Abstract
Leiomyosarcoma (LMS) is one of the most common subtypes of soft tissue sarcoma in adults and can occur in almost any part of the body. Uterine leiomyosarcoma is the most common subtype of uterine sarcoma. Increased awareness of this unique histology has allowed for the development of drugs that are specific to LMS and has begun to shed light on the similarities and possible unique aspects of soft tissue and uterine LMS. In this review, we summarize the current understanding of the epidemiology, diagnosis, genomics, and treatment options for LMS.
EPIDEMIOLOGY
Leiomyosarcoma (LMS) is one of the most common subtypes of malignant mesenchymal neoplasms and represents approximately 10% to 20% of all newly diagnosed soft tissue sarcomas (STSs).1 Common locations include the abdomen, retroperitoneum, larger blood vessels, and the uterus. LMS is less common in the extremities compared with other sarcoma subtypes, accounting for 10% to 15% of limb sarcomas, with preference for the thigh. LMS of the uterus (uLMS) is the most common subtype of uterine sarcoma and likely accounts for the single largest site-specific group of LMS.2
As in STSs in general, the overall incidence of LMS increases with age and peaks at the seventh decade of life. In contrast, uLMS occurs from the third decade into old age, but is most common in the perimenopausal age group—the fifth decade of life.3 The sex incidence greatly depends on tumor location, with women comprising a clear majority of patients with retroperitoneal and inferior vena cava LMS, whereas there is a mild male predominance in noncutaneous soft tissue sites and cutaneous LMS.3
ETIOLOGY AND PREDISPOSING FACTORS
Most patients have no clear predisposing factors for the development of LMS. Patients with hereditary retinoblastoma have a cumulative risk of 13.1% for developing any STS as a secondary malignancy, including LMS,4 which is in agreement with the relevance of RB1 loss in sporadic LMS (see below). Similarly, patients with inherited TP53 mutations—Li-Fraumeni syndrome—are at risk for developing sarcomas, including LMS, of any site.5 In one study of patients with Li-Fraumeni syndrome, 7% to 8% developed LMS at a median age of 44 years.
Radiation exposure also increases the risk of developing sarcomas, including LMS.6 Whereas tamoxifen use is most closely associated with an increased risk for endometrial carcinomas, there are reports of uLMS associated with tamoxifen exposure.7
PATHOLOGY AND TUMOR BIOLOGY
Histopathology
LMS is a malignant mesenchymal tumor composed of cells that show distinct features of the smooth muscle lineage. The typical histologic pattern of LMS of any origin is that of intersecting, sharply marginated fascicles of spindle cells with abundant eosinophilic cytoplasm and elongated and hyperchromatic nuclei.3 Focal pleomorphism is common, and some patients show extensive pleomorphism that resembles any undifferentiated STS.8 The majority of LMS are reactive for α-smooth muscle actin, desmin, and h-caldesmon on immunohistochemistry, although none of these markers is specific for smooth muscle differentiation.
Stanford criteria are commonly used for histologic diagnosis of uLMS, incorporating the presence of histologic atypia, tumor cell necrosis, and an elevated mitotic rate.9 Estrogen receptors (ERs) and/or progesterone receptors (PRs) have been reported to be positive in 40% to 70% of patients and may have prognostic significance.10 Because of the nuances of histologic diagnosis—particularly in light of additional complex smooth muscle tumors of the uterus, including atypical leiomyomas and smooth muscle tumors of uncertain malignant potential—expert review by gynecologic and/or sarcoma pathologists is recommended for LMS of any site.
Tumor Biology
Standard karyotyping and fluorescent in situ hybridization techniques as well as more recent DNA copy number variation studies have demonstrated that cytogenetic and molecular changes in LMS are complex.11,12 The most consistent changes that have been detected across several studies are losses that involve two tumor suppressor genes, RB1 (10q) and PTEN (13q).11,12 Recent whole-exome sequencing of LMS and uLMS has confirmed this highly heterogeneous genomic landscape and has demonstrated frequent alterations in TP53, RB, ATRX, and MED12.13,14
Recently, The Cancer Genome Atlas performed a multiplatform molecular characterization of multiple subtypes of soft tissue sarcoma, including 53 soft tissue LMS and 27 uLMS.60 Overall, sarcomas were noted to have low mutational burdens compared with other tumors in the Cancer Genome Atlas project. In addition, analysis confirmed mutations and deletions in RB1, p53, and PTEN as common events in LMS of any site. Somatic copy number alterations were commonly observed without clear distinction between sites of LMS; however, distinct methylation and mRNA signatures were noted between uLMS and soft tissue LMS, some of which have prognostic implication. Of interest, uLMS was noted to have a higher DNA damage response score, whereas soft tissue LMS was noted to have a more prominent hypoxia-inducible factor-1α signaling signature. Gene expression studies have been performed previously in LMS and identified three reproducible molecular subtypes that are overall distributed similarly over LMS and uLMS.15 Subtype I LMS expresses most genes associated with smooth muscle differentiation, tends to be the conventional LMS subtype, and demonstrates improved outcome compared with subtype II LMS, which, in turn, represents a less differentiated form of LMS and partially overlaps in a subset of patients with undifferentiated pleomorphic sarcoma. Subtype III LMS is the only subtype that displays a preference for a specific anatomic site and was more likely to be from the uterus, although uLMS is evenly distributed over the three subtypes. Although intriguing from a research perspective, gene expression remains an investigational technique and has not yet been applied to clinical practice in the care of patients with LMS of any site.15
Several pathways and signal intermediates have been investigated in LMS, and the relevance of the activation of the phosphatidylinositol 3-kinase/AKT pathway has been consistently demonstrated throughout several studies.16 Indeed, genomic deletion of chromosome 10q targets the PTEN tumor suppressor gene and leads to the hyperactivation of phosphatidylinositol 3-kinase/AKT, which is a common finding in LMS. Although approximately one half of LMS expresses insulin-like growth factor (IGF)-1R and IGF-II,13,17 the relevance of IGFR in the proliferation and survival of LMS is yet to be elucidated.
