Abstract
Background
Evidence concerning the efficacy and safety of extracorporeal membrane oxygenation (ECMO) in patients with influenza A (H7N9) has been was limited to case reports. Our study is aimed to investigate the current application, efficacy and safety of ECMO in for severe H7N9 pneumonia-associated acute respiratory distress syndrome (ARDS) in the Chinese population.
Methods
A multicentre retrospective cohort study was conducted at 20 hospitals that admitted patients with avian influenza A (H7N9) viral pneumonia patients’ admission from 9 provinces in China between October 1, 2016, and March 1, 2017. Data from the National Health and Family Planning Commission of China, including general conditions, outcomes and ECMO management, were analysed. Then, successfully weaned and unsuccessfully weaned groups were compared.
Results
A total of 35 patients, aged 57 ± 1 years, were analysed; 65.7% of patients were male with 63% mortality. All patients underwent invasive positive pressure ventilation (IPPV), and rescue ventilation strategies were implemented for 23 cases (65.7%) with an average IPPV duration of 5 ± 1 d, PaO2/FiO2 of 78 ± 23 mmHg, tidal volume (VT) of 439 ± 61 ml and plateau pressure (Pplat) of 29 ± 8 cmH2O pre-ECMO. After 48 h on ECMO, PaO2 improved from 56 ± 21 mmHg to 90 ± 24 mmHg and PaCO2 declined from 52 ± 24 mmHg to 38 ± 24 mmHg. Haemorrhage, ventilator-associated pneumonia (VAP) and barotrauma occurred in 45.7%, 60% and 8.6% of patients, respectively. Compared with successfully weaned patients (n = 14), the 21 unsuccessfully weaned patients had a longer duration of IPPV pre-ECMO (6 ± 4 d vs. 2 ± 1 d, P < 0.01) as well as a higher Pplat (25 ± 5 cmH2O vs. 21 ± 3 cmH2O, P < 0.05) and VT (343 ± 96 ml vs. 246 ± 93 ml, P < 0.05) after 48 h on ECMO support. Furthermore, the unsuccessfully weaned group had a higher mortality (100% vs. 7.1%, P < 0.01) with more haemorrhage (77.3% vs. 28.6%, P < 0.01).
Conclusions
ECMO is effective at improving oxygenation and ventilation of patients with avian influenza A (H7N9) induced severe ARDS. Early initiation of ECMO with appropriate IPPV settings and anticoagulation strategies are necessary to reduce complications.
Background
Avian influenza A (H7N9) viral pneumonia can manifest with varying degrees of dyspnea and is associated with a mortality of ~30% [1]. In particular, 97% of patients develop rapidly progressive pneumonia and 71% progress to acute respiratory distress syndrome (ARDS). The mortality of severe ARDS is as high as 62% [2].
Timely and effective respiratory support is particularly important to treat severe ARDS caused by avian influenza A (H7N9) pneumonia. However, severe ARDS induced by avian influenza A (H7N9) pneumonia might manifest as refractory hypoxaemia even with appropriate invasive positive pressure ventilation (IPPV) support. Extracorporeal membrane oxygenation (ECMO) is the ultimate respiratory support method and directly improves the oxygenation and ventilation of patients as well as enables implementation of the “lung protective ventilation strategy” [3]. ECMO was the breakthrough treatment for the severe avian influenza A (H1N1) outbreak of 2009 and reduced mortality from this outbreak [4–6]. Therefore, we believe that ECMO could also be effective for other types of severe viral pneumonia.
Existing studies of ECMO treatment for avian influenza A (H7N9) pneumonia are primarily limited to case reports [7–9], and no study has systematically reviewed the efficacy or safety of ECMO to treat such diseases. Therefore, it is particularly important to understand the current application of ECMO for avian influenza A (H7N9) pneumonia-induced severe ARDS, investigate the application timing and management strategies of ECMO, and explore the possible reasons for treatment failure. Based on the current study, we expect to standardize the management of ECMO and provide a description of our experiences using ECMO to treat patients with avian influenza A (H7N9) pneumonia-induced severe ARDS.
Methods
Study population and data collection
Patients who had laboratory-confirmed avian influenza A (H7N9) virus-induced pneumonia were included in this study. Patients were admitted to 20 hospitals in 9 provinces of China between October 1, 2016, and March 1, 2017, and were reported to the National Health and Family Planning Commission of China.
We included patients aged >14 ys who were supported by ECMO. Patients who were lacking key detailed records of parameters during ECMO, such as ventilator or laboratory findings, were excluded.
The included patients were divided into 2 groups, namely, the “successfully weaned group” and “unsuccessfully weaned group”. The former refers to a group of patients whose condition improved and were weaned from ECMO for at least 48 h; the “unsuccessfully weaned group” refers to those who died or voluntarily discontinued treatment due to lack of improvement during ECMO support.
General conditions
The general conditions included age, gender, pregnancy status, underlying disease, time from onset to antiviral drug administration, vasoactive drug administration pre-ECMO, duration of IPPV pre-ECMO, whether rescue ventilation strategies (including lung recruitment maneuvre, prone-position ventilation, and high-frequency oscillation ventilation) were implemented pre-ECMO, disease severity score, total duration of ECMO and IPPV.
Conditions during ECMO
We collected the ECMO blood flow at 24, 48, 72, and 96 h on ECMO. Improvement in circulatory and respiratory physiological indicators were considered, as well as IPPV parameters at 6 h pre-ECMO and 24, 48, and 72 h on ECMO. Furthermore, anticoagulation indicators during ECMO, including the types of anticoagulant drugs and methods of use; the maximum and minimum values of the activated coagulation time (ACT) and activated partial thromboplastin time (APTT); and the differences between the maximum and minimum ACT and APTT at 24, 48, and 72 h on ECMO were recorded. Finally, data regarding complications during ECMO therapy, including ECMO and IPPV-related complications and nosocomial infections, were collected.
