Abstract
Astrocytes, the most abundant type of glial cells in the brain, play critical roles in supporting neuronal development and brain function. While astrocytes have been frequently detected in brain tumors, including medulloblastoma (MB), their functions in tumorigenesis are not clear. Here we demonstrate that astrocytes are essential components of the MB tumor microenvironment. Tumor-associated astrocytes (TAA) secreted the ligand sonic hedgehog (Shh), which is required for maintaining MB cell proliferation despite the absence of its primary receptor Patched-1 (Ptch1). Shh drove expression of Nestin in MB cells through a Smoothened-dependent, Gli1-independent mechanism. Ablation of TAA dramatically suppressed Nestin expression and blocked tumor growth. These findings demonstrate an indispensable role for astrocytes in MB tumorigenesis and reveal a novel Ptch1-independent Shh pathway involved in MB progression.
Keywords: Nestin, astrocytes, Sonic hedgehog, Patched-1, medulloblastoma
Introduction
Astrocytes are specialized glial cells, distributed ubiquitously throughout the brain, that play essential roles in microcirculation, provision of energy metabolites to neurons, and homeostasis maintenance of extracellular ions and neurotransmitters (1, 2). Antibodies against glial fibrillary acid protein (GFAP) are often used to detect astrocytes (3), sometimes in conjunction with antibodies specific for other biomarkers such as S100β (4), astrocyte cell surface antigen 2 (ACSA-2) (5) and brain lipid binding protein (BLBP) (6) in immunohistochemistry assays. Astrocytes in the cerebellum, often referred to as Bergmann glial cells, provide critical functions that support proliferation and migration of cerebellar granule neuron precursors (GNPs) (7). Under pathological brain conditions, such as trauma or malignant growth, astrocytes undergo astrogliosis, up-regulating GFAP expression, increasing proliferation and enduring pronounced hypotrophy (8, 9). This process has been linked to elevated expression of survival genes in tumor cells that have been proposed to provide protection from chemotherapeutic agents (10). In addition, astrocytes release numerous growth factors and cytokines that potentially provide a supportive microenvironment for brain tumor growth and metastasis.
Medulloblastoma (MB) is the most common malignant brain tumor in children. Approximately 30% of human MBs result from aberrant activation of the hedgehog signaling pathway (11–13). The hedgehog pathway plays fundamental roles during normal development by regulating multiple processes involved in tissue patterning, proliferation and differentiation (14). Three mammalian hedgehog homologues, Sonic hedgehog (Shh), Indian hedgehog and Desert hedgehog, activate the hedgehog pathway by binding to and inhibiting the receptor Patched-1 (Ptch1). This relieves the inhibitory effect of Ptch1 on Smoothened (Smo), which then translocates to the primary cilium in an active form. Activated Smo then suppresses the function of Suppresser-of-Fused (Sufu) allowing Gli1 and Gli2 to translocate to the nucleus and increase transcription of a set of target genes, including Ptch1 and Gli1. Gli3, an additional Gli family member that is positively regulated by Sufu, predominately serves as a negative regulator of hedgehog signaling (15, 16). Elevated expression of Gli1 is a hallmark of hedgehog pathway activation. However, substantial evidence indicated that not all hedgehog activity requires Gli1 (17, 18). Shh has been shown to induce cytoskeletal rearrangements in mouse fibroblasts independently of Gli1 (19). Furthermore, activation of Src family kinases by Shh in commissural neurons, occurs via a Gli1-independent mechanism (20).
Previously, we showed that deletion of Ptch1 in mouse cerebellar GNPs results in MB formation with 100% penetrance confirming GNPs as the cell of origin for hedgehog group MB(21). However, after a prolonged period of proliferation, the majority of GNPs ultimately differentiated, despite the loss of Ptch1. Only a small subset of Ptch1-deficient GNPs developed into tumors, suggesting that while loss of Ptch1 alone is necessary for tumor formation, it is not sufficient for malignant transformation of cerebellar GNPs (21). Recently, we reported that expression of Nestin, a type VI intermediate filament protein, is required for Ptch1-deficient GNPs to form MB. Nestin augmented hedgehog pathway activity by binding to Gli3, thereby abolishing its inhibitory function (22). These data reveal that Nestin expression is indispensable for MB tumorigenesis. However, the mechanism underlying Nestin expression in MB cells has not been identified.
Here, we demonstrate that astrocytes, enriched in both human and mouse MB tissue, represent a key functional component of the tumor microenvironment. Tumor-associated astrocytes (TAA) were found to express and secrete Shh to promote MB cell proliferation. Astrocyte-derived Shh induced Nestin expression in MB cells through a Smo-dependent, but Gli1-independent mechanism. Genetic ablation of TAA dramatically inhibited Nestin expression in MB cells, resulting in reduced proliferation and a block in tumor growth. Thus, astrocytes play a critical role in supporting MB growth by secreting the mitogen Shh into the tumor microenvironment.
Materials and Methods
Mice
Ptch1fl/fl mice, Nestin-CFP mice have been described previously (22). Math1-Cre Mice, Ptch1-lacZ mice, GFAP-GFP mice, GFAP-TK mice and Rosa26-SmoM2 mice were from Jackson Labs (Bar Harbor, ME). All animals were maintained in the LAF at Fox Chase Cancer Center and all experiments were performed in accordance with procedures approved by the Fox Chase Cancer Center Animal Care and Use Committee.
Cell isolation, Flow cytometry and Cell culture
GNPs were isolated from cerebella of P4–P7 mice, and MB cells from adult cerebella as previously described (23). Briefly, Cerebella were digested in a solution containing 10U/ml papain (Worthington, Lakewood, NJ), 200ug/ml L-cysteine (Sigma) and 250 U/ml DNase (Sigma) to obtain a single cell suspension, and then centrifuged through a 35%–65% Percoll gradient (Sigma). Cells from the 35–65% interface were suspended in NB-B27 (Neurobasal with B27 supplement, 1mM sodium pyruvate, 2mM L-glutamine, and Pen/Strep, all from Invitrogen). CFP-negative GNPs from p4 Math1-Cre/Ptch1fl/fl/Nestin-CFP cerebella were then purified using a FACS Aria II (BD Bioscience). GNPs and MB cells were suspended in NB-B27 and plated on Poly-D-lysine (PDL)-coated 24-well plates for further experiment.
Histology, Immunohistochemistry and Western blotting
Primary antibodies used in this study include: anti-Nestin (1:1000; Abcam), anti-GFAP (1:500, BD), anti-S100 (1:500, Sigma), anti-BLBP (1:500, Millipore), anti-Ki67 (1:500, BD), anti-NeuN (1:200, millipore), anti-GFP (1:500, millipore), anti-Cre (1:500, Novagen), anti-BrdU (1:500, Sigma), anti-Shh (1:500, BD), anti-Gli1 (1:1000, CST), anti-GAPDH (1:2000, Sigma) and anti-HA (1:200, Covance). Secondary antibodies include: Alexa Fluor-594 anti-rabbit IgG (1:200), Alexa Fluor-594 anti-mouse IgG (1:200), Alexa Fluor-594 anti-chicken IgG (1:200), FITC Fluor-488 anti-rabbit IgG (1:200) FITC Fluor-488 anti-mouse IgG (1:200), FITC Fluor-488 anti-mouse chicken IgG (1:200) from Invitrogen.
For immunohistochemistry, mice were perfused with PBS followed by 4% paraformaldehyde (PFA). Cerebella were removed and fixed overnight in 4% PFA, cryoprotected in 30% sucrose, frozen in Tissue Tek-OCT (Sakura Finetek, CA) and cut into 10–12µm sagittal sections. Immunofluorescent staining for sections and cultured cells was carried out according to standard protocols. Briefly, after fixation in 4% PFA, sections or cells were blocked and permeabilized for 1 hr with PBS containing 0.1% Triton X-100 and 10% normal goat serum, stained with primary antibodies overnight at 4°C, and incubated with secondary antibodies for 2 hrs at room temperature. Sections were counterstained with DAPI and mounted with Fluoromount G (Southern Biotechnology, AL) before being visualized using a Nikon Eclipse Ti microscope.
For western blot analysis, cells were lysed in RIPA buffer (Thermo Scientific, IL) supplemented with protease and phosphatase inhibitors. Total lysate containing equal amount of protein were separated by SDS-PAGE gel and subsequently transferred onto PVDF membrane. Membranes were then subjected to probe with antibodies. Western blot signals were detected by using SuperSignal West Pico Chemiluminescent substrate (Thermo Scientific, IL).
