Abstract
Tuberculosis (TB) and depression act synergistically via social, behavioral, and biological mechanisms to magnify the burden of disease. Clinical depression is a common, under-recognized, yet treatable condition that, if comorbid with TB, is associated with increased morbidity, mortality, community TB transmission, and drug resistance. Depression may increase risk of TB reactivation, contribute to disease progression, and/or inhibit the physiological response to anti-tuberculosis treatment because of poverty, undernutrition, immunosuppression, and/or negative coping behaviors, including substance abuse. Tuberculous infection and/or disease reactivation may precipitate depression as a result of the inflammatory response and/or dysregulation of the hypothalamic-pituitary-adrenal axis. Clinical depression may also be triggered by TB-related stigma, exacerbating other underlying social vulnerabilities, and/or may be attributed to the side effects of anti-tuberculosis treatment. Depression may negatively impact health behaviors such as diet, health care seeking, medication adherence, and/or treatment completion, posing a significant challenge for global TB elimination. As several of the core symptoms of TB and depression overlap, depression often goes unrecognized in individuals with active TB, or is dismissed as a normative reaction to situational stress. We used evidence to reframe TB and depression comorbidity as the ‘TB–depression syndemic’, and identified critical research gaps to further elucidate the underlying mechanisms. The World Health Organization’s Global End TB Strategy calls for integrated patient-centered care and prevention linked to social protection and innovative research. It will require multidisciplinary approaches that consider conditions such as TB and depression together, rather than as separate problems and diseases, to end the global TB epidemic.
Keywords: comorbidity, poverty, biomarkers, immunosuppression, social determinants
ALTHOUGH it is a treatable condition, tuberculosis (TB) remains one of the leading causes of death worldwide.1 Treatment for drug-susceptible TB is nearly 100% effective and, even with the emergence of drug-resistant TB (DR-TB) and multidrug-resistant TB (MDR-TB), treatment using second- and third-line drugs can achieve moderate rates of success. Medication adherence is essential to achieve cure, minimize community TB transmission, and prevent the emergence of drug resistance, especially MDR-TB and, more recently, extensively drug-resistant TB (XDR-TB). Unfortunately, most TB cases occur in low-resource settings, where overburdened health systems and impoverished patients face multiple competing priorities and demands that negatively impact treatment outcomes.
Depression is a treatable condition that can affect as many as half of all individuals undergoing treatment for TB,2 and is associated with greater morbidity, mortality,3,4 drug resistance,5 and community transmission.2 Understanding and addressing the social and biological complexities of TB, including the negative synergies caused by co-occurring poverty and mental illness, is critical to curbing the TB, MDR-TB and XDR-TB epidemics globally.6
A syndemic is defined as the convergence of two or more conditions that act synergistically to magnify the burden of disease.7 Syndemics of co-occurring mental and physical health conditions, such as TB and depression, often interact with social and economic disparities that reduce access to health care, increasing both the odds and severity of negative outcomes among affected vulnerable populations. Whereas poverty is widely recognized as a driver of the TB epidemic,8 it is also a risk factor for clinical depression.9 People with TB who are depressed are more likely to abuse alcohol or drugs,10 and are less likely to adhere to medications and/or complete treatment.11 These individuals therefore not only carry a greater risk for negative TB outcomes, such as drug resistance5 and death,3,4 they may be infectious for longer periods of time in the community.2 The TB–depression syndemic is the product of complex, bidirectional negative synergies between social, biological, and behavioral factors that collectively increase the risk for negative health and mental health outcomes (Figure 1).
Figure 1.
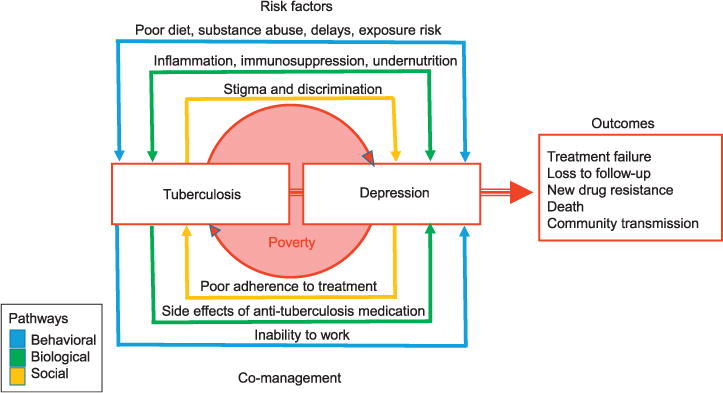
The tuberculosis-depression syndemic model. This image can be viewed online in color at http://www.ingentaconnect.com/content/iuatld/ijtld/2017/00000021/00000008/art000.…
The purpose of this review is to reframe TB and depression comorbidity as the ‘TB–depression syndemic’, rather than two separate and co-occurring conditions. The World Health Organization’s (WHO’s) Global End TB Strategy calls for integrated patient-centered care and prevention linked to social protection and innovative research. It thus needs to be responsive to the educational, psychological and material needs of those affected by TB,12 and will require a multidisciplinary approach to address the associated complexities underlying the syndemic, including screening for and treating comorbid depression.
SYNERGIES BETWEEN TUBERCULOSIS AND DEPRESSION
Depression is associated with an increased risk for several physical illnesses, including coronary heart disease, diabetes mellitus, cerebrovascular diseases, chronic obstructive pulmonary disease, cancer and asthma.13 Data from the Korean Institute of Tuberculosis suggest that the same may be true for TB; in a nationwide population-based cohort involving 64 000 individuals, persons with depression had a 2.63 times greater risk of TB over one decade, and the risk of TB was proportional to the severity of depression.14 Depression comorbidity among individuals with TB is extremely high;2,15,16 however, bidirectional risk factors often make it difficult to know which condition arose first.
Here, we describe a complex web of synergistic associations between biological, social, and behavioral factors that may contribute to these observations, while highlighting critical research gaps that require further investigation.
Inflammation and immunosuppression
Compromised immune function is implicated in both depression and TB, and the interrelationships are extremely complex, owing in part to different stages of immune responses to TB that comprise innate vs. cellular vs. humoral immunity, and differences between acute and chronic depression. Analogous to studies on cancer and human immunodeficiency virus (HIV) infection, in which depression has been associated with an inhibited response to pharmacological treatment and/or weakened response to vaccines,17,18 depression may worsen the prognosis for TB.19 This could occur because the glucocorticoids, catecholamines, histamine, and opioids released during chronic stress and depression antagonize several aspects of cellular-mediated immunity, including activation of natural killer cells, cluster of differentiation-8 T-cells, tissue macrophages, dendritic cells, and T helper 1 (Th1) lymphocytes,20,21 leading to a shift of Th1 (cellular immunity) to Th2 (humoral immunity) (Figure 2). This suppression of cellular immunity can result in TB reactivation and loss of TB containment in granulomas.22
Figure 2.
