Abstract
Familial aggregation in inflammatory bowel disease (IBD) has been established for several decades, reflecting shared genetic and environmental susceptibility. A positive family history remains the strongest recognizable risk factor for the development of IBD and is reported in around 8-12% of IBD patients. Crohn’s disease shows a more frequent familial pattern than ulcerative colitis. The risk of developing IBD in first-degree relatives of an affected proband is increased 4- to 8-fold. The risk for twins and children born from couples who both have IBD is also substantially higher; a cumulative effect of the number of family members affected has been described, with the highest incidence being described for families with three or more affected members. Herein, we review the available evidence regarding familial IBD, and briefly discuss the variation of IBD across different races and ethnicities, hoping to provide a useful update and a practical guide that can serve clinicians as a guide for counseling.
Keywords: Inflammatory bowel disease, familial risk, ethnic risk, first-degree relatives, phenotype, counseling
Introduction
Inflammatory bowel diseases (IBD), encompassing Crohn’s disease (CD) and ulcerative colitis (UC) are chronic, relapsing, inflammatory diseases of the gastrointestinal tract with an unknown etiology [1,2]. Currently, the strongest identifiable risk factor for the development of IBD is a positive family history [3]. Familial aggregation of IBD may be due to genetic factors and/or to shared putative environmental factors [1,2]. The genetic influence is supported by higher concordance rates in monozygotic (MZ) twins and the increased incidence of IBD in first-degree relatives (FDRs) of affected probands [4]. In contrast, the presence of discordant MZ twins suggests that other acquired factors either trigger IBD in the affected twin or “protect” the unaffected one [4]. In fact, genome-wide association studies have identified more than 200 alleles associated with disease; however, it is estimated that only 8.2-13.1% of disease heritability is explained by genetic variation, suggesting that other shared environmental and/or epigenetic factors may be involved [5,6].
Family studies provide information for counseling of unaffected relatives naturally concerned about their risk of developing the disease. Herein, we analyze the most important studies describing familial and ethnic IBD in the last decades. We hope this review will provide a useful update and a practical guide for counseling.
Assessing the risk of IBD when there is a positive family history
Over the last decades, several studies have been conducted to predict the risk of developing IBD in relatives of an affected proband. The increased risk in relatives is difficult to quantify with precision, despite many published studies. The large variation can be explained by markedly heterogeneous methodologies and possible bias caused by differences in population selection, study design and ascertainment of diagnosis. Furthermore, different studies tend to use different estimates to report on risk [7,8].
A positive family history of IBD has been reported in 1.5-28% and 1.5-24% of CD and UC probands, respectively, highlighting the study variation [7,9-28]. White populations have a higher prevalence of IBD family history (26-33%) compared to African American (9-18%), Hispanic (9-16%) or Asian (5.9%) populations [29-33].
FDRs
Studies on familial risk in IBD have consistently reported an increased risk of CD and UC in the FDRs of affected probands. Tables 1 and 2 show the results from familial studies of CD and UC, respectively, and include the most relevant studies published since the 1980s that reported on the prevalence and/or the estimated risk of IBD in FDRs. The prevalence of IBD in FDRs of a CD and UC proband ranges from 0.35-4.5% and 0.3-2.7%, respectively [7,10-16,22,24,25,28,34]. The risk of developing IBD is higher in FDRs of patients with CD compared to those with UC. The highest prevalence of IBD in FDRs has been reported in studies in Ashkenazi Jewish populations (4-4.5% and 2.6-2.7% for CD and UC probands, respectively) [11,16,24,25], who per se have a higher baseline risk for developing disease. In fact, Yang et al compared familial aggregation between Jewish and non-Jewish patients and found the lifetime risk for IBD among FDRs was higher in Jews (7.8% vs. 5.2% for CD probands and 4.5% vs. 1.6% for UC probands, respectively) [16].
Table 1.
Studies of FDRs in probands with CD
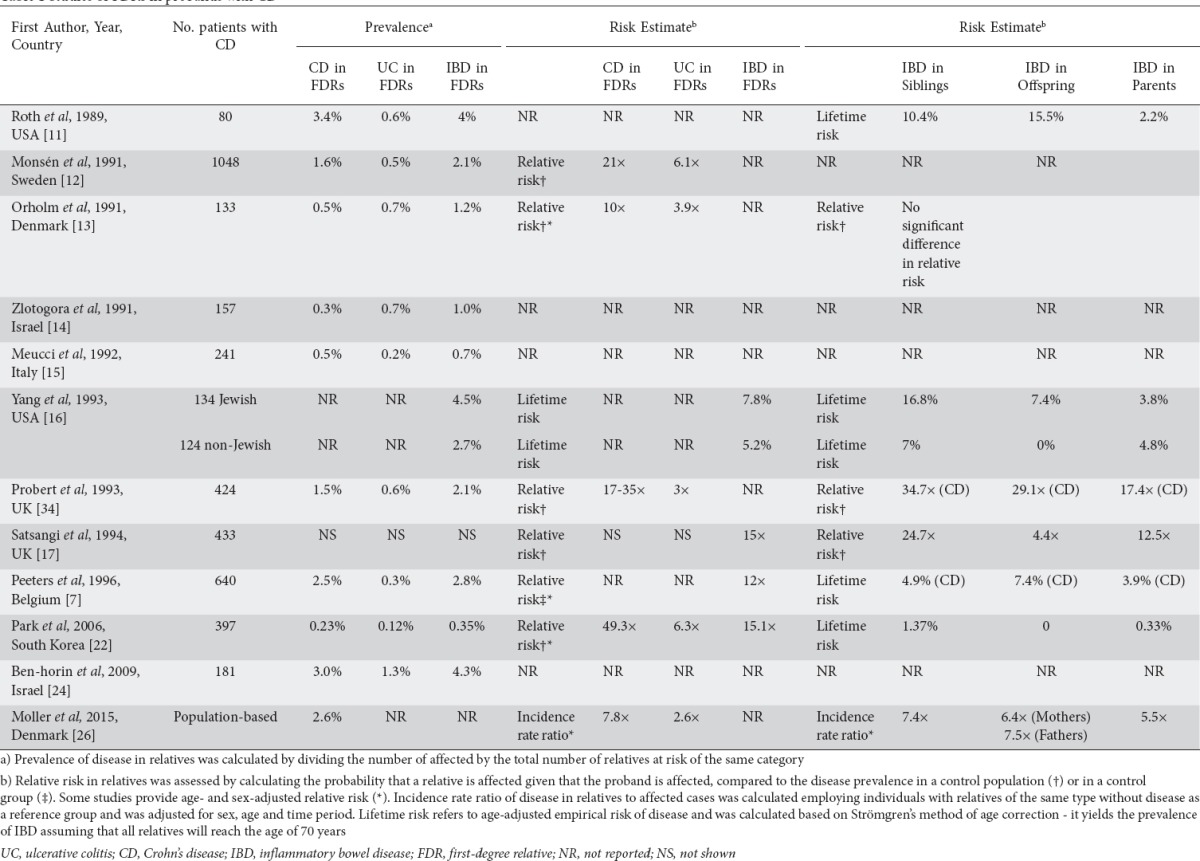
Table 2.
