Abstract
Angiogenesis is a normal biological process wherein new blood vessels form from the growth of pre-existing blood vessels. Preventing angiogenesis in solid tumours by targeting pro-angiogenic factors including vascular endothelial growth factor (VEGF), angiopoietin-1 (Ang-1), basic fibroblast growth factor (bFGF), hepatocyte growth factor, and platelet-derived growth factor (PDGF) is currently under investigation for cancer treatment. Concurrently targeting the cell signalling pathways involved in the transcriptional and post-translational regulation of these factors may provide positive therapeutic results. One such pathway is the Wnt signalling pathway. Wnt was first discovered in mice infected with mouse mammary tumour virus, and has been crucial in improving our understanding of oncogenesis and development. In this review, we summarise molecular and cellular aspects of the importance of Wnt signalling to angiogenesis, including β-catenin-dependent mechanisms of angiogenic promotion, as well as the study of Wnt antagonists, such as the secreted frizzled-related protein family (SFRPs) which have been shown to inhibit angiogenesis. The growing understanding of the underlying complexity of the biochemical pathways mediating angiogenesis is critical to the identification of new molecular targets for therapeutic applications.
Angiogenesis and Cancer
Angiogenesis is the process whereby new vessels form by the budding and sprouting of pre-existing blood vessels into an avascular area.1–4 Stages of angiogenic growth include the degradation of the basement membrane, migration and proliferation of endothelial cells to form a vessel sprout, tube formation, and recruitment of pericytes or smooth muscle cells to form a mature vessel.3,5–7 Angiogenesis occurs during embryogenesis, menstruation, and pathological processes that include tumour growth.2,5,6 Remodelling is common in angiogenesis and includes the growth and regression of vessels, which adapt to meet the demands of the local tissues.1 Vessel diameter and thickness is dependent on the needs of local tissue, often beginning as a plexus of primitive tubes that is modified to form a more complex vascular network in the adult.1 The control of apoptosis is critical for the formation and maintenance of the vasculature.3 An increase in endothelial apoptosis occurs during remodelling and/or regression of the vascular network.3 It is important for vessel formation and maintenance to be tightly regulated, otherwise pathologies may arise, such as the development of tumours.
There are many angiogenic growth factors that prevent the apoptosis of endothelial cells and stimulate proliferation to optimise vessel growth.3 Growth factors that regulate angiogenesis include vascular endothelial growth factor (VEGF), angiopoietin-1 (Ang-1), basic fibroblast growth factor (bFGF), hepatocyte growth factor, and platelet-derived growth factor (PDGF).1,3,7,8 These angiogenic factors and their respective receptors have been implicated in the progression of many cancers, and have been extensively researched as potential therapeutic targets.9 Pro-angiogenic factors also promote the sprouting of capillaries toward the tumour that is secreting the growth factor, therefore vascularising the region. Anti-angiogenic factors include thrombospondin-1, angiostatin, endostatin, and tumstatin.5,7,8 and they suppress vascular cell recruitment and growth of the vasculature.
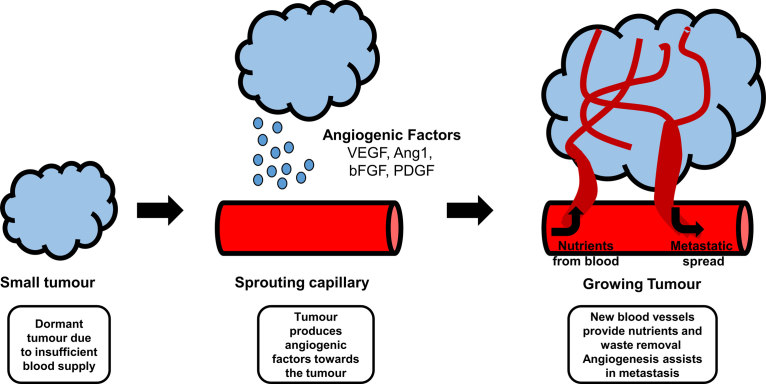
The formation and remodelling of vessels are prominent processes in a variety of pathological states.3 There is a shift towards pro-angiogenic activities during tumour growth that is mediated by factors such as VEGF, which is produced and secreted by the tumour cells.8,10,11 Excessive vessel growth contributes to tumour progression and, therefore, tumour growth is angiogenesis-dependent.3,7,8,11 Angiogenesis is necessary for a tumour to grow beyond 1–2 mm3 in size and for metastasis to occur.5,7,8 Without a new and functional vasculature being present, a tumour’s ability to grow and proliferate would be limited.12 The demand for oxygen is met by transcription of hypoxia-inducible genes for proteins that turn on the angiogenic switch to stimulate the production of more VEGF.5 The new vasculature also aids in the removal of waste products and facilitates metastasis.7 The process of angiogenesis in tumours is shown in Figure 1. There are many signalling pathways implicated in the control of angiogenesis, including Wnt signalling.13
Figure 1.
A schematic diagram of tumour angiogenesis.
Wnt Signalling
Wnts are potent angiogenic factors, and their signalling pathways play an important role in normal development and in the pathogenesis of a variety of diseases, including cancer.3,14,15 Wnt pathways are involved in cellular proliferation, survival, differentiation, migration, and apoptosis.3,16–18 The development of a number of organ systems depends on Wnt signalling, including the kidney, female reproductive tract, and placenta.3 The Wnt pathway plays an important role in angiogenesis and vessel remodelling.3,16
Wnt proteins are a family of secreted cysteine-rich glycoproteins that are highly conserved among species.2,3,19,20 There are 19 Wnt genes that have been identified in humans.3,19 The majority of human Wnt genes contain four coding exons, with exon 1 containing the initiator methionine,19 and exert their effects through modulating cellular events at the post-translational and transcriptional level.21 Wnt proteins transduce cellular signals by binding to a range of cell-surface co-receptor molecules: the transmembrane Frizzled (Fzd) receptors and members of the lipoprotein receptor-related proteins 5 or 6 (LRP5/6)3,19,20 are perhaps the most important of these. LRPs are made up of an ectodomain with four β-propeller repeats, each of which is interspersed with a single EGF repeat, culminating in three low-density lipoprotein receptor-A (LDLR-A) domains. The cytoplasmic tail contains PPP[S/T]PxS motifs that are phosphorylated upon Wnt ligand stimulation.22
The majority of Wnt proteins share about 35% amino acid sequence identity, with an increased sequence identity if they are members of a subgroup.19 Human Wnt proteins are very similar in size, ranging in molecular weight from 39 kDa (Wnt7a) to 46 kDa (Wnt10a).19 Wnt proteins are modified with palmitoleate lipids by Porcupine, a Wnt-specific acyltransferase, at a conserved serine (in Wnt3a, at S209).23 This modification facilitates both secretion and the interaction with Fzd,24–26 although it is unknown if lipidation facilitates interaction with LRP co-receptors; recent structural evidence suggests that the Wnt lipid facilitates dimerisation of Fzd CRDs, which may imply it does not facilitate co-receptor interaction.27,28 The structure of Wnt proteins has been recently revealed (Figure 2), as complexed with the cysteine-rich domain (CRD) of a Fzd receptor.29 Wnt proteins feature 23 or 24 highly conserved cysteine residues, the majority of which were revealed in the crystal structure to form an intricate network of disulphide bonds to stabilise the unusual fold of the Wnt protein; the disulphide bond network is critical for Wnt signalling.30 The CRD utilises two binding sites to bind Wnt; a hydrophobic site to bind the Wnt lipid (often referred to as the Wnt ‘thumb’; formed within the N-terminal domain), and a region on the opposite face of the CRD binding a moderately conserved, cysteine-rich, two-stranded β-sheet (often referred to as the Wnt ‘index finger’; formed within the C-terminal domain). Some Wnts have been shown to display selectivity for specific Fzds.31 Specific regions of Wnts and Fzd CRDs frequently involved in mediating Wnt-Frizzled interactions have recently been proposed computationally.32
Figure 2.
