daily activities but could not perform any exertional physical activity. Subsequent multigated acquisition scan confirmed reduced LVEF of 45%, and dobutamine stress test was negative for inducible ischemia.
Definitive treatment
This patient did not desire breast conservation and underwent modified radical mastectomy. Histopathology report showed a pathological tumor size of 3 cm with negative margins, IDC Grade II, and Modified Richardson Bloom Score of 7. Lymphovascular emboli were present while perineural invasion and ductal carcinoma in situ were absent. Two out of 14 lymph nodes dissected were positive for metastasis. The patient also underwent L1 vertebroplasty at the same time and the bone biopsy obtained was negative for metastatic involvement. This patient presented to us for consideration of adjuvant chemotherapy.
Principles of Evaluation for Adjuvant Treatment
Estimating the overall benefit of adjuvant chemotherapy regimens
As per the 2012 Early Breast Cancer Trialists' Collaborative Group (EBCTCG) meta-analysis, standard anthracycline-taxane adjuvant polychemotherapy reduces the relative risk of breast cancer-related mortality at 10 years by 33% compared to no chemotherapy, with an overall absolute 10% benefit.[1] Similar relative benefit of standard chemotherapy was obtained in the subgroup analysis in the 55–69 years' age group. Dose-dense chemotherapy, which was not included in the EBCTCG meta-analysis, would add to this benefit. Although the relative benefit of chemotherapy appears to be independent of age (at least up to 70 years), nodal status, tumor diameter, differentiation and receptor status, the absolute benefit is proportional to recurrence risk. Therefore, the absolute benefit of chemotherapy may be higher in patients with larger, higher grade, node-positive, TNBC subgroups.
Due to higher rate of comorbidities and decrease in physiological organ reserve, the toxic impact of chemotherapy on daily function and quality of life may be greater and benefits are possibly lower than younger women. Only a few randomized adjuvant trials have included elderly patients, especially those more than 70 years of age. A retrospective analysis of four CALGB prospective trials (7851, 8082, 8541, and 9344, respectively) suggested that the overall survival (OS) and recurrence-free survival (RFS) benefits that older women derive from more aggressive adjuvant chemotherapy regimen are similar to that conferred on their younger counterparts.[2] For women older than 65, there was a 42% reduction in relapse rate and a 27% reduction in the overall mortality rate associated with more intensive as opposed to less intensive chemotherapy. However, patients aged ≥65 years, who comprised 8% of total population in this analysis, were found to have significantly more treatment-related deaths (0.2% for ≤50 years, 0.7% for 51–64 years, and 1.5% for ≥65 years, P < 0.001).
CALGB-49907 trial is the largest prospective study that evaluated adjuvant chemotherapy regimens in elderly patients with breast cancer.[3] In this study, 633 patients of operable breast cancer with age ≥65 years, PS 0–2, tumor diameter >1 cm, adequate organ function, and clear surgical margins were randomized to receive either standard adjuvant chemotherapy (6 cycles of cyclophosphamide + methotrexate + 5-fluorouracil [CMF] or 4 cycles of adriamycin + cyclophosphamide [AC]) or 6 cycles of capecitabine. Sixty-five percent of the patients were ≥70 years of age and about 5% were ≥80 years of age. Ninety-seven percent had a PS of 0 or 1 and only 3% were PS 2. Results showed that capecitabine was inferior but less toxic than standard chemotherapy. Three-year RFS and OS were 68% and 86% in capecitabine group versus 85% and 91%, respectively, in the standard chemotherapy group, while moderate-to-severe toxicity was seen in 33% and 64% of patients in the two groups, respectively.
Two other randomized trials have compared a nonanthracycline regimen of docetaxel-cyclophosphamide (taxotere and cyclophosphamide [TC]) with anthracycline regimens. The US Oncology Group Trial showed that 4 cycles of TC were superior to 4 cycles of AC in both disease-free survival (DFS) (81% vs. 75%, respectively, Hazard ratio [HR] = 0.74, P = 0.033) and OS (87% vs. 82%, respectively, HR = 0.69, P = 0.032) at a median follow-up of 7 years.[4] In the preplanned subset analysis of DFS in patients ≥65 years age also, TC was superior compared to AC (70% vs. 65%, respectively, HR = 0.70) as was OS (78% vs. 70%, respectively). The rate of cardiac toxicity was lower in TC (two deaths due to cardiac illness) compared to AC (five cardiac deaths), but that of febrile neutropenia (FN) was higher (TC vs. AC, 5% vs. 2.5% overall and 8% vs. 4%, respectively, in elderly age group). A more recent study compared 6 cycles of TC with several standard full anthracycline-taxane regimen (TaxAC) in high-risk Her2 negative breast cancer.5 TC was proven inferior with 4-year invasive DFS as 88.2% for 6 TC vs. 90.7% for TaxAC (P = 0.04).
