Abstract
Background
Drivers of change in dairy herd health management include the significant increase in herd/farm size, quota removal (within Europe) and the increase in technologies to aid in dairy cow reproductive management.
Main body
There are a number of key areas for improving fertility management these include: i) handling of substantial volumes of data, ii) genetic selection (including improved phenotypes for use in breeding programmes), iii) nutritional management (including transition cow management), iv) control of infectious disease, v) reproductive management (and automated systems to improve reproductive management), vi) ovulation / oestrous synchronisation, vii) rapid diagnostics of reproductive status, and viii) management of male fertility. This review covers the current status and future outlook of many of these key factors that contribute to dairy cow herd health and reproductive performance.
Conclusions
In addition to improvements in genetic trends for fertility, numerous other future developments are likely in the near future. These include: i) development of new and novel fertility phenotypes that may be measurable in milk; ii) specific fertility genomic markers; iii) earlier and rapid pregnancy detection; iv) increased use of activity monitors; v) improved breeding protocols; vi) automated inline sensors for relevant phenotypes that become more affordable for farmers; and vii) capturing and mining multiple sources of “Big Data” available to dairy farmers. These should facilitate improved performance, health and fertility of dairy cows in the future.
Keywords: Dairy cattle, Herd health management, Cow fertility, Reproduction, Breeding
Background
Dairy herd health management is undergoing a period of radical change worldwide. The drivers of this change are many and include the massive increase in technologies to aid in dairy cow reproductive management, quota removal (within Europe) and the significant increase in herd / farm size. Following the removal of quotas in Europe many countries are expanding dairy production, for example Ireland has ambitious plans to expand dairy output by 50%, this is to be achieved by a combination of increased herd size and greater milk output per cow [1, 2]. The present paper aims to identify some of the changes that will facilitate increased output, enhanced dairy-cow-herd health and reproductive management.
Within Europe, dairy herd size and numbers have been largely static from 1984 until 2015. As from April 2015, quotas have been removed allowing the opportunity for expansion to occur, this is likely to take the form of both increases in cow yields and increases in cow numbers. In Ireland, the Food Harvest 2020 report [1] (and underpinned by the Foodwise 2025 report [2]) discusses the expansion of dairy output by 50% between 2015 and 2020. Similar expansion is occurring in the Netherlands, although total phosphate limits are posing a challenge to expansion of cow numbers in that country. This review will focus on developments in the areas of data management, nutritional strategies, genetic strategies, disease control, precision livestock farming (hormonal treatments and sensor technologies) and male fertility that will have potential impact on increased milk production, cow health and cow fertility.
Genetic strategies to improve reproduction
Up to the early 2000s, dairy genetic selection programmes in dairy producing countries traditionally selected predominantly for milk yield often at the expense of other dairy relevant traits, including fertility and health [3–5]. Breeding programmes in the early part of this century started to include fertility (e.g., by including traits such as longevity and calving intervals) and health as part of the selection traits. Inclusion of these traits has served to reverse some of the earlier trends that gave rise to reduced fertility. Over the last 15 years it is now recognised that trends in both longevity (increased) and calving intervals (decreased) have improved [5]. A major challenge for breeding programmes in terms of incorporation of fertility traits has been to develop phenotypes that have reasonable heritability. For example many fertility traits have typically only low heritability estimates (e.g., 0.1, compared with many growth and carcass traits where the heritability is 0.25–0.5). A second major issue for many fertility traits is to have easily measured phenotypic traits or genomic markers (single-nucleotide polymorphisms; SNPs) that correlate to appropriate fertility traits.
Opportunities may now be arising for selection of new traits that could be incorporated into breeding programmes. An EU funded project “Genotype plus Environment” (GplusE) has amongst its many objectives the identification of new novel milk based phenotypes that may be used as predictors for the traditional, but as well as the difficult to measure, record and select traits such as conception rates and uterine health (www.gpluse.eu). This project aims to develop, amongst other things, novel milk based traits that correlate and predict health and fertility traits in dairy cows. The strategies being used include the measurement of Mid-infrared spectra (MIR) in milk, metabolites in milk and glycans on the immuno-gamma globulin (IgG) fraction of milk. The project is relating these new measurable traits to fertility and health traits [6–9], and then relating both novel and traditional traits to novel genomic markers (SNPs) eventually facilitating improved selection strategies in the future. This project and work from other labs should result in further fertility SNPs that may enhance genetic selection for additional improvements in fertility.
New tools and applications to new phenotypes that may be used in the dairy sector
Recent work in University College Dublin has led to the development of glycan markers for uterine health. This has been developed into a patent application (PCT/EP2014/068734: “Methods for predicting, diagnosing or monitoring infections or conditions”). Indeed milk-based glycan markers have also been developed that can predictively identify cows having retained placental membranes [6]. Such biomarkers that are easily measured in milk would allow animal breeders to select for cows with a propensity for improved uterine health and therefore move towards cows that would have increased fertility.
While in-vitro fertilisation (IVF) and embryo transfer are now significant tools to increase genetic selection on the female side with Bos indicus cattle [10], currently multiple ovulation and embryo transfer remains the more cost-effective method for Bos Taurus cattle (including all significant dairy breeds: Holstein-Friesian, Brown Swiss, Jersey etc) at population level. This is because the Bos Taurus breeds only produce between 5 to 20 follicles per follicle wave emergence event [11], which is insufficient numbers to allow adequate numbers of ova for effective culture for IVF.
Nutritional strategies to improve reproduction
Modern dairy cows have been predominantly selected for a high milk yield in early lactation that is associated with a very high capacity to mobilize body reserves during this period. In a study by Tamminga et al. [12] with 5 production trials using 295 cows, calculations showed that cows can produce as much as between 120 and 550 kg of milk from body reserves on the basis of energy (average 324 kg). Maximum mobilisation in 8 weeks amounted to 41.6 kg empty body weight, 30.9 kg fat and 4.6 kg protein [12]. Most cows can cope with this metabolic load which is defined as: ‘the total energy burden imposed by the synthesis and secretion of milk, which may be met by mobilisation of body reserves’ [13]. Metabolic stress however is defined as ‘the amount of metabolic load that cannot be sustained by this mobilisation, leading to the down-regulation of some energetic processes, including those that maintain general health’ [13]. Hence, the ‘over’ mobilisation of body reserves during the period of NEB is a key factor for disease susceptibility in modern dairy cattle. Furthermore, in addition to post calving energy balance, pre-calving loss in body condition also has significant consequences for metabolic status, milk composition and subsequent health [14] and should be acknowledged.
The genetically and hormonally driven body mobilisation is further aggravated by a serious mismatch between the energy need and the cow’s capacity to take in energy [15]. The latter often being even further negatively affected by an inadequate adaptation of both the gastro-intestinal tract and the overall intermediary metabolism and often an elevated incidence of diseases in the period after calving [15]. Maximal feed intake occurs commonly at 6 to 8 weeks in lactation, which is much later than peak production, causing cows typically to be in negative energy balance for 5–7 weeks post partum [12].
Components of reduced fertility in modern dairy cows include delayed resumption of normal ovarian cyclicity [16–18], uterine health [17–19], lower expression of heat symptoms and lower pregnancy rates to first and subsequent inseminations. The latter mainly being caused by an increased incidence of embryonic and foetal death [20]. Relevant review papers have been published about the mechanistic backgrounds of the relationship between metabolic stress and impaired fertility in modern postpartum dairy cows [21, 22].
Management strategies for transition cows are mainly focused on helping the cows to cope with the metabolic load by optimizing health, minimizing stress (e.g., by minimising the changes in group or ration), stimulating dry matter intake and immune function. There are great opportunities for the veterinary practitioner to regularly monitor and adapt the herd management in order to do so. LeBlanc [23] and Mulligan et al. [15] identified the key issues that should be covered by the practitioner to optimally guide farmer clients to optimize their transition-cow management.
Furthermore, application of diets specifically designed to improve fertility by counteracting mechanisms related to the negative energy balance (NEB) or by supporting a specific pathway that is necessary for successful fertility, has always been a very attractive way to circumvent the impairment of reproduction during early lactation [24]. Although the reproductive system is known to be influenced by multiple hormones that are also involved in the adaptation towards high milk production (e.g., growth hormone; GH, insulin-like growth factor I; IGF-I and leptin), only insulin is known to be relatively responsive to changes in the composition of the ration [25]. Ovarian follicles contain insulin receptors [26] and cows with lower peripheral insulin levels in the immediate postpartum period suffer from retarded postpartum ovarian resumption and normal cyclicity among others by a higher risk to suffer from cystic ovarian disease [27]. Therefore, glucogenic diets have been advocated in the immediate postpartum period aiming to enhance the peripheral insulin concentrations and advance normal ovarian resumption [25]. However, insulin has been shown to have detrimental effects on oocyte and embryo competence [28] and has been shown to stimulate enzymatic catabolism of progesterone (P4) in the liver [29]. The latter suggests glucogenic diets only being of advantage when offered in the immediate postpartum period, while they should be avoided when cows are inseminated.
Rations leading to high peripheral urea levels are generally mentioned to be associated with lower pregnancy rates due to its detrimental effects on the embryo [30]. However, the mechanistic pathways by which this detrimental effect may be caused and the threshold peripheral urea concentrations, are both still matters of debate. Special attention in this respect should be given to the supplementation of soyabean meal as the main protein source in the ration. In a recent study it was demonstrated that commercially available soyabean meal contains isoflavones in concentrations that are able to induce increases in the blood concentration of oestrogenically active isoflavone metabolites (equol, O-desmethylangolensin, dihydrodaidzein) in high yielding dairy cows post partum, even when supplemented in relatively low amounts (1.72 kg per day on average) [26]. When compared with rapeseed meal, soya supplementation was furthermore associated with a decreased angio- and steroidogenesis at the level of the corpus luteum (CL) based on biopsy sampling at day 9 of the oestrous cycle [31]. However, it was not possible to demonstrate any effect on the peripheral progesterone concentration during the first 3 oestrous cycles after calving [31]. Therefore, although the results of that study suggest negative effects of soya feeding on CL function in recently calved dairy cows, the contribution of this effect on the peripheral progesterone concentration and consequently on overall fertility of supplemented cows warrants further research [31].
