Summary
Acetylcholine in the neocortex is critical for executive function [1–3]. Degeneration of cholinergic neurons in aging and Alzheimer’s Dementia is commonly treated with cholinesterase inhibitors[4–7], however, these are modestly effective and are associated with side-effects, which preclude effective dosing in many patients [8]. Electrical activation of the Nucleus Basalis (NB) of Meynert, the source of neocortical acetylcholine [9,10] provides a potential method of improving cholinergic activation [11,12]. Here we tested whether NB stimulation would improve performance of a working memory task in a non-human primate model. Unexpectedly, intermittent stimulation proved to be most beneficial (60 pulses per second, for 20 seconds every minute), whereas continuous stimulation often impaired performance. Pharmacological experiments confirmed that the effects depended on cholinergic activation. Donepezil, a cholinesterase inhibitor, restored performance in animals impaired by continuous stimulation but did not improve performance further during intermittent stimulation. Intermittent stimulation was rendered ineffective by either nicotinic or muscarinic receptor antagonists. In the months after stimulation began, performance also improved in sessions without stimulation. Our results reveal that intermittent NB stimulation can improve working memory, a finding that has implications for restoring cognitive function in aging and Alzheimer’s Dementia.
Keywords: Deep brain stimulation, Nucleus Basalis of Meynert, working memory, nonhuman primate, executive function
Results and Discussion
Five adult Rhesus monkeys were trained with a delayed match to sample task, requiring them to remember a stimulus presented on a touch screen, and after a delay period, to select the stimulus that was initially presented (Figure 1). Once the animals were proficient with the task, they were implanted bilaterally with NB stimulation electrodes. Placement and anatomical verification are described in the STAR Methods. The zone of stimulation was broad, on the order of a 4 mm diameter sphere [13], and the accuracy of placement of the electrode tip within the nucleus was not critical in this experiment. Experiments in the first two animals (K and S) primarily guided proper positioning of stimulation leads in the next three (CH, DI, and PU). No histological confirmation of electrode placement occurred in these three animals, and electrode positioning is presumptive based on stereotaxic targeting.
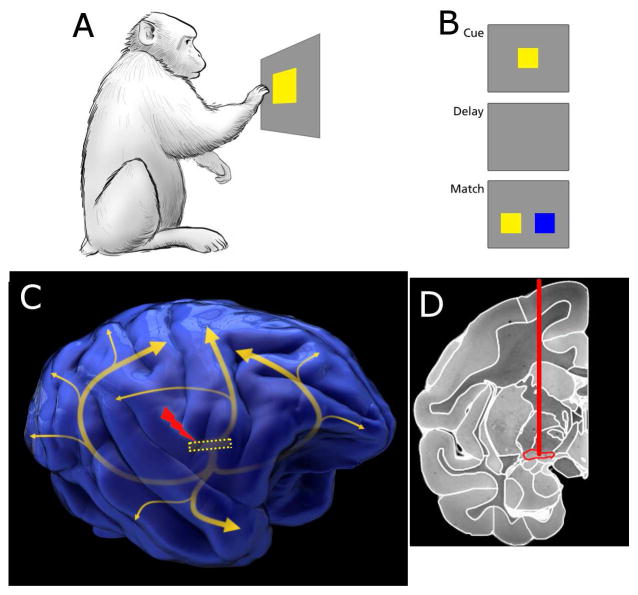
Figure 1. Task paradigm and electrical stimulation position in the brain.
A. Macaque monkeys were trained to interact with a touchscreen. The first step in the task is touching the cue. B. After the cue is touched, the screen blanks during the delay period, followed by presentation of two potential matches. Three colors were used in training, and two were randomly selected as cue/match and distractor on each trial. C. Stimulation of the Nucleus Basalis of Meynert was used in experimental sessions. The position of the nucleus is shown by the dotted yellow rectangle, with the curved arrows approximating the pathways from the nucleus to neocortices[52]. D. An MRI coronal section of a Rhesus monkey brain with the implantation target outlined in red. The MRI was taken from the Macaque Scalable Brain Atlas[53,54]. See also Figure S1–S2.
Effects of electrical stimulation parameters
We initially tested the hypothesis that NB stimulation improves working memory performance by applying a continuous train of stimulation pulses in blocks of 100 trials interleaved with blocks of 100 trials without stimulation. Contrary to our expectations, we found that continuous stimulation always impaired performance, and effects were larger at higher stimulation rates (Figure 2A). Results for continuous stimulation at 80Hz reached statistical significance (binomial tests, Animal CH: n=800 trials, p < 0.0001; Animal DI: n=1000, p <0.001).
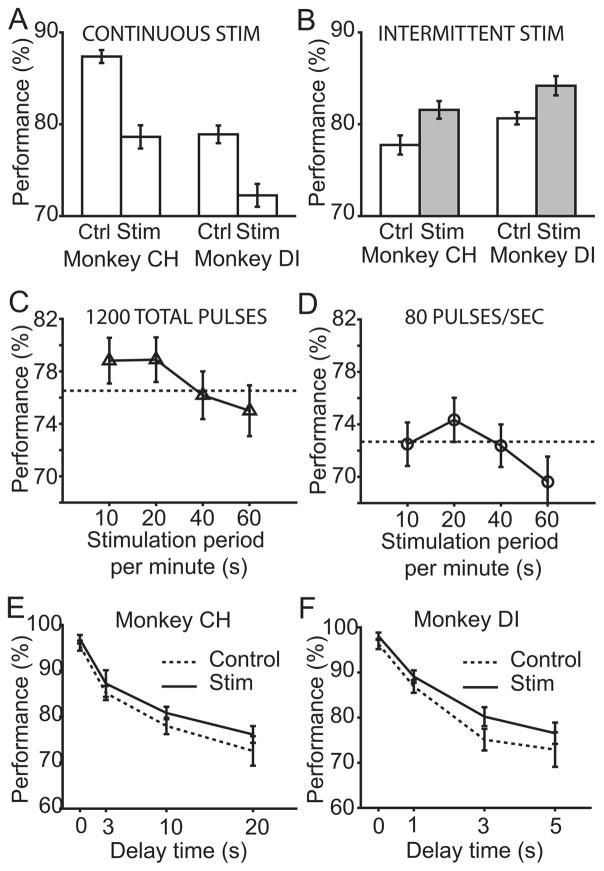
Figure 2. Effects of NB stimulation on working memory behavior.
A. The impact of continuous stimulation on performance. Monkeys performed the task with concurrent 80 Hz continuous stimulation, or under a control condition. B. The impact of intermittent stimulation on performance. The gray bars indicated intermittent stimulation condition distinct from continuous stimulation condition indicated by empty bars in A. C. Both monkeys were tested with 1200 stimulation pulses per minute, delivered in 10 seconds (120 Hz), 20 seconds (60 Hz), 40 seconds (30 Hz) or 60 seconds (20 Hz). The performance under the control, no stimulation, condition for this week is indicated with the dashed line, and its standard error is 1.8%. D. Performance as both monkeys were tested with 80 pulses per second for different fractions of a minute. The standard error in control condition is 1.7%. E. Delay performance curve with and without intermittent stimulation in Monkey CH. F. Delay performance curve with and without intermittent stimulation in Monkey DI. Up to 1000 trials were used to determine each point. See also Figure S3 –S4.