Despite the increase in descriptive knowledge of genomic abnormalities and mutated signaling pathways in LMS, none of these has been proven to serve as effective therapeutic targets. Studies are ongoing to increase our understanding of the complex biologic underpinnings of LMS.
PRINCIPLES OF GENERAL MANAGEMENT
Diagnosis
Clinical presentation of LMS, as with other STSs, is often associated with nonspecific symptoms caused by the displacement of structures, rather than invasion, in specific anatomic locations of the primary tumor and its metastases. Pretreatment biopsy is mandatory in extrauterine sites, with core biopsy the preferred technique. Detailed pathologic evaluation is typically performed after complete resection. Although endometrial biopsy may rarely yield a diagnosis of uLMS, it is far more common to have a negative endometrial biopsy or curettage as LMS is a disease of the myometrium. In many cases the diagnosis of uLMS is made at the time of hysterectomy.
Imaging approaches include magnetic resonance imaging in soft tissue extremity/truncal tumors and uterine masses, and contrast-enhanced computed tomography scan for retroperitoneal lesions. Chest and abdominal computed tomography scan is required in the initial workup, as hematogenous spread is a frequent event in LMS, with the lung and liver as two common sites of metastases.
Unfortunately, in uLMS, no single imaging criterion can reliably distinguish a benign uterine tumor from one that is malignant. Intrauterine masses that had been considered to be benign fibroids, but that continue to increase in size after menopause, should raise suspicion for malignancy. One study of preoperative magnetic resonance imaging for patients with uterine mesenchymal neoplasms demonstrated poor accuracy in distinguishing leiomyomas with atypical features from malignant mesenchymal neoplasms.18 A more recent study identified combinations of qualitative magnetic resonance features that could accurately differentiate LMS from atypical leiomyoma.19
Prognostic Factors
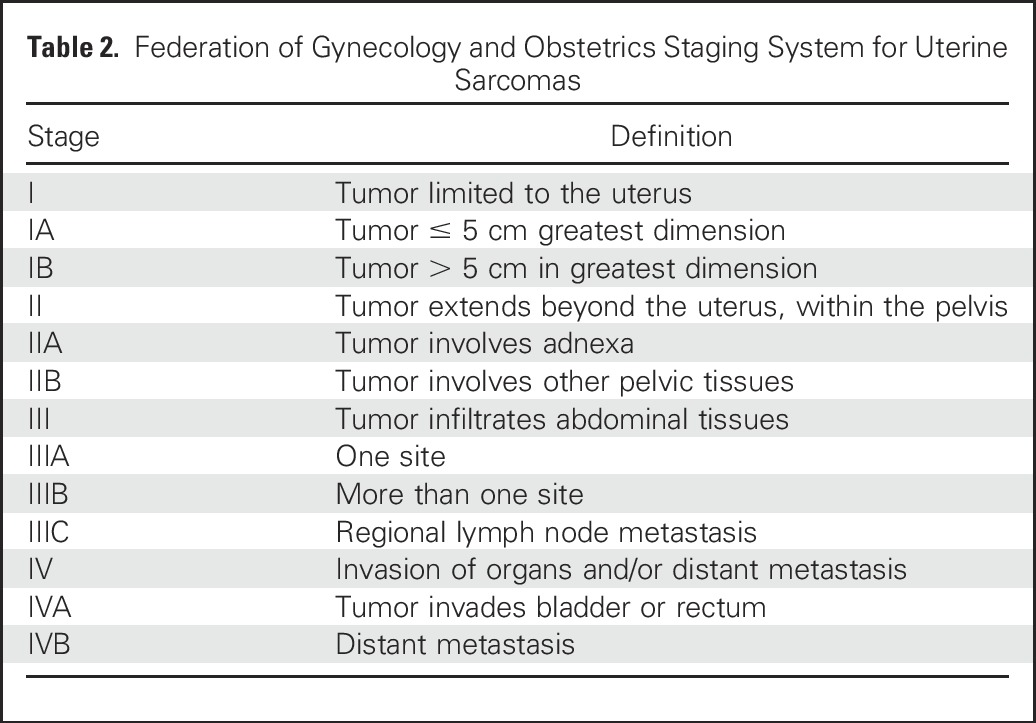
Histologic grade, tumor size, and tumor depth are the three major clinicopathologic prognostic factors for STSs, including extra-uLMS,20,21 and all are included in the American Joint Committee of Cancer staging system (Table 1). LMS has substantial intrinsic aggressiveness as one of the sarcoma subtypes with the highest risk of distant recurrence and decreased disease-specific survival.20 In contrast, uLMSs are staged by using the Federation of Gynecology and Obstetrics (FIGO) 2009 staging system (Table 2), which does not include tumor grading. Five-year survival estimates of uLMS by FIGO stage for stage I is 76%; stage II, 60%; stage III, 45%; and stage IV, 29%. Other factors that have been evaluated for their potential prognostic effect include tumor fragmentation,22 extrauterine spread, mitotic index, and tumor grade, although tumor grade in uLMS remains an area of controversy and is not routinely applied to diagnostic or staging procedures.23
Table 1.
