Abstract
Background
Fucoxanthin is a carotenoid present in the chloroplasts of brown seaweeds. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a cytokine that selectively induces apoptosis in many tumor cells and is an attractive candidate for antitumor therapies.
Material/Methods
After human cervical cancer cell lines HeLa, SiHa, and CaSki were treated with fucoxanthin or TRAIL. Cell viability was determined by 2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-2-tetrazolium 5-carboxanilide (XTT) method. Apoptosis was measured by flow cytometry (FCM). Protein expression of phosphatidylinositol 3 kinase (PI3K), protein kinase B (Akt), phosphated Akt (p-Akt), NF-κB nuclear factor-k-gene binding (NF-κB). Phosphated nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (p-IκBa), was measured by Western blot analysis. mRNA expression of Bax and Bcl2 was measured by RNA preparation and quantitative reverse transcription polymerase chain reaction (RT-PCR).
Results
In the present study, the effectiveness in terms of apoptosis was as follows: TRAIL plus fucoxanthin>fucoxanthin>TRAIL, indicating the combination of fucoxanthin and TRAIL, produced a strong synergistic effect on apoptosis in human cervical cancer cells. Additionally, we found that upstream signaling PI3K/Akt and NF-κB pathways-mediated cell apoptosis was activated by TRAIL and suppressed by fucoxanthin. By using PI3K and NF-κB inhibitors LY49002 and PDTC, we found that fucoxanthin- or TRAIL-induced apoptosis of human cervical cancer cells was obviously down-regulated.
Conclusions
Taken together, these findings suggest that fucoxanthin and TRAIL increased the apoptosis in human cervical cancer cells by targeting the PI3K/Akt/NF-κB signaling pathway.
MeSH Keywords: Apoptosis, Chlorophyll Binding Proteins, Tumor Necrosis Factor Decoy Receptors
Background
Cervical cancer (CC), one of the 4 most malignant neoplasms arising from cells in the cervix uteri, is among the top causes of death from cancer in women in many developing countries [1]. The prevention of cervical cancer, including human papillomavirus (HPV) vaccination and HPV-screening programs, has been developed, resulting in increased protection against cervical cancer [2]. Furthermore, during the last decades, significant progress has been made in the treatment of CC using novel agents to inhibit the angiogenesis process. Unfortunately, around 15–61% of patients develop a recurrence in lymph nodes or distant sites within the first 2 years of completing treatment and the prognosis for these patients remains poor [3].
Fucoxanthin is obtained from the chloroplasts of brown seaweeds, and can be metabolized to fucoxanthinol by digestive enzymes in the gastrointestinal tract. Previous studies have highlighted the role of fucoxanthin in exerting anti-mutagenic, anti-obesity, anti-diabetic, anti-inflammatory, anti-oxidation, and anti-neoplastic actions [4]. Biological activities of fucoxanthin in cancers have been investigated by many researchers [5–10]. Numerous studies on fucoxanthin in cancers have considered fucoxanthin as an effective anticancer drug due to its anti-proliferative behavior in various types of cancer, such as glioma cancer [11], breast cancer [12], lung cancer [13], colon cancer [14], and colorectal cancer [15]. However, few studies have investigated molecules and pathways that can be modulated and regulated by fucoxanthin in cancer cells.
TRAIL is a member of the TNF superfamily, which is mainly expressed in the immune system [16]. TRAIL can selectively trigger apoptosis in tumor cells without influencing the functions of normal cells [17]. TRAIL has the ability to circumvent the chemoresistance of conventional therapeutics and to interact with self-renewal pathways in cancer stem cells (CSCs). Then, the therapeutic efficacy is severely compromised in cancer cells because of low sensitivity of cancer cells to TRAIL [18]. Therefore, it is important to identify the mechanisms underlying this lack of sensitivity and to develop strategies that increase the sensitivity of cancer cells to TRAIL.
Our previous study has shown that fucoxanthin suppresses tumor growth in vivo and induces significant apoptosis in HeLa cells [19]. The present study was undertaken to evaluate the molecular mechanisms of fucoxanthin and TRAIL against human cervical cancer cells. We found that fucoxanthin could improve the sensitivity of human cervical SiHa cells to TRAIL. These findings have also improved our understanding of the role of fucoxanthin and TRAIL in human cervical cancer cells, and revealed a potential mechanism of fucoxanthin-mediated PI3K/Akt and NF-κB suppression in human cervical cancer cells.
Material and Methods
Cell culture
The human cervical cancer cell lines HeLa, SiHa, and CaSki (ATCC, Manassas, VA, USA) were cultured in RPMI 1640 medium containing 10% (v/v) fetal bovine serum, 10 mmol/L hydroxyethyl piperazine ethanesulfonic acid, 2 mmol/L L-glutamine, 50 μmol/L β-mercaptoethanol, 1μmol/L sodium pyruvate, 10 μg/mL streptomycin, and 100 U/mL penicillin (Gibco, New York, NY, USA) at 37°C in a humidified atmosphere of 5% CO2 in air.
