Abstract
Background
Idiopathic pulmonary fibrosis (IPF) can severely damage lung function, which may result in death. Emodin is a major ingredient of rhubarb and has been proven to protect against lung disruptions. Our study focused on the potential medicinal effect of emodin against IPF.
Material/Methods
The experiment subjects were fully-grown male Sprague-Dawley rats with average weight of 180–220 kg. Histological analyses, Western blotting analysis, quantitative real-time PCR, and statistical analysis were used in the study.
Results
We found that emodin significantly reduced lung structural distortion, collagen overproduction, massive inflammatory cells infiltration, proinflammatory cytokines expansion, and injuries caused by administration of bleomycin (BLM). Additionally, emodin suppressed the accumulation of p-IκBα and NF-κB, while stimulating the Nrf2-antioxidant signaling process in damaged lungs. Emodin inhibited epithelial-mesenchymal transition (EMT) induced by BLM in the lungs. Moreover, emodin suppressed the TGF-β1 expression and the downstream signal molecules p-Smad-2 and p-Smad-3, which are reinforced by BLM. Emodin can also reverse EMT-like shifts induced by recombinant TGF-β1 in alveolar epithelial cultured cells.
Conclusions
The effect of emodin in fibrotic lung injury is closely related to its favorable properties of anti-inflammation and anti-oxidation.
MeSH Keywords: Emodin, Epithelial-Mesenchymal Transition, Idiopathic Pulmonary Fibrosis
Background
As a chronic disease, idiopathic pulmonary fibrosis (IPF) has the following characteristics: fibroblasts over-accumulation, widespread deposition of extracellular matrix (ECM), alveolar structure damages, and the gradual decline of pulmonary function [1,2]. Over the years, researchers have endeavored to explore and develop appropriate preventive and therapeutic strategies for IPF [3]. Despite these efforts, an effective anti-fibrotic remedy has not yet been found. The current survival rate (for the long-term) of patients with IPF is still very short [4]. Recently, the U.S. Food and Drug Administration (FDA) has recognized 2 medicines for IPF: pirfenidone, which inhibits TGF-β and TGF-β-induced collagen production; and nintedanib, which inhibits tyrosine-kinase [5,6]. Nevertheless, these drugs have inevitable adverse effects such as gastrointestinal disorders, skin-related abnormalities, and unusual increases in the amount of alanine and aspartate aminotransferase, and they only postpone pulmonary function failure, not reverse the process [7]. Considerable efforts are still needed in the ongoing study of IPF and the exploration of its effective therapeutants.
Previous studies indicated that there is a correlation between IPF and epithelial-mesenchymal transition (EMT) through losing the epithelial markers (E-cadherin) and expression of mesenchymal markers (vimentin and fibronectin), suggesting the influence of EMT on IPF progression [8,9]. As a major fibrogenic cytokine, TGF-β1 controls many cell functions, such as adhesion, migration, proliferation, and apoptosis [10], which was demonstrated in lungs with IPF [11]. TGF-β1 not only induces the change from fibroblasts to myofibroblasts and maintains contractile phenotype, but also causes EMT in alveolar epithelial cells (AECs) and play a key role in organ fibrosis by controlling canonical and non-canonical Smad signaling [12,13]. These 2 methods can both subdue the expression of E-cadherin in the form of zinc-finger transcription factors like Slug and Snail [14]. Therefore, we believe that the anti-EMT pathway or the method of inhibiting of TGF-β1 signaling could effectively assist the treatment of IPF [15].
Extracted from the plant Rheum palmatum L., emodin (1,3,8-trihydroxy-6-methylanthraquinone) is an anthraquinone with antivirus, anticancer, and anti-inflammation actions, as well as having immunosuppressive and pro-apoptotic effects [16]. Recent studies reported emodin can stimulate apoptosis through caspase-dependent processing and achieve suppression of EMT, thus inhibiting proliferation [17–19]. It was also reported that emodin can moderate pancreatic and hepatic fibrosis by suppressing TGF-β1 expression level [20,21]. Emodin has also been reported to restrain BLM-triggered pulmonary fibrosis in experiments with laboratory mice [22]. However, although emodin has demonstrated certain positive therapeutic effects on pulmonary fibrosis, the underlying pharmacology and mechanism remain unclear.
The present study explored the therapeutic effect of emodin in lung fibrotic disease. Experiments were conducted in vivo and in vitro. Our results suggest that emodin has a therapeutic effect on fibrotic lung injury via its anti-oxidation, anti-fibrosis, and anti-inflammation properties.
Material and methods
Animals
The experiment subjects were fully-grown male Sprague-Dawley rats with average weight of 180–220 kg, obtained from the Experimental Animal Center of the Chinese Academy of Sciences in Shanghai. They were raised in a constant environmental condition of 12/12 h light/dark cycle and a temperature of around 25(±2)°C with ad libitum feeding. With the approval of the Animal Care and Use Committee of Tongji Medical College of Huazhong University of Science and Technology, the animal experiments were carried out strictly following the guidelines given by the committee.
