Abstract
Importance
It is unclear if female carriers of the Apolipoprotein E (APOE) ε4 allele are at greater risk of developing Alzheimer’s disease (AD) than men, and the sex-dependent association of mild cognitive impairment (MCI) and APOE has not been established.
Objective
To determine how sex and APOE genotype affect the risks for developing MCI and AD.
Data Sources
Twenty-seven independent research studies in the Global Alzheimer’s Association Interactive Network with data on nearly 58,000 subjects.
Study Selection
Non-Hispanic Caucasians with clinical diagnostic and APOE genotype data.
Data Extraction and Synthesis
Homogeneous data sets were pooled in case-control analyses, and logistic regression models were used to compute risks.
Main Outcome(s) and Measure(s)
Age-adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for developing MCI and AD were calculated for men and women across APOE genotypes.
Results
APOE ε3/ε4 men (OR, 3.09; CI, 2.79 – 3.42) and women (OR, 3.31; CI, 3.03 – 3.61) across the lifespan of 55 to 85 years of age did not show a difference in AD risk; however, women had an increased risk compared to men between the ages of 65 and 75 (p-value = 0.002). APOE ε3/ε4 men had an increased risk of AD compared to APOE ε3/ε3 men (p-value < 0.001). The APOE ε2/ε3 genotype conferred a protective effect on women (OR, 0.51; CI, 0.43 – 0.61) decreasing their risk of AD more (p-value = 0.01) than men (OR, 0.71; CI, 0.60 – 0.85). There was no difference between APOE ε3/ε4 men (OR, 1.55; CI, 1.36 – 1.76) and women (OR, 1.60; CI, 1.43 – 1.81) in their risk of developing MCI between the ages of 55 and 85, but women had an increased risk between 55 and 70 (p-value = 0.05). There were no significant differences between men and women in their risks for converting from MCI to AD between the ages of 55 and 85. APOE ε4/ε4 individuals showed increased risks over ε3/ε4 individuals for developing AD and MCI and converting from MCI to AD.
Conclusions and Relevance
Contrary to long-standing views, men and women with the APOE ε3/ε4 genotype have nearly the same odds of developing AD across the age span of 55 to 85 years, but women have an increased risk at younger ages.
Introduction
For nearly twenty years, the prevalent view has been that women who carry copies of the ε4 allele of the Apolipoprotein E (APOE) gene have a greater risk of developing Alzheimer’s disease (AD) than men with the same number of copies.1 The ε4 allele is the main genetic risk factor for late-onset Alzheimer’s disease (AD)2, and sex-based differences in AD risk have important implications for treatment trials, diagnostics, and therapeutics3. Additionally, the sex-dependent relationship between APOE and mild cognitive impairment (MCI), which is often a transitional phase from cognitively normal (NL) aging to dementia,4 is unclear. Studies are in general agreement that the APOE ε4 allele is a risk factor for developing MCI,5,6,7,8,9,10,11 but there is controversy as to whether it increases10,12,13,14 or does not increase9,11,15,16 the risks of transitioning from MCI to AD or dementia. The three most common alleles of the APOE gene are ε2, ε3, and ε4; whereas carrying the ε4 allele increases one’s risk of developing AD, the ε2 allele conversely has a putative protective effect that is associated with longevity and a lower AD risk.17
Studies of participants with a family history of late-onset AD have reported that women with one copy of ε4 have a greater risk than male heterozygote ε4 carriers, who in turn have about the same risk as male ε3 homozygotes18,19. This sex dependence was also found in first-degree (parents and siblings) relatives of individuals with AD,20,21 and in the meta-analysis of Farrer et al.,1 which aggregated data from 40 independent research studies. Among studies of residents in city suburbs and communities, there is general agreement that elderly female ε4 carriers have an increased risk of AD, dementia, and cognitive decline over male ε4 carriers.22,23,24,25. However, when subjects are randomly recruited from hospitals, retirement homes, and aging consortiums, most studies have found no sex-specific difference between men and women in the risks of AD and dementia associated with the APOE ε4 allele26,27,28,29. The sex-dependent role of APOE ε4 in the risks of developing MCI and in MCI conversions to AD has been recently investigated,30,3 and there is evidence that women are at greater risk than men.
Methods
We collected data sets from 27 independent research studies totaling nearly 58,000 subjects. Information was collected on each subject’s APOE genotype, sex, race, ethnicity, diagnosis (NL, MCI, AD), and age at diagnosis. From these data sets we included only Caucasian subjects that were mostly non-Hispanic.
GAAIN Data Sets
Prospective participants for this meta-analysis were identified using resources1 from the31,32 Global Alzheimer’s Association Interactive Network (GAAIN). As shown in Table 1, we utilized multiple data sets from 12 research institutions in GAAIN, with two institutions (NIAGADS, CAMD) managing data from several independent studies. Details of the data sets obtained through GAAIN are given in the Data Set Description section in the Supplement.
Table 1.
Characteristics of APOE data sets from the Global Alzheimer’s Association Interactive Network (GAAIN)
| Data Set | Name | No. of Subjects | Diagnosis | Race/Country | Ethnicity | Ascertainment |
|---|---|---|---|---|---|---|
| ACE | Fundació ACE50 | 1,243 | MCI,AD | 99% Spain; 1% O | 100% U | Mostly residents of Barcelona, Spain |
| ADNI | Alzheimer’s Disease Neuroimaging Initiative51 | 2,065 | NL,MCI,AD | 93% C; 5% B; 2% O | 96% N; 3% H; 1% U | 59 acquisition sites across United States and Canada |
| AIBL | The Australian Imaging, Biomarkers & Lifestyle Flagship Study of Ageing52 | 834 | NL,MCI,AD | 100% Australia | 100% U | 2 acquisition centers in Australia |
| ARWIBO | Alzheimer’s Disease Repository Without Borders53,54 | 1,201 | NL,MCI,AD | 100% C | 100% N | Mostly residents of Brescia, Italy |
| CAMD | Coalition Against Major Diseases55 | 2,382 | MCI,AD | 91% C; 5% A; 3% B; 1% O | 58% N; 36% U; 6% H | |
| - 1009 | Clinical Trial 1009 | 162 | AD | 99% C; 1% O | 100% U | Canada and several European countries |
| - 1056 | Clinical Trial 1056 | 493 | AD | 92% C; 7% A; 1% B | 95% N; 3% H; 2% U | Several countries |
| - 1057 | Clinical Trial 1057 | 500 | AD | 88% C; 9% A; 3% O | 81% N; 19% H | Europe, Japan, and Argentina |
| - 1058 | Clinical Trial 1058 | 166 | AD | 76% C; 22% A; 2% O | 90% N; 10% H | Several countries |
| - 1105 | Clinical Trial 1105 | 266 | - | 98% C; 1.5% B; 0.5% O | 100% U | United States, Canada, Europe, South Africa |
| - 1132 | Clinical Trial 1132 | 286 | MCI | 93% C; 5% B; 2% O | 100% U | Multiple U.S. states |
| - 1136 | Clinical Trial 1136 | 141 | AD | 100% C | 100% U | Scandinavia |
| - 1142 | Clinical Trial 1142 | 368 | AD | 87% C; 9% B; 2% A; 2% O | 93% N; 6% H; 1% U | Multiple U.S. states |
| EDSD | European Diffusion Tensor Imaging Study in Dementia56 | 196 | NL,MCI,AD | 100% C | 100% N | 9 memory assessment clinics in 4 European countries |
| FHS | Framingham Heart Study57 | 5,402 | NL,MCI,AD | 99% C; 1% O | 99% N; 1% H | Residents of Framingham, Massachusetts |
| LMRR | Laboratory of Magnetic Resonance Research | 113 | NL,MCI,AD | 100% A | 100% N | Residents of Taiwan |
| NACC | National Alzheimer’s Coordinating Center58 | 23,999 | NL,MCI,AD | 83% C; 10% B; 5% O; 2% A | 92% N; 7% H; 1% U | 34 centers in United States |
| NIAGADS | National Institute on Aging Genetics of Alzheimer’s Disease Data Storage Site59 | 18,869 | NL,AD | 91% C; 8% O, 1% B | 51% U; 46% N; 3% H | |
| - UPitt | University of Pittsburgh study60 | 2,436 | NL,AD | 90% C; 10% O | 100% U | Recruited subjects from one center at University of Pittsburgh |
| - TGEN2 | Translational Genomics Research Institute study61,62,63 | 1,599 | AD | 64% C; 36% O | 100% U | Brain donors and healthy controls with first-degree relative with AD |
| - ROS/MAP | Religious Orders Study/Rush Memory and Aging Project64,65,66,67 | 1,571 | AD | 99.9% C; 0.1% B | 99% N; 1% H | 40 religious groups from 12 states in mid-west United States/40 retirement communities in northeastern Illinois |
| - WashU | Washington University study | 670 | NL,AD | 100% C | 100% U | |
| - MIRAGE | Multi Institutional Research of Alzheimer Genetic Epidemiology study68 | 1,245 | NL,AD | 94% C; 6% O | 100% U | 17 clinical centers in the United States, Canada, Germany, and Greece |
| - NIA-LOAD | National Institute on Aging LOAD Family Study69 | 5,220 | NL,AD | 85% C; 10% O; 5% B | 88% N; 11% H; 1% U | Recruited families with 2 or more AD siblings |
| - ACT | Adult Changes in Thought70 | 2,432 | NL,AD | 100% C | 100% N | Random subjects from a Seattle HMO |
| - UMVUMSSM | University of Miami, Vanderbilt University, Mount Sinai School of Medicine study71 | 1,632 | NL,AD | 100% C | 100% U | |
| - MAYO GWAS | Mayo Clinic GWAS study72 | 2,064 | NL,AD | 100% C | 100% U | 3 Mayo Clinics in the United States |
| PharmaCog (E-ADNI) | Prediction of cognitive properties of new drug candidates for neurodegenerative diseases in early clinical development73 | 143 | MCI | 100% C | 100% N | 9 memory assessment clinics in 4 European countries |
| WRAP | Wisconsin Registry for Alzheimer’s Prevention74 | 1,532 | NL,MCI | 91% C; 8% B; 1% O | 98% N; 2% H | Persons with or without parental history of sporadic AD recruited throughout Wisconsin |
| TOTAL: | 57,979 |
Abbreviations: A, Asian; AD, Alzheimer’s disease; B, Black; C, Caucasian; GWAS, genome-wide association study; H, Hispanic; HMO, health maintenance organization; LOAD, late onset Alzheimer’s disease; MCI, mild cognitive impairment; N, non-Hispanic; NL, normal cognitive; O, other races; U, unknown ethnicity.