Cell proliferation assay
The endogenous effects of TRAIL on cell viability were evaluated using the XTT Cell Viability Assay Kit (Sigma, USA). Briefly, human cervical cancer cell lines HeLa, SiHa, and CaSki at a density of 2.0×104/mL was seeded in medium on 96-well plates. When cells attained 65% confluency, they were treated with TRAIL at concentrations of 0, 5, 10, 50, and 100 ng/mL. After 48 h of culture, cells in each well were added with 100 μL fresh medium and 25 μL XTT solution. After 5 h of incubation, cell viability in each well was estimated at a wavelength of 450 nm using a microplate reader (Bio-Rad, Hercules, CA, USA).
Flow cytometry
Human SiHa cervical cancer cells seeded onto a 96-well plate were treated with fucoxanthin (0.5 μmol/L), TRAIL (100 ng/mL), and fucoxanthin (0.5 μmol/L) plus TRAIL (100 ng/mL) for 48 h, and cell apoptotic rate was measured by flow cytometry method according to the manufacturer’s instructions. Briefly, human SiHa cervical cancer cells were collected and fixed in 70% ethanol for 30 min. Then, cells were stained with 50 μg/mL FITC, Annexin V, and PI (BD Biosciences, San Jose, CA, USA), respectively. Cell apoptotic rate was analyzed using a FACScanVantage SE (BD Biosciences, San Jose, CA, USA).
Western Blot analysis
Western blot was used to analyze protein expression of PI3K, Akt, p-Akt, NF-κB (p65), and pIκBα after human SiHa cervical cancer cells were treated with fucoxanthin (0.5 μmol/L), TRAIL (100 ng/mL), and fucoxanthin (0.5 μmol/L) plus TRAIL (100 ng/mL) for 48 h. In brief, proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes. Then, the membranes were blocked in 5% non-fat milk for 2 h at room temperature and incubated at 4°C overnight with polyclonal anti-PI3K (1: 1000 diluted), Akt (1: 1000 diluted), pAkt (1: 2000 diluted), NF-κB (p65) (1: 1000 diluted), and pIκBα (1: 500 diluted). Five different antibodies were purchased from Cell Signaling Technology (MA, USA). After antibody incubation, the membranes were washed and immunoblotted with HRP-conjugated anti-rabbit IgG antibody (diluted 1: 1000, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 37°C for 30 min. The membranes were then exposed to X-ray film. β-actin was used to ensure adequate sample loading for all Western blots and its antibody was purchased from R&D SYSTEMS INC (Minneapolis, MN, USA). Band density was quantitated using Image J software (National Institutes of Health, Bethesda, MD, USA).
Real-time RT-PCR
After human SiHa cervical cancer cells were treated with fucoxanthin (0.5 μmol/L), TRAIL (100 ng/mL), and fucoxanthin (0.5 μmol/L) plus TRAIL (100 ng/mL) for 48 h, the expressions of Bax and Bcl2 in cells were measured by RNA preparation and quantitative reverse transcription polymerase chain reaction (RT-PCR). Total cellular RNA isolation (Invitrogen, Carlsbad, CA, USA) and Real-time RT-PCR analysis (Qiagen, Valencia, CA, USA) was conducted according to the manufacturer’s instructions. The reaction was run at 1 cycle of 50°C for 2 min and 94°C for 15 min, followed by 40 cycles of 94°C for 15 s, 58°C for 30 s, and 72°C for 30s. Specific primer sequences were synthesized in BIOSUNE Biological Technology Corp (Shanghai, China), and shown as follows: Bax, forward: 5′-GAGAGGTCTTTTTCCGAGTGG-3′, reverse: 5′-CCTTGAGCACCAGTTTGCTG-3′; Bcl2, forward: 5′-GGCGCACGCTGGGAGAAC-3′, reverse: 5′-TAGCGGCGG GAGAAGTCGTC-3′; and GADPH, forward: 5′-CAAGGTCATCCATG ACAACTTTG-3′, reverse: 5′-GTCCACCACCCTGTTGCTGTAG-3′.
Caspase-3 activity analysis
After human SiHa cervical cancer cells were treated with fucoxanthin (0.5 μmol/L), TRAIL (100 ng/mL) and fucoxanthin (0.5 μmol/L) plus TRAIL (100 ng/mL) for 48 h, caspase-3 activity was analyzed using the Caspase-3 Activity Assay Kits according to the manufacturer’s instructions. Briefly, the reaction buffer and the specific enzyme DEVD-pNA were added to each cell plate and further cultured in an incubator for 1 h at 37°C. The developed colorimetric reaction was measured at 405 nm in a 96-well Bio-Rad 680 microplate reader (Bio-Rad, Hercules, CA, USA).
Statistical analysis
Statistical analysis was carried out with one-way analysis of variance (ANOVA) and t test using SPSS17.0 software (SPSS, Chicago, IL, USA). Values are expressed as means ± standard deviation (SD). The mean values and standard deviations were calculated from 3 independent experiments. Differences were considered statistically significant at P<0.05.