Thus, taking the available evidence into account, CMF and TC are both appropriate choices when anthracyclines are contraindicated as in our patient, although the strongest evidence, especially in elderly patients, is available for the former.
Utilizing clinical prognostic tools in estimating the benefit of adjuvant chemotherapy
Multivariable prognostic tools have been developed which utilize clinical and tumor characteristics to estimate the benefit of adjuvant chemotherapy. Adjuvant! Online incorporates age and comorbidities to estimate their impact on treatment benefit and survival,[6] and PREDICT also estimates the added survival benefit of adjuvant systemic therapies.[7] Both tools have been validated in large cohorts and help patients as well as clinicians to consider the realistic benefits of various adjuvant therapies, especially chemotherapy. On the basis of these tools, guidelines suggest consideration of adjuvant chemotherapy if there is >10% risk of mortality from breast cancer at 10 years and if chemotherapy decreases 10-year mortality by ≥5%. Adjuvant chemotherapy is generally not offered if the benefit is <3% and may be given as per patient preference if benefit is between 3%–5%.[7,8]
Toxicities of chemotherapy
The boundary of therapeutic and toxic effect of adjuvant chemotherapy is very narrow. Therefore, ascertaining the expected toxicity and tolerance of a regimen becomes very important, especially in older patients with concurrent comorbidities. Life-threatening late toxicities include 0.5%–1.5% risk of anthracycline-induced cardiac dysfunction and 0.5% risk of secondary myelodysplastic syndrome (MDS)/leukemia.[9] The 5- and 10-year rates of congestive heart failure in a multivariate Cox regression analysis of SEER data from 1992 to 2002 for women aged 66-70 years receiving chemotherapy were 19% and 38.4% for anthracycline cohort, 18% and 32.5% for nonanthracycline cohort, and 15% and 29% for no-chemotherapy cohorts, respectively.[10] A retrospective analysis of three CALGB studies reported 1% of cardiac deaths and 1.8% of acute myeloid leukemia-/MDS-related deaths in patients ≥65 years of age.[11] Other long-term toxicities include 25%–60% chance of taxane-induced persistent symptomatic neuropathy. The rate of Grade III–IV neurotoxicity in patients ≥65 year age has been reported as 9%.[11] Chemotherapy is also related to metabolic syndrome and weight gain with 60% chances of being overweight and 30% chances of obesity after chemotherapy, which increases cardiovascular morbidity and mortality.[12] Early menopause, distress, depression, anxiety, and cognitive impairment are other significant and often underestimated after-effects of chemotherapy which contribute to long-term morbidity.
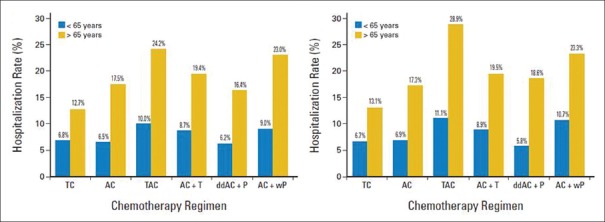
Acute life-threatening toxicities include 5%–25% risk of FN requiring admission and 1%–2% risk of severe hypersensitivity reaction, with an overall average mortality risk of 1%, for different chemotherapy regimens and age groups. Patients of age ≥65 years are 66% more likely to have Grade 4 hematologic toxicity than those aged ≤50 years and a 6% chance of treatment discontinuation compared to 2% in the latter age group.[11] A large retrospective study analyzed the risk of hospitalization according to chemotherapy regimen in early breast cancer.[13] Among patients aged <65 years, hospitalization rates ranged from 6.2% for dose-dense AC + wP (AC followed by weekly-paclitaxel) to 10% for TAC (docetaxel + adriamycin + cyclophosphamide). Those who received TAC and AC + T (AC followed by docetaxel) had significantly higher rates of hospitalization than did patients who received TC. Among patients aged >65 years, these rates ranged from 12.7% (TC) to 24.2% (TAC), and the rates of hospitalization of patients who received TAC, AC + T, AC, or AC + wP were higher than those of patients who received TC [Figure 1]. Apart from infection, FN and hematological toxicities, acute nonhematological toxicities such as nausea and vomiting, mucositis, diarrhea, constipation, dyspepsia, fatigue, and alopecia lead to distress, dropout, decreased quality of life, and increased cost of treatment.
Figure 1.