Studies of FDRs in probands with UC
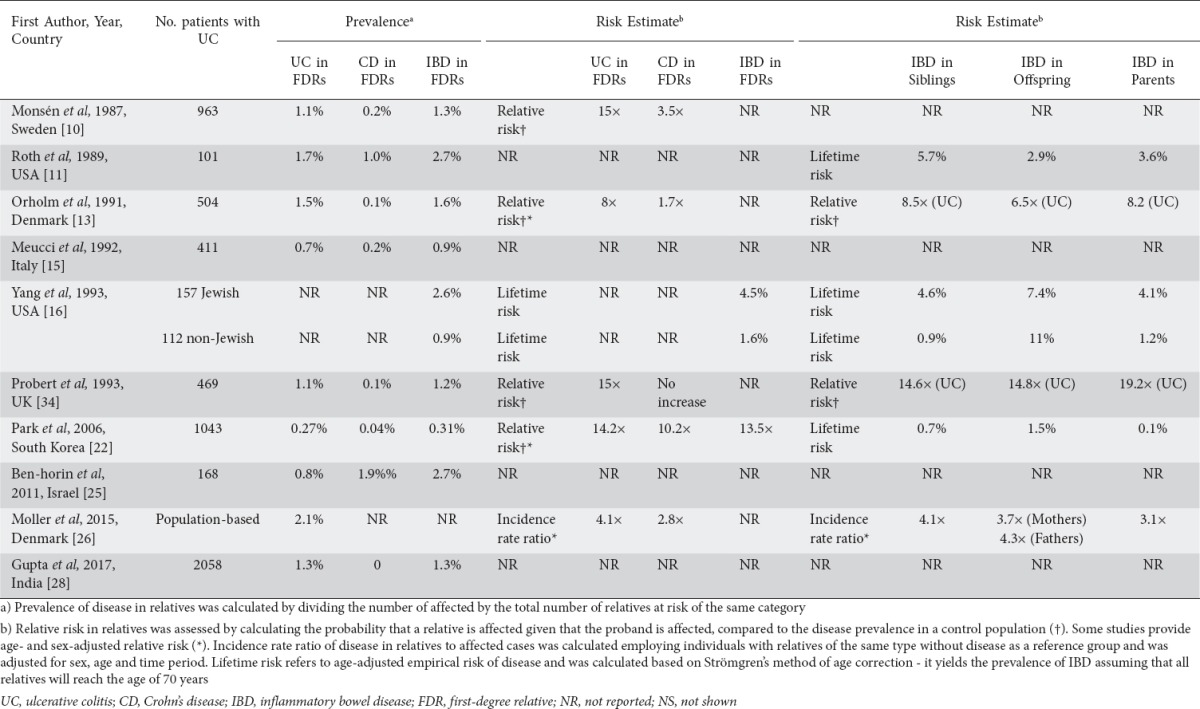
To our knowledge, only one study assessed the relative risk of IBD among FDRs in Asian populations [22]. The authors found a risk of IBD 13.5-15 times higher in FDRs than in the general population, concluding that familial clustering is a common feature of IBD in both low- and high-prevalence areas.
The most powerful study of familial IBD has recently been published by Moller et al [26]. The main strengths of this study were the inclusion of unselected IBD patients from the entire Danish population, and the long follow-up period of up to 34 years. Furthermore, to ensure the validity of the diagnosis, only patients who had at least two independent contacts with the healthcare system resulting in an IBD diagnosis were included. With this methodology the authors were able to assess the incidence rate ratio (IRR) adjusted for age, sex and time period, and to estimate the familial risk in different age groups. The risk of CD was almost 8-fold higher in FDRs of a CD case, whereas the risk of UC in FDRs of a UC proband was increased 4-fold. Having a relative with UC also increased the risk of CD and vice versa. Additionally, the risk among FDRs was dependent on age: the increased risk was particularly pronounced in younger relatives, especially those below the age of 20 years, and decreased progressively with age.
Twins, siblings, offspring, and parents
Among the FDRs of patients with IBD, twins are the group at highest risk for developing disease [26]. The relative risk among MZ twins is significantly higher for CD than for UC [35-37]. Studies on twins with IBD reported concordance rates in MZ twins ranging from 20-56% for both developing CD and from 6-19% for UC. In dizygotic (DZ) twins concordance rates are lower, varying from 0-7% for both CD and UC [4,35,36,38-44]. Twins are also at increased risk of developing the opposite IBD phenotype of their twin in up to 10% of cases [4,35,44]. Original studies reporting on the relative risk of CD in twins estimated this to be 95-738 times higher in MZ twins and 42-72 times in DZ twins with CD, compared to the prevalence rates in the background population. The corresponding risk of UC in MZ and DZ twins with UC was 50-203 and 20-22 [35-37]. Likewise, a recently published population-based study from Denmark confirmed that twins with an affected co-twin have the highest risk among FDRs, although it was less pronounced than previously reported: the IRR of CD was 51.4 (95% confidence interval [CI] 29.12-90.71) in twins with a CD co-twin, and the risk of UC was 11.29 (95%CI 6.18-20.61) when the co-twin had UC. Differences between MZ and DZ twins were not evaluated [26].