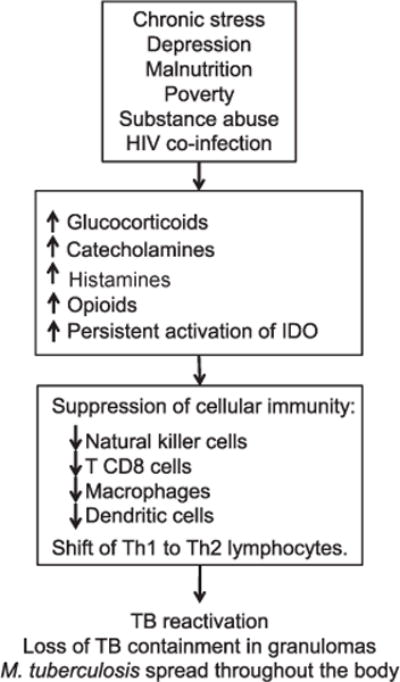
Hypothesized mechanisms through which depression-related immunosuppression increases the risk of TB reactivation and/or weakens the physiological response to treatment. IDO = indoleamine 2,3-dioxygenase; Th = T helper; TB = tuberculosis.
Conversely, increased production of pro-inflammatory cytokines in response to medical illness can trigger depression, even in individuals with no history of psychiatric disorders.23–25 Tuberculous infection and/or disease reactivation produce inflammatory responses and dysregulation of the hypothalamic-pituitary-adrenal axis.26 If an individual is exposed to TB, Th1 cells produce interferon-gamma, tumor necrosis factor-alpha and cytokines such as interleukin-2, which interact to activate anti-mycobacterial macrophage functions.22 These cytokines, essential for the cell-mediated immune response to TB,22,27 may precipitate depression28,29 via several biological mechanisms,23,30,31 as shown in Figure 3 and described below.
Figure 3.
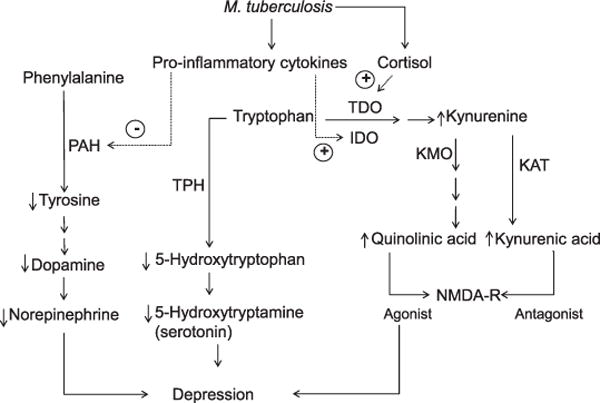
Effects of M. tuberculosis hypothesized to promote excitotoxicity and reduced monoaminergic neurotransmission, leading to depression. Infection with M. tuberculosis activates expression of pro-inflammatory cytokines, which stimulate the production of kynurenine and its metabolites, quinolinic acid and kynurenic acid, with opposing effects on NMDA-Rs (right side of diagram), and may lower production of serotonin (5-hydroxytryptamine), dopamine, and norepinephrine (left side). Tryptophan and kynurenine cross the blood-brain barrier, and can undergo the metabolic steps pictured here in the periphery or central nervous system. Kynurenine can be converted to kynurenic acid in astroglia or to quinolinic acid in infiltrating macrophages and microglia. Quinolinic acid increases glutamate release and leads to lipid peroxidation, thus contributing to excitotoxicity, oxidative stress and, ultimately, neurodegeneration. TDO = tryptophan 2,3-dioxygenase; IDO = indoleamine 2,3-dioxygenase; PAH = phenylalanine 4-hydroxylase; KMO = kynurenine-3-monooxygenase (also known as kynurenine-3-hydroxylase); KAT = kynurenine aminotransferases; TPH = tryptophan hydroxylase; NMDA-R = N-methyl-D-aspartate receptor.
Neurotransmitters such as 5-hydroxytryptamine (5-HT), dopamine (DA), and norepinephrine (NE) play significant roles in the pathogenesis of depressive symptoms25,32 and interact with inflammatory cascades. For example, pro-inflammatory cytokines reduce the bioavailability of DA and NE by inhibiting the enzyme phenylalanine 4-hydroxylase (PAH),33 which promotes the synthesis of tyrosine (Tyr), the precursor of DA and NE. Lower bioavailability of DA and NE exacerbates somatic depressive symptoms such as fatigue and weakness.23,34 5-HT affects macrophage polarization (M1 vs. M2 activation states) via 5-HT2B and 5-HT7 receptors to induce anti-inflammatory processes.35 In opposition, pro-inflammatory cytokines activate the enzymes indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO),30 which divert tryptophan (Trp) to the production of kynurenine (Kyn) instead of 5-HT. As dietary Trp-depletion paradigms have been shown to cause depression relapse, presumably due to decreased 5-HT levels,36 it was initially postulated that IDO activation may be depressogenic because it reduces 5-HT production in favor of Kyn.37 An alternative hypothesis is that inflammation-associated depression may stem from neurotoxic metabolites of Trp via the Kyn pathway, particularly quinolinic acid (QUIN), an agonist of the N-methyl D-aspartate (NMDA) receptor.34,38,39 This hypothesis is supported by findings showing depressive symptoms in patients administered therapeutic cytokines to correlate with higher levels of Kyn and QUIN in blood and cerebrospinal fluid (CSF),40 and lower blood proportions of a neuroprotective Kyn metabolite, the NMDA antagonist kynurenic acid (KA).38
However, in studies with patients administered therapeutic cytokines,38,40 reduced levels of Trp in blood did not correspond to reduced levels of Trp in CSF. As an alternative to the 5-HT-depletion hypothesis, inflammation-associated depression may stem from immunosuppressive and neurotoxic metabolites of Trp via the Kyn pathway, KA and QUIN, which are respectively antagonist and agonist to the NMDA receptor.34,38,39 The relevance of IDO and the Kyn pathway for depression in the context of tuberculous infection is instantiated by a mouse model in which TB vaccination with bacille Calmette-Guérin (BCG), an attenuated form of TB, induces IDO activation and triggers chronic depressive-like behaviors via the Kyn pathway, whereas knockout mice lacking IDO do not develop depressive-like behaviors.41 Similarly, if inflammation is induced in mice by lipopolysaccharide administration, depressive-like behaviors can be prevented by pretreatment with IDO blockers.42
Although acute infection with Mycobacterium tuberculosis causes IDO activation43 to help the organism to fight off tuberculous infection by triggering innate immunity in barrier tissues such as the gut, skin, and lung,44 persistent IDO activation inhibits T-lymphocyte function,45 contributing to a shift of Th1 to Th2 and TB activation or reactivation, as described above. This hypothesis is in accordance with evidence showing TB patients with active pulmonary disease to have significantly higher Kyn and lower Trp concentrations, greater IDO activity, and higher mortality.46
Poverty and undernutrition
Poverty is a risk factor for TB and depression, and the negative synergies between them can be deadly. TB disproportionately affects people living in poverty and other socially vulnerable populations such as the severely mentally ill, homeless, incarcerated, institutionalized, drug-addicted, and/or those co-infected with HIV.11,47 Several of the mechanisms through which poverty increases risk for TB are well understood.8 People living in poverty have a greater risk of exposure to TB due to overcrowded and poorly ventilated living conditions that favor TB transmission. Secondary factors such as substance abuse and HIV co-infection can compromise the immunological response to exposure, greatly increasing the risk for developing active TB. Instead of being encapsulated and contained in granulomas, M. tuberculosis overcomes the immune response and spreads throughout the body.22 In acutely ill individuals, poverty can negatively affect outcomes through treatment delays and/or inadequate treatment adherence, which increases the risk of resistance to antibacterial drugs, morbidity and mortality.