In an effort to determine if stimulation during a particular interval of the task was disrupting working memory, the task was altered to stimulate only during the inter-trial period, or to stimulate only during trials. Unexpectedly, either condition resulted in supranormal performance. We established an intermittent stimulation condition which provides 20 seconds of pulses at a rate of 60 per second followed by 40 seconds without pulses. Results from this condition are shown in Figure 2B. Trials with stimulation resulted in better performance than the trials with no stimulation in the animals tested (binomial test, n=1000, p<0.01). Note that a longer delay period was used in Figure 2B than in Figure 2A to avoid ceiling effects. We conclude that intermittent stimulation results in supranormal working memory performance.
We explored the parameters for optimal stimulation in two ways. First, the number of pulses per minute was fixed, and the duration and rate of stimulation was altered. The 1200 pulses per minute were delivered in 10 seconds (i.e. at a rate of 120 Hz), 20 seconds, 40 seconds, or 60 seconds. Results are shown in Figure 2C for Animals CH and DI. A two way ANOVA found a significant effect of these conditions on performance (test for animal number and condition, F(4,4)=12.92 for main effect of condition, p<0.02; F(1,4)=1.48 for main effect of animal p>0.2). Post hoc comparisons showed that performance was significantly better at 10 or 20 second period than the 60 second condition of continuous stimulation (binomial statistic, ~1050 trials for each condition, Bonferroni corrected p<0.005).
In a second experiment, the rate of stimulation was fixed at 80 pulses per second but the duration was varied to 10, 20, 40, or 60 seconds per minute (Figure 2D). A two-way ANOVA found a significant effect of condition and animal (F(1,4)=15.09 for animal p<0.02, F(4,4)=8.48 for condition p<0.04). The highest performance occurred for the 20 second stimulation condition which was significantly better than the 60 second stimulation in post hoc tests (binomial statistic, ~1350 trials each, Bonferroni corrected p<0.005). The significant ANOVA finding on the animal variable occurred as Animal CH averaged higher performance in the 80Hz stimulation conditions than Animal DI. These parametric tests revealed that the condition of 1200 pulses being delivered in 15–20 seconds per minute were optimal within the parameters tested.
The effect of intermittent stimulation across varying delay periods can be seen in Figure 2E–F. The performance change, expressed as change in percent correct, was larger for longer delays, up to 5% improvement, than for shorter delays. However, the variability of the measure is a function of the percent correct, and if the percent correct is closer to 100, the variance is lower. To enable a fair comparison of the performance change caused by stimulation as a function of delay length, these percent change differences were converted to changes in signal size using the signal detection theory metric d′ (see STAR Methods), which measured how far above the noise the signal size, or memory, rose. This metric inferred the signal size from the behavioral responses, and it was normalized by the noise standard deviation. Using either signal detectability metrics or percent correct, no statistically significant changes in stimulation effect were found as a function of delay length using ANOVAs (p>0.1). Stimulation improved performance across the entire working memory delay curve, and the change in d′ ranged from 0.13 to 0.39, with an average at the longest delay of 0.16. Alternately, if the animals were at zero delay and 96% correct, improvements were 1.4 to 1.7%, while at longer delays animals improved 3.6–4% above 75%. Changes of this magnitude reached statistical significance in two to four behavioral sessions.
Insight into the speed with which these effects occurred after stimulation began was determined by analyzing the performance shortly after a condition change. If effects appeared slowly, then performance shortly after stimulation began should be lower than performance later in the trial block. Similarly, if the effects decreased slowly once stimulation stopped, the no-stimulation blocks should have had lower performance later in the block. Performance compared to trial position in the block is shown in Figure S3A and B for continuous and intermittent stimulation. No significant trends based on position within the blocks were found, which indicated effects occurred in less than 3 minutes, which was the average time to complete 10 trials.
The interactions of stimulation effects with cholinergic pharmacology
To test the hypothesis that the improvement of working memory performance after intermittent stimulation depended on cholinergic pathways, stimulation was delivered with and without the cholinesterase inhibitor Donepezil. When the Donepezil dose was combined with intermittent stimulation, performance was no different from the Donepezil alone, or stimulation alone, as shown in Figure 3A. The impact of combining Donepezil with 80 pulses per second continuous stimulation is shown on the right in Figure 3A. Continuous stimulation significantly impaired performance, but Donepezil reversed this impairment and resulted in supranormal performance. In Figure 3A, all comparisons relative to the control condition were significant (binomial test, n is >3000 for each condition, p<0.005 each). The result suggested that intermittent stimulation achieved a ceiling effect on cholinergic pathways. It also suggested that continuous stimulation degraded behavior by decreasing acetylcholine levels.
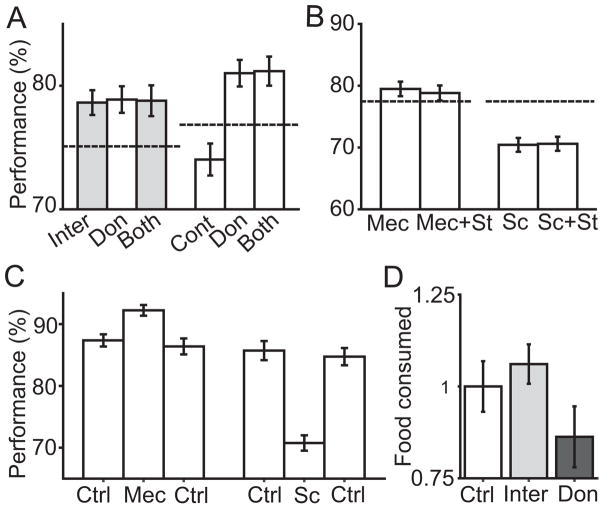
Figure 3. Pharmacological interactions with stimulation and effects on the animals’ appetite.
A. On the left, the experimental conditions of intermittent stimulation (Inter) are compared to control (dashed line), donepezil (Don), and both stimulation and donepezil (Both). On the right, in a separate experiment, the same comparisons are applied with continuous stimulation (Cont). The gray bars indicated intermittent stimulation involved. Standard errors for control conditions (dashed lines) on the left and right are 1% and 1.1%, respectively. B. Effects of stimulation and acetylcholine receptor blockers. Mecamylamine (Mec) was given to animals prior to behavior, and working memory performance was assessed during intermittent stimulation, and without stimulation. Scopolamine (Sc) was similarly tested. Average of the two animals tested in each condition is displayed. Dashed line shows the baseline performance in control condition, and standard error is 1.6%. C. Effects of Mecamylamine and Scopolamine. Animals performed behavior in control condition (left Ctrl), then were given Mecamylamine (Mec), or Scopolamine (Sc). Then, animals performed washout behavior (right Ctrl, 72 h after drug application). D. Effects of stimulation and Donepezil on appetite. Food consumption under the experimental conditions of Control, Intermittent Stimulation, or Donepezil. Individual animal consumption under all conditions was normalized by mean Control consumption to allow pooling across animals for this plot. Food consumption data was collected from animal CH and DI.