Risk of hospitalization according to different chemotherapy regimens in early breast cancer.[13] “Reprinted with permission © (2017) American Society of Clinical Oncology. All rights reserved”
Predicting chemotherapy toxicity – risk assessment tools
Various risk assessment tools are available which utilize patient- and chemotherapy-related factors to assess fitness for chemotherapy and estimate the expected toxicities. These tools may be useful in elderly patients and those with significant medical comorbidities where even low-grade toxicities may be enough to cause functional decline, deterioration in quality of life, and even death. There is wide heterogeneity in health and functional status among the elderly population. Chronological age alone provides only limited information regarding an individual's potential tolerance to cancer treatment. Apart from the traditional PS scales, KPS (Karnofsky PS) or ECOG such as and organ function status, several other domains of geriatric assessment need to be evaluated as they impact treatment decisions and outcomes. A multidimensional interdisciplinary comprehensive geriatric assessment (CGA) offers a systematic approach to evaluating the fitness of older cancer patients for various adjuvant therapies.[14] Consensus guidelines from both the National Comprehensive Cancer Network and the International Society for Geriatric Oncology (SIOG) recommend the routine use of a geriatric assessment scale for patients who are >65 years.[15] The key domains that are included in CGA are functional status, comorbidities, cognition, nutrition, psychological state, social support, and medication review. This information can be used to anticipate and manage toxicity, predict survival, uncover unexpected health problems, improve nutrition, and improve pain control.[16] Geriatric assessment allows for characterization of a patient into one of the three following groups: fit, vulnerable, and frail, to assist in clinical decision-making. While a frail patient may be offered palliative care, vulnerable patients can be optimized using rehabilitative strategies to make them fit for standard chemotherapy or be offered modified chemotherapy using extra precautions. Others have developed a nomogram based on elements of CGA for Asian patients that predicts OS in elderly patients with cancer.[17] A full CGA is time-consuming and requires specialized personnel and resources. Hence, simplified geriatric screening tools are required to identify vulnerable patients for whom a CGA could potentially optimize their cancer treatment. These include French G8 tool and the Flemish version of the Triage Risk Screening Tool (fTRST).[18,19]
Models based on CGA have been developed to predict chemotherapy toxicity. In a pivotal study, Hurria et al. investigated factors associated with chemotherapy toxicity in 500 patients of different cancers aged ≥65 years. In addition to demographic and standard clinical variables such as age, cancer type, chemotherapy dose, and creatinine clearance, their study found that deficits identified by CGA such as hearing impairment, limited physical function, falls, and decreased social activity predicted risk of Grade 3-5 chemotherapy toxicity in older patients. These parameters were combined into a Cancer and Aging Research Group (CARG) model and patients were stratified into high-, mid-, and low-risk groups which predicted 83%, 52%, and 30% risk of severe toxicity, respectively. This approach was significantly better than the clinician assessment of KPS for predicting chemotherapy toxicity.[20] In contrast to CGA, CARG tool can be administered in a much shorter time frame. Another tool, Chemotherapy Risk Assessment Scale for High-Age Patient (CRASH) score, which was developed in patients >70 years, can provide reasonable accurate estimates of risk of chemotherapy toxicity and help tailor therapy.[21]
Evaluating risk of other-cause mortality
Life expectancy calculation
Patients are at risk of dying not only from cancer (cause-specific mortality) but also from other causes (other-cause mortality). Adjuvant systemic cancer therapy will obviously only reduce cause-specific mortality in most instances. It could either have no effect or increase other-cause mortality. Thus, one way of considering the real benefit of adjuvant systemic therapy could be as follows: net gain in all-cause mortality = (gain in cause-specific mortality) – (increase in other-cause mortality). However, this equation does not account for “competing causes of mortality.” The risk of other-cause mortality keeps increasing with age because of progressively increasing comorbidities. The implication is that, with increasing age, the number of breast cancer patients who are at risk of dying from breast cancer goes on decreasing.[22] Since adjuvant systemic therapy (and radiotherapy) can only potentially prevent breast cancer deaths, the absolute mortality benefit of these treatments declines with age. The preceding formulation is not self-evident to clinicians and is not often considered. However, the end point of OS in clinical trials incorporates all causes of death, including competing causes, and is therefore the most robust measure of benefit from a treatment.
One thought example would be useful to illustrate this point: consider a group of 100 women with clinically T1–T2, N0, breast cancer, all of whom also have New York Heart Association (NYHA) class IV dilated cardiomyopathy and are between the ages of 65 and 75 years. A cardiologist estimates that the 1-year cardiac-cause survival of this cohort is likely to be 30% and 3-year survival is approximately 5%. It is evident that, in this particular breast cancer cohort, no cancer-directed treatment (including definitive surgery) is likely to result in any substantial reduction in all-cause mortality because the overwhelming majority are likely to die of cardiac failure, on which this treatment is likely to have no or detrimental impact. Persisting with this thought example, what would be the overall mortality gain from adjuvant chemotherapy in a group of patients aged between 65 and 75 years, of whom 3% have end-stage renal disease, 6% NYHA class I-II cardiac disease, 1% NYHA class III-IV cardiac disease, 16% poorly controlled hypertension, 14% well-controlled hypertension, 8% poorly controlled diabetes, 15% well-controlled diabetes, 7% more than one comorbidity, and 30% free of any comorbidities? It is evident that the probability of dying of a cause other than breast cancer over time is real and substantial in all these hypothetical groups of patients but is complex to compute, especially in the last group. Despite obvious difficulty, it is important to appreciate that the absolute all-cause mortality gain from any cancer intervention is likely to be less in a group of patients with higher other-cause mortality compared to a group with lower other-cause mortality, given the same efficacy as an anticancer treatment in both groups. The Prostate Cancer Community has appreciated this concept for a long time and incorporated it in their clinical trials and decision-making, but this has not been the case with breast cancer. Therefore, the patient's estimated remaining life expectancy, independent of her breast cancer diagnosis and inclusive of comorbidities, aids in decision-making.