Animal treatment
The rats were randomly divided into the following groups (5 rats per group): a) a control group, with saline plus 0.5% sodium carboxymethyl cellulose (0.5% CMC-Na); b) a BLM group, BLM plus 0.5% CMC-Na i.g.; c) BLM+emodin group, BLM plus 20 mg/kg emodin in 0.5% CMC-Na i.g. BLM in saline and saline alone were all intragastrically injected into the subjects. After 10 days of BLM administration, we administrated emodin or 0.5% CMC-Na by gavage once a day for 21 consecutive days. The lungs were harvested for experiments immediately after euthanizing the rats.
Cell culture
The human A549 cell line (Sigma Aldrich, Shanghai, China) was obtained and maintained in DMEM (Thermo Fisher Scientific) complemented with 10% FBS (Hyclone, Logan, Utah, USA) in 95% air and 5% CO2 at 37°C. Type II AECs were extracted from neonatal rat pulmonary tissues following the protocols as previously described [23]. Recombinant TGF-β1 in volume of 10 ng/mL (Creative Biomart, NY, USA) and emodin (60 μM) were collectively administrated to the rat primary AECs and the A549 cell line for 2 days.
Bronchoalveolar lavage (BAL)
We ligated the right bronchus of rats and administered BAL to the left part via a tracheal tube with 1.5 mL normal saline. A cell pellet was then retrieved from the bronchoalveolar lavage fluids (BALFs), which was then suspended in cool PBS, mounted on a microscope slide, and stained with Giemsa (Jian Cheng Bioengineering Institute) to differentiate various types of cells for examination under a microscope. A hemocytometer was used to calculate the total cell numbers. The proinflammatory cytokines of the cell-free BALF, including tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), and interleukin 6 (IL-6), were measured using USCN ELISA kits (Wuhan USCN Business Co., Ltd., Wuhan, China), which was used in accordance with the protocols provided by the producer. The result values are presented in picograms (pg) per milliliter (ml) of BALF.
Evaluation of collagen deposition and oxidative stress
We used kits manufactured by JanCheng Bioengineering Institute to assess superoxide dismutase (SOD), malondialdehyde (MDA), glutathione peroxidase (GSH-Px), hydroxyproline, and glutathione (GSH) in rat pulmonary tissues.
Histological analyses
The pulmonary tissues were prepared by being submerged in paraformaldehyde (4%) and embedded in paraffin for histological and immunohistochemical studies. We stained 4-μm sections with hematoxylin and eosin (H&E). All measurements were performed following the manufacturers’ instructions.
Western blot analysis
The pulmonary cells were lysed in a solution of 20 mM Tris-HCL (pH 7.4), 4% sodium dodecyl sulphate (SDS), and 10% glycerol, which was then boiled for 10 min at 100°C. The protein of cell supernatants was obtained using Amicon® Ultra-4 (Merck Millipore) according to protocol (AG-CR1-3951); nuclear extraction was obtained by utilizing the NE-PER Nuclear and Cytoplasmic Extracts Reagents kits (Pierce Biotechnology, IL, USA). The Bicinchoninic Acid (BCA) Protein Assay Kit (Pierce Biotechnology) was used to determine the protein concentrations. For Western blot analysis, 20–40 μg lysate was isolated from 10% or 12% SDS-polyacrylamide gel under reducing conditions and delivered onto polyvinylidene difluoride membranes (Merck Millipore). We pre-incubated the membranes in 1× Tris-buffered-saline with 5% milk for 1 h at room temperature. The membranes were then incubated with primary antibodies at 4°C overnight. The primary antibodies were anti-p-NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells), anti-p-IκBα, anti-p-Smad2, anti-p-Smad3, anti-vimentin, anti-Nrf2, anti-GAPDH, anti-Histone-H3, and anti-NADPH/quinone-oxidoreductase (Cell signaling Technology, MA, USA).The rabbit polyclonal antibodies were anti-E-cadherin, anti-fibronectin, anti-TGF-β1, anti-hemoxygenase-1 (anti-HO-1), and a mouse antibody anti-α-smooth muscle actin (anti-α-SMA) from Santa Cruz Biotechnology (CA, USA).The secondary antibody was a goat anti-rabbit antibody (Sigma-Aldrich). Bands were detected with chemiluminescence kit (SuperSignal West Pico substrate; IL, USA).
Quantitative real-time PCR
Total RNA of the pulmonary tissues was isolated using Trizol (Takara, Shiga, Japan). We used the RT-qPCR Kit by Toyobo (Japan) to process first-strand cDNA synthesis with 0.5 μg of total RNA. The mRNA of E-cadherin and TGF-β1 was evaluated using the iCyler iQ Real-time PCR Detection System (Bio-Rad Laboratories Inc., USA) with SYBR Green Real-time PCR Master Mix (Toyobo, Japan) in the volume of 20 μL. We calculated the CT values of each gene in samples and used the 2−ΔΔCt method to measure the transcript levels. Measurement were repeated 3 times and then standardized with GAPDH mRNA. The primers used for qPCR assays were: E-cadherin F, 5′ CAC CTG GAG AGA GGC CAT GT3′ and E-cadherin R, 5′ TGG GAA ACA TGA GCA GCT CT3′; TGF-β1 F, 5′ GGC TTT CGC CTT AGC GCC CA3′ and TGF-β1 R, 5′ CTC GGC GGC CGG TAG TGA AC3′; GAPDH F, 5′-CAG CCT CAA GAT CAT CAG CA-3′ and GAPDH R, 5′-TGT GGT CAT GAG TCC TTC CA-3′.