Adding fats is another strategy that has been extensively tested to reduce the impaired reproductive capacity of dairy cows. A study aiming to minimize the negative energy balance by decreasing the milk fat synthesis and hence limiting energy output via milk by supplementing the ration with exogenous fats, was not successful since cows simply produced more milk when reducing the NEB [32]. Omega-6 fatty acids are believed to have pro-inflammatory and thus prostaglandin F2alpha (PGF)-stimulating properties rendering them of extra value early post-partum, while omega-3 fatty acids can weaken this inflammatory potency, leading to a higher chance of survival of the embryo when supplemented during the periconceptual period [33]. Unfortunately, research results rarely provide a consensus in this topic. The consequences of these fat-feeding strategies on oocyte and embryo quality remain an intriguing issue for debate. Fat feeding may alter the microenvironment of the growing and maturing oocyte of the early and older embryo and thus may affect reproductive outcome [34]. Research has shown that dietary-induced hyperlipidaemic conditions can be harmful for embryo development and metabolism [35]. However, to date, research results remain somewhat conflicting most probably due to differences in fat sources used, in diet and duration of supplementation and in experimental set-up in general [35]. Furthermore, peripheral blood in lactating dairy cows will contain a mixture of fatty acids of dietary origin and from body-tissue breakdown, the latter being largely abundant in the immediate postpartum period and containing a high proportion of saturated fatty acids [34, 36]. Especially the latter have been shown to have a significantly detrimental effect on both the oocyte as well as embryo quality [34].
Adding extra vitamins and minerals to the diet has often been suggested as a “golden bullet” solution to reduce declines in cow fertility by various commercial interests, while requirements for optimal reproductive efficiency in modern dairy cattle deserve careful re-evaluation based on well-designed scientific research [37]. Usually farmers readily adopt these “proposed supplement solutions” since they don’t involve extra labour which is often their paramount constraint. Stating whether the amount of these compounds is sufficient in the ration is often very difficult for the practitioner since it is usually impossible to even estimate the content of these substances present in the basic roughage ration. In herds in which cows are given high quantities of concentrates to sustain peak yield in the immediate postpartum period, the risk of suffering from specific deficiencies is lower due to the fact that concentrates are usually highly supplemented with vitamins and minerals [37]. In terms of their effect on immune response and embryo quality, special attention should be given to vitamin E and selenium. The latter was supported by recent finding that in herds that were tocopherol deficient during the dry period, treatment with injectable vitamin E of 1000 IU each week for the last 3 weeks of gestation not only reduced the incidence of retained placenta and stillbirth but also significantly decreased pregnancy loss (20.5% vs. 12.5%; P < 0.01) [38].
Controlling infectious diseases
Veterinarians managing fertility in dairy herds should regularly evaluate the herd health status for pathogens known to compromise reproductive efficiency. Infections with pathogens like Leptospira hardjo, bovine viral diarrhoea or herpes viruses are known to reduce conception rates, while infections with Neospora caninum and emerging viruses like the bluetongue virus may cause foetal losses and abortions. Bovine herpes virus 4 is reported to have a tropism for endometrial cells and therefore should be specifically monitored and controlled in herds suffering from uterine diseases, particularly where other risk factors are controlled or ruled out [39]. In addition to continuing careful monitoring and appropriate biosecurity plans inclusion of appropriate vaccination protocols may be required to prevent the introduction of new agents into the herd and to prevent spread within the herd [40].
Of special interest among bacterial diseases, is the minimisation of uterine disease. In cattle, bacterial contamination of the uterus is ubiquitous at parturition. However, this does not automatically imply the establishment of uterine disease and subsequent fertility problems. It is generally a suppression in uterine immune function in addition to pathogen presence that allows a shift in bacterial populations and establishment of disease in up to 20% of animals [19, 42]. Despite the fact that several papers have been published aiming to come to a general agreement about the definitions of postpartum uterine diseases based on mainly clinical symptoms [41, 42], there is still a lot of confusion about these definitions among practitioners. This confusion in definitions gives rise to a wide variety of preventative and curative treatment protocols being applied in the field, many of which are not scientifically proven to be efficacious. Recent literature underlines the high incidence of especially subclinical endometritis in high yielding herds [43]. Diagnosis of this impairment is based on intra-uterine sampling for cytology, which is not routinely done at the moment. Therefore, work by Pascottini et al. [44] reported the use of the cytotape that allows sampling early post partum and during insemination, and facilitates profiling of uterine cytology in repeat breeder cows. The generally accepted necessity to minimise the use of antibiotics in cows should be extended to treatment of uterine infections. It is important to determine the risk factors for the different uterine diseases, and design prevention and control programmes to reduce the incidence of disease.
Use of precision livestock farming
Oestrous detection
Traditional approaches to reproductive management and use of artificial insemination have included either visual observation of oestrous behaviour, or the use of fixed time insemination protocols (e.g., OVSYNC [45]).
To achieve high submission rates to artificial insemination (AI), which are critical to achieve a 365-d calving interval in seasonal calving herds, requires an effective, practical means of identifying each cow in oestrus. Standing to be mounted is considered the main behavioral sign identifying an oestrous period and is used to determine the correct time to inseminate [46]. Both the physical activity and mounting activity induced by increased oestradiol production during the preovulatory follicular phase can be monitored in various ways. Heat detection rates (submission rate) vary from herd to herd with between 30 and 70% of cows exhibiting oestrous behaviour usually being detected in oestrus. With optimal visual observation of mounting activity for 20 min 5 times per day heat detection rates of 90 to 95% may be achieved [47] but is considered laborious and time consuming. With lower frequency of observation, lower rates of oestrous detection are achieved especially with higher yielding cows (e.g., only 70% of cows detected in oestrus with two or three observation periods of 30 min duration [48]).
Furthermore, in high-yielding Holstein-Friesian dairy cows, the percentage of cows that display standing to be mounted by other cows has decreased, leaving it more difficult to detect oestrus [49]. Roelofs et al. [49] found that only 58% of cows were observed in standing oestrus. This, in turn, decreases submission rate to AI and thereby contributes significantly to reduced reproductive efficiency [50].
Successful reproductive performance based on detection of oestrous behaviour requires the need to accurately detect oestrous onset in the majority of cows, and then inseminate 4 to 16 h later [51]. This led to the common practice of breeding cows according to the am-pm rule which requires that cows are observed for oestrus five-times per day, those commencing oestrus in the morning get inseminated that evening and those commencing oestrus after 12.00 noon are inseminated the next morning (onset of oestrus defined as the first observation period where the cow is observed to stand to be mounted by other herd mates or a teaser bull).
The approach of oestrous observation has served well for herds prepared to invest the time and effort into good and accurate oestrous detection. However, it requires a significant commitment of labour, good cow identification and personnel trained in detection of oestrus in cows.
Sensors for oestrous detection
Over the last 2 decades various systems for automation of oestrous detection have been developed to various degrees of success.
Pressure sensors
The characteristic oestrous behaviour of standing to be mounted can be monitored through the use of systems such as, scratch cards (e.g., Estrotect; Rockway Inc., Spring Valley, WI), colour ampoules (Kamar Products Inc., Zionsville, IN), vasectomized bulls fitted with a chin-ball marker, the use of tail-painting methods or the electronic device HeatWatch [47, 48, 52].
Activity monitors
One labour-saving technology available to farmers to help increase submission rate and decrease labour requirements for oestrous detection is the use of a monitor of physical activity. The pedometer, attached to a leg, detects an increase in the number of steps taken per hour during oestrus (e.g., S.A.E. Afikim, Kibbutz Afikim, Israel) [52], whereas the use of a neck collar (e.g., Alpro; DeLaval International AB, Tumba, Sweden; Heatime, SCR, Netanya, Israel; MooMonitor; Dairy Master, Ireland) [53] identifies increased physical activity (walking, mounting, getting up and lying down) expressed as an activity cluster (AC) and alerts the farmer as to when the AC started (when the cows next enter the milking parlour). It can therefore identify for the farmer the optimum time to AI, which is during a 12- to 18-h window before the predicted time of ovulation. A recent study, using the neck collar activity monitor Heatime (SCR Engineers Ltd., Netanya, Israel), identified that the odds of an AC being in a pre-ovulatory follicular phase rather than a luteal phase improved by 29% for every 1-unit increase in peak activity and by 91% for every 2-h increase in duration of an AC (Fig. 1, Fig. 2) [54]. Using one such activity monitor (Heatime) the optimum time to inseminate was between 9 and 15 h after the activity cluster was triggered [55].
Fig. 1.
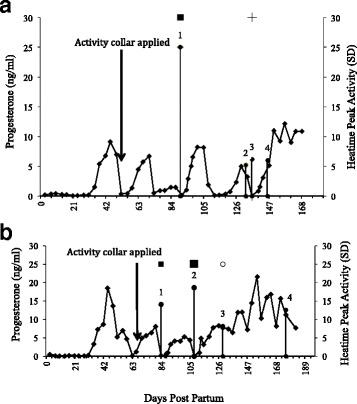
Milk progesterone profiles and activity clusters(*) associated with different reproductive states for two representative post partum dairy cows (a and b). Heatime™ activity clusters are labelled 1–4. Insemination with conception = symbol ■. Insemination and full term pregnancy resulting = symbol +. Insemination whilst pregnant and still went full term = symbol O. Aungier et al. [48]
Fig. 2.
