Abstract
Objective
Preadolescent loss-of-control-eating (LOC-eating) is a risk factor for excess weight gain and binge-eating-disorder. We evaluated feasibility and acceptability of a preventive family-based interpersonal psychotherapy (FB-IPT) program. FB-IPT was compared to family-based health education (FB-HE) to evaluate changes in children’s psychosocial functioning, LOC-eating, and body mass.
Method
A randomized, controlled pilot trial was conducted with 29 children, 8 to 13 years who had overweight/obesity and LOC-eating. Youth-parent dyads were randomized to 12-week FB-IPT (n=15) or FB-HE (n=14) and evaluated at post-treatment, six-months, and one-year. Changes in child psychosocial functioning, LOC-eating, BMI, and adiposity by dual-energy-X-ray-absorptiometry were assessed. Missing follow-up data were multiply imputed.
Results
FB-IPT feasibility and acceptability were indicated by good attendance (83%) and perceived benefits to social interactions and eating. Follow-up assessments were completed by 73% FB-IPT and 86% FB-HE at post-treatment, 60% and 64% at six-months, and 47% and 57% at one-year. At post-treatment, children in FB-IPT reported greater decreases in depression (95% CI −7.23, −2.01, Cohen’s d=1.23) and anxiety (95% CI −6.08, −0.70, Cohen’s d=.79) and less odds of LOC-eating (95% CI −3.93, −0.03, Cohen’s d=.38) than FB-HE. At six-months, children in FB-IPT had greater reductions in disordered-eating attitudes (95% CI −0.72, −0.05, Cohen’s d=.66) and at one-year, tended to have greater decreases in depressive symptoms (95% CI −8.82, 0.44, Cohen’s d=.69) than FB-HE. There was no difference in BMI gain between the groups.
Discussion
Family-based approaches that address interpersonal and emotional underpinnings of LOC-eating in preadolescents with overweight/obesity show preliminary promise, particularly for reducing internalizing symptoms. Whether observed psychological benefits translate into sustained prevention of disordered-eating or excess weight gain requires further study.
Keywords: interpersonal psychotherapy, loss-of-control eating, overweight, obesity, preadolescents
Infrequent (~1 episode/month) loss-of-control-eating (LOC-eating) is common (~30%) in preadolescents (8–13y) with overweight/obesity (Tanofsky-Kraff, 2008). Symptomatology consistent with binge-eating-disorder is observed in ~5% of non-treatment-seeking and ~11% of treatment-seeking preadolescents with overweight (Morgan et al., 2002; Wildes et al., 2010). Children who report any LOC-eating are at-risk for worsening depressive/anxiety symptoms, disordered-eating, and future binge-eating-disorder (Tanofsky-Kraff et al., 2011). In samples enriched for children at-risk for adult obesity, youth with LOC-eating are more likely gain adiposity excessively, develop obesity, and manifest worsening in metabolic functioning (Sonneville et al., 2013; Tanofsky-Kraff et al., 2012; Tanofsky-Kraff et al., 2009). Thus, amelioration of LOC-eating in children with overweight/obesity has been proposed to prevent increased disordered-eating, further excessive weight gain, and deteriorations in metabolic health (Tanofsky-Kraff, Wilfley, et al., 2007).
Interpersonal psychotherapy (IPT; Weissman, Markowitz, & Klerman, 2000) addresses interpersonally-driven depressive/anxiety symptoms posited to elicit LOC-eating (Tanofsky-Kraff, Wilfley, et al., 2007). Theoretically, youth’s social problems increase negative affect, triggering LOC-eating to soothe or avoid adverse emotions (Tanofsky-Kraff, Wilfley, et al., 2007). In adolescents with LOC-eating and high body mass index (BMI; kg/m2), IPT in a group format led to fewer binge-episodes one-year later compared to health education (Tanofsky-Kraff et al., 2014). IPT also significantly decreased excess gains in BMI-metrics and adiposity three-years later in adolescents with high baseline social problems or anxiety (Tanofsky-Kraff et al., 2017).
IPT has not been evaluated for intervening with LOC-eating to prevent exacerbated disordered-eating and to prevent excess weight gain in preadolescents with overweight/obesity.1 Preadolescents with disordered-eating may be at particularly high-risk for adverse eating/weight-related outcomes (Larsen, Strandberg-Larsen, Micali, & Andersen, 2015; Pearson & Smith, 2015). Traditional lifestyle-based weight management shows limited long-term success in children, necessitating development of alternative targeted approaches (Field, Camargo, & Ogino, 2013). Preliminary data suggest that lifestyle-based weight management in preadolescents may be less effective for reducing excess weight in children with LOC-eating, compared to preadolescents without LOC-eating (Wildes et al., 2010).
IPT for family-based delivery (FB-IPT) was developed as a treatment approach for preadolescent depression (Dietz, Mufson, Irvine, & Brent, 2008). FB-IPT demonstrated preliminary efficacy for acutely decreasing depressive/anxiety symptoms in preadolescents with depressive disorders, compared to active treatments (Dietz et al., 2008; Dietz, Weinberg, Brent, & Mufson, 2015). We adapted FB-IPT for preadolescents with LOC-eating. We selected FB-IPT for several reasons. Compared to preadolescents without LOC, preadolescents with LOC-eating have less healthy familial communication (Czaja, Hartmann, Rief, & Hilbert, 2011), which relates to children’s overeating and may be one early contributor to LOC-eating maintenance and/or exacerbation (Czaja et al., 2011; Hilbert, Tuschen-Caffier, & Czaja, 2010; Saltzman & Liechty, 2016). Further, children typically experience LOC-eating as secretive and report frequent emotional eating-eating in response to negative affect (Tanofsky-Kraff, Goossens, et al., 2007). Family involvement in weight management is essential in preadolescence (Wilfley, Vannucci, & White, 2010). Unlike traditional weight management programs for preadolescents, FB-IPT explicitly addresses underlying parent-child communication problems that may contribute to LOC-eating.
We conducted a randomized, comparison pilot trial to evaluate feasibility and acceptability of FB-IPT adapted for preadolescents with LOC-eating and overweight/obesity. Based upon preliminary data in depressed preadolescents (Dietz et al., 2008), we hypothesized that an FB-IPT program adapted for preadolescents with LOC-eating and overweight/obesity would be feasible and acceptable. We anticipated that children in FB-IPT would perceive benefits to elements of the interpersonal model, including better social interactions and greater confidence in resisting emotional and LOC-eating. Also, we sought to estimate the effects of FB-IPT on children’s social functioning, depressive/anxiety symptoms, disordered-eating, BMI and adiposity, compared to a family-based health education (FB-HE) standard-of-care comparison program (Wilfley et al., 2007). In exploratory analyses for this pilot study, we anticipated that, compared to FB-HE, FB-IPT would show trends toward greater decreases in social problems, negative affect, disordered-eating, and less BMI gain from baseline through one-year.