A further test of the cholinergic basis of effects was conducted using acetylcholine receptor antagonists. Mecamylamine was used to block nicotinic acetylcholine receptors in one set of experiments, and scopolamine was used to block muscarinic acetylcholine receptors in the second set of experiments. In either case (Figure 3B), intermittent stimulation did not improve performance (p>0.1). The result suggested that both receptor sub-types were necessary for the performance increase caused by intermittent stimulation. As shown in Figure 3C, administration of mecamylamine and scopolamine alone reproduced known effects [14,15]. Scopolamine impaired working memory (binomial test, n=550, p<0.001). Mecamylamine, at the dose tested, improved working memory (binomial test, n=2700, p<0.001).
Donepezil is reported to cause side effects including nausea which limits its clinical efficacy in some patients [8,16,17]. We plotted food consumption of the animals under control conditions, days in which intermittent stimulation was delivered in blocks, and days in which Donepezil was given. The results (Figure 3D) showed a significant effect of condition (unbalanced ANOVA, F(2,56)=3.4 for condition p=0.04, F(1,56)=1.85 for animal p=0.179).). Post hoc testing found that monkeys consumed less food on days in which Donepezil were given relative to days in which stimulation was given (t-test, t=2.81, df=35, p<0.009).
The reaction times of Monkey CH and DI to the Cue and Match are shown in Figure S4. These reaction times were measured in the same data shown in Figure 2E–F. Under no conditions were there significant effects of stimulation on reaction times (paired t-tests, p>0.1 for all), which argued against stimulation causing a general effect on arousal or alertness.
Prior work stimulating the Nucleus Basalis in anesthetized animals has demonstrated consistent spectrum shifts of cortical electroencephalogram (EEG) recordings [18–21]. The low frequency portions of the EEG, under 12 Hz, tend to decrease in energy, or desynchronize, while the higher frequency gamma range increases under some experimental conditions. We recorded the subcortical local field potential (LFP), via the stimulating electrode, immediately before and after stimulation during periods of intermittent and continuous stimulation in the awake animal. As shown in Figure S2A–D, consistent reductions in LFP power spectrum occurred as a result of intermittent stimulation. Similar effects occurred after continuous stimulation at 80 Hz, shown in Figures S2E–F. All effects were significant (t-tests, p<0.001, frequency bands <4Hz, 4–8Hz, 8–12Hz, 12–20Hz, 20–40Hz)
Long term changes caused by stimulation
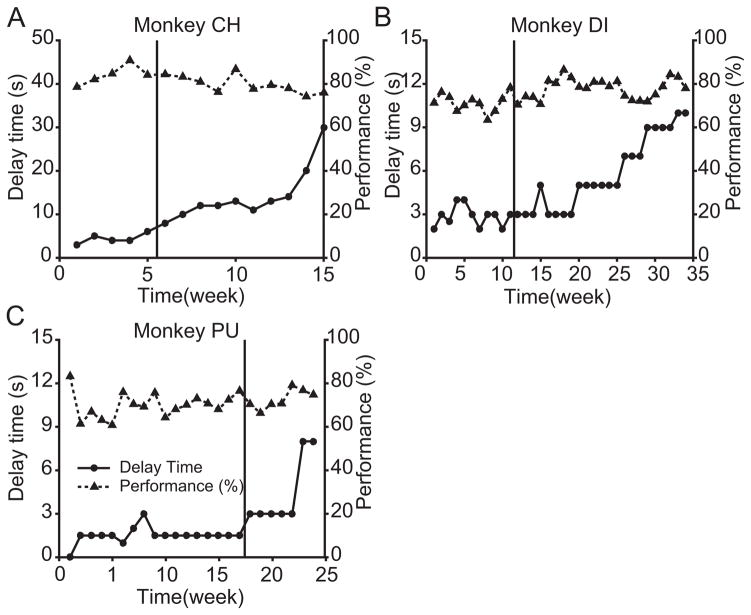
Unexpected improvements in working memory performance were observed in the three animals that were tested with stimulation of NB for months. In each animal, the delay interval was adjusted to maintain performance between 75 and 80 percent. The first indication of this change is shown in Figure 4A, in Animal CH. The working memory delay duration had been increased from 6 seconds to 30 seconds over 9 weeks. Animal CH was improving shortly before stimulation was introduced, so further improvement could have been caused by a long term stimulation effect or a continuation of an improvement trend.
Figure 4. Changes in working memory over time.
A. The working memory delay and performance for Animal CH. Delay and performance are plotted against time relative to initiating intermittent stimulation. The delay was chosen to maintain performance range. The vertical line before week 6 indicates when intermittent stimulation was introduced. Left axis illustrates the delay which is marked by the circle symbols, while the right axis illustrated the performance plotted as triangles. All data in this figure are defined under no-stimulation control conditions. B. Data for Animal DI. C. Data for Animal PU.
Figure 2E–F gives a chance to convert stimulation effects into equivalent changes in delay length. If the working memory delay chosen caused an animal to perform close to 75% correct, and the delay were halved, performance would have increased 5–6%. This increase is slightly larger than the impact of intermittent stimulation shown in Figure 2B. A five-fold decrease in the length of the delay period corresponds to a 12–14% increase in percent correct. In signal detection terms, a 14% increase in performance, from 75% to 89%, corresponds to a d′ change of 0.78, which is 4.9 times greater than the average acute d′ change caused by stimulation at long delays in Figure 2E–F.
Animal DI was allowed to reach asymptotic performance in the absence of stimulation for a longer period, and performance was stable over 11 weeks with a working memory delay of 2 to 3 seconds. Over the ensuing five months, the working memory delay increased from 3 seconds to 10 seconds, which is shown in Figure 4B. Under the null hypothesis that working memory ability was stable, this change was highly significant (Binomial Model, see STAR Methods, p<10−5). Animal PU (Figure 4C) performed the task for 17 weeks prior to initiating stimulation. Its performance was stable in this pre-stimulation period. In the following 7 weeks, the 1.5 second delay increased to 8 seconds, more than five-fold, which again was significant (p<10−5). The results from Animals DI and PU showed that stimulation increased delay duration. All the performance data used in this analysis were collected under control conditions i.e., without the acute boost in performance shown in Figure 2B. For all the data shown in Figure 4, the stimulation delivered in the several month long period of stimulation was mixed while the experiments of Figures 2–4 were conducted.