Country-specific and age-adjusted life expectancy tables for both males and females, provided by population census data or life insurance data, can be utilized. Such updated life expectancy tables are also provided by the World Health Organization (WHO) and calculators based on these data can be used.[23,24] However, different factors which influence life expectancy need to be considered for realistic estimates and several tools are available to aid us in a variety of different patient populations (e.g., community dwelling or nursing home). Many of these tools are available in an online calculator format on the “ePrognosis” website,[25] which itself is based on the meta-analysis of individual participant data from five cohorts of older adults in developed and developing countries.[26] This index is based on age, gender, comorbidity status (diabetes, cardiac, pulmonary, cancer), habits (smoking, alcohol), BMI, several easily ascertained functional status inputs (physical activity, bathing, walking, and orientation in time), and individual's self-assessed health status, all of which were derived from a Cox regression model. Importantly, estimates of 10-year mortality derived from this model have proven to be highly accurate in a validation dataset. Another index, the Lee–Schonberg index, takes into consideration age, gender, BMI, comorbidities, cigarette smoking, physical activity, previous hospitalizations, and limitations in activities of daily living due to physical, mental, emotional, or cognitive problems to estimate the patient's remaining life expectancy.[27,28] None of these tools are perfect but they provide reasonable estimates of the probability of patient surviving long enough to benefit from cancer treatments and are generally better than subjective guessing.
Impact of comorbidities on life expectancy
The detrimental effect of comorbidities on survival needs to be estimated if not already considered while estimating life expectancy. A longitudinal observational study of 936 women with breast cancer aged 40–84 years showed that patients who had three or more comorbid medical conditions had a 20-fold higher rate of mortality from causes other than breast cancer and a 4-fold higher all-cause mortality rate compared to those who had no comorbid medical conditions.[29] In addition to its effect on survival, the presence of comorbid medical conditions also affects a patient's ability to tolerate cancer therapy as well as the choice of therapy. A patient with significant diabetic neuropathy does not merit taxanes, just like someone with cardiac disease will not merit anthracyclines. An assessment of comorbidities can also provide information that is independent of the individual's functional status.[30]
The most commonly used general comorbidity measure is the Charlson Comorbidity Index (CCI). It was developed to predict 1-year mortality in medical inpatients and was subsequently validated in a population of breast cancer patients.[31] Nineteen comorbid conditions are assigned weights of 1, 2, 3, or 6, based on the ratio of the mortality risk for patients with the comorbidity of interest versus the mortality risk for those without. The sum of the weights of all the conditions is calculated to create a comorbidity index for each patient. The 10-year mortality estimates for comorbid disease for the different scores were: “0”–8%, “1”–25%, “2”–48%, and “≥3”–59%. Another example of comorbidity measure is the Cumulative Illness Rating Scale for Geriatrics. This scoring system measures the chronic medical illness (morbidity) burden while taking into consideration the severity of chronic diseases in 14 items representing individual body systems.[32]
Disease-specific comorbidity measures have also been developed and tested in a single disease population and intended for use only in that disease. In heart failure, the two most commonly used tools for predicting disease-specific mortality are the MAGIC meta-analysis model and the Seattle Heart Failure Model (SHFM). The MAGIC meta-analysis model includes 13 highly significant independent predictors of mortality in the following order of predictive strength: age, lower ejection fraction, NYHA class, serum creatinine, diabetes, not prescribed β-blocker, lower systolic BP, lower body mass, time since diagnosis, current smoker, chronic obstructive pulmonary disease, male gender, and not prescribed angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blockers. Based on these factors, an integer score is calculated which has been shown to predict 1- and 3-year mortality.[33] This score can be calculated in clinic by an easy-to-use web-based tool.[34] The SHFM is another validated prediction model that estimates total mortality in patients with heart failure using commonly obtained clinical, laboratory, medication, and device variables.[35]
Application of Principles to Our Patient: Mrs. X
Benefit of adjuvant chemotherapy in Mrs. X – PREDICT score
Applying the principles of evaluation discussed above, the absolute benefit of adjuvant chemotherapy was estimated using the PREDICT score [Figure 2].[7] The breast cancer-related mortality at 5 and 10 years was estimated in Mrs. X as 42% and 56%, respectively. The predicted absolute 5- and 10-year reduction in mortality with second-generation chemotherapy regimen such as standard 4AC followed by 4P or 4TC was 10% and 11.3%, respectively. An additional mortality benefit of 4.5% at 5 years and 5.3% at 10 years was expected with third-generation regimens such as dose-dense, wP or 6 cycles of TAC.