We did not receive information about clinical diagnoses for all subjects, and in some cases the ages of elderly subjects were truncated downward to 90 years to protect their identities. We excluded subjects with missing information and/or 90 year-truncated ages from all data sets. In many data sets, birth dates were rounded to the nearest year as an extra measure to protect subject confidentiality. We excluded data from subjects in the NACC data set who were also known to have participated in the ADNI study; however, the full extent of the subject overlap between NACC and ADNI has not currently been established, but is estimated to be at most 3%. Across data sets most subjects were Caucasian, and for many subjects, ethnic information was either not collected or not known. Due to insufficient numbers of other races, we only included subjects of the Caucasian race (along with subjects from the ACE and AIBL data sets) with non-Hispanic or unknown ethnicities. Through our correspondences with data set providers, we estimate that Hispanic subjects make up no more than 5% of all Caucasian subjects with unknown ethnicities. After applying exclusion criteria, these data sets were representative of non-Hispanic Caucasians in North America and Europe.
The descriptions of the clinical diagnoses we received were unstandardized32 and the levels of detail varied across different data sets according to how each disease was defined (e.g., “mild” or “moderate” AD) and how it was recorded (e.g., “AD associated with cerebrovascular disease”). We worked directly with each data set provider to translate each set of diagnoses into our three general preplanned diagnoses: NL, MCI, and AD. In addition, we excluded all subjects with a clinical history of stroke, cerebrovascular disease, Lewy bodies, APP or presenilin gene mutations, or comorbidity with any other known neurological disease. All subtypes of MCI (e.g., amnestic and non-amnestic) were combined into a single MCI diagnosis.
For longitudinal data sets (e.g., NACC and FHS) that had multiple diagnoses per subject, we assigned each subject a single diagnosis as follows. Each subject without a history of MCI or AD was assigned a NL diagnosis, each subject with a history of MCI and no history of AD was assigned an MCI diagnosis, and each subject with a history of AD and no history of MCI was assigned an AD diagnosis. Subjects with a history of both MCI and AD were randomly assigned either an MCI or AD diagnosis. We used the latest examination age for the diagnosis age of NL subjects and the earliest recorded age of MCI or AD for MCI and AD subjects, respectively. With the exception of the FHS data set, no subjects were followed more than 10 years; therefore, our NL diagnosis ages were not significantly skewed towards very old ages. We used these diagnosis assignments to form three case-control study groups containing 22 AD-NL, 10 MCI-NL, and 7 AD-MCI data sets.
Statistical Analysis
Meta-analyses of the case-control study groups were conducted using the Mantel-Haenszel fixed-effects method to calculate odds ratios for each sex and APOE genotype using the APOE ε3/ε3 genotype as the referent. We imputed missing NL data in the ACE, CAMD, TGEN2, and ROS/MAP data sets using available NL subject data as follows. The Mann-Whitney U test was used to compare the age distributions of NL subjects from each research study, and dissimilar NL subject data was excluded. In particular, we excluded the ARWIBO and WRAP data sets because the median age of their NL subjects was relatively young (mid-fifties to mid-sixties) and that of the ACT data set was comparatively older (lower eighties). Variations in the total numbers of ε2, ε3, and ε4 alleles of NL subjects were then compared using the chi-square test of homogeneity ( ) to exclude correspondingly heterogeneous data sets. The resultant NL subject data contained men ( ) and women ( ) with NL diagnoses from the ADNI, AIBL, NACC, and WashU data sets, respectively. The NL subjects used for imputation were in Hardy-Weinberg equilibrium (males: , p-value = 0.39; females: , p-value = 0.75), their ages were normally distributed (male mean = 73.5 y [SD, 7.0 y]; female mean = 74.6 [SD, 7.1 y]), and their APOE genotype frequencies were consistent with those reported for the general population of the United States33 (eTable 4 in the Supplement). Forest plots of the log odds ratios for the APOE ε3/ε4 genotype by sex are shown in eFigures 1, 2, and 3 in the Supplement. Separate meta-analyses were also performed in three age ranges (55 to 65 years, 65 to 75 years, 75 to 85 years).
The meta-analyses were repeated after removing ascertainment-biased studies from the case-control study groups. Community-based studies (ACE, ARWIBO, FHS) that recruited participants in localized geographic regions and disease-biased studies (NIA-LOAD, TGEN2) that recruited participants with family histories of AD were excluded. The ROS/MAP study was also excluded because we did not have enough information to definitively remove subjects with comorbidities from its data set.
Data from each ascertainment-adjusted case-control study group was then pooled together and logistic regression was used to calculate odds ratios for each sex and APOE genotype (Table 2). For each sex, a continuous age variable and five indicator (values of one or zero) variables representing the five APOE genotypes (ε2/ε2, ε2/ε3, ε2/ε4, ε3/ε4, and ε4/ε4) were used with the APOE ε3/ε3 genotype as the referent. We also conducted another pooled analysis where we added a sex indicator variable and five additional covariates that were products of the sex variable with each APOE genotype variable in order to test for sex interactions. The age-dependent curves shown in Figure 2 were derived by adding several quadratic covariate products to the logistic regression that were created by combining APOE genotype, sex, and age. Because the NACC data set was predominantly larger (48% - 85%) than other data sets in the pooled analysis, we separated it from the pooled data and repeated the analyses without it and exclusively with it. Results of all the above analyses are listed in eTables 1, 2, and 3 in the Supplement for the APOE ε3/ε4 genotype.
Table 2.