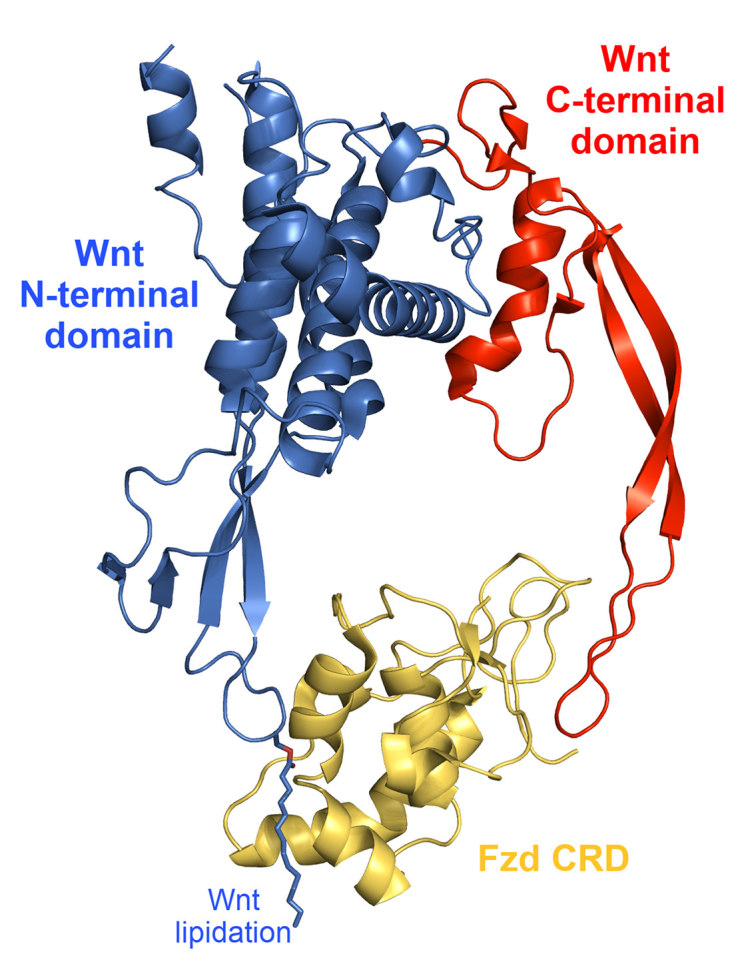
Crystal structure complex of the Xenopus Wnt8 with the mouse Fzd8 cysteine-rich domain (PDB 4F0A). The N-terminal domain (blue) contains the Wnt ‘thumb’, featuring lipid modification and is bound by a site generally conserved among Frizzled cysteine-rich domains. The C-terminal domain (red) contains the Wnt ‘index finger’, a moderately conserved, cysteine-rich, two-stranded β-sheet, which is bound by a site on the opposite face of Frizzled cysteine-rich domains to that bound by the thumb.
There are at least three Wnt signalling pathways: the canonical Wnt/β-catenin pathway, and the two non-canonical pathways: the Wnt/calcium (Wnt/Ca2+) pathway, and the Wnt/planar cell polarity pathway (Wnt/PCP).19 In addition, there are endogenous Wnt antagonists, including the secreted Frizzled-related proteins (SFRP1-5), the Dickkopf (DKK) family of proteins, the Wnt inhibitory-factors (WIFs), and Cerebus.3
Wnt/β-catenin Signalling Pathway
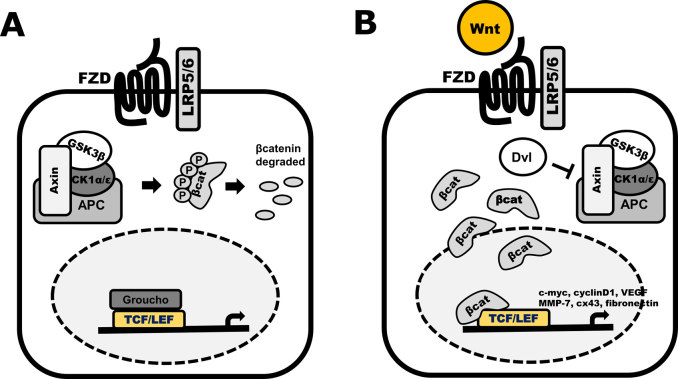
The canonical Wnt/β-catenin signalling pathway targets the key cellular regulatory molecule β-catenin in signalling (Figure 3).33 Signalling through a β-catenin-dependent mechanism relies on the post-translational modification status of β-catenin. This is regulated through the cytoplasmic destruction complex, comprising glycogen synthase kinase-3β (GSK3β), Axin, Casein kinase1/2 (CK1/2), Protein phosphatase 2A (PP2A), and adenomatous polyposis coli (APC).33 In the absence of Wnt signalling, cytosolic β-catenin is phosphorylated by CK1 at Thr41, and GSK3β at Ser33 and Ser37.34 Phosphorylated β-catenin is then primed for ubiquitination by the E3 ligase, β-TrCP, and targeted for proteosomal degradation.34 Wnt binding to Fzd recruits co-receptor LRP5/6, which results in the activation of the cytoplasmic phosphoprotein Dishevelled (Dvl).35,36 These events lead to the inhibition of GSK3β, thereby promoting the accumulation of unphosphorylated (or active) β-catenin and its subsequent translocation to the nucleus, where it binds to a variety of transcription factors, including T-cell factor/lymphocyte enhancing factor (TCF/LEF)37 and forkhead box (in particular, the FOXO subtypes)38 family proteins. TCF/LEF are able to bind and facilitate the transcription of a number of Wnt target genes involved in cell proliferation, Wnt signal transduction and vascular growth, including CCND1 (cyclin D1),39 AXIN2,40 and VEGF.41
Figure 3.
Schematics of the Wnt/β-catenin signalling pathway. A) The inactive canonical pathway (OFF state). Without Wnt ligand stimulation through the FZD-LRP5/6 receptor complex, no signal is transduced through Dvl. This results in stabilisation of the destruction complex consisting of Axin, APC, GSK3 and CK1, resulting in phosphorylation of β-catenin and subsequent degradation. B) Active canonical Wnt signalling (ON state). Stimulation by a Wnt ligand activates the FZD receptor and LRP5/6 co-receptors. A signal is transduced through Dvl leading to destabilisation of the destruction complex. Non-phosphorylated (active) β-catenin translocates to the nucleus where is binds to TCF/LEF transcription factors, initiating the transcription of Wnt target genes.