Methods
Participants
Preadolescent children and parents were recruited for a study testing a family-based program for prevention of children’s exacerbated disordered-eating and excess weight gain. The study was part of a broader genetic protocol, which influenced inclusion criteria. Recruitment strategies included newspapers advertisements, mailings to parents, flyers, and notices to school e-mail listservs. Boys and girls’ inclusion criteria were: (i) 8–13y, (ii) good general health, assessed by physical exam/medical history conducted by an endocrinologist or nurse practitioner and meaning that children did not have significant health problems or obesity-related comorbidities, (iii) BMI≥85th percentile, (iv) ≥1 LOC-eating episode/past month, and (v) English-speaking. All participants had an English-speaking biological parent with overweight/obesity (BMI≥25 kg/m2), willing to participate in study procedures. Consistent with previous behavioral trials for excess weight gain prevention (e.g., Tanofsky-Kraff et al., 2014), children were excluded for: (i) major renal, hepatic, gastrointestinal, endocrinologic, hematological, or pulmonary disorders; (ii) untreated obesity-related medical complication requiring more aggressive intervention (e.g., dyslipidemia); (iii) medication use affecting weight or appetite; (iv) current psychotherapy or structured weight loss; (v) reported weight loss in past two-months>3% of total body weight; (vi) in older preadolescent females, pregnancy; (vii) past or current full-syndrome eating disorder (other than binge-eating-disorder); or (viii) current major psychiatric disorder necessitating more intensive treatment. We did not include families if the participating parent had a major mental health diagnosis such as major depressive disorder (except binge-eating-disorder) determined by clinical interview or if a female parent was pregnant.
Procedure
Overview
Study procedures took place at the NIH Clinical Research Center and were approved by the NICHD Institutional Review Board. The trial was registered in clinicaltrials.gov (NCT00263536). After a telephone screen to evaluate eligibility, preadolescents and parents were scheduled for a screening. Parents and preadolescents provided written consent and assent, respectively. Eligible youths were randomized to 12-week FB-IPT or a 12-week FB-HE. Outcomes were evaluated directly after the program ended and six-months and one-year following the program’s start. Allocation was concealed from assessors of body measurements; LOC-eating interviewers were not consistently blinded to assignment.
Baseline and Follow-up Assessments
Physical Assessments
Following an overnight fast, children’s height was measured three times to the nearest millimeter with a calibrated electronic stadiometer. Weight was measured to the nearest 0.1 kg with a calibrated digital scale. Shoes and outerwear were removed. Weight and averaged heights were used to calculate BMI (kg/m2). U.S. Centers for Disease Control and Prevention growth standards were used to derive BMI-z and percentile, standardized for age and sex (Kuczmarski et al., 2002). Body fat (kg) was determined by dual-energy X-ray absorptiometry (DXA) using a Hologic QDR-4500A or Discovery instrument (Bedford, MA). An endocrinologist or nurse practitioner conducted a medical history/physical exam, during which puberty was assessed by breast development (girls) and testicular volume (boys) (Tanner, 1981). BMI-metrics were assessed at all intervals. DXA was collected at baseline, six-months, and one-year.
Interviews
All sections of the Eating Disorder Examination adapted for Children (ChEDE) (Bryant-Waugh, Cooper, Taylor, & Lask, 1996) were administered to assess presence (≥1 episode/past month) of LOC-eating and binge-eating and disordered-eating attitudes (global score; average of all items, with values ranging from 0–6 in increasing severity). LOC-eating included subjective and/or objective binge-eating, whereas binge-eating included LOC over an unambiguously large amount. Youth were assessed with the entire ChEDE except at post-treatment when only the overeating section was administered to determine presence/absence of LOC/binge-eating. Among preadolescents of all weight strata, ChEDE global score has excellent interrater reliability (>.95, p<.001); Cohen’s kappa for presence of different eating episodes was 1.00 (p<.001) (Tanofsky-Kraff et al., 2004). In this study, internal consistency for the global score was adequate (α=.77).
At screening only, a trained interviewer administered the Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS) to rule out presence of a major psychiatric disorder that would warrant study exclusion and a referral (Kaufman et al., 1997). Interviewers were psychologists or advanced clinical psychology graduate students with 2–6y prior clinical training. Interviewers were trained by the K-SADS developer.
Questionnaires
Youth completed a modified version of the Social Adjustment Scale–Self-Report (SAS-SR) to assess social functioning in family, peer, and school domains (Garber, Kriss, Koch, & Lindholm, 1988; Weissman & Bothwell, 1976), removing the dating domain and using minor language modifications to the adolescent form to increase accessibility for preadolescents. The total score was calculated as the average of all 20 items rated on a 1–5 Likert-scale, with higher scores reflecting more social problems (current sample α=.69). Youth completed the Children’s Depression Inventory (CDI), a widely-used, reliable, and valid 27-item questionnaire for school-aged youth (Kovacs, 1985). The total score, calculated as the sum of all items rated 0, 1, or 2, ranges from 0–54 (α=.73). Children completed the State-Trait Anxiety Inventory for Children (STAIC)–Trait Version, a psychometrically-sound and widely-used, 20-item measure of anxiety symptoms (Spielberger, 1983). STAIC total score (α=.81) represents the sum of all items rated on a 3-point Likert-scale; the total score ranges from 20–60.
Feasibility and acceptability
Feasibility and acceptability were operationalized as median session attendance. We also determined percentage who attended ≥80% of the program (10/12 sessions). Youth completed an adapted Treatment Process Questionnaire at the outset of sessions 1 (start-of-treatment), 6 (mid-treatment), and 12 (end-of-treatment) to evaluate perceived benefits to social relationships, emotional and LOC-eating (Hilbert et al., 2007). We evaluated ratings on three items: i) “Rate how you have gotten along with other people (friends, family members, peers) during the past week?” from 0=not at all good to 6=very good; ii) “How sure have you been during the past week that you would be able to keep from eating when in a bad mood (worried, sad, angry)?” from 0=not at all sure to 6=very sure; and iii) “How sure have you been during the past week that you would be able to keep from loss-of-control eating?” from 0=not at all sure to 6=very sure.
Interventions
A computer program using a random number generator was used to assign dyads to FB-IPT or FB-HE. Randomization was performed by a study coordinator and investigator uninvolved in intervention delivery.
FB-IPT is a manualized intervention of 12 weekly, 45-minute sessions delivered to parent-child dyads. FB-IPT was adapted from a combination of: (i) FB-IPT for treatment of preadolescent depressive disorders (Dietz et al., 2008) and (ii) IPT for Prevention of Excess Weight Gain, an IPT group for adolescents with LOC-eating and above-average BMI (Tanofsky-Kraff et al., 2014). FB-IPT focuses on psycho-education about the IPT model of LOC-eating and general skill-building applied to improve communication, increase support, and resolve conflict within the parent-child relationship. Initial treatment (sessions 1–3) includes education and information-gathering in a combination of individual/joint parent-child meetings. Treatment goals, developed in collaboration among facilitator, parent, and child, center on interpersonal problem areas that trigger or maintain children’s LOC-eating. The next phase (sessions 4–9) involves the child learning communication skills (e.g., “I” statements, timing of conversations, perspective-taking) that are practiced with the parent during the final 15-minutes of each session. During this phase, facilitators connect week-to-week changes in interpersonal problem areas to symptom reductions in LOC-eating. The final phase (sessions 10–12) focuses upon solidification of skills and planning for continued use following termination. Facilitators were 5 clinical psychologists and 7 advanced clinical psychology graduate students trained in FB-IPT. Sessions were audio-recorded. Facilitators received weekly supervision from a licensed clinical psychologist with IPT expertise (MTK).
