Abstract
Background
Incretin-based therapies are used in the treatment of type 2 diabetes mellitus (T2DM) and obesity. We investigated the changes in arterial stiffness and left ventricular (LV) myocardial deformation after 6-month treatment with the GLP-1 analogue liraglutide in subjects with newly diagnosed T2DM.
Methods
We randomized 60 patients with newly diagnosed and treatment-naive T2DM to receive either liraglutide (n = 30) or metformin (n = 30) for 6 months. We measured at baseline and after 6-month treatment: (a) carotid-femoral pulse wave velocity (PWV) (b) LV longitudinal strain (GLS), and strain rate (GLSR), peak twisting (pTw), peak twisting velocity (pTwVel) and peak untwisting velocity (pUtwVel) using speckle tracking echocardiography. LV untwisting was calculated as the percentage difference between peak twisting and untwisting at MVO (%dpTw–UtwMVO), at peak (%dpTw–UtwPEF) and end of early LV diastolic filling (%dpTw–UtwEDF) (c) Flow mediated dilatation (FMD) of the brachial artery and percentage difference of FMD (FMD%) (d) malondialdehyde (MDA), protein carbonyls (PCs) and NT-proBNP.
Results
After 6-months treatment, subjects that received liraglutide presented with a reduced PWV (11.8 ± 2.5 vs. 10.3 ± 3.3 m/s), MDA (0.92 [0.45–2.45] vs. 0.68 [0.43–2.08] nM/L) and NT-proBNP (p < 0.05) in parallel with an increase in GLS (− 15.4 ± 3 vs. − 16.6 ± 2.7), GLSR (0.77 ± 0.2 vs. 0.89 ± 0.2), pUtwVel (− 97 ± 49 vs. − 112 ± 52°, p < 0.05), %dpTw–UtwMVO (31 ± 10 vs. 40 ± 14), %dpTw–UtwPEF (43 ± 19 vs. 53 ± 22) and FMD% (8.9 ± 3 vs. 13.2 ± 6, p < 0.01). There were no statistically significant differences of the measured markers in subjects that received metformin except for an improvement in FMD. In all subjects, PCs levels at baseline were negatively related to the difference of GLS (r = − 0.53) post-treatment and the difference of MDA was associated with the difference of PWV (r = 0.52) (p < 0.05 for all associations) after 6-month treatment.
Conclusions
Six-month treatment with liraglutide improves arterial stiffness, LV myocardial strain, LV twisting and untwisting and NT-proBNP by reducing oxidative stress in subjects with newly diagnosed T2DM.
ClinicalTrials.gov Identifier NCT03010683
Keywords: Arterial stiffness, Augmentation index, Glp-1 analogue, Liraglutide, Metformin, Left ventricular function, Oxidative stress
Background
Type-2 diabetes mellitus (T2DM) is associated with cardiac dysfunction [1]. Subclinical LV dysfunction is present in patients with T2DM and it is caused by factors such as insulin resistance, microvascular disease and cardiac autonomic dysfunction [2].
Glucagon-like peptide 1 (GLP-1) is an incretin hormone secreted mainly by the intestinal L-cells in response to the presence of nutrients [3]. GLP-1 analogues are a class of antidiabetic medications that mimic the actions of the endogenous incretin GLP-1 through supraphysiological blood concentrations that are resistant to degradation by DPP-4. These drugs lower glucose levels by inhibiting the secretion of glucagon, by promoting the release of insulin in a glucose-dependent manner, by slowing gastric emptying, and by acting at the hypothalamus causing an anorexigenic effect and thus regulating energy balance [4]. Recent large scale study has demonstrated that in addition to improving glycaemic control and promoting weight loss, GLP-1 analogues may improve cardiovascular outcomes in patients at high cardiovascular risk [5].
Liraglutide, an analogue of human GLP-1, has been approved for the treatment of T2DM and obesity [6, 7]. The long-term effects of liraglutide on cardiovascular outcomes were assessed in a recent study involving a high cardiovascular risk population including subjects with heart failure [5]. In the LEADER trial, cardiovascular benefit was observed after 1-year treatment with liraglutide, suggesting a beneficial effect in atherosclerosis, rather than an immediate effect on the haemodynamic parameters or the acute metabolic changes observed in the EMPA-REG trial [8].
Established prognostic markers of vascular integrity and function namely carotid-femoral pulse wave velocity (PWV), augmentation index (AI) and brachial artery flow-mediated dilation (FMD) have been found to be impaired in T2DM [9–11]. Myocardial deformation of the left ventricle (LV), as assessed by speckle tracking echocardiography, allows a sensitive assessment of LV function. Strain imaging is superior to LV ejection fraction in the detection of early type 2 diabetic myocardial disease [12, 13]. Abnormal PWV is related to impaired longitudinal LV function in hypertensive patients [14]. Measurement of natriuretic peptides like NT-proBNP contribute to the detection of left ventricular dysfunction [15]. Increased oxidative stress is a widely accepted participant in the development and progression of diabetes and its complications [16].
Study have shown that addition of GLP-1 analogue in patients with T2DM well controlled on metformin monotherapy improves several markers of vascular function [17]. However, the impact of treatment with GLP-1 analogue on vascular dysfunction, LV function, NT-proBNP and oxidative stress burden have not been clearly defined in patients with T2DM and no CVD history.
In the present study we hypothesized that a 6-month treatment with liraglutide in patients with newly diagnosed T2DM causes greater improvement in vascular function, LV myocardial strain, twisting–untwisting, oxidative stress burden as assessed by malondialdehyde (MDA) and protein carbonyls (PCs) and NT-proBNP concentrations compared to the standard treatment with metformin.
The aim of the present study was to investigate the early differences in vascular function, LV myocardial strain and twisting–untwisting and oxidative stress 6 months after treatment with either liraglutide or metformin in a population with a short disease duration and no prior therapeutic intervention.
Methods
We examined 60 newly diagnosed and treatment-naive patients with type 2 diabetes mellitus. Patients were randomly assigned in a 1:1 ratio, to gradually receive either 1.8 mg of liraglutide once daily (with a weekly dose escalation as instructed by the SPC) as a subcutaneous injection or matching metformin 1000 mg twice daily for 6 months. Patients were recruited consecutively from the Diabetes Centre outpatient clinic. Exclusion criteria were history or clinical evidence of coronary or valvular heart disease, congestive heart failure, peripheral vascular disease, liver or kidney failure, history of alcohol or drug abuse, and treatments able to modify glucose metabolism. All women were premenopausal and their investigations were undertaken during the first week of their menstrual cycles. None of them were taking oral contraceptives. Dyslipidaemia was defined as total cholesterol > 220 mg/dl (LDL > 100 mg/dl and/or HDL < 40 mg/dl in men and HDL < 50 mg/dl in women) and/or triglycerides > 150 mg/dl. Hypertension was defined as clinic BP > 140/90 mmHg. Height, weight, body mass index (BMI) waist, and hips circumference were determined for all participants. 10 of the patients presented with various degrees of dyspnea at baseline and were classified as NYHA I stage of heart failure.
Primary and secondary endpoints
The primary endpoint was a change in global longitudinal strain 6 months after treatment with either liraglutide or metformin.