Astrocytes isolation and culture
Tumor-associated astrocytes were isolated from MB tissues from Math1-Cre/Ptch1fl/fl/GFAP-GFP mice at 8 weeks of age. Briefly, MB tissues were digested using papain dissociation system to obtain a single cell suspension as mention above, the cells were suspended in DPBS plus 0.5%BSA, and stained with anti-ACSA-2-APC (1:500, Miltenyi Biotec), TAA were collected by harvesting GFP+/ACSA2-APC+ cells using fluorescence-activated cell sorting (FACS). For co-culture of TAA with GNPs and MB cells, isolated TAA were culture in PDL-coated wells for 3 days, then purified GNPs or MB cell were added on top of TAA at a ratio of 5 to 1, and co-cultured for indicated time points.
For detection of Shh ligand by ELISA assay, the culture medium for astrocytes was replaced with serum-free DMEM medium after being cultured with serum for 3 days. Conditioned culture medium was harvested 2 days later, the concentration of Shh ligand was measured using the mouse Shh-N ELISA kit (Sigma, MO).
For Luciferase assay, shh-light II cells (ATCC® CRL-2795™) were cultured in DMEM with 10% FBS. After the cells reached 70–80% confluence, the medium was replaced with DMEM without FBS (naïve culture medium), 50% Shh-CM or TAA-CM for 2 days, then luciferase levels in shh-light II cells were measured using Dual-Luciferase® Reporter Assay (Promega).
Magnetic resonance imaging (MRI)
Magnetic resonance microimaging was performed using intense magnetic fields on a 7 Tesla wide bore system (ESPREE, Germany) according to the standard protocol. MRI image analysis and tumor volume calculation was performed using Image J software (version 1.46; National Institutes of Health, Bethesda, MD), which has Bruker plugins that can reads reconstructed date files (2dseq) from Bruker Biospec spectrometer. The regions of interest were drawn manually covering the entire tumor area on each slice of the MRI images, and the areas of the individual slices were summed afterwards. The tumor volume was calculated by multiplying summated areas by slice thickness.
Slice culture
Cerebellar were harvested from Math1-Cre/Ptch1fl/fl/Nestin-CFP mice. 250µm slices were prepared using a VT1000S vibratome (Leica Microsystems, IL) as previously described (24). Tumor tissue slices were transferred onto a Millicell cell culture membrane inserts (0.4 µm, 30 mm diameter, Merck Millipore, Ireland) in a 6-well plate with 1ml DMEM culture medium containing 25mM HEPES. Tumor tissue slices receives medium from below and air from above, and cultured in an incubator with 5% CO2 at 37°C for indicated times.
RT-PCR
RNA was extracted from GNPs or MB cells using RNeasy mini kit (Qiagen, Germany). For real time PCR, first strand cDNA was synthesized from equal amounts of RNA using Superscript III Reverse Transcriptase (Invitrogen, Switzerland). Triplicate reactions were prepared using TaqMan probe real-time polymerase chain reaction, and real-time quantification was performed on a BIORAD iCycler iQ system (BioRad). Primer sequences are available upon request.
In Situ Hybridization
Tumor-bearing mice were perfused with 4% PFA; brains were embedded in OCT and cut into 12-µM sections. Sections were fixed in 4% PFA, acetylated and incubated for 1h at room temperature in pre-hybridization buffer (50% formamide, 5× SSC, 1× Denhardt’s solution, 10% dextran sulfate, 1 mg/ml yeast tRNA, 40 µg/ml salmon sperm DNA). Digoxigenin (DIG)-UTP–labeled probes for Shh were denatured and added to this mix on the section, hybridization was performed at 59°C for 40 hours. Probes were synthesized using a DIG labeling kit (Roche). After hybridization, sections were incubated overnight at 4°C with alkaline phosphatase-conjugated anti-DIG antibodies (Roche). Bound probe was visualized by incubating sections in NBT/BCIP (Roche) overnight in the dark. Coverslips were mounted with Aqua- Polymount (Polysciences, Warrington, PA).
Statistical analysis
Unpaired t test was performed to determine the statistical significance of the difference. p<0.05 was considered statistically significant. Error bars represent the SEM. Data handling and statistical processing was performed using Graphpad Prism Software.
Results
Astrocytes are enriched in MB tissue
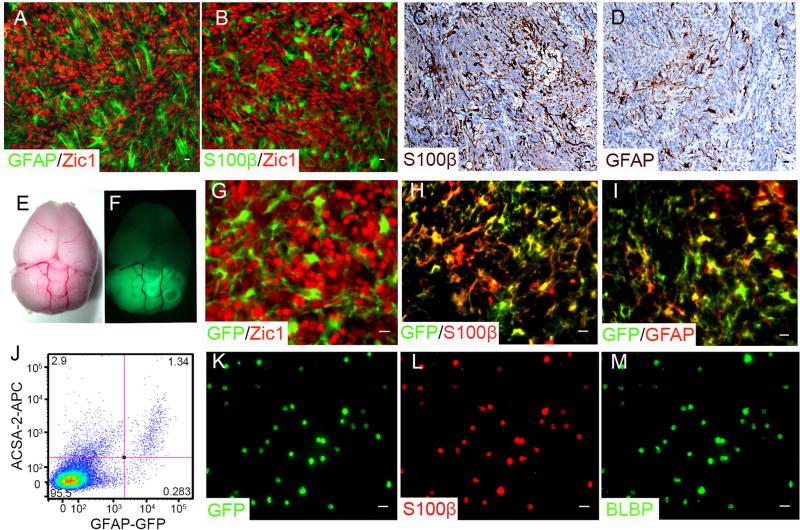
Human MB comprises at least 4 subgroups: Wnt, Shh, group 3 and group 4, with the Shh group accounting for approximately 30% of cases (11, 25, 26). Conditional genomic deletion of ptch1 in cerebellar GNPs led to MB formation in Math1-Cre/Ptch1fl/fl mice (21). We examined the presence of astrocytes in MB tissues from Math1-Cre/Ptch1fl/fl mice by immunohistochemistry. As shown in figure 1A and 1B, a significant number of TAA (GFAP+ or S100β+) were identified, intermingled with MB cells (Zic1+) (27) throughout the tumor mass. Similarly, astrocytes were also detected in human Shh type MB tissue (Fig. 1C and 1D) as well as in MB derived from Ptch1 heterozygous mice (28) and mice carrying constitutively activated Smo in cerebellar GNPs (29) (Supplementary Fig. S1A–D). These data suggest that astrocytes are abundant in the MB tumor microenvironment.
Figure 1. Astrocytes are enriched in Shh type MB.
A and B, Sagittal sections from Math1-Cre/Ptch1fl/fl cerebella at 8 weeks of age, were immunostained for GFAP and Zic1 (A) or S100β and Zic1 (B). C and D, Astrocytes in human MBs were examined by immunohistochemistry for GFAP (C) and S100β (D). E and F, Whole-mount images of mouse brain from a Math1-Cre/Ptch1fl/fl/GFAP-GFP mouse at 8 weeks of age in bright field (E) and GFP channel (F). G, H and I, Sagittal sections from Math1-Cre/Ptch1fl/fl/GFAP-GFP cerebella, were immunostained for GFP and Zic1 (G), GFP and S100β (H) or GFP and GFAP (I). J, Cells harvested from Math1-Cre/Ptch1fl/fl/GFAP-GFP cerebella at 8 weeks of age were stained for ACSA2, and analyzed for ACSA2 (APC) and GFAP (GFP) expression by flow cytometry. K, L and M, Purified TAA were immunostained for GFP (K), S100β (L) and BLBP (M) at 0 hr (right after being plated). Scale bar, 10 µm.