Statistical analysis
Standard deviation was considered in the measurement of data, and statistical analysis was conducted with Student’s t-test or one-way analysis of variance (ANOVA) for continuous variables and a p<0.05 was considered statistically significant.
Results
Emodin alleviates rat BLM-triggered lung injuries
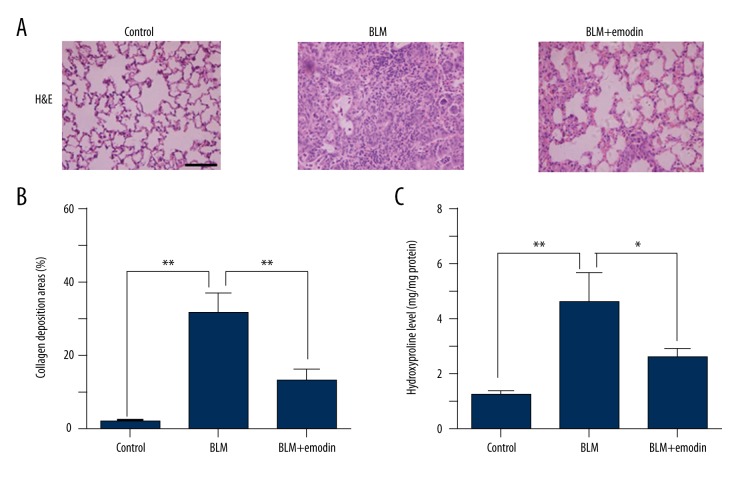
To examine the anti-fibrotic ability of emodin in vivo, the BLM-treated rats were administrated emodin (20 mg/kg) for 3 consecutive weeks in total. The fibrosis-injured tissues in rats were stained with H&E to distinguish it from normal tissue. Histopathological results showed that BLM treatment induced a high degree of structural distortion (Figure 1A, 1B). Additionally, there was considerable increase in hydroxyproline compared with the non-BLM-treated group in the fibrotic pulmonary tissues (Figure 1C). Our data show that emodin moderated the BLM-triggered pulmonary fibrosis (Figure 1A, 1B), and emodin significantly decreased the hydroxyproline level in comparison with the control group (Figure 1C). These data prove that emodin inhibits the BLM-induced fibrotic lesions of rats.
Figure 1.
Emodin ameliorates BLM-triggered pulmonary fibrosis in rats. Rats received intratracheal injection of BLM and then were administrated with emodin 20 mg/kg each day by gavage for 21 days, with 5 rats per group. (A) Fractions of pulmonary tissue of control group, BLM group, and BLM + emodin group were stained with H&E. Scale bars 100 μm. (B) Extent of lung fibrosis was assessed and presented in percentages to the total area under evaluation. (C) Hydroxyproline levels of 3 groups were measured and compared with each other 3 weeks after BLM treatment. All data are presented as mean ±SD (n=5), * P<0.05; ** P<0.01.
Emodin has anti-inflammatory effects in BLM-damaged lungs of rats
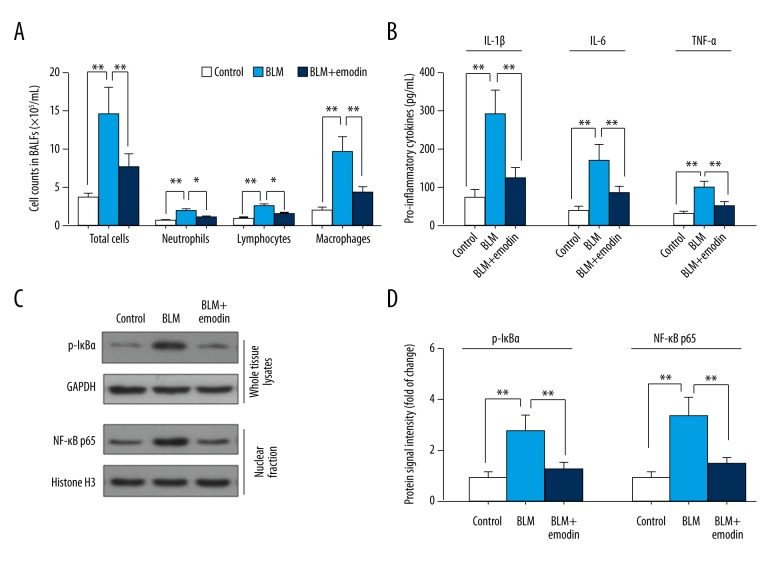
To assess the spread of BLM-caused inflammation, total cell number in 1 mL BALF was calculated. Giemsa staining was used to help differentiate and examine different cell types. As demonstrated in Figure 2A, massive inflammatory cells, including neutrophils, lymphocytes, and macrophages, entered into the lung with the injection of BLM compared with the control group, and macrophages were the prevailing cell type. The harmful progression of inflammatory infiltration was hindered by emodin treatment (Figure 2A), and the excessive production of proinflammatory IL-1β, IL-6, and TNF-α was significantly reduced by emodin compared with the group not treated with BLM (Figure 2B). IκBα phosphorylation and NF-κB (p65) accumulation in nuclei were increased by BLM treatment, but they were decreased by emodin treatment (Figure 2C, 2D). These data suggest that emodin has positive anti-inflammatory effects on lung lesions, in which NF-κB signaling pathways have an important role.