Study definitions and design
Outcomes
The primary outcome was in-hospital mortality. The secondary outcomes were the length of stay in the intensive care unit (ICU) and total length of hospitalization.
Definition of avian influenza A (H7N9) virus infection
Three methods were used for a laboratory diagnosis, namely, the real-time reverse transcription-polymerase chain reaction (RT-PCR), viral isolation, and serological testing for the avian influenza A (H7N9) virus using a modified haemagglutinin inhibition assay [10–12].
Definition of acute respiratory syndrome (ARDS)
We defined ARDS according to the Berlin definition in 2012 [13, 14].
Definitions of pneumonia and severe pneumonia
Pneumonia was diagnosed as an acute illness with fever, cough, or dyspnea/tachypnea, and at least one new focal chest sign that was supported by a finding of lung shadowing on a chest radiograph and without other non-infectious causes.
The primary criteria for severe pneumonia were as follows: <1 > need for tracheal intubation and mechanical ventilation (MV) and <2 > need for vasoactive drugs after the active fluid resuscitation due to septic shock. The secondary criteria were as follows: <1 > respiratory rate ≥ 30 times/min; <2 > PaO2/FiO2 ≤ 250 mmHg; <3 > multiple lobe infiltration; <4 > disturbances of consciousness, disorientation, or both; <5 > blood urea nitrogen ≥ 7.14 mmol/L; and <6 > systolic blood pressure ≤ 90 mmHg that required active fluid resuscitation. Patients who met one primary criterion or at least three secondary criteria were diagnosed as having severe pneumonia [15].
Definitions of ventilator-associated pneumonia (VAP)
The criteria for the diagnosis of VAP are in accordance with the European Centre for Disease Prevention and Control [16] and included the following: <1 > two or more sequential chest x-rays or CT scans with a suggestive image of pneumonia for patients with underlying cardiac or pulmonary disease, or one definitive chest x-ray or CT scan in patients without underlying cardiac or pulmonary disease; <2 > a fever greater than 38 °C and/or leukocytosis greater than or equal to 12,000 WBC/mm3 or leukopenia less than or equal to 4000 WBC/mm3; and <3 > at least one of the following: <a > new onset of purulent sputum or change in the characteristics of the sputum; <b > cough, dyspnea, or tachycardia; <c > auscultatory findings, such as rales, bronchial breath sounds, ronchi, or wheezing; or <d > worsening gas exchange (e.g., oxygen desaturation or increased oxygen requirements or increased ventilation demand).
For all included patients, we first described the general conditions, ECMO model and parameters, IPPV parameters, the changes in circulation and respiratory physiological indicators from pre-ECMO to on ECMO status, anticoagulation on ECMO, and complications during ECMO therapy in all included patients. Then, we compared patients who were successfully or unsuccessfully weaned from ECMO with regard to above items.
Statistical analysis
All of the analyses were performed using SPSS 17.0 software. Normally distributed continuous variables are expressed as the means ± SD and were compared using the t-test or chi-square test. Non-normally distributed continuous variables are expressed as medians and quartiles and were compared using the Wilcoxon rank-sum test. Categorical variables were compared using the x2 test. P-values < 0.05 were considered significant.
Results
A total of 473 patients were diagnosed with avian influenza A (H7N9) virus-related pneumonia. Patients were admitted to 20 hospitals in 9 provinces of China between October 1, 2016, and March 1, 2017, and were reported to the National Health and Family Planning Commission of China. The medical records of 216 patients were available, and 36 patients were reported to be supported by ECMO. One of the 36 patients lacked IPPV and ECMO parameters pre-ECMO and on ECMO and was eliminated as a participant; therefore, 35 patients were ultimately selected (Fig. 1).
Fig. 1.
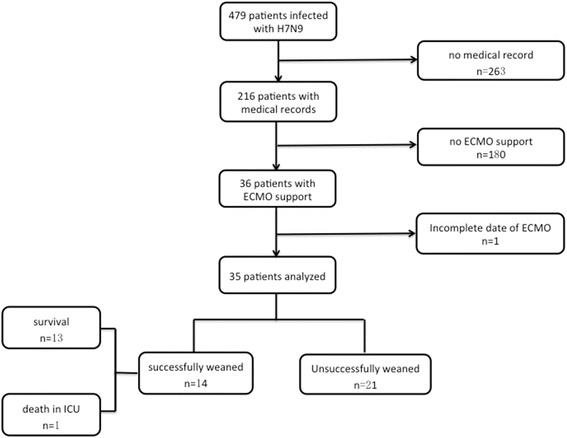
Flowchart. A flowchart illustrating the enrolment of patients with avian influenza A (H7N9) virus-induced pneumonia who were reported to the National Health and Family Planning Commission of China; patients were admitted to 20 hospitals in 9 provinces of China between October 1, 2016, and March 1, 2017
General conditions and outcomes
Data from 35 patients (65.7% males), with an average age of 57 ± 1 years, were analysed. There was no patient under the age of 16. A total of 22 patients had underlying diseases, 8 patients were treated with steroids and immunosuppressive agents within 1 month of admission to the hospital, and 1 patient was pregnant. The Sequential Organ Failure Assessment (SOFA) score was 9 ± 3 points, and the Murray score was 3.6 ± 0.4 points. The time from onset to antiviral drug administration was approximately 9 ± 5 d, and the time from onset to ECMO support was approximately 10 ± 3 d. High-dose vasoactive drugs [17] were needed to maintain blood pressure in 20 patients (57.1%). The duration of IPPV pre-ECMO was approximately 5 ± 1 d. Rescue ventilation strategies, including the recruitment manoeuvre (RM), prone-position ventilation (PP) and high frequency oscillatory ventilation (HFOV), were needed for 23 patients (65.7%) pre-ECMO. The total durations of IPPV and ECMO were approximately 20 ± 8 d and 8 d (5–13 d), respectively. Of the 35 patients, 14 (40%) were successfully weaned from ECMO, and the other 21 patients died due to an uncontrolled haemorrhage (5 patients), septic shock (6 patients due to progressive lung infection, 3 patients due to bloodstream infection), heart failure (2 patients) and discontinuation of treatment because of no improvement (5 patients). One of 14 patients showed an aggregated lung infection after weaning and eventually died due to septic shock. The in-hospital mortality was 63%. The length of ICU stay was 27 ± 14 d, and the total length of hospitalization was 31 ± 14 d (Table 1).