Figure 2.
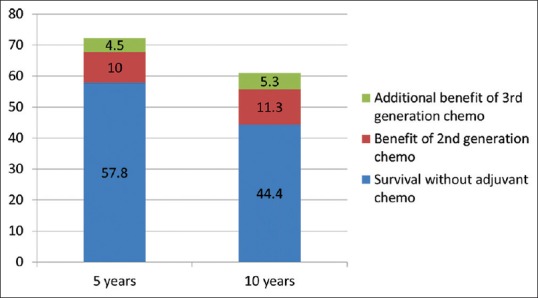
Estimated 5- and 10-year mortality rates with or without adjuvant chemotherapy in patient Mrs. X as per PREDICT[7]
Fitness for chemotherapy and toxicity risk
The geriatric risk assessment of Mrs. X using the fTRST tool was scored zero. Hence, she did not qualify for CGA.[19] Applying the CARG model of toxicity prediction, she scored 7 and was in medium risk strata which meant up to 52% chance of Grade III or higher toxicity from standard adjuvant polychemotherapy.[20]
Life expectancy of Mrs. X at 65 years of age
As per the WHO life tables for women in India, her remaining life expectancy was calculated at 14.5 years without cancer or any comorbidity.[23,24] The combined Lee–Schonberg index estimated her survival with and without breast cancer at 5 years as 96% and 98%, respectively, and at 10 years as 77% and 90%, respectively.[25] The ePrognosis tool estimated her 10-year mortality as 25% with cancer and 15% without cancer.[25]
Impact of cardiac compromise and hypertension on life expectancy
The CCI predicted an independent 10-year mortality risk due to congestive heart failure as 25% and combined with breast cancer as 59% in Mrs. X.[31] The MAGIC meta-analysis model predicted 1- and 3-year mortality due to heart failure as 5.8% and 14.6% (considering her as having NYHA Class I symptoms), respectively. If treated with ACE inhibitors or angiotensin receptor blockers and β-blockers, the mortality decreased to 3.9% and 10.2% at 1- and 5-year time points, respectively.[34] The SHFM similarly predicted a 5-year mortality of 24% due to heart failure which decreased to 13% if ACE inhibitors and β-blockers were given.[35]
Evaluating competing causes of mortality and risk-benefit assessment of chemotherapy in Mrs. X
Table 1 shows an overview of the patient's life expectancy and the mortality risks due to breast cancer, heart failure, natural causes, and the absolute benefit of chemotherapy, considering no overlap between the probabilities. We can see that the patient's chances of dying due to breast cancer is much higher than dying naturally or due to heart failure at 5 years (42% vs. 15%, respectively) and 10 years (56% vs. 40%, respectively). Therefore, adjuvant chemotherapy will be beneficial for this patient as it will decrease the breast cancer-related mortality. We can also understand that the benefit of adjuvant chemotherapy will decrease after 5 years due to age and comorbidities. Adjuvant chemotherapy was expected to decrease mortality by 16.5% at 10 years as per PREDICT. However, as per competing risk model, the cumulative probability of survival at 10 years without chemotherapy with competing mortality risks of cancer (56%), heart failure (25%), and natural death (15%) will be ([1–0.56] × [1–0.25] × [1–0.15]) which is 28%. With chemotherapy leading to 16.5% decrease in cancer-related mortality and 2% increase due to treatment-related deaths, the cumulative probability of survival at 10 years will be ([1–0.395] × [1–0.25] × [1–0.15] × [1–0.02]) which is 38%. Hence, with competing risks of dying due to causes other than breast cancer at 10 years, chemotherapy would best reduce the mortality by 10% only.
Table 1.