Results
Effects of TRAIL on cell viability in human cervical cancer cells
The human cervical cancer cell lines Hela, SiHa, and CaSki were treated with TRAIL at concentrations of 0, 5, 10, 50, or 100 ng/mL for 48 h, and cell viability was detected using the XTT assay. We found that different concentrations of TRAIL could reduce cell viability of Hela, SiHa, and CaSki in a dose-dependent manner, but the effects were different. Treatment of SiHa cells with TRAIL at a concentration of 100 ng/mL (the highest level used in this study) decreased cell viability by approximately 14.6%. In contrast, treatment of Hela and CaSki cells with TRAIL, even at the lowest level of 5 ng/mL, dramatically reduced cell viability, and 100 ng/mL TRAIL showed a 68.5% and 63.3% decrease in cell viability, respectively (P<0.05) (Figure 1). The results above indicate that SiHa cells are highly resistant to TRAIL-induced cell death, which were selected and used in the following studies.
Figure 1.
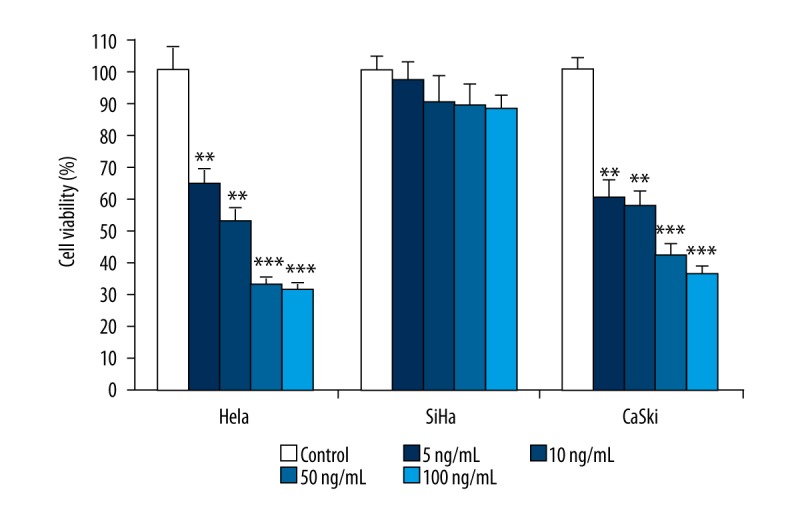
Effect of various concentrations of TRAIL on cell viability of human cervical cancer cells. The human cervical cancer cell lines Hela, SiHa, and CaSki were treated with TRAIL at concentration of 0, 5, 10, 50, and 100 ng/mL for 48 h, and cell viability was measured by MTT method. Data are expressed as mean ±SD of 3 independent experiments in triplicate. ** P<0.01, *** P<0.001, or P>0.05 vs. control.
Fucoxanthin increased the apoptosis induced by TRAIL in human cervical cancer SiHa cells
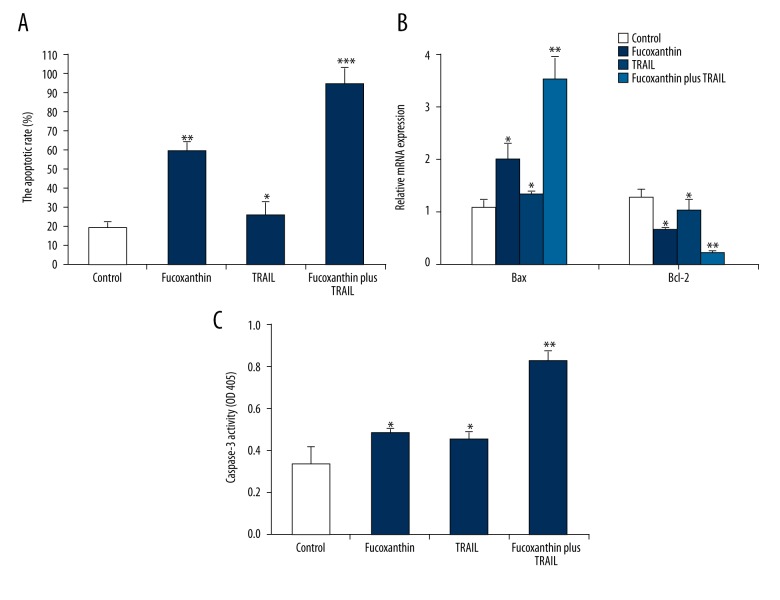
To evaluate the biological effect of fucoxanthin on TRAIL-treated human SiHa cells, cell apoptosis was determined by flow cytometry. We found that the apoptotic rate of TRAIL-treated SiHa cells (14.29%) was higher than that in the control cells (10.68%). Co-treatment with TRAIL and fucoxanthin caused about a 3.6-fold increase in the proportion of apoptotic cells in comparison with TRAIL-treated SiHa cells (Figure 2A).
Figure 2.
Fucoxanthin promoted the apoptosis induced by TRAIL in human cervical cancer SiHa cells. SiHa cells were treated with fucoxanthin, TRAIL, and fucoxanthin-TRAIL for 48 h. Cell apoptosis was determined by flow cytometry (A), mRNA expression of Bax and Bcl-2 was measured by RT-PCR (B), and caspase-3 activity was further examined by use of the Caspase-3 kit (C). Data are expressed as mean ±SD of 3 independent experiments in triplicate. * P<0.05, ** P<0.01, or P>0.05 vs. control.