To investigate the possible role of astrocytes in MB tumorigenesis, we purified TAA from Math1-Cre/Ptch1fl/fl mice which had been crossed with GFAP-GFP mice that express green fluorescent protein (GFP) in astrocytes (30). We observed a robust GFP signal in MBs arising in Math1-Cre/Ptch1fl/fl/GFAP-GFP mice at 8 weeks of age (Fig. 1E–1F), but there were no MB cells (Zic1+) positive for GFP (Fig. 1G). In contrast, the majority of GFP+ cells expressed the astrocyte makers, S100β (Fig. 1H) and GFAP (Fig. 1I). Based on flow cytometry analyses, 1–2% of cells dissociated from MB tissue in Math1-Cre/Ptch1fl/fl/GFAP-GFP mice were positive for GFP. Approximately, 80–85% of the GFP+ cells also expressed ACSA2 (Fig. 1J), a cell surface marker for astrocytes (31). Purified GFP+/ACSA2+ cells exclusively expressed S100β and BLBP (Fig. 1K–M), and no Zic1+ were detected in the GFP+ population (data not shown). When cultured, purified GFP+/ACSA2+ cells solely gave rise to astrocytes based on marker expression and star-like morphology (supplementary Fig. S1E–G). These data suggest that TAAs in MB tissue from Math1-Cre/Ptch1fl/fl/GFAP-GFP mice represent GFP+/ACSA2+ cells. Therefore, for the following experiments, TAA were harvested by FACS purifying GFP+/ ACSA2+ cells from MBs of Math1-Cre/Ptch1fl/fl/GFAP-GFP mice.
TAA in MB tissue secrete Shh ligand
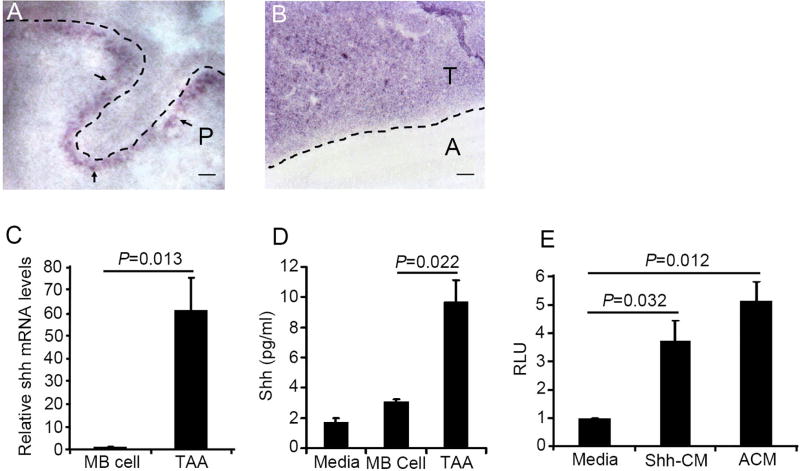
Previously, astrocytes in the cortex were reported to secrete Shh to support brain development (32). Therefore, we looked for the presence of Shh mRNA in MB tissue by in situ hybridization. At postnatal day 8 (P8), Shh mRNA was detected in the Purkinje layer of the cerebellum (Fig. 2A), consistent with previous reports demonstrating that Purkinje neurons secrete Shh to promote GNPs proliferation in neonatal mice (33). Abundant expression of Shh mRNA was found in MB tissue from Math1-Cre/Ptch1fl/fl mice at 8 weeks of age (Fig. 2B), but not in adjacent normal tissue, suggesting that Shh-expressing cells exist in the MB microenvironment. To investigate whether Shh transcripts in the MB microenvironment are derived from astrocytes, we isolated TAA and MB cells from Math1-Cre/Ptch1fl/fl/GFAP-GFP mice and examined Shh mRNA expression by q-PCR. As shown in figure 2C, elevated levels of Shh mRNA expression were detected in TAA compared to MB cells, indicating that TAAs highly expressed Shh mRNA. Having observed increased expression of Shh mRNA in TAAs, we then tested whether TAA secreted Shh. MB cells and TAA isolated from Math1-Cre/Ptch1fl/fl/GFAP-GFP mice were cultured in vitro for 3 days. Conditioned culture medium (CM) was collected to examine the presence of Shh by ELISA, and regular culture medium was used a negative control. As shown in figure 2D, markedly increased levels of Shh ligand were detected in TAA CM, compared to MB cell CM and regular culture medium. These data indicate that TAA, but not MB cells, secrete Shh in culture.
Figure 2. TAA secrete Shh.
A and B, Sagittal sections from wild type cerebella at P4 (A) and Ptch1+/− MB (B) were examined for Shh mRNA expression by in situ hybridization. Note that Shh mRNA was found in the purkinje cell layer of the wild type cerebellum (A) and MB tissue (B). P, Purkinje cell layer; T, tumor tissue; A, adjacent normal cerebellum. The dotted line in A indicates the molecular layer of wild type cerebellum. C, Expression of Shh mRNA in MB cells and TAA was examined by q-PCR. D, Concentrations of Shh ligand in conditioned culture media collected from control (naïve culture medium), MB cell culture, or TAA culture, were measured by an ELISA kit. E, Relative luciferase level (RLU) in Shh-light II cells cultured with recombinant Shh or TAA conditioned culture medium (TAA-CM) for 2 days. The values in figure C, D and E are the means ± SEM from three independent experiments. Scale bar, 20 µm.
To determine whether TAA-derived Shh is functional, we investigated activation of the Shh pathway by TAA CM in Shh-light II cells (34). TAA CM was prepared as mentioned above, and Shh-light II cells were treated with TAA CM or naïve culture medium with and without recombinant Shh as controls. As expected, the luciferase level was significantly elevated in Shh-light II cells treated with recombinant Shh, compared to treatment with control culture medium (Fig. 2E). Enhanced luciferase levels were also observed in Shh-light II cells after treatment with TAA CM, suggesting that TAA-derived Shh markedly stimulated the hedgehog pathway in Shh-light II cells. Taken together, the above data demonstrate that TAA efficiently export functional Shh into the MB microenvironment.
TAA-derived Shh supports MB cell proliferation
We next investigated the possible contribution of Shh to MB cell proliferation in the tumor microenvironment. MB cells from Math1-Cre/Ptch1fl/fl mice were cultured in vitro and cells were examined at different time points to determine the level of proliferation. As shown in figure 3A, a majority of MB cells were proliferating after 24 hrs in culture. However, MB cells started exiting the cell cycle after 48 hrs in culture, with only 10% still dividing at 96 hrs. These data suggest that MB cells gradually cease proliferation in vitro. The expression of Gli1 also progressively declined in cultured MB cells during the same time frame (Supplementary Fig. S2A), indicating that hedgehog pathway activity is rapidly down-regulated when MB cells are cultured in vitro, as shown previously (35). To examine whether exogenous Shh can support tumor cell proliferation in vitro, MB cells were cultured in the presence of 1ug/ml recombinant Shh. As shown in figure 3B–3F, approximately 15% of MB cells were BrdU positive after 72 hrs in the control culture. However, almost 30% of MB cells were BrdU positive when cultured in the presence of Shh, indicating that exogenous Shh enhances MB cell proliferation in vitro.
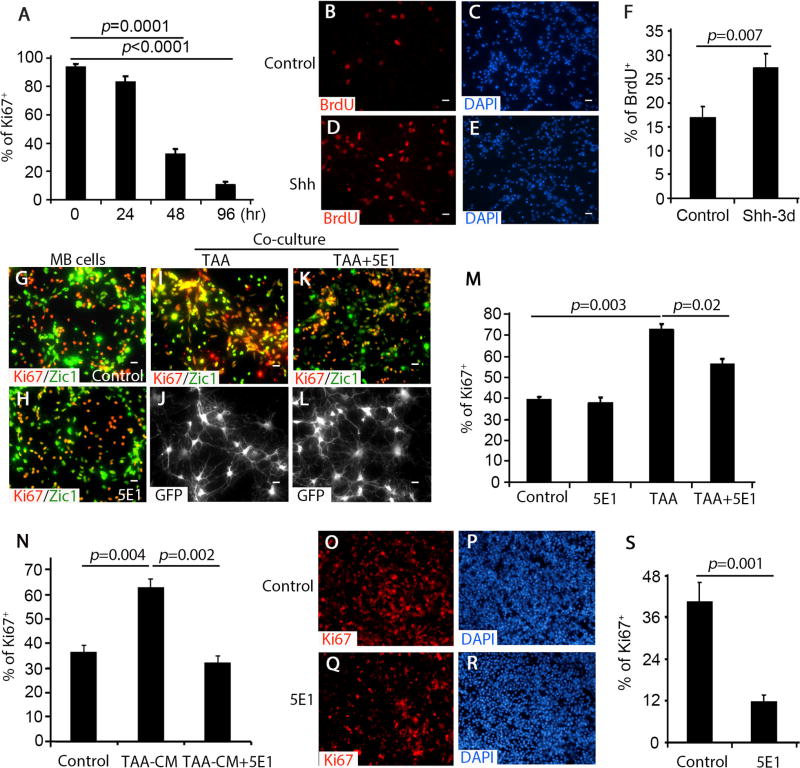
Figure 3. TAA-derived Shh supports MB cell proliferation.