Poverty is also an independent risk factor for depression.9 People living in poverty have a greater risk of exposure to precipitating factors such as violence, social exclusion, discrimination, and abuse, while simultaneously lacking adequate access to health care, education, and other services.48 This constellation of chronic stressors can lead to sustained autonomic and neuroendocrine responses, increased production of pro-inflammatory cytokines and, consequently, depression.49
Malnutrition, commonly associated with poverty, can increase the risk of TB50,51 as well as depression.52 A recent systematic review of cohort studies confirmed a higher risk of TB disease among individuals with a body mass index <18.5 kg/m2.53 Furthermore, patients treated for pulmonary TB who maintain moderate-to-severe protein-caloric malnutrition up to the second month have an increased risk of relapse.54 TB can lead to reduced appetite, as well as nutrient malabsorption and altered metabolism, leading to wasting.51 Malnutrition can also be the cause or consequence of depression.52 Undernutrition is commonly observed among individuals with depression, and nutritional supplements have been effective in reducing symptoms.55 Depression can also affect the appetite and diet, leading to either a severe restriction of food due to loss of appetite, or excess intake of foods with limited nutritional content.52 Impoverishment resulting from TB and/or depression can worsen nutritional status, thereby weakening the immune system and inhibiting recovery from either condition.
Taken separately, TB and depression can each worsen poverty through functional impairment that can make it difficult to work and earn a living, compounded by the costs associated with treatment.56 Taken together, TB and depression comorbidity in the context of poverty can lead to a spiral of worsening symptoms of both illnesses and ever-increasing poverty.
Stigma, discrimination and trauma
TB, poverty and depression are all stigmatized conditions that can have compounding deleterious impacts on health via biological, social, and behavioral pathways.57,58 It is interesting to note that TB was not always stigmatized. Up until the early nineteenth century, TB was considered a ‘romantic’ disease associated with noble character, creative genius and heightened sensitivity among intellectuals and artists.59 When rapid industrialization and urbanization not only brought TB to epidemic levels but elucidated the link between TB and poverty, it became a ‘shameful’ condition.59 With the discovery of the TB bacillus in 1882 and along with it an increased understanding of germ transmission, fear of contagion led to social stigma associated with TB, which continues to this day.59–61
Ample evidence from other stigmatized conditions suggests that social stigma is an important risk factor for depression,62,63 and TB-related stigma may also contribute to depression.64 Stigma-related stress can lead to sustained autonomic and neuroendocrine responses that simultaneously increase risk for depression while weakening the immune system.57 TB-related stigma may also have social, emotional, and financial consequences. Negative attitudes towards TB can lead to internalized feelings of shame, disgust, and guilt in individuals with TB, which can contribute to external consequences such as discrimination and social isolation,61 resulting in depression. Finally, TB-related stigma can have behavioral consequences such as treatment delays and poor treatment adherence,11,60 which may, at least in part, be mediated or exacerbated by clinical depression.65
Individuals undergoing treatment with second- and third-line drugs for MDR- or XDR-TB have an even greater risk for stigma, discrimination, poverty, and depression.66 In settings with high levels of anti-tuberculosis drug resistance and limited access to drug susceptibility testing and treatment, prolonged infectiousness while receiving ineffective treatments can lead to clustering of cases within households or institutional settings, often resulting in catastrophic loss of life and financial burden.64 Shame, blame, and discrimination are amplified in such situations, as well as compounded trauma if multiple individuals in the same family or household fail treatment and/or die before accessing adequate treatment.64 Among a sample of 285 psychologically and socially vulnerable MDR- and XDR-TB patients who participated in a psychosocial support group intervention in Peru, most had failed multiple treatment regimens before initiating individualized treatment (an average of three previous treatments, range 0–9) and had been living with MDR-TB for a median of 4.5 years (range 7.2 months–24.7 years).64 Over half of the patients (52.6%) had at least one close family member with a history of TB (range 1–8 family members), and among those, about one quarter (24.2%) had at least one deceased family member due to TB (range 1–4 family members). Not surprisingly, more than half (52.2%) of the first cohort of 75 patients who completed treatment through this program were diagnosed with clinical depression before initiating treatment.15 Whereas depression resolved in most patients (66.7%), an additional 12.0% experienced a new onset of depression over the course of the 2-year treatment, resulting from physiological processes, side effects of medication and/or psychosocial stress associated with treatment.15 Although this psychosocial group support intervention was not evaluated against a control group, only 3.5% of the 285 MDR-TB patients purposively selected based on significant psychological need and social vulnerability discontinued treatment.64
Side effects of anti-tuberculosis medications
Several anti-tuberculosis agents have been associated with psychiatric side effects: cycloserine (CS), isoniazid (INH), ethionamide, ciprofloxacin, ethambutol, rifampicin (RMP), and the fluoroquino-lones.11,15,16,67,68 Although sufficient data from randomized controlled studies are not available to conduct a meta-analysis of the psychiatric side effects associated with anti-tuberculosis medications, recent reviews by Doherty et al.16 and Pachi et al.11 provide detailed summaries of evidence based on numerous case studies and other reviews. Whereas psychiatric side effects associated with most of the anti-tuberculosis agents mentioned above are relatively rare, numerous case reports have described severe psychiatric side effects and increased suicide risk among individuals receiving INH.16 Although CS has been considered to be the anti-tuberculosis agent with the highest psychiatric side effects (as high as 50%),11,15,16,68 a recent meta-analysis assessing the safety of CS in drug-resistant tuberculosis treatment found a significantly more modest rate of 5.7%.67