Next, the expression of the apoptotic indicators Bax and Bcl-2 was determined by RT-PCR, and the activity of caspase-3 were further examined by Caspase-3 Activity Assay Kits. Results in Figure 2B suggest that mRNA expression of Bax was promoted while the expression level of Bcl-2 was inhibited by fucoxanthin or TRAIL alone in comparison with the control (P<0.05). After SiHa cells were co-treated with fucoxanthin and TRAIL, up-regulation of Bax expression and down-regulation of Bcl-2 expression were more significant compared to fucoxanthin or TRAIL-treated cells (P<0.01). Furthermore, the activity of Caspase-3 was significantly increased in fucoxanthin, TRAIL, and fucoxanthin plus TRAIL-treated groups compared to the control group (P<0.05), and the effect in fucoxanthin plus TRAIL was more significant (P<0.01) (Figure 2C). Fucoxanthin promoted TRAIL-induced apoptosis in TRAIL-resistant human cervical cancer SiHa cells.
Fucoxanthin inhibited the activation of the PI3K/AKT/NF-κB pathway induced by TRAIL in human cervical cancer SiHa cells
To understand the molecular mechanism involved in TRAIL and fucoxanthin functions, the alterations in protein expression of PI3K, AKT, p-AKT, NF-κB, and p-IκBα in SiHa cells treated with fucoxanthin, TRAIL, or fucoxanthin plus TRAIL for 48 were assessed using Western blot analysis (Figure 3A). Relative band ratio analysis found that fucoxanthin, TRAIL, or fucoxanthin plus TRAIL showed no obvious effects on protein expression of PI3K, AKT, and NF-κB (p65). TRAIL alone promoted the protein expression level of p-AKT and p-IκBα, suggesting activation of the PI3K/AKT/NF-κB pathway. Moreover, fucoxanthin induced a marked decrease in protein expression of p-AKT and p-IκBαin SiHa cells (P<0.05), and a TRAIL-induced decrease was less significant after SiHa cells were co-treated with fucoxanthin and TRAIL (Figure 3B). We found that fucoxanthin attenuated phosphorylation of AKT and IκBα expression activated by TRAIL, indicating that fucoxanthin suppressed the PI3K/AKT/NF-κB pathway activated by TRAIL in SiHa cells.
Figure 3.
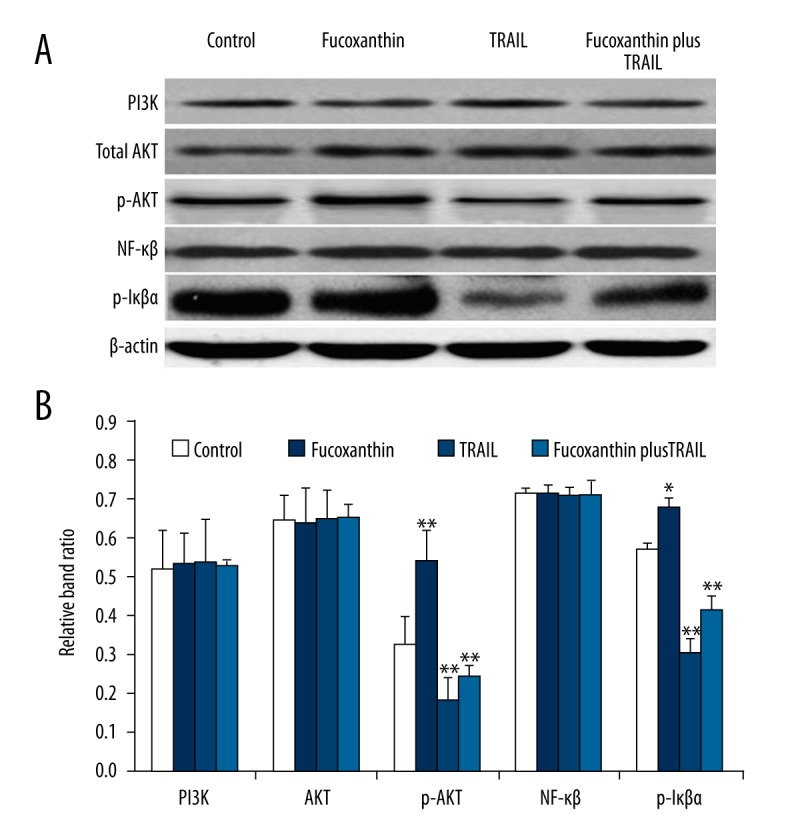
Fucoxanthin inhibited activation of the PI3K/AKT/NF-κB pathway induced by TRAIL in human cervical cancer SiHa cells. SiHa cells were treated with fucoxanthin, TRAIL, and fucoxanthin-TRAIL for 48 h, and protein expression of PI3K, AKT, p-AKT, NF-κB, and p-IκBα was assessed using Western blot analysis (A). Band ratio was analyzed by Image J software (B). Data are expressed as mean ±SD of 3 independent experiments in triplicate. * P<0.05, ** P<0.01, or P>0.05 vs. control.
