Abstract
As a composite concept, negative affect comprises various aversive emotional experiences, such as irritability and nervousness. It is a critical motivational factor that helps maintain smoking behavior, and contributes significantly to smoking cessation failure as a core withdrawal symptom. Prior research has indicated an important role of nicotinic mechanisms in negative affect processing. The most effective smoking cessation medication, varenicline, targets nicotinic acetylcholine receptors (nAChRs) as a partial agonist, while another first-line cessation medication, bupropion, has shown antagonistic effects on nAChRs. Therefore, it is possible that both medications work to reduce smoking behavior through modulating negative affect processing. To evaluate this hypothesis, we examined the impact of varenicline tartrate and bupropion hydrochloride sustained-release on electrophysiological responses to affective, cigarette-related, and neutral cues before and during smoking cessation treatment in a randomized placebo-controlled clinical trial. The participants were 206 smokers, a subset of 294 participants that were enrolled in a larger smoking cessation clinical trial who were randomly assigned to one medication group for 12 weeks. Orbicularis oculi (startle eyeblink response) and corrugator supercilii facial electromyographic (EMG) reactivity toward emotional pictures (i.e., pleasant and unpleasant) in a picture-viewing task were measured before treatment and 2 and 6 weeks after treatment was started. The startle and corrugator EMG activities increase with the exposure to unpleasant cues, and served as indices for negative emotional reactivity (NER). We found that after 6 weeks, drug reduced startle-related NER in the varenicline group, but not in the bupropion or placebo group. Independent of medication treatment, lower baseline NER, as measured by the corrugator EMG activity, predicted a higher likelihood of smoking abstinence 1 and 3 months after quitting smoking. These findings indicate the important roles of varenicline in negative affect processing and negative emotional reactivity in the course of smoking cessation.
Keywords: Bupropion, Varenicline, Smoking Cessation, Startle Response, Corrugator EMG
1. Introduction
Varenicline tartrate (referred to as varenicline) and bupropion hydrochloride sustained-release (referred to as bupropion) have been recommended by the Food and Drug Administration as first-line pharmacotherapies for nicotine dependence in the United States (Fiore et al., 2008). Unfortunately, only about 14% treated with bupropion and 22% treated with varenicline maintain continuous abstinence throughout the first year (Cahill et al., 2012). Understanding these medications' therapeutic mechanisms can help researchers improve the treatment efficacy of these pharmacologic interventions.
Bupropion, as well as its active metabolite, (2S,3S)-hydroxybupropion, is a noncompetitive antagonist on nicotinic acetylcholine receptors (nAChRs), particularly those containing α4β2 and α3β2 subunits (Damaj et al., 2004; Carroll et al., 2014). It is also a dopaminergic and noradrenergic reuptake inhibitor (Stahl et al., 2004). Varenicline is a selective α4β2-containing nAChR partial agonist and a full agonist at the homomeric α7-containing nAChR (Aubin et al., 2014) and also exerts some antagonistic properties on these receptors with nicotine coadministration (Mihalak et al., 2006). Bupropion's nicotinic antagonism and varenicline's partial agonism on α4β2 nAChR have been suggested to be particularly important for their therapeutic effects (Aubin et al., 2014; Carroll et al., 2014). As nicotine's primary molecular targets, nAChRs mediate smoking's rewarding effects (Picciotto et al., 1998; Tapper et al., 2004) and withdrawal symptoms during nicotine deprivation (Salas et al., 2004).
Clinically, withdrawal symptoms are recognized as a major component of nicotine dependence (American Psychiatric Association, 2013). Among the various aspects of smoking withdrawal, negative affect is considered to be the core symptom (Baker et al., 2004). Broadly speaking, negative affect captures subjective distress and unpleasant engagement and is a general dimension that includes various aversive emotional experiences, such as irritability, fear, disgust, nervousness, contempt, guilt, and stress (Watson et al., 1988). Negative affect has been found to play an important role in precipitating smoking relapse. Smokers are more vulnerable to relapse if they have higher negative affect levels before they quit smoking (Ginsberg et al., 1995; Killen et al., 1996; Kenford et al., 2002; Cinciripini et al., 2003) or after they quit smoking (Kenford et al., 2002). Consistent with their superior therapeutic efficacies in improving smoking abstinence, both bupropion and varenicline reduce negative affect levels more than placebo (Gonzales et al., 2006; Jorenby et al., 2006; West et al., 2008; Cinciripini et al., 2013).
The reformulated negative reinforcement model of drug addiction (Baker et al., 2004), has provided a theoretic framework on elucidating the role of negative affect in drug addiction, particularly with nicotine dependence. As the key to continued nicotine use, control of negative affect begins with a preconscious level of processing of negative affect information — a signal of an incipient increase in negative affect levels. Repeated nicotine use and withdrawal lead smokers to be able to preconsciously process this negative affect information by detecting its interoceptive cues during the early stages of nicotine withdrawal. By responding to this interoception, smokers self-administer nicotine to reduce negative affect and the increase of nicotine levels will reverse this early withdrawal process. The reduction of negative affect and other unpleasant withdrawal symptoms contributes to the reinforcement of smoking behavior and the development and maintenance of nicotine addiction. This negative reinforcement model of nicotine addiction suggests that understanding the biological processes associated with the early and preconscious stages of negative affect processing will help elucidate the relationship among nicotine use, nicotine withdrawal, and negative affect.
Many studies using rodent models and human neuroimaging technique have investigated nicotinic mechanisms in modulating various aspects of negative affect. Different classes of nAChR (e.g., α4β2, α7) are expressed widely in the brain, including the hippocampus, the ventral tegmental area, and the striatum, and stimulate and regulate the release of various types of neurotransmitters, including glutamate, GABA, dopamine, and serotonin (Dani and Bertrand, 2007). Local infusion and use of pharmacological agents (e.g., nicotine, nAChR antagonists) have suggested the importance of stress hormones, serotonergic, and GABAergic pathways in mediating nicotine's effect on anxiety-related behaviors using various behavioral paradigms (e.g., elevated plus maze testing) in rodents (Costall et al., 1989; Brioni et al., 1993; Cao et al., 1993; For more, see review by Picciotto et al., 2002). Genetic studies that involved evaluating polymorphism of the α4 subunit and β2 null mutation have found that these genetic variants modulate nicotine's effects on fear-related acoustic startle response in mice (Tritto et al., 2002; Owens et al., 2003).
Given that both bupropion (Damaj et al., 2004) and varenicline (Mihalak et al., 2006) target nAChRs, both should also be expected to play a role in regulating the activities of the above-mentioned nAChR-expressing brain regions and neurotransmitters that are regulated by nicotinic activities. This postulation is supported by several functional neuroimaging studies (Menossi et al., 2013). For example, compared with placebo, bupropion treatment reduced brain activation of the left ventral striatum when smokers were instructed to resist craving actively (Culbertson et al., 2011), and it also reduced smoking cue-related activation in the anterior cingulate cortex (Brody et al., 2004). Several neuroimaging studies found that varenicline treatment reduced the blood oxygen level-dependent (BOLD) activity of the amygdala during a face emotion identification task (Loughead et al., 2011) and in the resting state (Franklin et al., 2011), and the resting state connectivity between amygdala and insula (Sutherland et al., 2013). Despite both medications modulating nAChR-expressing brain regions, it should be noted that bupropion and varenicline appear to be associated with different activation patterns (e.g., anterior cingulate cortex by bupropion vs. amygdala by varenicline), which suggests that bupropion and varenicline may have differential neurophysiological mechanisms. Importantly, research has consistently suggested that both the amygdala and anterior cingulate cortex play critical roles in fear and other emotional processing (Phelps and LeDoux, 2005; Mechias et al., 2010; Shackman et al., 2011). Thus, it can be expected that by regulating these affect-related brain regions, bupropion and varenicline should modulate emotional processing. However, this hypothesis has not been tested clinically.
Using psychophysiological approaches to study negative affect, one can measure immediate physiological changes in response to a negatively-valenced stimulus (Bylsma et al., 2008), which we refer to as negative emotional reactivity (NER). NER can be indexed by the startle eyeblink response, measured from the orbicularis oculi, and by corrugator supercilii electromyographic (EMG) activity using the picture-viewing paradigm (Bradley et al., 2001). The startle response itself is a reflexive reactivity to an abrupt aversive stimulus (e.g., loud noise), and basic research has extensively characterized its neural pathways (Davis et al., 1982; Koch and Schnitzler, 1997; Swerdlow and Geyer, 1999; Lang et al., 2000; Grillon and Baas, 2003). In addition, startle response can be modulated by presenting the startle stimulus within the context of a pre-existing ambient emotional cue, such as an unpleasant picture (e.g., gun threat), and the more unpleasant and arousing the ambient cue, the larger the startle response (Bradley et al., 2001), a paradigm that is termed affect-modulated startle response.