Figure 2.
Emodin suppressed inflammatory infiltration in BLM-induced pulmonary tissues. (A) Total number of cells and number of differentiate cell types in 1 mL BALFs was counted (n=5). (B) IL-1β, IL-6, and TNF-α level in cell-free BALFs supernatant were measured with ELISA kits. (C) The levels of protein expression of p-IκBα and nuclear translocation of NF-κB p65 were determined by Western blot analysis. (D) Quantitation of Western blot signal intensities. The study used endogenous GAPDH as a control for p-IκBα, and Histone H3 as a control for p65. All data are presented as mean ±SD (n=5), * P<0.05; ** P<0.01.
Emodin displays anti-oxidative effects in BLM-damaged lungs of rats
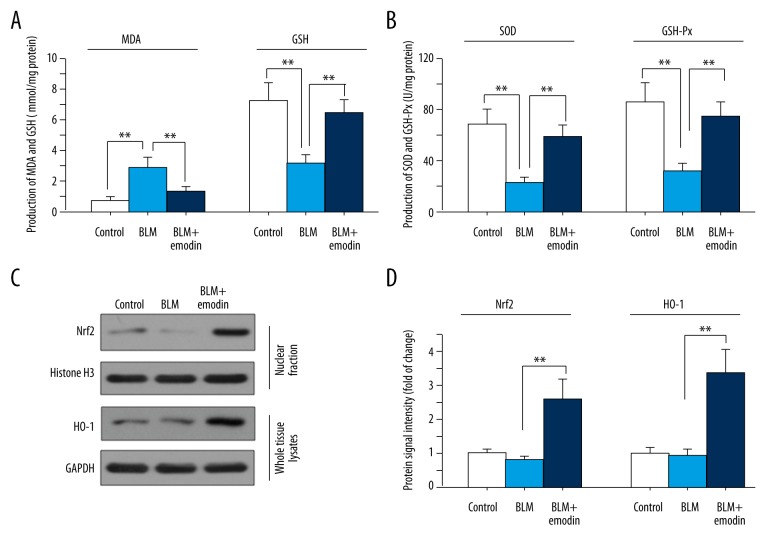
We further investigated the anti-oxidative effects of emodin in BLM-damaged lungs of rats. As shown in Figure 3A, BLM treatment caused higher MDA production than in the control group, while emodin decreased MDA production. On the contrary, the levels of antioxidants like GSH, SOD, and GSH-Px was decreased under BLM influence, and emodin treatment increased the levels of GSH, SOD, and GSH-Px compared with the BLM group (Figure 3B). The accumulation of Nrf2 in cell nuclei was greatly enhanced by emodin, along with a rise in HO-1, an Nrf2-inducible antioxidant (Figure 3C, 3D). These results indicate that emodin displays anti-oxidative effects on lung lesions, with the Nrf2 signaling pathway playing an important role.
Figure 3.
Emodin has anti-oxidative function and stimulates Nrf2 signaling in BLM-treated pulmonary tissues of rats. Activities of MDA, GSH (A), SOD, and GSH-Px (B) in control group, BLM group, and BLM + emodin group were measured using commercial kits. (C) Protein expression levels of nuclear Nrf2 and HO-1 were assessed by Western blot assay. (D) Quantitation of Western blot signal intensities. Histone H3 was used as a control for Nrf2 and GAPDH for HO-1. All data are presented as mean ±SD (n=5), ** P<0.01.