Wnt/Ca2+ Signalling Pathway
In the Wnt/Ca2+ pathway, the activation of Fzd stimulates the release of calcium (Ca2+).3 The Wnt/Ca2+ pathway mobilises intracellular Ca2+ to activate a pertussis toxin-sensitive subclass of heterotrimeric G protein, leading to an increase in intracellular Ca2+ and subsequent activation of Ca2+/calmodulin-dependent protein kinase II (CaMKII) and protein kinase C (PKC).3,19,21 After CaMKII activation of calcineurin (Cn), a serine/threonine phosphatase, Nuclear factor of activated T-cells (NFAT) transcription factors are activated through dephosphorylation and initiate transcription.42 Calcium activation of PKC is able to initiate a variety of cellular responses.43 Stimulation of the Wnt/Ca2+ pathway has also been shown to antagonise Wnt/β-catenin signal transduction through Wnt5a-induced activation of the TAK1-NLK pathway.44 NLK is able to negatively regulate TCF/LEF transcription through phosphorylation of TCF-4, which interferes with its ability to bind to DNA.45 This demonstrates some of the complex cross-talk between Wnt signalling pathways.
Wnt Planar Cell Polarity (PCP) Pathway
The Wnt/PCP pathway regulates the morphogenesis of both vertebrates and invertebrates. Polarisation during cell division, migration, and cytoskeletal rearrangements are governed, in part, by the Fzd-Wnt/PCP pathway.46 Regulation of this pathway is synchronised through Dvl upon Wnt activation. Signal transduction is then mediated through Rho activation of Rho-associated protein kinase (ROCK) or Rac activation of the c-Jun N-terminal kinase (JNK) pathway, leading to actin polymerisation and changes in cell and tissue polarity.47,48
Wnt and Angiogenesis
Recent evidence suggests that both the canonical and non-canonical Wnt signalling pathways are implicated in angiogenesis in a variety of organs in both normal and pathological conditions.49–51 Wnt signalling, both canonical and non-canonical, appears to be essential in vascular endothelial cells and functions through a variety of regulators, including R-spondin352 and Fzd7.53 Transcriptional regulation of VEGF by Wnt/β-catenin signalling has been demonstrated, where seven TCF binding sites have been found in the VEGF gene promoter.54 Furthermore, defects in APC resulting in constitutively active β-catenin and Wnt signalling can lead to over-expression of VEGF.54 The β-catenin/TCF complex is also responsible for the transcriptional regulation of interleukin-8 and matrix metalloproteinases (MMPs), which are key drivers and regulators of angiogenesis.17,20 More recent evidence also suggests that Wnt7a and Wnt7b, both canonical Wnt ligands, are able to activate β-catenin-mediated signalling through Gpr124.51,55 The role of SFRP family proteins has been demonstrated to regulate angiogenesis via regulating both canonical and non-canonical Wnt signalling.
VEGF
VEGF is produced and secreted by mesenchymal and stromal cells in a paracrine manner.1,56 It is a potent mitogen and chemoattractant for endothelial cells.1,5,10,56 Mutations in the Wnt signalling pathway have resulted in up-regulation of VEGF expression.10 VEGF induces neovascularisation of malignant cells and has an increased expression in tumour cells.5 VEGF is a stimulator of endothelial proliferation, migration, and survival, and has critical roles in physiological and pathological angiogenesis.3,10 Tyrosine kinases Flt-1 and Flk-1/KDR are receptors for VEGF that are expressed primarily by the endothelium.1 Flk-1/KDR is the main receptor that allows for the mitogenic, angiogenic, and permeability-enhancing effects of VEGF.10 VEGF is capable of inducing the expression of the anti-apoptotic protein B-cell leukaemia/lymphoma 2 (Bcl-2).10 VEGF-activated endothelial cells have also been demonstrated to increase the expression of pro-angiogenic proteins, MMPs.7 Inhibition of VEGF signalling inhibits both angiogenesis and the growth in tumour size.56
There are numerous small molecules targeting VEGF and VEGFR undergoing clinical trials, as well as the anti-VEGF monoclonal antibody, bevacizumab, which has been approved as a first line treatment for colorectal and non-small cell lung cancer.57 Bevacizumab was originally approved by the FDA for combinatorial treatment with 5-fluorouracil-based chemotherapies for colon cancer.58 Following its initial success, it was approved for use in glioblastoma multiforme,59 ovarian carcinoma,60 renal cell carcinoma,61 cervical cancer62 and HER2-negative breast cancer.63 While initial reports of success were overwhelming, the use of bevacizumab was revoked for use in breast cancer, after improvement in progression-free survival was unable to be replicated in subsequent cohorts.64 The effectiveness of bevacizumab has been questioned due to its toxicity profile and the high number of adverse reactions associated with its addition to treatment regimens.65,66 Currently, there are no FDA-approved drugs for cancer that directly target the Wnt pathway.67
MMPs
MMPs create and maintain a microenvironment that facilitates the growth and angiogenesis of tumours by mediating the degradation and remodelling of the extracellular matrix (ECM), thereby facilitating the sprouting of new vessels.3,6,68,69 Matrilysin (MMP-7) plays an important role in cancer metastasis and is expressed by endothelial cells of the vasculature adjacent to the MMP-7-producing tumour, thereby promoting angiogenesis by accelerating the migration of endothelial cells.68,69 MMP-7 promotes cancer invasion and angiogenesis by proteolytic cleavage of ECM substrates.69 MMP-7 promotes the secretion of soluble FasL, increasing apoptosis in surrounding cells by activating Fas.69 In response to Wnt signalling, MMP-7 is upregulated in colon cancer cells and can stimulate the proliferation of endothelial cells in culture.3 MMP-7 is upregulated in invasive cancers. Some MMP inhibitors are thought to act as cancer metastasis suppressors and exhibit the ability to inhibit tumour angiogenesis.68 Tissue inhibitors of metalloproteinases (TIMP) inhibit angiogenesis by downregulating the activity of MMPs.4,6,68 It has also been demonstrated that TIMPs inhibit angiogenesis in an MMP-independent fashion by blocking signalling through α3β1 integrins.70
GPR124
A recently discovered essential regulator of Wnt signalling and angiogenesis is the G-protein coupled receptor, Gpr124. It was initially linked to vascular development in colorectal tumour models.71 It is now known that Gpr124 is crucial for angiogenesis and vascularisation in the central nervous system through direct regulation of canonical Wnt signalling. Gpr124 has been demonstrated to act as a receptor for Wnt7a and Wnt7b, and may enhance the formation of a Wnt signalosome, consisting of Wnt7a/b, Gpr124, Fzd4 and LRP5 (Figure 4).51 Studies have indicated that Wnt7a and Wnt7b signal transduction through Gpr124 is dependent on leucine-rich repeats (LRRs) in the ectodomain of Gpr124 and an intracellular PDZ-binding motif.55 Recent evidence also suggests that Gpr124 works synergistically with Reversion-inducing-cysteine-rich protein with kazal motifs (Reck) to co-activate Wnt7a and Wnt7b signalling.50 The structure of Gpr124 is likely similar to the Leucine-rich repeat-containing G-protein coupled receptor family of GPCRs, which also feature LRRs in their ectodomain,72 and can also activate Wnt signalling. The likely structure of Gpr124 suggests it may potentially be an LGR family member; however, it is currently unknown if Gpr124 interacts with the R-spondins, which are the major mediators for Wnt signalling via the LGRs.73
Figure 4.