Furthermore, there may be interactions between some anti-tuberculosis drugs and psychiatric medications.11,16 Although the number of case studies in the literature are limited, two anti-tuberculosis medications, INH and linezolid, have mild monoamine oxidase inhibitor properties and may cause 5-HT syndrome (the accumulation of excess levels of serotonin which can cause symptoms ranging from mild agitation to high fever, seizures, and unconciousness) when combined with selective 5-HT receptor inhibitors (SSRIs), triptan painkillers or anti-headache medications, and the herbal supplement Saint John’s wort. SSRIs, the most commonly used class of antidepressants, may therefore be relatively contraindicated in combination with these two medications and require monitoring.11,16,69 Conversely, several antipsychotic medications, including phenothiazine and thioridazine, may have a beneficial impact. Evidence from several animal, in vitro, and small clinical trials suggest that these medications may not only increase antibacterial activity, they also have inherent anti-tuberculosis properties, and have been used experimentally to treat XDR-TB.70,71 There is limited evidence that RMP may reduce the plasma concentrations of antipsychotic medications, potentially requiring dose adjustments.16
Several second- and third-line drugs may also cause indirect psychological impacts on patients due to their physical effects, particularly among individuals receiving treatment for MDR- or XDR-TB, where significant side effects are common. Among a cohort of 60 patients treated for MDR- and XDR-TB in Lima, Peru, all experienced mild gastritis, many of whom described the unpleasant experience of vomiting repeatedly following administration of as many as 20 pills every day.64,68 Many patients (43.3%) experienced dermatological effects, including darkening of skin pigmentation due to clofazamine,68 which was both distressing and made concealing their illness to avoid stigmatization increasingly difficult.64 New onset of depression, anxiety, and psychosis occurred in respectively 18.3%, 11.7%, and 10% of patients.68 Other temporary side effects from the anti-tuberculosis medications included the inability to walk due to peripheral neuropathy (16.7%), dysregulation of the central nervous system including seizures and/or intractable headaches (8.3%), arthritis (6.7%), renal toxicity (3.3%), hepatitis (1.7%), and severe gastritis (1.7%). Four patients (6.7%) experienced irreversible hearing loss. Most patients received daily injections for a minimum of 6 months, which many reported to be both painful and psychologically distressing.64
Evidence from other medical disorders suggests that individuals with comorbid depression or anxiety may be less able to adapt to the adverse symptoms of chronic disease symptoms and often have a heightened awareness of physical symptoms.72 A systematic review of comorbid depression and chronic medical illnesses, including diabetes mellitus, congestive heart failure, coronary heart disease, osteoarthritis, rheumatoid arthritis, asthma, and chronic obstructive pulmonary disease, showed that depression was significantly associated with more physical symptoms after controlling for the severity of medical illness.72 This may be attributed (at least in part) to dysregulation of the endogenous pain modulatory system.72 Psychosocial support groups can provide an important opportunity for patients to commiserate and share coping strategies to minimize the mental distress associated with these experiences.64
Behavioral consequences of depression
People who are depressed are more likely to use negative coping such as alcohol and drug abuse,10 which can exacerbate poverty, undernutrition, and immunosuppression. As many as one third of individuals with depression struggle with comorbid substance abuse,10 which has been associated with TB reactivation,73,74 delays in seeking anti-tuberculosis treatment,75 poor medication adherence,76 reinfection,74 drug resistance,74 loss to follow-up,74,77 and mortality.78 Depression may also affect eating behaviors, such as skipping meals and/or consuming excess foods with low nutritional value.52 Because of these combined risk factors, people with depression are more likely to have more advanced disease upon diagnosis, remain infectious for longer periods of time, and therefore amplify the risk for community transmission of TB, MDR- and XDR-TB.2 Once in treatment, individuals with TB and depression are less likely to take all their medications and/or complete treatment,11 amplifying the risk of drug resistance,5 treatment failure and death.3,4
RESPONDING TO THE TUBERCULOSIS–DEPRESSION SYNDEMIC
There are multiple challenges in addressing the TB–depression syndemic. First is that depression often goes unrecognized or undiagnosed. As systemic inflammation induces symptoms of ‘sickness behavior’, some TB symptoms may resemble depression (e.g., low appetite, fatigue, insomnia, irritability, and loss of interest in one’s physical and social environment), which can lead to a missed diagnosis of comorbid depression.23 It may be difficult for non-mental health specialists to distinguish between situational distress and clinical depression. Depressed mood in the context of TB is also frequently conflated with being sick and/or poor, and not recognized as a clinically significant and treatable condition. Another barrier is that even if a psychiatric disorder is evident, many providers may be reluctant to ask patients about their mental health in settings where few treatment options are available.
Ample evidence suggests that people with chronic medical illnesses have a two to three times higher risk for depression than healthy controls, which is associated with higher health risk behaviors, medical symptom burden, functional impairment, non-adherence to medication regimens, increased costs, medical complications, and mortality.13,17,65 While there is every reason to believe that the same would be true for TB, methodological rigor to truly understand and document these associations is lacking. In a review of 31 studies from 11 countries, the mean weighted prevalence of depression was 48.9% among individuals with TB.2 However, most of these studies used brief screening instruments (which tend to overestimate prevalence), sample sizes were generally small, none included population-representative samples (mostly hospitals and clinics), and only two studies included control groups.2 Samples were diverse and not necessarily comparable, including in-patients and out-patients at different stages of treatment, with both drug-susceptible TB and DR-TB. Therefore, although preliminary evidence suggests that TB and depression comorbidity is high, and has been associated with negative TB outcomes, until more studies are conducted that involve a systematic diagnosis of depression, and do not rely merely on screening tools, it will not be possible to truly grasp the scope and impact of this comorbidity.