Discussion
The delayed match to sample task was used because it is a well-studied test of executive function in the nonhuman primate [22]. It embodies several mnemonic processes that are critical to human cognition including attention, stimulus discrimination, encoding, and memory for recent events. [23]. The task is sensitive to cholinergic modulation as indicated by its enhancement by cholinesterase inhibitors [24,25], as well as cholinergic receptor agonists [26,27], and it is sensitive to impairments of performance by cholinergic antagonists [15,28]. Intermittent stimulation of the Nucleus Basalis led to short term improvement in the task, while continuous stimulation suppressed performance. Pharmacological administration of cholinesterase inhibitors and acetylcholine receptor blockers demonstrated that the effects occur through modulation of acetylcholine levels and require activation of both classes of acetylcholine receptors. Unlike cholinesterase inhibitors, appetite is not suppressed by cholinergic modulation through deep brain stimulation. Even though stimulation was delivered in varied amounts each week, each of three animals increased their working memory delay duration three to five-fold in the months after stimulation began. Two of those animals had extended periods of stable task performance before stimulation began. Future work may address the specificity of these effects, as working memory only comprises one component of executive function.
A major unexpected finding in this study is the form of stimulation that produced optimal results: 60 pulses per second delivered for 20 seconds and followed by 40 seconds without stimulation. Deep brain stimulation’s mechanisms have been a subject of debate [29]. Locally, a depolarization block and possibly activation of local inhibitory circuits dominates to prevent somatic activation, while recording and imaging evidence suggests axonal efferent outflow is activated by each stimulation pulse. Accordingly, the NB axons should be activated each time a pulse is delivered. Why the intermittent pattern results in more cortical acetylcholine, as inferred from pharmacological experiments, than continuous stimulation is an obvious question for subsequent studies. We speculate that intermittent, not continuous, neuronal stimulation results in higher levels of acetylcholine synthesis and release. The rate-limiting step in acetylcholine synthesis is the uptake of choline from the synaptic cleft via the high affinity choline transporter hCHT [30]. Before release, the proton exchanging acetylcholine transporter VAChT brings more acetylcholine into the vesicle, and the high affinity choline transporter, which would transport choline to the cytosol, is turned off by the low vesicular pH[31,32]. After release, the vesicular membrane becomes part of the cell membrane, and the decreased proton concentrations turn the acetylcholine transporter off, and the choline transporter on. If frequent vesicular release from high frequency stimulation alters post-release activation of the high affinity choline transporter by changing local pH, overall acetylcholine synthesis and release could be reduced.
A similar curiosity is that the stimulation does not have any temporal relation to the behavioral task or to known cholinergic kinetics other than the recycle time [33,34]. Donepezil’s effects on behavioral performance are arguably boosting the efficacy after normal cholinergic release, which would preserve the normal temporal function of acetylcholine [24]. However, some experiments with subtype selective agonists also improve working memory function [35–37], and these would similarly lack any temporal specificity.
Improvement in cognition from cholinergic system activation could be caused by cholinergic modulation of neurons, or by cholinergic induced increases in blood flow, and these mechanisms are not mutually exclusive. In neocortex, cholinergic activation of neurons leads to increased thalamic input into layer IV while suppressing other cortical inputs [38,39]. This, at least partly, explains how the activation of the cholinergic system desynchronizes the EEG and local field potential (Figure S2), which was also reported by previous studies [19,20,40,41]. The coupling of desynchronization to behavioral improvements suggests direct cholinergic modulation of neurons improves the behavior.
At the same time, we cannot exclude the possibility that indirect mechanisms such as increases of blood flow played a role in the improved cognitive performance. In the brain, acetylcholine is acting on both neurons and glia adjacent to blood vessels [42,43]. Cholinesterase inhibitors in humans increase cerebral blood flow of 10–15% [44], and this blood flow is typically reduced in Alzheimer’s [45–47]. Reductions in cerebral blood flow likely contribute to the cognitive impairments [48]. Future work may shed light on the relative contributions of neural modulation and blood flow regulation in these effects, as well as on the question of their independence.
The long-term effects observed indicate working memory duration is increased three to five-fold in each of three animals tested over periods of up to five months. The changes even occurred after long stable performance periods prior to stimulation. These results suggest that cholinergic modulation combined with executive function behavior causes brain plasticity to support behavioral improvements. Prior work has associated phasic stimulation of Nucleus Basalis with neural plasticity in sensory cortex [18,20,49].
Several clinical trials have been conducted on deep brain stimulation of cholinergic pathways [50,51]. None have found consistent cognitive improvement using continuous stimulation. Similarly, animal work with continuous stimulation finds weak to no effects on learning and memory [11]. We measured analogous effects using continuous stimulation, which also suggest that high frequency continuous stimulation suppresses acetylcholine levels. Our data show that making the stimulation intermittent is critical to turning cognitive suppression into cognitive enhancement. Animals had stronger appetites while receiving stimulation than while receiving Donepezil. The use of the intermittent stimulation parameters outlined in this work reach efficacies as high as those of high doses of cholinesterase inhibitors without peripheral side effects, and point towards new candidates for therapeutic treatment of Alzheimer’s Dementia.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to, and will be fulfilled by the Lead Contact, David T Blake (dblake.mcg@gmail.com).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animal Model
Five rhesus monkeys (Macaca mulatta) were used in this study. Two initial animals (K and S) were used to optimize the placing of the stimulation electrodes. These were a 7 years old male and a 12 years old female weighing 7 and 6 kg. The three subsequent animals (animals CH, DI, and PU), used for data in Figures 2–4, were male, 6 years old and weighed 8 –11 kg. The monkeys were pair-housed in 12-h light/dark cycle rooms. All monkeys were task naive at the start of the experiments. All animals were chaired during task performance, with one arm restrained to both establish consistency in the arm used to perform the task, and to prevent spinning in the primate chair. Animals were not head fixed during task performance.
All animal studies comply with the Guide for the Care and Use of Animals, 8th Edition, and were approved by the IACUC at Augusta University.
METHOD DETAILS
Behavioral task
All behavioral software was based on prior work [26], and available from PBTLI Prime Behavior Testing Laboratories, Inc, http://www.pbtli.com/, Augusta, GA. The behavioral task, shown in Figure 1, initiated with a colored square cue in the center-top part of the touchscreen. After the animal touched the square, the cue disappeared, and the screen remained blank for the delay period. In the match phase, two colored squares were presented in the lower right, and lower left, of the touchscreen. One of the two squares, randomized in location, had the same color as the cue square. Correctly touching the target square resulted in delivery of food slurry reward, while incorrect responses resulted in doubling the inter trial period as a brief timeout. The animal reaction times were not strongly constrained. Animals had 10 seconds to make a response. Percent correct and reaction times were recorded. Animals required several months of daily training to achieve stable performance at the task. Three colors (red, blue and yellow) were used. In each trial, two were randomly chosen as cue/match, and as the distractor.