FB-HE
The comparative condition was an adapted version of family-based weight management education (Wilfley et al., 2007). The original program was less effective than social-facilitation for children’s weight maintenance (Wilfley, Kass, & Kolko, 2011; Wilfley et al., 2007). To match FB-IPT, FB-HE was administered as 12 weekly, 45-minute parent-child sessions. FB-HE provides basic information on healthy eating (e.g., details about fiber benefits) and practicing exercises (e.g., stretching) that can be done at home. To ensure no overlap with FB-IPT, communication skills or emotional/LOC-eating were not discussed. Conversely, FB-IPT did not include FB-HE eating and exercise content. Facilitators were also clinical psychologists or advanced psychology students. To control for facilitator effects, interventionists alternated between administering FB-IPT and FB-HE.
Statistical Analysis
A target recruitment goal of 40 dyads was planned to determine feasibility/acceptability and to estimate comparative efficacy of FB-IPT versus FB-HE on child outcomes. Outliers were recoded a priori to 1.5 times the interquartile range <25th or >75th percentile, resulting in satisfactory skew and kurtosis. Independent samples t-tests and chi-square were used to compare children and parents randomized to FB-IPT versus FB-HE on baseline characteristics. To describe attrition, chi-square was used to compare attrition by condition. Logistic regression was used to evaluate baseline characteristics as attrition predictors. For the primary outcome, median session attendance was compared with a non-parametric test between FB-IPT and FB-HE. Chi-square was used to compare the percentage who attended ≥80% sessions by condition. To assess the primary outcome of acceptability, ANOVA/ANCOVA were used to describe intervention differences in children’s acceptability of treatment process (social relationships, emotional and LOC-eating) at start-of-treatment, and changes from start-of-treatment to mid-treatment and end-of-treatment in perceived benefits, adjusting for start-of-treatment ratings. For secondary outcomes, ANCOVA was used to predict post-treatment, six-month, and one-year changes in child outcomes (social problems, depressive/anxiety symptoms, disordered-eating, BMI metrics, adiposity)2 by condition, adjusting for baseline level of the outcome. We accounted for children’s sex, baseline age and BMI-z. Instead of BMI-z, baseline BMI or adiposity were used in models predicting those outcomes. Logistic regression was used to predict presence/absence of LOC/binge-eating, adjusting for baseline episodes. Missing data were handled with multiple imputation with the full intent-to-treat sample, including all families randomized regardless of withdrawal. Twenty multiply-imputed data sets were created with SAS Proc MI; results were combined using standard methods in SAS Proc MIANALYZE. Separate imputation models were estimated for each set of variables (all times for same outcome) because there were too many parameters to estimate in a single model with the small sample. Imputation models included treatment, children’s age, sex, and BMI-z as auxiliary variables along with the baseline value of the variable being imputed. Continuous variables were imputed using the Monte-Carlo Markov chain method and dichotomous variables with the monotone discriminant function method. Effect sizes for between-group differences were estimated with Cohen’s d, interpreted as small (0.2), medium (0.5), or large (0.8).
Results
Baseline Characteristics and Study Flow
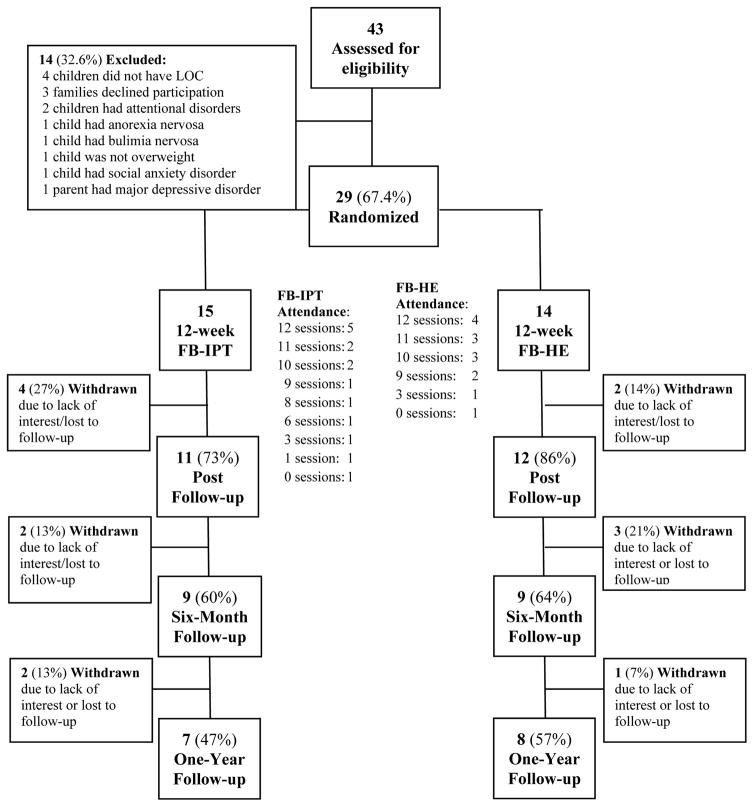
Recruitment took place from September 2012-July 2014. Two-hundred-sixty-eight families responded to advertisements; 43 attended screenings. Three (7% screened) withdrew prior to randomization due to lack of interest. Twenty-nine dyads (73% of targeted recruitment goal of N=40) were randomized to FB-IPT (n=15) or FB-HE (n=14; Figure 1). Preadolescents and parents did not significantly differ by condition in any baseline continuous or binary/categorical characteristic (Table 1). Retention exceeded 70% at post-intervention (FB-IPT:73% versus FB-HE:86%, p=.41). Six-month follow-up (FB-IPT:60% versus FB-HE:64%, p=.81) and one-year retention (FB-IPT:47% versus FB-HE:57%, p=.57) were lower, with no difference between programs. No baseline demographic, psychosocial, or disordered-eating characteristic significantly predicted attrition at any interval (ps>.10).
FIGURE 1.
Study flow
TABLE 1.