Table 1.
General conditions and outcomes of patients with ECMO therapy
| Variable | Total (n = 35) | Successfully weaned group (n = 14) | Unsuccessfully weaned group (n = 21) | P value |
|---|---|---|---|---|
| Gender, number (%) | ||||
| Male | 23(65.7) | 9(64.3) | 14(66.7) | 1.00 |
| Age, years, mean ± SD | 57 ± 1 | 51 ± 10 | 60 ± 12 | 0.02* |
| Underlying Diseases, number (%) | ||||
| Hypertension | 19(54.3) | 7(50) | 12(57.1) | 0.74 |
| Diabetes | 13(37.1) | 1(7.1) | 12(57.1) | < 0.01* |
| Cerebrovascular disease | 5(14.3) | 3(21.4) | 2(9.5) | 0.37 |
| History of steroid and immunosuppressant within 1 month | 8(22.9) | 4(28.6) | 4(19.0) | 0.69 |
| Pregnancy, number (%) | 1(2.9) | 0(0.0) | 1(47.6) | 1.00 |
| Onset to NAI | 9 ± 5 | 7 ± 3 | 11 ± 6 | 0.04* |
| Onset to ECMO | 10 ± 3 | 10 ± 3 | 10 ± 3 | 0.97 |
| Organ support pre-ECMO | ||||
| High-dose vasoactive drugsa, number (%) | 20(57.1) | 6(42.9) | 14(63.6) | 0.31 |
| Rescue ventilation strategiesb, number (%) | 23(65.7) | 6(43.9) | 17(81.0) | 0.03* |
| Duration of IPPV, days, mean ± SD | 5 ± 1 | 2 ± 1 | 6 ± 4 | < 0.01* |
| SOFA Score | 9 ± 3 | 7 ± 2 | 10 ± 4 | 0.02* |
| Murray Score | 3.6 ± 0.4 | 3.6 ± 0.5 | 3.6 ± 0.4 | 0.95 |
| PaO2/FiO2 pre-ECMO, mmHg, mean ± SD | 78 ± 23 | 89 ± 24 | 71 ± 20 | 0.04* |
| Duration of ECMO, days, median (IQR) | 8(5–13) | 10(7–13) | 7(3–16) | 0.48 |
| Total Duration of IPPV, days, mean ± SD | 20 ± 8 | 23 ± 13 | 18 ± 15 | 0.36 |
| Clinical outcomes | ||||
| Mortality, number (%) | 22 (63) | 1 (7.1) | 21 (100) | < 0.01* |
| Length of stay in ICU, days, mean ± SD | 27 ± 14 | 33 ± 12 | 24 ± 14 | 0.081 |
| Length of hospitalization, days, mean ± SD | 31 ± 14 | 39 ± 10 | 27 ± 14 | 0.010* |
Note: ahigh-dose vasoactive drugs [17] infer to: noradrenaline > 0.5 μg/kg/min or dopamine > 20 μg/kg/min; brescue ventilation strategies includes recruitment maneuver (RM), prone position ventilation (PP) and high frequency oscillatory ventilation (HFOV)
NAI neuraminidase inhibitors, IPPV invasive positive pressure ventilation, ECMO extracorporeal membrane oxygenation, SOFA score sequential organ failure score, ICU intensive care unit
We made comparison between successfully weaned and unsuccessfully weaned group using t-test, x2 test or Wilcoxon rank-sum test, and P values <0.05 were considered significant, marked as *
Conditions during ECMO
ECMO mode and information concerning the equipment
Of the 35 patients, 33 were treated using the veno-venous ECMO (V-V ECMO) model. A total of 2 patients with severe cardiac insufficiency and cardiogenic shock were treated using the venous-arterial ECMO (V-A ECMO) model. The ECMO equipment was mainly provided by MAQUET (Shanghai) Medical Equipment Co., Ltd. and SORIN (Shanghai) Medical Equipment Co., Ltd. The pump from SORIN was the Stockert Centrifugal Pump System (SCP/SCPC), and the oxygenator was the D905 EOS ECMO. The pump from MAQUET was the ROTAFLOW, and the oxygenator was the QUADROX PLS.
ECMO management
Changes in IPPV parameters and physiological indicators in patients on ECMO
The ventilator parameters, including FiO2, positive end-expiratory pressure (PEEP), Pplat, and VT, were significantly decreased in patients on ECMO. The vital signs, which included the heart rate, respiratory rate, and SpO2, and the arterial blood gas analysis (ABG), which included the pH, PaCO2, and PaO2 levels, were improved in patients after ECMO support (Table 2).
Table 2.