Corrugator activity represents outward facial expression when negative information is processed (Jäncke, 1996), as its EMG levels are significantly increased in response to unpleasant pictures, particularly mutilations and contamination, compared with neutral pictures, as opposed to the startle response, which is more sensitive to animal and human attack (Bradley et al., 2001). Although both startle and corrugator reactivity towards unpleasant stimuli can index NER, they may reflect different subdomains of negative affect processing (e.g., corrugator in disgust, such as in response to mutilations and contamination vs. startle in fear, such as in response to gun threat). In addition, they appear to involve differential brain regions (Lang et al., 2000; Lee et al., 2012). Thus, taken together, measuring NER using both startle response and corrugator reactivity methods will allow us to evaluate the differential effects of bupropion and varenicline on negative affect-related biological processes.
In this study, we used startle response and corrugator EMG measures to evaluate whether bupropion and varenicline reduced NER in smokers who were undergoing smoking cessation treatment. We examined whether medication and abstinence modulated these NER-related measures during the cessation course and further tested if baseline NER predicted abstinence status. Specifically, we hypothesized that: (1) treatment with bupropion and varenicline would result in lower levels of NER than placebo, (2) abstinence would be associated with lower levels of NER than nonabstinence during post-quit time points, and (3) smokers with lower NER at baseline time point would be more likely to remain abstinent after they quit smoking. In addition to these three primary hypotheses related to NER, we conducted secondary analyses to examine whether bupropion and varenicline treatment would modulate smoking cue-related startle response, given that previous research has indicated that smoking-related cues reduce startle response relative to neutral cues (Geier et al., 2000; Cinciripini et al., 2006; Dempsey et al., 2007; Rehme et al., 2009).
2. Materials and Methods
2.1. Participants
Participants were recruited from the Houston metropolitan area as part of a larger clinical trial registered in the www.clinicaltrials.gov database (identifier: NCT00507728). The present report is a secondary analysis of the parent randomized placebo-controlled clinical trial (Cinciripini et al., 2013). To be enrolled in the trial, participants had to 1) be between 18 and 65 years old as we intended to primarily target the mid-age smokers; 2) have English fluency for communication purposes; 3) provide written consent for informed consent; 4) have a working telephone that allowed us to schedule visits and collect follow-up data; 5) smoke five or more cigarettes per day; 6) exhale 6 ppm or more of carbon monoxide (CO) at baseline as a biochemical index of smoking intensity (Marrone et al., 2010); and 7) have no uncontrolled chronic medical illnesses. Unlike some clinical smoking trials (Gonzales et al., 2006; Jorenby et al., 2006) that used 10 cigarettes per day and 10 ppm of CO as the inclusion cutoff, we chose cutoffs of 5 cigarettes per day and 6 ppm of CO which allowed us to better reflect the trend toward lighter smoking in the United States (Jamal et al., 2016). Exclusion criteria included 1) taking psychotropic medications; 2) having a lifetime history of a psychotic disorder; 3) having a psychiatric hospitalization within the past year; 4) having a current psychiatric disorder other than nicotine dependence; 5) being involved in any other concurrent smoking cessation activities; 6) having moderate or severe suicidality as determined by the Mini-International Neuropsychiatric Interview (Sheehan et al., 1998); and 7) having contraindications for varenicline or bupropion. The last criterion was used because participants in the parent clinical trial were randomly assigned to varenicline, bupropion, or placebo, they should not have any contraindications for either medication. The other exclusion criteria were used because we intended to study how smokers in the general population without psychiatric comorbidities responded to these pharmacological interventions.
The Institutional Review Board at The University of Texas MD Anderson Cancer Center approved the parent clinical trial and all its procedures. Among the 294 participants enrolled (Cinciripini et al., 2013), only 206 are presented in this report. Thirty-seven participants were assigned to the functional neuroimaging arm of the parent trial (the results will be reported elsewhere) and did not have psychophysiological data being reported herein, 42 participants did not yield useful psychophysiological data (i.e., < 66% good startle trials), and 9 participants' psychophysiological data were not collected due to technical difficulties.
2.2. Study timeline
After an initial telephone interview, all eligible participants were invited to attend a baseline visit for further screening and final determination of study eligibility. Eligible participants completed the first laboratory session and were then assigned randomly to one of three drug groups: varenicline tartrate, bupropion hydrochloride sustained-release, or placebo, all in conjunction with standard smoking cessation counseling, as detailed in the main outcome manuscript (Cinciripini et al., 2013). Participants were asked to start taking the medication the day after their baseline visit. They took the medications for 12 weeks with the following dosage scheme: the varenicline group took 0.5 mg a day for 3 days, 0.5 mg twice a day for 4 days, and 1 mg twice a day thereafter; the bupropion group took 150 mg a day for 3 days and 150 mg twice a day thereafter. The titration and full strength dose was based on the pivotal clinical trials that evaluated the treatment effects of varenicline on smoking abstinence (Gonzales et al., 2006; Jorenby et al., 2006). The patients were asked to bring any unused medications during their in-person visits, when their use of medication was collected using self-report. About 90% patients adhered to the treatment, and the adherence rates did not differ among the three medication groups.
On their scheduled quit date, the participants were asked to quit smoking. On average, the interval between the beginning of drug treatment and scheduled quit date was 13.2 days (SD = 1.6). One day before the scheduled quit date, participants received telephone-based counseling to prepare them for quitting. The first laboratory session occurred 1 day before participants started taking medication. The second laboratory session occurred 1 day after the scheduled quit date (i.e., after 2 weeks of medication). The third laboratory session occurred 4 weeks after the quit date (i.e., after 6 weeks of medication). Participants also had follow-up visits to our clinic 12 and 24 weeks after their scheduled quit date. In total, there were 50 and 77 drop-outs for 12- and 24-week post-quit visits, respectively, and the drop-out rates did not differ among three treatment groups for both time points (p > 0.6).
2.3. Abstinence assessment
For the post-quit visits, each participant's abstinence status was assessed via self-report and biochemical verification with an expired CO < 10 ppm (Gonzales et al., 2006; Jorenby et al., 2006) and salivary cotinine levels < 15 ng/mL (Benowitz et al., 2002), criteria previously identified for verifying smoking abstinence (Benowitz et al., 2002). For the second laboratory session (i.e., 1 day after the quit date), abstinence status was established by self-reported nonsmoking in the past 24 hours and expired CO < 10 ppm. For the other post-quit time points, abstinence status was defined as self-reported nonsmoking in the past 7 days (i.e., 7-day point prevalence) and expired CO < 10 ppm. Using the intention-to-treat definition, those patients lost to follow-up were considered as smoking.
2.4. Questionnaires
The Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991) was used to measure the severity of nicotine dependence. Depressive symptoms were measured using the Center for Epidemiologic Studies Depression Scale (CES-D; Radloff, 1977). The Positive and Negative Affect Schedule (PANAS; Watson et al., 1988) was used to measure positive affect (the positive affect subscale) and negative affect (the negative affect subscale). The FTND questionnaire was administered at the baseline screening visit, and the CES-D and PANAS were administered at all three laboratory sessions.
2.5. Laboratory assessment and data acquisition
During each laboratory session, participants viewed a different set of pictures that consisted of neutral and emotionally evocative pictures from the International Affective Picture System (IAPS; Lang et al., 2005) and cigarette-related pictures from our previous studies (Carter et al., 2006; Cinciripini et al., 2006; Versace et al., 2010; Robinson et al., 2015). We created three picture sets, each with 96 pictures. There were 24 pictures in each of four categories, including neutral, pleasant, unpleasant, and cigarette-related, matched on luminosity using the software Irfanview (v4, www.irfanview.com) and on complexity using subjective evaluation of image contents, such as the number and the size of objects/people. Additionally, the pleasant and unpleasant pictures were matched on their normative arousal levels (Lang et al., 2005). Detailed information regarding the normative ratings and the IAPS numbers of these pictures was provided in our previous report (Versace et al., 2012), and information regarding the cigarette-related images was provided in a different report of the present study (Robinson et al., 2013). In each session, the picture set was presented twice in separate blocks, with each having four sub-blocks of 24 pictures, resulting in 192 picture-viewing trials. The interval between the sub-blocks was 30 s. The pictures were presented on a 50-inch plasma TV screen with a refresh rate of 60 Hz approximately 1.5 meters away from the participants, forming a horizontal viewing angle of ∼24°. Each picture was on the screen for 4 s, and the inter-picture interval was random, ranging from 3 to 5 s.