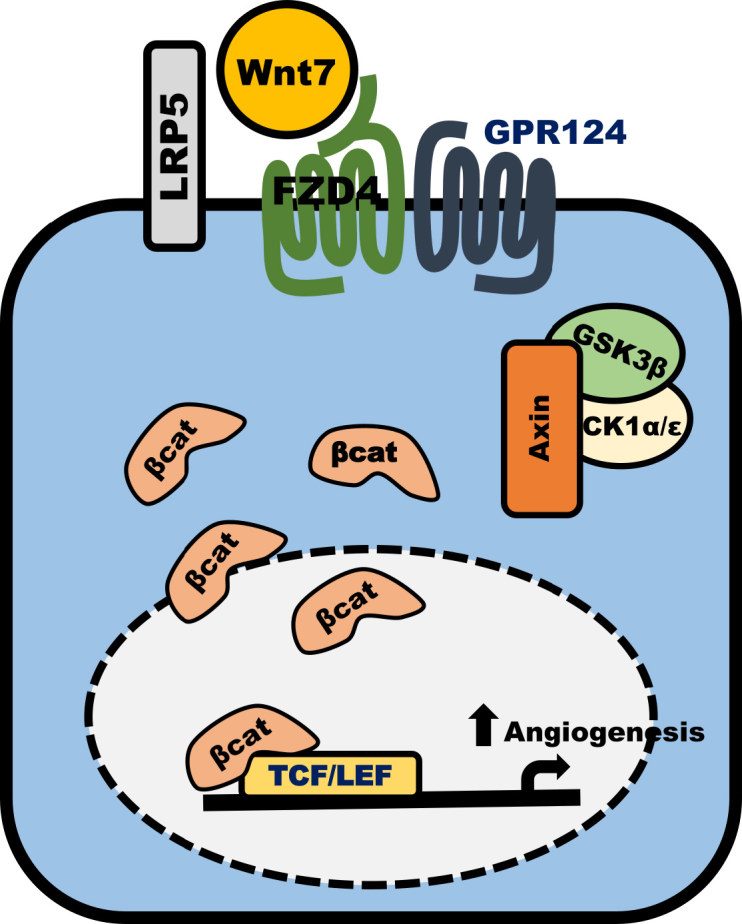
Wnt7/FZD4/GRP124/LRP5 signalling cascade. Activation of canonical Wnt/β-catenin signalling is initiated through Wnt7a/b binding to Fzd4. GPR124 and LRP5 act as co-receptors through which signal transduction is conveyed to destabilise the destruction complex (Axin, GSK3β, CK1α/ɛ). β-catenin is stabilised, allowing its translocation to the nucleus, where it can bind to TCF/LEF transcription factors, leading to vascularisation and angiogenesis.
Gpr124−/− embryos feature forebrain and ventral spinal cord angiogenic defects, similar to those seen in embryos deficient in Wnt7a and Wnt7b. These angiogenic defects were dramatically more pronounced in Fzd4−/−;Gpr124−/− embryos, which exhibited vascular leakage through up-regulation of plasmalemma vesicle-associated membrane protein and downregulation of claudin5.51 This phenotype was embryonically lethal by E10.5. The defects in angiogenesis and vasculature were able to be rescued through activating Wnt/β-catenin signalling by generating a Gpr124−/−;Ctnnb1flex3/+ phenotype with constitutively active β-catenin. This study demonstrates the role of Gpr124 in the regulation of angiogenesis and vascularisation in the central nervous system directly through canonical Wnt signalling.51
SFRPs
Proteins of the SFRP family are soluble inhibitors of Wnt signalling;3,74 they can also block endothelial cell proliferation.2 SFRPs feature a two-domain structure, consisting of a CRD, similar to that found in Fzd receptors,2,3,74 and a netrin-like domain (NLD), which likely bears structural similarity to the TIMP NLD.75 SFRPs may act as soluble modulators competing with Fzd to bind the Wnt ligands.76 SFRPs can also bind to Wnt proteins directly and antagonise Wnt function by preventing its interaction with Fzd receptors.2,19,74,77 The SFRPs may also interact with Fzds, which might antagonise Wnt signalling through the formation of a non-functional complex with the Fzd receptors.19,77
Early studies provided evidence for an anti-angiogenic role of SFRP-1.78 It was observed that bovine SFRP-1 was endogenously expressed in bovine endothelial cells and increased expression was associated with decreased proliferation rates.78 SFRP-1 inhibiting angiogenesis is logical as it likely antagonises Wnt signalling;79 however, more recent research contradicts this, describing a pro-angiogenic role for SFRP-1.80 Exposure to SFRP-1 activated the non-canonical Wnt signalling pathways, enhancing the velocity of endothelial cell spreading on laminin and collagen.81 Interestingly, SFRP-1 treatment was initially associated with a decrease in proliferation and then, after an extended period of time (15 days), a pro-angiogenic response was seen. This effect coincided with the up-regulation of Rac1 and RhoGTPases, and is probably due to endothelial cell cytoskeletal rearrangement involving the PCP/Wnt signalling pathway.81 Thus, it seems that endothelial cells have a biphasic response to SFRP-1: initial inhibition of the canonical pathway, followed by Rac-1 activation and angiogenesis promotion. Courtwright et al. discovered that SFRP-2 is pro-angiogenic. SFRP-2 was shown to increase endothelial cell migration and tube formation and protect against hypoxia-driven apoptosis is a dose-dependent fashion.82 Currently, there is only one study that has reported the effects of SFRP-4 on different endothelial cell lines to assess its role in angiogenesis.16 This study showed that SFRP-4 induced a significant reduction in endothelial cell migration. The authors suggest that SFRP-4 may be exerting its effects by inhibiting both proliferation and migration of endothelial cells. In the same study, the administration of SFRP-4 induced a significant reduction in the number of endothelial rings formed, a reduction in pseudopodia, and a loss of ring stability, all of which are important processes necessary for the initiation and development of angiogenesis. The effect of SFRP-4 on physiological and tumour-associated angiogenesis was also assessed using in vivo models, and they found there was a decrease in vascularisation and angiogenesis.
Stem Cells and Angiogenesis
Cancer Stem Cells and Angiogenesis
Cancer stem cells (CSCs) are a subpopulation of cells within the heterogenous tumour niche which have demonstrated higher angiogenic and tumourigenic potential than non-stem-like cells.83 CSCs derived from breast, colorectal, liver and glioblastoma tumours also demonstrate higher Wnt/β-catenin activity.84–87 Increased Wnt activity may also partly be responsible for the elevated levels of VEGF seen in CSCs.88–90
The relationship between CSCs and angiogenesis has not yet been conclusively established, although CD133+ CSCs from glioma cells show elevated expression of VEGF,91 and when compared with CD133− glioma cells demonstrated increased tumour initiation with increased vascularity, necrosis, and haemorrhage. CD133 expression has been linked to Wnt/β-catenin signalling in glioma stem cells. Decreased expression of CD133 was seen after the addition of the Wnt antagonist, SFRP-4. This was demonstrated to be a Fzd-dependent mechanism.92
In CD133+ glioma cells, CD31 staining showed increased vascular density and 10–20 fold up-regulated VEGF expression. Furthermore, bevacizumab treatment on CD133+ glioma cells depleted the vascular endothelial cell migration and tube formation. Additionally, bevacizumab in vivo inhibited tumour progression and down-regulated VEGFR in a CD133+ glioma cell-derived xenograft. Thus, targeting VEGFs may have direct implications on endothelial cells and tumour progression.91 These studies in glioma CSCs have also been replicated in vitro.93,94
In glioma cells, CSCs also contribute to tumour vascular development, suggesting the role of CSCs in recruiting endothelial progenitor cells (EPC). CSCs stimulate pro-angiogenic factors, such as VEGF and SDF-1, suggesting that CSCs promote local angiogenesis and EPC mobilisation.95
CSCs express proteins that induce neovascular growth and establish an aberrant vascular niche. Glioblastoma CSCs express Nestin, a major intermediate filament protein, which is located in close proximity to CD34+ capillaries, which are correlated to microvessel density.96 Nestin has also been shown to positively regulate Wnt/β-catenin signalling to promote invasiveness through up-regulation of MMP-2, MMP-7 and VEGF.97 The influence of vascular endothelial cells on CSCs was demonstrated when CSCs were co-cultured with endothelial cells. It was found that the interaction of endothelial cells with glioblastoma CSCs provides vasculogenic factors supporting self-renewal and differentiation capacity. Therapeutically targeting VEGF depleted the tumour vasculature and segregated the vascular niche of the CSCs.96 However, further investigation showed a dual effect of DC101 (a VEGFR-2 inhibitor) in C6 rat glioma xenograft tumours. The treatment depleted the pro-angiogenic factors secreted by CSCs and decreased tumour angiogenesis.95
These studies highlight the importance of targeting CSCs to inhibit tumour angiogenesis, as well as the close relationship between CSCs and Wnt/β-catenin in promoting angiogenesis.