Similarly, although what we know from other illnesses suggests that treating depression among individuals with TB could break the negative synergy between them, thereby improving the therapeutic response to anti-tuberculosis medications as well as improving health behaviors that favor positive outcomes,79 studies examining ways to do this in the context of TB are still woefully lacking. Until recently, only two intervention studies had sought to improve TB outcomes by treating depression. One small trial found eight sessions of an individual behavioral/motivational interview intervention to be associated with significant improvement in treatment adherence and TB outcomes in India.79 Another qualitative study of DR-TB patients found that psychosocial support groups, supplemented with psychotropic medications if indicated, were associated with low treatment default in Peru.64 More recently, a cluster randomized controlled trial was conducted in Ethiopia in which an intervention to change health behavior coupled with education was associated with significantly better medication adherence among all patients, regardless of depression diagnosis, when compared with directly observed therapy only.80 A prospective cohort study is underway in Ethiopia to assess the prevalence of depression among individuals with TB, and the evolution of symptoms over time, and to explore the associations with negative TB outcomes.81 Although not a trial, that study is being conducted in health settings that have trained nurses to follow the WHO’s Mental Health Action Program (mhGAP) guidelines82 for the treatment of depression, and psychotropic medications are given to patients free of charge; these activities will be systematically tracked.81
Although many TB treatment programs include ‘psychosocial support’ in their treatment protocols, the focus tends to be on the socio-economic challenges and reactive distress associated with the disease and/or treatment. This strategy is likely to be sufficient for individuals confronting multiple challenges related to poverty and adherence but, for individuals with clinically significant depression, a more intensive psychological intervention may be required. Contrary to popular belief, addressing poverty does not necessarily lead to better mental health outcomes, whereas the opposite (treating mental illness) has been consistently associated with socio-economic improvements.83 A recent systematic review by the WHO found that programs that included educational, psychological, and/or material support were associated with better TB outcomes, and can now be considered best practice.84
Collaborative care models in primary care are a pragmatic and cost-efficient strategy for incorporating mental health into general medical care in primary care settings,85 and can be used as a model for integrating treatment for depression into TB control programs. Such models have enhanced the control of medical diseases such as diabetes mellitus and coronary heart disease while improving depression symptoms, treatment adherence, satisfaction with care, functional status and overall quality of life.85–87 Treating depression has also been associated with greater suppression of viruses, reduction in cortisol levels and better treatment adherence, leading to higher overall survival rates.86 Collaborative care involves more than simply co-locating a mental health professional within a primary care setting; it is defined as ‘a team-based, multicomponent intervention to enact care delivery redesign by systematically improving coordination of patient care through organizational leadership support, evidence-based provider decision-making, and clinical information systems, as well as engaging patients in their care through self-management support and linkages to community resources’.85
Several randomized controlled trials have demonstrated that collaborative care models can work in low-resource settings if lay community health workers and nurses are trained to deliver evidence-based psychotherapeutic interventions, such as interpersonal psychotherapy, cognitive behavioral therapy, behavioral activation, problem-solving therapy, and monitor symptoms through care coordination with expert supervision.88 Brief psychiatric screening tools are a key element of non-specialist-led mental health management, but they must be adapted for use in diverse sociocultural and linguistic settings,89 and still need to be calibrated for use among TB patients, taking into account the high overlap in symptoms. The WHO mhGAP has developed numerous tools, guidelines, and intervention manuals to help integrate mental health treatment in primary care in low-resource settings (http://www.who.int/mental_health/mhgap/en/).
CONCLUSIONS
The WHO’s post-2015 Global End TB Strategy takes into account the often complex realities in which TB occurs, calling for ‘integrated patient-centered care’ to end the TB epidemic, which involves recognizing and addressing the psychosocial and economic needs of individuals with TB.12 We argue that approaching TB and depression comorbidity as a TB–depression syndemic, instead of two separate and co-occurring conditions, is essential for the achievement of this goal. The End TB Strategy also calls for ‘bold policies and supportive systems’ and ‘intensified research and innovation’ to achieve its ambitious global target to end the epidemic.12 More funding is needed to support research that can further elucidate the mechanisms underlying the TB–depression syndemic, the associated outcomes, and integrated treatment models that may help curb the TB and DR-TB epidemics globally.
There are five critical research gaps that must be addressed to support these efforts. First, given the strong overlap of TB and depressive symptoms, and the pragmatic need to rely on brief screening instruments in low-resource settings with few specialists, more research is needed to validate these scales against structured diagnostic assessments to improve their operational utility. Second, it is essential that all future TB trials seeking to explore the risk factors associated with negative treatment outcomes include assessment of both poverty and depression to better understand these links. Third, the ways in which psychological, social, and/or financial interventions independently and/or jointly impact adherence and outcomes must be researched. Fourth, limited resources allocated to disease-specific interventions in low-income settings need to be leveraged through integrated care models that include treatment of depression and substance abuse to improve both health and mental health outcomes. Finally, more basic science research is needed to better understand the temporal and physiological interactions underlying the TB–depression syndemic so as to improve patient-centered treatment. Addressing all of these five critical research gaps is consistent with the post-2015 End TB Strategy.12
The new TB and Mental Health Working Group established through the International Union Against Tuberculosis and Lung Disease has put together a research agenda and strategy to achieve these goals. This includes identifying and linking front-line TB providers with researchers to better understand the prevalence of depression among individuals undergoing treatment for TB, as well as the evolution of symptoms over time. Such a link could lead to development of an evidence base for integrated treatment models to improve health and mental health outcomes. Then, the TB and Mental Health Working Group will support efforts to disseminate best practices to national TB programs. Preliminary evidence from an ongoing global survey of national TB directors from more than 20 countries suggests very high receptivity to integrate TB and mental health treatment at the national level with increased capacity building in low-cost and effective treatment models using interventions led by non-mental-health specialists.88,90
Acknowledgments
Funding was provided, in part, by the US National Institute of Mental Health, Bethesda, MD, USA (T32 MH096724; K01 MH104514; T32-MH19139) and the Rio de Janeiro State Research Foundation (FAPERJ)/Columbia University Global Centers, Rio de Janeiro, RJ, Brazil (E-26/010.001765/2014).
MAO receives royalties for the commercial use of the Columbia-Suicide Severity Rating Scale. Her family owns stock in Bristol Myers Squibb (New York, NY, USA).
Footnotes
All other authors declare no conflicts of interest.