Age-adjusted odds ratios of developing Alzheimer’s disease and MCI for men and women across APOE genotypes between the ages of 55 and 85
| APOE Genotype | Sex | Control | Case | Odds Ratio (95% CI) | P Value | Tarone’s P Value |
|---|---|---|---|---|---|---|
| AD-NL | NLa (n=9279) | ADb (n=10485) | ||||
| ε2/ε2 | Male | 23 | 6 | 0.34 (0.14 to 0.84) | 0.02 | 0.24 |
| Female | 23 | 9 | 0.69 (0.32 to 1.51) | 0.35 | 0.12 | |
| ε2/ε3 | Male | 415 | 222 | 0.71 (0.60 to 0.85) | P<.001 | 0.35 |
| Female | 646 | 199 | 0.51 (0.43 to 0.61) | P<.001 | 0.07 | |
| ε2/ε4 | Male | 74 | 115 | 2.07 (1.54 to 2.79) | P<.001 | 0.82 |
| Female | 129 | 173 | 2.28 (1.80 to 2.88) | P<.001 | 0.02 | |
| ε3/ε4 | Male | 867 | 2002 | 3.09 (2.79 to 3.42) | P<.001 | 0.53 |
| Female | 1390 | 2639 | 3.31 (3.03 to 3.61) | P<.001 | 0.03 | |
| ε4/ε4 | Male | 86 | 733 | 11.7 (9.24 to 14.7) | P<.001 | 0.02 |
| Female | 158 | 809 | 9.67 (8.07 to 11.6) | P<.001 | P<.001 | |
| ε3/ε3 | Male | 2184 | 1642 | 1 | ||
| Female | 3284 | 1936 | 1 | |||
| MCI-NL | NLc (n=6471) | MCId (n=5077) | ||||
| ε2/ε2 | Male | 12 | 8 | 0.68 (0.28 to 1.68) | 0.41 | P>.99 |
| Female | 17 | 7 | 0.87 (0.36 to 2.11) | 0.76 | 0.91 | |
| ε2/ε3 | Male | 257 | 247 | 0.99 (0.82 to 1.19) | 0.89 | 0.44 |
| Female | 457 | 172 | 0.78 (0.65 to 0.95) | 0.01 | 0.46 | |
| ε2/ε4 | Male | 48 | 74 | 1.61 (1.11 to 2.34) | 0.01 | 0.81 |
| Female | 100 | 69 | 1.54 (1.12 to 2.11) | 0.007 | 0.008 | |
| ε3/ε4 | Male | 595 | 893 | 1.55 (1.36 to 1.76) | P<.001 | 0.26 |
| Female | 1068 | 777 | 1.60 (1.43 to 1.81) | P<.001 | 0.66 | |
| ε4/ε4 | Male | 55 | 187 | 3.60 (2.64 to 4.91) | P<.001 | 0.56 |
| Female | 126 | 173 | 3.25 (2.55 to 4.15) | P<.001 | 0.22 | |
| ε3/ε3 | Male | 1407 | 1378 | 1 | ||
| Female | 2329 | 1092 | 1 | |||
| AD-MCI | MCIe (n=4496) | ADf (n=5228) | ||||
| ε2/ε2 | Male | 8 | 2 | 0.36 (0.08 to 1.69) | 0.19 | 0.92 |
| Female | 7 | 5 | 0.83 (0.26 to 2.63) | 0.75 | 0.98 | |
| ε2/ε3 | Male | 220 | 122 | 0.77 (0.61 to 0.97) | 0.03 | 0.19 |
| Female | 148 | 85 | 0.66 (0.49 to 0.87) | 0.004 | 0.93 | |
| ε2/ε4 | Male | 66 | 63 | 1.33 (0.93 to 1.90) | 0.12 | 0.83 |
| Female | 57 | 84 | 1.69 (1.19 to 2.39) | 0.003 | 0.38 | |
| ε3/ε4 | Male | 798 | 1098 | 1.90 (1.68 to 2.15) | P<.001 | 0.86 |
| Female | 680 | 1234 | 2.11 (1.84 to 2.40) | P<.001 | 0.46 | |
| ε4/ε4 | Male | 175 | 426 | 3.45 (2.83 to 4.20) | P<.001 | 0.13 |
| Female | 154 | 396 | 3.14 (2.54 to 3.87) | P<.001 | 0.77 | |
| ε3/ε3 | Male | 1235 | 892 | 1 | ||
| Female | 948 | 821 | 1 |
Abbreviations: AD, Alzheimer’s disease; APOE, Apolipoprotein E; CI, confidence interval; MCI, mild cognitive impairment; NL, normal cognition.
Male and female mean (SD) age of 73.4 (6.4) and 72.7 (6.7), respectively.
Male and female mean (SD) age of 73.6 (7.1) and 73.7 (7.1), respectively.
Male and female mean (SD) age of 72.6 (7.2) and 71.5 (7.5), respectively.
Male and female mean (SD) age of 73.1 (7.2) and 72.6 (7.5), respectively.
Male and female mean (SD) age of 73.6 (7.0) and 73.2 (7.3), respectively.
Male and female mean (SD) age of 74.0 (7.5) and 73.8 (7.7), respectively.
Figure 2.
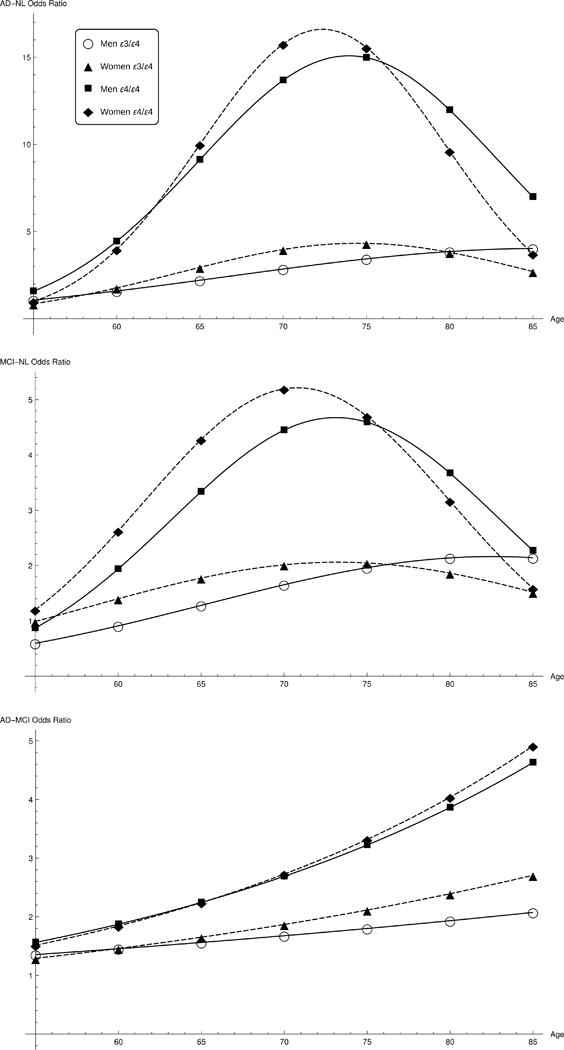
Alzheimer’s disease and mild cognitive impairment odds ratios for men and women with APOE ε3/ε4 genotypes between the ages of 55 and 85.
AD and MCI risk factors were calculated for men and women between the ages of 55 and 85 for each APOE genotype. Age-adjusted odds ratios are listed in Table 2 and shown in Figure 2 as a function of age for the APOE ε3/ε4 genotype. All male odds ratios were calculated relative to ε3/ε3 men, and all female odds ratios relative to ε3/ε3 women. Three conversion cases were considered: (1) developing AD from a cognitively normal (NL) status, (2) developing MCI from a NL status, and (3) transitioning from MCI to AD. Each conversion is labeled AD-NL, MCI-NL, and AD-MCI, respectively, in Table 2 and Figure 2.
Abbreviations: AD=Alzheimer’s disease; MCI=mild cognitive impairment; NL= normal cognition
Statistical analyses were performed in R2 (version 3.3.1) using the “metafor” meta-analysis package (version 1.9-9) along with the “glm” generalized linear model function. Mathematica3 (version 10.0) was used for curve fitting and plotting.
Results
From an aggregation of 27 independent research studies with a total of 57,979 subjects (Table 1), meta-analyses were performed on 31,340 non-Hispanic Caucasians with clinical diagnoses between the ages of 55 and 85 in three case-control analyses (Figure 1). After excluding ascertainment-biased studies, the data in each analysis was pooled and odds ratios for each sex and APOE genotype (Table 2) were calculated. In all case-control analyses, between-study heterogeneity was reduced after the removal of ascertainment-biased study data. However, p-values from Tarone’s34 test of heterogeneity (Table 2) still detected significant study heterogeneity in the female APOE ε3/ε4 data (p-value = 0.03) and in the APOE ε4/ε4 data (male p-value = 0.02; female p-value < 0.001) of the AD-NL analysis. Upon further investigation (eTable 1 of the Supplement), we found that the heterogeneity in the female APOE ε3/ε4 data was localized to the ages of 75 to 85 years (p-value = 0.003). This determination was supported after comparing the odds ratios in that age range from analyses without the NACC data set (OR, 2.67; 95% CI, 2.23-3.21) and with the NACC data set exclusively (OR, 4.12; 95% CI, 3.41-4.98). Otherwise, between the ages of 55 and 85, the 95% confidence intervals of the odds ratios calculated from pooled data without the NACC data set overlapped the confidence intervals of the odds ratios calculated using the NACC data set alone.