The PI3K/AKT/NF-κB signaling pathway was involved in fucoxanthin and TRAIL-induced cell apoptosis in human cervical cancer SiHa cells
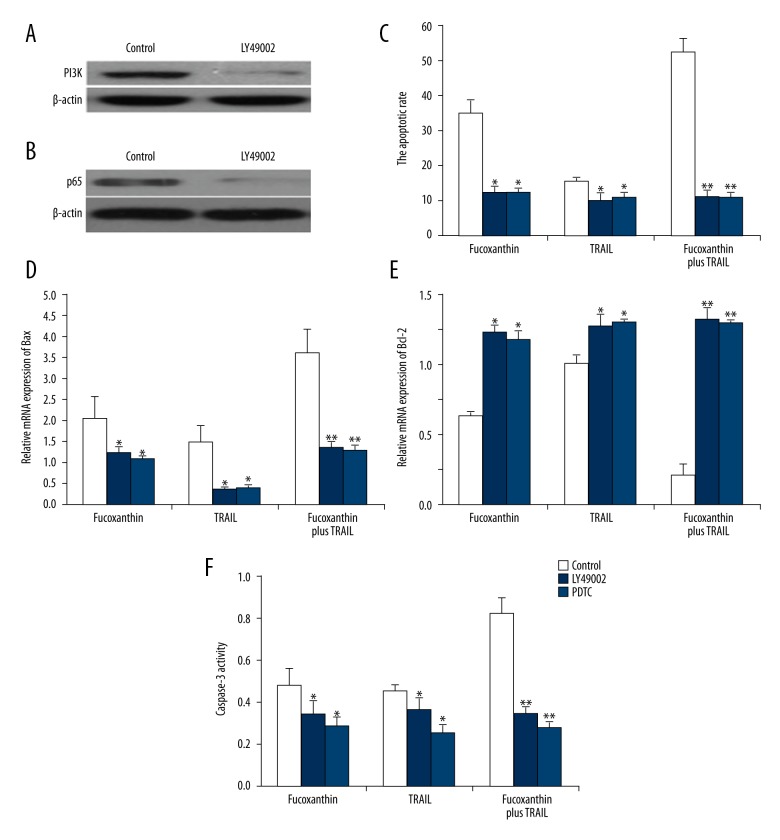
To assess whether fucoxanthin or TRAIL exerted its functions by altering the PI3K/AKT/NF-κB signaling pathway, cell viability and apoptosis were investigated after blocking the PI3K/AKT/NF-κB signaling pathway using inhibitors LY49002 and PDTC in human cervical cancer SiHa cells. The protein expression of PI3K (Figure 4A) and p65 (Figure 4B) was significantly down-regulated by LY49002 and PDTC, respectively (P<0.05), indicating the good efficiency of inhibitors LY49002 and PDTC. Furthermore, compared to the fucoxanthin-TRAIL-treated group without inhibitors, after inhibition of PI3K/AKT or NF-κB signaling, fucoxanthin, TRAIL, and fucoxanthin together with TRAIL showed little changes in the apoptotic rate (Figure 4C), Bax (Figure 4D) and Bcl-2 expression (Figure 4E), and Caspase-3 activity (Figure 4F) in human cervical cancer SiHa cells (P<0.05). Taken together, these results strongly indicate that the PI3K/AKT/NF-κB signaling pathway is required for fucoxanthin- and TRAIL-triggered inhibition of cell viability and promotion of cell apoptosis in human cervical cancer SiHa cells.
Figure 4.
The PI3K/AKT/NF-κB signaling pathway was required for the functions of fucoxanthin and TRAIL in human SiHa cervical cancer cells. SiHa cells were treated with LY49002 and PDTC for 24 h and Western blot analysis was performed to determine the efficiency of LY49002 (A) and PDTC (B). SiHa cells was blocked with LY49002 and PDTC for 12 h and then treated with fucoxanthin, TRAIL, and fucoxanthin-TRAIL for 48 h, then cell apoptosis (C), mRNA expression of Bax (D) and Bcl-2 (E), and caspase-3 activity (F) were measured. Data are expressed as mean ±SD of 3 independent experiments in triplicate. * P<0.05, ** P<0.01, or P>0.05 vs. control.
Discussion
Combination drug therapies have proved to be important in cancer therapy. Exploration of the underlying molecular mechanisms in the synergistic effects achieved by various chemotherapeutic agents would be useful in developing combination therapeutics to improve the prognosis of cancer patients. In this study, the effects of TRAIL and fucoxanthin combination treatment were investigated. We found that combination treatment of TRAIL and fucoxanthin resulted in increased apoptosis and enhanced TRAIL sensitivity in TRAIL-resistant human cervical cancer SiHa cells. The combination of TRAIL and fucoxanthin might overcome TRAIL resistance in human cervical cancer SiHa cells and would be an effective therapeutic strategy human cervical cancer.