During the picture presentation, we recorded electroencephalography (EEG; which will be reported elsewhere), orbicularis EMG, corrugator EMG, heart rate, and skin conductance. Because the focus of this study was on NER during smoking cessation and because of equipment issues related to skin conductance measurement (i.e., very weak signals and no valence-related changes), we report only the measures of orbicularis (i.e., startle response) and corrugator EMG as established indices of NER (Bradley et al., 2001). Startle probes were delivered during 12 of the 48 picture trials in each category by using a 50-ms 100-dB(A) acoustic startle noise with instantaneous rise time. Another 24 startle probes were delivered during the inter-picture intervals to increase the unpredictability of startle probe occurrence. The startle probes were delivered binaurally between 2.5 and 3.5 s after picture onset through insert earphones (model 3A, 10Ω E-A-R Auditory Systems, Indianapolis, IN, USA). Another six startle probes were delivered to habituate participants to the noise at the beginning of the picture presentation, and these habituation and inter-picture interval trials were excluded from further data reduction and analysis.
All experimental stimuli, including pictures and startle probes, were presented using the E-Prime software (v1.2; Psychology Software Tools, Sharpsburg, PA, USA). Recording electrodes (Ag-AgCl) were placed on the right orbicularis oculi and corrugator supercilii muscles. EMG activity was recorded and amplified with an EMG100C module connected to an MP150WSW amplifier at a sampling rate of 1000 Hz (BIOPAC Systems, Goleta, CA, USA). The EMG signals were filtered (28-500 Hz), rectified and smoothed by a five-sample boxcar filter, and displayed via AcqKnowledge III data acquisition software (v3.8.2; BIOPAC Systems).
2.6. Data reduction and scoring
2.6.1. Startle response
Startle magnitude was calculated as the difference between the maximum EMG activity within 20 to 120 ms after startle probe onset and the mean baseline activity within a 25-ms time window before probe onset. Startle trials were defined as unscorable if they contained excessive noise or no apparent response. If the data from a laboratory session had more than 33% unscorable startle responses (24 of 72 startle trials), the data were excluded from further analysis. Among all included data, the mean number of acceptable trials was 66.7 (SD = 6.1) per laboratory session. A more detailed description about the scoring of the startle responses can be found in our previous report (Cui et al., 2012). To minimize the inter-individual differences in startle response (Blumenthal et al., 2005), we computed standardized startle responses within each subject to generate T-scores (M = 50, SD = 10) using all the picture trials, and all trials that exceeded 3 SDs were considered as outliers and were excluded (328 trials, 1.2%).
Although not part of our hypotheses, previous research has indicated that startle response can reduce over time, a phenomenon termed habituation, and that such habituation varies as a function of picture valence (Bradley et al., 1993; Rehme et al., 2009). Thus, as exploratory analyses, we examined whether startle habituation could be modulated by medication and/or picture valence. To quantify startle habituation within a session, we calculated the mean startle difference between the first and eighth block for each participant, and found that there was startle habituation within the session (t = 12.43, p < 0.0001). Using this difference score as the dependent variable, we ran mixed models using valence, medication, and their interaction term as independent variables for the post-quit time points. However, we found no significant effects (all ps>0.05) for these terms, which suggest that picture valence and medication treatment did not affect startle habituation.
2.6.2. Corrugator EMG
The recorded BIOPAC files were first imported into MATLAB (v7.10.0.499; The MathWorks, Inc., Natick, MA) by using a modified script (Shen, 2011). To score the data, a 6-s segment, including 2 s before and 4 s after picture onset, was created for each trial. Next, 12 means were calculated for each 0.5-s epoch in the 6-s segment. Because of the impact of the startle probe on the EMG activity, we excluded all of the segments that contained startle probes (31.2%). Picture-related EMG reactivity was defined as the difference of the mean activity of 3 to 4 s after picture onset, when the EMG activity was stable, and the baseline activity, which was defined as the mean value of the first four means, which comprised the 2-s window prior to picture onset. To remove artifacts, we excluded outliers at the trial level if a score for a trial was greater than 3 SDs from the mean for each participant. A total of 1381 trials (2%) were identified as outliers and were excluded. Finally, we calculated the mean activity for each picture category for each participant.
2.6.3. Standardized d-score
To create the index for the NER for startle and corrugator activity, we calculated unpleasant-related d-scores using the formula for computing Cohen's d (Cohen, 1988): d-score = (M1 – M2)/SDp, where M1 and M2 are the startle or corrugator EMG mean values for unpleasant and neutral conditions and SDp is the pooled standard deviations of the unpleasant and neutral conditions. This standardized approach has been used in previous studies (Robinson et al., 2011; Cui et al., 2012) and is suggested to better reflect individual differences in the unpleasant-related responses than simply taking a difference score (Robinson et al., 2011).
2.7. Statistical analysis
The statistical analyses were conducted using SAS (v9.4; SAS Institute Inc., Cary, NC, USA). Participants' baseline characteristics were summarized using SAS PROC SQL. Before we tested our hypotheses, we examined the main effects of valence on startle and corrugator activities as a manipulation check to confirm that unpleasant pictures would result in greater startle and corrugator activities as previously reported (Bradley et al., 2001). In two separate models, the dependent variables were startle response amplitude (T-score) and corrugator EMG mean value in mV and the independent variable was Valence (neutral, pleasant, unpleasant, and cigarette-related). Because the measure of interest was NER using these two psychophysiological methods, to estimate their effect sizes, we calculated the Cohen's dz score for the comparisons between unpleasant and neutral conditions, using the formula: Cohen's where t was the t-statistic values for these contrasts and n was the sample size (Rosenthal, 1991). The dz values of 0.2, 0.5, and 0.8 are considered as small, medium, and large effects (Cohen, 1988).
To test our first two hypotheses, we used a mixed models approach (SAS PROC MIXED), with subject modeled as a random effect. For our first hypothesis (i.e., treatment with bupropion and varenicline would result in lower levels of NER than placebo), we tested the interaction of Drug (bupropion, varenicline, or placebo) and Valence (neutral, pleasant, unpleasant, and cigarette-related) on startle response and corrugator EMG activity. For our second hypothesis (i.e., abstinence would be associated with lower levels of NER than nonabstinence), we tested Abstinence × Valence interaction on startle response and corrugator EMG activity. All these mixed models were run separately for each post-quit session for each NER measure using FTND scores and their baseline NER values as covariates to account for possible pre-existing differences in nicotine dependence and negative affect reactivity among subjects.
To estimate the effect sizes of mixed models used in testing Hypotheses 1 and 2, we calculated the partial eta squared using the formula: , where F was F-statistics values, and dfeffect and dferror were degree of freedoms for the effect and error terms (Lakens, 2013). The values of 0.01, 0.06, and 0.14 are considered as small, medium, and large effects (Cohen, 1988).
For our third hypothesis (i.e., smokers with lower NER at the baseline would be more likely to be abstinent), we used a logistic regression approach with PROC LOGISTIC, in which the predictor variable was unpleasant-related d-scores for the startle response or corrugator EMG activity and the response variable was smoking abstinence status (7-day point prevalence, CO-verified) at 4 weeks, 12 weeks, and 24 weeks after the quit date (i.e., 6, 14, and 26 weeks after starting the drug). The pharmacological treatment and the FTND scores were included as covariates in these logistic models to account for drug and nicotine dependence severity effects on abstinence. The odds ratio (OR) values were considered to index effect size, and the OR values of 1.68, 3.47, and 6.71 (or reversed, 0.60, 0.29, and 0.15) are considered to be small, medium, and large effects (Chen et al., 2010).
We used a Type I error rate of 0.05 and adjusted for multiple comparisons using the Tukey-Kramer method as implemented in the SAS software package, where applicable.
3. Results
3.1. Sample characteristics
The baseline demographics and cigarette-related characteristics of the 206 participants are summarized in Table 1. Overall, this sample consisted of middle-aged smokers (mean age = 44.9, SD = 10.5) who were moderately dependent on nicotine (mean FTND = 4.5, SD = 2.1). Most were European- (58.7%) or African- (25.7%) American, and the majority were men (62.1%). No differences were noted between the three drug groups.