Figure 1.
PRISMA Flowchart.
Abbreviations: AD=Alzheimer’s disease; MCI=mild cognitive impairment; NL= normal cognition
As shown in Table 2, men and women with the APOE ε3/ε4 genotype had the same risks of developing AD (men OR, 3.09 [95% CI, 2.79 – 3.42]; women OR, 3.31 [95% CI, 3.03 – 3.61]; APOE-sex interaction p-value = 0.47) between the ages of 55 and 85. APOE ε3/ε4 men had an increased risk of AD compared to ε3/ε3 men (p-value < 0.001). The APOE ε2/ε3 genotype decreased the risk of AD more for women than for men (women OR, 0.51 [95% CI, 0.43 – 0.61]; men OR, 0.71 [95% CI, 0.60 – 0.85]; APOE-sex interaction p-value = 0.01). Men and women with the APOE ε3/ε4 genotype had the same risks of developing MCI between the ages of 55 and 85 (men OR, 1.55 [95% CI, 1.36 – 1.76]; women OR, 1.60 [95% CI, 1.43 – 1.81]; APOE-sex interaction p-value = 0.82).
Odds ratio curves for males and females with the APOE ε3/ε4 genotype are shown in Figure 2 between the ages of 55 and 85. The odds ratios calculated from the pooled data analyses in three age ranges (55 to 65 years, 65 to 75 years, 75 to 85 years) are plotted for each sex with error bars indicating their 95% confidence intervals. As shown in the top plot between the ages of 65 and 75, APOE ε3/ε4 women had an increased risk of AD compared to ε3/ε4 men (women OR, 4.37 [95% CI, 3.82 – 5.00]; men OR, 3.14 [95% CI, 2.68 – 3.67]; APOE-sex interaction p-value = 0.002). In the middle plot, the odds ratio curves suggested that APOE ε3/ε4 women were at higher risk for developing MCI than men between the ages of 55 and 70, which was confirmed in a separate analysis in that age range (women OR, 1.43 [95% CI, 1.19 – 1.73]; men OR, 1.07 [95% CI, 0.87 – 1.30]; APOE-sex interaction p-value = 0.05). No significant risk differences between men and women for MCI to AD transitions were found in the lower plot, but the odds ratio curves parallel a previous study that found that APOE ε4 increased the risk of transitioning from MCI to AD between the ages of 70 to 85, but not between the ages of 55 to 69.16
Discussion
When examining the entire age span from 55 to 85 years of age, men and women with the APOE ε3/ε4 genotype had nearly the same odds of developing MCI and AD, both in comparisons between data sets and in data set aggregation. Notably, women had an increased risk of MCI between the ages of 55 and 70 and an increased risk of AD between the ages of 65 and 75. These results are consistent with a previous study that found a significant association between APOE ε4 and cognitive decline between the ages of 70 to 80 in women only,24 and with another study that found that episodic memory was more impaired in APOE ε3/ε4 women than in ε3/ε4 men between the ages of 70 to 74.25 Mechanisms that underlie these sex differences may be linked to physiologic changes associated with menopause and estrogen loss that on average begin at 51 years of age35 just prior to our risk groups. Studies in animals and humans have reported an interaction between APOE ε4, menopause, and cognitive decline (for a review, see reference 36). Furthermore, other evidence suggests that carrying one copy of APOE ε4 shifts the age of onset in women, but not in men18. Collectively, our findings along with previous work warrant further investigation into a likely complex set of risk factors with consideration of sex-specific treatments for cognitive decline and Alzheimer’s disease. For example, if women are at increased risk for Alzheimer’s disease at younger ages, it is plausible that treatments for women may need to be initiated earlier, especially in those who carry an APOE ε4 allele. Both APOE ε3/ε4 men and women had an increased risk of AD compared to ε3/ε3 men and women, respectively. The APOE ε2/ε3 genotype conferred more of a protective effect on women, decreasing their risk of AD more than men. No significant sex-dependent differences were found for transitioning between MCI and AD. Our odds ratios for developing MCI are consistent with other studies.6,37
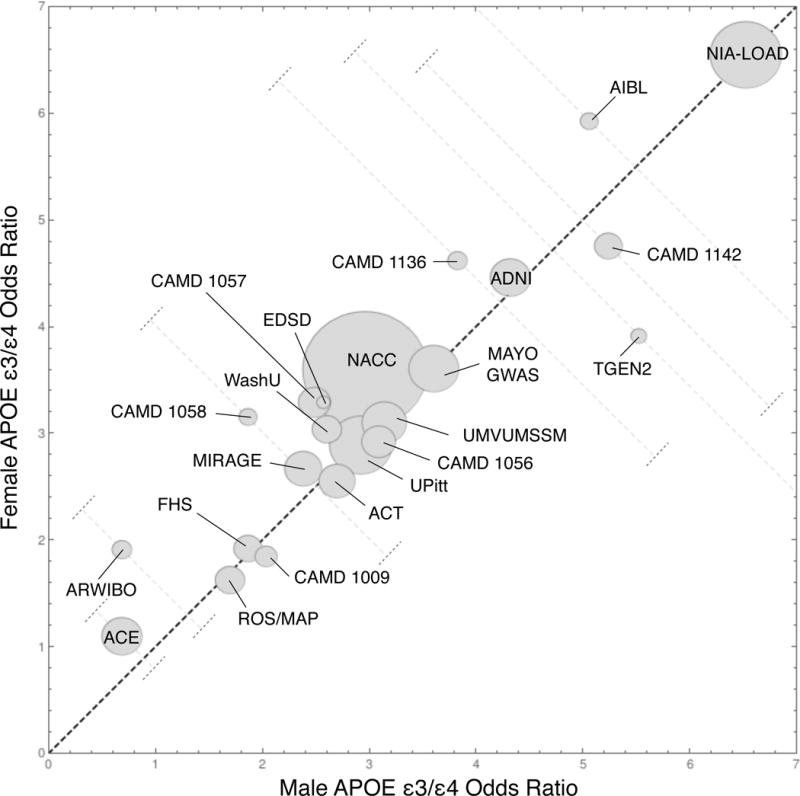
After adjusting for NL subject differences between AD studies by replacing NL subjects with the data set we used for imputation, there was significant variation of AD risk between data sets; the male and female ε3/ε4 odds ratios were near one for the ACE data set and nearly seven for the NIA-LOAD data set. In retrospect, high odds ratios were not remarkable for the NIA-LOAD study, which recruited families with two or more affected siblings with AD, because family history of AD is an AD risk factor and the probability of carrying a genetic mutation in a recognized AD gene increases with the number of first-degree relatives affected with AD.38 The lowest odds ratios tended to be associated with community-based studies (e.g., ACE, ARWIBO, and FHS) that ascertained subjects from geographically specific cities and suburbs. As shown in eFigure 4, most data points clustered around the NACC data point; these studies primarily recruited random subjects who were unrelated to one another.
These results are notably different from those of Farrer et al.1, who found that the relative odds of ε3/ε4 women compared to ε3/ε4 men for developing AD were about 1.5, and that ε3/ε3 and ε3/ε4 men had the same AD risks when subjects were ascertained from clinics/hospitals and autopsies/brain banks (n=6,305). Many of the subjects in their meta-analysis had family histories of AD, they noted differences with population-based studies, and they aggregated subjects with early-onset AD. Inclusion of the latter subjects could help explain why their AD odds ratio curves for ε3/ε4 individuals reached their maxima around ages 60 to 65, as opposed to ours which reached their maxima around the ages of 73 to 80. These results are in closer agreement with studies that have found ε3/ε4 carriers to have a mean age of clinical onset of 76 years, and the risk for developing late-onset AD to occur primarily between the ages of 60 to 79.26 We note that between the ages of 65 to 75, the odds ratios of APOE ε3/ε4 women and men differed by a factor of about 1.5, which is consistent with Farrer’s results across all ages. Our result that the APOE ε2/ε3 genotype decreased the risk of AD more for women than for men is the opposite of what they found; this is likely due to the fact that our analysis (n=1482) used more than three the number of subjects than they used (n=447).