Surgery and Deep Brain Stimulation
Surgery was performed in a sterile surgical suite under isoflurane anesthesia monitored by a clinical veterinarian. Stereotaxic measurements were made to target implantation. In our first two animals, targeting was 8mm lateral, 12 and 14 mm anterior interaural respectively, and 29 mm below the cortical surface in a vertical penetration. The final three animals were implanted at 16 mm anterior interaural with the same lateral and depth coordinates. The final lateral and anterior coordinates, and depth, were chosen to correspond to the center of the anterior portion of the Nucleus Basalis of Meynert, which would contain the highest density of projections to the prefrontal cortex [10,55,56]. At the targeted brain position, the stimulation region, which is estimated to be a 4 mm diameter sphere[29], should include the Nucleus Basalis of Meynert as well as portions of the anterior amygdala including the central nucleus, the anterior commissure, and the inferior internal globus pallidus[57]. A skin flap was performed, and two holes were drilled above the location of each implant. A small cylindrical titanium chamber (5-mm inner diameter and 7-mm outer) was mounted on the cranium and the skin was sutured into place leaving the chamber exposed. A 26 ga. hypodermic guide tube was lowered and the tip advanced 5 mm below the dura mater. The electrode was inserted into the guide tube, and a stylus was used to push it to the appropriate depth. The use of the guide tube and stylus followed published methods [58,59], which are analogous to methods used in human neurosurgical deep brain electrode implantation. The guide tube was then raised while the stylus depth maintained. The chamber was evacuated of fluid, flushed with ceftriaxone, and thereafter fluid evacuated a second time. Silicone was poured into the chamber to seal the fenestrations in the skull and the inside of the chamber. The electrode was fixed in depth with a drop of cyanoacrylate, and its rear wire was stripped and soldered to a connector that was fixed on the chamber outer wall. One week after the surgery, the animals returned to behavioral studies.
The stimulation pulses were created by a multiple functional I/O device (USB-6211, National Instruments, Austin, TX), which was controlled by a custom programed software. Impedances of electrodes were checked daily prior to each behavioral session so that stimulation voltages could be tailored to deliver the appropriate currents (200 μA). Continuous stimulation was performed from 20 pulses per second to 120. Intermittent stimulation was initially applied at 60 pulses per second only in-between trials, or only during trials, which were roughly 3 and 5 second periods. Thereafter, experiments standardized on intermittent stimulation using a one-minute cycle length, and stimulation for 20 seconds at 60 pulses per second, followed by 40 seconds with no stimulation. In these one-minute cycle stimulation experiments, no attempt was made to synchronize the stimulation with the behavioral trials.
Electrodes were custom manufactured in our laboratory based on published specifications[58,59]. Conductors were 50 μm Pt/Ir, Teflon-insulated wire (A-M systems, Seattle, WA) embedded within a 30 ga. hypodermic tube, which was encased in a 28 ga. polyimide sheath. The wire extended from the end of the sheath into the brain tissue by roughly 1 mm, and the last 0.7 mm of insulation was stripped to achieve impedances of 5–10 kOhm at 1 kHz. The far end of the electrode was soldered to an extracranial connector fixed on the chamber outer wall. Preliminary experiments on electrode placement in the two pilot animals tested the effects of short periods of stimulation on EEG desynchronization. Stimulation was delivered with biphasic, negative first, unipolar 200 μA pulses with 100 μS per phase, and 10 pulses were delivered in 100 msec. Under anesthesia, this resulted in EEG desynchronization when averaged over 10 trials when the electrode was at a depth corresponding to the atlas position of Nucleus Basalis. EEG was recorded with ×1000 gain from two bone screws at the same M-L position, with one near vertex, and one lateralized. An electrode movement vertically in either direction of more than 1 mm was adequate to make desynchronization not possible using the same protocol. After our second pilot animal, we determined the depth for our A-P and M-L coordinates was 29 mm below the cortical surface. We further recovered our electrode track from our second pilot animal, shown in Figure S1. It was implanted 2 mm more caudally than the electrodes from the three monkeys presented in the results. The tip of the electrode was centered 1 mm medial to Nucleus Basalis at 14 mm anterior interaural. After our pilot animals, we implanted to the same depth in all animals, and 2 mm more rostrally, for the current study, and measured LFP desynchronization in the awake animals using the stimulation electrode as the positive lead (Figure S2).
In these first two pilot animals, we frequently moved electrodes vertically to new positions. At this point in the study, we understood that the electrode depth at which EEG desynchronization occurred after brief phasic stimulation precisely matched that at which 80 Hz continuous stimulation resulted in significant performance decrements. The rear ends of the electrodes were always visible, protruding from the implanted titanium cylinder, which gave an absolute depth measurement.
Pharmacology and stimulation studies administration
We used donepezil, mecamylamine and scopolamine (Sigma-Aldrich, St. Louis, MO) in this study. The drugs were dissolved in a 0.5 cc saline vehicle to an amount specified by the experiments (donepezil: 200 μg/kg, mecamylamine: 300 μg/kg and scopolamine: 6.2 μg/kg). Donepezil was given via I.M. administration 15 minutes before behavior testing on the mornings in which data was collected. The rationale for the doses selected for Donepezil was based on previous behavioral and functional brain imaging data (dose range 50–250 μg/kg) in rhesus monkeys [25,60]. Data from days without donepezil were at least 72 hours after the last administration. Doses higher than 200 μg/kg could not be used in this study, as the animals administered these doses refused to engage in behavioral trials for food rewards. Nausea is a well-known, clinical side effect of the drug in humans. Each week, control performance was evaluated. Then, performances under the intermittent stimulation alone, donepezil alone, and donepezil+stimulation were evaluated on successive days. Similar weekly plans were used for continuous stimulation, and mecamylamine (300 μg/kg) and scopolamine (6.2 μg/kg) testing. This weekly testing schedule was chosen over an intraday comparison because an intraday design would compare drug vs no-drug conditions at different times, and with different appetitive motivations. The recovery test sessions from drug were recorded 72 h after drug application. The doses of mecamylamine and scopolamine used in this study refer to previous publications [14,28].
Initial deep brain stimulation studies, shown in Figure 2A–B, provided stimulation in blocks. For continuous stimulation, 100 trials without stimulation were interleaved with 100 trials with stimulation. For intermittent stimulation, blocks were 50 trials. For Figure 2C–D, all stimulation conditions were executed randomly block by block and interleaved with each other. Each block also contained 50 trials. For Figure 3A–C, we collected animals’ performance data in control conditions for 1–2 days first, then collected performance data after administering pharmacological agents. Drug and Stim+Drug conditions were interleaved with each other in blocks of 50 trials. The recording procedures for the pharmacology experiments were 2–3 times to improve statistical power. For Figure 2E–F and S4, which includes performance at different delays with and without stimulation, we tested the animals’ performance with interleaved blocks in control and stimulation conditions. In each block, variable delay times were pseudo-randomly chosen and arranged in the test sequence. Software was administered directly from PBTLI software while a second computer was used to generate stimulation pulses. Data for Figure 4 were selected from all available data in which no drug or stimulation condition occurred.