Table 1. Baseline sample characteristics.
| Measure | PLA (n = 70) | BUP (n = 64) | VAR (n = 72) | Total (n = 206) |
|---|---|---|---|---|
| Age, M (SD) | 45.3 (11.3) | 45.7 (9.3) | 43.9 (10.7) | 44.9 (10.5) |
| Women, N (%) | 27 (38.6) | 23 (35.9) | 28 (38.9) | 78 (37.9) |
| Race/ethnicity, N (%) | ||||
| African American | 18 (25.7) | 17 (26.6) | 18 (25.0) | 53 (25.7) |
| White, non-Hispanic | 44 (62.9) | 39 (60.9) | 38 (52.8) | 121 (58.7) |
| Hispanic | 6 (8.6) | 4 (6.3) | 8 (11.1) | 18 (8.7) |
| Other | 2 (2.9) | 4 (6.3) | 8 (11.1) | 14 (6.8) |
| FTND score, M (SD) | 4.5 (2.1) | 4.5 (1.9) | 4.5 (2.3) | 4.5 (2.1) |
| Years of smoking, M (SD) | 24.6 (12.2) | 26.1 (10.6) | 23.5 (11.7) | 24.7 (11.6) |
| Cigarettes per day, M (SD) | 18.8 (8.8) | 18.5 (7.7) | 18.9 (8.6) | 18.7 (8.3) |
| Carbon monoxide, M (SD) | 25.3 (12.5) | 26.7 (15.5) | 25.3 (14.9) | 25.7 (14.3) |
| CES-D score, M (SD) | 8.4 (7.5) | 7.0 (5.9) | 6.9 (6.4) | 7.4 (6.7) |
FTND, Fagerström Test for Nicotine Dependence; CES-D, Center for Epidemiologic Studies' Depression Scale; PLA, placebo; BUP, Bupropion Hydrochloride Sustained-release; VAR, Varenicline Tartrate
3.2. Picture valence manipulation check
The overall effects of picture valence on startle response (F(3, 513) = 39.96, p < 0.0001, Cohen's dz = 0.40) and on corrugator EMG activity (F(3, 615) = 82.49, p < 0.0001, Cohen's dz = 0.86) were significant. As shown in Fig. 1A, unpleasant pictures were significantly associated with an increased startle response compared with neutral pictures (t(513) = 5.79, p < 0.0001). Both pleasant (t(513) = -3.92, p < 0.001) and cigarette-related (t(513) = -3.42, p < 0.005) pictures were significantly related to a reduction in startle magnitude compared with neutral pictures. No significant differences were noted in startle magnitude between the pleasant and cigarette-related pictures (t(513) = 0.50, p > 0.9). Similarly, unpleasant pictures were also associated with a significantly higher corrugator EMG activity in comparison to the other three picture categories (all ts > 11, ps < 0.0001). EMG corrugator activity associated with both pleasant (t(615) = 1.91, p > 0.2) and cigarette-related (t(615) = 1.24, p > 0.6) pictures did not differ from neutral pictures. However, cigarette-related pictures resulted in increased corrugator EMG activity in comparison to pleasant pictures (t(615) = 3.15, p < 0.01).
Fig. 1.
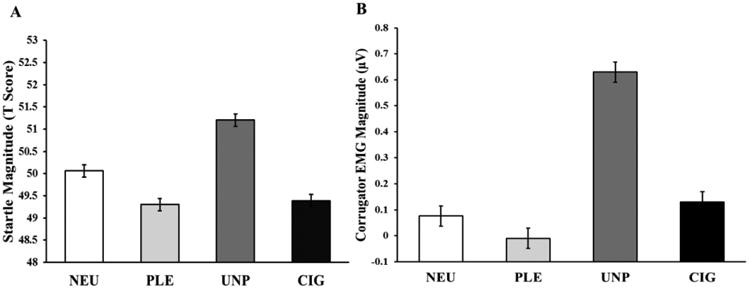
Modulation of startle response (A) and corrugator electromyographic (EMG) activity (B) by picture category, expressed as estimated means from the mixed models. Unpleasant pictures resulted in greater magnitude in both startle response and corrugator EMG activity than did other picture categories. NEU, neutral; PLE, pleasant; UNP, unpleasant; CIG, cigarette-related.
3.3. Effects of drug on startle response and corrugator EMG
To examine whether bupropion and varenicline would reduce NER more than placebo (Hypothesis 1), we tested the Drug × Valence interaction on startle response and corrugator EMG activity after 2 and 6 weeks of drug while controlling for smoking abstinence status and their baseline (prior to drug onset) startle and corrugator values. As expected, no significant interactions were observed for either measure at baseline (both ps > 0.7). When baseline values were controlled, there were no significant interactions for both measures after 2 weeks of drug (both ps > 0.1). However, 6 weeks after drug onset, a significant Drug × Valence interaction (F(6, 236) = 3.12, p < 0.01, was observed for the startle response but not for corrugator EMG activity (F(6, 383) = 0.58, p > 0.7). Post-hoc analyses revealed that varenicline attenuated the expected potentiation of the startle response to unpleasant stimuli, whereas placebo and bupropion did not (F(2, 306) = 6.83, p < 0.005, Fig. 2). Importantly, this varenicline-related modulation was specific to the unpleasant pictures as the three groups did not differ in their startle magnitude in response to other types of pictures (all ps > 0.1), particularly including cigarette-related condition, which suggests that bupropion and varenicline did not modulate smoking cue-related startle response.
Fig. 2.
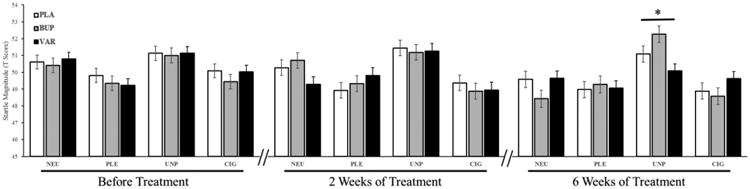
Estimated values of the startle response from the mixed models as a function of drug and valence before treatment and after 2 and 6 weeks of treatment. Startle responses did not differ between medication groups before treatment and after 2 weeks of treatment, but after 6 weeks, VAR treatment resulted in significant reduction in UNP-related startle response compared to the PLA and BUP conditions. * = p < 0.05. PLA, placebo; BUP, bupropion; VAR, varenicline; NEU, neutral; PLE, pleasant; UNP, unpleasant; CIG, cigarette-related.
Consistent with this observation, we found that the main effect of Valence was nonsignificant in the varenicline group (F(3, 111) = 1.08, p > 0.3), but significant Valence effects were noted in the placebo (F(3, 93) = 4.91, p < 0.005, and bupropion (F(3, 102) = 14.97, p < 0.0001, groups. We noted that the estimated values (Fig. 2) appeared to show that 6-week bupropion treatment resulted in an increase in its unpleasant-related startle response relative to placebo treatment. We tested this possibility and found that the interaction of Valence and Group (i.e., placebo and bupropion) was nonsignificant (F(3,195) = 1.47, p > 0.2, suggesting that bupropion and placebo did not differ in their unpleasant-related startle response after 6 weeks of treatment. Although there was a trend of startle potentiation induced by bupropion treatment with an effect size of 0.3 (t = 1.32, p > 0.1), this nonsignificant result may be due to the inherent variation of startle methodology, even after T-score standardization.
3.4. Effects of abstinence on startle response and corrugator EMG
To examine whether abstinence would reduce NER compared with non-abstinence (Hypothesis 2), we tested the Abstinence × Valence interaction on startle response and corrugator EMG activity at two time points, 2 or 6 weeks of drug treatment (1 day and 4 weeks after the quit date, respectively), while controlling for drug and participants' baseline startle and corrugator values. However, none of these interactions was significant (all ps > 0.3).
To examine whether smokers treated with different medications would show different startle responses and corrugator activities as function of their abstinence status, we examined the Drug × Abstinence × Valence three-way interactions on startle response and corrugator EMG activity at both post-quit time points (1 day and 4 weeks after quitting) while controlling for their baseline values. Examining this interaction would allow us to examine if smokers treated with different medications would show different startle responses and corrugator activities as function of their abstinence status. However, no significant interaction was observed (all ps > 0.4).