In agreement with previous studies1,39, we found that individuals with two copies of the APOE ε4 allele were at greater risk for developing AD than individuals with only one copy. No significant differences between ε4/ε4 men and women were seen in their risks for developing AD, which is consistent with the results reported by Farrer. APOE ε4 homozygotes also had increased risks compared to ε4 heterozygotes for MCI and for transitioning from MCI to AD.
Ascertainment biases are known to modify the true effects of APOE on the risks of developing AD, and they may have played a role in the variations we found between data sets. Men have higher rates of cardiovascular disease and stroke than women, so men who live to old age may be healthier than women of the same age and therefore have lesser risks of developing AD.40,41 On average women live longer than men, which makes it difficult to locate older men with AD in sufficient numbers to study. There may be increased study participation rates among individuals with a family history of AD,42 which is an established risk factor for developing AD.43,44,45 Population-based studies can oversample participants from families in areas where widows outnumber widowers.23 Non-responders are generally burdened with higher rates of illness than responders to surveys and they require extra effort to participate.46 Biases may occur when recruitment and dropout occur continuously throughout studies,29 or when individuals do not consent to or are not available for genotyping. A notable example of ascertainment bias occurred in a study that compared subjects sampled from a research clinic with subjects recruited through a health maintenance organization; they found that the research-based cohort contained younger subjects, more severe AD cases, and a higher APOE ε4 allele frequency.47
Variability in the methodologies used to define AD and MCI across data sets could have affected our results. We relied upon the expertise of each data set provider to translate their diagnostic definitions into our general AD and MCI diagnoses independently of other data set providers. Although it would have been preferable to use MCI subtypes (e.g., amnestic, non-amnestic), that level of diagnostic detail was mostly unavailable. We could not adjust for known AD risk factors such as the number of years of education and family history of AD/dementia because in many data sets that information was not provided. Nor could we account for sex-dependent differences due to factors such as cigarette smoking, hormonal changes with age, and alcohol usage48. As was previously mentioned, in some data sets the birth dates of subjects were rounded to the nearest year, and that limited the accuracy in determining the onset ages of AD and MCI. Finally, we were not able to fully exclude all Hispanic subjects from our meta-analysis because in many cases information about ethnicity was not collected. Although we believe the percentage of Hispanic subjects to be less than 5%, this could have affected our results since the odds of developing AD is different among Hispanics than in Caucasians.1 Taken together, limited information on risk factors were not modeled in our analysis due to our large pooled cohort approach. Of particular note, lifestyle factors such as lower educational attainment and vascular risk factors are well-documented contributors to Alzheimer’s risk49 and could have influenced our findings.
Supplementary Material
Key Points.
Question
Are female carriers of the Apolipoprotein E ε4 allele at greater risk of developing Alzheimer’s disease than men?
Findings
In this meta-analysis of 27 independent research studies with 58,000 subjects, women and men with one copy of Apolipoprotein E ε4 did not show a difference in risk of Alzheimer’s disease across the lifespan of 55 to 85 years of age. However, these women were at increased risk over men between the ages of 65 and 75.
Meaning
Sex-specific treatments for cognitive decline and Alzheimer’s disease may need to be initiated a younger age, especially those who carry an Apolipoprotein E ε4 allele.
Tweet.
Women with one copy of APOE ε4 have same lifetime risk of Alzheimer’s disease as men except between ages 65 and 75
Acknowledgments
Dr. Neu had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
This work was supported by the Global Alzheimer’s Association Interactive Network (GAAIN) initiative of the Alzheimer’s Association (GAAIN-14-244631) and National Institutes of Health grants U54-EB020406 and P41-EB015922.
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH- 12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous con- tributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol- Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neuro- track Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Data used in the preparation of this article was obtained from the Australian Imaging Biomarkers and Lifestyle flagship study of ageing (AIBL) funded by the Commonwealth Scientific and Industrial Research Organisation (CSIRO) which was made available at the ADNI database (www.loni.usc.edu/ADNI). The AIBL researchers contributed data but did not participate in analysis or writing of this report. AIBL researchers are listed at www.aibl.csiro.au.
The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIAfunded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Steven Ferris, PhD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG016570 (PI Marie-Francoise Chesselet, MD, PhD), P50 AG005131 (PI Douglas Galasko, MD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosen- berg, MD), P50 AG005136 (PI Thomas Montine, MD, PhD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), and P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
Data for this study were prepared, archived, and distributed by the National Institute on Aging Alzheimer’s Disease Data Storage Site (NIAGADS) at the University of Pennsylvania (U24-AG041689-01), funded by the National Institute on Aging.
Data used in preparation of this article were obtained from the Coalition Against Major Diseases (CAMD) database (http://codr.cpath.org). A complete listing of CAMD members can be found at: http://c-path.org/programs/camd/. Funding of the CAMD database is made possible by membership dues and a by grant number 1U18FD005320 from the U.S. Food and Drug Administration’s Critical Path Public Private Partnerships Grant Program.
ARWIBO, EDSD, and PharmaCOG (alias E-ADNI) data used in the preparation of this article was obtained from NeuGRID4You initiative (www.neugrid4you.eu) funded by the European Commission (FP7/2007-2013) under grant agreement no.283562.
Data used in this study was supported by the Framingham Heart Study’s National Heart, Lung, and Blood Institute contract (N01-HC-25195), by grants (R01-AG016495, R01-AG008122, R01-AG033040) from the National Institute on Aging, and by grant (R01-NS017950) from the National Institute of Neurological Disorders and Stroke. The authors thank the participants for their extraordinary dedication.
We thank all patients for their participation in this project. We also thank Trinitat Port-Carbó and her family for their support of the Fundació ACE research programs. Fundació ACE collaborates with the Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas (CIBERNED, Spain), and is one of the participating centers of the Dementia Genetics Spanish Consortium (DEGESCO). CIBERNED is an Instituto de Salud Carlos III ISCIII Project. AR is supported by Grant PI13/02434 (Acción Estratégica en Salud, Instituto de Salud Carlos III (ISCIII), Ministerio de Economía y Competitividad, Spain). Genetic Research project at Fundació ACE (GR@ACE) is funded by Fundación Bancaria ‘La Caixa’ (Barcelona, Spain), GRIFOLS S.A and intramural funds.
Data used in this study was supported by the National Institutes of Health grant R01-AG027161.
The author (Wang) would like to acknowledge the support from Health Ageing Research Center, ChangGung University, and Center for Advanced Molecular Imaging and Translation, Chang Gung Memorial Hospital, Linkou
This work was supported by grants UL1TR001855 and UL1TR000130 from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health.
Footnotes
Contributor Information
Scott C. Neu, Laboratory of Neuro Imaging, Stevens Institute for Neuroimaging and Informatics, Keck School of Medicine, University of Southern California, Los Angeles, CA.
Judy Pa, Laboratory of Neuro Imaging, Stevens Institute for Neuroimaging and Informatics, Keck School of Medicine, University of Southern California, Los Angeles, CA.
Walter Kukull, National Alzheimer’s Coordinating Center, University of Washington, Seattle, Wa.
Duane Beekly, National Alzheimer’s Coordinating Center, University of Washington, Seattle, Wa
Amanda Kuzma, Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia, PA.
Prabhakaran Gangadharan, Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia, PA.
Li-San Wang, Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia, PA.
Klaus Romero, Critical Path Institute, Coalition Against Major Disease, Tucson, AZ.
Stephen P. Arneric, Critical Path Institute, Coalition Against Major Disease, Tucson, AZ.
Alberto Redolfi, IRCCS Fatebenefratelli, The National Centre for Alzheimer’s Disease, Brescia, Italy.
Daniele Orlandi, IRCCS Fatebenefratelli, The National Centre for Alzheimer’s Disease, Brescia, Italy.
Giovanni B. Frisoni, University Hospitals and University of Geneva, Switzerland; IRCCS Fatebenefratelli, The National Centre for Alzheimer’s Disease, Brescia, Italy.