Baseline characteristics of study participants and their parents randomized to family-based interpersonal psychotherapy (FB-IPT) or family-based health education (FB-HE)
| Characteristic | FB-IPT n=15 |
FB-HE n=14 |
Between-group 95% CI | Cohen’s d |
|---|---|---|---|---|
| M (SD), Range | M (SD), Range | |||
| Child | ||||
| Age, y | 11.7 (1.6), 9.2–13.4 | 11.0 (1.9), 8.4–13.5 | −.65, 1.98 | .40 |
| BMI, kg/m2 | 28.4 (3.4), 23.2–32.3 | 27.2 (4.9), 21.1–36.6 | −1.92, 4.43 | .28 |
| BMI, z-score | 2.1 (.3), 1.4–2.6 | 2.0 (.4), 1.3–2.6 | −.15, .36 | .28 |
| Body fat, kg | 26.7 (5.6), 15.0–36.2 | 26.6 (11.4), 9.2–53.6 | −6.89, 7.10 | .01 |
| Social problems | 1.6 (.3), 1.1–2.1 | 1.6 (.4), 1.1–2.4 | −.24, .32 | .00 |
| Depression symptoms | 7.0 (4.1), 2.0–13.0 | 8.4 (4.8), 3.0–18.0 | −4.76, 2.04 | .31 |
| Anxiety symptoms | 30.8 (5.9), 23.0–46.0 | 34.7 (5.9), 24.0–45.0 | −8.40, .57 | .66 |
| LOC episodes, past month | 3.1 (1.8), 1.0–6.0 | 2.6 (1.4), 1.0–6.0 | −.65, 1.81 | .31 |
| % (n) | % (n) | |||
| Sex, female | 46.7 (7) | 78.6 (11) | ||
| Race/ethnicity | ||||
| Hispanic | 40.0 (6) | 7.1 (1) | ||
| Non-Hispanic Black | 33.3 (5) | 64.3 (9) | ||
| Non-Hispanic White | 20.0 (3) | 21.4 (3) | ||
| Multiple/Other | 6.7 (1) | 7.1 (1) | ||
| Puberty1 | ||||
| Prepubertal | 14.3 (2) | 28.6 (4) | ||
| Early pubertal | 50.0 (7) | 35.7 (5) | ||
| Mid-pubertal | 21.4 (3) | 14.3 (2) | ||
| Late-pubertal | 7.1 (1) | 7.1 (1) | ||
| Completed puberty | 7.1 (1) | 14.3 (2) | ||
| Objective binge presence | 40.0 (6) | 42.9 (6) | ||
| M (SD), Range | M (SD), Range | |||
| Parent | ||||
| Age, y | 42.8 (7.5), 27.0–53.0 | 45.9 (4.8), 40.0–58.0 | −7.90, 1.76 | .49 |
| BMI, kg/m2 | 34.3 (7.4), 25.1–48.7 | 35.6 (5.2), 30.9–47.1 | −6.26, 3.58 | .20 |
| % (n) | % (n) | |||
| Sex, female | 86.7 (13) | 92.9 (13) | ||
| Race/ethnicity | ||||
| Hispanic | 33.3 (5) | 7.1 (1) | ||
| Non-Hispanic Black | 33.3 (5) | 57.1 (8) | ||
| Non-Hispanic White | 33.3 (5) | 21.4 (3) | ||
| Multiple/Other | 0 (0) | 14.3 (2) | ||
| Median, Range | Median, Range | |||
| SES, Hollingshead | 3.0, 1.0–4.0 | 3.0, 1.0–4.0 | ||
Note:
Prepubertal: Tanner I breast development for girls or testis volume ≤3 mL for boys; early pubertal: Tanner II breast development or testis volume 4–8 mL; Mid-pubertal: Tanner III breast development or testis volume >8–12 mL; Late-pubertal: Tanner IV breast development or testis volume >12–20 mL; Completed puberty: Tanner V breast development or testis volume >20 mL.
BMI, z-score=body mass index standard score for age and sex. BMI=body mass index. Children’s social problems assessed with the Social Adjustment Scale. Symptoms of depression assessed with the Children’s Depression Inventory. Symptoms of anxiety assessed with the State-Trait Anxiety Inventory-Trait Version. Loss-of-control (LOC; subjective and/or objective binge episodes) and binge-eating (presence ≥1 objective binge episode in the past month) on the Eating Disorder Examination adapted for Children.
SES=socioeconomic status as assessed by the Hollingshead Index (Hollingshead, 1975).
Feasibility and Acceptability
Among randomized participants, FB-IPT dyads attended a median 10.0/12.0 sessions, and FB-HE a median 10.5 sessions (Figure 1; p=1.00). Sixty-percent in FB-IPT and 71% FB-HE attended ≥80% sessions (p=.52).
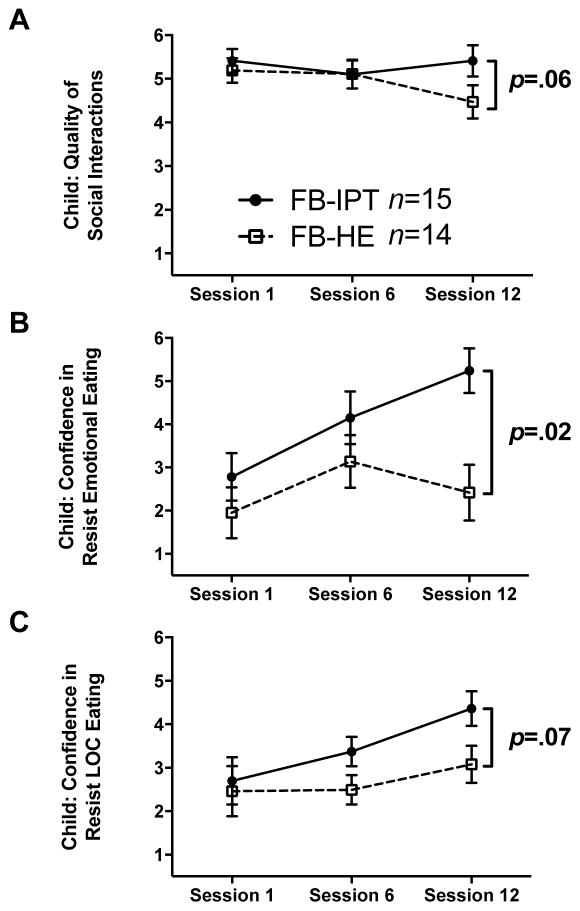
Figure 2 displays changes throughout FB-IPT and FB-HE in children’s perceptions of program process on relationships, emotional and LOC-eating. With respect to relationships, children in both conditions perceived positive social interactions at start-of-treatment (FB-IPT: Mean±SD, 5.39±.25 versus FB-HE:5.19±.29), which persisted at mid-treatment (Figure 2A; p=.60). At end-of-treatment, FB-HE reported a trend toward decreased relationship quality from start-of-treatment (Δ=−.72±.36, p=.06), with no change in FB-IPT (Δ=.00±.36, p=1.00; between-condition difference: .72±.51, p=.16). For emotional eating, children in FB-IPT (2.78±.55) versus FB-HE (1.95±.59) reported low confidence in resisting emotional eating at start-of-treatment (Figure 2B; p=.29). By mid-treatment, confidence in resisting emotional eating increased in FB-IPT (Δ=1.37±.61, p=.03) and FB-HE (Δ=1.19±.61, p=.05), with no between-condition difference (p=.84). By end-of-treatment, children in FB-IPT reported continued increased confidence in resisting emotional eating (Δ=2.46±.52, p<.001), whereas those in FB-HE reported no change by end-of-treatment (Δ=.47±.65, p=.47; between-condition difference: 1.99±.83, p=.018). For LOC-eating, children in both conditions perceived low-to-moderate confidence in resisting LOC-eating at start-of-treatment (FB-IPT:2.70±.55 versus FB-HE:2.46±.58, p=.75; Figure 2C). At mid-treatment, FB-IPT tended to gain confidence in resisting LOC-eating (Δ=.67±.34, p=.05), whereas no change was observed in FB-HE (Δ=.03±.34, p=.93; between-condition difference: .64±.49, p=.19). By end-of-treatment, FB-IPT continued to gain confidence in resisting LOC-eating (Δ=1.66±.40, p<.001); those in FB-HE had no significant change (Δ=.62±.43, p=.15; between-condition difference: 1.04±.57, p=.07).
