Abstract
Background:
Sulforaphane (SFN), a phytochemical found in abundance in broccoli sprouts, potently induces a variety of antioxidant enzymes, and thereby protects cells from injury induced by various kinds of oxidative stresses. It has been suggested that both H. pylori infection and intake of non-steroidal anti-inflammatory drugs (NSAIDs) induce chronic oxidative stress in gastrointestinal (GI) mucosa, thereby causing mucosal injury in the GI tract. Therefore, it would be a reasonable assumption that SFN protects GI mucosa against oxidative injury induced by H. pylori or NSAIDs.
Methods:
We examined the effects of SFN on H. pylori viability in vitro, levels of gastritis in H.pylori-infected mice in vivo, and in H.pylori-infected human subjects. We also examined the effects of SFN on NSAID-induced small intestinal injury in mice.
Results:
Our data from the H. pylori infection study clearly demonstrated that SFN inhibited H. pylori viability both in vitro and in vivo, and mitigated H. pylori-induced gastritis in mice and humans. Similar-ly, our study on NSAID-induced small intestinal injury showed that SFN not only mitigated aspirin-induced injury of small intestinal epithelial cells in vitro, but also ameliorated indomethacin-induced small intestinal injury in mice in vivo.
Conclusions:
These data strongly suggest that SFN contributes to the protection of GI mucosa against oxidative injury induced by H. pylori or NSAIDs.
Keywords: Sulforaphane, Helicobacter pylori, stomach, aspirin, indomethacin, small intestine
1. Introduction
Sulforaphane (SFN), an abundant antioxidant phytochemical found in broccoli sprouts (BS), potently induces a variety of antioxidant enzymes, which protect cells and organs against various kinds of oxidative stresses [1]. Recent studies have shown the antibacterial activity of SFN against Helicobacter pylori (H. pylori) in vitro [2]. We have recently shown that SFN induces antioxidant enzymes in the gastro-intestinal (GI) tract of mice, and protects GI mucosa against injuries induced by H. pylori and NSAIDs [3-5]. Our studies show that SFN not only enhances the antioxidant activity of GI mucosa, but also demonstrates antibacterial activity against H. pylori in gastric mucosa and anaerobic bacteria in the small intestine of mice [5]. We have conducted clinical trials with H. pylori-infected human subjects who consume BS, and have shown that SFN clearly inhibits H. pylori activity and mitigates H. pylori-induced gastritis [4]. This review introduces our recent data on the protective effects of SFN, which demonstrate that SFN prevented H. pylori- and NSAID-induced GI mucosal injury.
2. Role of SFN in Protection of the Cells Against Oxidative Stress
It has been suggested that environmental factors, especially dietary factors, are more important in the development of GI cancer than genetic factors [6, 7]. Recent studies have clearly associated the development of gastric cancer not only with H. pylori infection [8, 9], but also with the intake of a high salt diet [10, 11]. In contrast, it has also been reported that daily intake of fruits and vegetables decreased the risk of developing GI cancers [6, 12]. However, the mechanisms by which intake of fruits and vegetables decreased the risk of GI cancers were not clarified until recently. It is naturally important to identify the protective substances from the various phytochemicals in fruits and vegetables. Furthermore, it is necessary to clarify the mechanisms by which each substance protects the GI tract against oxidative injury. Finally, the effects of such substances on human health should be examined through well-designed clinical trials.
SFN has been studied extensively in the past two decades, and several studies have suggested the possibility that SFN may contribute to cancer chemoprotection [13]. SFN, a member of the Isothiocyanate (ITC) family, is abundant in cruciferous vegetables, in particular Broccoli Sprouts (BS) [13, 14]. Previous studies have shown that the unique molecular functional group -N=C=S is common to ITCs, which accounts for the pungency of these vegetables [15] (Fig. 1). Different ITCs have been reported in a variety of foods. For example, Wasabi and mustard oils are rich in allyl ITCs [15]. Phenethyl ITC and 4-(methylthio)-3-butenyl ITC are found abundantly in Daikon and Cresson, respectively [16]. As previously stated, BS are rich in SFN. SFN potently induces various antioxidant (or phase 2) enzymes, such as glutathione S-transferase (GST), heme oxygenase-1 (HO-1), and NAD(P)H: quinone oxidoreductase 1 (NQO1), via Nf-E2 related factor 2-Kelch-like ECH-associated protein 1 (Nrf2-Keap1)-dependent pathways (17), thereby enhancing the antioxidant activity of the cells in the GI tract [4, 5] (Fig. 2).
Fig. (1).
Broccoli Sprouts Contain High Concentration of Sulforaphane (SFN)
Fig. (2).
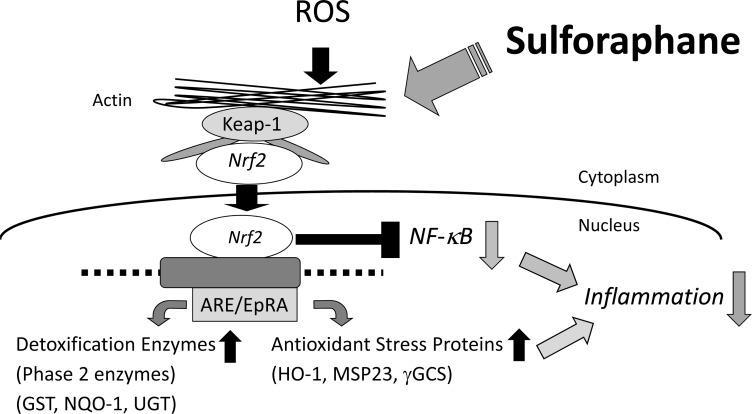
Sulforaphane (SFN) Enhances Antioxidant Activity via Nrf2-Keap1 System.
In raw BS, SFN exists in the biologically inactive form of sulforaphane glucosinolates (SGS) [14]. The transformation of SGS to SFN occurs during the chewing process, where SGS is subjected to the action of myrosinase, also a component of BS [14]. SGS is also transformed to SFN in the intestinal lumen, by myrosinase of the intestinal microflora [14]. As previously stated, SFN upregulates
SFN, a member of the isothiocyanate (ITC) family, is abundant in cruciferous vegetables, especially broccoli sprouts (BS). (A) Fifty grams of raw BS contain 128 mg of sulforaphane glucosinolate, a precursor of SFN. (B) SFN possesses -N=C=S, which is a common chemical structure found in the ITC family. This molecular structure accounts for the pungency of the cruciferous vegetables.
phase 2 enzymes via Nrf2-Keap1-dependent mechanisms [17]. Under basal conditions, Nrf2 proteins are located in the cytoplasm and are biologically inactive as Nrf2 is bound to the Keap1 protein. Following exposure of the cells to oxidative stresses or SFN, Nrf2 proteins become dissociated from Keap1 and translocate into the nucleus. After entering the nucleus, Nrf2 protein binds to the antioxidant response element, and upregulates expression of a variety of xenobiotic and antioxidant enzymes [18-20] (Fig. 2). It has been shown that induction of the phase 2 enzymes by SFN lasts almost for 72 h [17]. In addition to activation of the Nrf2-Keap1 pathway within cells, SFN has also been shown to inhibit H. pylori viability in vitro; these effects were demonstrated in both the clarithromycin-sensitive and resistant strains [2]. Furthermore, other studies have shown that SFN decreases colonization in mice stomach ex vivo [21], and in a small number of human cases [22].
SFN potently induces various antioxidant (or phase 2) enzymes, such as glutathione S-transferase (GST), heme oxygenase-1 (HO-1), and NAD(P)H: quinone oxidoreductase 1 (NQO1), via Nf-E2 related factor 2 (Nrf2) - Kelch-like ECH-associated protein 1 (Keap1) dependent pathways. These are responsible for the enhanced antioxidant activity of the cells.
Based on these results, we aimed to determine if oral intake of SFN contributed to the protection of GI mucosa against GI diseases induced by oxidative stresses, such as those induced by H. pylori and NSAIDs.
3. Roles of SFN in Protection Against H. pylori-Induced Gastric Mucosal Inflammation
H. pylori infection induces chronic oxidative stress in gastric mucosa, which eventually induces gastric cancer. However, SFN has been shown to mitigate various kinds of oxidative stresses. In this study, we aimed to determine if SFN inhibited H. pylori activity in vitro, and if SFN mitigated H. pylori-induced oxidative injury in gastric mucosa in mice and humans in vivo.