Several studies have shown that siblings are the FDRs with the highest risk of developing IBD, when compared with parents or children, especially if the proband has CD [11,13,16,17,22,34]. Studies published in the previous decades have reported a relative risk of developing CD of 23-35 in siblings of a CD proband, and of 5-15 in siblings with an UC proband [13,34,37]. These findings were not confirmed in the population-based cohort study by Moller et al, which found that the risk of developing IBD was similar between siblings and offspring. The estimated risk for CD in patients with a CD-affected sibling was 7.36 (95%CI 6.25-8.67) and the IRR for UC with an UC-affected sibling was 4.08 (95%CI 3.60-4.63) [26].
The frequency of IBD in the offspring of affected parents has also been studied. Two studies specifically assessed whether being born from a mother with IBD conferred a higher risk for the progeny compared to being born from a father with the disease [45,46]. In the first study, out of 135 families identified where both a parent and a child had IBD, parent-to-child transmission was due to the mother in 69% of cases; this was observed only for non-Jewish CD pairs [45]. In a more recent study, familial IBD cases were compared with sporadic IBD. Not only was there a higher proportion of females in familial IBD, but also a significantly higher number of mother-to-child transmissions (63%) were observed, which again was specifically related to CD [46]. These results were not confirmed in the recent population-based study by Moller et al, which reported a slightly higher risk for the offspring when the affected parent was the father: for CD the IRR was 7.53 (95%CI 6.36-8.91) vs. 6.37 (95%CI 5.07-8.00) if the mother was affected; for UC the risk was 4.25 (95%CI 3.70-4.87) compared to 3.71 (95%CI 3.24-4.25) when the mother had the disease [26].
Two studies have reported the risk for the progeny when both parents are affected with IBD [47,48]. In a first study by Bennet et al, 19 couples were identified where both parents had IBD. The diagnosis of IBD was established in 12 of 33 (36%) living offspring from these couples [47]. In a more recent study, the prevalence of CD among 54 children from 25 couples with both parents affected with IBD was 17% (9/54) and the probability of developing disease increased with age, with a 33% risk estimated by 28 years of age [48]. Interestingly, in both studies CD predominated in offspring regardless the type of IBD in the parents, while all children born from couples where both members had CD eventually developed CD.
Finally, among FDRs, parents of children with IBD are the ones with the lowest risk of developing IBD, especially if the proband has CD [7,11,16,22,34]. Moller et al showed that the risk of CD and UC in parents of offspring with CD and UC is slightly lower than for other FDRs (IRR 5.52, 95%CI 4.43-6.88; and IRR 3.05, 95%CI 2.64-3.54, respectively) [26].
Second- (SDRs) and third-degree relatives (TDRs)
The risk for SDRs and TDRs has not been so extensively studied as in FDRs. The prevalence of IBD in SDRs of patients with CD and UC is about 0.2% and 0.3%, respectively [13]. The study by Moller et al showed that the risk of CD and UC was significantly increased in SDRs of probands with the same disease compared to the general population (IRR 2.44, 95%CI 2.01-2.96; and IRR 1.85, 95%CI 1.60-2.13, respectively). The risk in uncles, aunts, nephews, nieces, grandchildren, and grandparents was similar to the overall second-degree familial risk. Interestingly, the risk of IBD was higher in half siblings from the same mother, although the difference was not statistically significant. In previous studies the prevalence of IBD among SDRs seems to be increased only for the same type of IBD as the proband, whereas the prevalence of the opposite IBD phenotype in SDRs was similar than in the general population [13]. Moller et al did not confirm these findings [26].
TDRs also have an increased risk of IBD, although it is considerably less pronounced than the risk for FDRs. The IRR of CD was 1.88 (95%CI 1.30-2.71) and the risk of UC was 1.51 (95%CI 1.07-2.12) if the proband had CD and UC, respectively [26].
Multiplex families
Studies on multiplex IBD families (usually defined as at least three FDRs affected) [49-51] reported up to 7 FDRs affected by the disease within the same nuclear family [51]. Unaffected relatives from multicase families are described as the ones that bear the highest risk for developing IBD, with the highest incidence being described for families with three or more FDRs affected by IBD [51]. Joossens et al found that there is a cumulative effect of the number of family members affected with an increased risk of CD of approximately 10% per additional FDR affected: the odds ratio for developing CD increased from 1.53 (95%CI 1.27-1.84) to 2.33 (95%CI 1.62-3.37) and 3.57 (95%CI 2.06-6.19) if the unaffected FDR had 1, 2 or 3 CD-affected members within its nuclear family, respectively. Furthermore, the authors found a 57-fold increase in the incidence within multiplex families as compared to that of the general population. Moller et al also showed an increased risk of IBD in individuals with two or more affected FDRs: the IRR of CD was 9.77 (95%CI 7.07-13.50) and the IRR of UC was 6.63 (95%CI 5.02-8.75) in individuals with two or more affected FDRs. The same pattern, although less pronounced, was observed for individuals with two or more SDRs affected by IBD [26].
Familial phenotypes
Studies of familial IBD have tried to assess the degree of concordance for disease type, phenotypic features and indicators of disease severity in pairs of relatives concordant for both CD and UC. Most studies have measured concordance using the simple concordance index (i.e., the proportion of agreements against the total number of pairs). Two studies evaluated Cohen’s Kappa (κ) index [52], which provides a more conservative estimate of concordance [7,53]. Phenotypic characteristics and disease severity have also been compared between familial and sporadic disease in several studies.
Type of IBD
Several studies have reported high concordance rates for the type of IBD. For CD and UC familial studies have shown concordance rates of 67-89% [7,9,15,20,22,23,49,53,54] and 69-89%, respectively [9,15,20,22,23,49,53,54]. A recent study published by Cabré et al showed a moderate concordance rate according to the κ index (κ=0.58; 95%CI 0.42-0.73; P<0.01) [53]. These results are in line with studies reporting on familial risk that observed a lower risk of developing the opposite IBD phenotype as in the proband, in both FDRs and SDRs [26]. Likewise, the reported rates for different IBD phenotypes in MZ twins are very low (<10%), as mentioned above [4,35,44].
Phenotype
The age at onset/diagnosis has been compared in familial and sporadic disease and has been found to be significantly lower in familial IBD, ranging from 22-27 vs. 27-33 years in CD and 22-33 vs. 29-37 years in UC, respectively [10,12,16,23,27,55-57]. However, in other studies no differences were identified [7,15,19-21,24,58,59].