Secondary endpoints were changes in endothelial function, arterial stiffness as assessed by pulse wave velocity and augmentation index. LV twisting and untwisting, NT-proBNP and oxidative stress post-treatment with either liraglutide or metformin.
Blood pressure measurement
Each patient rested in a supine position for 10 min in a quiet room at 23 °C before the baseline haemodynamic measurements were recorded. Brachial blood pressure (BP) and heart rate (HR) were measured in the right arm with an automated digital oscillometric sphygmomanometer (TensioMed, Budapest Hungary, Ltd). Two sequential measurements separated by 2-min interval were obtained and the mean was used for statistical analysis.
Assessment of arterial stiffness
At baseline and after 6-month treatment we measured the carotid-femoral pulse wave velocity (PWVc), central systolic blood pressure (cSBP), central pulse pressure (cPP) and augmentation index (AI). PWVc (m/s) was measured using tonometry by Complior (Alam Medical, Vincennes, France). Two non-invasive pressure sensors were used to record the carotid and femoral waveforms and the distance between the two arterial sites was measured with a tape measure. PWV was calculated as the distance divided by transit time between waves (m/s). Normal values for PWV < 10 m/s [18]. AI was defined as 100 × (P2 − P1)/PP where P2 = late backward systolic wave, P1 = early forward systolic wave, PP = pulse pressure, and represents the pressure boost that is induced by the return of the reflected waves at the aorta. AI75 was calculated to adjust the AI for a heart rate of 75 beats/min using the formula: AI75 = ([heart rate − 75] × 0.39) + AI [19]. The inter-and intra-observer variabilities for PWV were 6 and 5% and for Aix 12 and 10%, respectively.
Endothelial function
Flow-mediated dilation (FMD) of the brachial artery was determined according to a previously published method and was expressed as a percentage change of the arterial diameter from the baseline vessel size [20]. Interobserver and intraobserver variability of the brachial artery diameter was, respectively, 0.08 ± 0.19 and 0.1 ± 0.12 mm, and the day-to-day variability of FMD was 1.1 ± 1%.
Echocardiography
Studies were performed using a Vivid 7 (GE Medical Systems, Horten, Norway) ultrasound system. All studies were digitally stored in a computerized station (Echopac GE, Horten, Norway) and were analyzed by two observers blinded to clinical and laboratory data. For the determination of interobserver variability, data from the first 20 patients were analyzed by the 2 readers. Interobserver and intraobserver variabilities were calculated as the SD of the differences between the first and second measurements and expressed as a percentage of the average value.
Doppler echocardiography
Using pulse-wave Doppler, E and A waves of the mitral inflow velocity were measured and the ratio E/A was calculated [20].
2D strain and strain rate analysis
In all patients, we measured longitudinal systolic strain (LS) and systolic strain rate (LSR) from standard 2-dimensional acquisitions (frame rate: 60–80/s) with the use of a dedicated software (EchoPac PC 201, GE Healthcare) [20]. Global longitudinal strain (GLS) and global longitudinal strain rate (GLSR) was calculated using the 17 LV segment model imaged from apical chamber views (4, 2 and 3 chamber view), as previously published [20, 21]. The intra- and inter-observer variability for LV strain parameters were 8 and 9% respectively. Normal values for GLS: − 20% [22].
LV twisting and untwisting
Left ventricular rotation and twisting–untwisting were assessed using parasternal short axis views at basal and apical level [20, 21]. Subsequently, torsional deformation and velocity curves along with time were generated (EchoPac PC 201, GE Healthcare). Utilizing the pulse wave Doppler recording of mitral valve inflow, we measured time interval between the onset of the QRS of the simultaneous ECG recording and the onset, peak and end of the E wave of the mitral inflow waveform, respectively [20, 21]. Based on the above measured time intervals, utilizing as a starting point the onset of the QRS of the ECG recording in the generated torsional deformation curve along with time, we estimated peak twisting (pTw, deg), as well as untwisting at the time of mitral valve opening (UtwMVO), peak (UtwPEF) and end of left ventricular early filling (UtwEDF), respectively. The degree of LV untwisting during diastole was calculated as the percentage difference between peak twisting and untwisting at MVO (%dpTw–UtwMVO), at the peak (%dpTw–UtwPEF) and end of early filling (%dpTw–UtwEDF), using our previously published methodology [20, 21]. Furthermore, we measured peak twisting (pTw, deg), peak twisting velocity (pTwVel, deg/s) and peak untwisting velocity (pUtwVel, deg/s). The inter- and intra-observer variability these measurements were 8 and ≤ 10% respectively for all markers.
Laboratory assays
Plasma glucose was measured by the enzymatic in vitro test (Roche, automatic chemistry clinical analyzer). Serum insulin concentration was determined by a chemiluminescense-based assay (Roche Diagnostics).
Malondialdehyde (MDA) and protein carbonyls (PCs) were determined spectrophotometrically with a commercial kit (Oxford Biomedical Research, Rochester Hills, MI) of colorimetric assay for lipid peroxidation (measurements range, 1–20 nmol/l) [23]. For the qualification of protein carbonyl content, we based on spectrophotometric measurement of 2,4-dinitrophenylhydrazine derivatives of protein carbonyls, as previously published [24] and results expressed as nmol/mg protein. Using commercially available enzyme-linked immunosorbent assay kits, we measured serum concentrations of amino-terminal Pro-B-Type Natriuretic Peptide (NT-proBNP) (ABNOVA, Taipei, Taiwan; sensitivity 2 pg/ml).
Statistical analysis
All comparisons were performed with the Statistical Package for Social Sciences 21.0 for Windows (SPSS Inc., Chicago, Illinois, USA). Categorical data were compared between patients by contingency tables. Continuous variables were tested for normality using the Kolmogorov–Smirnov test. Normally distributed variables are given as mean ± standard deviation. Data with a non-gaussian distribution are expressed as median (interquartile range) and were analyzed after transformation into ranks. Differences in mean values for each of the measured variables were compared by t-test or paired t-test for continuous variables. For non-normally distributed data Mann–Whitney or Wilcoxon signed-rank test was used. We used parametric (Pearson r) and non-parametric (Spearman rho) correlation coefficients to examine cross-sectional associations. ANOVA (general linear model, SPSS 22, SPSS Inc, Chicago, Ill) for repeated measurements was applied (a) for measurements of the examined markers at baseline, 6 months after treatment used as a within-subject factor (b) for the effects of treatment (liraglutide vs. metformin), as a between-subject factors. The F and P values of the interaction between time of measurement of the examined markers and type of treatment were calculated. The F and P values of the comparison between treatments were calculated. The Greenhouse–Geisser correction was used when the sphericity assumption, as assessed by Mauchly’s test, was not met. Post hoc comparisons were performed with Bonferroni correction.
Comparisons between baseline or post-treatment values of measured markers between the two treatment groups were performed using factorial ANOVA. Post hoc comparisons were performed with Bonferroni correction.
Logistic regression analysis was performed to examine the association of the median values of GLS, PWV and FMD post-treatment with the type of study medication (liraglutide or metformin). Statistical significance was considered as p < 0.05. Baseline variables that were statistically different (p < 0.05) among the 2 study groups or were of clinical significance (HbA1c, weight, BMI and waist circumference) were included in multivariate models as covariates.