A, Percentage of Ki67+ cells among cultured MB cells harvested at designated time points (0–96 hrs) was quantified. B–E, MB cells were treated with PBS control (B and C) or Shh (D and E) for 3 days, and immunostained for BrdU after a 2 hour pulse with 100µM BrdU. F, The percentage of BrdU+ cells among control (DMSO) or Shh-treated MB cells was quantified. G–N, MB cells cultured alone (G and H) or co-cultured with TAA (I and J) together with 1% 5E1 (K and L) were immunostained for Ki67, Zic1 or GFP. MB cells were treated with NB-B27 (control), TAA-CM, or together with 5E1 for 48hrs and immunostained for Ki67 (N). The percentage of Ki67+ cells among cultured MB cells was quantified in M and N. O–S, MB slices were immunostained for Ki67 after being culture without treatment (O and P) or 5E1 (Q and R) for 4 days. The percentage of Ki67+ cells among MB cells in MB slices was quantified (S). DAPI was used to counterstain cell nuclei in C, E, O and Q. Data in figure A, F, M, N and S represent means ± SEM from three independent experiments. Scale bar, 10 µm.
To examine whether TAA promote MB cell proliferation by secreting Shh, we treated co-cultures of TAA and MB cells with the Shh-neutralizing antibody 5E1 (36). As a control, we treated cerebellar GNPs with Shh in the presence and absence of 5E1. As shown in supplementary figure S2B, 5E1 significantly repressed up-regulation of Gli1 and cyclin D1 in GNPs following Shh treatment, indicating that 5E1 is capable of antagonizing Shh. MB cells and TAA were isolated from Math1-Cre/Ptch1fl/fl/GFAP-GFP mice at 8 weeks of age, then the purified MB cells were cultured alone, or with TAA, in the presence and absence of 5E1. At 48 hrs after treatment, cells were examined by immunostaining with Ki67 and Zic1 antibodies (Fig. 3G and 3H). When cultured alone, approximately 40% of MB cells were proliferative (Ki67+) and no alterations in MB cell proliferation were found after treatment with 5E1. However, MB cell proliferation was dramatically enhanced in the presence of TAA (Fig. 3I and 3J). TAA-enhanced proliferation of MB cells was significantly inhibited by 5E1 treatment (Fig. 3K, 3L and 3M), suggesting that TAA stimulated MB cell proliferation through Shh secretion. There still remains a possibility that Shh could stimulate proliferation of MB cells through upregulation of other factors in astrocytes. To address this, we cultured TAA for 48hrs before collecting conditioned medium (TAA-CM). MB cells were treated with NB-B27 (control), TAA-CM (NB-B27 + TAA-CM [1:1]), or TAA-CM + 5E1. 48hrs after treatment, MB cells were harvested for immunocytochemistry with an antibody against Ki67. TAA-CM dramatically increased MB cell proliferation, compared with NB-B27. Enhanced proliferation of MB cells in the presence of TAA-CM, was effectively repressed by 5E1 (Fig. 3N). These data indicate that Shh from TAA directly stimulate MB cell proliferation.
To further investigate the supporting role of Shh in the tumor microenvironment, we used organotypic slice cultures (24), to examine the blocking activity of 5E1. Tumor slices were prepared from MB developed in Math1-Cre/Ptch1fl/fl mice at 8 weeks of age as previously described (24), and treated without and with 5E1 for 4 days. Following treatment, tumor slices were examined by immunohistochemistry to determine the effects of shh abrogation on proliferation. As shown in figure 3O and 3P, there was robust proliferation of MB cells in the tumor slices, with 40% of MB cells positive for Ki67 after 4 days. The level of proliferation was significantly elevated in tumor slices compared to cell culture, highlighting the important role of the tumor microenvironment in MB cell proliferation. This enhanced level of MB cells proliferation was reduced to 12% by 5E1 treatment of the tumor slices (Fig. 3Q, 3R and 3S). No increase in apoptosis or cell death was observed in tumor slices after 5E1 treatment (supplementary figure S3A–C). These data demonstrate that Shh, present in the tumor microenvironment, is required for MB cell proliferation.
Shh induces Nestin expression in Ptch1-deficient GNPs
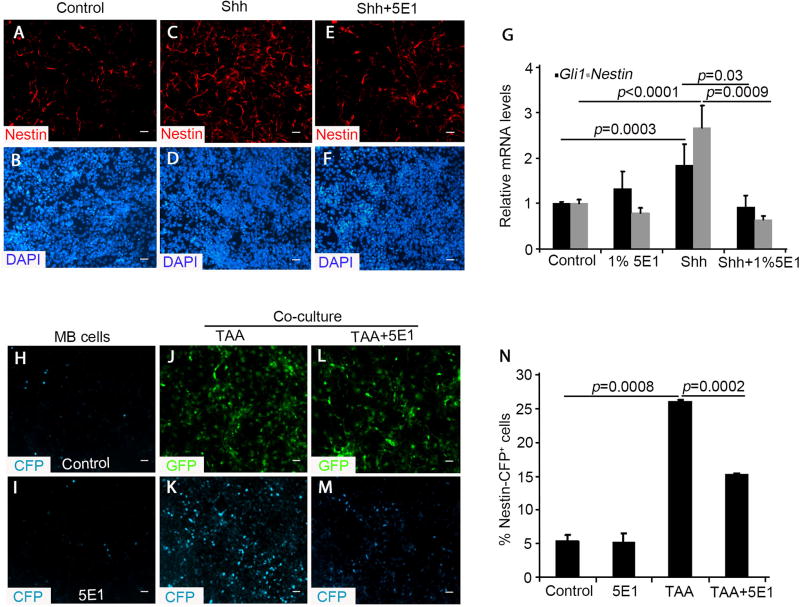
Recently we reported that Nestin expression level increase during tumor progression and that it plays a key role in enhancing hedgehog pathway activity and MB cell proliferation in vivo (22). Having observed that Shh can promote MB cell proliferation, we examined whether Shh affects Nestin expression in MB cells. For this purpose, we utilized Math1-Cre/Ptch1fl/fl/Nestin-CFP mice, in which MB cells gradually increase the levels of Nestin, presented by cyan fluorescent protein (CFP) expression as tumors develop (22). Nestin negative (CFP−) cells were purified by FACS from Math1-Cre/Ptch1fl/fl/Nestin-CFP mice at P7. At this age, only 20% of Ptch1-deficient GNPs express Nestin (CFP+) in the cerebellum (22). Ptch1 deletion, as well as activation of the hedgehog pathway in CFP− cells, was previously demonstrated by q-PCR and western analyses (22). CFP− cells were treated with recombinant Shh alone or together with 5E1. After 48 hrs culture, only a few Nestin-expressing cells were present in the cultures based on immunocytochemistry (Fig. 4A and 4B). However, Shh treatment significantly increased Nestin expression among Ptch1-deficient GNPs (Fig. 4C and 4D). Moreover, Shh-induced Nestin expression was effectively blocked by 5E1 (Fig. 4E and 4F). Based on qRT-PCR analysis, Shh significantly enhanced the level of Nestin mRNA expression in Ptch1-deficient GNPs, whereas Shh-induced Nestin expression was markedly reduced following 5E1 treatment (Fig. 4G), No alteration in the expression of Nestin or Gli mRNA was observed in Ptch1-deficient GNPs after treatment with 5E1 alone (Fig. 4G). These data reveal that Shh is capable of inducing Nestin mRNA expression in Ptch1-deficient GNPs.
Figure 4. TAA-derived Shh induces Nestin expression in MB cells.
A–F, CFP-negative Ptch1-deficient GNPs from Math1-Cre/Ptch1fl/fl/Nestin-CFP mice at P7 were immunostained with Nestin after treatment with PBS control (A and B), 1µg/mL Shh (C and D), or Shh plus 1% 5E1 (E and F) for 48 hours. G, Expression of Gli1 and Nestin mRNA in CFP-negative Ptch1-deficient GNPs treated with 1% 5E1, 1µg/mL Shh or Shh plus 5E1 for 48 hours, were examined by q-PCR. H–M, CFP-negative Ptch1-deficient GNPs were cultured alone (H and I) or co-cultured with TAA (GFP+, J–M) in the absence and presence of 5E1. N, The percentage of Nestin-CFP + cells among Ptch1-deficient GNPs was quantified. Data in figure G and N represent means ± SEM from three independent experiments. Scale bar, 10 µm.