Several studies have reported a difference in the age onset/diagnosis of between 15 and 23 years among different generations [7,8,54,56,60-64]. However, the concept of genetic anticipation, i.e., the disease being diagnosed at an earlier age in younger generations, has been questioned and could be related to several biases inherent to the parent–child study design (e.g., ascertainment bias, selection bias). In fact, in most studies within the same generation (i.e., sibling–sibling combination) no differences in the mean age at onset/diagnosis were found [7,24,54,60]. A recent study found that the youngest members in the pairs were diagnosed at an earlier age than the oldest ones, both for pairs of the same (23.5 vs 28, P<0.0005) and different generations (21.2 vs 43.5, P<0.0005); again, this could just be attributable to a higher awareness for early symptoms of IBD, leading to a shorter delay in diagnosis [53].
CD
Studies have tried to assess whether, besides disease, there is transmission of phenotype within affected pairs from the same family. The concordance rates for disease location and disease behavior in pairs of relatives with CD range from 16-86% and 17-82%, respectively, across studies [7,18,49,53-55]. One study found a greater than expected concordance for both CD location and behavior [18], whereas other studies observed a higher than expected concordance only for small bowel/ileal location [54,55] and for perianal disease, and lower concordance for colonic location [55]. Two studies reported that concordance rates for both disease location and behavior increased within families with more than two affected members [7,55]. However, studies reporting κ index showed a poor (κ=0.11) [53] to mild concordance (κ=0.285) [7] for CD location and a mild concordance for disease behavior (κ=0.26) [53]. Likewise, for perianal disease and extra-intestinal manifestations (EIM) concordance rates vary from 44-63% [7,55] and 67%, [54], respectively; however, a poor overall concordance has been reported according to the κ index (κ=0.07 [53] and κ=0.123 [7] for perianal disease and κ=-0.04 for EIM [53]).
In studies comparing phenotypic features between familial and sporadic disease, no significant differences were found for CD location [21,23,24,49,59], behavior [20,21,23,24,54,56,58,59], or for the presence of perianal disease [19,20,24,59] and EIM [19,56,59]. Nonetheless, Colombel et al, in a study comparing 1377 CD patients with sporadic disease and 176 CD patients with familial disease, showed that familial cases had a higher frequency of both small bowel and colonic involvement (61% vs. 49%, P<0.05) and perianal disease (39% vs. 19%, P<0.001) [48]. Later, Carbonnel et al evaluated 1316 patients with CD (1164 sporadic and 152 familial CD) and also observed an increased frequency of both small bowel and colonic involvement (43% vs. 34%, P=0.04), as well as a higher rate of perforating complications (46% vs. 34%, P=0.04) [19]. Likewise, a recently published study by Andreu et al including 6200 patients with CD from a Spanish database (5329 sporadic and 871 familial) found that ileocolonic location (51.7% vs 42.7, P<0.0001), penetrating phenotype (21% vs. 17.6%, P=0.01), perianal disease at CD onset (32% vs. 27.1%, P=0.003) and EIM (30.1% vs. 23.6%, P<0.0001) were significantly more frequent in the familial than in the sporadic group, suggesting that familial aggregation in IBD is associated with a more severe phenotype in CD. This pattern was seen whether patients had FDRs or more distant relatives affected by the disease [57].
UC
In pairs with UC concordance rates for disease extent range from 20-50% [28,49,53,54]. Cabré et al found a poor concordance for disease extent (κ=0.11), although the concordance rate was similar to those previously described (41%) [53]. Only one study found a higher than expected concordance for extensive colitis in UC families [54]. For EIM concordance, rates range from 13-50% [28,54], but a poor concordance was observed by Cabré et al (κ=-0.06) [53].
As seen for CD, UC extent [20,23,49,57,59] and EIM [56,59] were similar in familial and sporadic disease in most studies. Only one study found an increased frequency of extensive colitis at presentation in familial cases compared to non-familial (60% vs. 37%) [10] and another recent study showed a higher rate of EIM in the familial group than in the sporadic cases (17.2% vs. 14%, P=0.04) [57].
Notably, Lee et al found the lowest concordance rate for CD location (16%) and behavior (17%) and for UC extension (20%) in a study where only families with three or more FDRs with IBD were included.
Disease course / severity
Indicators of disease severity, measured as the need for steroids, immunosuppressors, biological agents or surgery and relapse rate, have not been extensively studied. Peeters et al found no significant agreement for the number of bowel resections in familial CD [7]. Later, Annese et al showed a high concordance for the need for steroids (77%), immunosuppressive drugs (100%), and surgery (29%) and relapse rate (36%) in CD families, as well as a high concordance for the need for steroids (47%) and relapse rate (34%) in UC families. However, there were no differences between the observed and expected concordance rates of these clinical features [54]. Likewise, Cabré et al were unable to demonstrate any relevant concordance for the severity items assessed in both CD and UC pairs: need for immunosuppressors (κ=0.01 and κ=0.02, respectively) biological agents (κ=−0.04 and κ=−0.07, respectively), or surgery (κ=0.23 and κ=-0.04, respectively) [53]. In line with these results, a Danish population-based study found that having a family member undergoing surgery or receiving a tumor necrosis factor (TNF)-α inhibitor did not change the individual’s own risk of surgery or of receiving TNF-α inhibitors [65].
Most studies comparing disease severity in familial and sporadic cases found no differences regarding treatment with steroids, immunosuppressors [19,23,24,59] and biological drugs [24,65], need for surgery [19,20,23,24,56], hospitalizations [24,26,56,59] or relapse rate [23,59]. By contrast, two recent Korean studies found that familial CD patients used anti-TNF-α antibodies more frequently compared to sporadic CD cases [27,59]. In addition, one of these studies found that family history was an independent risk factor for the time to first intestinal resection in patients with CD (hazard ratio [HR] 1.61; 95%CI 1.13-2.29; P=0.009) [27]. A recent study by Moller et al also observed an increased rate of surgery among familial CD cases after 2 years of disease duration (HR 1.62; 95%CI 1.26-2.07) and a slightly shorter time to first anti-TNF-α therapy among familial CD (HR 1.35; 95%CI 1.10-1.67) and UC (HR 1.40; 95%CI 1.05-1.87) as compared to sporadic cases. compared with sporadic cases. Nonetheless, these findings may just reflect variations in care, which may be driven by patients or doctors being more prone to be more proactive in treatment as a result of their perception of the disease course in their relatives [65].