QUANTIFICATION AND STATISTICAL ANALYSIS
d′ Calculation
The signal detection theoretic d′ is the salience of the target relative to the distractor as inferred from the animals’ behavioral choices. In our two alternative forced choice framework, d′ is calculated using only the percent correct with the Matlab (Mathworks, Natick, MA) norminv function, as [61,62]. This metric measures the amount of the memory that is retained, on average. It assumes the memory can be measured in terms of a mean and a noise standard deviation. As time passes, that mean, or memory, regresses towards zero. The metric measures the magnitude, normalized by the noise standard deviation, of the mean at the end of the delay period. The following table details how d′ relates to percent correct in the task.
| d′ | Percent Correct |
|---|---|
| 0.25 | 57 |
| 0.5 | 64 |
| 0.75 | 70 |
| 1.0 | 76 |
| 1.25 | 81 |
| 1.5 | 85 |
| 1.75 | 89 |
| 2.0 | 92 |
Binomial statistical model
We relied on a binomial statistic model to test if Nucleus Basalis stimulation, over weeks and months, produced long-term improvements in working memory that could be measured which resulted in ability to perform the task at high performance with a longer delay interval (Figure 4). Prior to stimulation, animals performed 3000 trials weekly at a performance level averaging 78% correct, with a delay interval ranging between 1.5–6 s for the three animals. For each animal, we asked what performance improvement would occur if the delay, at which performance level is 78% correct, were doubled? In each animal’s delay curves, shown in Figure 2E–F, halving the delay would result in changes in percent correct of 5.4 and 5.1 percent. In other words, if we time scaled the graphs in Figure 2E–F to the same percentage correct at twice the delays, animal performance at the original delay would be predicted, based on the delay curves in Figure 2E–F, to increase by just over 5%. We conservatively rounded this percentage to a 5 percent change. Next, the two-tailed probabilities were calculated that a binomial process with a fraction correct of 0.78 over 3000 trials could result in more correct trials than a process with a fraction correct 5 percent higher or lower. This probability is less than 10−5. The implication of the binomial model is that if an animal performs at 78% correct for duration 2X, then it would be expected to perform at 83%, or higher, at duration X, and that this performance would be significantly better than its pre-stimulation performance of 78% at duration X.
DATA AND SOFTWARE AVAILABILITY
Data used to generate these figures are archived at https://figshare.com/s/43301bcb03202ec0f086
Supplementary Material
Table 1.
Dates of data collection for each animal for each figure.
| Experiment | Animal CH | Animal DI | Animal PU |
|---|---|---|---|
| Figure 2A–B and S3 | 8/15-10/15 | 11/15-1/16 | |
| Figure 2C–D | 6/16-7/16 | 6/16-6/16 | |
| Figure 2E–F and S4 | 10/15-11/15 | 11/15-12/15 | |
| Figure 3 | 2/16-8/16 | 6/16-8/16 | 8/2016 |
| Figure 4 | 7/15-11/15 | 3/15-11/15 | 12/15-8/16 |
Table 2.
Delay times used in each experiment.
Acknowledgments
Veterinary support provided by N. Rodriguez and P. Otovic. Artwork in Figure 1 was done by D. Bliss. This work funded by NIH grant 5R01MH097695.
Footnotes
Conflict of interest: The authors declare no competing financial interests.
Author Contributions: Conceptualization and Funding Acquisition: D.T.B and C.C. Project Administration: D.T.B. Pharmacology methodology: A.V.T. and P.M.C. Investigation and Analysis: R.L. and J.C and D.T.B performed experiments. Software: R.L. and D.T.B. Writing: D.T.B. and C.C. and R.L. Review and Editing: All authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References list
- 1.Bartus RT. On Neurodegenerative Diseases, Models, and Treatment Strategies: Lessons Learned and Lessons Forgotten a Generation Following the Cholinergic Hypothesis. Exp Neurol. 2000;163:495–529. doi: 10.1006/exnr.2000.7397. [DOI] [PubMed] [Google Scholar]
- 2.Terry AV, Jr, Buccafusco JJ. The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: recent challenges and their implications for novel drug development. J Pharmacol Exp Ther. 2003;306:821–827. doi: 10.1124/jpet.102.041616. [DOI] [PubMed] [Google Scholar]
- 3.Sarter M, Bruno JP. Cognitive functions of cortical acetylcholine: toward a unifying hypothesis. Brain Res Rev. 1997;23:28–46. doi: 10.1016/s0165-0173(96)00009-4. [DOI] [PubMed] [Google Scholar]
- 4.Birks JS. Cholinesterase inhibitors for Alzheimer’s disease. In: Birks JS, editor. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hogan DB, Bailey P, Carswell A, Clarke B, Cohen C, Forbes D, Man-Son-Hing M, Lanctôt K, Morgan D, Thorpe L. Management of mild to moderate Alzheimer’s disease and dementia. Alzheimer’s Dement. 2007;3:355–384. doi: 10.1016/j.jalz.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Qaseem A, Snow V, Cross JT, Forciea MA, Hopkins R, Shekelle P, Adelman A, Mehr D, Schellhase K, Campos-Outcalt D, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2008;148:370–8. doi: 10.7326/0003-4819-148-5-200803040-00008. [DOI] [PubMed] [Google Scholar]
- 7.Sabbagh M, Cummings J. Progressive cholinergic decline in Alzheimer’s Disease: consideration for treatment with donepezil 23 mg in patients with moderate to severe symptomatology. BMC Neurol. 2011;11:21. doi: 10.1186/1471-2377-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farlow MR, Salloway S, Tariot PN, Yardley J, Moline ML, Wang Q, Brand-Schieber E, Zou H, Hsu T, Satlin A. Effectiveness and tolerability of high-dose (23 mg/d) versus standard-dose (10 mg/d) donepezil in moderate to severe Alzheimer’s disease: A 24-week, randomized, double-blind study. Clin Ther. 2010;32:1234–1251. doi: 10.1016/j.clinthera.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hendry SHC, Jones EG, Killackey HP, Chalupa LM. Choline acetyltransferase-immunoreactive neurons in fetal monkey cerebral cortex. Dev Brain Res. 1987;37:313–317. doi: 10.1016/0165-3806(87)90252-5. [DOI] [PubMed] [Google Scholar]