American Joint Committee of Cancer Version 7 Staging System for Soft Tissue Sarcoma
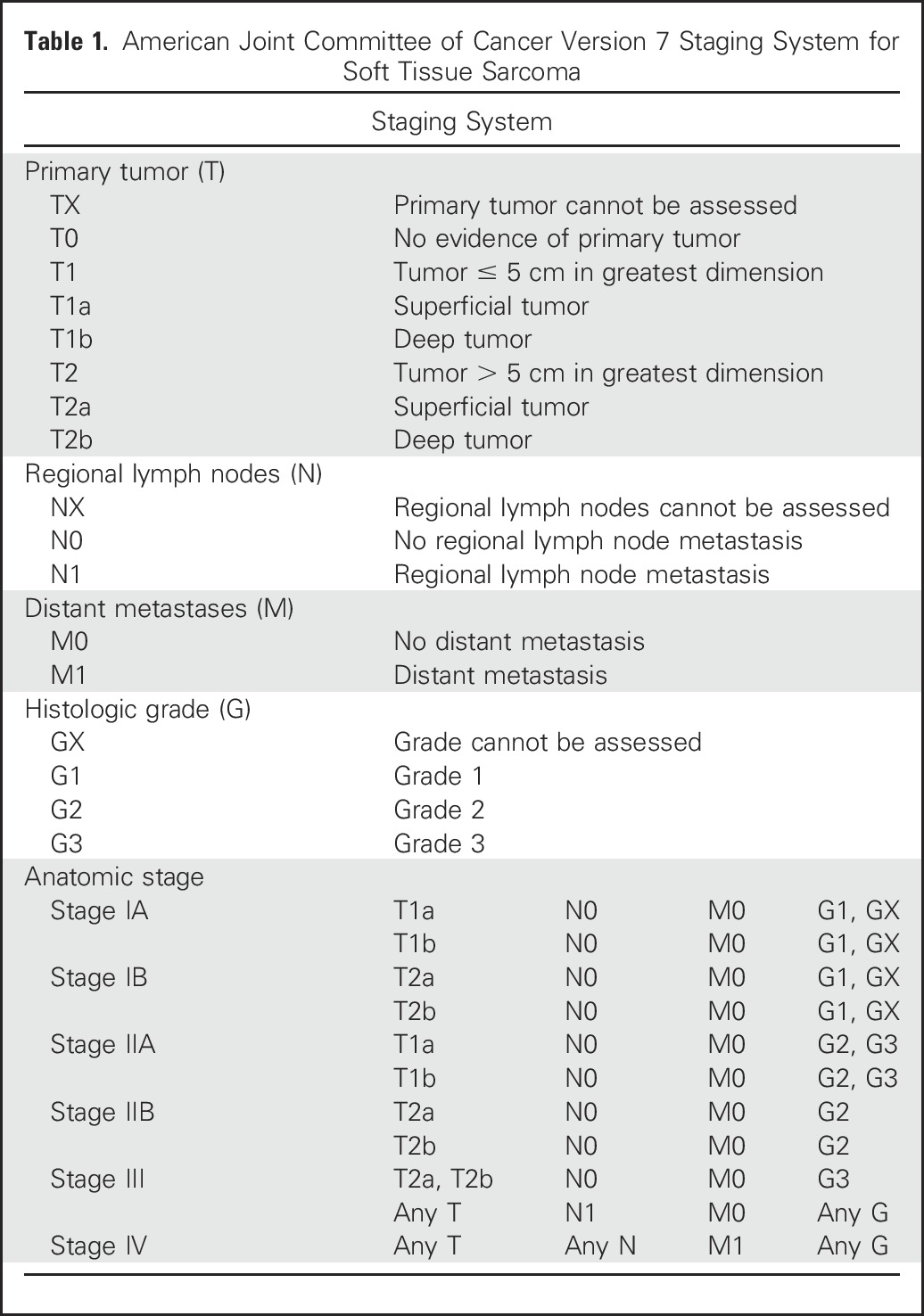
Table 2.
Federation of Gynecology and Obstetrics Staging System for Uterine Sarcomas
TREATMENT OF LMS AND uLMS
Localized Disease
Surgical resection is the cornerstone treatment for patients with localized LMS, independent of the site of origin. The standard surgical procedure involves a complete excision with wide negative margins (R0 resection), which offers the best chance of cure; however, because many LMSs are large and located in the retroperitoneum, achieving surgical R0 resection is challenging as a result of anatomic constraints. The ability to perform a complete surgical resection at the time of initial presentation is the most important prognostic factor for survival.24
For uLMS, hysterectomy is recommended for patients whose disease seems to be limited to the uterus. Uterine morcellation procedures have been associated with the intra-operative spread of malignant tissue and poorer survival outcomes.22 Routine lymph node dissection is not generally required because the risk of occult metastatic disease to lymph nodes is low. Lymph nodes that appear enlarged and/or suspicious for malignant involvement should undergo resection.25 Bilateral salpingo-oophorectomy is reasonable in perimenopausal and postmenopausal women, although it is recognized that there are no data to indicate that oophorectomy improves survival outcomes.25 For uLMS disease that appears locally advanced but potentially completely resectable, an attempt to resect all visible disease is reasonable. Retrospective data have demonstrated longer overall survival (OS) among women whose disease is completely resected compared with those with residual disease.26
Adjuvant Radiotherapy
Radiation therapy, either pre- or postoperatively, leads to improved local control rates in localized STS of the extremity and trunk, but there are no randomized data that address this question in retroperitoneal sarcomas, which leads to institutional variation on the use of radiation in this setting. A large, multinational trial that addresses this question (ClinicalTrials.gov identifier: NCT01344018) has recently completed accrual, and results will inform future practice standards.