Life expectancy and adjuvant chemotherapy risk/benefit estimates of Mrs. X
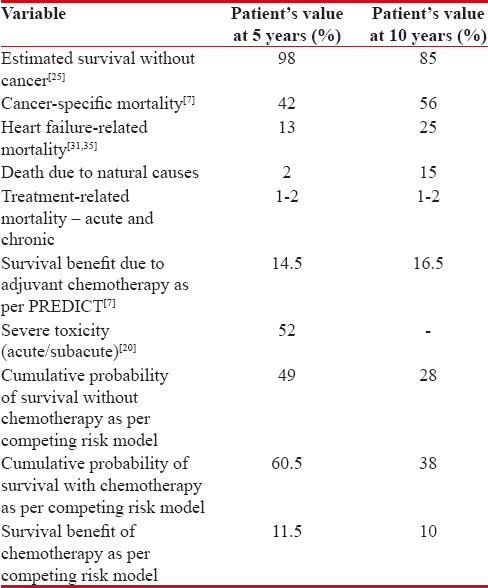
Hence, this patient may not derive the extra benefit of the intensified third-generation chemotherapy regimens over the second-generation ones but would definitely have to bear the extra toxicity, if third-generation regimen is used. This needs to be taken into account for selecting the appropriate chemotherapy regimen. Although chemotherapy carries 52% risk of Grade III or worse toxicities, the mortality reduction benefit clearly outweighs the risks in this patient. The comorbidity status of this patient, especially the reduced cardiac function, has to be carefully considered while selecting chemotherapy regimen. Clearly, anthracyclines are contraindicated in this patient. She may also need granulocyte-colony-stimulating factor support to prevent FN if a chemotherapy regimen with high likelihood of this complication is chosen.
Our plan of adjuvant chemotherapy regimen
In view of her baseline subclinical but significant cardiac dysfunction, the patient was not considered fit for any anthracycline-based chemotherapy. Hence the two options of chemotherapy for her included 4 cycles of TC or 6 cycles of CMF followed by taxanes. Due to her node-positive status, TC was thought to be an inferior chemotherapy regimen. Hence, we started adjuvant chemotherapy with classical CMF regimen with a plan to start wP after 6 cycles, followed by locoregional radiotherapy. The patient was also referred to a cardiologist for optimizing and initiating cardiovascular preventive treatment. She was started on atorvastatin due to high low-density lipoprotein and ramipril.
Discussion
The case of Mrs. X is not an uncommon scenario in a medical oncology clinic and yet it can be particularly challenging to devise an optimal adjuvant strategy for such patients. This review outlines an optimal approach toward risk assessment in such patients and estimates the pros and cons of adjuvant chemotherapy so that a well-informed decision can be taken. Numerous web-based tools are currently available and some validated ones should be used in the clinic. These tools help in framing a realistic picture of the benefits and harms of chemotherapy and its relative efficacy or failure in the context of patient's disease, comorbidities, and functional status.
The first step is to assess the benefit from adjuvant chemotherapy. Apart from defining recurrence risk and consequent benefit as per classical clinicopathologic features, web-based prognostic indices (e.g., Adjuvant! Online or PREDICT) can also be utilized for obtaining quantitative estimates. For patients aged 65 years or more, brief geriatric assessment tools should be utilized to identify important deficits which may affect treatment tolerance, guide multidisciplinary intervention, and aid treatment selection. Next, tools such as CARG or CRASH should be utilized to estimate the risk of chemotherapy toxicities in older patients. We then suggest estimating life expectancy and the independent impact of cancer and comorbidities on survival so that the competing causes of mortality can be compared and goals of care can be prioritized.
A recent study compared a novel generalized competing event (GCE) model based on similar principles as discussed above with standard Cox proportional hazards model for stratifying elderly patients with cancer.[36] The authors calculated risk scores for cancer-specific and all-cause mortalities in 84,319 senior patients (age over 66 years) with nonmetastatic prostate, head and neck, and breast cancers, identified from the SEER database. In the breast cancer subgroup (n = 22,929) diagnosed from 2004 to 2009, the 5-year cumulative incidences of all-cause, cancer-specific, second cancer, and noncancer mortality were 20.1%, 4.8%, 3.1%, and 12.2%, respectively. With the GCE model, effect estimates for stage, grade, tumor size, and nodal status were comparable to those obtained from Cox model in magnitude and direction. However, the GCE estimates for effect of age, teaching hospital, and CCI were opposite of those obtained from Cox model. The authors concluded that GCE models created greater separation in area under the curve for cancer-specific mortality versus noncancer mortality (P < 0.001), indicating better discriminatory ability between these competing events.
Before selecting the chemotherapy regimen, a discussion on benefit and risks of each regimen is warranted within the context of patient's values and preferences. Among multiple chemotherapy regimens, a detailed risk-benefit analysis would not only help in selecting the appropriate regimen but also define the optimal supportive care required. Nevertheless, classical tumor clinicopathologic features would remain the most important guide for tailoring the choice of regimen. For example, in our patient, the node-positive status of her tumor influenced the choice of chemotherapy.