Parameters on ECMO, IPPV parameters and physiological indicators pre and on ECMO
| Variable | 6 h pre-ECMO | 24 h on ECMO | 48 h on ECMO | 72 h on ECMO | P value |
|---|---|---|---|---|---|
| Blood flow (L/min) | – | 3.84 ± 0.87 | 4.48 ± 0.77 | 4.18 ± 1.00 | 0.023* |
| IPPV parameters | |||||
| FiO2 | 94 (55–100) | 50 (40–80) | 45 (40–60) | 46 (40–50) | <0.001* |
| PEEP (cmH2O) | 14 ± 4 | 12 ± 5 | 11 ± 3 | 11 ± 2 | 0.022* |
| VT (ml) | 439 ± 61 | 285 ± 94 | 317 ± 103 | 308 ± 128 | <0.001* |
| Pplat (cmH2O) | 29 ± 8 | 23 ± 6 | 23 ± 5 | 24 ± 7 | 0.004* |
| Physiological indicators | |||||
| Heart Rate (/min) | 103 ± 27 | 90 ± 24 | 85 ± 22 | 90 ± 25 | 0.025* |
| MAP (mmHg) | 84 ± 15 | 87 ± 15 | 81 ± 21 | 94 ± 17 | 0.057 |
| Respiratory Rate (/min) | 31 ± 7 | 19 ± 6 | 16 ± 6 | 16 ± 6 | <0.001* |
| pH | 7.33 ± 0.10 | 7.42 ± 0.10 | 7.39 ± 0.10 | 7.43 ± 0.10 | 0.006* |
| PaCO2 (mmHg) | 52.4 ± 16.6 | 34.1 ± 9.8 | 38.3 ± 10.5 | 38.2 ± 7.8 | <0.001* |
| PaO2 (mmHg) | 56.3 ± 20.9 | 90.0 ± 35.9 | 94.8 ± 45.7 | 104.4 ± 57.5 | <0.001* |
| Lactate (mmol/L) | 2.8 ± 1.9 | 3.1 ± 3.7 | 4.0 ± 4.8 | 2.9 ± 2.7 | 0.625 |
Note: ECMO extracorporeal membrane oxygenation, IPPV invasive positive pressure ventilation, FiO2 fraction of inspiration, PEEP positive end-expiratory pressure, VT tidal volume, Pplat plateau pressure, MAP mean arterial pressure, SpO2 fingertip pulse oxygen saturation, PaCO2 partial pressure of arterial carbon dioxide, PaO2 partial pressure of arterial oxygen
*P < 0.05
Monitoring of anticoagulation
All 35 patients received a continuous infusion of unfractionated heparin for anticoagulation. However, heparin was discontinued for 2 patients with cerebral haemorrhage and 3 with active gastrointestinal haemorrhage. The ACT was 144 ± 61 to 220 ± 66 s at 24 h, 16 ± 37 to 234 ± 37 s at 48 h, and 147 ± 26 to 221 ± 40 s at 72 h on ECMO. The APTT was 58 ± 23 to 84 ± 28 s and 53 ± 15 to 75 ± 23 s at 48 and 72 h on ECMO, respectively.
Complications during ECMO therapy
In this study, the rates of gastrointestinal haemorrhage, cerebral haemorrhage, brain death, renal insufficiency, disseminated intravascular coagulation (DIC), hyperglycaemia, and ECMO oxygenator thrombosis were higher compared to the relevant data from the ECLS Registry Report [18, 19]. New cases of VAP developed in 21 patients during ECMO, with an incidence rate of 60%. New cases of barotrauma occurred in 3 patients, accounting for 8.6% of cases. In addition, 6 patients had a urinary infection, with an incidence rate of 17.1%, and 10 patients had a catheter-related blood stream infection (CRBSI), with an incidence rate of 28.6% (Table 3).
Table 3.
Complications During ECMO
| Complications | Our study (%) | ECLS Registry Report (%) [18, 19] |
|---|---|---|
| ECMO Mechanical Complications | ||
| Oxygenator failure | 0.0 | 18 |
| Oxygenator thrombosis | 14.3 | 12 |
| Other sites thrombosis | 2.9 | 7.7 |
| Hemorrhage | 45.7 | – |
| Gastrointestinal hemorrhage | 28.6 | 4.6 |
| Cerebral hemorrhage | 8.6 | 4–8 |
| Other site hemorrhage | 31.4 | – |
| Organ Failure | ||
| Brain death | 8.6 | 3.8 |
| Cerebral infarction | 2.9 | – |
| Epilepsy | 2.9 | – |
| Renal insufficiency | 48.6 | 33.5 |
| Heart failure | 62.9 | 61.8 |
| Arrhythmia with unstable hemodynamics | 5.7 | 18.2 |
| Cardiac arrest | 11.4 | 9.8 |
| Liver failure | 25.7 | – |
| DIC | 8.6 | 3.8 |
| Hemolysis | 2.9 | 7.1 |
| Severe thrombocytopenia | 11.4 | – |
| Nosocomial Infection | ||
| CRBSI | 28.6 | 21.2 |
| Bacteremia of other sources | 5.7 | – |
| VAP | 60.0 | – |
| Urinary infections | 17.1 | – |
| Barotrauma | 8.6 | – |
| Metabolic | ||
| Hyperbilirubinemia | 4 (11.4) | 7.3 |
| Hyperglycemia | 17 (48.6) | 18.2 |
Note: DIC disseminated intravascular coagulation, CRBSI catheter related bloodstream infection, VAP ventilator associated pneumonia; severe thrombocytopenia refers to thrombocyte less than 20*10^9/L; hyperglycemia refers to blood glucose more than 13.3 mmol/L
Comparison between the patients successfully and unsuccessfully weaned from ECMO
Group 1 contained 14 patients who were successfully weaned from ECMO, and group 2 included 21 patients who were unsuccessfully weaned from ECMO.