FIGURE 2.
Children’s perceptions of social relationship quality (panel A), confidence in resisting emotional eating (panel B), and confidence in resisting loss-of-control-eating (LOC-eating) (panel C) throughout the 12 sessions of family-based interpersonal psychotherapy (FB-IPT; n=15) or family-based health education (FB-HE; n=14)
Secondary Outcomes
See Table 3 for intervention effects on secondary outcomes.
Social adjustment
There were no significant changes in children’s self-reported social adjustment in either condition at post-treatment, six-months, or one-year, and no between-condition differences in social adjustment (ps>.10).
Depression/anxiety symptoms
At post-treatment, FB-IPT demonstrated decreased depressive symptoms (p<.001), whereas FB-HE showed no change (p=.49; between-condition difference p<.001). At six-months, all children had decreased depressive symptoms from baseline (ps<.05), with no treatment effect (p=.47). Yet, at one-year, FB-IPT showed sustained reductions in depressive symptoms (p<.01). Children in FB-HE had no baseline to one-year change in depressive symptoms (p=.88). The between-condition difference in one-year depressive symptoms represented a moderate effect (Cohen’s d=.64; p=.08).
Anxiety symptoms decreased in FB-IPT at post-treatment (p<.001), and to a greater extent than FB-HE youth, who showed no change (p=.80; between-condition difference p=.01). Anxiety continued to decrease at six-months in FB-IPT (p=.01), with no change in FB-HE (p=.85), but the between-condition six-months difference did not reach significance (p=.16). At one-year, neither FB-IPT nor FB-HE showed significant change from baseline in anxiety (ps>.43).
Disordered-eating
At post-treatment, LOC-eating persisted in fewer children who received FB-IPT than FB-HE (38% versus 77%; odds ratio (SE):−1.99 (.99), p<.05). LOC-eating persistence did not differ at six-months or one-year (ps>.20). Prevalence of binge-eating did not differ between conditions at any follow-up interval (ps>.45). Global disordered-eating attitudes decreased from baseline to six-months in both conditions (ps<.001), with a more pronounced decrease among FB-IPT compared to FB-HE (between-condition difference p=.02). At one-year, all children decreased global disordered-eating (ps<.001), with no between-condition difference (p=.97).
BMI/adiposity
As expected in growing children, BMI increased in FB-IPT and FB-HE at post-intervention, six-months, and one-year. The between-condition difference did not reach significance at any interval. For descriptive purposes, the pattern pointed to greater BMI gain in FB-HE compared to FB-IPT. The difference was most pronounced at one-year, with youth in FB-HE gaining twice as many BMI-units as FB-IPT (between-condition difference p=.11), representing a moderate effect (Cohen’s d=.69). BMI-z showed stability throughout one-year, with no significant changes within either condition and no between-condition differences (ps>.10). Adiposity increased at six-months and one-year (ps<.05), with no difference between conditions (ps>.54).
Discussion
In this randomized comparison pilot trial, we evaluated an FB-IPT program adapted for coordinated prevention of disordered-eating and excess weight gain in high-risk preadolescents. FB-IPT was feasible and acceptable throughout treatment. Some patterns suggested that FB-IPT may be more promising than FB-HE for psychosocial, eating, and weight outcomes. FB-IPT was more effective than FB-HE in improving symptoms of depression, anxiety, and LOC-eating at post-treatment, global disordered-eating at six-months, and depression at one-year. Anxiety and disordered-eating benefits were not maintained at one-year. Similarly, BMI/adiposity outcomes were not significantly different between conditions at any time.
FB-IPT attendance was comparable to FB-HE and provides objective support for program feasibility and acceptability. Further, there was a pattern that children perceived FB-IPT program-specific benefits in social interactions and eating. Youth in FB-IPT sustained positive, self-reported social interactions throughout the intervention. FB-HE tended to report decreased quality of social interactions from beginning to end-of-treatment, consistent with developmental trends in increasing social problems as children age (Qualter et al., 2015). Yet, there was no significant between-condition effect. All preadolescents rated their baseline social interactions positively, which, in combination with the small sample, likely affected our ability to detect treatment-related changes in social adjustment. Moreover, children in FB-IPT increased confidence in resisting emotional and LOC-eating over 12-weeks. These preliminary findings suggest that youth perceived improvements in key components of the theoretical model upon which FB-IPT is predicated.
In exploratory analyses, we found that FB-IPT, compared to FB-HE, had greater decreases in anxiety symptoms and LOC-eating at post-treatment, greater reductions in disordered-eating attitudes at six-months, and greater decreases in depressive symptoms at post-treatment and one-year (ps<.05). Although this pilot study was designed to obtain preliminary data, these initial findings suggest that in preadolescents with LOC-eating and overweight/obesity, FB-IPT may prove to be an effective intervention for mood and disordered-eating. Consistent with findings in depressed preadolescents (Dietz et al., 2008), FB-IPT appears to be a promising approach to reduce depressive symptoms in youth with LOC-eating and overweight/obesity. Given that participants did not meet threshold criteria for any mood disorder, FB-IPT may offer a preventative approach to depression in preadolescents, just as group IPT is an effective strategy for preventing adolescent depression (Young, Mufson, & Davies, 2006). As FB-HE does not address mood symptoms or LOC-eating, FB-IPT may be a useful targeted approach for children with overweight/obesity that is driven by negative affective states (Tanofsky-Kraff, Wilfley, et al., 2007). Nonetheless, treatment differences in anxiety and disordered-eating were no longer apparent at one-year, suggesting maintenance efforts are necessary. Another consideration is that LOC-eating is not the only underlying psychosocial contributor to excess weight gain; LOC-eating remits naturally in ~half of youth at-risk for excess gain (Tanofsky-Kraff et al., 2011). Negative affect, including depressive symptoms that purportedly trigger LOC-eating, predict obesity onset and may promote excess weight gain through multiple pathways, other than LOC-eating alone (Blaine, 2008).