Adjuvant pelvic radiation is not recommended for patients with FIGO stage I uLMS as a prospective randomized trial demonstrated no improvement in local recurrence rates and OS outcomes in uterine sarcomas, including uLMS.27
Adjuvant Systemic Therapy
Despite complete, intact resection of uterus-limited, high-grade LMS, the risk of recurrence after complete resection of uLMS approaches 50% to 70%,28 and is approximately 40% in extra-uLMS.29 Subsequent efforts have been pursued to evaluate adjuvant therapy, although its role remains poorly understood.
There have been no specific trials of adjuvant chemotherapy in LMS that originates outside of the uterus, although LMS has been included in more comprehensive trials of adjuvant chemotherapy in STS. There is institutional variation in the use of adjuvant chemotherapy for LMS on the basis of the interpretation of data from trials of systemic therapy for high-grade, extremity-localized STS in general. Combination docetaxel and gemcitabine has been proven as an effective regimen in patients with metastatic uLMS; therefore, it has been investigated as an adjuvant regimen. A prospective phase II study of adjuvant gemcitabine plus docetaxel for four cycles, followed by doxorubicin for four cycles, in women with uterus-limited uLMS demonstrated a 2-year progression-free survival (PFS) rate of 78% and a 3-year PFS rate of 58%30; however, this PFS rate did not seem to be superior to outcomes among women who were observed in informal cross-trial comparison. An international, randomized, phase III trial of observation versus adjuvant chemotherapy failed to recruit patients and definitively closed in 2016. Whether there is benefit from adjuvant docetaxel plus gemcitabine in patients with FIGO stage I uLMS remains unanswered, and observation is a standard approach. Similarly, there are no prospective data that address adjuvant chemotherapy for more advanced, completely resected disease.
Metastatic Disease
Surgery. Patients with metastatic LMS of any site should be evaluated to determine whether the resection of metastases may be appropriate. In general, resection should be considered for patients with a relatively long disease-free interval and an isolated site of disease that is amenable to complete resection, with an acceptably low risk of morbidity.31 There are no data that evaluate adjuvant systemic treatment after metastasectomy, and the standard approach is surveillance.
Systemic Treatment of Unresectable Metastatic Disease
Chemotherapy.
The different STS subtypes have recognized variable patterns of chemosensitivity, and LMS show moderate sensitivity to chemotherapy, whereas uLMS seems to be overall more responsive.32 There is no established best first-line chemotherapy treatment. Regimens to consider for first-line therapy are listed in Table 3 and include doxorubicin-based therapies such as doxorubicin plus ifosfamide33 and doxorubicin plus olaratumab,34 as well as gemcitabine plus docetaxel.35-37
Table 3.
Early-Line Systemic Therapies Evaluated in STS and LMS/uLMS
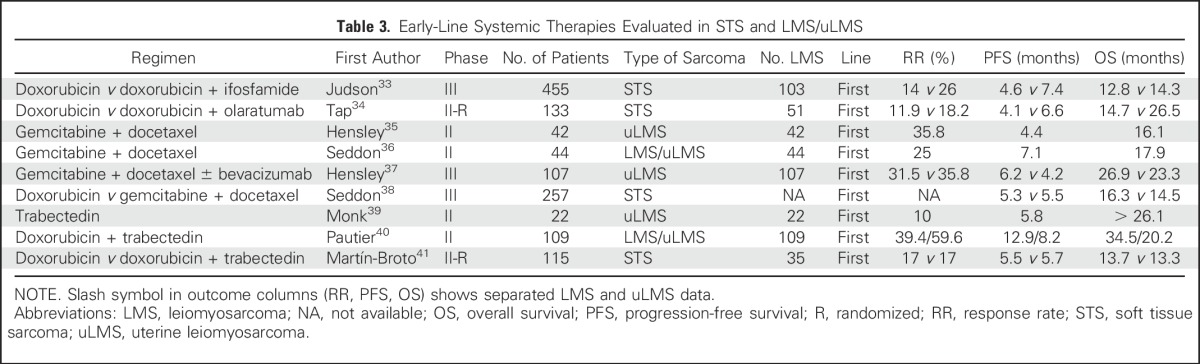
Combination docetaxel plus gemcitabine, unlike other STSs, seems to be particularly effective in LMS as both first-line and second-line treatments in patients who have previously received anthracycline-based therapy. The phase II study, Comparison of Gemcitabine Versus Gemcitabine Plus Docetaxel in Unresectable Soft Tissue Sarcoma (SARC002) demonstrated improved objective response, PFS, and OS with gemcitabine plus docetaxel compared with gemcitabine alone in advanced, previously treated STS.42 This study also confirmed the higher sensitivity of LMS and uLMS to this regimen, which had been observed previously in nonrandomized clinical trials.43,44 In contrast, the study, Randomized Multicenter and Stratified Phase II Study of Gemcitabine Alone Versus Gemcitabine and Docetaxel in Patients With Metastatic or relapsed Leiomyosarcomas: A Federation Nationale des Centres de Lutte Contre le Cancer French Sarcoma Group Study (TAXOGEM) only observed benefit of the combination in uLMS, but this was not statistically significant.45 These encouraging results in second-line treatment and beyond were explored to the first line. The phase III multicenter trial, Gemcitabine and Docetaxel Versus Doxorubicin as First-Line Treatment in Previously Untreated Advanced Unresectable or Metastatic Soft-Tissue Sarcoma (GeDDiS) did not observe differences in response rate and PFS from first-line docetaxel plus gemcitabine treatment compared with single-agent doxorubicin, with both regimens demonstrating activity in both LMS and uLMS.38 Only doxorubicin in combination with olaratumab has demonstrated an OS benefit; when compared with doxorubicin alone, however, the number of patients with LMS was relatively small, and a confirmatory phase III trial is pending. Given the broad number of regimens with activity in first-line therapy, treatment recommendations for an individual patient should take into consideration individual patient circumstances.