To investigate whether astrocytes can stimulate Nestin expression in Ptch1-deficient GNPs through Shh secretion, we purified CFP− GNPs as mentioned above, and isolated TAA from Math1-Cre/Ptch1fl/fl/GFAP-GFP mice. CFP− GNPs were cultured alone, or with TAA, at a ratio (MB cells to TAA) of 5:1 in the presence and absence of 5E1 for 48hrs. We then examined Nestin induction in Ptch1-deficient GNPs using CFP expression as a marker. As shown in figure 4H, spontaneous CFP+ cells were identified at a low level in Ptch1-deficient GNPs cultured alone. This low-level spontaneous expression was not dependent on exogenous Shh, as 5E1 treatment had no effect on CFP expression (Fig. 4I). In the presence of TAA that already extended elongated processes (Fig. 4J), the number of CFP+ cells dramatically increased in the Ptch1-deficient GNP population (Fig. 4K). Treatment of these co-cultures with 5E1 suppressed the number of CFP+ cells (Fig. 4L, 4M and 4N). These data indicate that TAA-induced Nestin expression in Ptch1-deficient GNPs is mediated by Shh secretion.
Shh-induced Nestin expression relies on Smo activation, but is independent of Gli1
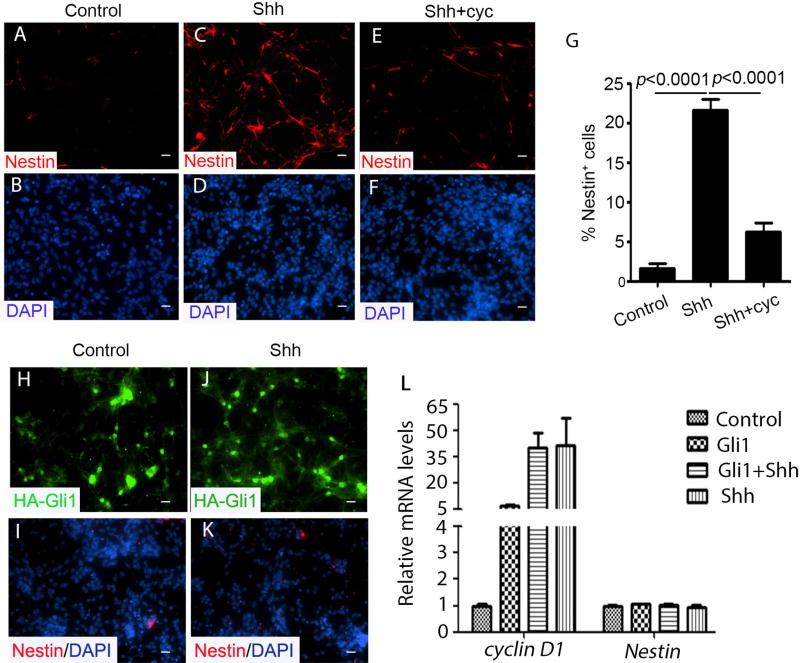
Shh-induced Nestin expression in Ptch1-deficient GNPs, reveals a distinct function of Shh that is independent of binding to Ptch1. To further characterize the mechanism involved in Shh-induced Nestin expression, we isolated CFP−, Ptch1-deficient GNPs from Math1-Cre/Ptch1fl/fl/GFAP-GFP mice and treated them with 1µg/ml recombinant Shh, in the presence and absence of cyclopamine, a potent naturally occurring plant alkaloid that antagonizes Smo (37). As shown in figure 5A and 5B, nearly no Nestin expression was detected in Ptch1-deficient GNPs at control conditions. In contrast, approximately 22% of Ptch1-deficient GNPs exhibited Nestin expression after Shh treatment (Fig. 5C and 5D). However, cyclopamine dramatically blocked the increase in Nestin mRNA and protein in Ptch1-deficient GNPs following Shh treatment (Fig. 5E, 5F and 5G), indicating that Smo activation is required for Shh-induced Nestin expression.
Figure 5. Shh-induced Nestin expression requires Smo but not Gli1.
A–F, CFP-negative Ptch1-deficient GNPs from Math1-Cre/Ptch1fl/fl/Nestin-CFP mice at P7 were immunostained with Nestin after treatment with PBS (A and B), 1µg/mL Shh (C and D) or Shh plus 1µM cyclopamine (cyc) (E and F) for 48 hours. G, The percentage of Nestin+ cells among Ptch1-deficient GNPs treated with PBS control, Shh and Shh plus cyc was quantified. E–H, wild type GNPs infected with a lentivirus carrying HA-Gli1 were treated without (H and I) or with Shh (J and K) for 2 days, and immunostained for HA or Nestin. I, Expressions of cyclin D1 and Nestin mRNAs in infected GNPs were examined by qRT-PCR. The values in figure G and L are the means ± SEM from three independent experiments. Scale bar, 10 µm.
We next investigated whether Shh-induced Nestin expression is mediated by Gli1. Cerebellar GNPs isolated from p8 wild type mice, were infected with a lentiviral vector expressing HA-tagged Gli1. Approximately, 48 hrs after infection, cultured GNPs were harvested for immunocytochemistry and qRT-PCR analysis. As shown in figure 5H and 5I, no Nestin was detected in Gli1-infected GNPs, regardless of the presence of Shh (Fig. 5J and 5K). In contrast, it was clear that exogenous Gli1 induced expression of cyclin D1, a Shh pathway target gene (33) (Fig. 5L). Nevertheless, no induction of Nestin mRNA was detected in GNPs after Gli1 overexpression and Shh treatment (Fig. 5L). In addition, Nestin negative cells purified from Math1-Cre/Ptch1fl/fl/Nestin-CFP mice at P7, were infected with a lentivirus carrying a HA-tagged Gli1 vector or a HA-tagged empty vector. 48hrs after infection, no Nestin expression was observed in Gli1-overexpressed or the control cells (Supplementary Fig. S4A–D). These data suggest that Gli1 is not sufficient for Shh-induced Nestin expression in cerebellar GNPs.
Taken together, the above data indicate that, while Shh-induced Nestin expression in Ptch1-deficient GNPs requires Smo activation, it is independent of Gli1.
TAA are required for MB progression
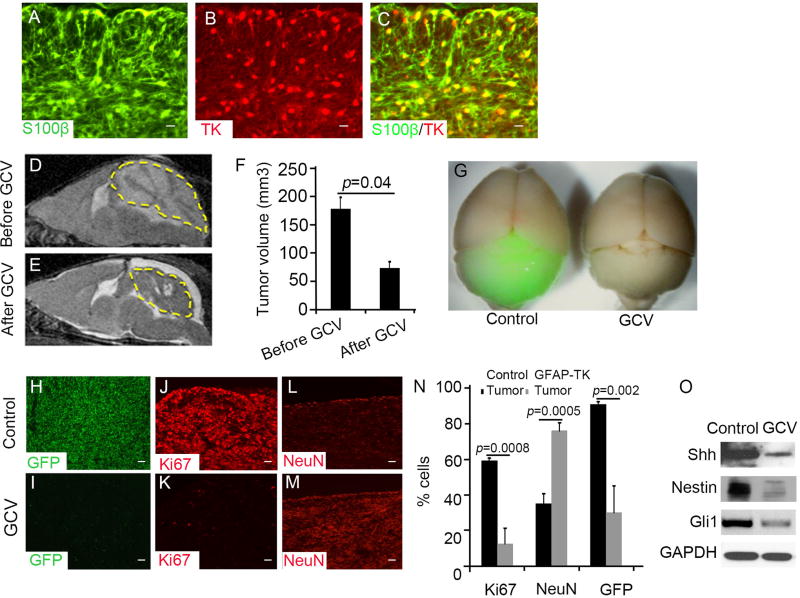
To investigate the role of TAA in MB progression, we examined MB growth after ablation of TAA in GFAP-TK mice. In these mice, astrocytes express thymidine kinase (TK), which renders them sensitive to ganciclovir (GCV) treatment (38). GFAP-TK mice were crossed with Math1-Cre/Ptch1fl/fl mice. As shown in figure 6A–6C, over 95% of TAA (S100β+) were positive for TK, indicating that Math1-Cre/Ptch1fl/fl/GFAP-TK mice could be used to target TAA by GCV treatment. We treated Math1-Cre/Ptch1fl/fl/GFAP-TK mice at 8 weeks of age with GCV or saline vehicle control once daily for 7 days. Before and after GCV treatment, tumor volume was examined by MRI. As shown in figure 6D and 6E, the tumor volume decreased dramatically in Math1-Cre/Ptch1fl/fl/GFAP-TK mice following GCV treatment, compared with that prior to GCV treatment (Fig. 6F). These data demonstrate that ablation of TAA significantly suppresses MB growth in vivo.