Children in FB-IPT and FB-HE had stable BMI-z from baseline to one-year. This pattern is noteworthy because most children with overweight/obesity, and particularly preadolescents who also experience LOC-eating, would be anticipated to gain excessively as they grow (Field, Cook, & Gillman, 2005; Tanofsky-Kraff et al., 2009; Whitaker, Wright, Pepe, Seidel, & Dietz, 1997). As expected in developing children, youth gained raw BMI and adiposity over time, with no significant difference between conditions. In our previous adolescent IPT effectiveness trial, we similarly found no difference between IPT and HE on BMI and adiposity at one- or three-years (Tanofsky-Kraff et al., 2017; Tanofsky-Kraff et al., 2014). However, treatment effects on BMI-metrics in adolescents with high social problems and anxiety became increasingly more pronounced with a longer follow-up (~three-years) that allowed for individual differences in growth trajectories to manifest (Tanofsky-Kraff et al., 2017). In the current pilot study, there was a pattern for an increasing difference in BMI change between conditions over time; by one-year, FB-HE had gained twice as many BMI units than FB-IPT. Even small reductions in BMI are associated with reductions in cardiometabolic risk factors (Ho et al., 2012). Although the pattern in BMI change was not statistically significant, the effect size was moderate, pointing to the potential of FB-IPT to offer benefits for excess weight gain prevention in high-risk preadolescents. The achieved pilot study sample size was only sufficient to detect large between-group differences (Cohen’s d>1.0). In future studies, 120-dyads per condition would need to be recruited, after accounting for observed attrition, to detect a moderate effect size at one-year.
Study strengths include a randomized design and comparison to an active behavioral condition matched for delivery, attention, time, and facilitator expertise, lending confidence to observed differences between conditions. We used interview assessment of eating pathology and objective measures of body composition. Historically disadvantaged racial/ethnic groups at disproportionate risk for obesity and cardiometabolic disease were highly represented, providing support for generalizability to populations in high-need of binge-eating-disorder and excess weight gain prevention efforts (Ogden, Carroll, Kit, & Flegal, 2014). We did not study preadolescents who had parents with major psychopathology, limiting generalizability to families with major mental health disorders. The SAS-SR requires validation in preadolescents. Although treatment fidelity was monitored throughout the trial through clinical supervision and feedback on audio-recorded sessions, we did not collect formal fidelity ratings, which would be necessary in a subsequent trial. Despite adequate participation in the interventions, we had study execution difficulties in recruiting and retaining families for one-year follow-up assessments in both conditions, reflecting challenges in conducting research with at-risk preadolescents and their families. In just under two-years, we recruited 73% of our targeted sample size. Furthermore, in contrast to our previous trials supporting ≥85% one-year retention of similar populations (Tanofsky-Kraff et al., 2014; Tanofsky-Kraff et al., 2010), retention of families for six-month and one-year follow-ups was low. Recruitment and retention were likely affected by a number of factors: (i) relatively high participant burden of research procedures; (ii) as part of a larger genetic protocol, youth who did not live with a biological parent or were adopted were excluded; (iii) unlike our adolescent trials for which many participants attended weekly sessions alone, the current protocol required involvement of parent and child, which could have deterred recruitment; (iv) families were highly diverse in terms of race/ethnicity and many had economic strain, making travel to an academic center and time away from work a challenge; and (v) we studied youth presenting with sub-clinical depression/anxiety symptoms and LOC-eating, which might have made a family-based intervention feel burdensome or unnecessary. It is possible that targeting a sample with more elevated depressive/anxiety symptoms, in addition to LOC-eating and high BMI, would have led to more initial interest and more engagement following the treatment. In future studies, a more symptomatic sample also may increase the ability to detect stronger treatment effects (e.g., in social adjustment). Alternatively, less restrictive inclusion/exclusion criteria, such as not requiring that parents have overweight/obesity, would make the program more generalizable with broader reach. Weight management studies have reported significant difficulties in retaining families of disadvantaged racial/ethnic groups (Parikh, Mason, & Williams, 2016). Despite good program participation, in future trials study execution could be improved by reducing burden (e.g., fewer visits and/or at-home assessments) and enhancing contact with families throughout follow-up. Although we were able to compensate youth in the current study for their time and to provide some support for transportation, retention would be bolstered by greater incentives to families for follow-up participation (Parikh et al., 2016). While children reported perceived benefits throughout treatment and families attended the majority of sessions, we did not collect parents’ perceptions of treatment process in their preadolescents or a qualitative post-intervention interview, which could have provided valuable feedback on program development and enhancing follow-up assessment retention. In a larger trial, it would also be important to include parent outcomes. Program delivery of family-based weight management interventions in primary care settings and/or adapting such programs for electronic administration might improve recruitment and follow-up assessment retention.
We observed feasibility and acceptability of FB-IPT for addressing psychosocial factors that may drive LOC-eating and, in turn, exacerbated disordered-eating and excess weight gain in some children. FB-IPT may be particularly suitable for addressing psychological symptoms that frequently accompany overweight/obesity in children (Blaine, 2008). The impact on weight/fat gain remains to be determined. Future tests of FBT for preadolescents require additional procedures aimed at bolstering recruitment, as well as retention during follow-up assessments, so that the effectiveness of this approach for eating disorder and excess weight gain prevention may be established.
TABLE 2.
Intervention effects: Changes at post-intervention, six-months, and one-year of children’s psychosocial, disordered-eating, and BMI/adiposity outcomes
| Outcome | FB-IPT | FB-HE | Difference2 | Cohen’s d | p |
|---|---|---|---|---|---|
| Post-Intervention1 | |||||
|
|
|||||
| Δ Social problems | .01 (−.34, .36) | .20 (−.16, .57) | −.19 (−.71, .33) | .30 | .47 |
| Δ Depression | −3.96 (−5.73, −2.19)* | .66 (−1.22, 2.54) | −4.62 (−7.23, −2.01) | 1.23 | .0005 |
| Δ Anxiety | −3.64 (−5.39, −1.88)* | −.25 (−2.19, 1.69) | −3.39 (−6.08, −.70) | .79 | .01 |
| Δ BMI, z-score | −.01 (−.06, .04) | .01 (−.04, .07) | −.02 (−.10, .05) | .27 | .53 |
| Δ BMI, kg/m2 | 6.54 (5.33, 8.53)* | 7.92 (4.04, 11.79)* | −1.38 (−6.88, 4.13) | .19 | .62 |
|
|
|||||
| Six-Month Follow-up1 | |||||
|
|
|||||
| Δ Social problems | −.11 (−.42, .20) | .18 (−.13, .48) | −.28 (−.73, .16) | .53 | .21 |
| Δ Depression | −3.33 (−5.36, −1.30)* | −2.27 (−4.34, −.20)* | −1.06 (−3.98, 1.86) | .29 | .48 |
| Δ Anxiety | −6.07 (−10.60, −1.54)* | −.42 (−4.96, 4.12) | −5.64 (−13.56, 2.27) | .73 | .16 |
| Δ Disordered-eating | −.82 (−1.05, −.59)* | −.43 (−.66, −.20)* | −.39 (−.72, −.05) | .66 | .02 |
| Δ BMI, z-score | .00 (−.07, .07) | .02 (−.06, .09) | −.02 (−.13, .09) | .16 | .71 |
| Δ BMI, kg/m2 | 6.31 (2.58, 10.04)* | 8.47 (4.61, 12.33)* | −2.16 (−7.62, 3.29) | .28 | .44 |
| Δ Body fat, kg | 2.09 (.29, 3.89)* | 2.88 (1.16, 4.60)* | −.79 (−3.34, 1.76) | .29 | .54 |
|
|
|||||
| One-Year Follow-up1 | |||||
|
|
|||||
| Δ Social problems | −.16 (−.50, .18) | −.10 (−.42, .20) | −.05 (−.56, .45) | .10 | .83 |
| Δ Depression | −4.42 (−7.54, −1.30)* | −.20 (−3.32, 2.86) | −4.19 (−8.82, .44) | .69 | .08 |
| Δ Anxiety | 2.97 (−9.23, 15.17) | −4.61 (−16.55, 7.33) | 7.58 (−13.81, 28.97) | −.54 | .48 |
| Δ Disordered-eating | −.92 (−1.14, −.70)* | −.91 (−1.13, −.69)* | −.01 (−.32, .31) | .01 | .97 |
| Δ BMI, z-score | .01 (−.10, .12) | −.04 (−.15, .06) | .05 (−.11, .21) | −.26 | .53 |
| Δ BMI, kg/m2 | 5.94 (.12, 11.77)* | 11.92 (6.45, 17.40)* | −5.98 (−13.36, 1.40) | .64 | .11 |
| Δ Body fat, kg | 3.36 (1.00, 5.71)* | 3.82 (1.86, 5.78)* | −.46 (−3.52, 2.59) | .17 | .76 |
Note:
Values displayed are changes from baseline (Δ); Mean (95% CI) are shown.