Emodin reduces TGF-β1-Smad-2-Smad-3 signaling in BLM-induced rat pulmonary tissues
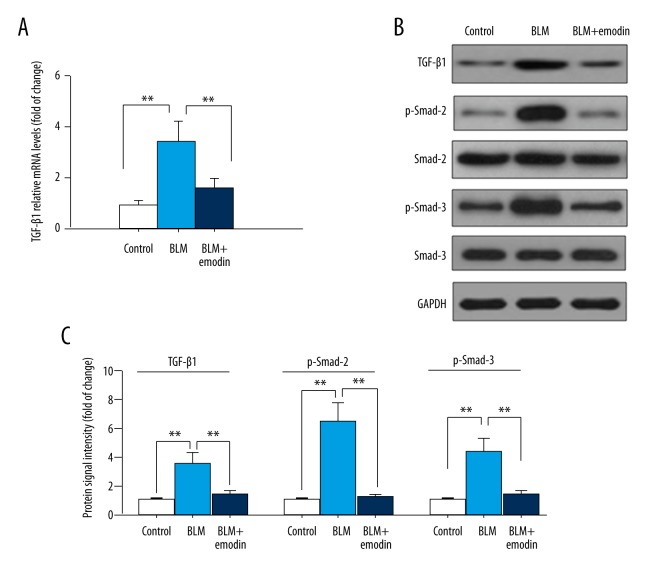
Because the TGF-β-Smad signaling pathway was activated during BLM-induced fibrotic injuries [24], we proceeded with the exploration of whether emodin has any influence on TGF-β signaling. The quantitative RT-PCR test results indicated an obvious improvement in mRNA expression of TGF-β1 in the BLM-induced rat lungs, and the level was then reduced by treatment with emodin (Figure 4A). The suppressive effects of emodin on expression of TGF-β1 was also double-validated by Western blot analysis (Figure 4B, 4C). Emodin also reduced the level of BLM-stimulated phosphorylation of Smad-3 and Smad-2 without altering the expressions of total proteins among various groups (Figure 4B, 4C). These data demonstrated the capacity of emodin to inhibit BLM-triggered TGF-β1/Smad-2/Smad-3 signaling in rat pulmonary tissues.
Figure 4.
Emodin prohibits TGF-β1/Smad-2/-3 signaling transduction in BLM-damaged pulmonary tissue. (A) The mRNA expression levels of TGF-β1 were measured by qRT-PCR. (B) Protein expression levels of TGF-β1, smad2, p-smad2, smad3, and p-smad3 were evaluated with Western blot assay. (C) Quantitation of Western blot signal intensities. All data are presented as mean ±SD (n=5), ** P<0.01.
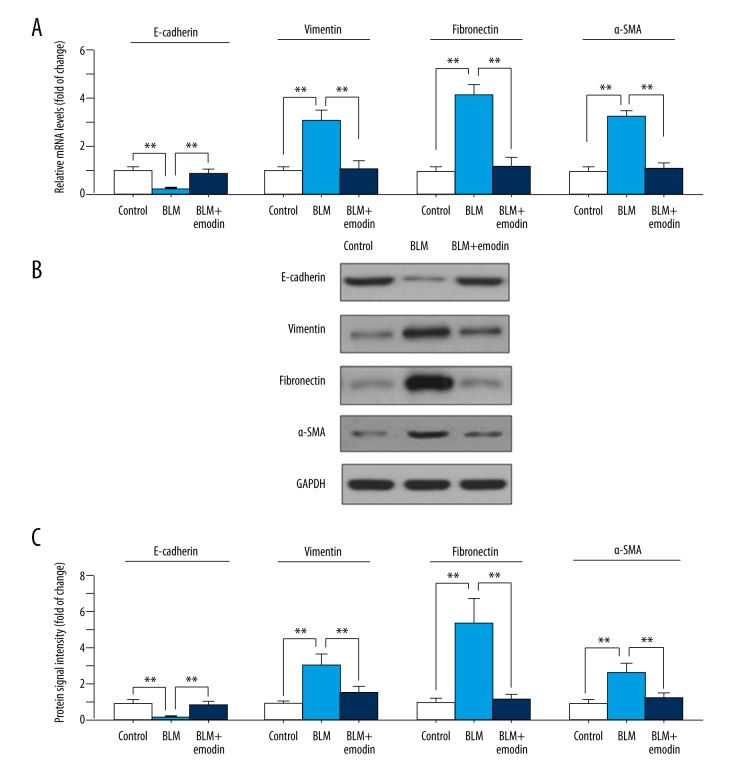
Emodin suppresses BLM-induced EMT of rat pulmonary tissues
E-cadherin loss usually signifies EMT-like responses in cells [25]. To determine if BLM treatment would induce EMT in lungs, the E-cadherin expression levels were detected by qRT-PCR and Western blot analysis. We found a significant down-regulation of expression of both protein and mRNA of E-cadherin in BLM-induced pulmonary tissues compared with control (Figure 5A–5C). Notably, emodin treatment inhibited the decreasing expression of E-cadherin in BLM stimulation (Figure 5A–5C). In addition, protein and mRNA expression of vimentin, fibronectin, and α-SMA as EMT markers were elevated by BLM and suppressed by emodin (Figure 5A–5C). These results show the therapeutic effects of emodin on rats with BLM-induced EMT in pulmonary tissues.
Figure 5.
Emodin subdues BLM-induced EMT in rat pulmonary tissues. (A) Relative mRNA expression levels of indicate genes were measured with qRT-PCR. (B) Protein expression levels of E-cadherin, fibronectin, vimentin, and α-SMA were assessed with Western blot assay. (C) Quantitation of Western blot signal intensities. All data are presented as mean ±SD (n=5), ** P<0.01.