3.1. Effect of SFN on Urease Activity and H. pylori Viability In Vitro
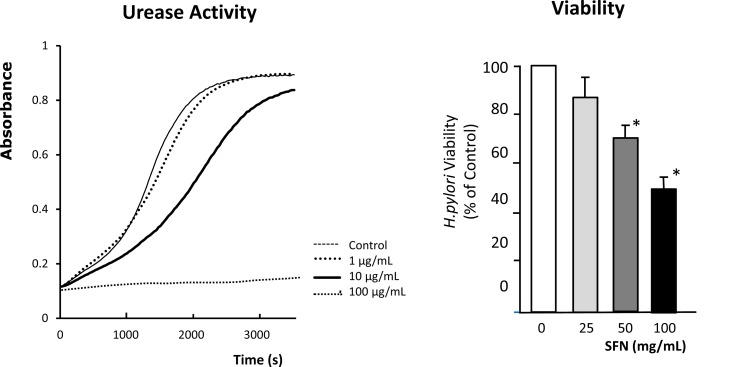
In this series of experiments, we examined whether SFN showed direct antibacterial activity against H. pylori in vitro [23]. The H. pylori strain Sydney Strain-1 (SS-1) was used in this study. The viability of H. pylori was determined by evaluating the number of Colony Forming Units (CFU) after incubation of the H. pylori in the absence or presence of various concentrations of SFN for 3 h. The urease activity of H. pylori was assessed by measurement of the concentration of ammonia released into the medium during incubation with 5 mM urea for 1 h. The effects of SFN on urease activity and viability of H. pylori were examined at ambient pH 7.4 in vitro. At doses from 1-100 μg/mL, SFN dose-dependently decreased urease activity and viability of H. pylori (Fig. 3). These results suggest that SFN shows antibacterial activity against H. pylori in vitro.
Fig. (3).
Sulforaphane (SFN) Markedly Inhibits Urease Activity and Viability of H. pylori
The H. pylori strain Sydney Strain-1 (SS-1) was used in this study. The urease activity of H. pylori was assessed by measurement of the concentration of ammonia released into the medium during a 1-h incubation period with 5 mM urea. The viability of H. pylori was determined by the number of colony forming units (CFU) after incubation of the H. pylori in the absence or presence of various concentrations of SFN for 3 h. The effects of SFN on H. pylori viability and urease activity were examined at ambient pH 7.4 in vitro. At doses of 1-100 μg/mL, SFN dose-dependently decreased the viability of H. pylori. The representative trace in left panel shows that SFN dose-dependently inhibits H. pylori urease activity. The right panel shows that SFN dose-dependently decreased H. pylori viability. n: number of experiments. *P < 0.05; significant difference from the corresponding values in the absence of SFN.
3.2. Effect of SFN on H. pylori Colonization and Gastric Mucosal Inflammation in H. pylori-Infected Mice In Vivo
Based on the in vitro experimental data, we conducted the next series of studies to determine if SFN inhibited colonization of H. pylori and mitigated inflammation in H. pylori-infected gastric mucosa in mice in vivo.
Gastric mucosal infections with H. pylori Sydney Strain-1 were established in 6-week-old female C57BL/6 mice of both wild-type (Nrf2+/+) and Nrf2 knockout (Nrf2-/-) strains by inoculation with 5 × 107 CFU of H. pylori [24]. The H. pylori-infected mice were fed a high-salt diet (7.5% NaCl) for 2 months in order to exacerbate inflammation in gastric corpus mucosa [11]. The wild-type and Nrf2-/- mice were fed the homogenized BS (+BS), or no BS (−BS). Approximately 3 μmol/mouse/day of SGS was administered to the +BS group. Eight weeks later, all mice were sacrificed. Gastric mucosal preparations were fixed with formalin, stained with hematoxylin and eosin, and examined using light microscopy. The degree of gastric mucosal inflammation was measured using the updated Sydney system [25]. Activities of the gastric mucosal phase 2 detoxification enzymes, NQO1 and GST, were measured by ELISA. H. pylori colonization of mice gastric mucosa was assessed as described in our previous report [4].
3.2.1. Effect of BS Treatment on Gastric Mucosal Inflammation
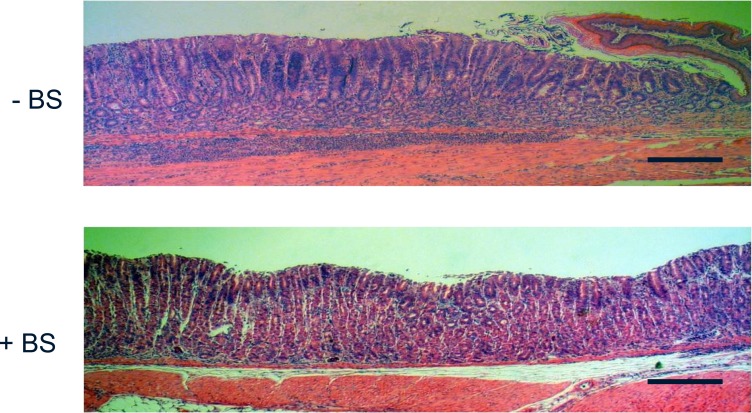
Morphological examination by light microscopy of the gastric mucosae in H. pylori-infected Nrf2+/+ mice showed massive infiltration of inflammatory cells in the mice not receiving BS, while the mice fed BS showed less inflammation (Fig. 4).
Fig. (4).
Broccoli Sprouts Markedly Attenuate Corpus Gastritis in H. pylori- infected Mice Fed High Salt Diet
Gastric mucosal infections with H. pylori Sydney strain-1 were established in 6-week-old female C57BL/6 mice of both the wild-type (Nrf2+/+) and knockout (Nrf-/-) mice by inoculation of 5 × 107 CFU of H. pylori [24]. The H. pylori-infected mice were fed for 2 months with a high-salt diet (7.5% NaCl) in order to exacerbate inflammation in gastric corpus mucosa. The mice were fed with the homogenized Broccoli Sprouts (+BS), or without BS (−BS). Approximately 3 μmol/mouse/day of SGS were administered into the +BS group. All the mice were sacrificed at 8 weeks later. Gastric mucosal preparations, fixed with formalin and stained with hematoxylin and eosin, were examined by light microscopy.
A representative histology of gastric mucosa of the H. pylori-infected mice without BS treatment shows massive infiltration of inflammatory cells (upper panel), while the histology of the mice fed with BS shows less inflammation (lower panel).
Scale bar: 200 μm.
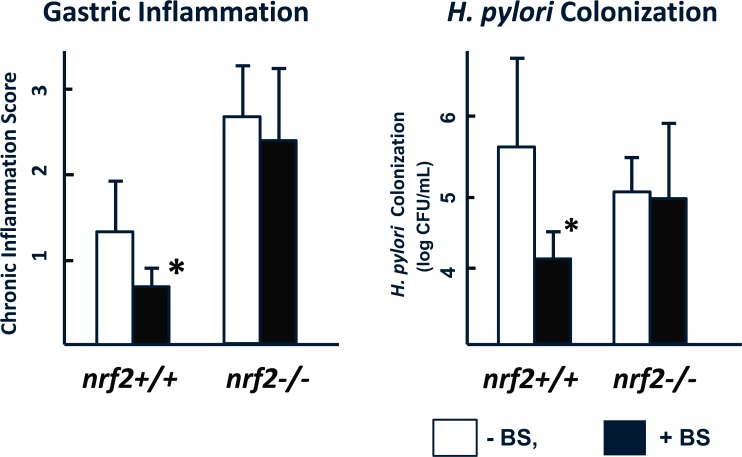
Following the administration of BS, activation of the antioxidant enzymes NQO1 and GST increased significantly in Nrf2+/+, but not Nrf2-/- mice, as expected [26]. In agreement with these findings, inflammation of the gastric corpus mucosa in H. pylori-infected mice was substantially attenuated by treatment with BS in Nrf2+/+, but not in Nrf2-/- mice (Fig. 4, Fig. 5).
Fig. (5).
Broccoli Sprouts Attenuate Corpus Gastritis and Inhibits H. pylori Colonization in Nrf2+/+, but not in Nrf2-/- mice
3.2.2. Effect of BS Treatment on H. pylori Colonization
BS treatment induced an almost 2-log reduction in H. pylori colonization in wild-type mice but not in Nrf2-/- mice (Fig. 5), thus confirming the integral role of the SFN-induced Nrf2 activation in protection against H. pylori-induced gastric inflammation.
The wild-type (Nrf2+/+) and the Nrf2 knockout (Nrf2-/-) mice infected with H. pylori were fed with broccoli sprouts (BS) (+BS; ■), or without BS (-BS; □) (as described in Fig. 4 legend). The degree of inflammation in gastric corpus mucosa was expressed as the inflammation score as defined in the updated Sydney system [25]. H. pylori colonization was expressed as the number of H. pylori Colony Forming Units (CFU) after incubation of the mucosal homogenates in the specific medium for H. pylori culture, described in our previous report [4]. The left panel shows that feeding with BS significantly mitigated both the inflammation score (left panel) and the colonization (right panel) in Nrf2+/+, but not in Nrf2-/- mice (*P < 0.05; significant difference from the corresponding values in the absence of BS; n=number of animals).