We planned to study the percent change (Δ) of GLS after treatment from independent control (patients on metformin) and experimental subjects (patients on liraglutide) with 1 control per experimental subject. In a pilot study of 10 patients treated with metformin and 10 treated with liraglutide, the response within each group was normally distributed with standard deviation 10%. The true difference between patients treated with metformin and those treated with liraglutide in the means of ΔGLS was 7.3%. Therefore, we would need to study 30 patients treated with metformin and 30 treated with liraglutide, to be able to reject the null hypothesis that the population means for ΔGLS post-treatment of the metformin and liraglutide groups are equal with probability (power) 0.8 and type I error probability 0.05.
Results
The baseline characteristics for the study groups are shown in Table 1. Prior to the enrolment in the study, patients were not receiving diabetes medication and were being treated with diet and lifestyle therapy solely.
Table 1.
Baseline characteristics of the study groups
| Total (n = 60) | Liraglutide (n = 30) | Metformin (n = 30) | p | |
|---|---|---|---|---|
| Age, years | 51 ± 12 | 51 ± 10 | 50 ± 12 | 0.594 |
| Male sex, % (n) | 66.7 (40) | 66.7 (20) | 66.7 (20) | 0.896 |
| Smoking, % (n) | 36.6 (22) | 36.7 (11) | 36.7 (11) | 0.782 |
| Hypertension, % (n) | 55 (33) | 56.7 (17) | 53.3 (16) | 0.270 |
| Dyslipidemia, % (n) | 51.6 (31) | 53.3 (16) | 50 (15) | 0.410 |
| Family history CAD, % (n) | 20 (12) | 20 (6) | 20 (6) | 0.804 |
| Creatinine, mg/dl | 1.0 ± 0.2 | 1.1 ± 0.2 | 1.0 ± 0.3 | 0.733 |
| eGFR, ml/min | 85 ± 9 | 85 ± 8 | 83 ± 11 | 0.315 |
| Medication | ||||
| Beta blockers, % (n) | 18.3 (11) | 16.7 (5) | 20 (6) | 0.492 |
| Calcium antagonists, % (n) | 30 (18) | 33.3 (10) | 26.7 (8) | 0.231 |
| ACE-I, ARB, % (n) | 31.7 (19) | 30 (9) | 33.3 (10) | 0.913 |
| Diuretics, % (n) | 13.3 (8) | 13.3 (4) | 13.3 (4) | 0.984 |
| Statins, % (n) | 43.3 (26) | 46.7 (14) | 40 (12) | 0.292 |
Data are expressed as the mean (SD) or n (%). p: p of the model of the ANOVA for comparisons between groups
Changes in metabolic parameters and vascular markers after 6-month treatment
Treatment with liraglutide resulted in a greater weight loss, reduction in BMI, waist circumference, and HbA1c in comparison to metformin (p < 0.05, Table 2). Furthermore, treatment with liraglutide caused a significant reduction in PWV, AI, systolic blood pressure and central systolic blood pressure (p < 0.05, Tables 2 and 3) while these changes were not evident in patients treated with metformin (p > 0.05) after adjusting for HbA1c, weight, BMI and waist circumference. Thus, post treatment patients that received liraglutide had lower PWV, AI, systolic blood pressure and central systolic blood pressure than those on metformin (p < 0.05).
Table 2.
Effect of treatment with liraglutide vs. metformin on anthropometric, biochemical and vascular variables
| Time, months | Liraglutide (n = 30) | Metformin (n = 30) | ||||
|---|---|---|---|---|---|---|
| 0 | 6 | p | 0 | 6 | p | |
| Weight, Kg | 98 ± 16 | 92 ± 16* | 0.031 | 78 ± 12 | 77 ± 14 | 0.655 |
| BMI, Kg/m2 | 32.9 ± 5 | 30.9 ± 5# | 0.009 | 27.7 ± 2 | 26.9 ± 3 | 0.176 |
| Waist, cm | 109 ± 15 | 104 ± 14* | 0.065 | 97 ± 12 | 95 ± 13 | 0.401 |
| Fasting Glucose, mg/dl | 165 ± 45 | 135 ± 33* | 0.027 | 163 ± 40 | 143 ± 45 | 0.042 |
| HbA1c, % (mmol/mol) | 8.6 ± 2 (70 ± 22) | 7 ± 1.2 (53 ± 13)* | 0.006 | 8.4 ± 1.2 (68 ± 13) | 7.7 ± 1 (61 ± 11) | 0.012 |
| SBP, mmHg | 142 ± 15 | 138 ± 19* | 0.024 | 142 ± 19 | 141 ± 16 | 0.632 |
| DBP, mmHg | 90 ± 8 | 87.5 ± 12 | 0.092 | 89 ± 9 | 88 ± 8 | 0.122 |
| cSBP, mmHg | 143 ± 20 | 138 ± 19* | 0.035 | 142 ± 18 | 140 ± 18 | 0.574 |
| HR, bpm | 74 ± 12 | 80 ± 11* | 0.043 | 71 ± 12 | 68 ± 10 | 0.075 |
Data are presented as mean ± SD values
BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, cSBP central systolic blood pressure, HR heart rate
* p < 0.05; #p < 0.01—both for liraglutide vs. metformin post treatment
Table 3.
Effect of treatment with liraglutide vs. metformin on arterial stiffness, LV function, and oxidative stress
| Time, months | Liraglutide (n = 30) | Metformin (n = 30) | ||||
|---|---|---|---|---|---|---|
| 0 | 6 | p | 0 | 6 | p | |
| PWV, m/s | 11.8 ± 2.5 | 10.3 ± 3.3* | 0.019 | 11.2 ± 3 | 11 ± 3 | 0.719 |
| AI75, % | 18 (− 1 to 31) | 13 (− 2 to 31)* | 0.032 | 14 (− 9 to 24) | 15 (− 8 to 24) | 0.503 |
| GLS, % | − 15.4 ± 3 | − 16.6 ± 2.7* | 0.043 | − 15.5 ± 2.9 | − 15.7 ± 3.2 | 0.721 |
| GLSR, 1/s | 0.77 ± 0.2 | 0.89 ± 0.2* | 0.038 | 0.79 ± 0.3 | 0.82 ± 0.3 | 0.212 |
| pTw, deg | 15.5 ± 4 | 13.2 ± 6* | 0.029 | 16.2 ± 5 | 15.0 ± 6 | 0.313 |
| pUtw velocity, deg/s | − 97 ± 49 | − 112 ± 52* | 0.033 | − 100 ± 41 | − 98 ± 43 | 0.576 |
| %dpTw–UtwMVO | 31 ± 10 | 40 ± 14* | 0.021 | 29 ± 18 | 30 ± 18 | 0.787 |
| %dpTw–UtwPEF | 43 ± 19 | 53 ± 22 | 0.018 | 45 ± 19 | 50 ± 16 | 0.874 |
| E/A | 0.92 ± 0.2 | 0.98 ± 0.3 | 0.555 | 0.99 ± 0.3 | 1.1 ± 0.4 | 0.679 |
| FMD % | 8.9 ± 3 | 13.2 ± 6* | 0.003 | 8.8 ± 5 | 11.8 ± 6 | 0.033 |
| MDA, nM/L | 0.92 (0.45–2.45) | 0.68 (0.43–2.08)# | 0.006 | 0.78 (0.55–1.58) | 0.86 (0.1–1.88) | 0.09 |
| PCs, nmol/mg protein | 0.023 (0.011–0.026) | 0.013 (0.008–0.017) | 0.04 | 0.015 (0.006–0.019) | 0.013 (0.009–0.017) | 0.08 |
| NT-proBNP, pg/ml | 432 (154–2921) | 282 (80–2302)* | 0.03 | 490 (202–2670) | 400 (98–2083) | 0.08 |
Data are presented as mean ± SD values. Values for AI75 and biomarkers are median and interquartile range. PWV: pulse wave velocity; AI75 was calculated to adjust the AI for a heart rate of 75 beats/min using the formula: AI75 = ([heart rate − 75] × 0.39) + AI
GLS global longitudinal strain, GLSR global longitudinal strain rate, pTw peak twisting, pUtw velocity peak untwisting velocity, %dpTw–UtwMVO percentage difference between peak twisting and untwisting at MVO, %dpTw–UtwPEF percentage difference between peak twisting and untwisting at peak of left ventricular early filling, E/A ratio ratio of E to A waves of the mitral inflow velocity, FMD% percentage difference of flow mediated dilatation, MDA malondialdehyde, PCs protein carbonyls, NT-proBNP N-terminal pro-brain natriuretic peptide
ANOVA was adjusted for HbA1c, weight, BMI and waist circumference. * p < 0.05; #p < 0.01—both for liraglutide vs. metformin post treatment
Heart rate was increased after liraglutide treatment in contrast to subjects who received metformin (p < 0.05, Table 2).