Several regimens have shown activity in LMS as second-line treatment or later and are listed in Table 4. Trabectedin is approved in patients with unresectable or metastatic liposarcoma or LMS/uLMS who received a prior anthracycline-containing regimen on the basis of a phase III trial that demonstrated trabectedin superiority over dacarbazine in PFS, but not in objective response rate (ORR) or OS.46 The efficacy and safety of trabectedin in patients with uLMS was confirmed in a later subgroup analysis from the phase III trial.51 Several studies, including data from 431 patients with LMS of any origin who were treated in a trabectedin expanded access program demonstrated an ORR of 7.5% in patients with LMS compared with 5.9% among patients with all-type STS.52 Likewise, patients with L-sarcomas (LMS and liposarcomas) obtained a higher clinical benefit rate (54%) and median OS (16.2 months) than did patients with non–L-sarcomas (38% and 8.4 months, respectively). Trabectedin also demonstrated activity as a first-line treatment in LMS or uLMS, either alone or in combination with doxorubicin in two nonrandomized phase II trials.39,40 However, a subsequent randomized trial of trabectedin plus doxorubicin compared with doxorubicin alone demonstrated no improvement in response rates (17% in both arms) or PFS (5.7 months v 5.5 months; Table 3).41
Table 4.
Later-Line Systemic Therapies Evaluated in STS and LMS/uLMS
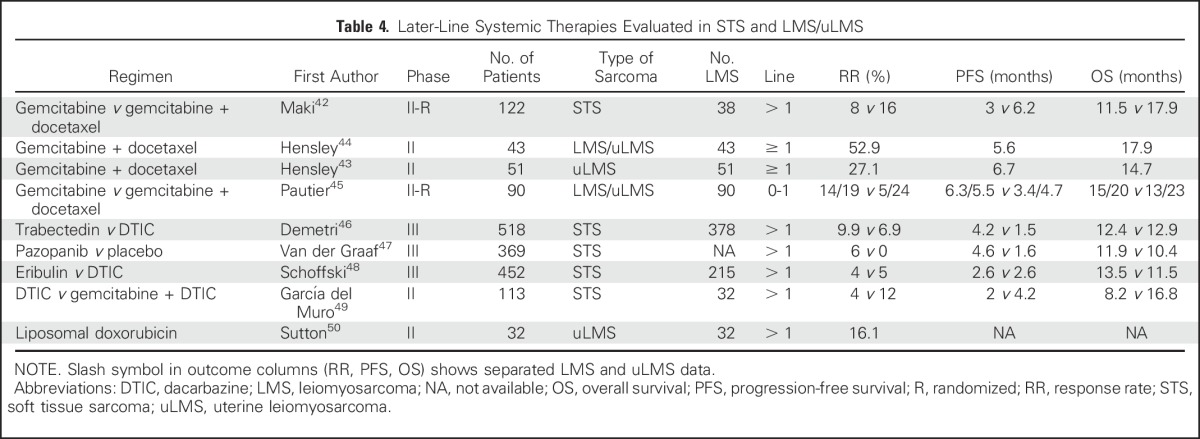
The small-molecule inhibitor pazopanib also has activity in LMS and uLMS. Pazopanib improved PFS (4.6 months v 1.6 months) compared with placebo in STS, although there was no difference in OS, and ORR was observed in only 4% of patients.47 Pazopanib demonstrated modest efficacy in a subgroup analysis of patients with uLMS (response rate, 11%; PFS, 3 months; OS, 17.5 months; Table 4).53
In another phase III trial for L-sarcomas, eribulin demonstrated superior OS benefit compared with dacarbazine, but this was not the case for PFS or response rate.48 These data led to US Food and Drug Administration approval of eribulin for liposarcoma, but not for LMS on the basis of preplanned subgroup analysis. Finally, other agents or regimens have been reported to have activity in LMS, such as dacarbazine,46,48 gemcitabine monotherapy,42,45 gemcitabine plus dacarbazine,49 and liposomal doxorubicin.50
Antihormone therapies.
A small prospective study of the aromatase inhibitor letrozole in patients with ER- and/or PR-positive uLMS demonstrated a 12-week PFS rate of 50% with a median duration of treatment of 2.2 months.54 Of interest, three patients, all of whom had tumors that expressed ER and PR in > 90% of tumor cells, continued to receive letrozole for > 24 weeks. Thus, hormonal blockade may also be considered for patients with uLMS with low disease burden and indolent disease pace, particularly if their tumors are ER and PR positive.10 Of importance, prior retrospective data have suggested that high expression of ER/PR in uLMS is associated with an indolent clinical course, and, therefore, the effect of aromatase inhibition on PFS may be confounded by favorable tumor biology.55
Immunotherapy.