3.2.3. Effect of Daily Intake of SFN-Rich BS on H. pylori Infection in Human Subjects
Based on the in vivo experimental data from H. pylori-infected mice, we conducted the next experiments to determine if daily intake of SFN-rich BS mitigated H. pylori-induced gastritis in human subjects.
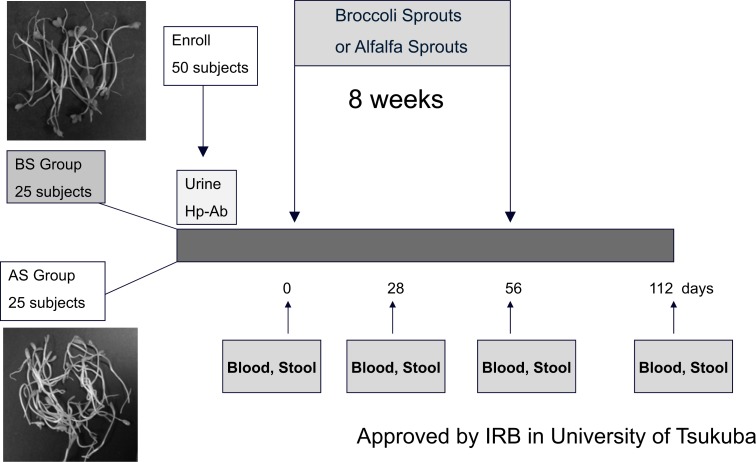
Fifty H. pylori-positive volunteers, whose endoscopy showed no abnormalities other than gastritis, were randomized to either the BS group (n=25) or the alfalfa sprouts (AS) group (n=25). In this study, AS were used as the placebo, since they do not contain SGS or other isothiocyanates (Fig. 6). Subjects were instructed to consume 70 g/day of SGS-rich 3-day-old BS (Broccoli Super Sprout®, Murakami Pharm Ltd, Japan) for 8 weeks; these sprouts were validated to have an SGS content of approximately 6 μmol/g [27, 28]. Subjects in the AS (placebo) group were instructed to consume an equivalent amount of AS for 8 weeks. All participants were required to attend the hospital for collection of blood and stool samples at 0, 4, 8, and 16 weeks (eight weeks after the completion of the intervention); the dates corresponded to study days 0, 28, 56, and 112, respectively. Stool samples were analyzed for H. pylori stool antigen (HpSA) using an HpSA-ELISA kit from Meridian Bioscience, Inc., as previously described [29]. Serum pepsinogens I and II (PGI and PGII) were measured in blood samples collected from volunteers at these same time points, and the PGI/PGII ratio was computed [30, 31]. All test results were compared using a Student's t-test. Error bars on all figures represent ±1 SD from the mean.
Fig. (6).
Protocol for the Broccoli Sprouts Clinical Trial on H. pylori Infection
The study protocol (outlined in Fig. 6) randomized 50 subjects to daily consumption of either 70 g of BS or a placebo (AS). The mean age of subjects at randomization was 54.5 years. There were more female (n=28) than male (n=19) subjects, but there was no difference in pre-intervention H. pylori infection and inflammation status for the two groups (Table 1). Two subjects in the AS group dropped out during the intervention period owing to acute viral infection. Thus, the data obtained from 25 subjects in the BS group and 23 in the AS group were analyzed.
Table 1.
Baseline data for H.pylori-infected subjects (n=50) after randomization to broccoli sprout and alfalfa sprout supplemented diets.
| Broccoli sprout (n=25) | Alfalfa sprout (n=23) | |||
|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | |
| Age (y) | 53.4±11.6 | 25 - 70 | 55.7±12.9 | 23 - 73 |
| UBT (‰) | 32.7±14.4 | 10.0 - 56.8 | 35.7±25.3 | 6.7 – 106.1 |
| PG I (ng/mL) | 70.3±21.4 | 33.3 - 118 | 69.4±26.7 | 31.5 - 135 |
| PG II (ng/mL) | 30.5±14.8 | 14.7 - 43.7 | 29.4±8.63 | 18.3 – 52.5 |
| PGI/PGII (ratio) | 2.41±1.00 | 0.68 - 4.10 | 2.38±0.70 | 1.03 – 3.93 |
Fifty H. pylori–positive volunteers were randomized to either the Broccoli Sprouts (BS) group (n=25), or the Alfalfa Sprouts (AS) group (n=25). In this study, AS was used as the placebo, since AS do not contain any sulforaphane glucosinolates (SGS), the precursor of SFN. Subjects were instructed to consume 70 g/day of either the SGS-rich 3-day-old BS that contains SGS of approximately 6 μmol/g dose [27, 28], or an equivalent amount of AS for 8 weeks. All participants were instructed to attend the hospital for collection of blood and stool samples at 0, 4, 8, and 16 weeks. Levels of serum pepsinogens I and II (PGI and PGII) were measured by a commercially available ELISA kit, and PGI/PGII ratios were calculated [30, 31]. Stool samples were analyzed for H. pylori stool antigen (HpSA) using a HpSA-ELISA kit, as previously described (29) All tests results were compared using a Student's t test for paired comparison. Error bars on all figures represent ±1 SD from the mean.
1) Effects of BS/AS Treatment on Serum PGI and PGII
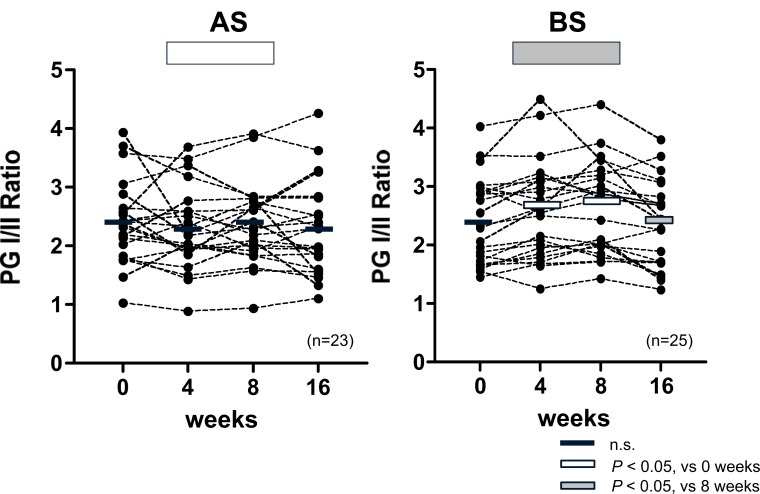
During the intervention period, significant reductions in both PGI and PGII (P < 0.05) compared with baseline levels were only observed in the BS group, and these returned to baseline values 2 months after the intervention. The ratio of PGI to PGII, used as a more robust indicator of changes in gastric inflammation (31), increased significantly (P < 0.05) during the intervention in the BS group (Fig. 7).
Fig. (7).
Effect of Broccoli Sprouts (BS) and/or Alfalfa Sprouts (AS) on Serum Pepsinogen I/II Ratio
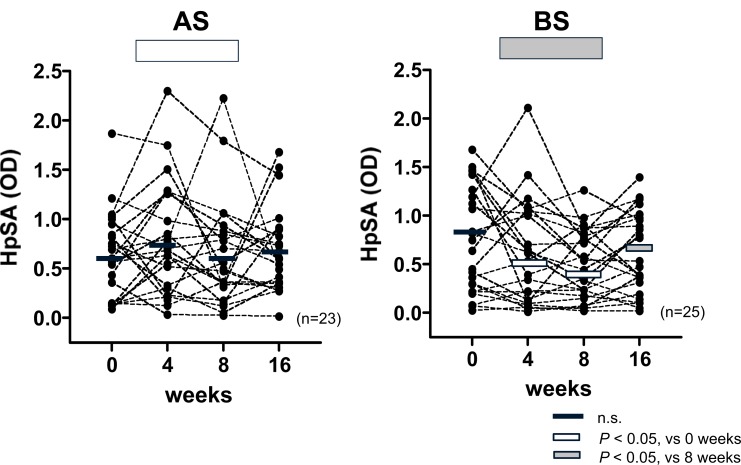
2) Effects of BS/AS Treatment on HpSA
The HpSA levels measured in the BS intervention arm were significantly lower (P < 0.05) during the intervention than at baseline, and returned to baseline levels (P < 0.05) at 16 weeks
There were significant reductions in both PGI and PGII compared with baseline levels during the 8-week intervention period in the BS group, and there was a return to baseline values at eight weeks after the intervention. Since the magnitude of the reduction in PGII was greater than PGI, the ratio of PGI to PGII (PGI/PGII) rose significantly during the intervention in the BS group, suggesting that BS treatment mitigates gastric inflammation. In contrast, there were no changes in PGI/PGII throughout the entire study period in AS group. Rectangular overlays represent means for each time period. n=number of participants.