Effect of treatment on endothelial function
Compared to baseline, FMD was increased in all subjects after 6-months treatment. However there was a significant interaction between type of treatment and change of FMD (p = 0.02). Among the two medications, liraglutide induced the greatest increase of percentage difference of FMD (Table 3) after adjusting for HbA1c, weight, BMI and waist circumference.
Markers of oxidative stress and NT-proBNP
Compared to baseline MDA, PCs and NT-proBNP were decreased in all subjects after 6-months treatment. However, there was a significant interaction between type of treatment and change of MDA, PCs and NT-proBNP (p = 0.01, p = 0.03 and p = 0.03 respectively). Thus, treatment with liraglutide caused the greatest decrease of MDA, PCs and NT-proBNP (p < 0.05), while these changes were of borderline significance after metformin (Table 3) after adjusting for HbA1c, weight, BMI and waist circumference. Thus, post treatment patients that received liraglutide had lower MDA and NT-proBNP than those on metformin (p < 0.05).
Speckle tracking analysis
Longitudinal strain
Compared to baseline, GLS and GLSR were increased after 6-month treatment with liraglutide (p < 0.05, Table 3, Fig. 1) after adjusting for HbA1c, weight, BMI and waist circumference. No significant changes were evident for GLS and GLSR in subjects that received metformin (p > 0.05, Table 3, Fig. 1). Thus, post treatment patients that received liraglutide had higher GLS than those on metformin (p = 0.03).
Fig. 1.
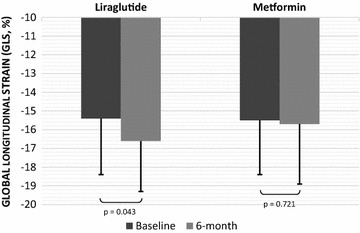
Changes in global longitudinal strain (GLS, %) after 6-month treatment with liraglutide or metformin. Data are presented as mean ± SD values
LV twisting and untwisting velocity
Compared to baseline, subjects under treatment with liraglutide showed a significant decrease in peak twisting and increase in peak untwisting velocity (p < 0.05) after adjusting for HbA1c, weight, BMI and waist circumference. In addition, the percentage changes between peak twisting and untwisting at mitral valve opening (%dpTw–UtwMVO) and peak (%dpTw–UtwPEF) of early diastolic LV filling were increased (p < 0.05, Table 3) suggesting a beneficial effect of liraglutide on LV twisting–untwisting. Metformin treatment did not cause any significant changes in the above parameter (p > 0.05, Table 3). Thus, post treatment patients that received liraglutide had lower peak twisting and peak untwisting velocity and higher %dpTw–UtwMVO than those on metformin (p < 0.05).
Interrelation between metabolic, vascular, and LV function markers
In all subjects, at baseline GLS was positively related with HbA1c (b = 0.391, p = 0.01), PWV (b = 0.473, p < 0.001), SBP (b = 0.273, p = 0.032), and DBP (b = 0.351, p = 0.005) and negatively with FMD% (b = − 0.299, p = 0.043). In addition, GLSR was inversely related with HbA1c (b = − 0.352, p = 0.022), PWV (b = − 0.498, p < 0.001), SBP (b = − 0.308, p = 0.015), and DBP (b = − 0.407, p < 0.001). Furthermore, cSBP was associated with %dpTw–UtwMVO (b = 0.293, p = 0.027).
After 6-month treatment, reduced PWV was positively related with GLS (b = 0.428, p = 0.026) and inversely with GLSR (b = − 0.488, p = 0.01) and FMD% (b = − 0.438, p = 0.047) in all subjects. In subjects that received liraglutide the percentage difference of HbA1c was associated with %dpTw–UtwPEF (b = 0.642, p = 0.033).
In all subjects PCs levels at baseline was negatively related with the difference of GLS after 6 months (b = − 0.527, p = 0.023). Furthermore, after 6-months treatment the difference of MDA was associated with the difference of PWV (b = 0.522, p = 0.032). In addition, the difference of NT-proBNP was negatively associated with the difference of GLS post-treatment (b = − 0.39, p = 0.04).
Table 4 reports univariate and multivariable association of a PWV > 10 m/s, GLS > − 15% and FMD < 11% post treatment with type of medication (liraglutide vs. metformin) HbA1c, weight, BMI, waist, and heart rate. Compared to metformin, liraglutide treatment was related with a lower odds ratio for elevated PWV, reduced GLS and FMD (p < 0.05) in model including the above covariates.
Table 4.