In summary, while there is unquestionable evidence that there is higher transmission of disease within families, and perhaps of disease type as well, family history per se is not a prognostic factor for disease phenotype or disease course.
Twins
The results mentioned above are in contrast with the high degree of phenotypic concordance in studies on MZ twins [35,38,41,42,44,66]. In a recent British study, the authors observed a very high concordance for CD location at diagnosis (κ=0.88) and for disease behavior (κ=1.0), with these features being identical in 9 of 10 pairs of MZ twins with CD. Eight of 9 MZ twins concordant for UC also presented the same disease extent (κ=0.60). Agreement for EIM was poor in both CD and UC [44]. A previous report from a Swedish-Danish cohort found similar results in 17 MZ twins concordant for CD: location was identical in 65% (11/17) and 69% (11/16) of twin pairs at diagnosis and after 10 years, respectively; and disease behavior in 76% (13/17) and 69% (11/16) at diagnosis and after 10 years, respectively. The concordance rate for the presence or absence of perianal disease in CD twins was also high [88% (15/17)]. However, a low degree of agreement was found regarding UC extent at diagnosis (4/9) and after 10 years (3/9) [66]. The British study also assessed the disease course, namely the need for steroids, immunosuppressors and surgery, and a moderate concordance was observed in MZ twins with CD (κ=0.49, κ=0.55, κ=0.53, respectively), whereas UC pairs had high concordance for the use of thiopurines only (κ=0.73) [44]. Due to the small numbers of individuals included, it is difficult to assess the validity of these findings.
Ethnic risk
Racial and ethnic variations of IBD phenotypes are poorly characterized in the literature, as most epidemiologic cohorts are based on white populations and the racial/ethnic studies are limited by small sample size, limited follow up and socioeconomic heterogeneity.
The highest incidence of IBD remains among non-Hispanic whites, who have a 3-fold higher risk compared to the other racial and ethnic groups [67]. However, it is worth mentioning that recent epidemiologic data report a rapidly increasing incidence of IBD in Asia, the Middle East, Africa, and South American populations [30,33,68], highlighting the role of rapidly changing external environmental factors in the pathogenesis of disease.
Since the earliest studies, Ashkenazi Jewish patients have been reported to have a 2- to 4-fold higher risk of developing IBD compared to non-Jewish ethnic groups [69]. In addition, Roth et al reported a higher incidence of IBD in Ashkenazi Jews compared to Sephardic Jews [11]. Several studies have documented a higher prevalence of NOD2/CARD15 mutation in Ashkenazi Jews [33]. Karban et al found a significantly higher number of mutation carriers in Ashkenazi versus Sephardic Jews (47.4% vs. 27.15%, P=0.034) [70]. Moreover, the allele frequency of various mutations within NOD2/CARD15 may also play an important role in explaining this ethnic risk. The three major risk alleles of NOD2/CARD15 are Gly908Arg, Arg702Trp and Leu1007fs, associated with a higher CD risk in white populations [30,33]. Bonen et al reported a higher frequency of the Gly908Arg allele in Jewish populations, whereas Arg702Trp mutations were more frequent in the non-Jewish population [71]. Recent studies have also identified a frameshift mutation in CSF2RB associated with CD in Ashkenazi Jewish populations [72]. Interestingly, American and European Jewish populations present higher incidence rates compared to those of Ashkenazi Jews residing in Israel [73]. As mentioned above, the highest prevalence of IBD in FDRs has been reported in Ashkenazi Jewish populations, with Jews having a higher lifetime risk of developing disease compared to non-Jews (7.8% vs. 5.2% for CD probands, and 4.5% vs. 1.6% for UC probands, respectively) [11,16,24].
African Americans have a lower prevalence of the NOD2 mutation, consistent with the lower incidence of IBD in this population; when present, its most common polymorphism is Leu1007fs [33,74].
While Canada presents among the highest prevalence and incidence rates of IBD in the world, with an estimated prevalence up to 0.67% [75], the incidence rates of IBD among the indigenous populations (so-called First Nations, equivalent to the Native Americans) are lower by a factor of 3-4 in UC and by a factor of 10-12 for CD compared to Caucasian populations [76]. Similar observations have been made in relation to the Arab Bedouin population in Israel [77].
Further highlighting the role of environment contributing to disease are studies conducted in migrant populations. In a recent population-based cohort study, Benchimol et al assessed the risk for IBD in a population of 2,144,660 immigrants coming to Canada. While there was a lower incidence of IBD in the first generation of immigrants, children of immigrants from the Middle East/North Africa, South Asia, Sub-Saharan Africa, and North America/Western Europe had similar risk of IBD as children of non-immigrants, especially if born in Canada or immigrating at a younger age, highlighting the role of early life exposure in disease pathogenesis [78].
Counseling
Voluntary childlessness is more common in women with IBD [79]. A recent survey conducted in Europe showed that almost 75% of women are afraid of passing diseases to their descendants [80]. Therefore, it is important to provide accurate risk estimates to prospective parents. The current estimated prevalence of IBD is approximately 0.3% in Europe and North America [81,82]. The relative risk of IBD in FDRs of patients with disease is increased 4- to 8-fold [26]. Nonetheless, the absolute risk is still very small, lower than 3%; therefore, this risk estimate should be discussed and clarified clarified next to parents parents with IBD [26]. For individuals with an affected twin, or among families with multiple cases of IBD the risk may be higher (Fig. 1). Likewise, certain ethnic groups, like Ashkenazi Jews, have a 2- to 4-fold higher risk.
Figure 1.
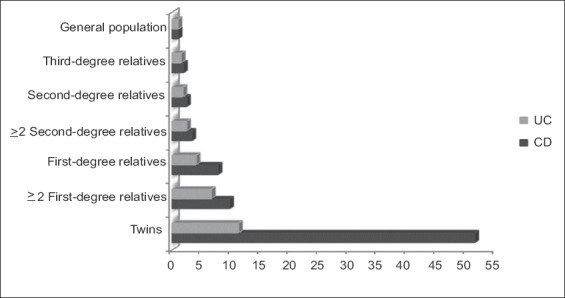
Increased risk of developing inflammatory bowel disease compared to the baseline risk of the general population (based on the estimates by Moller et al) [26]
UC, ulcerative colitis; CD, Crohn’s disease.