Compared with patients successfully weaned from ECMO, the unsuccessfully weaned group had a higher mortality (100% vs. 7.1%, respectively, P < 0.01), and was older (60 ± 12 years vs. 51 ± 10 years, respectively, P = 0.02), and more likely to have diabetes mellitus (57.1% vs. 7.1%, respectively, P < 0.01), had more frequent severe conditions (SOFA: 10 ± 4 points vs. 7 ± 2 points, respectively, P < 0.01) pre-ECMO. Meanwhile, they had a longer duration of IPPV (6 ± 4 d vs. 2 ± 1 d, respectively, P < 0.01), had lower PaO2/FiO2 levels (71.4 ± 20.0 mmHg vs. 89.3 ± 24.2 mmHg, respectively, P < 0.05), and higher rate of rescue ventilation strategies (81% vs. 43.9%, respectively, P < 0.05) before ECMO support. No significant differences were found in the total duration of IPPV, total duration of ECMO, length of ICU stay and length of hospitalization between the two groups (Table 1).
Blood flow in patients on ECMO
ECMO blood flow did not significantly differ between the two groups during the initiation of ECMO support. However, in the successfully weaned group vs. the unsuccessfully weaned group, a significant decrease in blood flow correlated with an increase in the duration of support, which was 3.65 ± 0.70 L/min vs. 4.57 ± 1.02 L/min, respectively, (P < 0.05) at 72 h on ECMO and 3.65 ± 0.86 L/min vs. 4.62 ± 0.90 L/min, respectively, (P < 0.01) at 96 h on ECMO (Additional files 1 and 2).
IPPV parameters in patients on ECMO
In the successfully weaned group compared to the unsuccessfully weaned group, FiO2 was 46 ± 13% vs. 74 ± 25%, respectively, (P < 0.01) at 48 h and 45 ± 11% vs. 78 ± 24%, respectively, (P < 0.01) at 72 h on ECMO; monitored Pplat was 21 ± 3 cmH2O vs. 25 ± 5 cmH2O, respectively, (P < 0.05) at 48 h, and 19 ± 4 cmH2O vs. 29 ± 6 cmH2O, respectively, (P < 0.01) at 72 h on ECMO; and monitored VT was 246 ± 93 ml vs. 343 ± 96 ml, respectively, (P < 0.05) at 48 h, and 236 ± 113 ml vs. 356 ± 116 ml, respectively, (P < 0.05) at 72 h on ECMO; all of the differences were statistically significant (Fig. 2, Additional file 1).
Fig. 2.
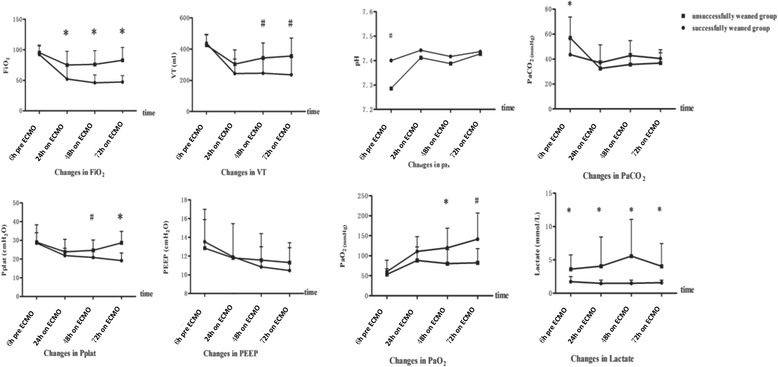
Comparison of IPPV parameters and ABGs between two groups of patients on ECMO. For the successfully weaned group compared to the unsuccessfully weaned group, FiO2 was 46 ± 13% vs. 74 ± 25%, respectively, at 48 h (P < 0.01) and 45 ± 11% vs. 78 ± 24%, respectively, at 72 h (P < 0.01). The monitored Pplat was 21 ± 3 cmH2O vs. 25 ± 5 cmH2O, respectively, at 48 h (P < 0.05) and 19 ± 4 cmH2O vs. 29 ± 6 cmH2O, respectively, at 72 h (P < 0.01). The monitored VT was 246 ± 93 ml vs. 343 ± 96 ml, respectively, at 48 h (P < 0.05) and 236 ± 113 ml vs. 356 ± 116 ml, respectively, at 72 h (P < 0.05) after ECMO support. However, there were no differences in PEEP during ECMO between the two groups. Patients who were in the unsuccessfully weaned group compared to patients in the successfully weaned group had severe acidosis (pH: 7.29 ± 0.14 vs. 7.40 ± 0.05, respectively, (P < 0.01), a higher PaCO2 (57.0 ± 16.7 mmHg vs. 43.6 ± 13.0 mmHg, respectively, (P < 0.05), and a higher lactate concentration (3.6 ± 2.1 mmol/L vs. 1.7 ± 0.8 mmol/L, respectively (P < 0.05), pre-ECMO. The pH and PaCO2 did not significantly differ between the two groups during ECMO therapy, while patients who eventually weaned successfully from ECMO had a gradual ascending tendency in PaO2 at 48 and 72 h on ECMO and a sustained low level of lactate after ECMO therapy
Improvement in circulatory and respiratory physiological indicators on ECMO
The vital signs were improved but did not significantly differ between the two groups pre-ECMO and during ECMO support (Additional file 3). Patients who were unsuccessfully weaned from ECMO compared to patients who were successfully weaned from ECMO had severe acidosis (pH: 7.29 ± 0.14 vs. 7.40 ± 0.05, respectively, (P < 0.01), a higher PaCO2 (57.0 ± 16.7 mmHg vs. 43.6 ± 13.0 mmHg, respectively, (P < 0.05), and a higher lactate concentration (3.6 ± 2.1 mmol/L vs. 1.7 ± 0.8 mmol/L, P < 0.05) pre-ECMO. pH and PaCO2 did not differ significantly between the two groups during ECMO support, while patients who were eventually successfully weaned from ECMO had a gradual ascending tendency of PaO2 at 48 and 72 h on ECMO and a sustained low level of lactate (Fig. 2, Additional file 1).