![]() P < 0.05; Significant difference from the corresponding values at week 0.
P < 0.05; Significant difference from the corresponding values at week 0.
![]() P <0.05; Significant difference from the corresponding values at week 8.
P <0.05; Significant difference from the corresponding values at week 8.
(2 months after intervention). The placebo group receiving AS showed no significant change in HpSA (Fig. 8). Of the 25 subjects in the BS treatment group, eight subjects had HpSA values below the cutoff (0.100) at the end of the 8-week BS treatment period. In six of these subjects, the HpSA values became positive again at 8 weeks after cessation of BS consumption, and the HpSA values of the remaining two subjects became positive again, 6 months after intervention, thus indicating that BS treatment reduced H. pylori colonization but did not result in complete eradication.
Fig. (8).
Effects of Broccoli Sprouts (BS) and/or Alfalfa Sprouts (AS) on H. pylori-Specific Stool Antigen (HPSA)
4. Discussion for H. pylori Study
This study was designed to determine whether regular dietary consumption of BS, which is rich in the SFN precursor SGS, inhibited H. pylori colonization and attenuated inflammation in the gastric mucosa of mice and humans.
There was a significant reduction in of H. pylori-Specific Stool Antigen (HpSA) compared with baseline levels during the 8-week intervention period in the BS group, and there was a return to baseline values at eight weeks after the intervention, suggesting that BS treatment reduces H. pylori colonization, but does not eradicate H. pylori completely. In contrast, there were no changes in the HpSA levels in the AS group, throughout the whole study period. Rectangular overlays represent means for each time period. n=number of participants.
![]() P < 0.05; Significant difference from the corresponding values at week 0.
P < 0.05; Significant difference from the corresponding values at week 0.
![]() P <0.05; Significant difference from the corresponding values at week 8.
P <0.05; Significant difference from the corresponding values at week 8.
In this study, we first established that oral intake of BS decreased gastric mucosal inflammation in vivo in a well-established H. pylori-infected animal model. Oral dosing of animals in this model (approximately 3 μmol/mouse/day) was consistent with the SFN dosages in a variety of other mouse experiments investigating carcinogenesis [21, 32-35].
Second, the findings strongly suggest that mitigation of gastritis by SFN was at least partially due to the induction of antioxidant enzymes via the Nrf2 signaling pathway. SFN is a well-known activator of antioxidant enzymes and we have shown that these enzymes are upregulated in BS-treated animals.
Third, we determined that H. pylori colonization decreased in SFN-treated wild-type mice but not in Nrf2-/- mice. This in vivo finding suggests that SFN may have a direct antibiotic effect on the level of H. pylori colonization. The primary effect may occur via the upregulation of the host's systemic protection against oxidative stress and inflammation, which results in reduced H. pylori colonization. The mechanisms for the detoxification effects of SFN have been studied extensively [1, 17, 36-38]. SFN induces cytoprotective, antioxidant, and anti-inflammatory enzymes via the transcription factor Nrf2, which activates the genes that control these endogenous protective responses [20]. H. pylori infection generates a variety of reactive oxygen species within the mucosa that enhance gastric mucosal injury and inflammation. Our data show that H. pylori-induced gastritis in human subjects is mitigated by BS treatment. The data suggest that this effect is induced either by the inhibition of H. pylori colonization (in vitro, SFN is a potent antibiotic against H. pylori), the upregulation of Nrf2-dependent antioxidant enzyme activity (a number of in vitro, animal, and clinical studies have reported this), or by a combination of these two factors. Nonetheless, the SFN-induced enhancement of Nrf2-dependent antioxidant enzyme activity in gastric mucosal cells reduced reactive oxygen species from gastric mucosa, which resulted in the mitigation of H. pylori infection-induced gastritis.
Fourth, daily intake of BS (70 g) for 2 months decreased serum levels of PGI and PGII and increased the PGI/PGII ratio during the 2-month intervention period, which was consistent with clinical observations correlating to increased PGI/PGII ratio with reduced inflammation of gastric mucosa [30, 31]. We also measured reductions in HpSA (an indicator of recent infection) after the intake of BS. All biomarkers returned to baseline levels after the intervention was discontinued.
In summary, our data on H. pylori-infected mice and humans clearly suggested that SFN may have a direct antibacterial effect on H. pylori, leading to reduced gastritis, and a systemic effect by increasing the antioxidant response. It is not possible to determine the relative contributions of these two mechanisms from this study; however, in light of other evidence which suggests a strong anti-H. pylori effect of SFN in vitro [2], the findings in this study suggest that SFN has promise both as an antibacterial agent directed against H. pylori and a dietary preventive agent against the development of human gastric cancer.
5. Roles of SFN in Protection of the Small Intestine against Aspirin/NSAID-induced Mucosal Damage
The current prevalence of the prescription of aspirin and/or other NSAIDs to prevent cardiovascular events and relieve pain from osteoarthritis [39-41] is rising. It is well-established that aspirin and/or NSAIDs frequently cause peptic ulcer disease and bleeding from the upper GI tract. In order to prevent aspirin and/or NSAID-induced GI mucosal injuries, potent acid inhibitors that significantly ameliorate these injuries, such as Proton Pump Inhibitors (PPI), have been prescribed [42]. However, recent advances in capsule video endoscopy have revealed that the ulcers caused by aspirin and/or NSAIDs were not only localized to the upper GI tract, but also the small intestine [43-45]. Studies in human volunteers using capsule endoscopy have shown PPIs do not offer effective protection of the small intestinal mucosa against injury [46]. A recent study in rat has shown that PPI exacerbates NSAID-induced ulcers in the small intestine [47]. Although misoprostol has some beneficial effects on NSAID-induced gastrointestinal ulcers [48, 49], it causes several adverse reactions such as abdominal pain, diarrhea, and abortion [50]. A recent study in rats has shown that geranylgeranylacetone (GGA), a mucosal protective agent known to induce HSP70 in gastrointestinal mucosa, prevents small intestinal mucosa from injury induced by loxoprofen [51]. A few clinical trials using a small number of human volunteers have shown that aspirin/NSAID-induced small intestinal injuries detected by capsule video endoscopy are ameliorated by GGA [52] or rebamipide [53, 54], both of which have been used as gastric mucosal protective agents. Currently, however, there is no direct evidence that such agents are clinically effective for protection against NSAID-induced small intestinal ulcers. In the present study, we investigated the phytochemical sulforaphane (SFN) as a possible candidate drug for prevention of NSAID-induced small intestinal injuries. Since activation of antioxidant enzymes by SFN persists for 48-72 h, the protective effects of SFN against oxidative stress may be more potent than those of other antioxidant substances are. As NSAIDs induce oxidative stress in GI mucosa, it is reasonable to assume that SFN may be useful for protection of the small intestinal mucosa against oxidative stress induced by NSAIDs. Recent studies have shown that NSAID exaggerates small intestinal injuries, at least in part by causing overgrowth of anaerobic bacteria in the intestinal lumen [55, 56]. Therefore, it seems reasonable to assume that SFN may inhibit colonization of anaerobic bacteria in the intestinal lumen, thereby preventing invasion of the bacteria into the mucosa and consequently mitigating small intestinal injuries. Therefore, we decided to determine if SFN could protect small intestinal mucosa from NSAID-induced small intestinal injuries.
5.1. Role of SFN in Protection of IEC6 cells against Aspirin-induced Injury in vitro
IEC6 cells, derived from rat small intestinal mucosa, were incubated with or without various doses of SFN. After incubation for 12 h, the cells were treated with various dosages of aspirin. In some experiments, the effect of zinc protoporphyrin-IX (ZnPP), an inhibitor of HO-1, was also examined. The viability of the IEC6 cells was assessed by the measurement of 3-[4,5-dimethylthiazol-2-yl]-2,5- diphenyltetrazolium bromide (MTT) incorporation into the cells. Expression of HO-1 protein by IEC6 cells were evaluated by western blot analysis, using a polyclonal antibody to HO-1 (Hsp 32) as the primary antibody.
5.1.1. Effects of Aspirin on Viability of IEC-6 Cells
Aspirin, at doses between 10 to 40 mM, dose-dependently decreased viability of IEC-6 cells after incubation for 12 h (data not shown). Therefore, in the next series of experiments, we decided to use 20 mM aspirin, which is a submaximal dose to cause injury in IEC-6 cells.