3.5. Prediction of abstinence using baseline NER
For Hypothesis 3, we found that unpleasant-related corrugator EMG activity d-scores at baseline significantly predicted abstinence status 4 weeks after the quit date (χ2 = 4.28, p < 0.05), such that participants with higher corrugator EMG activity induced by unpleasant pictures had a lower likelihood of being abstinent than those with lower corrugator EMG activity (OR estimate = 0.351, 95% CI: 0.130, 0.947). We extended our analyses to examine whether the pre-quit corrugator EMG activity predicted longer term abstinence status, and we found that higher unpleasant-related corrugator EMG activity d-scores predicted a lower probability of being abstinent 12 weeks after the scheduled quit date (OR estimate = 0.395, 95% CI: 0.171, 0.912) but not 24 weeks after the quit date (OR estimate = 0.734, 95% CI: 0.310, 1.739). For the drug and FTND covariates in these models, we found that FTND scores negatively predicted abstinence status at 4 weeks, but not at 12 or 24 weeks. However, neither baseline unpleasant-related startle response d-score nor self-reported negative affect predicted smoking abstinence status at any time point. The results are listed in Table 2.
Table 2.
Prediction of smoking abstinence status at 4, 12, and 24 weeks post-quit using baseline self-reported negative affect, unpleasant-related startle response and corrugator EMG activity d-scores.
| Measure | Time Point | ||
|---|---|---|---|
|
| |||
| 4 weeks | 12 weeks | 24 weeks | |
| Self-reported negative affect | 1.011 (0.942, 1.084) | 0.981 (0.930, 1.034) | 0.983 (0.928, 1.040) |
| Startle response | 0.945 (0.301, 2.968) | 1.496 (0.643, 3.482) | 1.625 (0.668, 3.949) |
| Corrugator EMG | 0.351* (0.130, 0.947) | 0.395* (0.171, 0.912) | 0.734 (0.310, 1.739) |
Values are estimated odds ratios, and their 95% CI are shown in the parentheses.
= p < 0.05
4. Discussion
As the first-line smoking cessation medications (Fiore et al., 2008), bupropion and varenicline have been suggested to exert their effects, in part, by reducing negative affect. However, it has been largely unclear regarding their psychophysiological effects on negative affect-related processes during the smoking cessation course. To address this issue, this study examined the effects of these two medications and smoking abstinence on startle response and corrugator EMG activity in smokers who were undergoing smoking cessation treatment. We found that after 6 weeks of treatment with varenicline, startle responses to unpleasant stimuli were reduced. In addition, we also showed that pre-quit unpleasant-related corrugator EMG activity was inversely related to smoking abstinence status at 4 and 12 weeks after the quit date, independent of medication treatment.
The association of varenicline with a reduction in the expected potentiation of the startle response to unpleasant stimuli is consistent with previous research. Several functional neuroimaging studies have consistently found that varenicline treatment reduced the BOLD activity of the amygdala in the resting state (Franklin et al., 2011) and during a face emotion identification task (Loughead et al., 2011), and amygdala's functional connectivity to insula (Sutherland et al., 2013). Notably, amygdala is particularly important as a brain region for the manifestation of the affect-modulated startle response (Lang et al., 1990; Patrick et al., 1993). Although it is speculative, the reduction of amygdalar activity induced by varenicline treatment as identified in these fMRI studies may account for varenicline-induced reduction in NER as shown by decreased unpleasant-related startle response in the current report. More specifically, we speculate that varenicline treatment may have ameliorated the reaction toward interoceptive cues for negative affect through regulating amygdala, as previous research has indicated a critical role of the amygdala in subconsciously processing fear and anger (Scott et al., 1997; Carlsson et al., 2004). However, varenicline has also been found to modulate neural activity of several other brain regions, such as prefrontal cortex and anterior cingulate cortex (Franklin et al., 2011; Loughead et al., 2011; Sutherland et al., 2013), both of which are involved in startle response (Pissiota et al., 2003; Sánchez-Navarro et al., 2014). Thus, it is equally plausible that varenicline's effects on reducing negative-related startle response may be also related to its regulation of these brain regions in addition to amygdala.
Further, it is worth noting that 6 weeks, but not 2 weeks, of varenicline treatment modulated affect-related startle response in our participants. This time-dependent effect of varenicline treatment is consistent with the findings of a previous study (Hong et al., 2011), which showed that varenicline reduced startle reactivity in patients with schizophrenia at week 8, but not at week 2, a timeline similar to that of our study. These findings suggest that it may take longer than 2 weeks for varenicline to produce neuroadaptations in the underlying affective circuitry (e.g. amygdala) that mediate startle-related NER, although the effects of varenicline on BOLD-measured amygdala activation have been observed with 2 to 3 weeks of treatment in several neuroimaging studies (Franklin et al., 2011; Loughead et al., 2011; Sutherland et al., 2013).
However, our finding of varenicline-related startle suppression in the negative valence condition is in contrast to a previous study (Mocking et al., 2013) that showed that varenicline potentiated startle response toward negative pictures. Two major reasons may account for this difference. First, our participants received longer varenicline treatment (6 weeks vs. 1 week) with higher dosage (1 mg twice a day vs. once a day) than their participants. Varenicline's effects have been reported to be dose-dependent (Faessel et al., 2006a; Nakamura et al., 2007). Second, their population (i.e., non-smoking young adults) is distinct from ours (i.e., mid-age smokers). Chronic exposure to nicotine in our older smokers may have resulted in elevated levels of the nAChRs and desensitization of these receptors (Fenster et al., 1999; Perry et al., 1999; Picciotto et al., 2008). The presumed differential receptor levels and desensitization statuses in these two populations may render them to have different tolerances to the partial nicotinic agonist varenicline (Faessel et al., 2006b). The presumed differential tolerability to varenicline, together with the different treatment regimens, may have led to the opposite findings in the current and the previous study (Mocking et al., 2013). Supportive of the possibility of distinct responsiveness to varenicline between these two populations, our shorter-term (i.e., 2 weeks) treatment of varenicline did not modulate startle response in our smokers, whereas startle modulation was observed with 1-week varenicline treatment in their nonsmokers.
We did not find any evidence that bupropion modulated NER-related psychophysiology at either 2 or 6 weeks. Although no similar studies have been conducted in smokers, this null finding is consistent with a study in depressed individuals. Using the same startle methodology, Dichter and colleagues (2004) found that 12 weeks' treatment with bupropion did not influence affective responses in depressed individuals. These null findings may indicate that the affect-related startle paradigm may be insensitive to capture bupropion-induced neurophysiological effects. As mentioned previously, the amygdala is particularly important for the manifestation of affect-modulated response (Lang et al., 1990; Patrick et al., 1993). However, previous studies provide little evidence that bupropion modulates amygdalar activity. Culbertson and colleagues (2011) found that, compared with placebo treatment, 8 weeks of treatment with bupropion reduced activation in the left ventral striatum and several other brain regions when nicotine-dependent smokers were instructed to actively resist smoking craving. Brody and colleagues (2004) found that patients treated with bupropion had reduced cigarette cue-induced activation in the anterior cingulate cortex. However, in a functional neuroimaging study of 10 patients with major depressive disorder, the authors found that patients treated with 8 weeks of bupropion had reduced BOLD signals in the right amygdala and several other brain regions (e.g., orbital frontal cortex, anterior cingulate cortex) associated with processing negative stimuli (Robertson et al., 2007). Future studies need to clarify the discrepancy regarding the effect of bupropion on amygdala and explore the relationships between startle response and amygdalar regulation with the treatment of bupropion.
We have previously discussed our results individually for each medication, and we want to synthesize the underlying reasons for such differential effects between these two medications. Most of previous research has found very similar results between varenicline and bupropion in various aspects. For example, both medications reduce negative affect to a similar extent in large-scale clinical trials (Gonzales et al., 2006; Jorenby et al., 2006). Both medications can reduce cue-related brain activation in several fMRI studies (Culbertson et al., 2011; Franklin et al., 2011). Despite these similarities, their difference in modulating unpleasant-startle response may be related to the different pharmacological natures of these two medications. Although both show antagonism against nicotinic receptors, varenicline is also a partial nicotinic agonist where as bupropion is a norepinephrine and dopamine reuptake inhibitor (Damaj et al., 2004; Stahl et al., 2004; Mihalak et al., 2006). Possibly reflecting their pharmacological differences, the above-mentioned fMRI indeed identified differential (e.g., amygdala by varenicline but not by bupropion) although somewhat overlapping activation patterns (e.g., ventral striatum, medial orbitofrontal cortex) induced by these two medications (Culbertson et al., 2011; Franklin et al., 2011).