Mesenchymal Stem Cells in Angiogenesis
Mesenchymal stem cells (MSCs) play a major role in postnatal angiogenesis, especially during tissue regeneration and repair. One of the mechanisms through which MSCs influence angiogenesis is through paracrine signalling via secreting various cytokines and growth factors, which can improve endothelial cell proliferation, migration, and functionality. For instance, when human amniotic or bone marrow-derived MSCs were co-cultured with human umbilical vein endothelial cells (HUVEC), the expression of platelet endothelial cell adhesion molecule and the vessel density were increased as compared to HUVEC monoculture.98 The observed pro-angiogenic effect was correlated to the increased activity of MMP-2 and MMP-9 in the co-culture as compared to the monoculture system.98
Secondly, MSCs can exploit their transdifferentiation potential into endothelial cells during vascular regeneration. In therapeutic angiogenesis, the benefits of using MSCs can arise from their transdifferentiation capabilities into either endothelial cells or smooth muscle cells.99 The potential clinical applications of MSCs in vascular regeneration have been demonstrated through various in vitro and in vivo studies. One study on myocardial ischaemia showed that rat foetal heart MSCs improved cardiac function by transdifferentiating into endothelial cells and smooth muscle cells.100 Similar results of improved cardiac function contributed by bone marrow-derived MSCs were reported in another in vivo study performed in swine animal models.101 Furthermore, MSCs overexpressed with VEGF/Hepatocyte growth factor have been reported to play a role in promoting angiogenesis and cardiac function of porcine heart after myocardial infarction.102
Wnt Signalling on the Angiogenic Potential of MSCs
Although the role of Wnt signalling in angiogenesis has been demonstrated, the role of the Wnt signalling pathway in the endothelial differentiation of MSCs has not been thoroughly studied. Wnt-inducing factors, such as Wnt1, VEGF, and the GSK3β inhibitor, CHIR99021, induced the vascular differentiation of MSCs derived from dental pulp-derived stem cells (DPSCs), as observed by an increased expression of VEGFR2 or Tie-2. On the other hand, the inhibition of Wnt signalling using the small molecule inhibitor JW67 or by silencing β-catenin inhibited the differentiation of DPSCs into endothelial cells.103 VEGF is one of the key factors promoting the endothelial differentiation of MSCs. However, in MSCs it has been reported to act through the Rho/ROCK pathway, wherein inhibition of the Rho/ROCK pathway suppressed the endothelial differentiation of human and rat MSCs.104
Another study by Zhang et al. demonstrated the role of Wnt signalling in MSC-mediated angiogenesis. Exosomes from human umbilical cord derived-MSCs enhanced endothelial functionality in terms of the proliferation, migration, and tube-formation potentials of endothelial cells. Wnt4 was identified in these exosomes, which was responsible for these pro-angiogenic effects in endothelial cells. This was evidenced by the nuclear translocation of β-catenin in endothelial cells, thereby up-regulating canonical Wnt signalling. They also observed an increase of in vivo angiogenesis in the rat skin burn model, where the rats were subcutaneously injected with MSC-derived exosomes. The observed pro-angiogenic effect was reversed with the β-catenin inhibitor ICG-001 or with a Wnt4 knock-down in these MSCs, demonstrating the role of Wnt signalling in promoting MSC-mediated angiogenesis.105
Wnt Antagonism on the Angiogenic Potential of MSCs
Amongst the few studies that reported the effect of Wnt antagonists on the angiogenic potential of MSCs, one demonstrated the role of Dkk-1 in MSC-derived angiogenesis. It was reported that interleukin-1β (IL-1β) enhanced the angiogenic potential of MSCs derived from bronchoalveolar lavage through up-regulation of miR-433, which in turn decreased the expression of the Wnt antagonist Dkk-1. To further confirm the role of the Wnt pathway in this, they also demonstrated that the exposure of MSCs to IL-1β as well as the over-expression of miR-433 in MSCs upregulated the expression of β-catenin.106 The decrease in Dkk-1 expression during an up-regulated angiogenesis indicates the need to have an activated Wnt signalling state to promote the angiogenic potential of MSCs.
A study by Dufourcq et al. demonstrated that over-expression of SFRP-1 in MSCs resulted in better vessel stability and maturation. SFRP-1 over-expression enabled the pro-angiogenic effects through different mechanisms. One of these was by promoting an elongated morphology of MSCs, permitting an efficient integration and closer contact of MSCs around the vessel walls. Secondly, SFRP-1 also increased the extent of cellular contact and communication between MSCs and endothelial cells via β-catenin-dependent cell-cell junctions around the vessel wall. Additionally, SFRP-1 increased the expression of platelet-derived growth factor-BB and α-smooth muscle actin in MSCs. All of these transformations induced by SFRP-1 over-expression in MSCs facilitated an enhanced stability, maturation, and organisation of the blood vessels.107
Conclusions
Wnt signalling appears to have an important role in mediating angiogenesis, influencing the role of many known angiogenic factors, including VEGF. However, further studies are needed to fully elucidate the role of Wnt signalling and its interplay with other molecular pathways in mediating angiogenesis. The relevance of angiogenesis to normal biological function, as well as cancer growth and metastasis, represents a formidable challenge in developing therapeutics that are both safe and specific. Targeting VEGF and VEGFR directly to block angiogenesis has been clinically successful, although the effects are typically short-lived, highlighting the need for alternative therapeutics. The up-regulation of the Wnt signalling pathway in many types of cancers, particularly in CSCs, provides a potential opportunity for the development of a reasonably safe and specific anti-angiogenic. However, caution is needed to identify and validate appropriate targets in the Wnt signalling pathway for this purpose, due to the complexity of the pathway and the great multitude of proteins and biological processes under its control.
Acknowledgements
Sebastian Öther-Gee Pohl is supported by funds from the Rotary Club of Belmont, Australian Rotary Health Research Fund and Curtin University School of Biomedical Sciences. Abhijeet Deshmukh is supported by a scholarship from the Curtin University Office of Research & Development, the School of Biomedical Sciences and Faculty of Health. Malini Visweswaran is supported by a scholarship from the Office of Research and Development, Faculty of Health Sciences, Curtin University. Mark Agostino is a recipient of a Raine Priming Grant, a Curtin Research Fellowship, and operational research support from the Curtin Institute for Computation. Arun Dharmarajan is supported by strategic research funds from the School of Biomedical Sciences (Curtin University), Commercialisation Advisory Board of Curtin University, Cancer Council of Western Australia and Actinogen Ltd, Perth, Western Australia.