5.1.2. Effect of Pretreatment with SFN on Aspirin-Induced Injury in IEC6 Cells
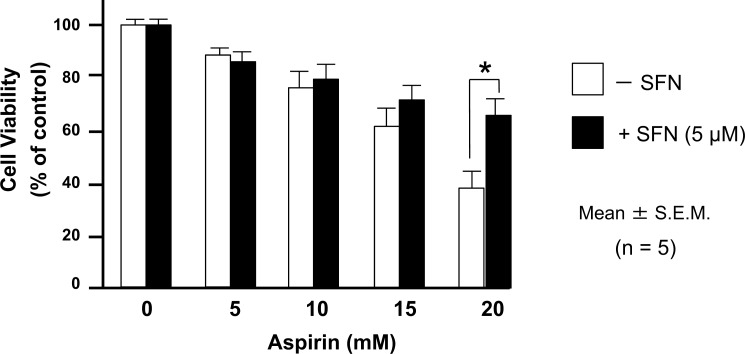
We confirmed that incubation of the cells with 5 μM SFN for 6 h did not cause injury to IEC-6 cells, but did induce HO-1 expression, as discussed later. Therefore, we decided to use 5 μM SFN in the following experiments. In this series of experiments, the cells were initially exposed to 5 μM SFN for 6 h, followed by incubation without SFN for a further 12 h. Then, the cells were exposed to various doses of aspirin. Pretreatment of the cells with SFN attenuated the 20 mM-aspirin-induced death of IEC6 cells (Fig. 9).
Fig. (9).
Sulforaphane (SFN) attenuates Aspirin-Induced Injury in IEC6 Cells
The cells were initially exposed to 5 μM SFN for 6 h, followed by incubation without SFN for a further 12 h. The cells were subsequently exposed to various doses of aspirin. Pretreatment with 5 μM SFN attenuated aspirin (20 mM)-induced decrease in the viability of IEC6 cells. The data are expressed as Mean ± SEM. n= number of experiments; * P < 0.05; statistically significant difference from the corresponding value in the absence of SFN.
5.1.3. Effect of Pretreatment with SFN on HO-1 Expression in IEC-6 Cells
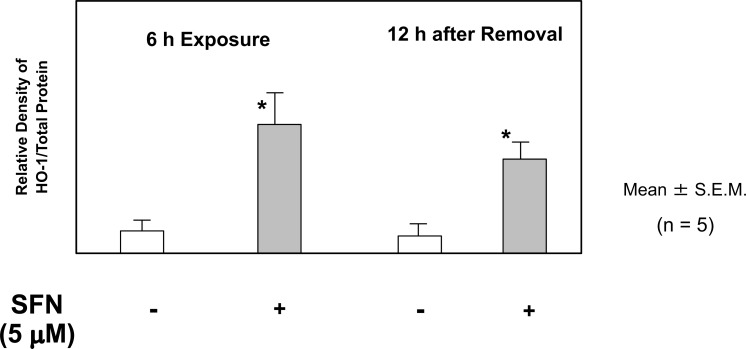
The cells were exposed to 5 μM SFN for 6 h, followed by incubation without SFN for a further 12 h. Western blot analysis showed that HO-1 expression in IEC-6 cells increased significantly after incubation with SFN for 6 h, and the effects persisted at 12 h after removal of SFN from the medium (Fig. 10). These results indicated that pretreatment with SFN upregulated HO-1 expression, and that the effect persisted during the subsequent exposure to aspirin.
Fig. (10).
Sulforaphane (SFN) Up-Regulates Heme Oxygenase (HO)-1 Expression in IEC6 Cells
5.1.4. Effects of HO-1 Inhibitor on the Protective Effects of SFN Against Aspirin-Induced Cell Injury
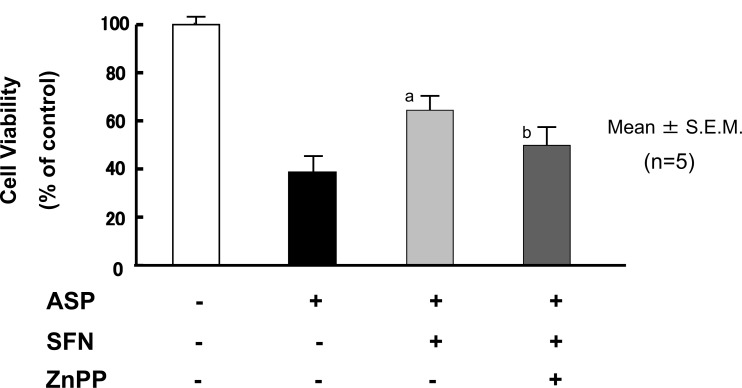
The cells were exposed for 6 h to 5 μM SFN in combination with 0.1 μM ZnPP, an HO-1 inhibitor, followed by incubation without SFN and ZnPP for a further 12 h. After this, the cells were exposed to 20 mM aspirin. Co-administration of ZnPP with SFN tended to attenuate the protective effects of SFN on the aspirin-induced IEC6 cell injury (Fig. 11), which indicated that HO-1 may contribute to the protective effect of SFN against aspirin-induced cell injury. Further experiments are required to confirm this assumption.
Fig. (11).
ZnPP, an Heme Oxygenase (HO)-1 inhibitor, Tends to Attenuate Protective Effects of Sulforaphane (SFN) against Aspirin-Induced Injury of IEC6 Cells
The cells were exposed to 5 μM SFN for 6 h, followed by incubation without SFN for a further 12 h. Western blot analysis shows that HO-1 expression increased significantly at 6 h after incubation with 5 μM SFN, and that this effect still persists at 12 h after removal of SFN from the medium. The data are expressed as Mean ± SEM. n=number of experiments; * P < 0.05; significant difference from the corresponding value in the absence of SFN.
The cells were initially exposed for 6 h to 5 μM SFN in combination with 0.1 mM ZnPP, an HO-1 inhibitor, followed by incubation without either agent for a further 12 h. The cells were then exposed to 20 mM aspirin. Co-administration of ZnPP with SFN attenuated the protective effects of SFN on aspirin-induced IEC6 cell injury. The data are expressed as Mean ± SEM. n=number of experiments; a, P < 0.05; significant difference from the corresponding value in the absence of SFN; b, P = 0.09; difference from the corresponding value in the absence of ZnPP.
5.2. Role of SFN in Protection of the Small Intestinal Mucosa against Indomethacin (IND)-Induced Injury in vivo
Male ddY mice aged 7 weeks were used in this study. Small intestinal injuries were induced by two subcutaneous injections of IND (20 mg/kg), as described elsewhere [57]. The time interval between the first and second IND injections was 24 h. The mice were sacrificed 6 h after the second IND injection. The small intestinal mucosa was carefully examined for lesions under a dissecting microscope.
MPO activity was measured by a previously described method [58]. Vascular permeability was assessed by measuring the amount of leakage of Evans blue into the small intestinal tissue, after intravenous injection of the dye at 1 h before sacrifice [59]. Enterobacteria within the small intestinal mucosa were quantified by a previously described method [60]. In brief, the homogenates of the small intestinal mucosa were placed on Gifu anaerobic medium agar and incubated for 24 h under anaerobic conditions. The number of enterobacteria was expressed as log CFU/g of tissue. SGS was orally administered at 0.5 h before and 6 h after the administration of IND.
5.2.1. Effects of IND on Small Intestinal Mucosae
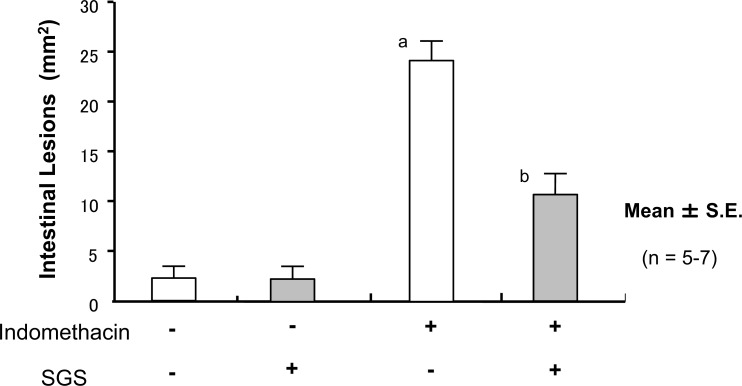
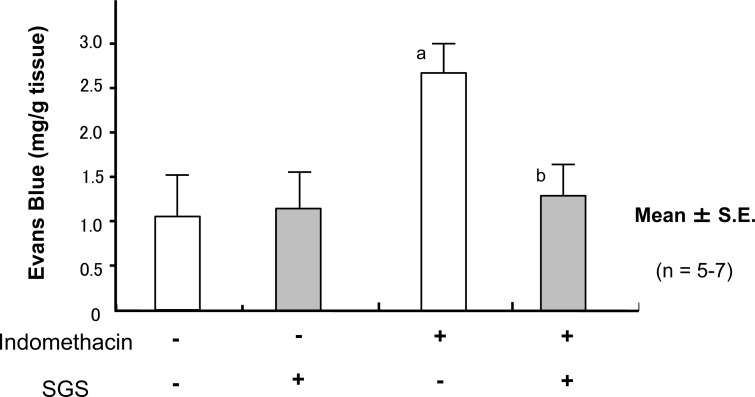
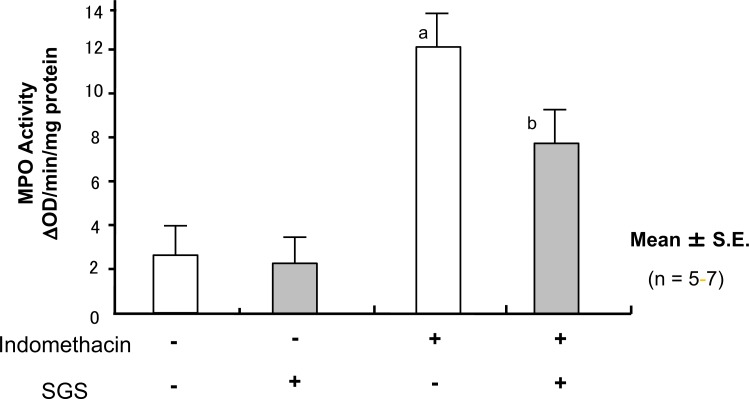
Treatment of the mice with IND induced small bowel shortening, increases in the lesion score (Fig. 12), vascular permeability (Fig. 13), and MPO activity (Fig. 14); this suggested that treatment with IND caused small intestinal mucosal injury in mice.