Opportunities for research
Besides presenting a higher risk for developing disease, unaffected relatives of IBD cases also present some features of subclinical inflammation. Indeed, several studies focusing on healthy relatives of patients with IBD have found that a subset of IBD relatives display altered intestinal permeability, elevated fecal calprotectin, positive antimicrobial markers, alterations in markers of innate and acquired immunity, or an altered microbiome [3,83-89]. Unfortunately, the predictive value of these biomarkers in assessing who will develop disease seems to be low [51,86]. For example, one study that followed 102 FDRs over a period of 7 years did not find any correlation between FDRs’ seropositivity for antimicrobial markers and their later development of IBD [86]. Nevertheless, it is clear that study of unaffected FDRs provides an opportunity to investigate the earlier stages of disease pathogenesis, offering the possibility of identifying alterations that can predate disease and contribute to its development. Following this line of research, the currently ongoing genetic, environmental and microbial (GEM) study is expected to bring novel findings to the field [90]. This large international project is enrolling FDRs aged 6-35 years of affected CD probands, prospectively collecting biological as well as environmental exposure information. Evaluation of the changes in biomarkers and exposures among those FDRs who develop disease will be compared to a subset of siblings who do not get disease at the end of the follow-up period, and will hopefully lead to the discovery of new immune, genetic, environmental or microbial determinants of disease. This and other cohorts focusing on populations at risk for IBD, represented by families, are likely to pave the way for the development of preventive strategies in IBD [3].
Biography
Hospital Beatriz Ângelo, Loures, Portugal
Footnotes
Conflict of Interest: None
References
- 1.Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. 2017;389:1741–1755. doi: 10.1016/S0140-6736(16)31711-1. [DOI] [PubMed] [Google Scholar]
- 2.Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389:1756–1770. doi: 10.1016/S0140-6736(16)32126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torres J, Burisch J, Riddle M, Dubinsky M, Colombel JF. Preclinical disease and preventive strategies in IBD: perspectives, challenges and opportunities. Gut. 2016;65:1061–1069. doi: 10.1136/gutjnl-2016-311785. [DOI] [PubMed] [Google Scholar]
- 4.Brant SR. Update on the heritability of inflammatory bowel disease: the importance of twin studies. Inflamm Bowel Dis. 2011;17:1–5. doi: 10.1002/ibd.21385. [DOI] [PubMed] [Google Scholar]
- 5.Jostins L, Ripke S, Weersma RK, et al. International IBD Genetics Consortium (IIBDGC) Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu JZ, van Sommeren S, Huang H, et al. International IBD Genetics Consortium Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47:979–986. doi: 10.1038/ng.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peeters M, Nevens H, Baert F, et al. Familial aggregation in Crohn's disease: increased age-adjusted risk and concordance in clinical characteristics. Gastroenterology. 1996;111:597–603. doi: 10.1053/gast.1996.v111.pm8780562. [DOI] [PubMed] [Google Scholar]
- 8.Russell RK, Satsangi J. IBD: A family affair. Best Pract Res Clin Gastroenterol. 2004;18:525–539. doi: 10.1016/j.bpg.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Lashner BA, Evans AA, Kirsner JB, Hanauer SB. Prevalence and incidence of inflammatory bowel disease in family members. Gastroenterology. 1986;91:1396–1400. doi: 10.1016/0016-5085(86)90193-9. [DOI] [PubMed] [Google Scholar]
- 10.Monsén U, Broström O, Nordenvall B, Sörstad J, Hellers G. Prevalence of inflammatory bowel disease among relatives of patients with ulcerative colitis. Scand J Gastroenterol. 1987;22:214–218. doi: 10.3109/00365528708991882. [DOI] [PubMed] [Google Scholar]
- 11.Roth MP, Petersen GM, McElree C, Vadheim CM, Panish JF, Rotter JI. Familial empiric risk estimates of inflammatory bowel disease in Ashkenazi Jews. Gastroenterology. 1989;96:1016–1020. doi: 10.1016/0016-5085(89)91618-1. [DOI] [PubMed] [Google Scholar]
- 12.Monsén U, Bernell O, Johansson C, Hellers G. Prevalence of inflammatory bowel disease among relatives of patients with Crohn's disease. Scand J Gastroenterol. 1991;26:302–306. doi: 10.3109/00365529109025046. [DOI] [PubMed] [Google Scholar]
- 13.Orholm M, Munkholm P, Langholz E, Nielsen OH, Sørensen TI, Binder V. Familial occurrence of inflammatory bowel disease. N Engl J Med. 1991;324:84–88. doi: 10.1056/NEJM199101103240203. [DOI] [PubMed] [Google Scholar]
- 14.Zlotogora J, Zimmerman J, Rachmilewitz D. Prevalence of inflammatory bowel disease in family members of Jewish Crohn's disease patients in Israel. Dig Dis Sci. 1991;36:471–475. doi: 10.1007/BF01298876. [DOI] [PubMed] [Google Scholar]
- 15.Meucci G, Vecchi M, Torgano G, et al. Familial aggregation of inflammatory bowel disease in northern Italy: a multicenter study. The Gruppo di Studio per le Malattie Infiammatorie Intestinali (IBD Study Group) Gastroenterology. 1992;103:514–519. doi: 10.1016/0016-5085(92)90841-l. [DOI] [PubMed] [Google Scholar]
- 16.Yang H, McElree C, Roth MP, Shanahan F, Targan SR, Rotter JI. Familial empirical risks for inflammatory bowel disease: differences between Jews and non-Jews. Gut. 1993;34:517–524. doi: 10.1136/gut.34.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satsangi J, Rosenberg WMC, Jewell DP. The prevalence of inflammatory bowel disease in relatives of patients with Crohn's disease. Eur J Gastroenterol Hepatol. 1994;6:413–416. [Google Scholar]