Footnotes
Competing Interests: None declared.
References
- 1.Beck L, Jr, DAmore PA. Vascular development: cellular and molecular regulation. FASEB J. 1997;11:365–73. [PubMed] [Google Scholar]
- 2.Maiese K, Li F, Chong ZZ, Shang YC. The Wnt signaling pathway: aging gracefully as a protectionist? Pharmacol Ther. 2008;118:58–81. doi: 10.1016/j.pharmthera.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodwin AM, DAmore PA. Wnt signaling in the vasculature. Angiogenesis. 2002;5:1–9. doi: 10.1023/a:1021563510866. [DOI] [PubMed] [Google Scholar]
- 4.Stetler-Stevenson WG. Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J Clin Invest. 1999;103:1237–41. doi: 10.1172/JCI6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shih T, Lindley C. Bevacizumab: an angiogenesis inhibitor for the treatment of solid malignancies. Clin Ther. 2006;28:1779–802. doi: 10.1016/j.clinthera.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 6.John A, Tuszynski G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol Oncol Res. 2001;7:14–23. doi: 10.1007/BF03032599. [DOI] [PubMed] [Google Scholar]
- 7.Nishida N, Yano H, Nishida T, Kamura T, Kojiro M. Angiogenesis in cancer. Vasc Health Risk Manag. 2006;2:213–9. doi: 10.2147/vhrm.2006.2.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Y, Adjei AA. Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor. Oncologist. 2015;20:660–73. doi: 10.1634/theoncologist.2014-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrara N, Hillan KJ, Gerber H-P, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 11.Gordon MS, Cunningham D. Managing patients treated with bevacizumab combination therapy. Oncology. 2005;69(Suppl 3):25–33. doi: 10.1159/000088481. [DOI] [PubMed] [Google Scholar]
- 12.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 13.Goodwin AM, Sullivan KM, DAmore PA. Cultured endothelial cells display endogenous activation of the canonical Wnt signaling pathway and express multiple ligands, receptors, and secreted modulators of Wnt signaling. Dev Dyn. 2006;235:3110–20. doi: 10.1002/dvdy.20939. [DOI] [PubMed] [Google Scholar]
- 14.Klein D, Demory A, Peyre F, Kroll J, Géraud C, Ohnesorge N, et al. Wnt2 acts as an angiogenic growth factor for non-sinusoidal endothelial cells and inhibits expression of stanniocalcin-1. Angiogenesis. 2009;12:251–65. doi: 10.1007/s10456-009-9145-5. [DOI] [PubMed] [Google Scholar]
- 15.Pohl SG, Brook N, Agostino M, Arfuso F, Kumar AP, Dharmarajan A. Wnt signaling in triple-negative breast cancer. Oncogenesis. 2017;6:e310. doi: 10.1038/oncsis.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muley A, Majumder S, Kolluru GK, Parkinson S, Viola H, Hool L, et al. Secreted frizzled-related protein 4: an angiogenesis inhibitor. Am J Pathol. 2010;176:1505–16. doi: 10.2353/ajpath.2010.090465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zerlin M, Julius MA, Kitajewski J. Wnt/Frizzled signaling in angiogenesis. Angiogenesis. 2008;11:63–9. doi: 10.1007/s10456-008-9095-3. [DOI] [PubMed] [Google Scholar]
- 18.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–19. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 19.Miller JR. The Wnts. Genome Biol. 2002;3:3001. doi: 10.1186/gb-2001-3-1-reviews3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parmalee NL, Kitajewski J. Wnt signaling in angiogenesis. Curr Drug Targets. 2008;9:558–64. doi: 10.2174/138945008784911822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kikuchi A, Yamamoto H. Tumor formation due to abnormalities in the β-catenin-independent pathway of Wnt signaling. Cancer Sci. 2008;99:202–8. doi: 10.1111/j.1349-7006.2007.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacDonald BT, He X. Frizzled and LRP5/6 receptors for Wnt/β-catenin signaling. Cold Spring Harb Perspect Biol. 2012;4:4. doi: 10.1101/cshperspect.a007880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao X, Hannoush RN. Single-cell imaging of Wnt palmitoylation by the acyltransferase porcupine. Nat Chem Biol. 2014;10:61–8. doi: 10.1038/nchembio.1392. [DOI] [PubMed] [Google Scholar]
- 24.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–52. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 25.Gao X, Hannoush RN. Single-cell imaging of Wnt palmitoylation by the acyltransferase porcupine. Nat Chem Biol. 2014;10:61–8. doi: 10.1038/nchembio.1392. [DOI] [PubMed] [Google Scholar]
- 26.Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, et al. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Nile AH, Mukund S, Stanger K, Wang W, Hannoush RN. Unsaturated fatty acyl recognition by Frizzled receptors mediates dimerization upon Wnt ligand binding. Proc Natl Acad Sci U S A. 2017;114:4147–52. doi: 10.1073/pnas.1618293114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeBruine ZJ, Ke J, Harikumar KG, Gu X, Borowsky P, Williams BO, et al. Wnt5a promotes Frizzled-4 signalosome assembly by stabilizing cysteine-rich domain dimerization. Genes Dev. 2017;31:916–26. doi: 10.1101/gad.298331.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC. Structural basis of Wnt recognition by Frizzled. Science. 2012;337:59–64. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacDonald BT, Hien A, Zhang X, Iranloye O, Virshup DM, Waterman ML, et al. Disulfide bond requirements for active Wnt ligands. J Biol Chem. 2014;289:18122–36. doi: 10.1074/jbc.M114.575027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dijksterhuis JP, Baljinnyam B, Stanger K, Sercan HO, Ji Y, Andres O, et al. Systematic mapping of WNT-FZD protein interactions reveals functional selectivity by distinct WNT-FZD pairs. J Biol Chem. 2015;290:6789–98. doi: 10.1074/jbc.M114.612648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agostino M, Pohl SÖ, Dharmarajan A. Structure-based prediction of Wnt binding affinities for Frizzled-type cysteine-rich domains. J Biol Chem. 2017;292:11218–29. doi: 10.1074/jbc.M117.786269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–80. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 34.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–47. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 35.He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131:1663–77. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- 36.Zeng X, Huang H, Tamai K, Zhang X, Harada Y, Yokota C, et al. Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development. 2008;135:367–75. doi: 10.1242/dev.013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shtutman M, Zhurinsky J, Simcha I, Albanese C, DAmico M, Pestell R, et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96:5522–7. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoogeboom D, Essers MA, Polderman PE, Voets E, Smits LM, Burgering BM. Interaction of FOXO with beta-catenin inhibits beta-catenin/T cell factor activity. J Biol Chem. 2008;283:9224–30. doi: 10.1074/jbc.M706638200. [DOI] [PubMed] [Google Scholar]
- 39.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–6. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 40.Yan D, Wiesmann M, Rohan M, Chan V, Jefferson AB, Guo L, et al. Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/beta -catenin signaling is activated in human colon tumors. Proc Natl Acad Sci U S A. 2001;98:14973–8. doi: 10.1073/pnas.261574498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X, Gaspard JP, Chung DC. Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Res. 2001;61:6050–4. [PubMed] [Google Scholar]