Changes in anticoagulation on ECMO
During the early stage of ECMO (24 and 48 h), the successful weaning group showed smaller differences between the ACTmax and ACTmin than the unsuccessful weaning group, which was 48 ± 20 s vs. 104 ± 35 s at 24 h (P < 0.05) and 40 ± 25 s vs. 98 ± 40 s at 48 h (P < 0.01). However, this trend was not found with regard to the difference between the maximum and minimum APTT.
Complications during ECMO
There were no differences between the two groups in mechanical complications associated with ECMO, VAP and barotrauma. The successfully weaned group compared to the unsuccessfully weaned group had a lower haemorrhage rate (28.6% vs. 77.3%, respectively, P < 0.01), lower rate of renal insufficiency (21.4% vs. 63.6%, respectively, P < 0.05), lower rate of liver failure (0% vs. 40.9%, respectively, P < 0.01) and lower heart failure rate (35.7% vs. 77.3%, respectively, P < 0.05).
Discussion
This study was the first to systematically and comprehensively discuss as well as elaborate on the current application of the efficacy and safety of ECMO in patients with H7N9 pneumonia-related ARDS.
A few studies [17, 20, 21, 22] have shown that the mortality of pH1N1-induced ARDS was reduced to 21–61% following ECMO treatment. Presently, no studies with large samples have investigated the mortality of H7N9-induced ARDS, while the in-hospital mortality was as high as 63% in our study. Late initiation of ECMO, inappropriate IPPV settings during ECMO, and more ECMO complications might explain the relatively high mortality. Moreover, as a multicentre collaboration study, the experiences of ECMO varied among the centres (Additional file 4), which might be another reason for the high mortality.
According to the Extracorporeal Life Support Organization (ELSO) data [23, 24], ECMO is indicated when death risk exceeds 80%, i.e., when PaO2/FiO2 < 80 mmHg on FiO2 > 90% and the Murray score is 3–4. Our patients met the indications for ECMO support. The duration of MV for more than 7 days pre-ECMO is an important prognostic factor for death [25]. For patients in the successfully weaned group, the duration of IPPV pre-ECMO was 5 ± 1 d; however, the duration was even longer among patients in the unsuccessfully weaned group (6 ± 4 d). Moreover, rescue ventilation strategies were implemented for most patients before ECMO, which partially delayed the timing of ECMO. In comparison, ECMO was initiated at 2 h (1–5 h) after IPPV among patients with pH1N1 in Australia and New Zealand in 2009 [5], which was significantly shorter than that in our cases. Therefore, we emphasized early implementation of ECMO in our patients.
The principle of IPPV during ECMO is the “lung rest strategy” [26]. The REVA registry study examined 123 patients with pH1N1-induced ARDS [6] and showed that the high Pplat (29 cmH2O) on day 1 of ECMO was related to high mortality. In our study, the pre-ECMO Pplat level was high (29 ± 8 cmH2O). High Pplat can lead to overdistension of the alveoli and cause lung volutrauma. The shear force between the overdistended and collapsed alveoli further aggregates VILI [27], which ultimately increases mortality. Although the Pplat values decreased to different degrees after ECMO, the Pplat of the unsuccessfully weaned group was significantly higher at 48 and 72 h during ECMO. The principle of low VT was similar in that we found a lower VT during ECMO in the successfully weaned group. A retrospective observational study of 168 patients with severe ARDS [28] showed that a high PEEP level within 3 d of being on ECMO was related to decreased mortality. Although no difference was observed in the PEEP levels between the two groups, we speculated that the down-regulation of PEEP during ECMO might have further aggravated the occurrence of collapse-induced injury, which led to atelectasis and sputum discharge obstacles. Therefore, the IPPV parameters, including high Pplat and VT levels and low PEEP settings, might have been unreasonable in our study; lung rest or the maintenance of open alveoli was not achieved.
The incidence of an ECMO oxygenator thrombus, haemorrhage, and organ failure in our study was high, which suggests that some problems existed in the anticoagulation management and organ supportive treatment of ECMO. We found that the unsuccessfully weaned group had larger fluctuations in ACT (the difference between ACTmax and ACTmin were larger) during the early stage of anticoagulation. This effect might suggest relatively unstable anticoagulation and a higher risk of haemorrhage. Moreover, the incidence rate of VAP during ECMO was as high as 60% and was partially attributed to the long course of H7N9 pneumonia and the prolonged duration of IPPV. Therefore, intensification of airway management was extremely necessary.
Our study had limitations. The nature of the study required the collection of data at multiple consecutive time points to evaluate the efficacy of ECMO. As a retrospective study with some missing data, we were unable to successfully collect data at 6 h pre-ECMO and 24, 48, and 72 h post-ECMO. Additionally, the number of subjects was too small to perform a multiple regression analysis to explore the risk factors for unsuccessful weaning from ECMO.
Conclusions
ECMO is effective at improving oxygenation and ventilation of patients with avian influenza A (H7N9)-induced severe ARDS. Early initiation of ECMO with appropriate IPPV settings and anticoagulation strategies are necessary to reduce complications.
Additional files
Blood flow during ECMO, changes in IPPV parameters and physiological indicators pre-ECMO and during ECMO. (DOCX 107 kb)
Blood flow during ECMO between the two groups. In the successfully weaned group vs. the unsuccessfully weaned group, a significant decrease in ECMO blood flow correlated with an increase in the duration of support, which was 3.65 ± 0.70 L/min vs. 4.57 ± 1.02 L/min, respectively, at 72 h (P < 0.05) and 3.65 ± 0.86 L/min vs. 4.62 ± 0.90 L/min, respectively, at 96 h (P < 0.01). (TIFF 185 kb)
Changes in vital signs pre-ECMO and during ECMO between the two groups. Vital signs were improved and did not significantly differ between the two groups during ECMO. (TIFF 204 kb)
ECMO Cases Per Year for Each Hospital. (DOCX 60 kb)
Acknowledgements
Not applicable.