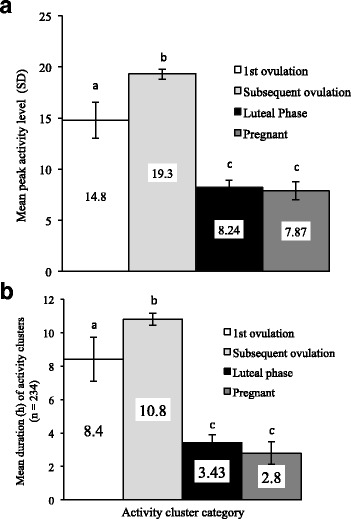
a The Mean ± SEM peak activity level of activity clusters was influenced by the endocrine state in which they occurred and b The Mean ± SEM duration of activity clusters was influenced by the endocrine state in which they occurred. a-cMeans within a bar chart with different superscripts differ (P < 0.0001). Aungier et al. [48]
Endocrine profiling
A commercially available in-line measurement system for endocrine profiling has been recently developed (Herd Navigator, Delaval) to detect metabolites and P4 concentration in milk [56]. Using algorithms P4 profiles may be used to predict oestrous events and potentially pregnancy status. However, to date this technology is still relatively expensive which is limiting its adoption. In addition, there are limits to its technical usefulness.
i) the system was initially developed assuming daily measurement of P4 in milk, however in the commercialised format it is often considered too expensive to use for daily measurements and generally only gets used twice or once weekly in herds equipped with this technology [56].
ii) The follicular phase in cattle can vary from 3 to 7 days and is highly variable, even with daily measurements the transition to the follicular phase (i.e., high P4 to low P4 is marked by the drop in P4) is not a good predictor of ovulation or onset of oestrus and is therefore not specific enough for timing of inseminations in practice. It can however identify follicular phase cows that should then be specifically observed for signs of oestrous behaviour (by other means) to then allow timing of insemination. Where measurement is only once or twice weekly this becomes much less useful and at weekly intervals the follicular phase can be inadvertently missed entirely.
iii) As a method for determining pregnancy status P4 is more reliable as a non pregnancy test than confirming pregnant positive cows. This is because a drop in P4 18–24 days after a correctly times insemination means non-pregnant. However high P4 18–24 days after an insemination can be due to pregnancy; or miss-timed initial insemination (meaning the cow is now in a non-pregnant luteal phase); or a persistent CL that appears as an early pregnancy profile, in the absence of a pregnancy (often associated with uterine infection); or initial pregnancy followed by embryo loss which will result in high progesterone, now in the absence of a pregnancy. In all of these cases a higher frequency of measurement (i.e. daily) will help reduce these problems, but do not completely overcome the limitations of the use of P4 as an indicator of pregnancy state.
Oestrous synchronisation and ovulation synchronisation
Traditional oestrous synchronisation methods (i.e., prostaglandin only programmes and 12-day progesterone programmes) were designed to synchronise oestrus, but generally still required observation of oestrus to optimize timing of mating and pregnancy rates. As an exception to this two injections of prostaglandin 11 days apart in maiden heifers can work with fixed-time insemination (FTAI) at 72 and 96 h or alternatively at 72 h, and then intensively observe for oestrus for a further 3–4 days and inseminate those late coming into oestrus, in response to standing oestrus (using the am-pm rule) [51]. This protocol in cows required observation for oestrus after the second prostaglandin injection.
Ovulation synchronisation programmes were designed to facilitate use of FTAI in herds without significant investment of time and labour into oestrous detection. These were developed from the early 1990s onwards [45]. They are more appropriate to large non-seasonal herds where calving to calving intervals are somewhat less relevant to economic performance of the herd and often calving intervals are allowed to extend beyond 400–420 days. The major issues for a basic ovulation synchronisation (OVSYNCH) programme is that conception rates to a single round of OVSYNCH are approximately only 30% [57, 58]; and in a European context are relatively expensive. Strategies to improve pregnancy rates have been developed (e.g., Double OVSYNCH and presynchronisation-ovulation synchronisation (PRESYNCH-OVSNCH) that are acceptable in many US herds (46 and 41% conception rates, respectively [59]), but incur substantial costs in terms or time, drug costs, compliance and public perceptions (around routine use of hormones in cattle production) that leave their use questionable in European dairy herds. For seasonal herds the treatment time required for OVSYNCH, PRESYNCH-OVSYNCH and Double OVSYNCH protocols are too long relative to the conception rates that can be achieved. Progesterone based programmes (e.g., 7 or 8 day protocol) using an intravaginal device incorporating GnRH at the start and PGF at the end (Day 7) gives better results in terms of synchronisation and pregnancy rates in healthy cows [60].
Pregnancy detection
Direct methods of pregnancy detection
Various methods are available to determine pregnancy status, these include return to oestrus [61], rectal palpation of the reproductive tract [62, 63] and ultrasound scanning to observe the reproductive tract [64, 65]. In practice return to oestrus is fraught by the difficulties associated with oestrous observation, so currently most pregnancy detection in cows is carried out by ultrasound scanning of the reproductive tract to detect the presence or absence of the early embryo and foetal fluid. Using this method pregnancy status is generally determined from day 28 onward of pregnancy. This method while routinely used, is too late to allow rebreeding at the optimal time (i.e., 18 to 24 days post initial AI) for non-pregnant cows as the normal oestrous cycle is 18 to 24 days [11].
Ideally an early pregnancy test would:
Have high sensitivity (ie correctly identify pregnant cows)
Have high specificity (ie correctly identify non-pregnant cows)
Be inexpensive to conduct
Be a simple cow-side test (ie usable in field conditions)
Determine pregnancy status in a timely manner (ideally at the time of performing the test); (list modified from Fricke et al. [66]).
Indirect methods for pregnancy detection in dairy cows
Indirect methods for early pregnancy diagnosis use qualitative or quantitative measures of hormones or conceptus-specific substances in maternal body fluids as indirect indicators of the presence of a viable pregnancy [67, 68]. Commercially available indirect methods for pregnancy diagnosis in dairy cows include milk progesterone tests and tests for pregnancy-associated glycoproteins (PAGs) in blood or milk [67, 68].
Progesterone assays are more useful as a non-pregnancy test on day 21 [67]. However, it is inaccurate as a test for pregnancy as reversion to low P4 in non-pregnant cows is highly variable due to early embryonic losses. It has been tried commercially, but has not survived due to these problems. In-line P4 testing (as mentioned earlier) has potential if the costs of repeated analyses can become competitive.
PAG measurement is a viable method of determination of pregnancy status in dairy cows [68], however, accuracy of PAG detection is only good after day 35 to 40. Interference may also occur from PAG carry over from previous pregnancy for 40–50 days giving rise to a risk of false positives. It also may give false positive results after embryo loss.
Work described in UK Patent Application No.1520248.4 has led to the development of a test based on glycan diagnostics using the IgG fraction in milk. This technology can detect pregnancy status from as early as day 16 and has led to a priority patent filing (filed 17 November 2015; UK Patent Application No.1520248.4). Importantly early detection of pregnancy status would allow a strategy to resynchronise and rebreed cows by day 21 post the initial unsuccessful insemination (Fig. 3).
Fig. 3.
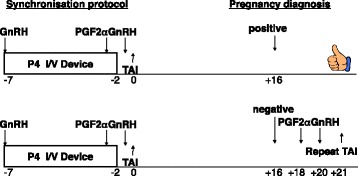
Potential strategy to re-synchronise and re-breed dairy cows after an early pregnancy diagnosis result (day 16; GnRH = gonadotrophin-releasing hormone; PGF2α = prostaglandin F2α; I/V = intravaginal; TAI = fixed-time artificial insemination)
The male side of the story
Although most evidence suggests the pressure on reproductive efficiency in modern dairy herds is primarily related to the high producing females, it is obvious that the role of the male should not be forgotten. While veterinary practitioners often overlook the importance of this side of the coin, farmers often blame issues like sperm quality and the expertise of the AI-technician, mostly because it is human nature to prefer blaming someone else instead of being critical against personal shortcomings. In a study examining the pregnancy outcome of 5883 inseminations, 1 of the 35 bulls that delivered semen was associated with a 2- to 2.5-fold increase in pregnancy rates [69]. In another study from the same group [70] in which the pregnancy results of 10,965 inseminations were scrutinized, not only a difference between bulls but also dramatic differences among inseminators were observed. The likelihood of a pregnancy were almost 4 times lower when a cow was inseminated by the worst compared with the best inseminator.
The success of an insemination depends amongst other factors on the deposition of appropriate numbers of sperm with a good fertilizing capacity at the appropriate site in the reproductive tract at the appropriate time in relation to ovulation. The fertility potential of an artificial insemination dose is a function of the quantity, quality, and health status of the semen contained therein. It is the task of the AI-industry to continue to maintain intense quality control programmes to ensure cryopreserved semen doses released for sale are disease free and meet the above mentioned criteria. A large survey of semen processing practices at AI companies in multiple countries reported that the average cryopreserved AI dose contains approximately 20 × 106 total spermatozoa [71]. This has been estimated to be on average 2 to 20 times greater than estimates of minimum threshold numbers required to guarantee normal fertilisation rates. Remarkably, bulls that are known to produce marginal quality semen often achieve below average fertility despite compensatory increases in number of spermatozoa per dose and vice versa [72].
In 2003 Pace [73] reviewed the technological advances that have been adopted by the AI industry since establishment in the late 1930s and concluded that ‘from a technological standpoint, the dairy industry is receiving the highest quality semen ever produced’. Technological advances in semen processing are reflected in fertilisation rates using cryopreserved semen in the late 1990s early 2000s comparable to those reported for non-frozen semen in the 1950s [72]. Innovative techniques recently tested in the AI industry are cryopreservation techniques that improve post-thaw sperm survival and thereby reduce sensitivity to the optimal timing of the insemination. In this regard, microencapsulation of spermatozoa for sustained time release [74] or techniques designed to reduce the magnitude of cryopreservation-induced capacitation [75] have been tested.