Our secondary analyses of the medications' effects on startle-related cue reactivity revealed that neither varenicline nor bupropion treatment modulated smokers' startle response to smoking-related cues. Previous studies have found that smoking-related cues reduce startle response (Geier et al., 2000; Cinciripini et al., 2006; Dempsey et al., 2007; Rehme et al., 2009; Cui et al., 2012). It is important to note that these laboratory methodologies measure acutely-induced cue reactivity, which is presumed to be very stable, as it can be measured years after cessation (Tiffany and Conklin, 2000). Thus, the presumed stability of cue reactivity may make it hard to be modulated by cessation medications. However, this explanation is inconsistent with other findings showing that smoking cue-elicited brain activation is diminished by treatment with varenicline (Franklin et al., 2011) and bupropion (Brody et al., 2004). Thus, our null finding using the startle methodology, combined with these positive findings using functional neuroimaging techniques, suggests that cessation medications may modulate smoking cue-elicited neural reactivity, which may be hard to be captured by peripheral physiological measures (e.g., startle response).
We found that smokers who showed higher reactivity to unpleasant stimuli before they quit smoking were more likely to be smoking in the short term after cessation treatment than those who had less unpleasant-related emotional reactivity. Importantly, this association was not modulated by pharmacological intervention, suggesting that baseline NER captures an endophenotype predictive of smoking cessation success. We speculate that smokers who react more to unpleasant stimuli at baseline may be more likely to experience negative affect in their daily lives than those who react less and that the increased levels of negative affect render these smokers more vulnerable to smoking relapse. This speculation is supported by the findings of previous studies in which pre-cessation levels of negative affect were positively associated with the likelihood of smoking treatment failure (Ginsberg et al., 1995; Cinciripini et al., 2003). This positive relationship supports the theoretical models of negative affect as a central withdrawal symptom that drives smoking relapse (Baker et al., 2004). These convergent findings both in underlying biological processes and subjectively-experienced negative affect on smoking relapse highlight the validity in studying preconscious NER to provide mechanistic insights into nicotine dependence. Additionally, the predictive value of corrugator EMG-related NER over pharmacologic intervention on smoking cessation is in line with previous research showing that a neural signal biomarker identified with EEG predicted smoking cessation independent of cessation medications (Versace et al., 2012).
Our study had several limitations. Our sample was a middle-aged treatment-seeking group of moderately to heavily nicotine-dependent smokers in their forties. Therefore, attempts to generalize our findings to other populations of smokers (e.g., less dependent smokers) would need to take these factors into consideration. In terms of negative stimuli used in the picture presentation, we did not balance fear and disgust among our picture sets, and thus we were unable to test whether medications would result in differential startle modulation in these two subcategories of negative stimuli, which have been shown to have different startle potentiation effects (Yartz and Hawk, 2002). Another limitation was that we relied on patients' self-reporting on medication taking and did not have biochemical validation to monitor treatment adherence in our patients. In addition, although we identified unpleasant-related psychophysiological response as a predictor of abstinence status, we found no indication that baseline self-reported negative affect predicted abstinence, in contrast to previous findings (Ginsberg et al., 1995; Cinciripini et al., 2003). Although these may highlight the sensitivity of psychophysiological measures compared with self-reported assessments, future studies need to address such discrepancy.
To conclude, our study represents an initial attempt to use psychophysiological approaches to identifying the shorter-term and longer-term effects of bupropion and varenicline on emotional reactivity, extending previous research in cessation medications' effects on cognitive processes (Rhodes et al., 2012; Austin et al., 2014) and cue reactivity (Brody et al., 2004; Culbertson et al., 2011). Research in various domains (e.g., affect and cognition) will help elucidate the therapeutic mechanisms of smoking cessation medications, allowing the development of more effective smoking cessation treatments.
Highlights.
Six weeks of varenicline treatment reduced smokers' responses to unpleasant stimuli.
Baseline negative emotional reactivity predicted long-term abstinence status.
Acknowledgments
The parent clinical study was supported by a grant (DA017073) awarded to Dr. Cinciripini from the National Institute on Drug Abuse and was supported partly by Cancer Center Support Grant CA016672 from the National Cancer Institute through The University of Texas MD Anderson Cancer Center. We also thank the handling editor and anonymous reviewers for their constructive feedback.
Dr. Paul Cinciripini served on the scientific advisory board of Pfizer, gave educational talks sponsored by Pfizer (2006–2008), and has received grant support from Pfizer.
Varenicline was provided to the parent clinical study by Pfizer. However, Pfizer had no roles in designing or conducting the parent clinical trial or the current study. The authors are solely responsible for the collection, management, analysis, and interpretation of the data, and the preparation, submission, and revision of the manuscript.
Footnotes
5. Conflict of interest: All the other authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth. American Psychiatric Association; Arlington, VA: 2013. [Google Scholar]
- Aubin HJ, Luquiens A, Berlin I. Pharmacotherapy for smoking cessation: Pharmacological principles and clinical practice. Br J Clin Pharmacol. 2014;77(2):324–336. doi: 10.1111/bcp.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin AJ, Duka T, Rusted J, Jackson A. Effect of varenicline on aspects of inhibitory control in smokers. Psychopharmacology (Berl) 2014;231(18):3771–3785. doi: 10.1007/s00213-014-3512-7. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychol Rev. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Ahijevych K, Hall S, Hansson A, Henningfield J, Hurt RD, Jacob PI, Jarvis MJ, LeHouezec J, Lichtenstein E, Tsoh JY, Velicer W. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42(1):1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion. 2001;1(3):276–298. doi: 10.1037/1528-3542.1.3.276. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ, Cuthbert BN. Emotion, novelty, and the startle reflex: Habituation in humans. Behav Neurosci. 1993;107:970–980. doi: 10.1037//0735-7044.107.6.970. [DOI] [PubMed] [Google Scholar]
- Brioni JD, O'Neill AB, Kim DJB, Decker MW. Nicotine receptor agonists exhibit anxiolytic-like effects on the elevated plus-maze test. Eur J Pharmacol. 1993;238:1–8. doi: 10.1016/0014-2999(93)90498-7. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Lee G, Smith E, Sadeghi M, Saxena S, Jarvik ME, London ED. Attenuation of cue-induced cigarette craving and anterior cingulate cortex activation in bupropion-treated smokers: a preliminary study. Psychiatry Res. 2004;130(3):269–281. doi: 10.1016/j.pscychresns.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylsma LM, Morris BH, Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clin Psychol Rev. 2008;28(4):676–691. doi: 10.1016/j.cpr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;4:CD006103. doi: 10.1002/14651858.CD006103.pub2. [DOI] [PubMed] [Google Scholar]
- Cao W, Burkholder T, Wilkins L, Collins AC. A genetic comparison of the behavioral actions of ethanol and nicotine in the mirrored chamber. Pharmacol Biochem Behav. 1993;45:803–809. doi: 10.1016/0091-3057(93)90124-c. [DOI] [PubMed] [Google Scholar]
- Carlsson K, Petersson KM, Lundqvist D, Karlsson A, Ingvar M, Ohman A. Fear and the amygdala: Manipulation of awareness generates differential cerebral responses to phobic and fear-relevant (but nonfeared) stimuli. Emotion. 2004;4(4):340–353. doi: 10.1037/1528-3542.4.4.340. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Blough BE, Mascarella SW, Navarro HA, Lukas RJ, Damaj MI. Bupropion and bupropion analogs as treatments for CNS disorders. Advances in pharmacology (San Diego, Calif) 2014;69:177–216. doi: 10.1016/B978-0-12-420118-7.00005-6. [DOI] [PubMed] [Google Scholar]