References
- 1.World Health Organization. Global tuberculosis report. Geneva, Switzerland: WHO; 2016. (WHO/HTM/TB/2016.13). 2016. [Google Scholar]
- 2.Sweetland A, Oquendo M, Wickramaratne P, Weissman M, Wainberg M. Depression: a silent driver of the global tuberculosis epidemic. World Psychiatry. 2014;13:325–326. doi: 10.1002/wps.20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duarte EC, Bierrenbach AL, Barbosa da Silva J, Jr, Tauil PL, de Fatima Duarte E. Factors associated with deaths among pulmonary tuberculosis patients: a case-control study with secondary data. J Epidemiol Community Health. 2009;63:233–238. doi: 10.1136/jech.2008.078972. [DOI] [PubMed] [Google Scholar]
- 4.Franke MF, Appleton SC, Bayona J, et al. Risk factors and mortality associated with default from multidrug-resistant tuberculosis treatment. Clin Infect Dis. 2008;46:1844–1851. doi: 10.1086/588292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson J, Kagal A, Bharadwaj R. Factors associated with drug resistance in pulmonary tuberculosis. Indian J Chest Dis Allied Sci. 2003;45:105–109. [PubMed] [Google Scholar]
- 6.Walker I, Baral S, Wei X, et al. Multidrug-resistant tuberculosis treatment programmes insufficiently consider co-morbid mental disorders. Int J Tuberc Lung Dis. 2017;21:603–609. doi: 10.5588/ijtld.17.0135. [DOI] [PubMed] [Google Scholar]
- 7.Singer M, Clair S. Syndemics and public health: reconceptualizing disease in bio-social context. Med Anthropol Q. 2003;17:423–441. doi: 10.1525/maq.2003.17.4.423. [DOI] [PubMed] [Google Scholar]
- 8.Figueroa-Munoz JI, Ramon-Pardo P. Tuberculosis control in vulnerable groups. Bull World Health Organ. 2008;86:733–735. doi: 10.2471/BLT.06.038737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. The WHO MIND Project: mental improvement for nations development. Geneva, Switzerland: WHO; 2017. Breaking the vicious cycle between mental ill-health and poverty. http://www.who.int/mental_health/policy/development/1_Breakingviciouscycle_Infosheet.pdf Accessed April 2017. [Google Scholar]
- 10.Davis L, Uezato A, Newell JM, Frazier E. Major depression and comorbid substance use disorders. Curr Opin Psychiatry. 2008;21:14–18. doi: 10.1097/YCO.0b013e3282f32408. [DOI] [PubMed] [Google Scholar]
- 11.Pachi A, Bratis D, Moussas G, Tselebis A. Psychiatric morbidity and other factors affecting treatment adherence in pulmonary tuberculosis patients. Tuberc Res Treat. 2013;2013:489865. doi: 10.1155/2013/489865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uplekar M, Weil D, Lönnroth K, et al. WHO’s new end TB strategy. Lancet. 2015;385:1799–1801. doi: 10.1016/S0140-6736(15)60570-0. [DOI] [PubMed] [Google Scholar]
- 13.Katon WJ. Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues Clin Neurosci. 2011;13:7–23. doi: 10.31887/DCNS.2011.13.1/wkaton. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh KH, Choi H, Kim E, Kim HJ, Cho S. Depression and risk of tuberculosis: a nationwide population-based cohort study. Int J Tuberc Lung Dis. 2017;21:000–000. doi: 10.5588/ijtld.17.0038. [DOI] [PubMed] [Google Scholar]
- 15.Vega P, Sweetland A, Acha J, et al. Psychiatric issues in the management of patients with multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2004;8:749–759. [PubMed] [Google Scholar]
- 16.Doherty AM, Kelly J, McDonald C, O’Dywer AM, Keane J, Cooney J. A review of the interplay between tuberculosis and mental health. Gen Hosp Psychiatry. 2013;35:398–406. doi: 10.1016/j.genhosppsych.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Katon WJ. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biol Psychiatry. 2003;54:216–226. doi: 10.1016/s0006-3223(03)00273-7. [DOI] [PubMed] [Google Scholar]
- 18.Kiecolt-Glaser JK, Glaser R. Depression and immune function: central pathways to morbidity and mortality. J Psychosom Res. 2002;53:873–876. doi: 10.1016/s0022-3999(02)00309-4. [DOI] [PubMed] [Google Scholar]
- 19.Stewart JC, Rand KL, Muldoon MF, Kamarck TW. A prospective evaluation of the directionality of the depression-inflammation relationship. Brain Behav Immun. 2009;23:936–944. doi: 10.1016/j.bbi.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elenkov IJ, Chrousos GP. Stress Hormones, Th1/Th2 patterns, pro/anti-inflammatory cytokines and susceptibility to disease. Trends Endocrinol Metab. 1999;10:359–368. doi: 10.1016/s1043-2760(99)00188-5. [DOI] [PubMed] [Google Scholar]
- 21.Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5:617–625. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- 22.Schluger NW, Rom WN. The host immune response to tuberculosis. Am J Respir Crit Care Med. 1998;157:679–691. doi: 10.1164/ajrccm.157.3.9708002. [DOI] [PubMed] [Google Scholar]
- 23.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benton T, Staab J, Evans DL. Medical co-morbidity in depressive disorders. Ann Clin Psychiatry. 2007;19:289–303. doi: 10.1080/10401230701653542. [DOI] [PubMed] [Google Scholar]
- 25.Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. doi: 10.1016/j.neuroscience.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bozza VV, D’Attilio L, Mahuad CV, et al. Altered cortisol/DHEA ratio in tuberculosis patients and its relationship with abnormalities in the mycobacterial-driven cytokine production by peripheral blood mononuclear cells. Scand J Immunol. 2007;66:97–103. doi: 10.1111/j.1365-3083.2007.01952.x. [DOI] [PubMed] [Google Scholar]
- 27.Biselli R, Mariotti S, Sargentini V, et al. Detection of interleukin-2 in addition to interferon-y discriminates active tuberculosis patients, latently infected individuals, and controls. Clin Microbiol Infect. 2010;16:1282–1284. doi: 10.1111/j.1469-0691.2009.03104.x. [DOI] [PubMed] [Google Scholar]
- 28.Maes M. Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:664–675. doi: 10.1016/j.pnpbp.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 30.Zunszain PA, Anacker C, Cattaneo A, Carvalho LA, Pariante CM. Glucocorticoids, cytokines and brain abnormalities in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:722–729. doi: 10.1016/j.pnpbp.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Capuron L, Fornwalt FB, Knight BT, Harvey PD, Ninan PT, Miller AH. Does cytokine-induced depression differ from idiopathic major depression in medically healthy individuals? J Affect Disord. 2009;119:181–185. doi: 10.1016/j.jad.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety. 2013;30:297–306. doi: 10.1002/da.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zoller H, Schloegl A, Schroecksnadel S, Vogel W, Fuchs D. Interferon-alpha therapy in patients with hepatitis C virus infection increases plasma phenylalanine and the phenylalanine to tyrosine ratio. J Interferon Cytokine Res. 2012;32:216–220. doi: 10.1089/jir.2011.0093. [DOI] [PubMed] [Google Scholar]