Rhoda Au, Depts of Anatomy & Neurobiology, Neurology & Epidemiology, Framingham Heart Study, Boston University Schools of Medicine & Public Health
Sherral Devine, Dept of Neurology/Framingham Heart Study, Boston University School of Medicine
Sanford Auerbach, Dept of Neurology/Framingham Heart Study, Boston University School of Medicine
Ana Espinosa, Research center and Memory clinic. Fundació ACE. Institut Català de Neurociències Aplicades. Barcelona. Spain.
Mercè Boada, Research center and Memory clinic. Fundació ACE. Institut Català de Neurociències Aplicades. Barcelona. Spain.
Agustín Ruiz, Research center and Memory clinic. Fundació ACE. Institut Català de Neurociències Aplicades. Barcelona. Spain.
Sterling C. Johnson, University of Wisconsin School of Medicine and Public Health.
Rebecca Koscik, University of Wisconsin School of Medicine and Public Health.
Jiun-Jie Wang, Department of Medical Imaging and Radiological Sciences, Chang Gung University; Neuroscience Research Center, Chang Gung Memorial Hospital, Linkou.
Wen-Chuin Hsu, Department of Neurology, Chang Gung Memorial Hospital, Linkou, Taiwan; Dementia Center, Chang Gung Memorial Hospital, Linkou, Taiwan.
Yao-Liang Chen, Department of Medical Imaging and Intervention, Chang Gung Memorial Hospital, Linkou, Taiwan; Department of Medical Imaging and Intervention, Chang Gung Memorial Hospital, Keelung, Taiwan.
Arthur W. Toga, Laboratory of Neuro Imaging, Stevens Institute for Neuroimaging and Informatics, Keck School of Medicine, University of Southern California, Los Angeles, CA.
References
- 1.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. Jama. 1997 Oct 22;278(16):1349–56. [PubMed] [Google Scholar]
- 2.Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nature Reviews Neurology. 2013 Feb 1;9(2):106–18. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ungar L, Altmann A, Greicius MD. Apolipoprotein E, gender, and Alzheimer’s disease: An overlooked, but potent and promising interaction. Brain imaging and behavior. 2014 Jun 1;8(2):262–73. doi: 10.1007/s11682-013-9272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen RC, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L. Mild cognitive impairment: a concept in evolution. Journal of internal medicine. 2014 Mar 1;275(3):214–28. doi: 10.1111/joim.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez OL, Jagust WJ, Dulberg C, Becker JT, DeKosky ST, Fitzpatrick A, Breitner J, Lyketsos C, Jones B, Kawas C, Carlson M. Risk factors for mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 2. Archives of neurology. 2003 Oct 1;60(10):1394–9. doi: 10.1001/archneur.60.10.1394. [DOI] [PubMed] [Google Scholar]
- 6.Tervo S, Kivipelto M, Hänninen T, Vanhanen M, Hallikainen M, Mannermaa A, Soininen H. Incidence and risk factors for mild cognitive impairment: a population-based three-year follow-up study of cognitively healthy elderly subjects. Dementia and geriatric cognitive disorders. 2004 Mar 17;17(3):196–203. doi: 10.1159/000076356. [DOI] [PubMed] [Google Scholar]
- 7.Kryscio RJ, Schmitt FA, Salazar JC, Mendiondo MS, Markesbery WR. Risk factors for transitions from normal to mild cognitive impairment and dementia. Neurology. 2006 Mar 28;66(6):828–32. doi: 10.1212/01.wnl.0000203264.71880.45. [DOI] [PubMed] [Google Scholar]
- 8.Boyle PA, Buchman AS, Wilson RS, Kelly JF, Bennett DA. The APOE e4 allele is associated with incident mild cognitive impairment among community-dwelling older persons. Neuroepidemiology. 2009 Nov 11;34(1):43–9. doi: 10.1159/000256662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barabash A, Marcos A, Ancin I, Vazquez-Alvarez B, de Ugarte C, Gil P, Fernández C, Encinas M, López-Ibor JJ, Cabranes JA. APOE, ACT and CHRNA7 genes in the conversion from amnestic mild cognitive impairment to Alzheimer’s disease. Neurobiology of aging. 2009 Aug 31;30(8):1254–64. doi: 10.1016/j.neurobiolaging.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Xu WL, Caracciolo B, Wang HX, Santoni G, Winblad B, Fratiglioni L. Accelerated progression from mild cognitive impairment to dementia among APOE ε4ε4 carriers. Journal of Alzheimer’s Disease. 2013 Jan 1;33(2):507–15. doi: 10.3233/JAD-2012-121369. [DOI] [PubMed] [Google Scholar]
- 11.Brainerd CJ, Reyna VF, Petersen RC, Smith GE, Kenney AE, Gross CJ, Taub ES, Plassman BL, Fisher GG. The apolipoprotein E genotype predicts longitudinal transitions to mild cognitive impairment but not to Alzheimer’s dementia: findings from a nationally representative study. Neuropsychology. 2013 Jan;27(1):86. doi: 10.1037/a0030855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersen RC, Smith GE, Ivnik RJ, Tangalos EG, Schaid DJ, Thibodeau SN, Kokmen E, Waring SC, Kurland LT. Apolipoprotein E status as a predictor of the development of Alzheimer’s disease in memory-impaired individuals. Jama. 1995 Apr 26;273(16):1274–8. [PubMed] [Google Scholar]
- 13.Samaranch L, Cervantes S, Barabash A, Alonso A, Cabranes JA, Lamet I, Ancín I, Lorenzo E, Martínez-Lage P, Marcos A, Clarimón J. The effect of MAPT H1 and APOE ε4 on transition from mild cognitive impairment to dementia. Journal of Alzheimer’s Disease. 2010 Jan 1;22(4):1065–71. doi: 10.3233/JAD-2010-101011. [DOI] [PubMed] [Google Scholar]
- 14.Aggarwal NT, Wilson RS, Beck TL, Bienias JL, Berry-Kravis E, Bennett DA. The apolipoprotein E ε4 allele and incident Alzheimer’s disease in persons with mild cognitive impairment. Neurocase. 2005 Feb 26;11(1):3–7. doi: 10.1080/13554790490903038. [DOI] [PubMed] [Google Scholar]
- 15.DeCarli C, Mungas D, Harvey D, Reed B, Weiner M, Chui H, Jagust W. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology. 2004 Jul 27;63(2):220–7. doi: 10.1212/01.wnl.0000130531.90205.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devanand DP, Pelton GH, Zamora D, Liu X, Tabert MH, Goodkind M, Scarmeas N, Braun I, Stern Y, Mayeux R. Predictive utility of apolipoprotein E genotype for Alzheimer disease in outpatients with mild cognitive impairment. Archives of neurology. 2005 Jun 1;62(6):975–80. doi: 10.1001/archneur.62.6.975. [DOI] [PubMed] [Google Scholar]
- 17.Suri S, Heise V, Trachtenberg AJ, Mackay CE. The forgotten APOE allele: a review of the evidence and suggested mechanisms for the protective effect of APOE ε2. Neuroscience & Biobehavioral Reviews. 2013 Dec 31;37(10):2878–86. doi: 10.1016/j.neubiorev.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Payami H, Montee KR, Kaye JA, Bird TD, Yu CE, Wijsman EM, Schellenberg GD. Alzheimer’s disease, apolipoprotein E4, and gender. Jama. 1994 May 4;271(17):1316–7. [PubMed] [Google Scholar]
- 19.Payami H, Zareparsi S, Montee KR, Sexton GJ, Kaye JA, Bird TD, Yu CE, Wijsman EM, Heston LL, Litt M, Schellenberg GD. Gender difference in apolipoprotein E-associated risk for familial Alzheimer disease: a possible clue to the higher incidence of Alzheimer disease in women. American journal of human genetics. 1996 Apr;58(4):803. [PMC free article] [PubMed] [Google Scholar]
- 20.Farrer LA, Adrienne Cupples L, Van Duijn CM, Kurz A, Zimmer R, Müller U, Green RC, Clarke V, Shoffner J, Wallace DC, Chui H. Apolipoprotein E genotype in patients with Alzheimer’s disease: implications for the risk of dementia among relatives. Annals of neurology. 1995 Nov 1;38(5):797–808. doi: 10.1002/ana.410380515. [DOI] [PubMed] [Google Scholar]