- Carter BL, Robinson JD, Lam CY, Wetter DW, Day SX, Tsan JY, Cinciripini PM. A psychometric evaluation of cigarette stimuli used in a cue reactivity study. Nicotine Tob Res. 2006;8(3):361–369. doi: 10.1080/14622200600670215. [DOI] [PubMed] [Google Scholar]
- Chen H, Cohen P, Chen S. How Big is a Big Odds Ratio?: Interpreting the Magnitudes of Odds Ratios in Epidemiological Studies. Communications in Statistics - Simulation and Computation. 2010;39(4):860–864. doi: 10.1080/03610911003650383. [DOI] [Google Scholar]
- Cinciripini PM, Robinson JD, Carter BL, Lam CY, Wu X, Moor CAde, Baile WS, Wetter DW. The effects of smoking deprivation and nicotine administration on emotional reactivity. Nicotine Tob Res. 2006;8(3):379–392. doi: 10.1080/14622200600670272. [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, Robinson JD, Karam-Hage M, Minnix JA, Lam CY, Versace F, Brown VL, Engelmann JM, Wetter DW. Effects of varenicline and bupropion sustained-release use plus intensive smoking cessation counseling on prolonged abstinence from smoking and on depression, negative affect, and other symptoms of nicotine withdrawal. JAMA Psychiatry. 2013;70(5):522–533. doi: 10.1001/jamapsychiatry.2013.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinciripini PM, Wetter DW, Fouladi RT, Blalock JA, Carter BL, Cinciripini LG, Baile WS. The effects of depressed mood on smoking cessation: Mediation by post-cessation self-efficacy. J Consult Clin Psychol. 2003;71(2):292–301. doi: 10.1037/0022-006X.71.2.292. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd. Lawrence Earlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Costall B, Kelly ME, Naylor RJ, Onaivi ES. The actions of nicotine and cocaine in a mouse model of anxiety. Pharmacol Biochem Behav. 1989;33:197–203. doi: 10.1016/0091-3057(89)90450-4. [DOI] [PubMed] [Google Scholar]
- Cui Y, Robinson JD, Versace F, Lam CY, Minnix JA, Karam-Hage M, Dani JA, Kosten TR, Wetter DW, Brown VL, Cinciripini PM. Differential cigarette-related startle cue reactivity among light, moderate, and heavy smokers. Addict Behav. 2012;37(8):885–889. doi: 10.1016/j.addbeh.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson CS, Bramen J, Cohen MS, London ED, Olmstead RE, Gan JJ, Costello MR, Shulenberger S, Mandelkern MA, Brody AL. Effect of bupropion treatment on brain activation induced by cigarette-related cues in smokers. Arch Gen Psychiatry. 2011;68(5):505–515. doi: 10.1001/archgenpsychiatry.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, Carroll FI, Eaton JB, Navarro HA, Blough BE, Mirza S, Lukas RJ, Martin BR. Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors. Mol Pharmacol. 2004;66(3):675–682. doi: 10.1124/mol.104.001313. [DOI] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: Lesion and stimulation studies. J Neurosci. 1982;2(6):791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JP, Cohen LM, Hobson VL, Randall PK. Appetitive nature of drug cues re-confirmed with physiological measures and the potential role of stage of change. Psychopharmacology (Berl) 2007;194(2):253–260. doi: 10.1007/s00213-007-0839-3. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Tomarken AJ, Shelton RC, Sutton SK. Early- and late-onset startle modulation in unipolar depression. Psychophysiology. 2004;41(3):433–440. doi: 10.1111/j.1469-8986.00162.x. [DOI] [PubMed] [Google Scholar]
- Faessel HM, Gibbs MA, Clark DJ, Rohrbacher K, Stolar M, Burstein AH. Multiple-dose pharmacokinetics of the selective nicotinic receptor partial agonist, varenicline, in healthy smokers. Journal of Clinical Pharmacology. 2006a;46(12):1439–1448. doi: 10.1177/0091270006292624. [DOI] [PubMed] [Google Scholar]
- Faessel HM, Smith BJ, Gibbs MA, Gobey JS, Clark DJ, Burstein AH. Single-dose pharmacokinetics of varenicline, a selective nicotinic receptor partial agonist, in healthy smokers and nonsmokers. J Clin Pharmacol. 2006b;46(9):991–998. doi: 10.1177/0091270006290669. [DOI] [PubMed] [Google Scholar]
- Fenster CP, Whitworth TL, Sheffield EB, Quick MW, Lester RA. Upregulation of surface alpha4beta2 nicotinic receptors is initiated by receptor desensitization after chronic exposure to nicotine. J Neurosci. 1999;19(12):4804–4814. doi: 10.1523/JNEUROSCI.19-12-04804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF, Froelicher ES, Goldstein MG, Healton CG, Henderson PN, Heyman RB, Koh HK, Kottke TE, Lando HA, Mecklenburg RE, Mullen PD, Orleans CT, Robinson LA, Stitzer ML, Tommasello AC, Villejo L, Wewers ME. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. 2008 PM:18807274. [Google Scholar]
- Franklin T, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y, Goldman M, Detre JA, O'Brien CP, Childress AR. Effects of varenicline on smoking cue-triggered neural and craving responses. Arch Gen Psychiatry. 2011;68(5):516–526. doi: 10.1001/archgenpsychiatry.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier A, Mucha RF, Pauli P. Appetitive nature of drug cues confirmed with physiological measures in a model using pictures of smoking. Psychopharmacology (Berl) 2000;150:283–291. doi: 10.1007/s002130000404. [DOI] [PubMed] [Google Scholar]
- Ginsberg D, Hall SM, Reus VI, Muñoz RF. Mood and depression diagnosis in smoking cessation. Exp Clin Psychopharmacol. 1995;3(4):389–395. doi: 10.1037/1064-1297.3.4.389. [DOI] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Nosek BA, Banaji MR. Understanding and using the implicit association test: I. An improved scoring algorithm. J Pers Soc Psychol. 2003;85(2):197–216. doi: 10.1037/0022-3514.85.2.197. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol. 2003;114(9):1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. Br J Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hong LE, Thaker GK, McMahon RP, Summerfelt A, RachBeisel J, Fuller RL, Wonodi I, Buchanan RW, Myers C, Heishman SJ. Effects of moderate-dose treatment with varenicline on neurobiological and cognitive biomarkers in smokers and nonsmokers with schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 2011;68(12):1195–1206. doi: 10.1001/archgenpsychiatry.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, Graffunder CM. Current Cigarette Smoking Among Adults - United States, 2005-2015. MMWR Morbidity and mortality weekly report. 2016;65(44):1205–1211. doi: 10.15585/mmwr.mm6544a2. [DOI] [PubMed] [Google Scholar]
- Jäncke L. Facial EMG in an anger-provoking situation: Individual differences in directing anger outwards or inwards. Int J Psychophysiol. 1996;23(3):207–214. doi: 10.1016/S0167-8760(96)00062-1. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: A randomized controlled trial. JAMA. 2006;296(1):56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Kenford SL, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Predicting relapse back to smoking: Contrasting affective and physical models of dependence. J Consult Clin Psychol. 2002;70:216–227. doi: 10.1037/0022-006X.70.1.216. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Kraemer HC, Varady AN, Davis L, Newman B. Interactive effects of depression symptoms, nicotine dependence, and weight change on late smoking relapse. J Consult Clin Psychol. 1996;64(5):1060–1067. doi: 10.1037/0022-006X.64.5.1060. [DOI] [PubMed] [Google Scholar]
- Koch M, Schnitzler HU. The acoustic startle response in rats--circuits mediating evocation, inhibition and potentiation. Behav Brain Res. 1997;89(1-2):35–49. doi: 10.1016/s0166-4328(97)02296-1. [DOI] [PubMed] [Google Scholar]
- Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Frontiers in Psychology. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychol Rev. 1990;97:377–395. doi: 10.1037/0033-295X.97.3.337. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report no A-6. University of Florida; Gainesville, FL: 2005. International Affective Picture System (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- Lang PJ, Davis M, Ohman A. Fear and anxiety: animal models and human cognitive psychophysiology. Journal of Affective Disorders. 2000;61:137–159. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- Lee H, Heller AS, van Reekum CM, Nelson B, Davidson RJ. Amygdala-prefrontal coupling underlies individual differences in emotion regulation. Neuroimage. 2012;62(3):1575–1581. doi: 10.1016/j.neuroimage.2012.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughead J, Ray R, Wileyto EP, Ruparel K, O'Donnell GP, Senecal N, Siegel S, Gur RC, Lerman C. Brain activity and emotional processing in smokers treated with varenicline. Addict Biol. 2011;18(4):732–738. doi: 10.1111/j.1369-1600.2011.00324.x. [DOI] [PubMed] [Google Scholar]