Checkpoint inhibitors have been explored as a therapeutic modality in both uLMS and extra-uLMS. There have been rare case reports of responses to single-agent programmed death-ligand 1 antibodies, although predictive markers of response remain undefined in this patient population.56 A one-arm study of single-agent nivolumab in patients with advanced uLMS, which closed after the first stage of accrual (12 patients) for lack of efficacy, demonstrated no objective responses and a median PFS of 1.8 months.57 Similarly, in a single-arm phase II trial that combined pembrolizumab with metronomic cyclophosphamide in STS, no patients with LMS demonstrated predefined clinical benefit, and PFS was only 1.4 months.58 A randomized phase II study evaluated nivolumab versus nivolumab plus ipilimumab in patients with STS.59 Patients with LMS comprised approximately one third of 85 enrolled patients. ORR was 3% in the nivolumab arm and 16% in the nivolumab plus ipilimumab arm. Among the eight of 85 patients who achieved a response, three had LMS. Observed PFS was 2.1 months with nivolumab and 4.4 months with nivolumab plus ipilimumab.
Although there may be rare case reports of response to checkpoint inhibition in LMS, use of these agents is not warranted outside of the clinical trial setting. It is hoped that biomarkers of response may be identified that will help to optimize treatment choices for patients with LMS.
SUMMARY
LMS is one of the more common subtypes of soft tissue sarcoma. A multidisciplinary approach is important for the diagnosis and treatment of these rare cancers. Surgery remains the mainstay of therapy for primary, localized disease. Doxorubin-based regimens and gemcitabine-based regimens are the backbone of early-line therapy for metastatic disease, but multiple newer agents have recently been approved for later-line advanced disease. Ongoing efforts to increase our understanding of the biologic underpinnings of the disease are critical to continued progress in improving the lives of patients with this disease. International consortia are essential to collect data and carry out clinical trials in patients with rare cancers.
Footnotes
Supported in part by Memorial Sloan Kettering Cancer Center Support Grant No. P30-CA008748 (to M.L.H.), Instituto de Salud Carlos III Grant No. PI16/01371 (to C.S.), and the Catherine England Leiomyosarcoma Fund and The Jill Effect (S.G.).
AUTHOR CONTRIBUTIONS
Conception and design: Suzanne George, Martee L. Hensley, Isabelle Ray-Coquard
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Soft Tissue and Uterine Leiomyosarcoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Suzanne George
Stock or Other Ownership: Abbott Laboratories, AbbVie (I)
Consulting or Advisory Role: Blueprint Medicines, Deciphera
Research Funding: Pfizer (Inst), Novartis (Inst), Bayer (Inst), ARIAD Pharmaceuticals (Inst), Blueprint Medicines (Inst), Deciphera (Inst)
César Serrano
No relationship to disclose
Martee L. Hensley
Employment: Sanofi (I)
Stock or Other Ownership: Sanofi (I)
Consulting or Advisory Role: EMD Serono, Janssen Pharmaceuticals, Tesaro, Eli Lilly, Onclive
Research Funding: Johnson & Johnson (Inst), Bristol-Myers Squibb (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Isabelle Ray-Coquard
No relationship to disclose
REFERENCES
- 1.National Cancer Institute : SEER cancer statistics review, 1975-2010. http://seer.cancer.gov/csr/1975_2010/
- 2.Amant F, Coosemans A, Debiec-Rychter M, et al. : Clinical management of uterine sarcomas. Lancet Oncol 10:1188-1198, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Miettinen M. (ed): Smooth muscle tumors, in Modern Soft Tissue Pathology (ed 1). New York, NY, Cambridge University Press, 2010, pp 460-490 [Google Scholar]
- 4.Venkatraman L, Goepel JR, Steele K, et al. : Soft tissue, pelvic, and urinary bladder leiomyosarcoma as second neoplasm following hereditary retinoblastoma. J Clin Pathol 56:233-236, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ognjanovic S, Olivier M, Bergemann TL, et al. : Sarcomas in TP53 germline mutation carriers: A review of the IARC TP53 database. Cancer 118:1387-1396, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Robinson E, Neugut AI, Wylie P: Clinical aspects of postirradiation sarcomas. J Natl Cancer Inst 80:233-240, 1988 [DOI] [PubMed] [Google Scholar]
- 7.Botsis D, Koliopoulos C, Kondi-Pafitis A, et al. : Myxoid leiomyosarcoma of the uterus in a patient receiving tamoxifen therapy: A case report. Int J Gynecol Pathol 25:173-175, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Chen E, O’Connell F, Fletcher CD: Dedifferentiated leiomyosarcoma: Clinicopathological analysis of 18 cases. Histopathology 59:1135-1143, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Bell SW, Kempson RL, Hendrickson MR: Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. Am J Surg Pathol 18:535-558, 1994 [PubMed] [Google Scholar]
- 10.Leitao MM, Jr, Hensley ML, Barakat RR, et al. : Immunohistochemical expression of estrogen and progesterone receptors and outcomes in patients with newly diagnosed uterine leiomyosarcoma. Gynecol Oncol 124:558-562, 2012 [DOI] [PubMed] [Google Scholar]