- 21.Martinez M, Campion D, Brice A, Hannequin D, Dubois B, Didierjean O, Michon A, Thomas-Anterion C, Puel M, Frebourg T, Agid Y. Apolipoprotein E ε4 allele and familial aggregation of Alzheimer disease. Archives of neurology. 1998 Jun 1;55(6):810–6. doi: 10.1001/archneur.55.6.810. [DOI] [PubMed] [Google Scholar]
- 22.Breitner JC, Wyse BW, Anthony JC, Welsh-Bohmer KA, Steffens DC, Norton MC, Tschanz JT, Plassman BL, Meyer MR, Skoog I, Khachaturian A. APOE-ε4 count predicts age when prevalence of AD increases, then declines The Cache County Study. Neurology. 1999 Jul 1;53(2):321–336. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- 23.Molero AE, Pino-Rami´rez G, Maestre GE. Modulation by age and gender of risk for Alzheimer’s disease and vascular dementia associated with the apolipoprotein E-ε4 allele in Latin Americans: findings from the Maracaibo Aging Study. Neuroscience letters. 2001 Jul 6;307(1):5–8. doi: 10.1016/s0304-3940(01)01911-5. [DOI] [PubMed] [Google Scholar]
- 24.Mortensen EL, Høgh P. A gender difference in the association between APOE genotype and age-related cognitive decline. Neurology. 2001 Jul 10;57(1):89–95. doi: 10.1212/wnl.57.1.89. [DOI] [PubMed] [Google Scholar]
- 25.Lehmann DJ, Refsum H, Nurk E, Warden DR, Tell GS, Vollset SE, Engedal K, Nygaard HA, Smith AD. Apolipoprotein E e4 and impaired episodic memory in community-dwelling elderly people: a marked sex difference. The Hordaland Health Study. Journal of Neurology. Neurosurgery & Psychiatry. 2006 Aug 1;77(8):902–8. doi: 10.1136/jnnp.2005.077818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bickeböller H, Campion D, Brice A, Amouyel P, Hannequin D, Didierjean O, Penet C, Martin C, Perez-Tur J, Michon A, Dubois B. Apolipoprotein E and Alzheimer disease: genotype-specific risks by age and sex. American journal of human genetics. 1997 Feb;60(2):439. [PMC free article] [PubMed] [Google Scholar]
- 27.Combarros O, Leno C, Oterino A, Berciano J, Fernandez-Luna JL, Fernandez-Viadero C, Pena N, Miro J, Delgado M. Gender effect on apolipoprotein E ε4 allele-associated risk for sporadic Alzheimer’s disease. Acta neurologica scandinavica. 1998 Jan 1;97(1):68–71. doi: 10.1111/j.1600-0404.1998.tb00611.x. [DOI] [PubMed] [Google Scholar]
- 28.Slooter AJ, Cruts M, Kalmijn S, Hofman A, Breteler MM, Van Broeckhoven C, van Duijn CM. Risk estimates of dementia by apolipoprotein E genotypes from a population-based incidence study: the Rotterdam Study. Archives of neurology. 1998 Jul 1;55(7):964–8. doi: 10.1001/archneur.55.7.964. [DOI] [PubMed] [Google Scholar]
- 29.Beydoun MA, Boueiz A, Abougergi MS, Kitner-Triolo MH, Beydoun HA, Resnick SM, O’Brien R, Zonderman AB. Sex differences in the association of the apolipoprotein E epsilon 4 allele with incidence of dementia, cognitive impairment, and decline. Neurobiology of aging. 2012 Apr 30;33(4):720–31. doi: 10.1016/j.neurobiolaging.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altmann A, Tian L, Henderson VW, Greicius MD. Sex modifies the APOE-related risk of developing Alzheimer disease. Annals of neurology. 2014 Apr 1;75(4):563–73. doi: 10.1002/ana.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neu SC, Crawford KL, Toga AW. Sharing data in the global Alzheimer’s Association Interactive Network. Neuroimage. 2016 Jan 1;124:1168–74. doi: 10.1016/j.neuroimage.2015.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toga AW, Neu SC, Bhatt P, Crawford KL, Ashish N. The Global Alzheimer’s Association Interactive Network. Alzheimer’s & Dementia. 2016 Jan 31;12(1):49–54. doi: 10.1016/j.jalz.2015.06.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raber J, Huang Y, Ashford JW. ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiology of aging. 2004 Jun 30;25(5):641–50. doi: 10.1016/j.neurobiolaging.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 34.Tarone Robert E. On heterogeneity tests based on efficient scores. Biometrika. 1985;72(1):91–95. [Google Scholar]
- 35.McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 1992 Jan;14(2):103–15. doi: 10.1016/0378-5122(92)90003-m. [DOI] [PubMed] [Google Scholar]
- 36.Riedel M, Thompson PM, Brinton RD. Age, APOE and sex: Triad of risk of Alzheimer’s disease. J of Steroid Biochem and Mol Biol. 2016 Jun;160:134–147. doi: 10.1016/j.jsbmb.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elias-Sonnenschein LS, Viechtbauer W, Ramakers IH, Verhey FR, Visser PJ. Predictive value of APOE-e4 allele for progression from MCI to AD-type dementia: a meta-analysis. Journal of Neurology, Neurosurgery & Psychiatry. 2011 Oct 1;82(10):1149–56. doi: 10.1136/jnnp.2010.231555. [DOI] [PubMed] [Google Scholar]
- 38.Loy CT, Schofield PR, Turner AM, Kwok JB. Genetics of dementia. The Lancet. 2014 Mar 7;383(9919):828–40. doi: 10.1016/S0140-6736(13)60630-3. [DOI] [PubMed] [Google Scholar]
- 39.Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nature genetics. 2007 Jan 1;39(1):17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 40.Chêne G, Beiser A, Au R, Preis SR, Wolf PA, Dufouil C, Seshadri S. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimer’s & Dementia. 2015 Mar 31;11(3):310–20. doi: 10.1016/j.jalz.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Appelros P, Stegmayr B, Terént A. Sex differences in stroke epidemiology a systematic review. Stroke. 2009 Apr 1;40(4):1082–90. doi: 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- 42.Hebert LE, Scherr PA, McCann JJ, Beckett LA, Evans DA. Is the risk of developing Alzheimer’s disease greater for women than for men? American journal of epidemiology. 2001 Jan 15;153(2):132–6. doi: 10.1093/aje/153.2.132. [DOI] [PubMed] [Google Scholar]
- 43.Fratiglioni L, Ahlbom A, Viitanen M, Winblad B. Risk factors for late-onset Alzheimer’s disease: A population-based, case-control study. Annals of neurology. 1993 Mar 1;33(3):258–66. doi: 10.1002/ana.410330306. [DOI] [PubMed] [Google Scholar]
- 44.Mayeux R, Sano M, Chen J, Tatemichi T, Stern Y. Risk of dementia in first-degree relatives of patients with Alzheimer’s disease and related disorders. Archives of Neurology. 1991 Mar 1;48(3):269–73. doi: 10.1001/archneur.1991.00530150037014. [DOI] [PubMed] [Google Scholar]
- 45.Lautenschlager NT, Cupples LA, Rao VS, Auerbach SA, Becker R, Burke J, Chui H, Duara R, Foley EJ, Glatt SL, Green RC. Risk of dementia among relatives of Alzheimer’s disease patients in the MIRAGE study What is in store for the oldest old? Neurology. 1996 Mar 1;46(3):641–50. doi: 10.1212/wnl.46.3.641. [DOI] [PubMed] [Google Scholar]
- 46.Norton MC, Breitner J, Welsh KA, Wyse BW. Characteristics of nonresponders in a community survey of the elderly. Journal of the American Geriatrics Society. 1994 Dec 1;42(12):1252–6. doi: 10.1111/j.1532-5415.1994.tb06506.x. [DOI] [PubMed] [Google Scholar]
- 47.Tsuang D, Kukull W, Sheppard L, Barnhart RL, Peskind E, Edland SD, Schellenberg G, Raskind M, Larson EB. Impact of sample selection on APOEε 4 allele frequency: A comparison of two Alzheimer’s disease samples. Journal of the American Geriatrics Society. 1996 Jun 1;44(6):704–7. doi: 10.1111/j.1532-5415.1996.tb01836.x. [DOI] [PubMed] [Google Scholar]