- Marrone GF, Paulpillai M, Evans RJ, Singleton EG, Heishman SJ. Breath carbon monoxide and semiquantitative saliva cotinine as biomarkers for smoking. Hum Psychopharmacol. 2010;25(1):80–83. doi: 10.1002/hup.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechias ML, Etkin A, Kalisch R. A meta-analysis of instructed fear studies: Implications for conscious appraisal of threat. Neuroimage. 2010;49(2):1760–1768. doi: 10.1016/j.neuroimage.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Menossi HS, Goudriaan AE, Azevedo-Marques Perico Cde, Nicastri S, Guerra de Andrade A, D'Elia G, Chiang-Shan RL, Castaldelli-Maia JM. Neural bases of pharmacological treatment of nicotine dependence-insights from functional brain imaging: a systematic review. CNS Drugs. 2013;27(11):921–941. doi: 10.1007/s40263-013-0092-8. [DOI] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline Is a Partial Agonist at alpha4beta2 and a Full Agonist at alpha7 Neuronal Nicotinic Receptors. Mol Pharmacol. 2006;70(3):801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Mocking RJT, Pflanz CP, Pringle A, Parsons E, McTavish SF, Cowen PJ, Harmer CJ. Effects of short-term varenicline administration on emotional and cognitive processing in healthy, non-smoking adults: a randomized, double-blind, study. Neuropsychopharmacol. 2013;38(3):476–484. doi: 10.1038/npp.2012.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Oshima A, Fujimoto Y, Maruyama N, Ishibashi T, Reeves KR. Efficacy and tolerability of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, in a 12-week, randomized, placebo-controlled, dose-response study with 40-week follow-up for smoking cessation in Japanese smokers. Clinical Therapeutics. 2007;29(6):1040–1056. doi: 10.1016/j.clinthera.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Owens JC, Balogh SA, McClure-Begley TD, Butt CM, Labarca C, Lester HA, Picciotto MR, Wehner JM, Collins AC. Alpha 4 beta 2* nicotinic acetylcholine receptors modulate the effects of ethanol and nicotine on the acoustic startle response. Alcohol Clin Exp Res. 2003;27(12):1867–1875. doi: 10.1097/01.ALC.0000102700.72447.0F. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Bradley MM, Lang PJ. Emotion in the criminal psychopath. J Abnorm Psychol. 1993;102:82–92. doi: 10.1037//0021-843x.102.1.82. [DOI] [PubMed] [Google Scholar]
- Perry DC, Davila-Garcia MI, Stockmeier CA, Kellar KJ. Increased Nicotinic Receptors in Brains from Smokers: Membrane Binding and Autoradiography Studies. J Pharmacol Exp Ther. 1999;289(3):1545–1552. [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: Activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84(4):329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Picciotto MRC, Brunzell DH, Caldarone BJ. Effect of nicotine and nicotinic receptors on anxiety and depression. [Review] Neuroreport. 2002;13(9):1097–1106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- Pissiota A, Frans O, Michelgard A, Appel L, Langstrom B, Flaten MA, Fredrickson M. Amygdala and anterior cingulate cortex activation during affective startle modulation: a PET study of fear. Eur J Neurosci. 2003;18(5):1325–1331. doi: 10.1046/j.1460-9568.2003.02855.x. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Rehme AK, Frommann I, Peters S, Block V, Bludau J, Quednow BB, Maier W, Schutz C, Wagner M. Startle cue-reactivity differentiates between light and heavy smokers. Addiction. 2009;104(10):1757–1764. doi: 10.1111/j.1360-0443.2009.02668.x. [DOI] [PubMed] [Google Scholar]
- Rhodes JD, Hawk LW, Jr, Ashare RL, Schlienz NJ, Mahoney MC. The effects of varenicline on attention and inhibitory control among treatment-seeking smokers. Psychopharmacology (Berl) 2012;223(2):131–138. doi: 10.1007/s00213-012-2700-6. [DOI] [PubMed] [Google Scholar]
- Robertson B, Wang L, Diaz MT, Aiello M, Gersing K, Beyer J, Mukundan S, Jr, McCarthy G, Doraiswamy PM. Effect of bupropion extended release on negative emotion processing in major depressive disorder: a pilot functional magnetic resonance imaging study. J Clin Psychiatry. 2007;68(2):261–267. doi: 10.4088/JCP.v68n0212. [DOI] [PubMed] [Google Scholar]
- Robinson JD, Lam CY, Carter BL, Minnix JA, Cui Y, Versace F, Wetter DW, Cinciripini PM. A multimodal approach to assessing the impact of nicotine dependence, nicotine abstinence, and craving on negative affect in smokers. Exp Clin Psychopharmacol. 2011;19(1):40–52. doi: 10.1037/a0022114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JD, Versace F, Engelmann JM, Cui Y, Slapin A, Oum R, Cinciripini PM. The motivational salience of cigarette-related stimuli among former never and current smokers. Exp Clin Psychopharmacol. 2015;23(1):37–48. doi: 10.1037/a0038467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JD, Versace F, Lam CY, Minnix JA, Engelmann JM, Cui Y, Karam-Hage M, Shete S, Tomlinson GE, Chen TTL, Wetter DW, Green CE, Cinciripini PM. The CHRNA3 rs578776 variant is associated with an intrinsic reward sensitivity deficit in smokers. Front Psychiatry. 2013;4(114):1–11. doi: 10.3389/fpsyt.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. Meta-Analytic Procedures for Social Research. 1st. Applied Social Research Methods SAGE Publications, Inc; 1991. [Google Scholar]
- Salas R, Pieri F, Biasi Mde. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. J Neurosci. 2004;24(45):10035–10039. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Navarro JP, Driscoll D, Anderson SW, Tranel D, Bechara A, Buchanan TW. Alterations of attention and emotional processing following childhood-onset damage to the prefrontal cortex. Behav Neurosci. 2014;128(1):1–11. doi: 10.1037/a0035415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SK, Young AW, Calder AJ, Hellawell DJ, Aggleton JP, Johnson M. Impaired auditory recognition of fear and anger following bilateral amygdala lesions. Nature. 1997;385(6613):254–257. doi: 10.1038/385254a0. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12(3):154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(20):22–33. [PubMed] [Google Scholar]
- Shen J. Load BIOPAC ACQ (AcqKnowledge for PC) data. 2011 http://www.mathworks.com/matlabcentral/fileexchange/14562-load-biopac-acq--acqknowledge-for-pc--data.
- Stahl SM, Pradko JF, Haight BR, Modell JG, Rockett CB, Learned-Coughlin S. A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Prim Care Companion J Clin Psychiatry. 2004;6(4):159–166. doi: 10.4088/pcc.v06n0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Hong LE, Stein EA. Down-regulation of amygdala and insula functional circuits by varenicline and nicotine in abstinent cigarette smokers. Biol Psychiatry. 2013;74(7):538–546. doi: 10.1016/j.biopsych.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA. Neurophysiology and neuropharmacology of short lead interval startle modification. In: Dawson ME, Schell AM, Böhmelt AH, editors. Startle modification Implications for neuroscience, cognitive science, and clinical science. Cambridge University Press; New York: 1999. pp. 114–133. [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. Nicotine activation of alpha4* receptors: Sufficient for reward, tolerance, and sensitization. Science. 2004;306(5698):1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Conklin CA. A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction (Abingdon, England) 2000;95(2):S145–53. doi: 10.1080/09652140050111717. [DOI] [PubMed] [Google Scholar]
- Tritto T, Stitzel JA, Marks MJ, Romm E, Collins AC. Variability in response to nicotine in the LS×SS RI strains: Potential role of polymorphisms in alpha4 and alpha6 nicotinic receptor genes. Pharmacogenetics. 2002;12(3):197–208. doi: 10.1097/00008571-200204000-00004. [DOI] [PubMed] [Google Scholar]
- Versace F, Lam CY, Engelmann JM, Robinson JD, Minnix JA, Brown VL, Cinciripini PM. Beyond cue reactivity: Blunted brain responses to pleasant stimuli predict long term smoking abstinence. Addict Biol. 2012;17(6):991–1000. doi: 10.1111/j.1369-1600.2011.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F, Robinson JD, Lam CY, Minnix JA, Brown VL, Carter BL, Wetter DW, Cinciripini PM. Cigarette cues capture smokers' attention: Evidence from event-related potentials. Psychophysiology. 2010;47(3):435–441. doi: 10.1111/j.1469-8986.2009.00946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS Scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology (Berl) 2008;197(3):371–377. doi: 10.1007/s00213-007-1041-3. [DOI] [PubMed] [Google Scholar]
- Yartz AR, Hawk LW. Addressing the specificity of affective startle modulation: Fear versus disgust. Biol Psychol. 2002;59(1):55–68. doi: 10.1016/s0301-0511(01)00121-1. [DOI] [PubMed] [Google Scholar]