Use of semen from sires with proven high-fertility is probably the most obvious and simple recommendation. However, when one starts to use super fertility bulls in a widespread manner on cows suffering from fertility problems, the question arises in what sense the latter may affect the fertility data reported for these bulls. Use of semen from other breeds in which the fertility decline is not as severe a problem as in the Holstein breed, may also be considered as an alternative to improve herd fertility especially if semen is used of bulls from appropriately progeny-tested sires from breeds with comparable production levels [76]. However we should keep in mind that cross-breeding is not per se a genetic improvement and that genetic selection is still strongly recommended within the breeds used.
Recently reports have been published showing that some sires perform better in timed AI scenarios than others [77]. The latter should stimulate the managers and veterinarians to analyze the fertility data of their herd in relation to the use of specific bulls. On the other hand, it is still unknown whether in this way we stimulate the selection towards animals that are better at coping with specific fixed time protocols instead of stimulating the selection towards enhanced fertility.
A further contributing factor is the site of semen deposition. Although the uterine body is generally accepted to be the proper site for semen deposition, in an experiment using contrast radiography to evaluate the accuracy of professional inseminators, the deposition of semen into the uterine body was successful in only 39% of the attempts, while in 25% of the cases, the semen was deposited in the cervix [78]. While several studies observed improved fertility in response to horn breeding (deep bicornual insemination in which the full insemination dose is divided among both uterine horns or deep unicornual insemination following a preceding ultrasound examination to detect the site of the ovulatory follicle in order to allow ipsilateral insemination), most comparative studies indicated no difference [79]. Recently, a new device that significantly facilitates deep intra-uterine insemination was developed [80], although authors were not able to demonstrate ameliorated pregnancy results using this device versus conventional insemination in the uterine body [81]. Deep intra-uterine insemination was tested for the insemination of low cell number doses of sex sorted sperm. Although flow cytometry/cell sorting has been shown to be a reliable procedure to differentiate X- versus Y-chromosome bearing spermatozoa, the use of sexed semen is often only recommended for nulliparous heifers because of disappointing fertility results in multiparous animals. As a conclusion, DeJarnette et al. [72] reviewing available papers, mentioned that the primary concern is to ensure that semen deposition takes place cranially of the internal cervical os. Remarkable in this context is the paper of López-Gatius and Hunter [82] in which authors report about the successful intrafollicular insemination in repeat breeder cows under heat stress. The latter study however needs confirmation with additional studies.
Usage and availability of “big data”
Common practice in dairy science
Before the “Big Data” era, dairy researchers successfully exploited randomised controlled trial data to explore the complex relationship between production and reproduction in dairy cattle [83, 84]. Multiple observational studies have been designed to identify (metabolic) risk factors that influence this relationship in dairy cattle [85]. Multiple studies have been conducted in this area and successfully published in high valued scientific publications [21, 22, 86]. However, as described by Leblanc [87], temporal associations that have been identified, do not imply causation. Many other aspects of the dairy industry have changed over the last decades confounding the relationship. Randomisation does not exclude confounding. The possibility remains that other variables than the treatment may independently be associated with the intervention and even the outcome. Although well-designed randomised controlled clinical trials remain the gold standard when evaluating experimental treatments, the potential of Big Data in dairy science lies in the combination of traditionally collected data with these new forms of data, both at an animal as well as at a population level. In human medicine, this type of data has been described as real-world evidence [88, 89]. The aforementioned abundance of real-world evidence in animals could potentially help unravel complex relationships such as the often described production-reproduction antagonism in dairy cows. A recent survey from Rutten et al. [90] exactly documents the lack of integrated information and decision-making support tools for current technology in dairy research. No single scientific publication was reported up to 2013 in the areas of metabolism and reproduction utilizing “Big Data”. The survey confirms the scientific methodological challenges observed in Big Data analytics.
Techniques used for analysis and visualisation of traditional dairy data are not adequate for Big Data. The volume, velocity, variety, distributed-ness and incremental nature of such data impose challenges on the traditional methods for data analysis.
Herd fertility and data management strategies
Historically, the emphasis in veterinary medicine has focused on the individual cow affected with a clinical disease. However, about 30 years ago, it was recognised that subclinical disease was the major cause of economic losses in dairy herds and veterinarians started to investigate the multifactorial nature of these sub-clinical diseases [91]. This turned out to be effective in improving the overall health status of the herd, and hence profitability. This approach was called herd health management and has been implemented in veterinary education for at least 3 decades. Over the same time period, internet and communication technology has emerged and integrated in herd health management to leverage the understanding of cow records. The generation and use of cow related data has occurred for in excess of 100 years. The first reporting of the recording and the collection of milk production data is from a union of dairy farmers in Denmark in 1895 [92]. In 1906, the first US milk recording association was founded [93]. Since the 1950’s, computers have been used as a management tool in dairy farming [94]. Over subsequent decades, dairy herd management software has evolved quickly and the personal computer has emerged as an important management tool to monitor production, reproduction and health [95]. Technologies to collect and store data have been evolving at a quicker pace compared with the speed at which new insights in dairy science have been discovered. The exponentially increased volume and speed at which data is now created, commonly referred to as Big Data, has brought new challenges for research in dairy science. The way researchers have to leverage the power of Big Data has been at the center of attention ever since the publication trend that started around 2009 [96, 97]. How to address these challenges will be the main scope for future research.
Available data for dairy practitioners
Official milk recording organisations are collecting 4 to 8 weekly milk samples to detect milk components. Novel analytical methods are detecting more metabolites to assess (re-) productive performance in milk. As an example, the entire mid-infra-red (MIR) spectrum of milk has been proposed as a predictor for disease in dairy cows [98]. MIR predictions are now readily available for milk composition traits such as milk fat, protein and fatty acids [98]; under development are additional prediction equations to allow prediction of greenhouse gases and novel performance and health traits [99, 100]. Diagnostic services are routinely analyzing a multitude of parameters in blood, milk and faecal samples from dairy cows. Recently, genomics information has become commercially available for both male and female animals creating a new set of data [101]. These so called secondary off-farm data centres, mainly containing milk recording data, genomic and diagnostics information, have been raised in different countries, each containing a subset of data representing the real world of dairy cows [101–103].
On farm, conventional and robotic milking systems are equipped with more and better sensors that collect information besides the produced amount of milk. Inline sensors are detecting milk composition, somatic cell counts, temperature and colour [104, 105]. Biosensors are collecting novel biomarkers such as progesterone (reproduction), L-lactate dehydrogenase (udder health), urea and beta-hydroxy-butyrate (metabolic health) [106, 107]. Weighing scales and 3 dimensional cameras [108, 109] are capturing the animal’s body weight and body condition score while milking [110, 111]. Ever since the beginning of sensor technology, cows have been equipped with pedometers and accelerometers that capture the animal’s movements in order to predict specific behaviour such as oestrus and disease in dairy cows [90, 112, 113]. Examples of early-stage innovations being applied to dairy cows are ruminal temperature and pH boluses [114, 115], intravaginal temperature sensors [116, 117] and heart rate measurements [118]. The volume or format of the data no longer presents a major constraint, hence the total volume of cow related data that is collected per day has increased rapidly [103, 119, 120].
New data sources in the dairy industry
The importance of environmental factors such as temperature and humidity in dairy reproduction are undeniable [121–124]. Location based data has become publically available over recent decades, creating the ability to layer physical maps and location-based insights on top of other available data. The approach of combining real-time Internet-of-Things (IoT) devices with historical data analysis are unexploited in dairy science. Thus data streamed from automated data loggers for environmental factors offers new applications in terms of big data collection and use to alter decision making and management [125, 126].
The speed and capacity of computer hardware has increased, while costs have decreased [119, 127]. This has led towards easier data recording through cheap mobile devices and high-availability cloud based data-centres that allows a more consistent and accurate capturing of manually entered reproduction, disease and treatment events at cow level. Using this data to build predictive models for anticipating disease outcomes from current treatment plans and refine those models in real time will improve scientific knowledge around treatment efficacy which is limited to observational studies at the moment [128].
Furthermore, mobile captured anecdotal and unstructured data from farmers, veterinarians’ notes and other sources is a giant frontier of untapped insights. Nowadays, it has been recognised by researchers that 85% of the world’s information is unstructured, comprised of free-form text, audio and video, rather than neatly organised recognisable fields [129]. Although the need for a standardised disease data input has already been recognized for a long time [130], effective implementation in current software is lacking [128]. Natural language processing consists of multiple computational techniques to process language human-like from machine-readable unstructured texts. This has been successfully applied in human medicine [131, 132], but not yet in dairy science to our knowledge. Capturing and exploiting this data will enrich analysis and insights immensely.
Conclusions
In conclusion, genetic trends for fertility are improving in dairy cow populations. Numerous future developments are likely over the next 5 to 10 years. These include: i) development of new and novel phenotypes that may be measurable in milk; ii) specific genomic markers; iii) early pregnancy detection; iv) increased use of activity monitors; v) improved breeding protocols; vi) automated inline sensors for relevant phenotypes that become more affordable for farmers; and vii) capturing and mining multiple sources of “big data” available to dairy farmers. These new developments should facilitate improved performance and health of dairy cows in the future.
Acknowledgements
This paper is based on a plenary presentation at the World Buiatrics Conference, Dublin 2016.
Funding
The authors are currently funded by EU FP7 grant number 613689 (GPlusE). MAC is also funded by SFI investigator award grant 13/IA/2025.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analysed during the current study as this is a review paper.