- 10.Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983;214:170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- 11.Lee JE, Jeong da U, Lee J, Chang WS, Chang JW. The effect of nucleus basalis magnocellularis deep brain stimulation on memory function in a rat model of dementia. BMC Neurol. 2016;16:6. doi: 10.1186/s12883-016-0529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinto L, Goard MJ, Estandian D, Xu M, Kwan AC, Lee SH, Harrison TC, Feng G, Dan Y. Fast modulation of visual perception by basal forebrain cholinergic neurons. Nat Neurosci. 2013;16:1857–63. doi: 10.1038/nn.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McIntyre CC, Grill WM, Sherman DL, Thakor NV. Cellular Effects of Deep Brain Stimulation3: Model-Based Analysis of Activation and Inhibition. J Neurophysiol. 2004;91:1457–1469. doi: 10.1152/jn.00989.2003. [DOI] [PubMed] [Google Scholar]
- 14.Terry AV, Buccafusco JJ, Prendergast MA. Dose-specific improvements in memory-related task performance by rats and aged monkeys administered the nicotinic-cholinergic antagonist mecamylamine. Drug Dev Res. 1999;47:127–136. [Google Scholar]
- 15.Buccafusco JJ, Terry AV, Webster SJ, Martin D, Hohnadel EJ, Bouchard KA, Warner SE. The scopolamine-reversal paradigm in rats and monkeys: The importance of computer-assisted operant-conditioning memory tasks for screening drug candidates. Psychopharmacology (Berl) 2008;199:481–494. doi: 10.1007/s00213-007-0887-8. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida T, Ha-Kawa S, Yoshimura M, Nobuhara K, Kinoshita T, Sawada S. Effectiveness of treatment with donepezil hydrochloride and changes in regional cerebral blood flow in patients with Alzheimer’s disease. Ann Nucl Med. 2007;21:257–265. doi: 10.1007/s12149-007-0022-2. [DOI] [PubMed] [Google Scholar]
- 17.Han SH, Lee JH, Kim SY, Park KW, Chen C, Tripathi M, Dash A, Kubota N. Donepezil 23 mg in Asian patients with moderate-to-severe Alzheimer’s disease. Acta Neurol Scand. 2016;135:252–256. doi: 10.1111/ane.12571. [DOI] [PubMed] [Google Scholar]
- 18.Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc Nat Acad Sci USA. 1996;93:11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bjordahl TS, Dimyan MA, Weinberger NM. Induction of long-term receptive field plasticity in the auditory cortex of the waking guinea pig by stimulation of the nucleus basalis. Behav Neurosci. 1998;112:467–479. doi: 10.1037//0735-7044.112.3.467. [DOI] [PubMed] [Google Scholar]
- 20.Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science (80-) 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- 21.McLin DE, 3rd, Miasnikov AA, Weinberger NM. Induction of behavioral associative memory by stimulation of the nucleus basalis. Proc Nat Acad Sci USA. 2002;99:4002–4007. doi: 10.1073/pnas.062057099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riley MR, Constantinidis C. Role of Prefrontal Persistent Activity in Working Memory. Front Syst Neurosci. 2015;9:181. doi: 10.3389/fnsys.2015.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez JS, Paule MG. Working memory delayed response tasks in monkeys. Methods Behav Anal Neurosci. 2009:1–11. [PubMed] [Google Scholar]
- 24.Buccafusco JJ, Terry AV. Donepezil-induced improvement in delayed matching accuracy by young and old rhesus monkeys. J Mol Neurosci. 2004;24:85–91. doi: 10.1385/JMN:24:1:085. [DOI] [PubMed] [Google Scholar]
- 25.Callahan PM, Hutchings EJ, Kille NJ, Chapman JM, Terry AV. Positive allosteric modulator of α7 nicotinic-acetylcholine receptors, PNU-120596 augments the effects of donepezil on learning and memory in aged rodents and non-human primates. Neuropharmacology. 2013;67:201–12. doi: 10.1016/j.neuropharm.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terry AV, Jr, Plagenhoef M, Callahan PM. Effects of the nicotinic agonist varenicline on the performance of tasks of cognition in aged and middle-aged rhesus and pigtail monkeys. Psychopharmacol (Berl) 2016;233:761–771. doi: 10.1007/s00213-015-4154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terry AV, Risbrough VB, Buccafusco JJ, Menzaghi F. Effects of (±)-4-{[2-(1-Methyl-2-pyrrolidinyl)ethyl]thio}phenol Hydrochloride (SIB-1553A), a Selective Ligand for Nicotinic Acetylcholine Receptors, in Tests of Visual Attention and Distractibility in Rats and Monkeys. J Pharmacol Exp Ther. 2002;301:284–292. doi: 10.1124/jpet.301.1.284. [DOI] [PubMed] [Google Scholar]
- 28.Zhou X, Qi XL, Douglas K, Palaninathan K, Kang HS, Buccafusco JJ, Blake DT, Constantinidis C. Cholinergic modulation of working memory activity in primate prefrontal cortex. J Neurophysiol. 2011;106:2180–8. doi: 10.1152/jn.00148.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vitek JL. Mechanisms of deep brain stimulation: Excitation or inhibition. Mov Disord. 2002;17:S69–S72. doi: 10.1002/mds.10144. [DOI] [PubMed] [Google Scholar]
- 30.Okuda T, Haga T, Kanai Y, Endou H, Ishihara T, Katsura I. Identification and characterization of the high-affinity choline transporter. Nat Neurosci. 2000;3:120–125. doi: 10.1038/72059. [DOI] [PubMed] [Google Scholar]
- 31.Iwamoto H, Blakely RD, De Felice LJ. Na+, Cl-, and pH dependence of the human choline transporter (hCHT) in Xenopus oocytes: the proton inactivation hypothesis of hCHT in synaptic vesicles. J Neurosci. 2006;26:9851–9859. doi: 10.1523/JNEUROSCI.1862-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen ML, Cox GD, Parsons SM. Kinetic parameters for the vesicular acetylcholine transporter: Two protons are exchanged for one acetylcholine. Biochemistry. 1998;37:13400–13410. doi: 10.1021/bi9802263. [DOI] [PubMed] [Google Scholar]
- 33.Trabucchi M, Cheney DL, Hanin I, Costa E. Application of principles of steady-state kinetics to the estimation of brain acetylcholine turnover rate: effects of oxotremorine and physostigmine. J Pharmacol Exp Ther. 1975;194:57–64. [PubMed] [Google Scholar]
- 34.Cheney DL, Trabucchi M, Racagni G, Wang C, Costa E. Effects of acute and chronic morphine on regional rat brain acetylcholine turnover rate. Life Sci. 1974;15:1977–1990. doi: 10.1016/0024-3205(74)90048-4. [DOI] [PubMed] [Google Scholar]
- 35.Terry AV, Buccafusco JJ, Borsini F, Leusch A. Memory-related task performance by aged rhesus monkeys administered the muscarinic M1-preferring agonist, talsaclidine. Psychopharmacology (Berl) 2002;162:292–300. doi: 10.1007/s00213-002-1105-3. [DOI] [PubMed] [Google Scholar]
- 36.Castner SA, Smagin GN, Piser TM, Wang Y, Smith JS, Christian EP, Mrzljak L, Williams GV. Immediate and sustained improvements in working memory after selective stimulation of3alpha-7 nicotinic acetylcholine receptors. Biol Psychiatry. 2011;69:12–18. doi: 10.1016/j.biopsych.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Buccafusco JJ, Jackson WJ. Beneficial effects of nicotine administered prior to a delayed matching-to-sample task in young and aged monkeys. Neurobiol Aging. 1991;12:233–238. doi: 10.1016/0197-4580(91)90102-p. [DOI] [PubMed] [Google Scholar]