Values displayed are difference estimates (95% CI) and corresponding p-values refer to the between-group difference in family-based interpersonal psychotherapy (FB-IPT, n=15 dyads) as compared to family-based health education (FB-HE n=14 dyads). Models adjusted for children’s sex, baseline age, the baseline level of the outcome, and baseline BMI-z (or baseline BMI or body fat for those outcomes).
p <.05 within-condition change.
Social problems assessed with the Social Adjustment Scale. Symptoms of depression assessed with the Children’s Depression Inventory. Symptoms of anxiety assessed with the State-Trait Anxiety Inventory for Children-Trait Version. Disordered-eating assessed as the global score on the Eating Disorder Examination adapted for Children (evaluated at six-month and one-year follow-ups only). BMI, z-score=body mass index standard score for age and sex. BMI=body mass index.
Acknowledgments
Support for this project was provided through the NIH Intramural Research Program Grant 1ZIAHD000641 (J. A. Yanovski) from NICHD and a Pilot Intramural Research Award 72ON-01 (M. Tanofsky-Kraff) from USUHS.
Footnotes
We use the terminology “prevention of excess weight gain” to refer to decreasing the likelihood of children gaining too much weight as they continue to grow. The concept is applicable to youth who have overweight/obesity, as these youth are particularly susceptible for continued excess weight gain.
We describe BMI and BMI-z change, although BMI has advantages for measuring for children’s change over time (Cole, Faith, Pietrobelli, & Heo, 2005).
Disclosure of Conflicts
None of the authors has a conflict of interest. J. A. Yanovski is a commissioned officer in the U.S. Public Health Service. The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of the Public Health Service, the Department of Health and Human Services, USUHS, or the U.S. Department of Defense.
ClinicalTrials.gov: NCT00263536
References
- Blaine B. Does depression cause obesity? A meta-analysis of longitudinal studies of depression and weight control. Journal of Health Psychology. 2008;13(8):1190–1197. doi: 10.1177/1359105308095977. [DOI] [PubMed] [Google Scholar]
- Bryant-Waugh RJ, Cooper PJ, Taylor CL, Lask BD. The use of the Eating Disorder Examination with Children: A pilot study. International Journal of Eating Disorders. 1996;19(4):391–397. doi: 10.1002/(SICI)1098-108X(199605)19:4<391::AID-EAT6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Cole TJ, Faith MS, Pietrobelli A, Heo M. What is the best measure of adiposity change in growing children: BMI, BMI %, BMI z-score or BMI centile? European Journal of Clinical Nutrition. 2005;59(3):419–425. doi: 10.1038/sj.ejcn.1602090. [DOI] [PubMed] [Google Scholar]
- Czaja J, Hartmann AS, Rief W, Hilbert A. Mealtime family interactions in home environments of children with loss of control eating. Appetite. 2011;56(3):587–593. doi: 10.1016/j.appet.2011.01.030. [DOI] [PubMed] [Google Scholar]
- Dietz LJ, Mufson L, Irvine H, Brent DA. Family-based interpersonal psychotherapy for depressed preadolescents: An open-treatment trial. Early Intervention in Psychiatry. 2008;2(3):154–161. doi: 10.1111/j.1751-7893.2008.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz LJ, Weinberg RJ, Brent DA, Mufson L. Family-based interpersonal psychotherapy for depressed preadolescents: examining efficacy and potential treatment mechanisms. Journal of the American Academy of Child and Adolescent Psychiatry. 2015;54(3):191–199. doi: 10.1016/j.jaac.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field AE, Camargo CA, Jr, Ogino S. The merits of subtyping obesity: One size does not fit all. JAMA. 2013;310(20):2147–2148. doi: 10.1001/jama.2013.281501. [DOI] [PubMed] [Google Scholar]
- Field AE, Cook NR, Gillman MW. Weight status in childhood as a predictor of becoming overweight or hypertensive in early adulthood. Obesity Research. 2005;13(1):163–169. doi: 10.1038/oby.2005.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber J, Kriss MR, Koch M, Lindholm L. Recurrent depression in adolescents: A follow-up study. Journal of the American Academy of Child and Adolescent Psychiatry. 1988;27(1):49–54. doi: 10.1097/00004583-198801000-00008. [DOI] [PubMed] [Google Scholar]
- Hilbert A, Saelens BE, Stein RI, Mockus DS, Welch RR, Matt GE, Wilfley DE. Pretreatment and process predictors of outcome in interpersonal and cognitive behavioral psychotherapy for binge eating disorder. Journal of Consulting and Clinical Psychology. 2007;75(4):645–651. doi: 10.1037/0022-006X.75.4.645. [DOI] [PubMed] [Google Scholar]
- Hilbert A, Tuschen-Caffier B, Czaja J. Eating behavior and familial interactions of children with loss of control eating: A laboratory test meal study. American Journal of Clinical Nutrition. 2010;91(3):510–518. doi: 10.3945/ajcn.2009.28843. [DOI] [PubMed] [Google Scholar]
- Ho M, Garnett SP, Baur L, Burrows T, Stewart L, Neve M, Collins C. Effectiveness of lifestyle interventions in child obesity: Systematic review with meta-analysis. Pediatrics. 2012;130(6):e1647–1671. doi: 10.1542/peds.2012-1176. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Four Factor Index of Social Status. Yale University Department of Psychology; New Haven, CT: 1975. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, … Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kovacs M. The Children’s Depression, Inventory (CDI) Psychopharmacology Bulletin. 1985;21(4):995–998. [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, … Johnson CL. 2000 CDC Growth Charts for the United States: Methods and development. Vital Health Statistics. 2002;11(246):1–190. [PubMed] [Google Scholar]
- Larsen PS, Strandberg-Larsen K, Micali N, Andersen AM. Parental and child characteristics related to early-onset disordered eating: A systematic review. Harvard Review of Psychiatry. 2015;23(6):395–412. doi: 10.1097/HRP.0000000000000073. [DOI] [PubMed] [Google Scholar]