Abbreviations
- AC
Activity cluster
- AI
Artificial insemination
- CL
Corpus luteum
- EU
European Union
- FTAI
Fixed-time artificial insemination
- GH
Growth hormone
- GnRH
Gonadotrophin-releasing hormone
- GplusE
Genotype plus Environment
- IGF-I
Insulin-like growth factor I
- IgG
Immune-gamma globulin
- IoT
Internet of things
- IVF
Invitro-fertilisation
- MIR
Mid-infra red spectra
- NEB
Negative energy balance
- OVSYNCH
Ovulation synchronisation
- P4
Progesterone
- PAGs
Pregnancy-associated glycoproteins
- PGF
Prostaglandin-F2alpha
- PRESYNCH-OVSYNCH
Presynchronisation-ovulation synchronisation
- SNPs
Single-nucleotide polymorphisms
Authors’ contributions
All three authors of this manuscript have contributed equally to this work in terms of drafting and reviewing the text contained in this review. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mark A. Crowe, Phone: 353 1 716 6255, Email: mark.crowe@ucd.ie
Miel Hostens, Email: Miel.Hostens@UGent.be.
Geert Opsomer, Email: Geert.Opsomer@UGent.be.
References
- 1.Department of Agriculture, Fisheries and Food, 2011. Food Harvest 2020, a vision for Irish agri-food and fisheries. Retrieved November 2011, from https://www.agriculture.gov.ie/media/migration/foodindustrydevelopmenttrademarkets/foodharvest2020/foodharvest2020/2020strategy/2020Foodharvest190710.pdf.
- 2.Department of Agriculture, Fisheries and Food, 2015. Foodwise 2025, Local Roots: A vision for growth for the Irish agricultural economy for the next ten years. Retrieved August 2017, from https://www.agriculture.gov.ie/media/migration/foodindustrydevelopmenttrademarkets/agri-foodandtheeconomy/foodwise2025/report/FoodWise2025.pdf
- 3.Crowe MA. Fertility in dairy cows – the conference in perspective. In Fertility in Dairy Cows bridging the gaps. Eds Royal MD, Friggens NC and Smith RF. UK: British Society of Animal Science / Cambridge University Press; 2007. pp. 156–60.
- 4.Wickham et al., 2008; Wickham BW, Cromie A, Kearney JF and Evans R 2008. A genetic solution to infertility in Irish dairy cattle. In Fertility in Dairy Cows bridging the gaps. Eds Royal MD, Friggens NC and Smith RF. British Society of Animal Science / Cambridge University Press 2007 pp 156–160.
- 5.Berry DP, Wall E, Pryce JE. Genetics and genomics of reproductive performance in dairy and beef cattle. Animal. 2014;8(s1):105–121. doi: 10.1017/S1751731114000743. [DOI] [PubMed] [Google Scholar]
- 6.Santoro A, Vandepitte J, Hostens M, Carter F, Matthews E, O’Flaherty R, Waegeman W, Fahey AG, Hermans K, Ferris C, Bell M, Sorensen MT, Höglund J, Rudd PM, Crowe MA. Potential for novel glycan measurements in milk as biomarker phenotypes for dairy traits. Proceedings EAAP Annual conference, Belfast, August 2016. pp. 300.
- 7.Höglund J, Sorensen MT, Larsen T, Foldager L, Ferris C, Bell M, Carter F, Santoro A, Crowe M, Ingvartsen KL. Potential for novel metabolite measurements in milk as biomarker phenotypes for dairy traits. Proceedings EAAP Annual conference, Belfast, August 2016. pp. 299.
- 8.Vanlierde A, Grelet C, Gengler N, Ferris C, Sorensen MT, Höglund J, Carter F, Santoro A, Hermans K, Hostens M, Dardenne P, Dehareng F. Potential of milk MIR spectra to develop new health phenotypes for dairy cows in the GplusE project. Proceedings EAAP Annual conference, Belfast, August 2016. pp. 299.
- 9.Marchitelli C, Signorelli F, Napolitano F, Buttazzoni L, Grelet C, Vanlierde A, Dehareng F, Soyeurt H, Crowe M, GplusE Consortium. Expression profiles of immune genes in milk somatic cells and MIR predicted mineral contents in milk as indicators of mastitis. 36th International Society for Animal Genetics conference 2017; Abstract MT153: 100.
- 10.Morotti F, SanchesBV PJHF, Basso AC, Siqueira ER, Lisboa LA, Seneda MM. Pregnancy rate and birth rate of calves from a large-scale IVF program using reverse-sorted semen in Bos indicus, Bos indicus-taurus, and Bos taurus cattle. Theriogenology. 2014;81:696–701. doi: 10.1016/j.theriogenology.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Forde N, Beltman ME, Lonergan P, Diskin M, Roche JF, Crowe MA. Oestrous cycles in Bos Taurus cattle. Anim Reprod Sci. 2011;124:163–169. doi: 10.1016/j.anireprosci.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 12.Tamminga S, Luteijn PA, Meijer RGM. Changes in composition and energy content of liveweight loss in dairy cows with time after parturition. Livest Prod Sci. 1997;52:31–38. doi: 10.1016/S0301-6226(97)00115-2. [DOI] [Google Scholar]
- 13.Knight CH, Beever DE, Sorensen A. Metabolic loads to be expected from different genotypes under different systems. Metabolic stress in dairy cows. British Society of Animal Science Occ Publication. 1999;24:37–36. [Google Scholar]
- 14.Sheehy MR, Fahey A, Aungier SPM, Carter F, Crowe MA, Mulligan FJ. A comparison of serum metabolic and production profiles of dairy cows that maintained or lost body condition 15 days before calving. J Dairy Sci. 2016;100:1–12. doi: 10.3168/jds.2016-11206. [DOI] [PubMed] [Google Scholar]
- 15.Mulligan FJ, O'Grady L, Rice DA, Doherty ML. A herd health approach to dairy cow nutrition and production diseases of the transition cow. Anim Reprod Sci. 2006;96:331–353. doi: 10.1016/j.anireprosci.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Opsomer G, Gröhn YT, Hertl J, Deluycker H, Coryn M, de Kruif A. Risk factors for postpartum ovarian dysfunction in high producing dairy cows in Belgium: a field study. Theriogenology. 2000;53:841–857. doi: 10.1016/S0093-691X(00)00234-X. [DOI] [PubMed] [Google Scholar]
- 17.Crowe MA, Diskin MG, Williams EJ. Parturition to resumption of ovarian cyclicity: comparative aspects of beef and dairy cows. Animal. 2014;8:1–14. doi: 10.1017/S1751731114000251. [DOI] [PubMed] [Google Scholar]
- 18.Crowe MA, Williams EJ, Mulligan FJ. Physiological and health factors affecting fertility in beef and dairy cows. Cattle Practice. 2015;23:47–61. [Google Scholar]
- 19.Crowe MA, Williams EJ. Triennial lactation symposium: effects of stress on postpartum reproduction in dairy cows. J Anim Sci. 2012;90:1722–1727. doi: 10.2527/jas.2011-4674. [DOI] [PubMed] [Google Scholar]
- 20.Diskin MG, Parr MH, Morris DG. Embryo death in cattle: an update. Reprod Fert Develop. 2012;24:244–251. doi: 10.1071/RD11914. [DOI] [PubMed] [Google Scholar]
- 21.Leroy JLMR, Opsomer G, Van Soom A, Goovaerts IGF, Bols PEJ. The importance of negative energy balance and altered corpus luteum function to the reduction of oocyte and embryo quality in high yielding dairy cows. Part I – the importance of negative energy balance and altered corpus luteum function to the reduction of oocyte and embryo quality in high-yielding dairy cows. Reprod Domest Anim. 2008;43:612–622. doi: 10.1111/j.1439-0531.2007.00960.x. [DOI] [PubMed] [Google Scholar]
- 22.Leroy JL, Van Soom A, Opsomer G, Goovaerts IG, Bols PE. Reduced fertility in high-yielding dairy cows: are the oocyte and embryo in danger? Part II. Mechanisms linking nutrition and reduced oocyte and embryo quality in high-yielding dairy cows. Reprod Domest Anim. 2008;43:623–632. doi: 10.1111/j.1439-0531.2007.00961.x. [DOI] [PubMed] [Google Scholar]
- 23.LeBlanc S. Monitoring metabolic health of dairy cattle in the transition period. J Reprod Dev. 2010;56:S29–S35. doi: 10.1262/jrd.1056S29. [DOI] [PubMed] [Google Scholar]
- 24.Garnswortthy PC, Sinclair KD, Webb R. Integration of physiological mechanisms that influence fertility in dairy cows. Animal. 2008;2:1144–1152. doi: 10.1017/S1751731108002358. [DOI] [PubMed] [Google Scholar]
- 25.Gong JG, Lee WJ, Garnsworthy PC, Webb R. Effect of dietary-induced increases in circulating insulin concentration during the early postpartum period on reproductive function in dairy cows. Reproduction. 2002;123:419–427. doi: 10.1530/rep.0.1230419. [DOI] [PubMed] [Google Scholar]
- 26.Bossaert P, DeCock H, Leroy JLMR, De Campeneere S, Bols PEJ, Filliers M, Opsomer G. Immunohistochemical visualization of insulin receptors in formalin-fixed bovine ovaries post mortem and in granulosa cells collected in vivo. Theriogenology. 2010;73:1210–1219. doi: 10.1016/j.theriogenology.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Vanholder T, Leroy JLMR, Dewulf J, Duchateau L, Coryn M, de Kruif A, Opsomer G. Hormonal and metabolic profiles of high-yielding dairy cows prior to ovarian cyst formation or first ovulation post partum. Reprod Domest Anim. 2005;40:460–467. doi: 10.1111/j.1439-0531.2005.00601.x. [DOI] [PubMed] [Google Scholar]
- 28.Fouladi-Nashta AA, Gutierrez CG, Garnsworthy PC, Webb R. Effect of dietary carbohydrate source on oocyte/embryo quality and development in high-yielding, lactating dairy cattle. Biol Reprod. (Special Issue) 2005;72:135–6.