- 42.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–32. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 43.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 44.Ishitani T, Kishida S, Hyodo-Miura J, Ueno N, Yasuda J, Waterman M, et al. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Mol Cell Biol. 2003;23:131–9. doi: 10.1128/MCB.23.1.131-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishitani T, Ninomiya-Tsuji J, Matsumoto K. Regulation of lymphoid enhancer factor 1/T-cell factor by mitogen-activated protein kinase-related Nemo-like kinase-dependent phosphorylation in Wnt/beta-catenin signaling. Mol Cell Biol. 2003;23:1379–89. doi: 10.1128/MCB.23.4.1379-1389.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Y, Mlodzik M. Wnt-Frizzled/planar cell polarity signaling: cellular orientation by facing the wind (Wnt) Annu Rev Cell Dev Biol. 2015;31:623–46. doi: 10.1146/annurev-cellbio-100814-125315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat Neurosci. 2005;8:34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- 49.Pate KT, Stringari C, Sprowl-Tanio S, Wang K, TeSlaa T, Hoverter NP, et al. Wnt signaling directs a metabolic program of glycolysis and angiogenesis in colon cancer. EMBO J. 2014;33:1454–73. doi: 10.15252/embj.201488598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vanhollebeke B, Stone OA, Bostaille N, Cho C, Zhou Y, Maquet E, et al. Tip cell-specific requirement for an atypical Gpr124- and Reck-dependent Wnt/β-catenin pathway during brain angiogenesis. Elife. 2015;4:4. doi: 10.7554/eLife.06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Y, Nathans J. Gpr124 controls CNS angiogenesis and blood-brain barrier integrity by promoting ligand-specific canonical wnt signaling. Dev Cell. 2014;31:248–56. doi: 10.1016/j.devcel.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scholz B, Korn C, Wojtarowicz J, Mogler C, Augustin I, Boutros M, et al. Endothelial RSPO3 Controls Vascular Stability and Pruning through Non-canonical WNT/Ca(2+)/NFAT Signaling. Dev Cell. 2016;36:79–93. doi: 10.1016/j.devcel.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 53.Ferreira Tojais N, Peghaire C, Franzl N, Larrieu-Lahargue F, Jaspard B, Reynaud A, et al. Frizzled7 controls vascular permeability through the Wnt-canonical pathway and cross-talk with endothelial cell junction complexes. Cardiovasc Res. 2014;103:291–303. doi: 10.1093/cvr/cvu133. [DOI] [PubMed] [Google Scholar]
- 54.Easwaran V, Lee SH, Inge L, Guo L, Goldbeck C, Garrett E, et al. beta-Catenin regulates vascular endothelial growth factor expression in colon cancer. Cancer Res. 2003;63:3145–53. [PubMed] [Google Scholar]
- 55.Posokhova E, Shukla A, Seaman S, Volate S, Hilton MB, Wu B, et al. GPR124 functions as a WNT7-specific coactivator of canonical β-catenin signaling. Cell Rep. 2015;10:123–30. doi: 10.1016/j.celrep.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–4. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 57.Meadows KL, Hurwitz HI. Anti-VEGF therapies in the clinic. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 59.Cohen MH, Shen YL, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14:1131–8. doi: 10.1634/theoncologist.2009-0121. [DOI] [PubMed] [Google Scholar]
- 60.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. ICON7 Investigators. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–96. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 61.Hainsworth JD, Sosman JA, Spigel DR, Edwards DL, Baughman C, Greco A. Treatment of metastatic renal cell carcinoma with a combination of bevacizumab and erlotinib. J Clin Oncol. 2005;23:7889–96. doi: 10.1200/JCO.2005.01.8234. [DOI] [PubMed] [Google Scholar]
- 62.Wright JD, Viviano D, Powell MA, Gibb RK, Mutch DG, Grigsby PW, et al. Bevacizumab combination therapy in heavily pretreated, recurrent cervical cancer. Gynecol Oncol. 2006;103:489–93. doi: 10.1016/j.ygyno.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 63.Robertson JD, Botwood NA, Rothenberg ML, Schmoll HJ. Phase III trial of FOLFOX plus bevacizumab or cediranib (AZD2171) as first-line treatment of patients with metastatic colorectal cancer: HORIZON III. Clin Colorectal Cancer. 2009;8:59–60. doi: 10.3816/CCC.2009.n.010. [DOI] [PubMed] [Google Scholar]
- 64.Sekeres MA. The avastin story. N Engl J Med. 2011;365:1454–5. doi: 10.1056/NEJMc1109550. [DOI] [PubMed] [Google Scholar]
- 65.Brandes AA, Bartolotti M, Tosoni A, Poggi R, Franceschi E. Practical management of bevacizumab-related toxicities in glioblastoma. Oncologist. 2015;20:166–75. doi: 10.1634/theoncologist.2014-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mohile SG, Hardt M, Tew W, Owusu C, Klepin H, Gross C, et al. Cancer and Aging Research Group. Toxicity of bevacizumab in combination with chemotherapy in older patients. Oncologist. 2013;18:408–14. doi: 10.1634/theoncologist.2012-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov. 2014;13:513–32. doi: 10.1038/nrd4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huo N, Ichikawa Y, Kamiyama M, Ishikawa T, Hamaguchi Y, Hasegawa S, et al. MMP-7 (matrilysin) accelerated growth of human umbilical vein endothelial cells. Cancer Lett. 2002;177:95–100. doi: 10.1016/s0304-3835(01)00772-8. [DOI] [PubMed] [Google Scholar]
- 69.Ii M, Yamamoto H, Adachi Y, Maruyama Y, Shinomura Y. Role of matrix metalloproteinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp Biol Med (Maywood) 2006;231:20–7. doi: 10.1177/153537020623100103. [DOI] [PubMed] [Google Scholar]
- 70.Seo D-W, Li H, Guedez L, Wingfield PT, Diaz T, Salloum R, et al. TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell. 2003;114:171–80. doi: 10.1016/s0092-8674(03)00551-8. [DOI] [PubMed] [Google Scholar]
- 71.St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, et al. Genes expressed in human tumor endothelium. Science. 2000;289:1197–202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 72.Zebisch M, Jones EY. Crystal structure of R-spondin 2 in complex with the ectodomains of its receptors LGR5 and ZNRF3. J Struct Biol. 2015;191:149–55. doi: 10.1016/j.jsb.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 74.Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 2008;121:737–46. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- 75.Jackson HW, Defamie V, Waterhouse P, Khokha R. TIMPs: versatile extracellular regulators in cancer. Nat Rev Cancer. 2017;17:38–53. doi: 10.1038/nrc.2016.115. [DOI] [PubMed] [Google Scholar]
- 76.Pohl S, Scott R, Arfuso F, Perumal V, Dharmarajan A. Secreted frizzled-related protein 4 and its implications in cancer and apoptosis. Tumour Biol. 2015;36:143–52. doi: 10.1007/s13277-014-2956-z. [DOI] [PubMed] [Google Scholar]
- 77.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–34. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 78.Duplàa C, Jaspard B, Moreau C, DAmore PA. Identification and cloning of a secreted protein related to the cysteine-rich domain of frizzled. Evidence for a role in endothelial cell growth control. Circ Res. 1999;84:1433–45. doi: 10.1161/01.res.84.12.1433. [DOI] [PubMed] [Google Scholar]