TRAIL has become a potential therapeutic drug for cancers, but effects are different between TRAIL-sensitive cancer cells and TRAIL-resistant cancer cells. TRAIL was sensitive to various types of cancer cells, including prostate and bladder cancer cells [20] and myeloma cell line RPMI 8226 [21], and significantly decreased cell proliferation rate in these cancer cells. Moreover, not all cancer cells are susceptible to TRAIL-mediated cell apoptosis. TRAIL is resistant to cancer cells such as myeloma cell line U266, triple-negative breast cancer cells [22], colorectal cancer HT-29 cells [23], hepatocellular carcinoma Huh-7 cells [24], and thyroid cancer cells [25]. In the present study, we found that TRAIL showed high sensitivity to human cervical cancer cell lines HeLa and CaSki, and heavily inhibited cell viability in 2 kinds of cell lines. In contrast, TRAIL had low sensitivity to SiHa cells. TRAIL-based combination therapy approaches have therefore been introduced as a novel strategy against resistance. Therefore, we used another antitumor drug, fucoxanthin, to promote the sensitivity of SiHa cells to TRAIL. The effects of fucoxanthin on cell viability in cancer cells, such as colon, bladder, lung, prostate, leukemia, and breast cancer cells, have been reported by numerous researchers [6,15,26–30]. MTT analysis results showed that fucoxanthin inhibited the proliferation of bladder cancer T24 cell line in a dose- and time-dependent manner by the up-regulation of p21 at low doses and by up-regulation of cleaved caspase-3 at high doses [13]. Fucoxanthin promoted apoptosis and reduced cell proliferation, migration, and invasion of human glioma cancer cell line U87 and U251 through Akt/mTOR and p38 suppression [11]. Antitumor activity of fucoxanthin isolated from U. pinnatifida against human leukemic HL-60 cells showed significant inhibitory effects on cell proliferation in a dose-dependent manner [26].
Apoptosis plays an important role in keeping the balance between cell proliferation and cell death. Drugs used to induce cell apoptosis have been considered as efficient for cancer therapy [30,31]. Activated caspase-3 plays a crucial role in the extrinsic and intrinsic pathways of apoptosis [32]. Bcl-2 is identified as a potent suppressor of apoptosis [33]. In this study, we also proved that fucoxanthin promoted cell apoptosis, showing as higher caspase-3 activity and Bax expression. Fucoxanthin arrested cell cycle and induced apoptosis by regulating the expression of Bcl-2, caspase-3/8, p53, p21, and Fas in human non-small-cell lung cancer cells [34]. After 48-h treatment with 20 μM fucoxanthin, the apoptotic rate of PC-3 prostate cancer cells was increased via caspase-3 activation associated with reduction in the expression of Bax and Bcl-2 proteins [26]. In another study, fucoxanthin isolated from U. pinnatifida revealed a remarkable inhibitory activity against the viability of human colon cancer cell lines Caco-2, DLD-1, and HT-29 through suppressing the level of Bcl-2 protein and peroxisome proliferator-activated receptor (PPAR) γ [35]. Additionally, in a separate study, Zhang et al. (2008) showed that fucoxanthin significantly reduced the viability of the urinary bladder cancer EJ-1 cell line in a dose- and time-dependent manner by activating caspase-3 activity. Our study also proved that fucoxanthin promoted cell apoptosis in human cervical cancer SiHa cells, and the effect of fucoxanthin and TRALL was synergistic.
Previous studies have reported that fucoxanthin exerts its apoptosis- and cancer-preventing effects meditated by different pathways including the caspases, Bcl-2 proteins, MAPK, NF-κB, and JAK/STAT, which are involved in cell growth and apoptosis, anti-angiogenesis, or inhibition of metastasis [36,37]. NF-κB, a family of closely related transcription factors, can be activated by many cytokines, growth factors, tyrosine kinases, tumor necrosis factor receptor families, and signaling pathways. NF-κB is often considered as a hallmark of cancer and is associated with several tumor/cancer related processes, such as cell proliferation and anti-apoptotic gene expression, as well as cancer therapy resistance [38,39]. Furthermore, the PI3K/AKT pathway is involved in the regulation of cell proliferation, differentiation, apoptosis, and DNA repair [40]. Breast cancer cell lines MCF-7 and MDA-MB-231 stimulated with 10 to 20 μM fucoxanthin showed reduced cell viability in a time-dependent manner as a result of increased apoptosis through reducing nuclear levels of NF-κB members p65, p52, and RelB [12]. Fucoxanthin at concentration of 50 μM and 75 μM reduced apoptosis in human gastric adenocarcinoma MGC-803 cells through the JAK/STAT signal pathway [41]. We found that fucoxanthin-TRAIL inhibited cell proliferation and promoted cell apoptosis in human cervical cancer SiHa cells via suppression of the PI3K/AKT/NF-κB signaling pathway.