Univariate and multivariable association of PWV, GLS and FMD % post treatment with parameters of the study population
| PWV > 10 m/s | GLS < -15% | FMD > 11% | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariable | Univariate | Multivariable | Univariate | Multivariable | |||||||
| Odds ratio | p | Odds ratio | p | Odds ratio | p | Odds ratio | p | Odds ratio | p | Odds ratio | p | |
| HbA1c | 1.499 (1.1–1.7) | 0.035 | 1.709 (1.1–1.9) | 0.047 | 1.104 (0.9–2) | 0.085 | 1.668 (1–2.1) | 0.033 | 1.456 (1.2–1.5) | 0.030 | 1.422 (1.1–1.4) | 0.048 |
| Weight | 1.037 (1–1.1) | 0.096 | 1.200 (0.9–1.3) | 0.059 | 1.099 (1–1.1) | 0.067 | 1.172 (0.9–1) | 0.029 | 1.225 (1–1.3) | 0.039 | 1.054 (0.9–1.3) | 0.057 |
| BMI | 1.142 (0.9–1.4) | 0.071 | 1.241 (1.1–2) | 0.092 | 1.325 (1–1.5) | 0.065 | 1.490 (0.9–1.6) | 0.014 | 1.198 (0.9–1.3) | 0.042 | 1.304 (0.9–1.5) | 0.072 |
| Waist | 1.252 (1–1.3) | 0.045 | 1.077 (0.9–1.4) | 0.062 | 1.132 (1–1.5) | 0.037 | 1.516 (1–2) | 0.043 | 1.427 (0.9–1.6) | 0.033 | 1.873 (1–1.9) | 0.011 |
| HR | 1.100 (1–1.2) | 0.010 | 1.210 (1–1.4) | 0.022 | 1.070 (1–1.3) | 0.053 | 1.094 (1–1.3) | 0.205 | 1.505 (1.1–1.6) | 0.069 | 1.783 (1.2–1.9) | 0.061 |
| Liraglutide | 0.333 (0.2–1.3) | 0.018 | 0.367 (0.2–1.7) | 0.046 | 0.815 (0.3–1.5) | 0.043 | 0.920 (0.4–1.4) | 0.046 | 0.650 (0.4–1.5) | 0.035 | 0.790 (0.6–1.7) | 0.049 |
The median values PWV, GLS and FMD of the makers were used in logistic regression analysis. PWV pulse wave velocity, GLS global longitudinal strain, FMD% percentage difference of flow mediated dilatation of brachial artery. Values are odd ratios (95% confidence intervals)
Effect of weight loss on vascular and LV function markers
After 6-months treatment, liraglutide induced a greater decrease of weight than metformin (6% vs. 1.3%, p < 0.05). Subjects that received liraglutide and had a weight reduction of 1.3% (n = 10), which is identical to that caused by metformin presented reduced PWV (p = 0.021), AI75 (p = 0.04) and cSBP (p = 0.029) in parallel with an increased in GLS (p = 0.048), %dTw–UtwMVO (p = 0.031) and FMD% (p = 0.023) compared to baseline. These results show that the better effects of liraglutide treatment compared to metformin on arterial stiffness, LV myocardial deformation and endothelial function are not simply an effect of weight loss.
Discussion
In the present study, treatment with liraglutide for 6 months led to a significant reduction of arterial stiffness, oxidative stress burden and NT-proBNP level in parallel with an improvement of LV longitudinal myocardial strain and strain rate, LV twisting–untwisting and endothelial function as assessed by FMD in newly diagnosed, treatment-naive T2DM patients. These changes were not evident after treatment with metformin. Thus patients that received liraglutide had better LV longitudinal deformation twisting and untwisting than those on metformin. Additionally, the reduction of oxidative stress was associated with improved arterial elasticity as assessed by PWV which in turn was associated with improved myocardial deformation after 6 months of treatment. Finally and the increase of LV longitudinal deformation post treatment was to a reduction of NT-proBNP levels.
The spatial organization of myocardial fibers has a major impact in cardiac mechanics. Longitudinal LV deformation is mainly attributed to subendocardial fibers [25]. In terms of LV twisting, the subepicardial fibers, by their larger radius, produce the dominant force for LV rotation, whereas the subendocardial torque partly counteracts this twist. Consequently, impaired function of subendocardial myocardial helix results in: (i) the predominance of the function of the subepicardial helix during systole leading to increased LV twisting, and (ii) the impairment of myocardial relaxation in diastole leading to delayed untwisting in diastole [20, 25]. Thus, improved longitudinal deformation by a direct effect of liraglutide on cardiomyocytes has led to the reduction of an abnormally augmented LV twisting as observed in our study.
Incretin-based therapies and direct cardiovascular effects
So far, the direct effects of incretin-based therapies on myocardial contractility and endothelial function have been controversial. Various theories have been put forward to explain the protective effects of GLP-1 on myocardium. The most upheld revolves around cardiac metabolism [26]. GLP-1 analogues, have been shown to ameliorate insulin resistance and inflammation [27, 28]. They also seem to promote an actual increase in glucose transporters GLUT-2 and GLUT-4, especially in cardiomyocytes [29, 30].
A 3-months’ exenatide delivery to sedentary patients with uncomplicated, although not treatment-naive, type 2 diabetes, has recently shown to improve cardiac function and reduce arterial stiffness, although it proved neutral in terms of functional exercise capacity and endothelial function [31]. Conversely, postprandial haemodynamics were assessed after acute and chronic treatment with GLP1 analogs (exenatide infusion vs. placebo and liraglutide vs. sitagliptin or placebo, respectively) in a double-blind, placebo-controlled randomized trial. The study showed that a 12-week liraglutide administration was neutral in terms of postprandial decrease in diastolic blood pressure, which is thought to impair coronary perfusion in type 2 diabetic patients, as well as in other indices of postprandial haemodynamics. However, the study was a substudy and not all patients had postprandial hypotension at baseline, nor did they all have measurements at week 12 due to protocol design. The treatment dose was not generally stable, and administration route was different among GLP1 agonists, which might also have interfered with the results [32].
In another randomized, double-blind, placebo-controlled crossover study, liraglutide failed to show benefit in the systolic function or the exercise capacity of patients with preserved ejection fraction, and stable coronary artery disease. However, the study population had already undergone revascularization leading to a low probability of abnormal stress response anyway. Interestingly, the largest improvement in ejection fraction in the present study was observed during recovery period after peak stress which agrees with our results [33].
In addition, a lack in FMD change with liraglutide was recently exhibited in a study of 16 DM patients already on antidiabetic treatment (e.g. DDP-4) during a 14 week follow up period compared to the respective FMD change of 15 patients treated with insulin [34]. The greater change of FMD post GLP-1 treatment in our study could be attributed: (a) to the longer treatment period of our study (6 months vs. 14 and 20 weeks, respectively) (b) to the fact that our patients were naïve to antidiabetic treatment and thus there was no carry on effect of previous medications on endothelial function while in the study by Nomoto more than 40% were receiving DDP4 inhibitor and all patients were treated with antidiabetic medication prior to inclusion in the study (c) the FMD examination in our study was performed within the tmax of liraglutide injection (9–12 h) while in the study by Nomoto et al. this was performed a day after the last injection of GLP-1 [34].
Sitagliptin has also failed to confirm a beneficial net effect on pulse wave velocity within 2 years of therapy. However, the results suggested that achievement of good glycaemic control inhibits the annual progression of the arterial stiffness [35].
GLP-1 analogues have additional direct natriuretic effect and action on endothelial vasodilatation [36, 37]. A meta-analysis assessing the effect of GLP-1 based therapy on FMD showed neutral results in longitudinal studies within a 20 weeks’ period though there was a trend of increased FMD values post-treatment [38]. Conversely, within the same study, when a meta-analysis of 7 cross-sectional studies was conducted, results showed a large increase in FMD values post GLP-1 based treatment [38].