- 11.El-Rifai W, Sarlomo-Rikala M, Knuutila S, et al. : DNA copy number changes in development and progression in leiomyosarcomas of soft tissues. Am J Pathol 153:985-990, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibault L, Pérot G, Chibon F, et al. : New insights in sarcoma oncogenesis: A comprehensive analysis of a large series of 160 soft tissue sarcomas with complex genomics. J Pathol 223:64-71, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Agaram NP, Zhang L, LeLoarer F, et al. : Targeted exome sequencing profiles genetic alterations in leiomyosarcoma. Genes Chromosomes Cancer 55:124-130, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mäkinen N, Aavikko M, Heikkinen T, et al. : Exome sequencing of uterine leiomyosarcomas identifies frequent mutations in TP53, ATRX, and MED12. PLoS Genet 12:e1005850, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo X, Jo VY, Mills AM, et al. : Clinically relevant molecular subtypes in leiomyosarcoma. Clin Cancer Res 21:3501-3511, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernando E, Charytonowicz E, Dudas ME, et al. : The AKT-mTOR pathway plays a critical role in the development of leiomyosarcomas. Nat Med 13:748-753, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Van der Ven LT, Roholl PJ, Gloudemans T, et al. : Expression of insulin-like growth factors (IGFs), their receptors and IGF binding protein-3 in normal, benign and malignant smooth muscle tissues. Br J Cancer 75:1631-1640, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornfeld D, Israel G, Martel M, et al. : MRI appearance of mesenchymal tumors of the uterus. Eur J Radiol 74:241-249, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Lakhman Y, Veeraraghavan H, Chaim J, et al. : Differentiation of uterine leiomyosarcoma from atypical leiomyoma: Diagnostic accuracy of qualitative MR imaging features and feasibility of texture analysis. Eur Radiol 27:2903-2915, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coindre JM, Terrier P, Guillou L, et al. : Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: A study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer 91:1914-1926, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Pisters PW, Leung DH, Woodruff J, et al. : Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol 14:1679-1689, 1996 [DOI] [PubMed] [Google Scholar]
- 22.George S, Barysauskas C, Serrano C, et al. : Retrospective cohort study evaluating the impact of intraperitoneal morcellation on outcomes of localized uterine leiomyosarcoma. Cancer 120:3154-3158, 2014 [DOI] [PubMed] [Google Scholar]
- 23.D’Angelo E, Prat J: Uterine sarcomas: A review. Gynecol Oncol 116:131-139, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Anaya DA, Lev DC, Pollock RE: The role of surgical margin status in retroperitoneal sarcoma. J Surg Oncol 98:607-610, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Leitao MM, Sonoda Y, Brennan MF, et al. : Incidence of lymph node and ovarian metastases in leiomyosarcoma of the uterus. Gynecol Oncol 91:209-212, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Leitao MM, Jr, Zivanovic O, Chi DS, et al. : Surgical cytoreduction in patients with metastatic uterine leiomyosarcoma at the time of initial diagnosis. Gynecol Oncol 125:409-413, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Reed NS, Mangioni C, Malmström H, et al. : Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: A European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Study (protocol 55874). Eur J Cancer 44:808-818, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Major FJ, Blessing JA, Silverberg SG, et al. : Prognostic factors in early-stage uterine sarcoma. A Gynecologic Oncology Group study. Cancer 71:1702-1709, 1993. (Suppl 4) [DOI] [PubMed] [Google Scholar]
- 29.Gladdy RA, Qin LX, Moraco N, et al. : Predictors of survival and recurrence in primary leiomyosarcoma. Ann Surg Oncol 20:1851-1857, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hensley ML, Ishill N, Soslow R, et al. : Adjuvant gemcitabine plus docetaxel for completely resected stages I-IV high grade uterine leiomyosarcoma: Results of a prospective study. Gynecol Oncol 112:563-567, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Temple LK, Brennan MF: The role of pulmonary metastasectomy in soft tissue sarcoma. Semin Thorac Cardiovasc Surg 14:35-44, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Grimer R, Judson I, Peake D, et al. : Guidelines for the management of soft tissue sarcomas. Sarcoma 2010:506182, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Judson I, Verweij J, Gelderblom H, et al. : Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: A randomised controlled phase 3 trial. Lancet Oncol 15:415-423, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Tap WD, Jones RL, Van Tine BA, et al. : Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: An open-label phase 1b and randomised phase 2 trial. Lancet 388:488-497, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hensley ML, Blessing JA, Mannel R, et al. : Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: A Gynecologic Oncology Group phase II trial. Gynecol Oncol 109:329-334, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seddon B, Scurr M, Jones RL, et al. : A phase II trial to assess the activity of gemcitabine and docetaxel as first line chemotherapy treatment in patients with unresectable leiomyosarcoma. Clin Sarcoma Res 5:13, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hensley ML, Miller A, O’Malley DM, et al. : Randomized phase III trial of gemcitabine plus docetaxel plus bevacizumab or placebo as first-line treatment for metastatic uterine leiomyosarcoma: An NRG Oncology/Gynecologic Oncology Group study. J Clin Oncol 33:1180-1185, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seddon BM, Whelan J, Strauss SJ, et al. : GeDDiS: A prospective randomised controlled phase III trial of gemcitabine and docetaxel compared with doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft tissue sarcomas (EudraCT 2009-014907-29). J Clin Oncol 33, 2015. (suppl 15; abstr 10500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monk BJ, Blessing JA, Street DG, et al. : A phase II evaluation of trabectedin in the treatment of advanced, persistent, or recurrent uterine leiomyosarcoma: A Gynecologic Oncology Group study. Gynecol Oncol 124:48-52, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pautier P, Floquet A, Chevreau C, et al. : Trabectedin in combination with doxorubicin for first-line treatment of advanced uterine or soft-tissue leiomyosarcoma (LMS-02): A non-randomised, multicentre, phase 2 trial. Lancet Oncol 16:457-464, 2015 [DOI] [PubMed] [Google Scholar]