- 34.Dantzer R, O’Connor JC, Lawson MA, Kelley KW. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology. 2011;36:426–436. doi: 10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Las Casas-Engel M, Corbi AL. Serotonin modulation of macrophage polarization: inflammation and beyond. Adv Exp Med Biol. 2014;824:89–115. doi: 10.1007/978-3-319-07320-0_9. [DOI] [PubMed] [Google Scholar]
- 36.Moore P, Landolt HP, Seifritz E, et al. Clinical and physiological consequences of rapid tryptophan depletion. Neuropsychopharmacology. 2000;23:601–622. doi: 10.1016/S0893-133X(00)00161-5. [DOI] [PubMed] [Google Scholar]
- 37.Wichers MC, Maes M. The role of indoleamine 2,3-dioxygenase (IDO) in the pathophysiology of interferon-alpha-induced depression. J Psychiatry Neurosci. 2004;29:11–17. [PMC free article] [PubMed] [Google Scholar]
- 38.Wichers MC, Koek GH, Robaeys G, Verkerk R, Scharpe S, Maes M. IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol Psychiatry. 2005;10:538–544. doi: 10.1038/sj.mp.4001600. [DOI] [PubMed] [Google Scholar]
- 39.Martinez P, Tsai AC, Muzoora C, et al. Reversal of the Kynurenine pathway of tryptophan catabolism may improve depression in ART-treated HIV-infected Ugandans. J Acquir Immune Defic Syndr. 2014;65:456–462. doi: 10.1097/QAI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raison CL, Dantzer R, Kelley KW, et al. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry. 2010;15:393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Connor JC, Lawson MA, Andre C, et al. Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. J Immunol. 2009;182:3202–3212. doi: 10.4049/jimmunol.0802722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Connor JC, Lawson MA, Andre C, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blumenthal A, Nagalingam G, Huch JH, et al. M. tuberculosis induces potent activation of IDO-1, but this is not essential for the immunological control of infection. PLOS ONE. 2012;7:e37314. doi: 10.1371/journal.pone.0037314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Memari B, Bouttier M, Dimitrov V, et al. Engagement of the aryl hydrocarbon receptor in Mycobacterium tuberculosis-infected macrophages has pleiotropic effects on innate immune signaling. J Immunol. 2015;195:4479–4491. doi: 10.4049/jimmunol.1501141. [DOI] [PubMed] [Google Scholar]
- 45.Li Q, Li L, Liu Y, Fu X, et al. Pleural fluid from tuberculous pleurisy inhibits the functions of T cells and the differentiation of Th1 cells via immunosuppressive factors. Cell Mol Immunol. 2011;8:172–180. doi: 10.1038/cmi.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki Y, Suda T, Asada K, et al. Serum indoleamine 2,3-dioxygenase activity predicts prognosis of pulmonary tuberculosis. Clin Vaccine Immunol. 2012;19:436–442. doi: 10.1128/CVI.05402-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deribew A, Tesfaye M, Hailmichael Y, et al. Common mental disorders in TB/HIV co-infected patients in Ethiopia. BMC Infect Dis. 2010;10:201. doi: 10.1186/1471-2334-10-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.World Health Organization. Mental health and development: targeting people with mental conditions as a vulnerable group. Geneva, Switzerland: WHO; 2010. [Google Scholar]
- 49.Berk M, Williams LJ, Jacka FN, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200. doi: 10.1186/1741-7015-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.World Health Organization. TB comorbidities and risk factors. Geneva, Switzerland: WHO; 2017. http://www.who.int/tb/areas-of-work/treatment/risk-factors/en/. Accessed April 2017. [Google Scholar]
- 51.Gupta KB, Gupta R, Atreja A, Verma M, Vishvkarma S. Tuberculosis and nutrition. Lung India. 2009;26:9–16. doi: 10.4103/0970-2113.45198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rao TSS, Asha MR, Ramesh BN, Rao KSJ. Understanding nutrition, depression and mental illnesses. Indian J Psychiatry. 2008;50:77–82. doi: 10.4103/0019-5545.42391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lönnroth K, Williams BG, Cegielski P, Dye C. A consistent log-linear relationship between tuberculosis incidence and body mass index. Int J Epidemiol. 2010;39:149–155. doi: 10.1093/ije/dyp308. [DOI] [PubMed] [Google Scholar]
- 54.Koethe JR, von Reyn CF. Protein-calorie malnutrition, macronutrient supplements, and tuberculosis. Int J Tuberc Lung Dis. 2016;20:857–863. doi: 10.5588/ijtld.15.0936. [DOI] [PubMed] [Google Scholar]
- 55.Lakhan SE, Vieira KF. Nutritional therapies for mental disorders. Nutrition J. 2008;7:2. doi: 10.1186/1475-2891-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanimura T, Jaramillo E, Weil D, Raviglione M, Lönnroth K. Financial burden for tuberculosis patients in low- and middle-income countries: a systematic review. Eur Respir J. 2014;43:1763–1775. doi: 10.1183/09031936.00193413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hatzenbuehler ML, Phelan JC, Link BG. Stigma as a fundamental cause of population health inequalities. Am J Public Health. 2013;103:813–821. doi: 10.2105/AJPH.2012.301069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Porto A. Social representations of tuberculosis: stigma and prejudice. Rev Saude Publica. 2007;41(Suppl 1):S43–S49. doi: 10.1590/s0034-89102007000800007. [Portuguese] [DOI] [PubMed] [Google Scholar]
- 60.Chang SH, Cataldo JK. A systematic review of global cultural variations in knowledge, attitudes and health responses to tuberculosis stigma. Int J Tuberc Lung Dis. 2014;18:168–173. i–iv. doi: 10.5588/ijtld.13.0181. [DOI] [PubMed] [Google Scholar]
- 61.Courtwright A, Turner AN. Tuberculosis and stigmatization: pathways and interventions. Public Health Rep. 2010;125(Suppl 4):34–42. doi: 10.1177/00333549101250S407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li L, Lee SJ, Thammawijaya P, Jiraphongsa C, Rotheram-Borus MJ. Stigma, social support, and depression among people living with HIV in Thailand. AIDS Care. 2009;21:1007–1013. doi: 10.1080/09540120802614358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cho J, Choi EK, Kim SY, et al. Association between cancer stigma and depression among cancer survivors: a nationwide survey in Korea. Psychooncology. 2013;22:2372–2378. doi: 10.1002/pon.3302. [DOI] [PubMed] [Google Scholar]