Even asymptomatic diabetic patients with preserved LV ejection fraction present with complications that are closely associated with systolic dysfunction [39]. Arterial stiffness is a determinant of LV longitudinal strain and twisting–untwisting by affecting perfusion of subendocardial myocardial fibres [21]. Ιn our study, a decreased afterload post-liraglutide treatment due to the reduced brachial systolic and central systolic blood pressure and decreased arterial stiffness may have contributed to the increased myocardial perfusion, reduced oxygen demand and consequently to improved LV function [20, 21, 23]. Indeed, lower values of PWV post treatment were related to the respective improvement of GLS and GLSR.
Previous reporting of elevated heart rate associated with liraglutide treatment was also observed in our study [7]. The chronotropic effect of GLP-1 analogues are believed to be mediated via GLP-1 receptors located to the sinoatrial node [40]. However, GLP-1 may exhibit an inhibitory effect on sympathovagal balance as well [41]. This increase in heart rate has not outweighed the cardioprotective effects of liraglutide as assessed with the significant improvement in myocardial deformation and reduction of NT-proBNP after liraglutide treatment in our study.
Effects of weight reduction and glycaemic control
There were no effects of metformin on vascular and LV myocardial deformation, in spite of a similar glycaemic control as assessed by a comparable HbA1c post-treatment between patients treated with liraglutide and metformin. Thus the observed changes in biochemical and vascular markers should not be attributed to differences in glycaemic status post treatment.
In our study liraglutide caused a greater decrease of weight than metformin. However, a subgroup of patients that received liraglutide and had a weight reduction identical to that caused by metformin treatment had also improved arterial stiffness and myocardial deformation post-treatment. These results show that the better effects of liraglutide treatment compared to metformin on arterial stiffness, LV myocardial deformation and endothelial function should not be attributed solely to weight loss. Indeed, in multivariable analysis including BMI, weight, waist circumference and HbA1c, liraglutide treatment predicted an improved GLS (> − 15%), FMD (> 11%) and PWV (< 10 m/s) post treatment.
Effects on oxidative stress markers
Malondialdehyde and PCs are valid biomarkers of lipid peroxidation and protein oxidation respectively [42]. Glycation has been reported to induce the formation of protein carbonyls, such as ketoamine derivatives, thus generating reactive radicals and perpetuating a vicious cycle [43]. Cytokines and nitro-oxidative stress have a direct negative inotropic effect and promote myocardial ischemia, apoptosis, and LV dysfunction [20, 44]. Therefore, increased oxidative stress leading to vascular dysfunction and fibrosis may explain the link between arterial stiffness and impairment of LV myocardial deformation [45]. The interplay between oxidative stress, inflammation, endothelial dysfunction and type 2 diabetes have recently been studied in a multicenter, longitudinal investigation of the evolution of cardiovascular disease risk starting in young adulthood. Biomarkers of inflammation and endothelial dysfunction were positively associated with incident type 2 diabetes. Specifically, ICAM-1 and E-selectin added to the prediction of T2D beyond a common risk score [46].
In line with the above, in a 26-week, randomized, open-label, two-arm, parallel-group study, compared treatment with insulin glargine vs. exenatide on top of other standard of care therapies. Although both agents improved glycaemia and endothelial function, incretin therapy was superior for weight management, and several cardiovascular biomarkers’ profile, mainly cytokines like hs-CRP, MCP-1, fibrinogen and endothelin-1, implying a more pronounced anti-inflammatory effect [47]. The same was true for teneligliptin, a novel DPP4 inhibitor, that was found to ameliorate endothelial function and to reduce renal and vascular oxidative stress in patients with type 2 diabetes and chronic kidney disease, irrespectively of reducing albuminuria or glycaemia [48].
In our study baseline PCs levels determined the percent reduction of PWV post treatment. Furthermore, the reduction of oxidative stress, as assessed by MDA, was associated with improved arterial elasticity as assessed by PWV which in turn was associated with improved myocardial deformation after 6 months of treatment. The decrease of MDA and PCs was greater after liraglutide than after metformin treatment. Thus a lower oxidative stress level resulted in a greater improvement of vascular function and LV myocardial deformation.
Effects on natriuretic peptides
Natriuretic peptides are produced primarily within the heart and released into the circulation in response to increased wall tension [49]. Circulating concentrations of N-terminal pro-brain natriuretic peptide (NT-proBNP) are raised in both symptomatic and asymptomatic patients with left ventricular dysfunction [50]. In our study, reduced NT-proBNP post treatment was associated with increased LV longitudinal strain. Again, the reduction of NT-proBNP was greater after liraglutide than after metformin treatment in parallel with the greater beneficial effect of liraglutide on LV longitudinal deformation.
Study limitations and strengths
Οur study has a modest number of subjects. Additional large scale studies are needed to expand our results. In addition, our study could be extended in time in order to investigate the long-term benefits from the use of GLP-1 analogues on the cardiovascular system and endothelial function and determine how long can these benefits be maintained.
The present study adds to the literature, as it involves diabetic patients early in the course of disease, without prior treatment to interfere with the results, and also without clinical cardiovascular disease. It shows a beneficial effect of liraglutide in specific cardiac biomarkers and non-invasive measurements of cardiac function as well as in oxidative stress within solely 6-months. The latter suggests that this treatment category might benefit younger patients with a classic cardiometabolic profile earlier, before overt cardiovascular disease develops. Of note, in the LEADER trial it took 1 year for the treatment to show an overall CVD benefit, but without a specific beneficial effect on myocardial infarction (silent infarctions were not included in the analysis) [5]. Thus, one could conclude that if type 2 diabetic patients with the classic atherogenic profile are treated earlier in the course of disease with such agents, myocardial and endothelial function could be preserved. As the current study is a mechanistic study, whose aim is to show the beneficial effects of liraglutide on strict surrogate markers of cardiovascular function it does not suggest that liraglutide is a first-line treatment in DM.
Conclusions
In the present study, 6-month treatment with liraglutide resulted in a greater improvement of endothelial function, arterial stiffness, LV myocardial strain, twisting and untwisting, NT-proBNP and oxidative stress than metformin in newly diagnosed and treatment-naive patients with T2DM. Thus, in diabetic patients with evidence of arterial stiffness and impaired LV deformation even subclinically, early targeted antidiabetic treatment which offers myocardial protection may retard the progression to diabetic heart disease.
Authors’ contributions
Authors contributions is as follows: (1) study conception and design (VL, GP, II); (2) laboratory measurements (FK, MV, DV, EM, JP); (3) data analysis and interpretation (DG, IA, HT), (4) drafting of the manuscript (JL, EI, GD). All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Consent for publication
All authors have read and agree to the publication of the manuscript.
Ethics approval and consent to participate
The study was approved by the hospital’s research committee, and all subjects provided written informed consent.
Funding
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- T2DM
type 2 diabetes mellitus
- LV
left ventricle
- GLP-1
glucagon like peptide-1
- PWV
pulse wave velocity
- GLS
global longitudinal strain
- GLSR
global longitudinal strain rate
- pTw
peak twisting
- pTwVel
peak twisting velocity
- pUtwVel
peak untwisting velocity
- %dpTw–UtwMVO
the percentage difference between peak twisting and untwisting at mitral valve opening
- %dpTw–UtwPEF
the percentage difference between peak twisting and untwisting at peak of early LV diastolic filling
- %dpTw–UtwEDF
the percentage difference between peak twisting and untwisting at end of early diastolic filling
- FMD
flow mediated dilatation
- FMD%
the percentage difference of flow mediated dilatation
- MDA
malondialdehyde
- PCs
protein carbonyls
- NT-proBNP
N-terminal pro-brain natriuretic peptide
Footnotes
Vaia Lambadiari and George Pavlidis equally contributed to this work
Contributor Information
Vaia Lambadiari, Phone: +30210 5831148, Email: vlambad@otenet.gr.