Fig. (12).
Oral Intake of Sulforaphane Glucosinolates (SGS) Ameliorates Indomethacin (IND)-Induced Injury of Small Intestine
Fig. (13).
Oral Intake of Sulforaphane Glucosinolates (SGS) Ameliorates Indomethacin (IND)-Induced Increase in Mucosal Permeability of Small Intestine in Mice
Fig. (14).
Oral Intake of Sulforaphane Glucosinolates (SGS) Blocks Indomethacin (IND)-Induced Increase in Myeloperoxidase (MPO) Activity of Small Intestinal Mucosa in Mice
Oral administration of 17 mg/kg SGS did not affect the lesion score in the IND-untreated mice. In contrast, pretreatment with SGS inhibited the increase in the lesion score in the IND-treated mice. The data are expressed as mean ± SEM. n=number of experiments; a, P<0.05; significant difference from the corresponding value without IND; b, P<0.05; significant difference from the corresponding value without SGS.
Oral administration of 17 mg/kg SDS did not influence the mucosal content of Evans blue in the IND-untreated negative control mice. In contrast, pretreatment of with SGS inhibited the increase in the mucosal content of Evans blue in the IND-treated mice. The data are expressed as mean ± SEM. n=number of experiments; a, P < 0.05; significant difference from the corresponding value without IND; b, P < 0.05; significant difference from the corresponding value without SGS.
5.2.2. Effect of SGS on the IND-induced Injuries in Small Intestinal Mucosa
Oral administration of SGS did not affect the lesion score, vascular permeability, or mucosal MPO activity in the IND-untreated negative control mice. In contrast, pretreatment of the mice with orally administrated SGS inhibited the increases in lesion score, vascular permeability, and MPO activity in the IND-treated mice (Fig. 12, 13, 14), suggesting that SGS attenuates IND-induced small intestinal injuries in mice.
Oral administration of 17 mg/kg SDS did not affect the mucosal MPO activity in the IND-untreated mice. In contrast, pretreatment with SGS inhibited the increase in the mucosal MPO activity in the IND-treated mice. The data are expressed as Mean ± SEM. n=number of experiments; a, P < 0.05; significant difference from the corresponding value without IND; b, P < 0.05; significant difference from the corresponding value without SGS.
5.2.3. Effect of SGS on Anaerobic Enterobacterial Count in Small Intestinal Mucosa of IND-Treated Mice
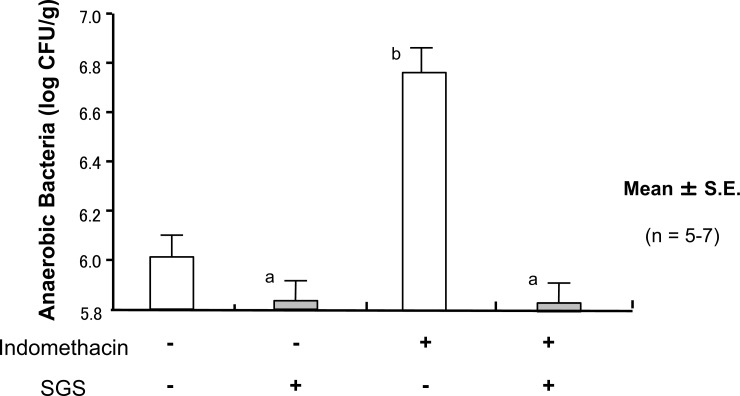
Oral administration of SGS significantly inhibited the overgrowth of anaerobic enterobacteria in IND-untreated mice. Furthermore, pretreatment of the mice with SGS completely abolished the increase in the overgrowth of anaerobic enterobacteria the IND-treated mice (Fig. 15).
Fig. (15).
Oral Intake of Sulforaphane Glucosinolates (SGS) Inhibits Indomethacin (IND)-Induced Increase in Anaerobic Bacteria of Small Intestinal Mucosa in Mice
Oral administration of 17 mg/kg SDS significantly inhibited amount of mucosal anaerobic enterobacteria in the IND-untreated mice. Pretreatment of the mice with SGS totally abolished the increase in the mucosal amount of anaerobic enterobacteria the IND-treated mice. The data are expressed as Mean ± SEM. n=number of experiments; a, P < 0.05; significant difference from the corresponding value without IND; b, P < 0.05; significant difference from the corresponding value without SGS.
6. Discussion for NSAID Study
The data from in vitro experiments show adaptive cytoprotection by SFN against aspirin in IEC6 cells. We have confirmed that SFN enhanced HO-1 expression in small intestinal mucosa in this experimental system. Furthermore, the present finding that the protective effects of SFN are mitigated by an HO-1 inhibitor, ZnPP, strongly support our hypothesis that SFN affords adaptive cytoprotection against aspirin via Nrf2-dependent pathways. It has been reported that aspirin causes oxidative stress in cells by damaging mitochondrial respiration [61]. Thus, we assume that pretreatment with SFN enhances the antioxidant system in the cells, thereby eliminating oxidative stress induced by aspirin. Our data from in vivo studies also showed that pretreatment with orally administrated SGS protects small intestinal mucosa against IND-induced injury, suggesting that SFN also provides adaptive cytoprotection to the small intestinal mucosa against IND-induced injury in vivo. The present study showed that SFN enhanced HO-1 expression in vitro. We also confirmed that SGS treatment enhanced HO-1 expression in small intestinal mucosa in mice in vivo (unpublished observations). In addition, the present study also indicated that SGS inhibited invasion of anaerobic enterobacteria into the mucosa in vivo. Thus, it seems reasonable to assume that SFN and/or SGS have twofold beneficial effects on small intestinal mucosa against IND-induced small intestinal injury.
It has been reported that mild irritants protect gastric mucosa from the ensuing severe stress by induction of endogenous prostaglandins, a phenomenon named “adaptive cytoprotection” by Andre Robert [62]. However, a number of other studies have shown that “adaptive cytoprotection” cannot be explained by endogenous prostaglandins. More recent studies have suggested possible candidates for adaptive cytoprotection. For example, it has been shown that mild stress induces heat shock proteins in gastric mucosa, thereby protecting the mucosa from severe stress, which also accounts for “adaptive cytoprotection” [63]. Alternatively, Yamamoto et al. have recently demonstrated that mild oxidative stress induces antioxidant enzymes via the Nrf2-Keap1 system, which strengthen antioxidant activity of the cells against more severe oxidative stress [64]. Since NSAIDs cause oxidative stress in cells [62, 65], it is reasonable to assume that SFN protects GI mucosa from NSAID-induced oxidative stress by strengthening Nrf2-Keap1 mediated antioxidant systems. Our findings strongly support this hypothesis [5]. In contrast, recent studies have suggested a role of anaerobic enterobacteria in the pathogenesis of NSAID-induced intestinal mucosa [47, 55, 56], which indicated that anaerobic bacteria generally exacerbate NSAID-induced injury in the small intestine, although the exact mechanisms remain to be elucidated. As SFN shows antibacterial activity against H. pylori [1, 4], it may be possible that SFN also demonstrates antibacterial activity against anaerobic enterobacteria in the small intestine, thereby ameliorating NSAID-induced small intestinal injury. In conclusion, we propose that SFN is a strong candidate for the prevention or treatment of aspirin/NSAID-induced small intestinal ulcers in clinical practice. Clinical trials of the effects of SFN on NSAID-induced injury in the small intestine of humans should be conducted imminently.