- 48.Mielke Michelle M, Vemuri Prashanthi, Rocca Walter A. Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin Epidemiol. 2014;6:37–48. doi: 10.2147/CLEP.S37929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barnes Deborah E, Yaffe Kristine. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. The Lancet Neurology. 2011;10(9):819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boada M, Tárraga L, Hernández I, Valero S, Alegret M, Ruiz A, Lopez OL, Becker JT, Center FA, Clinic M. Design of a comprehensive Alzheimer’s disease clinic and research center in Spain to meet critical patient and family needs. Alzheimer’s & Dementia. 2014 May 31;10(3):409–15. doi: 10.1016/j.jalz.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weiner MW, Aisen PS, Jack CR, Jagust WJ, Trojanowski JQ, Shaw L, Saykin AJ, Morris JC, Cairns N, Beckett LA, Toga A. The Alzheimer’s disease neuroimaging initiative: progress report and future plans. Alzheimer’s & Dementia. 2010 May 31;6(3):202–11. doi: 10.1016/j.jalz.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ellis KA, Bush AI, Darby D, De Fazio D, Foster J, Hudson P, Lautenschlager NT, Lenzo N, Martins RN, Maruff P, Masters C. The Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging: methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer’s disease. International Psychogeriatrics. 2009 Aug 1;21(04):672–87. doi: 10.1017/S1041610209009405. [DOI] [PubMed] [Google Scholar]
- 53.Frisoni GB, Prestia A, Zanetti O, Galluzzi S, Romano M, Cotelli M, Gennarelli M, Binetti G, Bocchio L, Paghera B, Amicucci G, Bonetti M, Benussi L, Ghidoni R, Geroldi C. Markers of Alzheimer’s disease in a population attending a memory clinic. Alzheimers Dement. 2009 Jul;5(4):307–17. doi: 10.1016/j.jalz.2009.04.1235. [DOI] [PubMed] [Google Scholar]
- 54.Galluzzi S, Testa C, Boccardi M, Bresciani L, Benussi L, Ghidoni R, Beltramello A, Bonetti M, Bono G, Falini A, Magnani G, Minonzio G, Piovan E, Binetti G, Frisoni GB. The Italian Brain Normative Archive of structural MR scans: norms for medial temporal atrophy and white matter lesions. Aging Clin Exp Res. 2009 Aug-Oct;21(4–5):266–76. doi: 10.1007/BF03324915. [DOI] [PubMed] [Google Scholar]
- 55.Neville J, Kopko S, Broadbent S, Avilés E, Stafford R, Solinsky CM, Bain LJ, Cisneroz M, Romero K, Stephenson D. Development of a unified clinical trial database for Alzheimer’s disease. Alzheimer’s & Dementia. 2015 Oct 31;11(10):1212–21. doi: 10.1016/j.jalz.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 56.Teipel SJ, Wegrzyn M, Meindl T, Frisoni G, Bokde AL, Fellgiebel A, Filippi M, Hampel H, Klöppel S, Hauenstein K, Ewers M. Anatomical MRI and DTI in the diagnosis of Alzheimer’s disease: a European multicenter study. Journal of Alzheimer’s Disease. 2012 Jan 1;31(s3) doi: 10.3233/JAD-2012-112118. [DOI] [PubMed] [Google Scholar]
- 57.Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham Study. Annals of the New York Academy of Sciences. 1963 May 1;107(2):539–56. doi: 10.1111/j.1749-6632.1963.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 58.Beekly DL, Ramos EM, Lee WW, Deitrich WD, Jacka ME, Wu J, Hubbard JL, Koepsell TD, Morris JC, Kukull WA. The National Alzheimer’s Coordinating Center (NACC) database: the uniform data set. Alzheimer Disease & Associated Disorders. 2007 Jul 1;21(3):249–58. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 59.Partch AB, Laufer D, Valladares O, Iodice J, Greenfest-Allen E, Childress DM, Malamon J, Gangadharan P, Arnold SE, Stoeckert CJ, Schellenberg GD. Nia genetics of Alzheimer’s disease data storage site (NIAGADS): 2015 update. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 2015 Jan 7;11(7):P362. [Google Scholar]
- 60.Kamboh MI, Minster RL, Demirci FY, Ganguli M, DeKosky ST, Lopez OL, Barmada MM. Association of CLU and PICALM variants with Alzheimer’s disease. Neurobiology of aging. 2012 Mar 31;33(3):518–21. doi: 10.1016/j.neurobiolaging.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reiman EM, Webster JA, Myers AJ, Hardy J, Dunckley T, Zismann VL, Joshipura KD, Pearson JV, Hu-Lince D, Huentelman MJ, Craig DW. GAB2 alleles modify Alzheimer’s risk in APOE ?4 carriers. Neuron. 2007 Jun 7;54(5):713–20. doi: 10.1016/j.neuron.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Caselli RJ, Reiman EM, Locke DE, Hutton ML, Hentz JG, Hoffman-Snyder C, Woodruff BK, Alexander GE, Osborne D. Cognitive domain decline in healthy apolipoprotein E e4 homozygotes before the diagnosis of mild cognitive impairment. Archives of neurology. 2007 Sep 1;64(9):1306–11. doi: 10.1001/archneur.64.9.1306. [DOI] [PubMed] [Google Scholar]
- 63.Webster JA, Gibbs JR, Clarke J, Ray M, Zhang W, Holmans P, Rohrer K, Zhao A, Marlowe L, Kaleem M, McCorquodale DS. Genetic control of human brain transcript expression in Alzheimer disease. The American Journal of Human Genetics. 2009 Apr 10;84(4):445–58. doi: 10.1016/j.ajhg.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002 Jul 23;59(2):198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 65.Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005 Mar 8;64(5):834–41. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- 66.Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005 Oct 28;25(4):163–75. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 67.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007 Dec 11;69(24):2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 68.Green RC, Cupples LA, Go R, Benke KS, Edeki T, Griffith PA, Williams M, Hipps Y, Graff-Radford N, Bachman D, Farrer LA. Risk of dementia among white and African American relatives of patients with Alzheimer disease. Jama. 2002 Jan 16;287(3):329–36. doi: 10.1001/jama.287.3.329. [DOI] [PubMed] [Google Scholar]
- 69.Lee JH, Cheng R, Graff-Radford N, Foroud T, Mayeux R. Analyses of the national institute on aging late-onset alzheimer’s disease family study: implication of additional loci. Archives of neurology. 2008 Nov 10;65(11):1518–26. doi: 10.1001/archneur.65.11.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kukull WA, Higdon R, Bowen JD, McCormick WC, Teri L, Schellenberg GD, van Belle G, Jolley L, Larson EB. Dementia and Alzheimer disease incidence: a prospective cohort study. Archives of neurology. 2002 Nov 1;59(11):1737–46. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- 71.Beecham GW, Martin ER, Li YJ, Slifer MA, Gilbert JR, Haines JL, Pericak-Vance MA. Genome-wide association study implicates a chromosome 12 risk locus for late-onset Alzheimer disease. The American Journal of Human Genetics. 2009 Jan 9;84(1):35–43. doi: 10.1016/j.ajhg.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carrasquillo MM, Zou F, Pankratz VS, Wilcox SL, Ma L, Walker LP, Younkin SG, Younkin CS, Younkin LH, Bisceglio GD, Ertekin-Taner N. Genetic variation in PCDH11X is associated with susceptibility to late-onset Alzheimer’s disease. Nature genetics. 2009 Feb 1;41(2):192–8. doi: 10.1038/ng.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Galluzzi S, Marizzoni M, Babiloni C, Albani D, Antelmi L, Bagnoli C, Bartres-Faz D, Cordone S, Didic M, Farotti L, Fiedler U, Forloni G, Girtler N, Hensch T, Jovicich J, Leeuwis A, Marra C, Molinuevo JL, Nobili F, Pariente J, Parnetti L, Payoux P, Del Percio C, Ranjeva JP, Rolandi, Rossini PM, Schönknecht P, Soricelli A, Tsolaki M, Visser PJ, Wiltfang J, Richardson JC, Bordet R, Blin O, Frisoni GB, PharmaCog Consortium Clinical and biomarker profiling of prodromal Alzheimer’s disease in workpackage 5 of the Innovative Medicines Initiative PharmaCog project: a ’European ADNI study’. J Intern Med. 2016 Jun;279(6):576–91. doi: 10.1111/joim.12482. [DOI] [PubMed] [Google Scholar]
- 74.Sager MA, Hermann B, La Rue A. Middle-aged children of persons with Alzheimer’s disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer’s Prevention. Journal of geriatric psychiatry and neurology. 2005 Dec 1;18(4):245–9. doi: 10.1177/0891988705281882. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