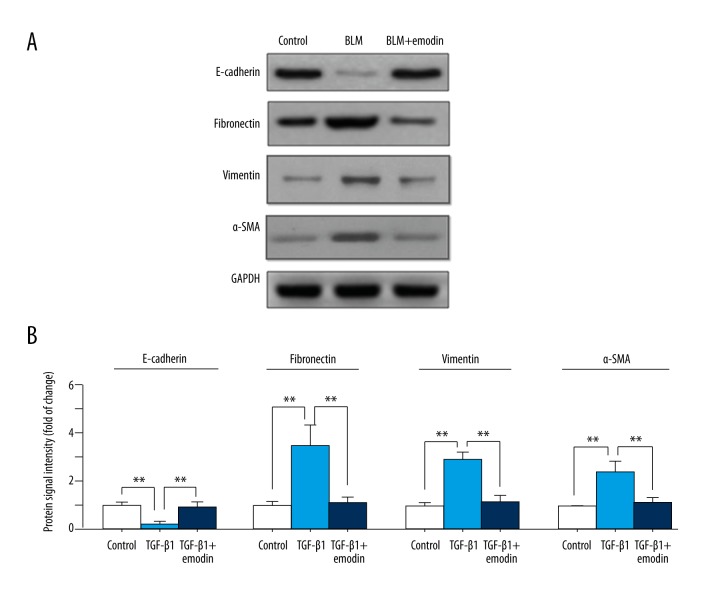
Emodin suppresses EMT induced by TGF-β1 in alveolar epithelial A549 cells
Due to the key role of EMT in the pathogenesis of fibrosis, we further assessed whether the anti-fibrosis effect of emodin was exerted through inhibiting EMT in vitro. Since TGF-β1 is a primary cytokine in inducing EMT [26], we examined the effect of emodin on TGF-β1-induced EMT in A549 cells. As shown in Figure 6A and 6B, decreased expression of E-cadherin and increased expression of fibronectin were found in TGF-β1-induced cells. Emodin successfully suppressed TGF-β1-induced EMT, which was defined by upregulating E-cadherin and downregulating fibronectin expression compared with the TGF-β1 treated group. These data indicate that the anti-fibrosis effects of emodin are at least partly mediated through suppressing EMT.
Figure 6.
Emodin inhibits TGF-β1-induced EMT in A549 cells. The A549 cells were treated with TGF-β1 (10 ng/mL) and emodin (60 μM), while the cells treated with DMSO was set up as the control group. (A) Expression levels of E-cadherin, fibronectin vimentin, and α-SMA were measured by Western blot assay. (B) Quantitation of Western blot signal intensities. All data are expressed as mean ±SD from 3 independent experiments. ** P<0.01.
Discussion
Fibrotic changes in lung injuries is the major factor that leads to the death of IPF patients. To advance IPF therapy, it is essential to identify a novel agent that has anti-fibrotic effects. In the present study, our results suggest that the therapeutic effect of emodin in fibrotic lung injury is closely correlated with its beneficial anti-oxidation, anti-fibrosis, and anti-inflammation properties.
As a conventional chemotherapy drug widely used in treating neoplasms, BLM is frequently applied in research to induce lung fibrosis [24]. The present study first explored the effect that emodin had on BLM-related pulmonary injury by evaluating the histological alterations in rats. The result are consistent with several previous studies [24,27], which revealed a considerable structural distortion in rat pulmonary tissues induced by BLM. The BLM-treated lungs were observed to have large inflammatory cell infiltrations, overproduction of IL-1β, IL-6, and TNF-α, and excessive collagens deposition, and these changes were all suppressed by emodin. Sufficient evidence shows that BLM stimulation triggers NF-κB signaling, which is able to contain inflammation during pulmonary fibrogenesis [28]. The results of our study provide evidence that emodin interferes with NF-κB signaling through subduing translocation of p-IκBα and p65 in nuclei.
Oxidative stress plays a major role in different pathological conditions [29]. BLM treatment was confirmed to be able to increase oxidative stress damage and stimulate reactive oxygen species (ROS) [30]. As ROS usually results in a production of lipid-peroxidated products [31], our study used MDA, a lipid peroxidation index, to examine oxidative tissue damage. Our results show that BLM treatment in rats leads to remarkable elevation of MDA, which was in turn fully controlled by emodin in rat pulmonary tissues. Apart from the abnormal increase in MDA, the BLM-stimulated oxidative injury may also be detected by observing the level of antioxidants, including GSH-Px, SOD, and GSH. Accordingly, activities of these antioxidants were evaluated in rat pulmonary tissues. Data reported declines of GSH-Px, SOD, and GSH in BLM-induced pulmonary tissues, and dramatic increases after being treated with emodin. Onset of Nrf2-mediated anti-oxidative protection signifies a new therapeutic strategy to stop the progression in fibrotic injury [32]. Unleashing (from the Keap1-Nrf2 complex) and accumulation of Nrf2 in nuclear stimulates the expression of anti-oxidation defense gene HO-1 by leveraging its association with certain antioxidant response factor [33,34]. Our study showed that emodin boosts Nrf2 accumulation in nuclei and promotes HO-1 expression during fibrotic lung injuries. Further research on pharmaceutical agents and gene therapies to suppress the activation of Nrf2 are needed to examine the regulatory pathway of emodin.