In summary, our data suggest that the activation of Nrf2-Keap1 dependent antioxidant enzyme activity by SFN contributed to protection of small intestinal mucosa against NSAID-induced oxidative stress. Our data also indicated that SFN ameliorates NSAID-induced small intestinal injuries by suppression of the invasion of anaerobic bacteria into the mucosa.
CONCLUSION
The present study strongly suggest that SFN contributes to the protection of GI mucosa against oxidative injury induced by H.pylori or NSAIDs.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Fahey J.W., Talalay P. Antioxidant functions of sulforaphane: a potent inducer of phase II detoxication enzymes. Food Chem. Toxicol. 1999;37:973–979. doi: 10.1016/s0278-6915(99)00082-4. [DOI] [PubMed] [Google Scholar]
- 2.Fahey J.W., Haristoy X., Dolan P.M., et al. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc. Natl. Acad. Sci. USA. 2002;99:7610–7615. doi: 10.1073/pnas.112203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yanaka A. Sulforaphane enhances protection and repair of gastric mucosa against oxidative stress in vitro, and demonstrates anti-inflammatory effects on Helicobacter pylori-infected gastric mucosae in mice and human subjects. Curr. Pharm. Des. 2011;17(16):1532–1540. doi: 10.2174/138161211796196945. [DOI] [PubMed] [Google Scholar]
- 4.Yanaka A., Fahey J.W., Fukumoto A., et al. Dietary sulforaphane rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-infected mice and humans. Cancer Prev. Res. (Phila.) 2009;2(4):353–360. doi: 10.1158/1940-6207.CAPR-08-0192. [DOI] [PubMed] [Google Scholar]
- 5.Yanaka A., Sato J., Ohmori S. Sulforaphane protects small intestinal mucosa from aspirin/NSAID-induced injury by enhancing host defense systems against oxidative stress and by inhibiting mucosal invasion of anaerobic enterobacteria. Curr. Pharm. Des. 2013;19(1):157–162. doi: 10.2174/13816128130120. [DOI] [PubMed] [Google Scholar]
- 6.Diet, nutrition, and the prevention of chronic diseases. Report of a Joint WHO/FAO Expert Consultation. WHO Technical Report Series, No. 916. Geneva: World Health Organization; 2003. Available from: http://whqlibdoc.who.int/trs/ WHO_TRS_916.pdf. 2003 [PubMed]
- 7.Fahey J.W., Kensler T.W. Role of dietary supplements/nutraceuticals in chemoprevention through induction of cytoprotective enzymes. Chem. Res. Toxicol. 2007;20:572–576. doi: 10.1021/tx7000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe T., Tada M., Nagai H., Sasaki S., Nakao M. Helicobacter pylori infection induces gastric cancer in Mongolian gerbils. Gastroenterology. 1998;115:642–648. doi: 10.1016/s0016-5085(98)70143-x. [DOI] [PubMed] [Google Scholar]
- 9.Uemura N., Okamoto S., Yamamoto S., et al. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 10.Lee S.A., Kang D., Shim K.N., Choe J.W., Hong W.S., Choi H. Effect of diet and Helicobacter pylori infection to the risk of early gastric cancer. J. Epidemiol. 2003;13:162–168. doi: 10.2188/jea.13.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox J.G., Dangler C.A., Taylor N.S., King A., Koh T.J., Wang T.C. High-salt diet induces gastric epithelial hyperplasia and parietal cell loss, and enhances Helicobacter pylori colonization in C57BL/6 mice. Cancer Res. 1999;59:4823–4828. [PubMed] [Google Scholar]
- 12.IARC . Cruciferous vegetables, isothiocyanates and indoles. International Agency for Research on Cancer, World Health Organization. Vol. 9. Lyon: IARC Press; 2004. p. 262. (IARC Handbooks of Cancer Prevention). [Google Scholar]
- 13.Fahey J.W., Zhang Y., Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fahey J.W., Zalcmann A.T., Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- 15.Hecht S.S., Kenney P.M., Wang M., Upadhyaya P. Benzyl isothiocyanate: an effective inhibitor of polycyclic aromatic hydrocarbon tumorigenesis in A/J mouse lung. Cancer Lett. 2002;187:87–94. doi: 10.1016/s0304-3835(02)00410-x. [DOI] [PubMed] [Google Scholar]
- 16.Conaway C.C., Wang C.X., Pittman B., et al. Phenethyl isothiocyanate and sulforaphane and their N-acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice. Cancer Res. 2005;65:8548–8557. doi: 10.1158/0008-5472.CAN-05-0237. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y., Tang L. Discovery and development of sulforaphane as a cancer chemopreventive phytochemical. Acta Pharmacol. Sin. 2007;28:1343–1354. doi: 10.1111/j.1745-7254.2007.00679.x. [DOI] [PubMed] [Google Scholar]
- 18.Ramos-Gomez M., Kwak M.K., Dolan P.M., et al. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in Nrf2 transcription factor-deficient mice. Proc. Natl. Acad. Sci. USA. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu X., Kensler T. Nrf2 as a target for cancer chemoprevention. Mutat. Res. 2005;591:93–102. doi: 10.1016/j.mrfmmm.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Wakabayashi N., Dinkova-Kostova A.T., Holtzclaw W.D., et al. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc. Natl. Acad. Sci. USA. 2004;101:2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haristoy X., Angioi-Duprez K., Duprez A., Lozniewski A. Efficacy of sulforaphane in eradicating Helicobacter pylori in human gastric xenografts implanted in nude mice. Antimicrob. Agents Chemother. 2003;47:3982–3984. doi: 10.1128/AAC.47.12.3982-3984.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galan M.V., Kishan A.A., Silverman A.L. Oral broccoli sprouts for the treatment of Helicobacter pylori infection: a preliminary report. Dig. Dis. Sci. 2004;49:1088–1090. doi: 10.1023/b:ddas.0000037792.04787.8a. [DOI] [PubMed] [Google Scholar]
- 23.Kameyama M., Mino K., Fukumoto A., Yanaka A. Sulforaphane suppress Helicobacter pylori activity and increases metronidazole susceptibility in metronidazole-resistant strains in vitro.; Annual Meeting in American Gastroenterological Association, Digestive Diseases Week; 2009. [Google Scholar]
- 24.Lee A., O’Rourke J., De Ungria M.C., Robertson B., Daskalopoulos G., Dixon M.F. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 25.Dixon M.F., Genta R.M., Yardley J.H., Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I., Yamamoto M., Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236(2):313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 27.Kensler T.W., Chen J.G., Egner P.A., et al. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People’s Republic of China. Cancer Epidemiol. Biomarkers Prev. 2005;14:2605–2613. doi: 10.1158/1055-9965.EPI-05-0368. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro T.A., Fahey J.W., Wade K.L., Stephenson K.K., Talalay P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: metabolism and excretion in humans. Cancer Epidemiol. Biomarkers Prev. 2001;10:501–508. [PubMed] [Google Scholar]
- 29.Adiloglu A.K., Isler M., Goren I., et al. Quantitative correlation of Helicobacter pylori stool antigen (HpSA) test with the severity of H. pylori-related gastritis. Tohoku J. Exp. Med. 2007;212:159–167. doi: 10.1620/tjem.212.159. [DOI] [PubMed] [Google Scholar]
- 30.Maconi G., Lazzaroni M., Sangaletti O., Bargiggia S., Vago L., Bianchi Porro G. Effect of Helicobacter pylori eradication on gastric histology, serum gastrin and pepsinogen I levels, and gastric emptying in patients with gastric ulcer. Am. J. Gastroenterol. 1997;92:1844–1848. [PubMed] [Google Scholar]
- 31.Ohkusa T, Miwa H, Nomura T, et al. 2004.