Conclusions
The present study investigated the anticancer effects of fucoxanthin and TRAIL using human cervical cancer cell lines. TRAIL was resistant to human cervical cancer SiHa cells. Mechanistically, fucoxanthin resulted in the promotion of cell apoptosis and enhanced the sensitivity human cervical cancer SiHa cells to TRAIL-induced apoptosis. Co-treatment with fucoxanthin and TRALL could be effective for the treatment and/or prevention of human cervical cancer.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Ronco G, Dillner J, Elfström KM, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: Follow-up of four European randomised controlled trials. Lancet. 2014;383:524–32. doi: 10.1016/S0140-6736(13)62218-7. [DOI] [PubMed] [Google Scholar]
- 3.Small W, Jr, Bacon MA, Bajaj A, et al. Cervical cancer: A global health crisis. Cancer. 2017;123(13):2404–12. doi: 10.1002/cncr.30667. [DOI] [PubMed] [Google Scholar]
- 4.Choi JH, Kim NH, Kim SJ, et al. Fucoxanthin inhibits the inflammation response in paw edema model through suppressing MAPKs, Akt, and NF-κB. J Biochem Mol Toxicol. 2016;30:111–19. doi: 10.1002/jbt.21769. [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa C, Tafuku S, Kadekaru T, et al. Antiadult T-cell leukemia effects of brown algae fucoxanthin and its deacetylated product, fucoxanthinol. Int J Cancer. 2008;123:2702–12. doi: 10.1002/ijc.23860. [DOI] [PubMed] [Google Scholar]
- 6.Kotake-Nara E, Asai A, Nagao A. Neoxanthin and fucoxanthin induce apoptosis in PC-3 human prostate cancer cells. Cancer Lett. 2005;220:75–84. doi: 10.1016/j.canlet.2004.07.048. [DOI] [PubMed] [Google Scholar]
- 7.Liu CL, Huang YS, Hosokawa M, et al. Inhibition of proliferation of a hepatoma cell line by fucoxanthin in relation to cell cycle arrest and enhanced gap junctional intercellular communication. Chem Biol Interact. 2009;182:165–72. doi: 10.1016/j.cbi.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Mise T, Yasumoto T. Simultaneous treatment of cancer cells lines with the anticancer drug cisplatin and the antioxidant fucoxanthin. Br J Pharmacol Toxicol. 2011;2:127–31. [Google Scholar]
- 9.Satomi Y, Nishino H. Fucoxanthin, a natural carotenoid, induces G1 arrest and GADD45 gene expression in human cancer cells. In Vivo. 2007;21:305–10. [PubMed] [Google Scholar]
- 10.Yamamoto K, Ishikawa C, Katano H, et al. Fucoxanthin and its deacetylated product, fucoxanthinol, induce apoptosis of primary effusion lymphomas. Cancer Lett. 2011;300:225–34. doi: 10.1016/j.canlet.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Zheng J, Zhang Y, et al. Fucoxanthin activates apoptosis via inhibition of PI3K/Akt/mTOR pathway and suppresses invasion and migration by restriction of p38-MMP-2/9 pathway in human glioblastoma cells. Neurochem Res. 2016;41:2728–51. doi: 10.1007/s11064-016-1989-7. [DOI] [PubMed] [Google Scholar]
- 12.Rwigemera A, Mamelona J, Martin LJ. Comparative effects between fucoxanthinol and its precursor fucoxanthin on viability and apoptosis of breast cancer cell lines MCF-7 and MDA-MB-231. Anticancer Res. 2015;35:207–19. [PubMed] [Google Scholar]
- 13.Wang L, Zeng Y, Liu Y, et al. Fucoxanthin induces growth arrest and apoptosis in human bladder cancer T24 cells by up-regulation of p21 and down-regulation of mortalin. Acta Biochim Biophys Sin (Shanghai) 2014;46:877–84. doi: 10.1093/abbs/gmu080. [DOI] [PubMed] [Google Scholar]
- 14.Kawee-Ai A, Kim SM. Application of microalgal fucoxanthin for the reduction of colon cancer risk: Inhibitory activity of fucoxanthin against beta-glucuronidase and DLD-1 cancer cells. Nat Prod Commun. 2014;9:921–24. [PubMed] [Google Scholar]
- 15.Takahashi K, Hosokawa M, Kasajima H, et al. Anticancer effects of fucoxanthin and fucoxanthinol on colorectal cancer cell lines and colorectal cancer tissues. Oncol Lett. 2015;10:1463–67. doi: 10.3892/ol.2015.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laussmann MA, Passante E, Hellwig CT, et al. Proteasome inhibition can impair caspase-8 activation upon submaximal stimulation of apoptotic tumor necrosis factor-related apoptosis inducing ligand (TRAIL) signaling. J Biol Chem. 2012;287:14402–11. doi: 10.1074/jbc.M111.304378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walczak H, Miller RE, Ariail K, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–63. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 18.Piggott L, Omidvar N, Perez SM, et al. Suppression of apoptosis inhibitor c-FLIP selectively eliminates breast cancer stem cell activity in response to the anti-cancer agent TRAIL. Breast Cancer Res. 2011;13:R88. doi: 10.1186/bcr2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye G, Lu Q, Zhao W, et al. Fucoxanthin induces apoptosis in human cervical cancer cell line HeLa via PI3K/Akt pathway. Tumour Biol. 2014;35:11261–67. doi: 10.1007/s13277-014-2337-7. [DOI] [PubMed] [Google Scholar]