Finally, the importance of detailed evaluation of cardiovascular risk factors and initiating preventive cardiovascular therapy in elderly patients with early breast cancer cannot be overstated. A recently published Canadian population-based cohort study in 98,999 women with early breast cancer found that, among women aged 66 years or older, the risks of breast cancer death and cardiovascular death at 10 years were 11.9% and 7.6%, respectively. Among patients with prior cardiovascular disease including hypertension and diabetes, the risks of death from breast cancer and cardiovascular disease were equivalent in the first 5 years, after which death from cardiovascular causes was more frequent (10-year cumulative incidence, 14.6% [95% confidence interval (CI), 13.7%–15.4%] for breast cancer vs. 16.9% [95% CI, 16.0%–17.8%] for cardiovascular disease). For women aged 66 years or older who survived 5 years or more after diagnosis of breast cancer, cardiovascular disease exceeded breast cancer as the leading cause of death at 10 years after diagnosis, when the cumulative incidence of each was 5%.[37]
Such a detailed multistep evaluation before adjuvant chemotherapy may not be warranted for young and fit patients with high-risk disease. However, amongst old patients with significant comorbidities, detailed evaluation as described above will help in selecting those who are likely to benefit from adjuvant chemotherapy while excluding patients with high chances of harm. This strategy upholds the fundamental principle that underlies the practice of good medicine -“Primum non nocere.”
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Peto R, Davies C, Godwin J, Gray R, Pan HC, et al. Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–44. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muss HB, Woolf S, Berry D, Cirrincione C, Weiss RB, Budman D, et al. Adjuvant chemotherapy in older and younger women with lymph node-positive breast cancer. JAMA. 2005;293:1073–81. doi: 10.1001/jama.293.9.1073. [DOI] [PubMed] [Google Scholar]
- 3.Muss HB, Berry DA, Cirrincione CT, Theodoulou M, Mauer AM, Kornblith AB, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360:2055–65. doi: 10.1056/NEJMoa0810266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones S, Holmes FA, O'Shaughnessy J, Blum JL, Vukelja SJ, McIntyre KJ, et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US oncology research trial 9735. J Clin Oncol. 2009;27:1177–83. doi: 10.1200/JCO.2008.18.4028. [DOI] [PubMed] [Google Scholar]
- 5.Blum JL, Flynn PJ, Yothers G, Asmar L, Geyer CE, Jacobs SA, et al. Anthracyclines in Early Breast Cancer: The ABC Trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology) J Clin Oncol. 2017;35:2647–55. doi: 10.1200/JCO.2016.71.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adjuvant! Online. [Last accessed on 2017 Mar 01]. Available from: http://www.adjuvantonline.com .
- 7.Predict. [Last accessed on 2017 Mar 01]. Available from: http://www.predict.nhs.uk .
- 8.Henry NL, Somerfield MR, Abramson VG, Allison KH, Anders CK, Chingos DT, et al. Role of patient and disease factors in adjuvant systemic therapy decision making for early-stage, operable breast cancer: American Society of Clinical Oncology Endorsement of Cancer Care Ontario Guideline Recommendations. J Clin Oncol. 2016;34:2303–11. doi: 10.1200/JCO.2015.65.8609. [DOI] [PubMed] [Google Scholar]
- 9.Azim HA, Jr, de Azambuja E, Colozza M, Bines J, Piccart MJ. Long-term toxic effects of adjuvant chemotherapy in breast cancer. Ann Oncol. 2011;22:1939–47. doi: 10.1093/annonc/mdq683. [DOI] [PubMed] [Google Scholar]
- 10.Pinder MC, Duan Z, Goodwin JS, Hortobagyi GN, Giordano SH. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol. 2007;25:3808–15. doi: 10.1200/JCO.2006.10.4976. [DOI] [PubMed] [Google Scholar]
- 11.Muss HB, Berry DA, Cirrincione C, Budman DR, Henderson IC, Citron ML, et al. Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: The Cancer and Leukemia Group B Experience. J Clin Oncol. 2007;25:3699–704. doi: 10.1200/JCO.2007.10.9710. [DOI] [PubMed] [Google Scholar]
- 12.Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, et al. American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. J Clin Oncol. 2016;34:611–35. doi: 10.1200/JCO.2015.64.3809. [DOI] [PubMed] [Google Scholar]
- 13.Barcenas CH, Niu J, Zhang N, Zhang Y, Buchholz TA, Elting LS, et al. Risk of hospitalization according to chemotherapy regimen in early-stage breast cancer. J Clin Oncol. 2014;32:2010–7. doi: 10.1200/JCO.2013.49.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Extermann M, Aapro M, Bernabei R, Cohen HJ, Droz JP, Lichtman S, et al. Use of comprehensive geriatric assessment in older cancer patients: Recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG) Crit Rev Oncol Hematol. 2005;55:241–52. doi: 10.1016/j.critrevonc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Decoster L, Van Puyvelde K, Mohile S, Wedding U, Basso U, Colloca G, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: An update on SIOG recommendations†. Ann Oncol. 2015;26:288–300. doi: 10.1093/annonc/mdu210. [DOI] [PubMed] [Google Scholar]