- 32.Hecht S.S., Kenney P.M., Wang M., Upadhyaya P. Benzyl isothiocyanate: an effective inhibitor of polycyclic aromatic hydrocarbon tumorigenesis in A/J mouse lung. Cancer Lett. 2002;187:87–94. doi: 10.1016/s0304-3835(02)00410-x. [DOI] [PubMed] [Google Scholar]
- 33.Conaway C.C., Wang C.X., Pittman B., et al. Phenethyl isothiocyanate and sulforaphane and their N-acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice. Cancer Res. 2005;65:8548–8557. doi: 10.1158/0008-5472.CAN-05-0237. [DOI] [PubMed] [Google Scholar]
- 34.Shen G., Khor T.O., Hu R., et al. Chemoprevention of familial adenomatous polyposis by natural dietary compounds sulforaphane and dibenzoylmethane alone and in combination in Apc Min/+ mouse. Cancer Res. 2007;67:9937–9944. doi: 10.1158/0008-5472.CAN-07-1112. [DOI] [PubMed] [Google Scholar]
- 35.Myzak M.C., Dashwood W.M., Orner G.A., Ho E., Dashwood R.H. Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apc-minus mice. FASEB J. 2006;20:506–508. doi: 10.1096/fj.05-4785fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fahey J.W., Kensler T.W. Role of dietary supplements/nutraceuticals in chemoprevention through induction of cytoprotective enzymes. Chem. Res. Toxicol. 2007;20:572–576. doi: 10.1021/tx7000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu X., Kensler T. Nrf2 as a target for cancer chemoprevention. Mutat. Res. 2005;591:93–102. doi: 10.1016/j.mrfmmm.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 38.Talalay P., Talalay P. The importance of using scientific principles in the development of medicinal agents from plants. Acad. Med. 2001;76:238–247. doi: 10.1097/00001888-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Williams A., Hennekens C.H. The role of aspirin in cardiovascular diseases--forgotten benefits? Expert Opin. Pharmacother. 2004;5(1):109–115. doi: 10.1517/14656566.5.1.109. [DOI] [PubMed] [Google Scholar]
- 40.Hennekens C.H. Aspirin in the treatment and prevention of cardiovascular disease: current perspectives and future directions. Curr. Atheroscler. Rep. 2007;9(5):409–416. doi: 10.1007/s11883-007-0053-0. [DOI] [PubMed] [Google Scholar]
- 41.Buffum M., Buffum J.C. Nonsteroidal anti-inflammatory drugs in the elderly. Pain Manag. Nurs. 2000;1(2):40–50. doi: 10.1053/jpmn.2000.7779. [DOI] [PubMed] [Google Scholar]
- 42.Klotz U. Proton pump inhibitors--their pharmacological impact on the clinical management of acid-related disorders. Arzneimittelforschung. 2009;59(6):271–282. doi: 10.1055/s-0031-1296397. [DOI] [PubMed] [Google Scholar]
- 43.Maiden L., Thjodleifsson B., Seigal A., et al. Long-term effects of nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 selective agents on the small bowel: a cross-sectional capsule enteroscopy study. Clin. Gastroenterol. Hepatol. 2007;5(9):1040–1045. doi: 10.1016/j.cgh.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 44.Fujimori S., Gudis K., Takahashi Y., et al. Distribution of small intestinal mucosal injuries as a result of NSAID administration. Eur. J. Clin. Invest. 2010;40(6):504–510. doi: 10.1111/j.1365-2362.2010.02290.x. [DOI] [PubMed] [Google Scholar]
- 45.Tachecí I., Kvetina J., Bures J., et al. Wireless capsule endoscopy in enteropathy induced by nonsteroidal anti-inflammatory drugs in pigs. Dig. Dis. Sci. 2010;55(9):2471–2477. doi: 10.1007/s10620-009-1066-z. [DOI] [PubMed] [Google Scholar]
- 46.Graham D.Y., Opekun A.R., Willingham F.F., Qureshi W.A. Visible small-intestinal mucosal injury in chronic NSAID users. Clin. Gastroenterol. Hepatol. 2005;3(1):55–59. doi: 10.1016/s1542-3565(04)00603-2. [DOI] [PubMed] [Google Scholar]
- 47.Wallace J.L., Syer S., Denou E., et al. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology. 2011;141(4):1314–1322. doi: 10.1053/j.gastro.2011.06.075. [DOI] [PubMed] [Google Scholar]
- 48.Fujimori S., Seo T., Gudis K., et al. Prevention of nonsteroidal anti-inflammatory drug-induced small-intestinal injury by prostaglandin: a pilot randomized controlled trial evaluated by capsule endoscopy. Gastrointest. Endosc. 2009;69(7):1339–1346. doi: 10.1016/j.gie.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 49.Watanabe T., Sugimori S., Kameda N., et al. Small bowel injury by low-dose enteric-coated aspirin and treatment with misoprostol: a pilot study. Clin. Gastroenterol. Hepatol. 2008;6(11):1279–1282. doi: 10.1016/j.cgh.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 50.Bianchi Porro G, Parente F. Side effects of anti-ulcer prostaglandins: an overview of the worldwide clinical experience. 1989. [DOI] [PubMed]
- 51.Iwai T., Ichikawa T., Kida M., et al. Protective effect of geranylgeranylacetone against loxoprofen sodium-induced small intestinal lesions in rats. Eur. J. Pharmacol. 2011;652(1-3):121–125. doi: 10.1016/j.ejphar.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 52.Niwa Y., Nakamura M., Miyahara R., et al. Geranylgeranylacetone protects against diclofenac-induced gastric and small intestinal mucosal injuries in healthy subjects: a prospective randomized placebo- controlled double-blind cross-over study. Digestion. 2009;80(4):260–266. doi: 10.1159/000236032. [DOI] [PubMed] [Google Scholar]
- 53.Fujimori S., Takahashi Y., Gudis K., et al. Rebamipide has the potential to reduce the intensity of NSAID-induced small intestinal injury: a double-blind, randomized, controlled trial evaluated by capsule endoscopy. J. Gastroenterol. 2011;46(1):57–64. doi: 10.1007/s00535-010-0332-3. [DOI] [PubMed] [Google Scholar]
- 54.Niwa Y., Nakamura M., Ohmiya N., et al. Efficacy of rebamipide for diclofenac- induced small-intestinal mucosal injuries in healthy subjects: a prospective, randomized, double-blinded, placebo-controlled, cross-over study. J. Gastroenterol. 2008;43(4):270–276. doi: 10.1007/s00535-007-2155-4. [DOI] [PubMed] [Google Scholar]
- 55.Watanabe T., Higuchi K., Kobata A., et al. Non-steroidal anti-inflammatory drug-induced small intestinal damage is Toll-like receptor 4 dependent. Gut. 2008;57(2):181–187. doi: 10.1136/gut.2007.125963. [DOI] [PubMed] [Google Scholar]
- 56.Hagiwara M., Kataoka K., Arimochi H., Kuwahara T., Ohnishi Y. Role of unbalanced growth of gram-negative bacteria in ileal ulcer formation in rats treated with a nonsteroidal anti-inflammatory drug. J. Med. Invest. 2004;51(1-2):43–51. doi: 10.2152/jmi.51.43. [DOI] [PubMed] [Google Scholar]
- 57.Miura N., Fukutake M., Yamamoto M., Ohtake N., Iizuka S., Imamura S., Tsuchiya N., Ishimatsu M., Nakamura Y., Ishige A., Watanabe K., Kase Y., Takeda S. An herbal edicine Orengedokuto prevents indomethacin-induced enteropathy. Biol. Pharm. Bull. 2007;30(3):495–501. doi: 10.1248/bpb.30.495. [DOI] [PubMed] [Google Scholar]
- 58.Bradley P.P., Priebat D.A., Christensen R.D., Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 1982;78(3):206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 59.Lange S., Delbro D.S., Jennische E. Evans blue permeation of intestinal mucosa in the rat. Scand. J. Gastroenterol. 1994;29(1):38–46. doi: 10.3109/00365529409090435. [DOI] [PubMed] [Google Scholar]
- 60.Deitch E.A., Ma L., Ma W.J., et al. Inhibition of endotoxin-induced bacterial translocation in mice. J. Clin. Invest. 1989;84:36–42. doi: 10.1172/JCI114164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raza H., John A., Benedict S. Acetylsalicylic acid-induced oxidative stress, cell cycle arrest, apoptosis and mitochondrial dysfunction in human hepatoma HepG2 cells. Eur. J. Pharmacol. 2011;668(1-2):5–24. doi: 10.1016/j.ejphar.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 62.Robert A., Nezamis J.E., Lancaster C., Davis J.P., Field S.O., Hanchar A.J. Mild irritants prevent gastric necrosis through “adaptive cytoprotection” mediated by prostaglandins. Am. J. Physiol. 1983;245(1):G113–G121. doi: 10.1152/ajpgi.1983.245.1.G113. [DOI] [PubMed] [Google Scholar]
- 63.Nakamura K., Rokutan K., Marui N., Aoike A., Kawai K. Induction of heat shock proteins and their implication in protection against ethanol-induced damage in cultured guinea pig gastric mucosal cells. Gastroenterology. 1991;101(1):161–166. doi: 10.1016/0016-5085(91)90473-x. [DOI] [PubMed] [Google Scholar]
- 64.Taguchi K., Motohashi H., Yamamoto M. Molecular mechanisms of the Keap1–Nrf2 pathway in stress response and cancer evolution. Genes Cells. 2011;16(2):123–140. doi: 10.1111/j.1365-2443.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- 65.Narushima S., Spitz D.R., Oberley L.W., Toyokuni S., Miyata T., Gunnett C.A., Buettner G.R., Zhang J., Ismail H., Lynch R.G., Berg D.J. Evidence for oxidative stress in NSAID-induced colitis in IL10-/- mice. Free Radic. Biol. Med. 2003;34(9):1153–1166. doi: 10.1016/s0891-5849(03)00065-0. [DOI] [PubMed] [Google Scholar]