- 79.Galli LM, Barnes T, Cheng T, Acosta L, Anglade A, Willert K, et al. Differential inhibition of Wnt-3a by Sfrp-1, Sfrp-2, and Sfrp-3. Dev Dyn. 2006;235:681–90. doi: 10.1002/dvdy.20681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–34. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 81.Dufourcq P, Leroux L, Ezan J, Descamps B, Lamazière JM, Costet P, et al. Regulation of endothelial cell cytoskeletal reorganization by a secreted frizzled-related protein-1 and frizzled 4- and frizzled 7-dependent pathway: role in neovessel formation. Am J Pathol. 2008;172:37–49. doi: 10.2353/ajpath.2008.070130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Courtwright A, Siamakpour-Reihani S, Arbiser JL, Banet N, Hilliard E, Fried L, et al. Secreted frizzle-related protein 2 stimulates angiogenesis via a calcineurin/NFAT signaling pathway. Cancer Res. 2009;69:4621–8. doi: 10.1158/0008-5472.CAN-08-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao Y, Bao Q, Renner A, Camaj P, Eichhorn M, Ischenko I, et al. Cancer stem cells and angiogenesis. Int J Dev Biol. 2011;55:477–82. doi: 10.1387/ijdb.103225yz. [DOI] [PubMed] [Google Scholar]
- 84.Wu M, Guan J, Li C, Gunter S, Nusrat L, Ng S, et al. Aberrantly activated Cox-2 and Wnt signaling interact to maintain cancer stem cells in glioblastoma. Oncotarget. 2017;8:82217–30. doi: 10.18632/oncotarget.19283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang R, Sun Q, Wang P, Liu M, Xiong S, Luo J, et al. Notch and Wnt/β-catenin signaling pathway play important roles in activating liver cancer stem cells. Oncotarget. 2016;7:5754–68. doi: 10.18632/oncotarget.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Basu S, Haase G, Ben-Zeev A. Wnt signaling in cancer stem cells and colon cancer metastasis. F1000Res. 2016;5:5. doi: 10.12688/f1000research.7579.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jang GB, Kim JY, Cho SD, Park KS, Jung JY, Lee HY, et al. Blockade of Wnt/β-catenin signaling suppresses breast cancer metastasis by inhibiting CSC-like phenotype. Sci Rep. 2015;5:12465. doi: 10.1038/srep12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–8. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 89.Plate KH, Breier G, Weich HA, Mennel HD, Risau W. Vascular endothelial growth factor and glioma angiogenesis: coordinate induction of VEGF receptors, distribution of VEGF protein and possible in vivo regulatory mechanisms. Int J Cancer. 1994;59:520–9. doi: 10.1002/ijc.2910590415. [DOI] [PubMed] [Google Scholar]
- 90.Majumder M, Xin X, Liu L, Tutunea-Fatan E, Rodriguez-Torres M, Vincent K, et al. COX-2 Induces Breast Cancer Stem Cells via EP4/PI3K/AKT/NOTCH/WNT Axis. Stem Cells. 2016;34:2290–305. doi: 10.1002/stem.2426. [DOI] [PubMed] [Google Scholar]
- 91.Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–8. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 92.Warrier S, Balu SK, Kumar AP, Millward M, Dharmarajan A. Wnt antagonist, secreted frizzled-related protein 4 (sFRP4), increases chemotherapeutic response of glioma stem-like cells. Oncol Res. 2013;21:93–102. doi: 10.3727/096504013X13786659070154. [DOI] [PubMed] [Google Scholar]
- 93.Pellegatta S, Poliani PL, Corno D, Menghi F, Ghielmetti F, Suarez-Merino B, et al. Neurospheres enriched in cancer stem-like cells are highly effective in eliciting a dendritic cell-mediated immune response against malignant gliomas. Cancer Res. 2006;66:10247–52. doi: 10.1158/0008-5472.CAN-06-2048. [DOI] [PubMed] [Google Scholar]
- 94.Yao XH, Ping YF, Chen JH, Xu CP, Chen DL, Zhang R, et al. Glioblastoma stem cells produce vascular endothelial growth factor by activation of a G-protein coupled formylpeptide receptor FPR. J Pathol. 2008;215:369–76. doi: 10.1002/path.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Folkins C, Shaked Y, Man S, Tang T, Lee CR, Zhu Z, et al. Glioma tumor stem-like cells promote tumor angiogenesis and vasculogenesis via vascular endothelial growth factor and stromal-derived factor 1. Cancer Res. 2009;69:7243–51. doi: 10.1158/0008-5472.CAN-09-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 97.Zhao Z, Lu P, Zhang H, Xu H, Gao N, Li M, et al. Nestin positively regulates the Wnt/β-catenin pathway and the proliferation, survival and invasiveness of breast cancer stem cells. Breast Cancer Res. 2014;16:408. doi: 10.1186/s13058-014-0408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jiang F, Ma J, Liang Y, Niu Y, Chen N, Shen M. Amniotic mesenchymal stem cells can enhance angiogenic capacity via MMPs in vitro and in vivo. BioMed Res Int. 2015;2015:324014. doi: 10.1155/2015/324014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tao H, Han Z, Han ZC, Li Z. Proangiogenic features of mesenchymal stem cells and their therapeutic applications. Stem Cells Int. 2016;2016:1314709. doi: 10.1155/2016/1314709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Garikipati VN, Jadhav S, Pal L, Prakash P, Dikshit M, Nityanand S. Mesenchymal stem cells from fetal heart attenuate myocardial injury after infarction: an in vivo serial pinhole gated SPECT-CT study in rats. PLoS One. 2014;9:e100982. doi: 10.1371/journal.pone.0100982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106:14022–7. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lu F, Zhao X, Wu J, Cui Y, Mao Y, Chen K, et al. MSCs transfected with hepatocyte growth factor or vascular endothelial growth factor improve cardiac function in the infarcted porcine heart by increasing angiogenesis and reducing fibrosis. Int J Cardiol. 2013;167:2524–32. doi: 10.1016/j.ijcard.2012.06.052. [DOI] [PubMed] [Google Scholar]
- 103.Zhang Z, Nör F, Oh M, Cucco C, Shi S, Nör JE. Wnt/β-Catenin Signaling Determines the Vasculogenic Fate of Postnatal Mesenchymal Stem Cells. Stem Cells. 2016;34:1576–87. doi: 10.1002/stem.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang N, Zhang R, Wang SJ, Zhang CL, Mao LB, Zhuang CY, et al. Vascular endothelial growth factor stimulates endothelial differentiation from mesenchymal stem cells via Rho/myocardin-related transcription factora signaling pathway. Int J Biochem Cell Biol. 2013;45:1447–56. doi: 10.1016/j.biocel.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 105.Zhang B, Wu X, Zhang X, Sun Y, Yan Y, Shi H, et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem Cells Transl Med. 2015;4:513–22. doi: 10.5966/sctm.2014-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sun J, Chen J, Cao J, Li T, Zhuang S, Jiang X. IL-1β-stimulated β-catenin up-regulation promotes angiogenesis in human lung-derived mesenchymal stromal cells through a NF-κB-dependent microRNA-433 induction. Oncotarget. 2016;7:59429–40. doi: 10.18632/oncotarget.10683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dufourcq P, Descamps B, Tojais NF, Leroux L, Oses P, Daret D, et al. Secreted frizzled-related protein-1 enhances mesenchymal stem cell function in angiogenesis and contributes to neovessel maturation. Stem Cells. 2008;26:2991–3001. doi: 10.1634/stemcells.2008-0372. [DOI] [PubMed] [Google Scholar]