Figure 6. TAA are required for MB progression.
A–C, Sagittal sections from Math1-Cre/Ptch1fl/fl/GFAP-TK mice at 8 weeks of age, were immunostained for S100β (A) and TK (B). A merged image (C) shows TK positive cells expressed S100β. D and E, Tumor volume of MB from a Math1-Cre/Ptch1fl/fl/GFAP-TK mouse before (D) and after treatment (E) with GCV for 7 days, was measured by MRI. The yellow dotted lines circle the tumor mass. F, MB tumor volume was quantified based on MRI images using Image J software. G, Whole mount brain images of Math1-Cre/Ptch1fl/fl/GFAP-TK/Nestin-CFP mice treated with saline (control) or GCV for 7 days. H–M, Sagittal sections of MB tissues from Math1-Cre/Ptch1fl/fl/Nestin-CFP/GFAP-TK mice following treatment with saline (H, J and L) or GCV (I, K and M) for 3 days, were immunostained for CFP (H and I), Ki67 (J and K) or NeuN (L and M). N, The percentage of Ki67, NeuN or Nestin-CFP positive cells in MB tissues after treatment with saline or GCV were quantified. O, Expression of Nestin, Gli1, Shh or GAPDH proteins in Math1-Cre/Ptch1fl/fl/Nestin-CFP/GFAP-TK MB tissue treated with saline or GCV for 3 days, was examined by western blotting. Data in figure F and N represent means ± SEM from three independent experiments. Scale bar, 10 µm.
To determine the basis for inhibition of MB growth after deletion of TAA, we crossed Math1-Cre/Ptch1fl/fl/GFAP-TK mice with Nestin-CFP mice, then examined MB cells proliferation and Nestin expression in MB cells. Math1-Cre/Ptch1fl/fl/GFAP-TK/Nestin-CFP mice were treated with GCV or saline by subcutaneous injection. Robust CFP expression was observed in tumors arising in Math1-Cre/Ptch1fl/fl/GFAP-TK/Nestin-CFP mice after saline treatment as expected (Fig. 6G). However, the level of CFP expression in MB tissue was dramatically decreased following GCV treatment (Fig. 6G), suggesting that ablation of TAA significantly reduced Nestin expression in MB tissue. Next, frozen sagittal frozen sections were prepared from MB obtained from Math1-Cre/Ptch1fl/fl/GFAP-TK/Nestin-CFP mice after the above treatment, to examine CFP expression and proliferation by immnuohistochemistry. As shown in supplementary figure S5A–B, the number of TK positive cells was significantly decreased after GCV treatment, compared with controls, suggesting that GCV treatment effectively ablated TAA from tumor tissue. Very few CFP-expressing cells remained in tumor tissue after GCV treatment. In contrast, over 95% of MB cells were positive for CFP (Fig. 6H and 6I) in mice treated with saline. Moreover, a significant reduction in the number of Ki67+ cells was found in GCV-treated MB tissue compared with the control (Fig. 6J and 6K), suggesting that MB cell proliferation was dramatically compromised by GCV treatment. No obvious increase in apoptosis was detected in GCV-treated MB tissue (Supplementary Fig. S5C–D), suggesting that the survival of MB cells was not affected by GCV treatment. Interestingly, increased differentiation (NeuN+) was observed in MB tissue after GCV treatment, whereas only a few differentiating MB cells were apparent in tumor tissue after saline treatment (Fig. 6L, 6M and 6N). These data indicate that ablation of TAA significantly inhibits tumor growth by blocking tumor cell proliferation while promoting differentiation. This is consistent with our previous finding regarding the functions of Nestin in MB tumorigenesis (22). After GCV or saline treatment, tumor tissue was harvested to determine the level of expression of Shh, Nestin and Gli1 by western analyses (Fig. 6O). The amount of Shh in MB tissue significantly declined following GCV treatment, which supports the hypothesis that Shh in MB tissue is predominately derived from TAA. The levels of Nestin and Gli1 also decreased dramatically after GCV treatment, consistent with our finding that TAA-derived Shh enhances activation of the hedgehog pathway in MB cells by induction of Nestin expression. These data demonstrate that TAA support MB cell proliferation and tumor growth by secretion of Shh.
Discussion
Solid tumors are no longer viewed as monoclonal collections of cancer cells. Instead, they are best described as multi-cellular organs harboring diverse, dynamic populations of neoplastic as well as normal cells (39). The relative proportions of the various cell populations change during tumor progression and over the course of therapy. Understanding the relationships among different cell types in the tumor microenvironment is key to understanding the biology of tumor initiation, progression and metastasis. It also has the potential to provide additional avenues for therapeutic intervention. The tumor microenvironment not only contributes to neoplastic growth, it also regulates responses to chemotherapy and radiation therapy (40). Although the molecular mechanisms underlying tumorigenesis of hedgehog pathway in MB has been studied extensively, the contributions of the tumor microenvironment to MB initiation and progression remain elusive. Here we demonstrate that astrocytes, a major cellular component of the MB microenvironment, promote tumor progression through Shh secretion. Ablation of astrocytes markedly prohibited MB growth by reducing proliferation of MB cells. These findings, for the first time, reveal an indispensable role for astrocytes in hedgehog-type MB, which highlight the importance of the tumor microenvironment in tumor progression.
Our previous studies revealed that, following Ptch1 loss, GNPs progressively express Nestin as an obligatory step in MB development (22). However, the mechanism responsible for increased Nestin expression in Ptch1-deficient GNPs was not known. Here, we demonstrate that astrocyte-derived Shh is responsible for induction of Nestin expression in Ptch1-deficient GNPs and MB cells. In the canonical hedgehog pathway, Shh binds to Ptch1 resulting in release of Smo that, in turn, inhibits Sufu, leading to activation of Gli1 and Gli2 and regulation of target gene expression. However, this does not appear to be the underlying mechanism whereby Shh induces Nestin expression in Ptch1-deficient GNPs. Neither deletion of Ptch1 nor overexpression of Gli1 was sufficient to stimulate Nestin expression in cerebellar GNPs. Nestin was only expressed in Ptch1-deficient GNPs after Shh treatment. This paradoxical effect of Shh in the absence of its receptor Ptch1 implies a role for additional Shh receptors such as Ptch2 or Boc. In particular, Ptch2 has been reported to mediate the response of fibroblasts to Shh in the absence of Ptch1 (41). Moreover, Ptch2 modulates MB tumorigenesis in mice with Ptch1 haploinsufficiency (42). While we attempted to examine the possible involvement of Ptch2 or Boc in Shh-induced Nestin expression, we failed to effectively knock down these two genes in MB cells using shRNA or siRNA, which is likely due to substantially increased expression of Ptch2 and Boc in MB cells (data not shown). Nevertheless, since cyclopamine blocked Shh-induced Nestin expression in Ptch1-deficient GNPs, activated Smo is still further required for this novel function of Shh. Nestin does not appear to be a classic Hh pathway target gene since it was not induced by overexpression of Gli1. These data indicate that Shh stimulates Nestin expression in GNPs and MB cells in a Smo-dependent, but Gli1-independent manner. Previously, we demonstrated that Nestin augments canonical Shh signaling by abolishing the inhibitory functions of Gli3 (22). Taken together, our findings reveal a novel, paradoxical signaling pathway in which Shh, Smo and Nestin, acting in concert, regulate hedgehog pathway activity in normal and neoplastic cells, independently of Ptch1 and Gli1.