Regarding the pathogenesis of IPF, researchers have reached a general consensus that EMT induced by severe initial epithelial injuries is highly associated with IPF progression [35]. TGF-β, a cytokine that promotes sclerosis, can stimulate EMT in fibrotic diseases [36]. The inhibition of fibroblast accumulation and even reversion of the established fibrosis can be achieved through interfering with TGF-β signaling [37]. Epithelial shifts triggered by BLM in rat pulmonary tissues were assessed by observing the expression levels of EMT-related biomarkers in our study. Results show that BLM decreases expression of major epithelial-specific biomarker E-cadherin and elevates levels of mesenchymal biomarkers (i.e., α-SMA, vimentin, and fibronectin) in lungs of rats. These shifts were attenuated by emodin, which suggests the inhibition of emodin on EMT-like changes in injured lungs. Phosphorylation of Smad-2 and Smad-3 triggered by the activation of TGF-β receptor is seen as a critical process in TGF-β signaling suppression [38]. Our study discovered that the activation of TGF-β-Smad signaling induced by BLM in rat lung issues was partially inhibited by emodin.
Some of the fibroblasts in pulmonary fibroblastic foci originate from epithelium in EMT [39]. Further experiments in vitro were conducted in recombinant TGF-β1-stimulated AECs to assess whether emodin effectively influences TGF-β-induced EMT alterations. Data from our research suggest that external TGF-β1 greatly depressed expression of E-cadherin in AECs. In agreement with conclusions of in vivo experiments, the decrease in E-cadherin was reversed by emodin. As fibrosis progresses in pulmonary tissues, fibroblasts can develop into myofibroblast phenotypes that produce ECM, increasing tissue stiffness and worsening injury [12,40]. However, pulmonary epithelial cells are not a major source of lung myofibroblasts during EMT [39]. Although our study showed that emodin inhibits EMT-like changes in pulmonary epithelial cells induced by TGF-β, how it modulates the differentiation and proliferation of myofibroblasts and activates myofibroblasts still needs further examination and clarification.
Conclusions
The present study proves that treatment with emodin can effectively alleviate the fibrotic lung injuries induced by BLM in laboratory rats. Its therapeutic effect was highly correlated to its actions in attenuating oxidation, inflammation, and fibrosis. Our study may improve IPF therapy by showing the potential therapeutic utility of emodin in clinical settings.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Camelo A, Dunmore R, Sleeman MA, Clarke DL. The epithelium in idiopathic pulmonary fibrosis: Breaking the barrier. Front Pharmacol. 2014;4:173. doi: 10.3389/fphar.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cottin V. Idiopathic interstitial pneumonias with connective tissue diseases features: A review. Respirology. 2016;21:245–58. doi: 10.1111/resp.12588. [DOI] [PubMed] [Google Scholar]
- 3.Spagnolo P, Maher TM, Richeldi L. Idiopathic pulmonary fibrosis: Recent advances on pharmacological therapy. Pharmacol Ther. 2015;152:18–27. doi: 10.1016/j.pharmthera.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Chakraborty S, Chopra P, Ambi SV, et al. Emerging therapeutic interventions for idiopathic pulmonary fibrosis. Expert Opin Investig Drugs. 2014;23:893–910. doi: 10.1517/13543784.2014.913569. [DOI] [PubMed] [Google Scholar]
- 5.King TE, Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–92. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 6.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–82. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 7.Kim HJ, Perlman D, Tomic R. Natural history of idiopathic pulmonary fibrosis. Respir Med. 2015;109:661–70. doi: 10.1016/j.rmed.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Milara J, Navarro R, Juan G, et al. Sphingosine-1-phosphate is increased in patients with idiopathic pulmonary fibrosis and mediates epithelial to mesenchymal transition. Thorax. 2012;67:147–56. doi: 10.1136/thoraxjnl-2011-200026. [DOI] [PubMed] [Google Scholar]
- 9.Olson N, van der Vliet A. Interactions between nitric oxide and hypoxia-inducible factor signaling pathways in inflammatory disease. Nitric Oxide. 2011;25:125–37. doi: 10.1016/j.niox.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willis BC, Borok Z. TGF-beta-induced EMT: Mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–34. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 11.Khalil N, O’Connor RN, Unruh HW, et al. Increased production and immunohistochemical localization of transforming growth factor-beta in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 1991;5:155–62. doi: 10.1165/ajrcmb/5.2.155. [DOI] [PubMed] [Google Scholar]
- 12.Hinz B, Phan SH, Thannickal VJ, et al. The myofibroblast: One function, multiple origins. Am J Pathol. 2007;170:1807–16. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King TE, Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–61. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 14.Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–66. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 15.Takemasa A, Ishii Y, Fukuda T. A neutrophil elastase inhibitor prevents bleomycin-induced pulmonary fibrosis in mice. Eur Respir J. 2012;40:1475–82. doi: 10.1183/09031936.00127011. [DOI] [PubMed] [Google Scholar]