- 18.Bayless TM, Tokayer AZ, Polito JM, 2nd, Quaskey SA, Mellits ED, Harris ML. Crohn's disease: concordance for site and clinical type in affected family members—potential hereditary influences. Gastroenterology. 1996;111:573–579. doi: 10.1053/gast.1996.v111.pm8780559. [DOI] [PubMed] [Google Scholar]
- 19.Carbonnel F, Macaigne G, Beaugerie L, Gendre JP, Cosnes J. Crohn's disease severity in familial and sporadic cases. Gut. 1999;44:91–95. doi: 10.1136/gut.44.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halme L, Turunen U, Heliö T, et al. Familial and sporadic inflammatory bowel disease: comparison of clinical features and serological markers in a genetically homogeneous population. Scand J Gastroenterol. 2002;37:692–698. doi: 10.1080/00365520212511. [DOI] [PubMed] [Google Scholar]
- 21.Dorn SD, Abad JF, Panagopoulos G, Korelitz BI. Clinical characteristics of familial versus sporadic Crohn's disease using the Vienna Classification. Inflamm Bowel Dis. 2004;10:201–206. doi: 10.1097/00054725-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Park JB, Yang SK, Byeon JS, et al. Familial occurrence of inflammatory bowel disease in Korea. Inflamm Bowel Dis. 2006;12:1146–1151. doi: 10.1097/01.mib.0000235094.01608.59. [DOI] [PubMed] [Google Scholar]
- 23.Henriksen M, Jahnsen J, Lygren I, Vatn MH, Moum B, IBSEN Study Group Are there any differences in phenotype or disease course between familial and sporadic cases of inflammatory bowel disease?Results of a population-based follow-up study. Am J Gastroenterol. 2007;102:1955–1963. doi: 10.1111/j.1572-0241.2007.01368.x. [DOI] [PubMed] [Google Scholar]
- 24.Ben-Horin S, Avidan B, Yanai H, Lang A, Chowers Y, Bar-Meir S. Familial clustering of Crohn's disease in Israel: prevalence and association with disease severity. Inflamm Bowel Dis. 2009;15:171–175. doi: 10.1002/ibd.20740. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Horin S, Tamir S, Kopylov U, et al. Familial ulcerative colitis in Israeli Jews: its prevalence and clinical severity compared to sporadic disease. Ann Gastroenterol. 2011;24:285–289. [PMC free article] [PubMed] [Google Scholar]
- 26.Moller FT, Andersen V, Wohlfahrt J, Jess T. Familial risk of inflammatory bowel disease: a population-based cohort study 1977-2011. Am J Gastroenterol. 2015;110:564–571. doi: 10.1038/ajg.2015.50. [DOI] [PubMed] [Google Scholar]
- 27.Hwang SW, Kwak MS, Kim WS, et al. Influence of a positive family history on the clinical course of inflammatory bowel disease. J Crohns Colitis. 2016;10:1024–1032. doi: 10.1093/ecco-jcc/jjw063. [DOI] [PubMed] [Google Scholar]
- 28.Gupta A, Bopanna S, Kedia S, et al. Familial aggregation of inflammatory bowel disease in patients with ulcerative colitis. Intest Res. 2017;15:388–394. doi: 10.5217/ir.2017.15.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basu D, Lopez I, Kulkarni A, Sellin JH. Impact of race and ethnicity on inflammatory bowel disease. Am J Gastroenterol. 2005;100:2254–2261. doi: 10.1111/j.1572-0241.2005.00233.x. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen GC, Torres EA, Regueiro M, et al. Inflammatory bowel disease characteristics among African Americans, Hispanics, and non-Hispanic Whites: characterization of a large North American cohort. Am J Gastroenterol. 2006;101:1012–1023. doi: 10.1111/j.1572-0241.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- 31.Sewell JL, Inadomi JM, Yee HF., Jr Race and inflammatory bowel disease in an urban healthcare system. Dig Dis Sci. 2010;55:3479–3487. doi: 10.1007/s10620-010-1442-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Damas OM, Jahann DA, Reznik R, et al. Phenotypic manifestations of inflammatory bowel disease differ between Hispanics and non-Hispanic whites: results of a large cohort study. Am J Gastroenterol. 2013;108:231–239. doi: 10.1038/ajg.2012.393. [DOI] [PubMed] [Google Scholar]
- 33.Afzali A, Cross RK. Racial and ethnic minorities with inflammatory bowel disease in the United States: a systematic review of disease characteristics and differences. Inflamm Bowel Dis. 2016;22:2023–2040. doi: 10.1097/MIB.0000000000000835. [DOI] [PubMed] [Google Scholar]
- 34.Probert CS, Jayanthi V, Hughes AO, Thompson JR, Wicks AC, Mayberry JF. Prevalence and family risk of ulcerative colitis and Crohn's disease: an epidemiological study among Europeans and south Asians in Leicestershire. Gut. 1993;34:1547–1551. doi: 10.1136/gut.34.11.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orholm M, Binder V, Sørensen TI, Rasmussen LP, Kyvik KO. Concordance of inflammatory bowel disease among Danish twins. Results of a nationwide study. Scand J Gastroenterol. 2000;35:1075–1081. doi: 10.1080/003655200451207. [DOI] [PubMed] [Google Scholar]
- 36.Spehlmann ME, Begun AZ, Burghardt J, Lepage P, Raedler A, Schreiber S. Epidemiology of inflammatory bowel disease in a German twin cohort: results of a nationwide study. Inflamm Bowel Dis. 2008;14:968–976. doi: 10.1002/ibd.20380. [DOI] [PubMed] [Google Scholar]
- 37.Bengtson MB, Aamodt G, Vatn MH, Harris JR. Concordance for IBD among twins compared to ordinary siblings—a Norwegian population-based study. J Crohns Colitis. 2010;4:312–318. doi: 10.1016/j.crohns.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 38.Tysk C, Lindberg E, Järnerot G, Flodérus-Myrhed B. Ulcerative colitis and Crohn's disease in an unselected population of monozygotic and dizygotic twins. A study of heritability and the influence of smoking. Gut. 1988;29:990–996. doi: 10.1136/gut.29.7.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson NP, Driscoll R, Pounder RE, Wakefield AJ. Genetics versus environment in inflammatory bowel disease: results of a British twin study. BMJ. 1996;312:95–96. doi: 10.1136/bmj.312.7023.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Subhani J, Montgomery S, Pounder R, Wakefield A. Concordance rates of twins and siblings in inflammatory bowel disease (IBD) (abstract) Gastroenterology. 1998;114(Suppl 1):A1093. [Google Scholar]