- 38.Metherate R, Ashe JH. Nucleus basalis stimulation facilitates thalamocortical synaptic transmission in the rat auditory cortex. Synapse. 1993;14:132–143. doi: 10.1002/syn.890140206. [DOI] [PubMed] [Google Scholar]
- 39.Aramakis VB, Metherate R. Nicotine selectively enhances NMDA receptor-mediated synaptic transmission during postnatal development in sensory neocortex. J Neurosci. 1998;18:8485–8495. doi: 10.1523/JNEUROSCI.18-20-08485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bakin JS, South DA, Weinberger NM. Induction of receptive field plasticity in the auditory cortex of the guinea pig during instrumental avoidance conditioning. Behav Neurosci. 1996;110:905–913. doi: 10.1037//0735-7044.110.5.905. [DOI] [PubMed] [Google Scholar]
- 41.McLin DE, Miasnikov AA, Weinberger NM. The effects of electrical stimulation of the nucleus basalis on the electroencephalogram, heart rate, and respiration. Behav Neurosci. 2002;116:795–806. [PubMed] [Google Scholar]
- 42.Vaucher E, Borredon J, Seylaz J, Lacombe P. Autoradiographic distribution of cerebral blood flow increases elicited by stimulation of the nucleus basalis magnocellularis in the unanesthetized rat. Brain Res. 1995;691:57–68. doi: 10.1016/0006-8993(95)00601-l. [DOI] [PubMed] [Google Scholar]
- 43.Vaucher E, Linville D, Hamel E. Cholinergic basal forebrain projections to nitric oxide synthase-containing neurons in the rat cerebral cortex. Neurosci. 1997;79:827–836. doi: 10.1016/s0306-4522(97)00033-x. [DOI] [PubMed] [Google Scholar]
- 44.Lojkowska W, Ryglewicz D, Jedrzejczak T, Minc S, Jakubowska T, Jarosz H, Bochynska A. The effect of cholinesterase inhibitors on the regional blood flow in patients with Alzheimer’s disease and vascular dementia. J Neurol Sci. 2003;216:119–126. doi: 10.1016/s0022-510x(03)00229-6. [DOI] [PubMed] [Google Scholar]
- 45.Sharp P, Gemmell H, Cherryman G, Besson J, Crawford J, Smith F. Application of iodine-123-labeled isopropylamphetamine imaging to the study of dementia. J Nucl Med. 1986;27:761–8. [PubMed] [Google Scholar]
- 46.Jagust WJ, Budinger TF, Reed BR. The diagnosis of dementia with single photon emission computed tomography. Arch Neurol. 1987;44:258–62. doi: 10.1001/archneur.1987.00520150014011. [DOI] [PubMed] [Google Scholar]
- 47.Schuff N, Matsumoto S, Kmiecik J, Studholme C, Du A, Ezekiel F, Miller BL, Kramer JH, Jagust WJ, Chui HC, et al. Cerebral blood flow in ischemic vascular dementia and Alzheimer’s disease, measured by arterial spin-labeling magnetic resonance imaging. Alzheimers Dement. 2009;5:454–62. doi: 10.1016/j.jalz.2009.04.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruitenberg A, den Heijer T, Bakker SLM, van Swieten JC, Koudstaal PJ, Hofman A, Breteler MMB. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol. 2005;57:789–94. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- 49.Bao S, Chang EF, Davis JD, Gobeske KT, Merzenich MM. Progressive degradation and subsequent refinement of acoustic representations in the adult auditory cortex. J Neurosci. 2003;23:10765–10775. doi: 10.1523/JNEUROSCI.23-34-10765.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R, Keren R, Wherrett J, Naglie G, Hamani C, Smith GS, et al. A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Ann Neurol. 2010;68:521–534. doi: 10.1002/ana.22089. [DOI] [PubMed] [Google Scholar]
- 51.Kuhn J, Hardenacke K, Lenartz D, Gruendler T, Ullsperger M, Bartsch C, Mai JK, Zilles K, Bauer A, Matusch A, et al. Deep brain stimulation of the nucleus basalis of Meynert in Alzheimer’s dementia. Mol Psych. 2015;20:353–360. doi: 10.1038/mp.2014.32. [DOI] [PubMed] [Google Scholar]
- 52.Selden NR, Gitelman DR, Salamon-Murayama N, Parrish TB, Mesulam MM. Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain. 1998;121(Pt 1):2249–2257. doi: 10.1093/brain/121.12.2249. [DOI] [PubMed] [Google Scholar]
- 53.Rohlfing T, Kroenke CD, Sullivan EV, Dubach MF, Bowden DM, Grant Ka, Pfefferbaum A. The INIA19 Template and NeuroMaps Atlas for Primate Brain Image Parcellation and Spatial Normalization. Front Neuroinform. 2012;6:27. doi: 10.3389/fninf.2012.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dubach MF, Bowden DM. Society for Neuroscience Abstr 199.5. 2009. BrainInfo online 3d macaque brain atlas: A database in the shape of a brain. [Google Scholar]
- 55.Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Atlas of cholinergic neurons in the forebrain and upper brainstem of the macaque based on monoclonal choline acetyltransferase immunohistochemistry and acetylcholinesterase histochemistry. Neuroscience. 1984;12:669–686. doi: 10.1016/0306-4522(84)90163-5. [DOI] [PubMed] [Google Scholar]
- 56.Gielow MR, Zaborszky L. The Input-Output Relationship of the Cholinergic Basal Forebrain. Cell Rep. 2017;18:1817–1830. doi: 10.1016/j.celrep.2017.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paxinos G, Huang XF, AWT . The Rhesus Monkey Brain in Stereotaxic Coordinates. Academic Press; San Diego, CA, USA: 2000. [Google Scholar]
- 58.McCairn KW, Turner RS. Pallidal stimulation suppresses pathological dysrhythmia in the parkinsonian motor cortex. J Neurophysiol. 2015;113:2537–2548. doi: 10.1152/jn.00701.2014. ID-ORCID: http://orcid.org/0000-0002-8546-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCairn KW, Turner RS. Deep brain stimulation of the globus pallidus internus in the parkinsonian primate: local entrainment and suppression of low-frequency oscillations. J Neurophysiol. 2009;101:1941–1960. doi: 10.1152/jn.91092.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsukada H, Nishiyama S, Fukumoto D, Ohba H, Sato K, Kakiuchi T. Effects of Acute Acetylcholinesterase Inhibition on the Cerebral Cholinergic Neuronal System and Cognitive Function: Functional Imaging of the Conscious Monkey Brain Using Animal PET in Combination with Microdialysis. Synapse. 2004;52:1–10. doi: 10.1002/syn.10310. [DOI] [PubMed] [Google Scholar]
- 61.Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: Wiley; 1966. [Google Scholar]
- 62.Smith DG, Duncan MJJ. Testing Theories of Recognition Memory by Predicting Performance Across Paradigms. J Exp Psychol Learn Mem Cogn. 2004;30:615–625. doi: 10.1037/0278-7393.30.3.615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.