George Pavlidis, Email: geo_pavlidis@yahoo.gr.
Foteini Kousathana, Email: f.kousathana@hotmail.com.
Maria Varoudi, Email: mvaroudi@gmail.com.
Dimitrios Vlastos, Email: dimitrisbvr@hotmail.com.
Eirini Maratou, Email: maratou@hotmail.com.
Dimitrios Georgiou, Email: digeorgiou@yahoo.gr.
Ioanna Andreadou, Email: jandread@pharm.uoa.gr.
John Parissis, Email: jparissis@yahoo.com.
Helen Triantafyllidi, Email: seliani@hotmail.com.
John Lekakis, Email: lekakisster@gmail.com.
Efstathios Iliodromitis, Email: iliodromitis@yahoo.gr.
George Dimitriadis, Email: gdimitr@uoa.med.gr.
Ignatios Ikonomidis, Email: ignoik@otenet.gr.
References
- 1.Authors Task Force, Members Rydén L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, ESC Committee for Practice Guidelines (CPG) Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD) Eur Heart J. 2013;34:3035–3087. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 2.Avery CL, Loehr LR, Baggett C, Chang PP, Kucharska-Newton AM, Matsushita K, et al. The population burden of heart failure attributable to modifiable risk factors: the ARIC (Atherosclerosis Risk in Communities) study. J Am Coll Cardiol. 2012;60:1640–1646. doi: 10.1016/j.jacc.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 4.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, LEADER Steering Committee. LEADER Trial Investigators et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du Q, Wang YJ, Yang S, Zhao YY, Han P. Liraglutide for the treatment of type 2 diabetes mellitus: a meta-analysis of randomized placebo-controlled trials. Adv Ther. 2014;31:1182–1195. doi: 10.1007/s12325-014-0164-2. [DOI] [PubMed] [Google Scholar]
- 7.Robinson LE, Holt TA, Rees K, Randeva HS, O’Hare JP. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ Open. 2013;3(1):e001986. doi: 10.1136/bmjopen-2012-001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. EMPA-REG OUTCOME investigators. empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 9.Schram MT, Henry RM, van Dijk RA, Kostense PJ, Dekker JM, Nijpels G, et al. Increased central artery stiffness in impaired glucose metabolism and type 2 diabetes: the Hoorn Study. Hypertension. 2004;43:176–181. doi: 10.1161/01.HYP.0000111829.46090.92. [DOI] [PubMed] [Google Scholar]
- 10.Cortigiani L, Rigo F, Gherardi S, Sicari R, Galderisi M, Bovenzi F, et al. Additional prognostic value of coronary flow reserve in diabetic and nondiabetic patients with negative dipyridamole stress echocardiography by wall motion criteria. J Am Coll Cardiol. 2007;50:1354–1361. doi: 10.1016/j.jacc.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 11.Naka KK, Papathanassiou K, Bechlioulis A, Kazakos N, Pappas K, Tigas S, et al. Determinants of vascular function in patients with type 2 diabetes. Cardiovasc Diabetol. 2012;11:127. doi: 10.1186/1475-2840-11-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng AC, Delgado V, Bertini M, van der Meer RW, Rijzewijk LJ, Shanks M, et al. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol. 2009;104:1398–1401. doi: 10.1016/j.amjcard.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 13.Holland DJ, Marwick TH, Haluska BA, Leano R, Hordern MD, Hare JL, et al. Subclinical LV dysfunction and 10-year outcomes in type 2 diabetes mellitus. Heart. 2015;101:1061–1066. doi: 10.1136/heartjnl-2014-307391. [DOI] [PubMed] [Google Scholar]
- 14.Ikonomidis I, Lekakis J, Papadopoulos C, Triantafyllidi H, Paraskevaidis I, Georgoula G, et al. Incremental value of pulse wave velocity in the determination of coronary microcirculatory dysfunction in never-treated patients with essential hypertension. Am J Hypertens. 2008;21:806–813. doi: 10.1038/ajh.2008.172. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto K, Burnett JC, Jr, Jougasaki M, Nishimura RA, Bailey KR, Saito Y, et al. Superiority of brain natriuretic peptide as a hormonal marker of ventricular systolic and diastolic dysfunction and ventricular hypertrophy. Hypertension. 1996;28:988–994. doi: 10.1161/01.HYP.28.6.988. [DOI] [PubMed] [Google Scholar]
- 16.Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 17.Forst T, Michelson G, Ratter F, Weber MM, Anders S, Mitry M, et al. Addition of liraglutide in patients with Type 2 diabetes well controlled on metformin monotherapy improves several markers of vascular function. Diabet Med. 2012;29:1115–1118. doi: 10.1111/j.1464-5491.2012.03589.x. [DOI] [PubMed] [Google Scholar]
- 18.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34:2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 19.Sung SH, Yu WC, Cheng HM, Chuang SY, Wang KL, Huang CM, et al. Pulsatile hemodynamics and clinical outcomes in acute heart failure. Am J Hypertens. 2011;24:775–782. doi: 10.1038/ajh.2011.26. [DOI] [PubMed] [Google Scholar]
- 20.Ikonomidis I, Tzortzis S, Andreadou I, Paraskevaidis I, Katseli C, Katsimbri P, et al. Increased benefit of interleukin-1 inhibition on vascular function, myocardial deformation, and twisting in patients with coronary artery disease and coexisting rheumatoid arthritis. Circ Cardiovasc Imaging. 2014;7:619–628. doi: 10.1161/CIRCIMAGING.113.001193. [DOI] [PubMed] [Google Scholar]
- 21.Raptis AE, Markakis KP, Mazioti MC, Ikonomidis I, Maratou EP, Vlahakos DV, et al. Effect of aliskiren on circulating endothelial progenitor cells and vascular function in patients with type 2 diabetes and essential hypertension. Am J Hypertens. 2015;28:22–29. doi: 10.1093/ajh/hpu119. [DOI] [PubMed] [Google Scholar]
- 22.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 23.Ikonomidis I, Makavos G, Papadavid E, Varoudi M, Andreadou I, Gravanis K, et al. Similarities in coronary function and myocardial deformation between psoriasis and coronary artery disease: the role of oxidative stress and inflammation. Can J Cardiol. 2015;31:287–295. doi: 10.1016/j.cjca.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Parissis JT, Kourea K, Andreadou I, Ikonomidis I, Markantonis S, Ioannidis K, et al. Effects of Darbepoetin Alfa on plasma mediators of oxidative and nitrosative stress in anemic patients with chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2009;103:1134–1138. doi: 10.1016/j.amjcard.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 25.Sengupta PP, Krishnamoorthy VK, Korinek J, Narula J, Vannan MA, Lester SJ, et al. Left ventricular form and function revisited: applied translational science to cardiovascular ultrasound imaging. J Am Soc Echocardiogr. 2007;20:539–551. doi: 10.1016/j.echo.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taegtmeyer H. Cardiac metabolism as a target for the treatment of heart failure. Circulation. 2004;110:894–896. doi: 10.1161/01.CIR.0000139340.88769.D5. [DOI] [PubMed] [Google Scholar]