- 41.Martin-Broto J, Pousa AL, de Las Peñas R, et al. : Randomized phase II study of trabectedin and doxorubicin compared with doxorubicin alone as first-line treatment in patients with advanced soft tissue sarcomas: A Spanish Group for Research on Sarcoma study. J Clin Oncol 34:2294-2302, 2016 [DOI] [PubMed] [Google Scholar]
- 42.Maki RG, Wathen JK, Patel SR, et al. : Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: Results of Sarcoma Alliance for Research Through Collaboration study 002 [corrected]. J Clin Oncol 25:2755-2763, 2007. [Erratum: J Clin Oncol 25: 3790, 2007] [DOI] [PubMed] [Google Scholar]
- 43.Hensley ML, Blessing JA, Degeest K, et al. : Fixed-dose rate gemcitabine plus docetaxel as second-line therapy for metastatic uterine leiomyosarcoma: A Gynecologic Oncology Group phase II study. Gynecol Oncol 109:323-328, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hensley ML, Maki R, Venkatraman E, et al. : Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: Results of a phase II trial. J Clin Oncol 20:2824-2831, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Pautier P, Floquet A, Penel N, et al. : Randomized multicenter and stratified phase II study of gemcitabine alone versus gemcitabine and docetaxel in patients with metastatic or relapsed leiomyosarcomas: A Federation Nationale des Centres de Lutte Contre le Cancer (FNCLCC) French Sarcoma Group Study (TAXOGEM study). Oncologist 17:1213-1220, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Demetri GD, von Mehren M, Jones RL, et al. : Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: Results of a phase III randomized multicenter clinical trial. J Clin Oncol 34:786-793, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Graaf WT, Blay JY, Chawla SP, et al. : Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 379:1879-1886, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Schöffski P, Chawla S, Maki RG, et al. : Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: A randomised, open-label, multicentre, phase 3 trial. Lancet 387:1629-1637, 2016 [DOI] [PubMed] [Google Scholar]
- 49.García-Del-Muro X, López-Pousa A, Maurel J, et al. : Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: A Spanish Group for Research on Sarcomas study. J Clin Oncol 29:2528-2533, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Sutton G, Blessing J, Hanjani P, et al. : Phase II evaluation of liposomal doxorubicin (Doxil) in recurrent or advanced leiomyosarcoma of the uterus: A Gynecologic Oncology Group study. Gynecol Oncol 96:749-752, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Hensley ML, Patel SR, von Mehren M, et al. : Efficacy and safety of trabectedin or dacarbazine in patients with advanced uterine leiomyosarcoma after failure of anthracycline-based chemotherapy: Subgroup analysis of a phase 3, randomized clinical trial. Gynecol Oncol 146:531-537, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samuels BL, Chawla S, Patel S, et al. : Clinical outcomes and safety with trabectedin therapy in patients with advanced soft tissue sarcomas following failure of prior chemotherapy: Results of a worldwide expanded access program study. Ann Oncol 24:1703-1709, 2013 [DOI] [PubMed] [Google Scholar]
- 53.Benson C, Ray-Coquard I, Sleijfer S, et al. : Outcome of uterine sarcoma patients treated with pazopanib: A retrospective analysis based on two European Organisation for Research and Treatment of Cancer (EORTC) Soft Tissue and Bone Sarcoma Group (STBSG) clinical trials 62043 and 62072. Gynecol Oncol 142:89-94, 2016 [DOI] [PubMed] [Google Scholar]
- 54.George S, Feng Y, Manola J, et al. : Phase 2 trial of aromatase inhibition with letrozole in patients with uterine leiomyosarcomas expressing estrogen and/or progesterone receptors. Cancer 120:738-743, 2014 [DOI] [PubMed] [Google Scholar]
- 55.O’Cearbhaill R, Zhou Q, Iasonos A, et al. : Treatment of advanced uterine leiomyosarcoma with aromatase inhibitors. Gynecol Oncol 116:424-429, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.George S, Miao D, Demetri GD, et al. : Loss of PTEN is associated with resistance to anti-PD-1 checkpoint blockade therapy in metastatic uterine leiomyosarcoma. Immunity 46:197-204, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ben-Ami E, Barysauskas CM, Solomon S, et al. : Immunotherapy with single agent nivolumab for advanced leiomyosarcoma of the uterus: Results of a phase 2 study. Cancer 123:3285-3290, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Toulmonde M, Penel N, Adam J, et al. : Use of PD-1 targeting, macrophage infiltration, and IDO pathway activation in sarcomas: A phase 2 clinical trial. JAMA Onco [epub ahead of print on June 29, 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.D'Angelo SP, Mahoney MR, Van Tine BA, et al. : A multi-center phase II study of nivolumab +/− ipilimumab for patients with metastatic sarcoma (Alliance A091401). J Clin Oncol 35, 2017. (suppl 15; abstr 11007) [Google Scholar]
- 60.Cancer Genome Atlas Research Network: Comprehensive and integrated genomic characterization of adult soft tissue sarcomas. Cell 171: 950-965.e28, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]