During cerebellar development, the Shh pathway drives a massive expansion of cerebellar GNPs (33). However, Shh-induced Nestin expression was found only in Ptch1-deficient GNPs but not in wild type GNPs (Fig. 5H–L). One possible explanation for this observation is that the extent of Smo activation may dictate whether Nestin expression can be induced by Shh in GNPs. As the predominant antagonizing partner of Smo, Ptch1 is also a target gene of hedgehog pathway activity. In wild type GNPs, Shh treatment also increases Ptch1 expression, subsequently restricting the activation of Smo. However, the negative feedback loop, mediated by Ptch1, is disrupted in Ptch1-deficient GNPs and in MB cells, resulting in over-activation of Smo. Future studies are warranted to further investigate the molecular events downstream of Smo that mediate Shh-induced Nestin expression in MB cells.
Previously, we reported that MB cells cannot maintain an active Shh pathway in vitro. However, if tumor tissue was never cultured but directly implanted in a flank allograft, the Shh pathway remains active in MB cells (35). Here, we provide an explanation for this conundrum. The addition of exogenous Shh, or co-culture with TAA, significantly augmented Shh pathway activity in cultured MB cells, resulting in continued proliferation in vitro. These findings indicate that astrocyte-derived Shh, secreted into the tumor microenvironment, is required for maintaining an active hedgehog pathway in MB cells. This support from the tumor microenvironment is lost when MB cells are cultured in vitro. In the case of allograft transplantation, host stromal cells may invade and rebuild the tumor microenvironment to maintain an active Shh pathway in MB cells. Several groups, including our own, have made many unsuccessful attempts to establish PDX models for Shh type MB. Only approximately 10% of human MB samples can be propagated in immunosuppressed mice, and the tumors that grow fail to maintain an active hedgehog pathway (data not shown). Our findings suggest an alterative approach, in which stromal cells, including astrocytes, could be co-injected with MB cells in an attempt to re-recreate the tumor microenvironment and provide support to enhance the survival and propagation of human MB cells after grafting into immunosuppressed mice. In addition, based on our studies, exogenous Shh may be required in the culture media to maintain hedgehog pathway activity in MB cells in vitro.
In our studies, tumor volume in Math1-Cre/Ptch1fl/fl/GFAP-TK/Nestin-CFP mice was dramatically decreased after GCV treatment, raising the possibility that the reduction in tumor size may be due to the “bystander effect”. The “bystander effect” is well documented in tumor models (43, 44), in which cells in the vicinity of TK-expressing cells are killed by GCV. It was attributed to the transfer of phosphorylated GCV from cell to cell through gap junction (45). However, no gap junctions between astrocytes and GNPs (MB cells) were found based on previous studies of cerebellar architecture (46). In addition, the previously reported “bystander effect” is associated with a significant amount of cell death, and the effect itself is to increase cell death. For example, more than 90% of cell death was detected in tumor tissues carrying the TK gene after GCV treatment (43, 44). However, no increase in cell death within the tumor tissue was found following GCV treatment in our studies (Fig. S4). The predominant effect of GCV treatment in Math1-Cre/Ptch1fl/fl/GFAP-TK/Nestin-CFP mice was induction of tumor cell differentiation and repression of Nestin expression in MB cells. Although we cannot completely rule out the “bystander effect”, we believe that the “bystander effect” is not the reason for reduced tumor volume observed in Math1-Cre/Ptch1fl/fl/GFAP-TK/Nestin-CFP mice after GCV treatment.
The Smo inhibitors, vismodegib and sonidegib, are currently approved for the treatment of advanced basal cell carcinoma (BCC) and they have demonstrated efficacy in clinical trials of Hh-MB (47–49). Despite the dramatic response to these inhibitors in the initial clinical trails, drug resistance readily arises in both human and animal models as a consequence of mutation in Smo (50), amplification of Gli2 (51) or cyclin D1 (52). Because of significant toxicities, many BCC patients cannot endure prolonged therapy and often come off drug only to go back on when tumors recur (53). In children, there is a greater concern for developmental toxicities since the Hh pathway is primarily active during development. Transient treatment of young mice with a Smo antagonist caused dramatic and permanent defects in bone development (54). These observations are consistent with the critical functions that the hedgehog pathway plays in bone growth (55). Recently, similar effects were seen in children and the FDA required modifications of the protocol excluding children in which bone growth is active unless there are no other treatment options. Here we demonstrate a major role for astrocyte-derived Shh in activation of the Shh pathway in MB cells through induction of Nestin expression. This effect was only observed in Ptch1-deficient GNPs and MB cells, but not in wild type GNPs. This suggests that targeting astrocytes, or the secretion of Shh, may represent tumor-specific strategies for therapeutic intervention in MB and other Hh pathway malignancies, such as BCC.
Supplementary Material
Acknowledgments
Financial Support:
This research was supported by funds from the US National Cancer Institute (CA178380, CA185504, Z.Yang. 2 T32 CA009035-45, L.W. Yuelling), American Cancer Society (RSG1605301NEC, Z. Yang), Pennsylvania Department of Health (CURE 4100068716, Z.Yang).
We thank J. Oesterling for flow cytometric analysis; E. Nicholas for qRT-PCR analysis; and Dr. R. Segal (Dana Farber Cancer Institute) for anti-Zic1 antibody; Dr. M. Sofroniew (University of California, Los Angeles) for anti-TK antibody. This research was supported by the US National Cancer Institute (CA178380, CA185504, Z.Yang), American Cancer Society RSG (RSG1605301NEC, Z. Yang), Pennsylvania Department of Health (CURE 4100068716, Z.Yang) and National Natural Science Foundation of China (81572724, Z.Yang).
References
- 1.Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60(3):430–40. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26(10):523–30. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Hol EM, Pekny M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Current opinion in cell biology. 2015;32:121–30. doi: 10.1016/j.ceb.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Ghandour MS, Labourdette G, Vincendon G, Gombos G. A biochemical and immunohistological study of S100 protein in developing rat cerebellum. Developmental neuroscience. 1981;4(2):98–109. doi: 10.1159/000112745. [DOI] [PubMed] [Google Scholar]
- 5.Sharma K, Schmitt S, Bergner CG, Tyanova S, Kannaiyan N, Manrique-Hoyos N, et al. Cell type- and brain region-resolved mouse brain proteome. Nat Neurosci. 2015;18(12):1819–31. doi: 10.1038/nn.4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurtz A, Zimmer A, Schnutgen F, Bruning G, Spener F, Muller T. The expression pattern of a novel gene encoding brain-fatty acid binding protein correlates with neuronal and glial cell development. Development. 1994;120(9):2637–49. doi: 10.1242/dev.120.9.2637. [DOI] [PubMed] [Google Scholar]
- 7.De Zeeuw CI, Hoogland TM. Reappraisal of Bergmann glial cells as modulators of cerebellar circuit function. Front Cell Neurosci. 2015;9:246. doi: 10.3389/fncel.2015.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myer DJ, Gurkoff GG, Lee SM, Hovda DA, Sofroniew MV. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006;129(Pt 10):2761–72. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- 9.Seifert G, Schilling K, Steinhauser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci. 2006;7(3):194–206. doi: 10.1038/nrn1870. [DOI] [PubMed] [Google Scholar]
- 10.Kim SJ, Kim JS, Park ES, Lee JS, Lin Q, Langley RR, et al. Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy. Neoplasia. 2011;13(3):286–98. doi: 10.1593/neo.11112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson MC, Fuller C, Hogg TL, Dalton J, Finkelstein D, Lau CC, et al. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(12):1924–31. doi: 10.1200/JCO.2005.04.4974. [DOI] [PubMed] [Google Scholar]
- 12.Kool M, Korshunov A, Remke M, Jones DT, Schlanstein M, Northcott PA, et al. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta neuropathologica. 123(4):473–84. doi: 10.1007/s00401-012-0958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Northcott PA, Nakahara Y, Wu X, Feuk L, Ellison DW, Croul S, et al. Multiple recurrent genetic events converge on control of histone lysine methylation in medulloblastoma. Nat Genet. 2009;41(4):465–72. doi: 10.1038/ng.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes & development. 2008;22(18):2454–72. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- 15.Humke EW, Dorn KV, Milenkovic L, Scott MP, Rohatgi R. The output of Hedgehog signaling is controlled by the dynamic association between Suppressor of Fused and the Gli proteins. Genes & development. 2010;24(7):670–82. doi: 10.1101/gad.1902910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aza-Blanc P, Ramirez-Weber FA, Laget MP, Schwartz C, Kornberg TB. Proteolysis that is inhibited by hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell. 1997;89(7):1043–53. doi: 10.1016/s0092-8674(00)80292-5. [DOI] [PubMed] [Google Scholar]