- 64.Acha J, Sweetland A, Guerra D, Chalco K, Castillo H, Palacios E. Psychosocial support groups for patients with multidrug-resistant tuberculosis: five years of experience. Glob Public Health. 2007;2:404–417. doi: 10.1080/17441690701191610. [DOI] [PubMed] [Google Scholar]
- 65.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 66.Thomas BE, Shanmugam P, Malaisamy M, et al. Psycho-socioeconomic issues challenging multidrug resistant tuberculosis patients: a systematic review. PLOS ONE. 2016;11:e0147397. doi: 10.1371/journal.pone.0147397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hwang TJ, Wares DF, Jafarov A, Jakubowiak W, Nunn P, Keshavjee S. Safety of cycloserine and terizidone for the treatment of drug-resistant tuberculosis: a meta-analysis. Int J Tuberc Lung Dis. 2013;17:1257–1266. doi: 10.5588/ijtld.12.0863. [DOI] [PubMed] [Google Scholar]
- 68.Furin JJ, Mitnick CD, Shin SS, et al. Occurrence of serious adverse effects in patients receiving community-based therapy for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2001;5:648–655. [PubMed] [Google Scholar]
- 69.Doyle ME, Hicks D, Aronson NE. Selective serotonin reuptake inhibitors and isoniazid: evidence of a potential adverse interaction. Military Med. 2001;166:1054–1056. [PubMed] [Google Scholar]
- 70.Amaral L, Martins A, Molnar J, et al. Phenothiazines, bacterial efflux pumps and targeting the macrophage for enhanced killing of intracellular XDRTB. In Vivo. 2010;24:409–424. [PubMed] [Google Scholar]
- 71.van Soolingen D, Hernandez-Pando R, Orozco H, et al. The antipsychotic thioridazine shows promising therapeutic activity in a mouse model of multidrug-resistant tuberculosis. PLOS ONE. 2010;5:e12640. doi: 10.1371/journal.pone.0012640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Katon W, Lin EH, Kroenke K. The association of depression and anxiety with medical symptom burden in patients with chronic medical illness. Gen Hosp Psychiatry. 2007;29:147–155. doi: 10.1016/j.genhosppsych.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 73.Lönnroth K, Williams BG, Stadlin S, Jaramillo E, Dye C. Alcohol use as a risk factor for tuberculosis: a systematic review. BMC Public Health. 2008;8:289. doi: 10.1186/1471-2458-8-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rehm J, Samokhvalov AV, Neuman MG, et al. The association between alcohol use, alcohol use disorders and tuberculosis (TB) A systematic review BMC Public Health. 2009;9:450. doi: 10.1186/1471-2458-9-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Storla DG, Yimer S, Bjune GA. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health. 2008;8:15. doi: 10.1186/1471-2458-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Munro SA, Lewin SA, Smith HJ, Engel ME, Fretheim A, Volmink J. Patient adherence to tuberculosis treatment: a systematic review of qualitative research. PLOS MED. 2007;4:e238. doi: 10.1371/journal.pmed.0040238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tola HH, Tol A, Shojaeizadeh D, Garmaroudi G. Tuberculosis treatment non-adherence and lost to follow up among TB patients with or without HIV in developing countries: a systematic review. Iran J Public Health. 2015;44:1–11. [PMC free article] [PubMed] [Google Scholar]
- 78.Alavi-Naini R, Moghtaderi A, Metanat M, Mohammadi M, Zabetian M. Factors associated with mortality in tuberculosis patients. J Res Med Sci. 2013;18:52–55. [PMC free article] [PubMed] [Google Scholar]
- 79.Janmeja AK, Das SK, Bhargava R, Chavan BS. Psychotherapy improves compliance with tuberculosis treatment. Respiration. 2005;72:375–380. doi: 10.1159/000086251. [DOI] [PubMed] [Google Scholar]
- 80.Tola HH, Shojaeizadeh D, Tol A, et al. Psychological and educational intervention to improve tuberculosis treatment adherence in Ethiopia-based on health belief model: a cluster randomized control trial. PLOS ONE. 2016;11:e0155147. doi: 10.1371/journal.pone.0155147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ambaw F, Mayston R, Hanlon C, Alem A. Depression among patients with tuberculosis: determinants, course and impact on pathways to care and treatment outcomes in a primary care setting in southern Ethiopia—a study protocol. BMJ Open. 2015;5:e007653. doi: 10.1136/bmjopen-2015-007653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.World Health Organization. mhGAP Mental Health Gap Action Programme: scaling up care for mental, neurological, and substance use disorders. Geneva, Switzerland: WHO; 2008. [PubMed] [Google Scholar]
- 83.Lund C, De Silva M, Plagerson S, et al. Poverty and mental disorders: breaking the cycle in low-income and middle-income countries. Lancet. 2011;378:1502–1514. doi: 10.1016/S0140-6736(11)60754-X. [DOI] [PubMed] [Google Scholar]
- 84.Alipanah N, Nguyen LN, Falzon D, et al. Systematic review and meta-analysis of adherence interventions in tuberculosis treatment; The 21st Annual Conference The Union North America Region; 23 February 2017; Vancouver, BC, Canada. [Google Scholar]
- 85.Goodrich DE, Kilbourne AM, Nord KM, Bauer MS. Mental health collaborative care and its role in primary care settings. Curr Psychiatry Rep. 2013;15:383. doi: 10.1007/s11920-013-0383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Katon WJ, Lin EHB, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363:2611–2620. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thota AB, Sipe TA, Byard GJ, et al. Collaborative care to improve the management of depressive disorders: a community guide systematic review and meta-analysis. Am J Prev Med. 2012;42:525–538. doi: 10.1016/j.amepre.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 88.Patel V, Belkin GS, Chockalingam A, Cooper J, Saxena S, Unutzer J. Grand challenges: integrating mental health services into priority health care platforms. PLOS Med. 2013;10:e1001448. doi: 10.1371/journal.pmed.1001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sweetland AC, Belkin GS, Verdeli H. Measuring depression and anxiety in sub-Saharan Africa. Depress Anxiety. 2014;31:223–232. doi: 10.1002/da.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sweetland A. TB and mental health: a survey of national TB programme directors. 47th Union World Conference on Lung Health: Confronting resistance: fundamentals to innovations, 26–29 October 2016, Liverpool, UK. [Symposium] Int J Tuberc Lung Dis. 2017;20(Suppl 1):S54. [Google Scholar]