Funding
Dr. Zhan was supported by a grant from the National Key Research and Development Programme—Major Chronic Non-Communicable Diseases’ Prevention and Control (QML 2016YFC1304300). Dr. Cao was supported by a grant from the National Science Fund for Distinguished Young Scholars (grant number 81425001/H0104) and grants from the National Natural Science Foundation of China (81271840/ H1904 and 81030032/H19), and the National Program for the Prevention and Control of Human Infections by Avian-Origin H7N9 Influenza A Virus (KJYJ-2013-01-05). The funding sources had no role in the design, conduct, or reporting of the study or the decision to submit the manuscript for publication. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- ABG
Arterial blood gas analysis
- ACT
Activated coagulation time
- APTT
Activated partial thromboplastin time
- ARDS
Acute respiratory distress syndrome
- CPAP
Continuous positive airway pressure
- CRBSI
Catheter-related blood stream infection
- DIC
Disseminated intravascular coagulation
- ECMO
Extracorporeal membrane oxygenation
- ELSO
Extracorporeal Life Support Organization
- FiO2
Fraction of inspiration
- HFOV
High frequency oscillatory ventilation
- IPPV
Invasive positive pressure ventilation
- MAP
Mean arterial pressure
- MV
Mechanical ventilation
- NAI
Neuraminidase inhibitors
- NPPV
Non-invasive positive pressure ventilation
- PaCO2
Partial pressure of arterial carbon dioxide
- PaO2
Partial pressure of arterial oxygen
- PEEP
Positive end-expiratory pressure
- PP
Prone position
- Pplat
Plateau pressure
- RM
Recruitment manoeuvre
- SOFA
Sequential Organ Failure Assessment
- SpO2
Fingertip pulse oxygen saturation
- VAP
Ventilation-associated pneumonia
- VILI
Ventilation-induced lung injury
- VT
Tidal volume
Authors’ contributions
All authors made substantial contributions to the conception and design of the study, data acquisition, analysis or interpretation of data, and review and approval of the final manuscript. Drs. LH, WZ, YY, WW and WL contributed equally to the article. Drs. BC and QZ assumed full responsibility for the integrity of the submission and publication and were involved in the study design. Drs. WZ, YY, WW, WL, HX, HZ, Yunfu Wu (YW), JS, LC, and LL were responsible for caring for the influenza A (H7N9) cases and have been involved in gathering data. Drs. LH, BC, QZ, CW, DL, and YW had full access to all of the data in the study, assume responsibility for the integrity of the data and the accuracy of the data analysis and were responsible for data verification and analysis, as well as the drafting of the manuscript.
Ethics approval and consent to participate
All patients gave written informed consent before ECMO treatment. As a highly pathogenic disease, the Chinese National Health and Family Planning Commission approved the collection of the data from the patients with H7N9 virus–induced pneumonia. The informed consent was waived to allow for exploration of the characteristics of the emerging infectious disease after rigorous contemplation and discussion by the Chinese National Health and Family Planning Commission.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12879-017-2903-x) contains supplementary material, which is available to authorized users.
Contributor Information
Linna Huang, Email: xuehuaalice@126.com.
Wei Zhang, Email: zhangweiliuxin@163.com.
Yi Yang, Email: yiyiyang2004@163.com.
Wenjuan Wu, Email: 1346801465@qq.com.
Weihua Lu, Email: lwh683@126.com.
Han Xue, Email: 2644257577@qq.com.
Hongsheng Zhao, Email: zxnt@163.com.
Yunfu Wu, Email: icuwu@163.com.
Jia Shang, Email: shangjia666@126.com.
Lihua Cai, Email: cailihua0912@163.com.
Long Liu, Email: liulong988@sina.com.
Donglin Liu, Email: m15890122166@163.com.
Yeming Wang, Email: wwyymm_love@163.com.
Bin Cao, Phone: +86 13911318339, Email: caobin1999@gmail.com.
Qingyuan Zhan, Phone: +86 13911785957, Email: drzhanqy@163.com.
Chen Wang, Email: cyh-birm@263.net.
References
- 1.Gao HN, Lu HZ, Cao B, et al. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med. 2013;368(24):2277–2285. doi: 10.1056/NEJMoa1305584. [DOI] [PubMed] [Google Scholar]
- 2.Costa EL, Amato MB. The new definition for acute lung injury and acute respiratory distress syndrome: is there room for improvement? Curr Opin Crit Care. 2013;19(1):16–23. doi: 10.1097/MCC.0b013e32835c50b1. [DOI] [PubMed] [Google Scholar]
- 3.Finney SJ. Extracorporeal support for patients with acute respiratory distress syndrome. Eur Respir Rev. 2014;23(133):379–389. doi: 10.1183/09059180.00005514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet (London, England) 2009;374(9698):1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 5.Australia, New Zealand Extracorporeal Membrane Oxygenation Influenza I. Davies A, et al. Extracorporeal membrane oxygenation for 2009 influenza A (H1N1) acute respiratory distress syndrome. JAMA. 2009;302(17):1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 6.Pham T, Combes A, Roze H, et al. Extracorporeal membrane oxygenation for pandemic influenza A (H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2013;187(3):276–285. doi: 10.1164/rccm.201205-0815OC. [DOI] [PubMed] [Google Scholar]
- 7.Nie Q, Zhang DY, Wu WJ, et al. Extracorporeal membrane oxygenation for avian influenza A (H7N9) patient with acute respiratory distress syndrome: a case report and short literature review. BMC Pulm Med. 2017;17(1):38. doi: 10.1186/s12890-017-0381-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang X, He H, Sun B, et al. ARDS associated with pneumonia caused by avian influenza A H7N9 virus treated with extracorporeal membrane oxygenation. Clin Respir J. 2015;9(3):380–384. doi: 10.1111/crj.12140. [DOI] [PubMed] [Google Scholar]
- 9.Wang G, Zhou Y, Gong S, et al. A pregnant woman with avian influenza A (H7N9) virus pneumonia and Ards managed with extracorporeal membrane oxygenation. Southeast Asian J Trop Med Public Health. 2015;46(3):444–448. [PubMed] [Google Scholar]
- 10.Cao B, Gao H, Zhou B, et al. Adjuvant corticosteroid treatment in adults with influenza A (H7N9) viral pneumonia. Crit Care Med. 2016;44(6):e318–e328. doi: 10.1097/CCM.0000000000001616. [DOI] [PubMed] [Google Scholar]
- 11.Li Q, Zhou L, Zhou M, et al. Epidemiology of human infections with avian influenza A (H7N9) virus in China. N Engl J Med. 2014;370(6):520–532. doi: 10.1056/NEJMoa1304617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao R, Cao B, Hu Y, et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;368(20):1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 13.Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38(10):1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 15.Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections--summary. Clin Microbiol Infect. 2011;17(Suppl 6):1–24. doi: 10.1111/j.1469-0691.2011.03602.x. [DOI] [PubMed] [Google Scholar]
- 16.Surveillance of nosocomial infections in Intensive Care Units: Hos- pital in Europe Link for Infection Control through Surveillance (HELICS) (Versión 6.1. September 2004). Available at: http://ecdc.europa.eu/en/publications/Publications/HAI-Net-ICU-protocol-v2.2.pdf. Accessed 1 Sept 2017.