- 17.Jenkins D. Hedgehog signalling: emerging evidence for non-canonical pathways. Cell Signal. 2009;21(7):1023–34. doi: 10.1016/j.cellsig.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 18.Chinchilla P, Xiao L, Kazanietz MG, Riobo NA. Hedgehog proteins activate pro-angiogenic responses in endothelial cells through non-canonical signaling pathways. Cell Cycle. 2010;9(3):570–79. doi: 10.4161/cc.9.3.10591. [DOI] [PubMed] [Google Scholar]
- 19.Bijlsma MF, Borensztajn KS, Roelink H, Peppelenbosch MP, Spek CA. Sonic hedgehog induces transcription-independent cytoskeletal rearrangement and migration regulated by arachidonate metabolites. Cell Signal. 2007;19(12):2596–604. doi: 10.1016/j.cellsig.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Yam PT, Langlois SD, Morin S, Charron F. Sonic hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron. 2009;62(3):349–62. doi: 10.1016/j.neuron.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Yang ZJ, Ellis T, Markant SL, Read TA, Kessler JD, Bourboulas M, et al. Medulloblastoma can be initiated by deletion of Patched in lineage-restricted progenitors or stem cells. Cancer Cell. 2008;14(2):135–45. doi: 10.1016/j.ccr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li P, Lee E, Du F, Gordon RE, Yuelling LW, Liu Y, et al. Nestin mediates hedgehog pathway tumorigenesis. Cancer research. 2016 doi: 10.1158/0008-5472.CAN-16-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li P, Du F, Yuelling LW, Lin T, Muradimova RE, Tricarico R, et al. A population of Nestin-expressing progenitors in the cerebellum exhibits increased tumorigenicity. Nat Neurosci. 2013;16(12):1737–44. doi: 10.1038/nn.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chadwick EJ, Yang DP, Filbin MG, Mazzola E, Sun Y, Behar O, et al. A Brain Tumor/Organotypic Slice Co-culture System for Studying Tumor Microenvironment and Targeted Drug Therapies. Journal of visualized experiments : JoVE. 2015;(105):e53304. doi: 10.3791/53304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gajjar AJ, Robinson GW. Medulloblastoma-translating discoveries from the bench to the bedside. Nat Rev Clin Oncol. 2014;11(12):714–22. doi: 10.1038/nrclinonc.2014.181. [DOI] [PubMed] [Google Scholar]
- 26.Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, et al. Medulloblastoma comprises four distinct molecular variants. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(11):1408–14. doi: 10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yokota N, Aruga J, Takai S, Yamada K, Hamazaki M, Iwase T, et al. Predominant expression of human zic in cerebellar granule cell lineage and medulloblastoma. Cancer research. 1996;56(2):377–83. [PubMed] [Google Scholar]
- 28.Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277(5329):1109–13. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 29.Schuller U, Heine VM, Mao J, Kho AT, Dillon AK, Han YG, et al. Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell. 2008;14(2):123–34. doi: 10.1016/j.ccr.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhuo L, Sun B, Zhang CL, Fine A, Chiu SY, Messing A. Live astrocytes visualized by green fluorescent protein in transgenic mice. Developmental biology. 1997;187(1):36–42. doi: 10.1006/dbio.1997.8601. [DOI] [PubMed] [Google Scholar]
- 31.Barry D, McDermott K. Differentiation of radial glia from radial precursor cells and transformation into astrocytes in the developing rat spinal cord. Glia. 2005;50(3):187–97. doi: 10.1002/glia.20166. [DOI] [PubMed] [Google Scholar]
- 32.Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 2011;334(6063):1727–31. doi: 10.1126/science.1206936. [DOI] [PubMed] [Google Scholar]
- 33.Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22(1):103–14. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 34.Taipale J, Chen JK, Cooper MK, Wang B, Mann RK, Milenkovic L, et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406(6799):1005–9. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 35.Sasai K, Romer JT, Lee Y, Finkelstein D, Fuller C, McKinnon PJ, et al. Shh pathway activity is down-regulated in cultured medulloblastoma cells: implications for preclinical studies. Cancer research. 2006;66(8):4215–22. doi: 10.1158/0008-5472.CAN-05-4505. [DOI] [PubMed] [Google Scholar]
- 36.Ericson J, Morton S, Kawakami A, Roelink H, Jessell TM. Two critical periods of Sonic Hedgehog signaling required for the specification of motor neuron identity. Cell. 1996;87(4):661–73. doi: 10.1016/s0092-8674(00)81386-0. [DOI] [PubMed] [Google Scholar]
- 37.Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes & development. 2002;16(21):2743–8. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delaney CL, Brenner M, Messing A. Conditional ablation of cerebellar astrocytes in postnatal transgenic mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16(21):6908–18. doi: 10.1523/JNEUROSCI.16-21-06908.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell. 2010;18(6):884–901. doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenvironment. Trends Genet. 2009;25(1):30–8. doi: 10.1016/j.tig.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 41.Alfaro AC, Roberts B, Kwong L, Bijlsma MF, Roelink H. Ptch2 mediates the Shh response in Ptch1−/− cells. Development. 2014;141(17):3331–9. doi: 10.1242/dev.110056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee Y, Miller HL, Russell HR, Boyd K, Curran T, McKinnon PJ. Patched2 modulates tumorigenesis in patched1 heterozygous mice. Cancer research. 2006;66(14):6964–71. doi: 10.1158/0008-5472.CAN-06-0505. [DOI] [PubMed] [Google Scholar]
- 43.Calvez V, Rixe O, Wang P, Mouawad R, Soubrane C, Ghoumari A, et al. Virus-free transfer of the herpes simplex virus thymidine kinase gene followed by ganciclovir treatment induces tumor cell death. Clinical cancer research : an official journal of the American Association for Cancer Research. 1996;2(1):47–51. [PubMed] [Google Scholar]
- 44.Freeman SM, Abboud CN, Whartenby KA, Packman CH, Koeplin DS, Moolten FL, et al. The "bystander effect": tumor regression when a fraction of the tumor mass is genetically modified. Cancer research. 1993;53(21):5274–83. [PubMed] [Google Scholar]
- 45.Culver KW, Ram Z, Wallbridge S, Ishii H, Oldfield EH, Blaese RM. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992;256(5063):1550–2. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- 46.Rakic P, Sidman RL. Organization of cerebellar cortex secondary to deficit of granule cells in weaver mutant mice. The Journal of comparative neurology. 1973;152(2):133–61. doi: 10.1002/cne.901520203. [DOI] [PubMed] [Google Scholar]
- 47.Ng JM, Curran T. The Hedgehog's tale: developing strategies for targeting cancer. Nature reviews Cancer. 2011;11(7):493–501. doi: 10.1038/nrc3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. The New England journal of medicine. 2012;366(23):2171–9. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Casey D, Demko S, Shord S, Zhao H, Chen H, He K, et al. FDA Approval Summary: Sonidegib for Locally Advanced Basal Cell Carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2017;23(10):2377–81. doi: 10.1158/1078-0432.CCR-16-2051. [DOI] [PubMed] [Google Scholar]
- 50.Yauch RL, Dijkgraaf GJ, Alicke B, Januario T, Ahn CP, Holcomb T, et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326(5952):572–4. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buonamici S, Williams J, Morrissey M, Wang A, Guo R, Vattay A, et al. Interfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Sci Transl Med. 2010;2(51):51ra70. doi: 10.1126/scitranslmed.3001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dijkgraaf GJ, Alicke B, Weinmann L, Januario T, West K, Modrusan Z, et al. Small molecule inhibition of GDC-0449 refractory smoothened mutants and downstream mechanisms of drug resistance. Cancer research. 2011;71(2):435–44. doi: 10.1158/0008-5472.CAN-10-2876. [DOI] [PubMed] [Google Scholar]
- 53.Tang JY, Mackay-Wiggan JM, Aszterbaum M, Yauch RL, Lindgren J, Chang K, et al. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. The New England journal of medicine. 2012;366(23):2180–8. doi: 10.1056/NEJMoa1113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kimura H, Ng JM, Curran T. Transient inhibition of the Hedgehog pathway in young mice causes permanent defects in bone structure. Cancer Cell. 2008;13(3):249–60. doi: 10.1016/j.ccr.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 55.St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes & development. 1999;13(16):2072–86. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.