- 29.Lemley CO, Butler ST, Butler WR, Wilson ME. Short communication: insulin alters hepatic progesterone catabolic enzymes cytochrome P450 2C and 3A in dairy cows. J Dairy Sci. 2008;91:641–645. doi: 10.3168/jds.2007-0636. [DOI] [PubMed] [Google Scholar]
- 30.Barton BA, Rosario HA, Anderson GW, Grindle BP, Caroll DJ. Effects of dietary crude protein, breed, parity, and health status on the fertility of dairy cows. J Dairy Sci. 1996;79:2225–2236. doi: 10.3168/jds.S0022-0302(96)76599-2. [DOI] [PubMed] [Google Scholar]
- 31.Cools S, Van den Broeck W, Vanhaecke L, Heyerick A, Bossaert P, Hostens M, Opsomer G. Feeding soybean meal increases the blood level of isoflavones and reduces the steroidogenic capacity in bovine corpora lutea, without affecting peripheral progesterone concentrations. Anim Reprod Sci. 2014;144:79–89. doi: 10.1016/j.anireprosci.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Hostens M, Fievez V, Vlaeminck B, Buyse J, Leroy J, Piepers S, De Vliegher S, Opsomer G. The effect of marine algae in the ration of high-yielding dairy cows during transition on metabolic parameters in serum and follicular fluid around parturition. J Dairy Sci. 2011;94:4603–4615. doi: 10.3168/jds.2010-3899. [DOI] [PubMed] [Google Scholar]
- 33.Silvestre FT, Carvalho TSM, Francisco N, Santos JEP, Staples CR, Jenkins TC, Thatcher WW. Effects of differential supplementation of fatty acids during the peripartum and breeding periods of Holstein cows: I. Uterine and metabolic responses, reproduction, and lactation. J Dairy Sci. 2011;94:189–204. doi: 10.3168/jds.2010-3370. [DOI] [PubMed] [Google Scholar]
- 34.Leroy JL, Vanholder T, Mateusen B, Christophe A, Opsomer G, de Kruif A, Genicot G, Van Soom A. Non-esterified fatty acids in follicular fluid of dairy cows and their effect on developmental capacity of bovine oocytes in vitro. Reproduction. 2005;130:485–495. doi: 10.1530/rep.1.00735. [DOI] [PubMed] [Google Scholar]
- 35.Leroy JLMR, Van Soom A, Opsomer G, Goovaerts IGF, Bols PEJ. Reduced fertility in high yielding dairy cows: are the oocyte and embryo in danger? (part II) Reprod Domest Anim. 2008;43:623–632. doi: 10.1111/j.1439-0531.2007.00961.x. [DOI] [PubMed] [Google Scholar]
- 36.Leroy JLMR, Opsomer G, De Vliegher S, Vanholder T, Goossens L, Geldhof A, Bols PEJ, de Kruif A, Van Soom A. Comparison of embryo quality in high-yielding dairy cows in dairy heifers and in beef cows. Theriogenology. 2005;64:2022–2036. doi: 10.1016/j.theriogenology.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Hurley WL, Doane RM. Recent developments in the roles of vitamins and minerals in reproduction. J Dairy Sci. 1989;72:784–804. doi: 10.3168/jds.S0022-0302(89)79170-0. [DOI] [PubMed] [Google Scholar]
- 38.Pontes GCS, Monteiro PLJ, Prata AB, Guardieiro MM, Pinto DAM, Fernandes GO. Effect of injectable vitamin E on incidence of retained fetal membranes and reproductive performance of dairy cows. J Dairy Sci. 2015;98:2437–2449. doi: 10.3168/jds.2014-8886. [DOI] [PubMed] [Google Scholar]
- 39.Donofrio G, Herath S, Sartori C, Cavirani S, Flammini F, Sheldon M. Bovine herpesvirus 4 is tropic for bovine endometrial cells and modulates endocrine function. Reproduction. 2007;134:183–197. doi: 10.1530/REP-07-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanderson MW, Gnad DP. Biosecurity for reproductive diseases. Vet Cl Nth Amer: Food Anim Pract. 2002;18:79–98. doi: 10.1016/s0749-0720(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 41.LeBlanc SJ, Duffield TF, Leslie KE, Bateman KG, Keefe GP, Walton JS, et a. Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows. J Dairy Sci, 2002;85:2223–2236. [DOI] [PubMed]
- 42.Sheldon IM, Lewis GS, LeBlanc S, Gilbert RO. Defining postpartum uterine disease in cattle. Theriogenology. 2006;65:1516–1530. doi: 10.1016/j.theriogenology.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 43.Sheldon IM, Cronin J, Goetze L, Donofrio G, Schuberth HJ. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol Reprod. 2009;81:1025–1032. doi: 10.1095/biolreprod.109.077370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pascottini OB, Dini P, Hostens M, Ducatelle R, Opsomer G. A novel cytological sampling technique to diagnose subclinical endometritis and comparison of staining methods for endometrial cytology samples in dairy cows. Theriogenology. 2015;84:1438–46. [DOI] [PubMed]
- 45.Pursley JR, Mee MO, Wiltbank MC. 1995. Synchronization of ovulation in dairy cows using PGF2α and GnRH. Theriogenology. 2015;44:915–923. doi: 10.1016/0093-691X(95)00279-H. [DOI] [PubMed] [Google Scholar]
- 46.Van Eerdenburg FJCM, Loeffler SH, Van Vliet JH. Detection of oestrus in dairy cows: a new approach to an old problem. Vet Q. 1997;32:137–141. doi: 10.1080/01652176.1996.9694615. [DOI] [PubMed] [Google Scholar]
- 47.Diskin MG, Sreenan JM. Expression and detection of oestrus in cattle. Reprod Nutr Dev. 2000;40:481–491. doi: 10.1051/rnd:2000112. [DOI] [PubMed] [Google Scholar]
- 48.Vliet JHV, Van Eerdenburg FJCM. Sexual activities and oestrus detection in lactating Holstein cows. Appl Anim Behav Sci. 1996;50:57–69. doi: 10.1016/0168-1591(96)01068-4. [DOI] [Google Scholar]
- 49.Roelofs JB, Van Eerdenburg FJCM, Soede NM, Kemp B. Various behavioral signs of estrous and their relationship with time of ovulation in dairy cattle. Theriogenology. 2005;63:1366–1377. doi: 10.1016/j.theriogenology.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 50.Diskin MG. Reproductive management of dairy cows: a review (part 1) Ir Vet J. 2008;61:326–332. [Google Scholar]
- 51.Trimberger GW, Davis HP. Conception rate in dairy cattle from artificial insemination at various stages of estrus. Nebraska Agric Exp Stn Res Bulletin. 1943;129:1–14. [Google Scholar]
- 52.Holman A, Thompson J, Routly JE, Cameron J, Grove-White D, Smith RF, Dobson H. Comparison of oestrus detection methods in dairy cattle. Vet Rec. 2011;169:47–53. doi: 10.1136/vr.d2344. [DOI] [PubMed] [Google Scholar]
- 53.Peralta OA, Pearson RE, Nebel RL. Comparison of three estrus detection systems during summer in a large commercial dairy herd. Anim Reprod Sci. 2005;87:59–72. doi: 10.1016/j.anireprosci.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 54.Aungier SPM, Roche JF, Sheehy M, Crowe MA. Effects of management and health on the use of activity monitoring for estrus detection in dairy cows. J Dairy Sci. 2012;95:2452–2466. doi: 10.3168/jds.2011-4653. [DOI] [PubMed] [Google Scholar]
- 55.Aungier SPM, Roche JF, Duffy P, Scully S, Crowe MA. The relationship between activity clusters detected by an automatic activity monitor and endocrine changes during the periestrous period in lactating dairy cows. J Dairy Sci. 2015;98:1666–1684. doi: 10.3168/jds.2013-7405. [DOI] [PubMed] [Google Scholar]
- 56.Friggens NC, Bjerring M, Ridder C, Højsgaard S, Larsen T. Improved detection of reproductive status in dairy cows using milk progesterone measurements. Reprod Domest Anim. 2008;43(Suppl 2):113–121. doi: 10.1111/j.1439-0531.2008.01150.x. [DOI] [PubMed] [Google Scholar]
- 57.Cordoba MC, Fricke PM. Initiation of the breeding season in a grazing-based dairy by synchronization of ovulation. J Dairy Sci. 2002;85:1752–1763. doi: 10.3168/jds.S0022-0302(02)74249-5. [DOI] [PubMed] [Google Scholar]
- 58.Jobst SM, Nebel RL, McGilliard ML, Pelzert KD. Evaluation of reproductive performance in lactating dairy cows with prostaglandin F2α, gonadotropin-releasing hormone, and timed artificial insemination. J Dairy Sci. 2000;83:2366–2372. doi: 10.3168/jds.S0022-0302(00)75125-3. [DOI] [PubMed] [Google Scholar]
- 59.Herlihy MM, Giordano JO, Souza AH, Ayres H, Ferreira RM, Keskin A. Nascimento AB, Guenther JN, Gaska JM, Kacuba SJ, Crowe MA, Butler ST and Wiltbank MC. Presynchronization with double-Ovsynch improves fertility at first postpartum artificial insemination in lactating dairy cows. J Dairy Sci 2012;95: 7003-7014. [DOI] [PubMed]
- 60.McNally JC, Crowe MA, Roche JF, Beltman ME. Effects of physiological and/or disease status on the response of postpartum dairy cows to synchronization of estrus using an intravaginal progesterone device. Theriogenology. 2014;82:1263–1272. doi: 10.1016/j.theriogenology.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 61.Senger PL. The estrus detection problem: new concepts, technologies, and possibilities. J Dairy Sci. 1994;77:2745–2753. doi: 10.3168/jds.S0022-0302(94)77217-9. [DOI] [PubMed] [Google Scholar]
- 62.Cowie TA. Pregnancy diagnosis tests: a review. Commonwealth agricultural bureaux joint publication No. 13, Oxford, UK; 1948. p. 11–7. 15.