- 17.Papadopoulos N, Ahmad Ael S, Marinos S, et al. Extracorporeal membrane oxygenation for influenza-associated acute respiratory distress syndrome. Thorac Cardiovasc Surg. 2013;61(6):516–521. doi: 10.1055/s-0032-1330923. [DOI] [PubMed] [Google Scholar]
- 18.Combes A, Pesenti A, Ranieri VM. Fifty years of research in ARDS. Is extracorporeal circulation the future of acute respiratory distress syndrome management? Am J Respir Crit Care Med. 2017;195(9):1161–1170. doi: 10.1164/rccm.201701-0217CP. [DOI] [PubMed] [Google Scholar]
- 19.Paden ML1, Conrad SA, Rycus PT, Thiagarajan RR; ELSO Registry.Extracorporeal Life Support Organization Registry Report 2012. ASAIO J. 2013;59(3):202–10. [DOI] [PubMed]
- 20.Wong I, Vuylsteke A. Use of extracorporeal life support to support patients with acute respiratory distress syndrome due to H1N1/2009 influenza and other respiratory infections. Perfusion. 2011;26(1):7–20. doi: 10.1177/0267659110383342. [DOI] [PubMed] [Google Scholar]
- 21.Stohr F, Emmert MY, Lachat ML, et al. Extracorporeal membrane oxygenation for acute respiratory distress syndrome: is the configuration mode an important predictor for the outcome? Interact Cardiovasc Thorac Surg. 2011;12(5):676–680. doi: 10.1510/icvts.2010.258384. [DOI] [PubMed] [Google Scholar]
- 22.Freed DH, Henzler D, White CW, et al. Extracorporeal lung support for patients who had severe respiratory failure secondary to influenza A (H1N1) 2009 infection in Canada. Can J Anaesth. 2010;57(3):240–247. doi: 10.1007/s12630-009-9253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paden ML, Conrad SA, Rycus PT, et al. Extracorporeal life support organization registry report 2012. ASAIO J. 2013;59(3):202–210. doi: 10.1097/MAT.0b013e3182904a52. [DOI] [PubMed] [Google Scholar]
- 24.Thiagarajan RR, Barbaro RP, Rycus PT, et al. Extracorporeal life support organization registry international report 2016. ASAIO J. 2017;63(1):60–67. doi: 10.1097/MAT.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 25.Hemmila MR, Rowe SA, Boules TN, et al. Extracorporeal life support for severe acute respiratory distress syndrome in adults. Ann Surg. 2004;240(4):595–605. doi: 10.1097/01.sla.0000141159.90676.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terragni P, Faggiano C, Ranieri VM. Extracorporeal membrane oxygenation in adult patients with acute respiratory distress syndrome. Curr Opin Crit Care. 2014;20(1):86–91. doi: 10.1097/MCC.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 27.Deans KJ, Minneci PC, Cui X, et al. Mechanical ventilation in ARDS: one size does not fit all. Crit Care Med. 2005;33(5):1141–1143. doi: 10.1097/01.CCM.0000162384.71993.A3. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt M, Stewart C, Bailey M, et al. Mechanical ventilation management during extracorporeal membrane oxygenation for acute respiratory distress syndrome: a retrospective international multicenter study. Crit Care Med. 2015;43(3):654–664. doi: 10.1097/CCM.0000000000000753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Blood flow during ECMO, changes in IPPV parameters and physiological indicators pre-ECMO and during ECMO. (DOCX 107 kb)
Blood flow during ECMO between the two groups. In the successfully weaned group vs. the unsuccessfully weaned group, a significant decrease in ECMO blood flow correlated with an increase in the duration of support, which was 3.65 ± 0.70 L/min vs. 4.57 ± 1.02 L/min, respectively, at 72 h (P < 0.05) and 3.65 ± 0.86 L/min vs. 4.62 ± 0.90 L/min, respectively, at 96 h (P < 0.01). (TIFF 185 kb)
Changes in vital signs pre-ECMO and during ECMO between the two groups. Vital signs were improved and did not significantly differ between the two groups during ECMO. (TIFF 204 kb)
ECMO Cases Per Year for Each Hospital. (DOCX 60 kb)
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.