- 16.Puts MT, Santos B, Hardt J, Monette J, Girre V, Atenafu EG, et al. An update on a systematic review of the use of geriatric assessment for older adults in oncology. Ann Oncol. 2014;25:307–15. doi: 10.1093/annonc/mdt386. [DOI] [PubMed] [Google Scholar]
- 17.Kanesvaran R, Li H, Koo KN, Poon D. Analysis of prognostic factors of comprehensive geriatric assessment and development of a clinical scoring system in elderly Asian patients with cancer. J Clin Oncol. 2011;29:3620–7. doi: 10.1200/JCO.2010.32.0796. [DOI] [PubMed] [Google Scholar]
- 18.Bellera CA, Rainfray M, Mathoulin-Pélissier S, Mertens C, Delva F, Fonck M, et al. Screening older cancer patients:First evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012;23:2166–72. doi: 10.1093/annonc/mdr587. [DOI] [PubMed] [Google Scholar]
- 19.Kenis C, Geeraerts A, Braes T, Milisen K, Flamaing J, Wildiers H. The Flemish version of the Triage Risk Screening Tool (TRST): A multidimensional short screening tool for the assessment of elderly patients. Crit Rev Oncol Hematol. 2006;60:S31. [Google Scholar]
- 20.Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J Clin Oncol. 2011;29:3457–65. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Extermann M, Boler I, Reich RR, Lyman GH, Brown RH, DeFelice J, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118:3377–86. doi: 10.1002/cncr.26646. [DOI] [PubMed] [Google Scholar]
- 22.Schairer C, Mink PJ, Carroll L, Devesa SS. Probabilities of death from breast cancer and other causes among female breast cancer patients. J Natl Cancer Inst. 2004;96:1311–21. doi: 10.1093/jnci/djh253. [DOI] [PubMed] [Google Scholar]
- 23.Global Health Observatory Data Repository: Life Tables by Country. [Last accessed on 2017 Mar 01]. Available from: http://www.who.int .
- 24.Life Expectancy by Age. [Last accessed on 2017 Mar 01]. Available from: http://www.worldlifeexpectancy.com/your-life-expectancy-by-age .
- 25.ePrognosis Calculators. [Last accessed on 2017 Mar 01]. Available from: http://www.eprognosis.com .
- 26.Suemoto CK, Ueda P, Beltrán-Sánchez H, Lebrão ML, Duarte YA, Wong R, et al. Development and validation of a 10-year mortality prediction model: Meta-analysis of individual participant data from five cohorts of older adults in developed and developing countries. J Gerontol A Biol Sci Med Sci. 2017;72:410–6. doi: 10.1093/gerona/glw166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cruz M, Covinsky K, Widera EW, Stijacic-Cenzer I, Lee SJ. Predicting 10-year mortality for older adults. JAMA. 2013;309:874–6. doi: 10.1001/jama.2013.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schonberg MA, Davis RB, McCarthy EP, Marcantonio ER. External validation of an index to predict up to 9-year mortality of community-dwelling adults aged 65 and older. J Am Geriatr Soc. 2011;59:1444–51. doi: 10.1111/j.1532-5415.2011.03523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satariano WA, Ragland DR. The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med. 1994;120:104–10. doi: 10.7326/0003-4819-120-2-199401150-00002. [DOI] [PubMed] [Google Scholar]
- 30.Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;16:1582–7. doi: 10.1200/JCO.1998.16.4.1582. [DOI] [PubMed] [Google Scholar]
- 31.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 32.Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, et al. Rating chronic medical illness burden in geropsychiatric practice and research: Application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–48. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 33.Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Køber L, Squire IB, et al. Predicting survival in heart failure: A risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013;34:1404–13. doi: 10.1093/eurheartj/ehs337. [DOI] [PubMed] [Google Scholar]
- 34.Heart Failure Risk Calculator. [Last accessed on 2017 Mar 01]. Available from: http://www.heartfailurerisk.org .
- 35.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, et al. The Seattle Heart Failure Model: Prediction of survival in heart failure. Circulation. 2006;113:1424–33. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 36.Carmona R, Zakeri K, Green G, Hwang L, Gulaya S, Xu B, et al. Improved method to stratify elderly patients with cancer at risk for competing events. J Clin Oncol. 2016;34:1270–7. doi: 10.1200/JCO.2015.65.0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdel-Qadir H, Austin PC, Lee DS, Amir E, Tu JV, Thavendiranathan P, et al. Apopulation-based study of cardiovascular mortality following early-stage breast cancer. JAMA Cardiol. 2017;2:88–93. doi: 10.1001/jamacardio.2016.3841. [DOI] [PubMed] [Google Scholar]