- 63.Wisnicky W, Cassida LE. A manual method for diagnosis of pregnancy in cattle. J Am Vet Med Assoc. 1948;113:451. [Google Scholar]
- 64.Fricke PM. Scanning the future – ultrasonography as a reproductive management tool for dairy cattle. J Dairy Sci. 2002;85:1918–1926. doi: 10.3168/jds.S0022-0302(02)74268-9. [DOI] [PubMed] [Google Scholar]
- 65.Griffin PG, Ginther OJ. Research applications of ultrasonic imaging in reproductive biology. J Anim Sci. 1992;70:953–972. doi: 10.2527/1992.703953x. [DOI] [PubMed] [Google Scholar]
- 66.Fricke PM, Ricci A, Giordano JO, Carvalho PD. Methods for and implementation of pregnancy diagnosis in dairy cows. Vet Clin N Am Food Anim Pract. 2016;32:165–180. doi: 10.1016/j.cvfa.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 67.Nebel RL. On-farm milk progesterone tests. J Dairy Sci. 1988;71:1682–1690. doi: 10.3168/jds.S0022-0302(88)79733-7. [DOI] [PubMed] [Google Scholar]
- 68.Cordoba MC, Sartori R, Fricke PM. Assessment of a commercially available early conception factor (ECF) test for determining pregnancy status of dairy cattle. J Dairy Sci. 2001;84:1884–1889. doi: 10.3168/jds.S0022-0302(01)74629-2. [DOI] [PubMed] [Google Scholar]
- 69.López-Gatius F, Santolaria P, Mundet I, Yániz JL. Walking activity at estrus and subsequent fertility in dairy cows. Theriogenology. 2005;63:1419–1429. doi: 10.1016/j.theriogenology.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 70.García-Ispierto I, López-Gatius F, Santolaria P, Yániz JL, Nogareda C, López-Béjar M. Factors affecting the fertility of high producing dairy herds in northeastern Spain. Theriogenology. 2007;67:632–638. doi: 10.1016/j.theriogenology.2006.09.038. [DOI] [PubMed] [Google Scholar]
- 71.Vishwanath R. Artificial insemination: the state of the art. Theriogenology. 2003;59:571–584. doi: 10.1016/S0093-691X(02)01241-4. [DOI] [PubMed] [Google Scholar]
- 72.DeJarnette JM, Marshall CE, Lenz RW, Monke DR, Ayars WH, Sattler CG. Sustaining the fertility of artificially inseminated dairy cattle: the role of the artificial insemination industry. J Dairy Sci. 2004;87(E. Suppl):E93–E104. doi: 10.3168/jds.S0022-0302(04)70065-X. [DOI] [Google Scholar]
- 73.Pace MM. Has the fertilizing capacity of bovine spermatozoa changed? Pages 13 to 21 in reproductive loss in dairy cows: is the trend reversible? Proc. II bi-annual W. E. Petersen Symp., University of MN—St. Paul Campus, Minneapolis, MN 2003.
- 74.Vishwananth R, Nebel RL, McMillan WH, Pitt CJ, Macmillan KL. Selected times of insemination with microencapsulated bovine spermatozoa affect pregnancy rates of synchronized heifers. Theriogenology. 1997;48:369–376. doi: 10.1016/S0093-691X(97)00248-3. [DOI] [PubMed] [Google Scholar]
- 75.Watson PF. Recent developments and concepts in the cryopreservation of spermatozoa and the assessment of their postthawing function. Reprod Fertil Dev. 1995;7:213–233. doi: 10.1071/RD9950871. [DOI] [PubMed] [Google Scholar]
- 76.Heins BJ, Hansen LB, Seykora AJ. Production of pure Holsteins versus crossbreds of Holstein with Normande, Montbeliarde, and Scandinavian red. J Dairy Sci. 2006;89:2799–2804. doi: 10.3168/jds.S0022-0302(06)72356-6. [DOI] [PubMed] [Google Scholar]
- 77.Hiers EA, Barthle CR, Dahms MKV, Portillo GE, Bridges GA, Rae DO, Thatcher WW, Yelich JV. Synchronization of Bos Indicus x Bos Taurus cows for timed artificial insemination using gonadotropin-releasing hormone plus prostaglandin F2alpha in combination with melengestrol acetate. J Anim Sci. 2003;81:830–835. doi: 10.2527/2003.814830x. [DOI] [PubMed] [Google Scholar]
- 78.Peters JL, Senger PL, Rosenberger JL, O’Connor ML. Radiographic evaluation of bovine artificial inseminating technique among professional and herdsman-inseminators using 0.5 and 0.25-ml French straws. J Anim Sci. 1984;59:1671–1683. doi: 10.2527/jas1984.5961671x. [DOI] [PubMed] [Google Scholar]
- 79.Lopez-Gatius F (2000). Site of semen deposition in cattle: a review. Theriogenology 2000; 53: 1407-1414. [DOI] [PubMed]
- 80.Verberckmoes S, Van Soom A, De Pauw I, Dewulf J, Vervaet C, de Kruif A. Assessment of a new utero-tubal junction insemination device in dairy cattle. Theriogenology. 2004;61:103–115. doi: 10.1016/S0093-691X(03)00186-9. [DOI] [PubMed] [Google Scholar]
- 81.Verberckmoes S, Van Soom A, Dewulf J, Thys M, de Kruif A. Low dose insemination in cattle with the Ghent device. Theriogenology. 2005;64:1716–1728. doi: 10.1016/j.theriogenology.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 82.López-Gatius F, Hunter RHF. Intrafollicular insemination for the treatment of infertility in the dairy cow. Theriogenology. 2011;75:1695–1698. doi: 10.1016/j.theriogenology.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 83.Nebel RL, McGilliard ML. Interactions of high milk yield and reproductive performance in dairy cows. J Dairy Sci. 1993;76:3257–3268. doi: 10.3168/jds.S0022-0302(93)77662-6. [DOI] [PubMed] [Google Scholar]
- 84.Lucy MC. Reproductive loss in high-producing dairy cattle: where will it end? J Dairy Sci. 2001;84:1277–1293. doi: 10.3168/jds.S0022-0302(01)70158-0. [DOI] [PubMed] [Google Scholar]
- 85.Walsh SW, Williams EJ, Evans ACO. A review of the causes of poor fertility in high milk producing dairy cows. Anim Reprod Sci. 2011;123:127–138. doi: 10.1016/j.anireprosci.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Opsomer G, Leroy JLMR, Vanholder T, Bossaert P, Cools S, Hostens M, et al. Proceedings of the 18th annual meeting of the Japanese veterinary medicine association. 2007. Major causes of declining fertility in dairy cows in Europe and some strategies to improve reproductive performance; pp. 97–99. [Google Scholar]
- 87.Leblanc S. Assessing the association of the level of milk production with reproductive performance in dairy cattle. J Reprod Develop. 2010;56:S1–S7. doi: 10.1262/jrd.1056S01. [DOI] [PubMed] [Google Scholar]
- 88.Khoury MJ, Ioannidis JP. Medicine. Big data meets public health. Science. 2014;346:1054–1055. doi: 10.1126/science.aaa2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krumholz HM. Big data and new knowledge in medicine: the thinking, training, and tools needed for a learning health system. Health Aff. 2014;33:1163–1170. doi: 10.1377/hlthaff.2014.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rutten CJ, Velthuis AGJ, Steeneveld W, Hogeveen H. Invited review: sensors to support health management on dairy farms. J Dairy Sci. 2013;96:1928–1952. doi: 10.3168/jds.2012-6107. [DOI] [PubMed] [Google Scholar]
- 91.de Kruif A, Opsomer G. Integrated dairy herd health management as the basis for prevention. Vlaams Diergeneeskundig Tijdschrift. 2004;73:44–52. [Google Scholar]
- 92.Mark T, Jakobsen JH, Jorjani H, Fikse WF, Philipsson J. International trends in recording and genetic evaluation of functional tratis in dairy cattle. Proc 56th Annual Meeting of EAAP, Upsalla, Sweden 2005;1–12. Available at http://old.eaap.org/Previous_Annual_Meetings/2005Uppsala/Papers/CG2.1_Mark.pdf
- 93.Bertrand J, Wiggans G. Proceedings of the 6th world congress on genetics applied to livestock production. 1998. Validation of data and review of results from genetic evaluation systems for US beef and dairy cattle; pp. 11–16. [Google Scholar]
- 94.Lissemore KD. The use of computers in dairy herd health program: a review. Can Vet J. 1989;30:631. [PMC free article] [PubMed] [Google Scholar]
- 95.Gloy BA, Akridge JT. Computer and internet adoption on large US farms. Internation Food Agribus Man Rev. 2000;3:323–338. doi: 10.1016/S1096-7508(01)00051-9. [DOI] [Google Scholar]
- 96.Chen HC, Chiang RHL, Storey VC. Business intelligence and analytics: from big data to big impact. Mis Quart. 2012;36:1165–1188. [Google Scholar]
- 97.Sagiroglu S, Sinanc D. Big data: A review. Collaboration Technologies and Systems (CTS), International Conference on: IEEE. 2013. p. 42–47.
- 98.Gengler N, Berry D, Bastin C 2013. Use of automated systems for recording of direct and indirect data with special emphasis on the use of MIR milk spectra (OptiMIR project). ICAR Technical Series no 2013;17:55.