- 20.Hao L, Zhao Y, Li ZG, et al. Tumor necrosis factor-related apoptosis-inducing ligand inhibits proliferation and induces apoptosis of prostate and bladder cancer cells. Oncol Lett. 2017;13:3638–40. doi: 10.3892/ol.2017.5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jian Y, Chen Y, Geng C, et al. Target and resistance-related proteins of recombinant mutant human tumor necrosis factor-related apoptosis-inducing ligand on myeloma cell lines. Biomed Rep. 2016;4:723–77. doi: 10.3892/br.2016.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Refaat A, Abdelhamed S, Saiki I, Sakurai H. Inhibition of p38 mitogen-activated protein kinase potentiates the apoptotic effect of berberine/tumor necrosis factor-related apoptosis-inducing ligand combination therapy. Oncol Lett. 2015;10:1907–11. doi: 10.3892/ol.2015.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trang KT, Kim SL, Park SB, et al. Parthenolide sensitizes human colorectal cancer cells to tumor necrosis factor-related apoptosis-inducing ligand through mitochondrial and caspase dependent pathway. Intest Res. 2014;12:34–41. doi: 10.5217/ir.2014.12.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luan Z, He Y, He F, Chen Z. Rocaglamide overcomes tumor necrosis factor-related apoptosis-inducing ligand resistance in hepatocellular carcinoma cells by attenuating the inhibition of caspase-8 through cellular FLICE-like-inhibitory protein downregulation. Mol Med Rep. 2015;11:203–11. doi: 10.3892/mmr.2014.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li LC, Jayarama S, Pilli T, et al. Down-modulation of expression, or dephosphorylation, of IG20/MADD in tumor necrosis factor-related apoptosis-inducing ligand-resistant thyroid cancer cells makes them susceptible to treatment with this ligand. Thyroid. 2013;23:70–78. doi: 10.1089/thy.2012.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hosokawa M, Wanezaki S, Miyauchi K, et al. Apoptosis-inducing effect of fucoxanthin on human leukemia cell line HL-60. Food Sci Technol Res. 1999;5:243–46. [Google Scholar]
- 27.Jaswir I, Noviendri D, Salleh HM, et al. Isolation of fucoxanthin and fatty acids analysis of Padina australis and cytotoxic effect of fucoxanthin on human lung cancer (H1299) cell lines. Afr J Biotechnol. 2011;10:18855–62. [Google Scholar]
- 28.Konishi I, Hosokawa M, Sashima T, et al. Halocynthiaxanthin and fucoxanthinol isolated from Halocynthia roretzi induce apoptosis in human leukemia, breast and colon cancer cells. Comp Biochem Physiol Part C. 2006;142:53–59. doi: 10.1016/j.cbpc.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z, Zhang P, Hamada M, et al. Potential chemoprevention effect of dietary fucoxanthin on urinary bladder cancer EJ-1 cell line. Oncol Rep. 2008;20:1099–103. [PubMed] [Google Scholar]
- 30.Wu L, Jin L, Zhang W, Zhang L. Roles of long non-coding RNA CCAT2 in cervical cancer cell growth and apoptosis. Med Sci Monit. 2016;22:875–79. doi: 10.12659/MSM.897754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–62. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 32.Cohen GM. Caspases: The executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young-Min H, Weon-Jong Y, Soo-Yeong P, et al. Quercitrin protects against oxidative stress-induced injury in lung fibroblast cells via up-regulation of Bcl-Xl. J Funct Foods. 2012;4:253–62. [Google Scholar]
- 34.Mei C, Zhou S, Zhu L, et al. Antitumor effects of laminaria extract fucoxanthin on lung cancer. Mar Drugs. 2017;15(2) doi: 10.3390/md15020039. pii: E39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosokawa M, Kudo M, Maeda H, et al. Fucoxanthin induces apoptosis and enhances the antiproliferative effect of the PPARγ ligand, troglitazone, on colon cancer cells. Biochim Biophys Acta. 2004;1675:113–19. doi: 10.1016/j.bbagen.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Kumar SR, Hosokawa M, Miyashita K. Fucoxanthin: A marine carotenoid exerting anti-cancer effects by affecting multiple mechanisms. Mar Drugs. 2013;11:5130–47. doi: 10.3390/md11125130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin LJ. Fucoxanthin and its metabolite fucoxanthinol in cancer prevention and treatment. Mar Drugs. 2015;13:4784–98. doi: 10.3390/md13084784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-κB in development and progression of human cancer. Virchows Arch. 2005;446:475–82. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- 39.Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-κB and IκB proteins: Implications in cancer and inflammation. Trends Biochem Sci. 2005;30:43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- 41.Yu RX, Hu XM, Xu SQ, et al. Effects of fucoxanthin on proliferation and apoptosis in human gastric adenocarcinoma MGC-803 cells via JAK/STAT signal pathway. Eur J Pharmacol. 2011;657:10–19. doi: 10.1016/j.ejphar.2010.12.006. [DOI] [PubMed] [Google Scholar]