- 27.Noyan-Ashraf MH, Shikatani EA, Schuiki I, Mukovozov I, Wu J, Li RK, et al. A glucagon-like peptide-1 analog reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation. 2013;127:74–85. doi: 10.1161/CIRCULATIONAHA.112.091215. [DOI] [PubMed] [Google Scholar]
- 28.Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, et al. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004;110:955–961. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- 29.Villanueva-Peñacarrillo ML, Puente J, Redondo A, Clemente F, Valverde I. Effect of GLP-1 treatment on GLUT2 and GLUT4 expression in type 1 and type 2 rat diabetic models. Endocrine. 2001;15:241–248. doi: 10.1385/ENDO:15:2:241. [DOI] [PubMed] [Google Scholar]
- 30.Vyas AK, Yang KC, Woo D, Tzekov A, Kovacs A, Jay PY, et al. Exenatide improves glucose homeostasis and prolongs survival in a murine model of dilated cardiomyopathy. PLoS ONE. 2011;6:e17178. doi: 10.1371/journal.pone.0017178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scalzo RL, Moreau KL, Ozemek C, Herlache L, McMillin S, Gilligan S, et al. Exenatide improves diastolic function and attenuates arterial stiffness but does not alter exercise capacity in individuals with type 2 diabetes. J Diabetes Complicat. 2017;31(2):449–455. doi: 10.1016/j.jdiacomp.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smits MM, Tonneijck L, Muskiet MH, Hoekstra T, Kramer MH, Diamant M, et al. The effects of GLP-1 based therapies on postprandial haemodynamics: two randomised, placebo-controlled trials in overweight type 2 diabetes patients. Diabetes Res Clin Pract. 2017;124:1–10. doi: 10.1016/j.diabres.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Kumarathurai P, Anholm C, Nielsen OW, Kristiansen OP, Mølvig J, Madsbad S, et al. Effects of the glucagon-like peptide-1 receptor agonist liraglutide on systolic function in patients with coronary artery disease and type 2 diabetes: a randomized double-blind placebo-controlled crossover study. Cardiovasc Diabetol. 2016;15(1):105. doi: 10.1186/s12933-016-0425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nomoto H, Miyoshi H, Furumoto T, Oba K, Tsutsui H, Miyoshi A, et al. A Comparison of the effects of the GLP-1 analogue liraglutide and insulin glargine on endothelial function and metabolic parameters: a randomized, controlled trial sapporo athero-incretin study 2 (SAIS2) PLoS ONE. 2015;10(8):e0135854. doi: 10.1371/journal.pone.0135854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomiyama H, Miwa T, Kan K, Matsuhisa M, Kamiya H, Nanasato M, et al. Impact of glycemic control with sitagliptin on the 2-year progression of arterial stiffness: a sub-analysis of the PROLOGUE study. Cardiovasc Diabetol. 2016;15(1):150. doi: 10.1186/s12933-016-0472-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lovshin JA, Barnie A, DeAlmeida A, Logan A, Zinman B, Drucker DJ. Liraglutide promotes natriuresis but does not increase circulating levels of atrial natriuretic peptide in hypertensive subjects with type 2 diabetes. Diabetes Care. 2015;38:132–139. doi: 10.2337/dc14-1958. [DOI] [PubMed] [Google Scholar]
- 37.Nyström T. The potential beneficial role of glucagon-like peptide-1 in endothelial dysfunction and heart failure associated with insulin resistance. Horm Metab Res. 2008;40:593–606. doi: 10.1055/s-0028-1082326. [DOI] [PubMed] [Google Scholar]
- 38.Song X, Jia H, Jiang Y, Wang L, Zhang Y, Mu Y, et al. Anti-atherosclerotic effects of the glucagon-like peptide-1 (GLP-1) based therapies in patients with type 2 diabetes mellitus: a meta-analysis. Sci Rep. 2015;5:10202. doi: 10.1038/srep10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mochizuki Y, Tanaka H, Matsumoto K, Sano H, Toki H, Shimoura H, et al. Clinical features of subclinical left ventricular systolic dysfunction in patients with diabetes mellitus. Cardiovasc Diabetol. 2015;14:37. doi: 10.1186/s12933-015-0201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pyke C, Heller RS, Kirk RK, Ørskov C, Reedtz-Runge S, Kaastrup P, et al. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155:1280–1290. doi: 10.1210/en.2013-1934. [DOI] [PubMed] [Google Scholar]
- 41.Griffioen KJ, Wan R, Okun E, Wang X, Lovett-Barr MR, Li Y, et al. GLP-1 receptor stimulation depresses heart rate variability and inhibits neurotransmission to cardiac vagal neurons. Cardiovasc Res. 2011;89:72–78. doi: 10.1093/cvr/cvq271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin Chem. 1997;43:1209–1214. [PubMed] [Google Scholar]
- 43.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-H. [DOI] [PubMed] [Google Scholar]
- 44.Prabhu SD. Cytokine-induced modulation of cardiac function. Circ Res. 2004;95:1140–1153. doi: 10.1161/01.RES.0000150734.79804.92. [DOI] [PubMed] [Google Scholar]
- 45.Paterson I, Mielniczuk LM, O’Meara E, So A, White JA. Imaging heart failure: current and future applications. Can J Cardiol. 2013;29:317–328. doi: 10.1016/j.cjca.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Odegaard AO, Jacobs DR, Jr, Sanchez OA, Goff DC, Jr, Reiner AP, Gross MD. Oxidative stress, inflammation, endothelial dysfunction and incidence of type 2 diabetes. Cardiovasc Diabetol. 2016;15:51. doi: 10.1186/s12933-016-0369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gurkan E, Tarkun I, Sahin T, Cetinarslan B, Canturk Z. Evaluation of exenatide versus insulin glargine for the impact on endothelial functions and cardiovascular risk markers. Diabetes Res Clin Pract. 2014;106(3):567–575. doi: 10.1016/j.diabres.2014.09.046. [DOI] [PubMed] [Google Scholar]
- 48.Sagara M, Suzuki K, Aoki C, Tanaka S, Taguchi I, Inoue T, et al. Impact of teneligliptin on oxidative stress and endothelial function in type 2 diabetes patients with chronic kidney disease: a case-control study. Cardiovasc Diabetol. 2016;15:76. doi: 10.1186/s12933-016-0396-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kinnunen P, Vuolteenaho O, Ruskoaho H. Mechanisms of atrial and brain natriuretic peptide release from rat ventricular myocardium: effect of stretching. Endocrinology. 1993;132:1961–1970. doi: 10.1210/endo.132.5.8477647. [DOI] [PubMed] [Google Scholar]
- 50.McDonagh TA, Robb SD, Murdoch DR, Morton JJ, Ford I, Morrison CE, et al. Biochemical detection of left-ventricular systolic dysfunction. Lancet. 1998;351:9–13. doi: 10.1016/S0140-6736(97)03034-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.