- 99.van Engelen S, Bovenhuis H, Mollenhorst H, Rademaker J, Visker M. Genetic background of predicted methane based on milk mid-infrared spectra of Dutch Holstein Friesian cows. 2017. [Google Scholar]
- 100.van Gastelen S, Dijkstra J. Prediction of methane emission from lactating dairy cows using milk fatty acids and mid-infrared spectroscopy. J Sci Food Agric. 2016;96:3963–3968. doi: 10.1002/jsfa.7718. [DOI] [PubMed] [Google Scholar]
- 101.Egger-Danner C, Cole JB, Pryce JE, Gengler N, Heringstad B, Bradley A, et al. Invited review: overview of new traits and phenotyping strategies in dairy cattle with a focus on functional traits. Animal. 2015;9:191–207. doi: 10.1017/S1751731114002614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Frost AR, Schofield CP, Beaulah SA, Mottram TT, Lines JA, Wathes CM. A review of livestock monitoring and the need for integrated systems. Comput Electron Agric. 1997;17:139–159. doi: 10.1016/S0168-1699(96)01301-4. [DOI] [Google Scholar]
- 103.Tomaszewski MA. Record-keeping systems and control of data flow and information retrieval to manage large high producing herds. J Dairy Sci. 1993;76:3188–3194. doi: 10.3168/jds.S0022-0302(93)77657-2. [DOI] [PubMed] [Google Scholar]
- 104.Viguier C, Arora S, Gilmartin N, Welbeck K, O'Kennedy R. Mastitis detection: current trends and future perspectives. Trends Biotechnol. 2009;27:486–493. doi: 10.1016/j.tibtech.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 105.Hovinen M, Pyorala S. Invited review: udder health of dairy cows in automatic milking. J Dairy Sci. 2011;94:547–562. doi: 10.3168/jds.2010-3556. [DOI] [PubMed] [Google Scholar]
- 106.Friggens NC, Chagunda MGG. Prediction of the reproductive status of cattle on the basis of milk progesterone measures: model description. Theriogenology. 2005;64:155–190. doi: 10.1016/j.theriogenology.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 107.Friggens NC, Chagunda MGG, Bjerring M, Ridder C, Hojsgaard S, Larsen T. Estimating degree of mastitis from time-series measurements in milk: a test of a model based on lactate dehydrogenase measurements. J Dairy Sci. 2007;90:5415–5427. doi: 10.3168/jds.2007-0148. [DOI] [PubMed] [Google Scholar]
- 108.Halachmi I, Polak P, Roberts DJ, Klopcic M. Cow body shape and automation of condition scoring. J Dairy Sci. 2008;91:4444–4451. doi: 10.3168/jds.2007-0785. [DOI] [PubMed] [Google Scholar]
- 109.Weber A, Salau J, Haas JH, Junge W, Bauer U, Harms J, et al. Estimation of backfat thickness using extracted traits from an automatic 3D optical system in lactating Holstein-Friesian cows. Livest Sci. 2014;165:129–137. doi: 10.1016/j.livsci.2014.03.022. [DOI] [Google Scholar]
- 110.Friggens NC, Ingvartsen KL, Emmans GC. Prediction of body lipid change in pregnancy and lactation. J Dairy Sci. 2004;87:988–1000. doi: 10.3168/jds.S0022-0302(04)73244-0. [DOI] [PubMed] [Google Scholar]
- 111.Bewley JM, Peacock AM, Lewis O, Boyce RE, Roberts DJ, Coffey MP, et al. Potential for estimation of body condition scores in dairy cattle from digital images. J Dairy Sci. 2008;91:3439–3453. doi: 10.3168/jds.2007-0836. [DOI] [PubMed] [Google Scholar]
- 112.Bewley JM, Boyce RE, Hockin J, Munksgaard L, Eicher SD, Einstein ME, et al. Influence of milk yield, stage of lactation, and body condition on dairy cattle lying behaviour measured using an automated activity monitoring sensor. J Dairy Res. 2010;77:1–6. doi: 10.1017/S0022029909990227. [DOI] [PubMed] [Google Scholar]
- 113.Chapinal N, de Passille AM, Rushen J, Wagner S. Automated methods for detecting lameness and measuring analgesia in dairy cattle. J Dairy Sci. 2010;93:2007–2013. doi: 10.3168/jds.2009-2803. [DOI] [PubMed] [Google Scholar]
- 114.De Nardi R, Marchesini G, Stefani AL, Barberio A, Andrighetto I, Segato S. Effect of feeding fine maize particles on the reticular pH, milk yield and composition of dairy cows. J Anim Physiol Anim Nutr. 2014;98:504–510. doi: 10.1111/jpn.12099. [DOI] [PubMed] [Google Scholar]
- 115.Humer E, Khol-Parisini A, Gruber L, Gasteiner J, Abdel-Raheem SM, Zebeli Q. Long-term reticuloruminal pH dynamics and markers of liver health in early-lactating cows of various parities fed diets differing in grain processing. J Dairy Sci. 2015;98:6433–6448. doi: 10.3168/jds.2015-9522. [DOI] [PubMed] [Google Scholar]
- 116.Burdick NC, Carroll JA, Dailey JW, Randel RD, Falkenberg SM, Schmidt TB. Development of a self-contained, indwelling vaginal temperature probe for use in cattle research. J Therm Biol. 2012;37:339–343. doi: 10.1016/j.jtherbio.2011.10.007. [DOI] [Google Scholar]
- 117.Hillman PE, Gebremedhin KG, Willard ST, Lee CN, Kennedy AD. Continuous measurements of vaginal temperature of female cattle using a data logger encased in a plastic anchor. Appl Eng Agric. 2009;25:291–296. doi: 10.13031/2013.26332. [DOI] [Google Scholar]
- 118.Laister S, Stockinger B, Regner AM, Zenger K, Knierim U, Winckler C. Social licking in dairy cattle-effects on heart rate in performers and receivers. Appl Anim Behav Sci. 2011;130:81–90. doi: 10.1016/j.applanim.2010.12.003. [DOI] [Google Scholar]
- 119.Spahr S. New technologies and decision making in high producing herds. J Dairy Sci. 1993;76:3269–3277. doi: 10.3168/jds.S0022-0302(93)77663-8. [DOI] [PubMed] [Google Scholar]
- 120.Pietersma D, Lacroix R, Wade K. A framework for the development of computerized management and control systems for use in dairy farming. J Dairy Sci. 1998;81:2962–2972. doi: 10.3168/jds.S0022-0302(98)75859-X. [DOI] [Google Scholar]
- 121.Bewley JM, Grott MW, Einstein ME, Schutz MM. Impact of intake water temperatures on reticular temperatures of lactating dairy cows. J Dairy Sci. 2008;91:3880–3887. doi: 10.3168/jds.2008-1159. [DOI] [PubMed] [Google Scholar]
- 122.Roth Z. Heat stress, the follicle, and its enclosed oocyte: mechanisms and potential strategies to improve fertility in dairy cows. Reprod Domest Anim. 2008;43:238–244. doi: 10.1111/j.1439-0531.2008.01168.x. [DOI] [PubMed] [Google Scholar]
- 123.Shehab-El-Deen MAMM, Leroy JLMR, Fadel MS, Saleh SYA, Maes D, Van Soom A. Biochemical changes in the follicular fluid of the dominant follicle of high producing dairy cows exposed to heat stress early post-partum. Anim Reprod Sci. 2010;117:189–200. doi: 10.1016/j.anireprosci.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 124.Tao S, Bubolz J, Do Amaral B, Thompson I, Hayen M, Johnson S, et al. Effect of heat stress during the dry period on mammary gland development. J Dairy Sci. 2011;94:5976–5986. doi: 10.3168/jds.2011-4329. [DOI] [PubMed] [Google Scholar]
- 125.Beng, L. T., P. B. Kiat, L. N. Meng, and P. N. Cheng. 2016. Field testing of IoT devices for livestock monitoring using Wireless Sensor Network, near field communication and Wireless Power Transfer. Pages 169–173 in Proc. Technologies for Sustainability (SusTech), 2016 IEEE Conference on. IEEE.
- 126.Kulatunga C, Shalloo L, Donnelly W, Robson E, Ivanov S. Opportunistic wireless networking for smart dairy farming. IT Professional. 2017;19:16–23. doi: 10.1109/MITP.2017.28. [DOI] [Google Scholar]
- 127.Etherington WG, Kinsel ML, Marsh WE. Options in dairy data management. Can Vet J. 1995;36:28. [PMC free article] [PubMed] [Google Scholar]
- 128.Wenz JR, Giebel SK. Retrospective evaluation of health event data recording on 50 dairies using dairy comp 305. J Dairy Sci. 2012;95:4699–4706. doi: 10.3168/jds.2011-5312. [DOI] [PubMed] [Google Scholar]
- 129.Hansen MM, Miron-Shatz T, Lau AY, Paton C. Big data in science and healthcare: a review of recent literature and perspectives. Contribution of the IMIA social media working group. Yearbook of medical informatics. 2014;9:21–26. doi: 10.15265/IY-2014-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.KeltonDF LKD, Martin RE. Recommendations for recording and calculating the incidence of selected clinical diseases of dairy cattle. J Dairy Sci. 1998;81:2502–2509. doi: 10.3168/jds.S0022-0302(98)70142-0. [DOI] [PubMed] [Google Scholar]
- 131.Ford E, Carroll JA, Smith HE, Scott D, Cassell JA. Extracting information from the text of electronic medical records to improve case detection: a systematic review. J Am Med Inform Assoc. 2016;23:1007–1015. doi: 10.1093/jamia/ocv180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liao KP, Cai TX, Savova GK, Murphy SN, Karlson EW, Ananthakrishnan AN, Gainer VS, Shaw SY, Xia ZQ, Szolovits P, Churchill S, Kohane I. Development of phenotype algorithms using electronic medical records and incorporating natural language processing. Brit Med J. 2015;350:h1885. doi: 10.1136/bmj.h1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study as this is a review paper.