- Morgan CM, Yanovski SZ, Nguyen TT, McDuffie J, Sebring NG, Jorge MR, … Yanovski JA. Loss of control over eating, adiposity, and psychopathology in overweight children. International Journal of Eating Disorders. 2002;31(4):430–441. doi: 10.1002/eat.10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. Journal of the American Medical Association. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh Y, Mason M, Williams K. Researchers’ perspectives on pediatric obesity research participant recruitment. Clinical and Translational Medicine. 2016;5(1):20. doi: 10.1186/s40169-016-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson CM, Smith GT. Bulimic symptom onset in young girls: A longitudinal trajectory analysis. Journal of Abnormal Psychology. 2015;124(4):1003–1013. doi: 10.1037/abn0000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualter P, Murphy SM, Abbott J, Gardner KJ, Japel C, Vitaro F, … Tremblay RE. Developmental associations between victimization and body mass index from 3 to 10 years in a population sample. Aggressive Behavior. 2015;41(2):109–122. doi: 10.1002/ab.21580. [DOI] [PubMed] [Google Scholar]
- Saltzman JA, Liechty JM. Family correlates of childhood binge eating: A systematic review. Eating Behaviors. 2016;22:62–71. doi: 10.1016/j.eatbeh.2016.03.027. [DOI] [PubMed] [Google Scholar]
- Sonneville KR, Horton NJ, Micali N, Crosby RD, Swanson SA, Solmi F, Field AE. Longitudinal associations between binge eating and overeating and adverse outcomes among adolescents and young adults: Does loss of control matter? JAMA Pediatrics. 2013;167(2):149–155. doi: 10.1001/2013.jamapediatrics.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger G, Lushene Vagg, Jacobs . Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologist Press; 1983. [Google Scholar]
- Tanner JM. Growth and maturation during adolescence. Nutrition Reviews. 1981;39(2):43–55. doi: 10.1111/j.1753-4887.1981.tb06734.x. [DOI] [PubMed] [Google Scholar]
- Tanofsky-Kraff M. Binge eating among children and adolescents. In: Jelalian E, Steele R, editors. Handbook of Child and Adolescent Obesity. New York: Springer Publishers; 2008. pp. 41–57. [Google Scholar]
- Tanofsky-Kraff M, Goossens L, Eddy KT, Ringham R, Goldschmidt A, Yanovski SZ, … Yanovski JA. A multisite investigation of binge eating behaviors in children and adolescents. Journal of Consulting and Clinical Psychology. 2007;75(6):901–913. doi: 10.1037/0022-006X.75.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Shomaker LB, Olsen C, Roza CA, Wolkoff LE, Columbo KM, … Yanovski JA. A prospective study of pediatric loss of control eating and psychological outcomes. Journal of Abnormal Psychology. 2011;120(1):108–118. doi: 10.1037/a0021406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Shomaker LB, Stern EA, Miller R, Sebring N, Dellavalle D, … Yanovski JA. Children’s binge eating and development of metabolic syndrome. International Journal of Obesity. 2012;36(7):956–962. doi: 10.1038/ijo.2011.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Shomaker LB, Wilfley DE, Young JF, Sbrocco T, Stephens M, … Yanovski JA. Excess weight gain prevention in adolescents: Three-year outcome following a randomized controlled trial. Journal of Consulting and Clinical Psychology. 2017;85:218–227. doi: 10.1037/ccp0000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Shomaker LB, Wilfley DE, Young JF, Sbrocco T, Stephens M, … Yanovski JA. Targeted prevention of excess weight gain and eating disorders in high-risk adolescent girls: A randomized controlled trial. American Journal of Clinical Nutrition. 2014;100(4):1010–1018. doi: 10.3945/ajcn.114.092536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Wilfley DE, Young JF, Mufson L, Yanovski SZ, Glasofer DR, Salaita CG. Preventing excessive weight gain in adolescents: Interpersonal psychotherapy for binge eating. Obesity. 2007;15(6):1345–1355. doi: 10.1038/oby.2007.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Wilfley DE, Young JF, Mufson L, Yanovski SZ, Glasofer DR, … Schvey NA. A pilot study of interpersonal psychotherapy for preventing excess weight gain in adolescent girls at-risk for obesity. International Journal of Eating Disorders. 2010;43(8):701–706. doi: 10.1002/eat.20773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Yanovski SZ, Schvey NA, Olsen CH, Gustafson J, Yanovski JA. A prospective study of loss of control eating for body weight gain in children at high risk for adult obesity. International Journal of Eating Disorders. 2009;42(1):26–30. doi: 10.1002/eat.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Yanovski SZ, Wilfley DE, Marmarosh C, Morgan CM, Yanovski JA. Eating-disordered behaviors, body fat, and psychopathology in overweight and normal-weight children. Journal of Consulting and Clinical Psychology. 2004;72(1):53–61. doi: 10.1037/0022-006X.72.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Bothwell S. Assessment of social adjustment by patient self-report. Archives of General Psychiatry. 1976;33(9):1111–1115. doi: 10.1001/archpsyc.1976.01770090101010. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Markowitz J, Klerman GL. Comprehensive guide to Interpersonal Psychotherapy. New York: Basic Behavioral Science Books; 2000. [Google Scholar]
- Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. New England Journal of Medicine. 1997;337(13):869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- Wildes JE, Marcus MD, Kalarchian MA, Levine MD, Houck PR, Cheng Y. Self-reported binge eating in severe pediatric obesity: Impact on weight change in a randomized controlled trial of family-based treatment. International Journal of Obesity. 2010;34(7):1143–1148. doi: 10.1038/ijo.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfley DE, Kass AE, Kolko RP. Counseling and behavior change in pediatric obesity. Pediatric Clinics of North America. 2011;58(6):1403–1424. x. doi: 10.1016/j.pcl.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfley DE, Stein RI, Saelens BE, Mockus DS, Matt GE, Hayden-Wade HA, … Epstein LH. Efficacy of maintenance treatment approaches for childhood overweight: A randomized controlled trial. Journal of the American Medical Association. 2007;298(14):1661–1673. doi: 10.1001/jama.298.14.1661. [DOI] [PubMed] [Google Scholar]
- Wilfley DE, Vannucci A, White EK. Early intervention of eating- and weight-related problems. Journal of Clinical Psychology in Medical Settings. 2010;17(4):285–300. doi: 10.1007/s10880-010-9209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JF, Mufson L, Davies M. Efficacy of Interpersonal Psychotherapy-Adolescent Skills Training: An indicated preventive intervention for depression. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2006;47(12):1254–1262. doi: 10.1111/j.1469-7610.2006.01667.x. [DOI] [PubMed] [Google Scholar]