- 16.Xu JD, Liu S, Wang W, et al. Emodin induces chloride secretion in rat distal colon through activation of mast cells and enteric neurons. Br J Pharmacol. 2012;165:197–207. doi: 10.1111/j.1476-5381.2011.01573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen T, Zheng LY, Xiao W, et al. Emodin ameliorates high glucose induced-podocyte epithelial-mesenchymal transition in-vitro and in-vivo. Cell Physiol Biochem. 2015;35:1425–36. doi: 10.1159/000373963. [DOI] [PubMed] [Google Scholar]
- 18.Wang C, Wu X, Chen M, et al. Emodin induces apoptosis through caspase 3-dependent pathway in HK-2 cells. Toxicology. 2007;231:120–28. doi: 10.1016/j.tox.2006.11.064. [DOI] [PubMed] [Google Scholar]
- 19.Way TD, Huang JT, Chou CH, et al. Emodin represses TWIST1-induced epithelial-mesenchymal transitions in head and neck squamous cell carcinoma cells by inhibiting the beta-catenin and Akt pathways. Eur J Cancer. 2014;50:366–78. doi: 10.1016/j.ejca.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 20.Wang CH, Gao ZQ, Ye B, et al. Effect of emodin on pancreatic fibrosis in rats. World J Gastroenterol. 2007;13:378–82. doi: 10.3748/wjg.v13.i3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhan Y, Li D, Wei H, et al. Emodin on hepatic fibrosis in rats. Chin Med J (Engl) 2000;113:599–601. [PubMed] [Google Scholar]
- 22.Chen XH, Sun RS, Hu JM, et al. Inhibitory effect of emodin on bleomycin-induced pulmonary fibrosis in mice. Clin Exp Pharmacol Physiol. 2009;36:146–53. doi: 10.1111/j.1440-1681.2008.05048.x. [DOI] [PubMed] [Google Scholar]
- 23.Abraham V, Chou ML, DeBolt KM, Koval M. Phenotypic control of gap junctional communication by cultured alveolar epithelial cells. Am J Physiol. 1999;276:L825–34. doi: 10.1152/ajplung.1999.276.5.L825. [DOI] [PubMed] [Google Scholar]
- 24.Della Latta V, Cecchettini A, Del Ry S, Morales MA. Bleomycin in the setting of lung fibrosis induction: From biological mechanisms to counteractions. Pharmacol Res. 2015;97:122–30. doi: 10.1016/j.phrs.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal. 2014;7:re8. doi: 10.1126/scisignal.2005189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–74. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 27.Arizmendi N, Puttagunta L, Chung KL, et al. Rac2 is involved in bleomycin-induced lung inflammation leading to pulmonary fibrosis. Respir Res. 2014;15:71. doi: 10.1186/1465-9921-15-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alvira CM. Nuclear factor-kappa-B signaling in lung development and disease: One pathway, numerous functions. Birth Defects Res A Clin Mol Teratol. 2014;100:202–16. doi: 10.1002/bdra.23233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birben E, Sahiner UM, Sackesen C, et al. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teixeira KC, Soares FS, Rocha LG, et al. Attenuation of bleomycin-induced lung injury and oxidative stress by N-acetylcysteine plus deferoxamine. Pulm Pharmacol Ther. 2008;21:309–16. doi: 10.1016/j.pupt.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Bocchino M, Agnese S, Fagone E, et al. Reactive oxygen species are required for maintenance and differentiation of primary lung fibroblasts in idiopathic pulmonary fibrosis. PLoS One. 2010;5:e14003. doi: 10.1371/journal.pone.0014003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kikuchi N, Ishii Y, Morishima Y, et al. Nrf2 protects against pulmonary fibrosis by regulating the lung oxidant level and Th1/Th2 balance. Respir Res. 2010;11:31. doi: 10.1186/1465-9921-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shelton P, Jaiswal AK. The transcription factor NF-E2-related factor 2 (Nrf2): A protooncogene? FASEB J. 2013;27:414–23. doi: 10.1096/fj.12-217257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walters DM, Cho HY, Kleeberger SR. Oxidative stress and antioxidants in the pathogenesis of pulmonary fibrosis: A potential role for Nrf2. Antioxid Redox Signal. 2008;10:321–32. doi: 10.1089/ars.2007.1901. [DOI] [PubMed] [Google Scholar]
- 35.Friedman SL, Sheppard D, Duffield JS, Violette S. Therapy for fibrotic diseases: Nearing the starting line. Sci Transl Med. 2013;5:167sr1. doi: 10.1126/scitranslmed.3004700. [DOI] [PubMed] [Google Scholar]
- 36.Jiang L, Xiao L, Sugiura H, et al. Metabolic reprogramming during TGFbeta1-induced epithelial-to-mesenchymal transition. Oncogene. 2015;34:3908–16. doi: 10.1038/onc.2014.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hashimoto N, Phan SH, Imaizumi K, et al. Endothelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2010;43:161–72. doi: 10.1165/rcmb.2009-0031OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 39.Tanjore H, Xu XC, Polosukhin VV, et al. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2009;180:657–65. doi: 10.1164/rccm.200903-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balestrini JL, Chaudhry S, Sarrazy V, et al. The mechanical memory of lung myofibroblasts. Integr Biol (Camb) 2012;4:410–21. doi: 10.1039/c2ib00149g. [DOI] [PubMed] [Google Scholar]