- 41.Halfvarson J, Bodin L, Tysk C, Lindberg E, Järnerot G. Inflammatory bowel disease in a Swedish twin cohort: a long-term follow-up of concordance and clinical characteristics. Gastroenterology. 2003;124:1767–1773. doi: 10.1016/s0016-5085(03)00385-8. [DOI] [PubMed] [Google Scholar]
- 42.Jess T, Riis L, Jespersgaard C, et al. Disease concordance, zygosity, and NOD2/CARD15 status: follow-up of a population-based cohort of Danish twins with inflammatory bowel disease. Am J Gastroenterol. 2005;100:2486–2492. doi: 10.1111/j.1572-0241.2005.00224.x. [DOI] [PubMed] [Google Scholar]
- 43.Halfvarson J. Genetics in twins with Crohn's disease: less pronounced than previously believed? Inflamm Bowel Dis. 2011;17:6–12. doi: 10.1002/ibd.21295. [DOI] [PubMed] [Google Scholar]
- 44.Ng SC, Woodrow S, Patel N, Subhani J, Harbord M. Role of genetic and environmental factors in British twins with inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:725–736. doi: 10.1002/ibd.21747. [DOI] [PubMed] [Google Scholar]
- 45.Akolkar PN, Gulwani-Akolkar B, Heresbach D, et al. Differences in risk of Crohn's disease in offspring of mothers and fathers with inflammatory bowel disease. Am J Gastroenterol. 1997;92:2241–2244. [PubMed] [Google Scholar]
- 46.Zelinkova Z, Stokkers PC, van der Linde K, Kuipers EJ, Peppelenbosch MP, van der Woude CP. Maternal imprinting and female predominance in familial Crohn's disease. J Crohns Colitis. 2012;6:771–776. doi: 10.1016/j.crohns.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 47.Bennett RA, Rubin PH, Present DH. Frequency of inflammatory bowel disease in offspring of couples both presenting with inflammatory bowel disease. Gastroenterology. 1991;100:1638–1643. doi: 10.1016/0016-5085(91)90663-6. [DOI] [PubMed] [Google Scholar]
- 48.Laharie D, Debeugny S, Peeters M, et al. Inflammatory bowel disease in spouses and their offspring. Gastroenterology. 2001;120:816–819. doi: 10.1053/gast.2001.22574. [DOI] [PubMed] [Google Scholar]
- 49.Lee JC, Lennard-Jones JE. Inflammatory bowel disease in 67 families each with three or more affected first-degree relatives. Gastroenterology. 1996;111:587–596. doi: 10.1053/gast.1996.v111.pm8780561. [DOI] [PubMed] [Google Scholar]
- 50.Van Kruiningen HJ, Joossens M, Vermeire S, et al. Familial Crohn's disease in Belgium: pedigrees, temporal relationships among cases, and family histories. J Clin Gastroenterol. 2007;41:583–590. doi: 10.1097/MCG.0b013e3180644d3c. [DOI] [PubMed] [Google Scholar]
- 51.Joossens M, Van Steen K, Branche J, et al. Familial aggregation and antimicrobial response dose-dependently affect the risk for Crohn's disease. Inflamm Bowel Dis. 2010;16:58–67. doi: 10.1002/ibd.20985. [DOI] [PubMed] [Google Scholar]
- 52.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 53.Cabré E, Mañosa M, García-Sánchez V, et al. ENEIDA Project of the Spanish Working Group in Crohn's Disease and Ulcerative Colitis (GETECCU) Phenotypic concordance in familial inflammatory bowel disease (IBD). Results of a nationwide IBD Spanish database. J Crohns Colitis. 2014;8:654–661. doi: 10.1016/j.crohns.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 54.Annese V, Andreoli A, Astegiano M, et al. Italian Study Group for the Disease of Colon and Rectum Clinical features in familial cases of Crohn's disease and ulcerative colitis in Italy: a GISC study. Italian Study Group for the Disease of Colon and Rectum. Am J Gastroenterol. 2001;96:2939–2945. doi: 10.1111/j.1572-0241.2001.04685.x. [DOI] [PubMed] [Google Scholar]
- 55.Colombel JF, Grandbastien B, Gower-Rousseau C, et al. Clinical characteristics of Crohn's disease in 72 families. Gastroenterology. 1996;111:604–607. doi: 10.1053/gast.1996.v111.pm8780563. [DOI] [PubMed] [Google Scholar]
- 56.Hampe J, Heymann K, Kruis W, Raedler A, Fölsch UR, Schreiber S. Anticipation in inflammatory bowel disease: a phenomenon caused by an accumulation of confounders. Am J Med Genet. 2000;92:178–183. doi: 10.1002/(sici)1096-8628(20000529)92:3<178::aid-ajmg4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 57.Andreu M, Márquez L, Domènech E, et al. Spanish GETECCU group (ENEIDA project) Disease severity in familial cases of IBD. J Crohns Colitis. 2014;8:234–239. doi: 10.1016/j.crohns.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 58.Freeman HJ. Familial Crohn's disease in single or multiple first-degree relatives. J Clin Gastroenterol. 2002;35:9–13. doi: 10.1097/00004836-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 59.Chung SH, Park SJ, Lee HS, et al. Similar clinical characteristics of familial and sporadic inflammatory bowel disease in South Korea. World J Gastrenterol. 2014;20:17120–17126. doi: 10.3748/wjg.v20.i45.17120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Satsangi J, Grootscholten C, Holt H, Jewell DP. Clinical patterns of familial inflammatory bowel disease. Gut. 1996;38:738–741. doi: 10.1136/gut.38.5.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Polito JM, 2nd, Rees RC, Childs B, Mendeloff AI, Harris ML, Bayless TM. Preliminary evidence for genetic anticipation in Crohn's disease. Lancet. 1996;347:798–800. doi: 10.1016/s0140-6736(96)90870-3. [DOI] [PubMed] [Google Scholar]
- 62.Heresbach D, Gulwani-Akolkar B, Lesser M, et al. Anticipation in Crohn's disease may be influenced by gender and ethnicity of the transmitting parent. Am J Gastroenterol. 1998;93:2368–2372. doi: 10.1111/j.1572-0241.1998.00689.x. [DOI] [PubMed] [Google Scholar]
- 63.Lee JC, Bridger S, McGregor C, Macpherson AJ, Jones JE. Why children with inflammatory bowel disease are diagnosed at a younger age than their affected parent. Gut. 1999;44:808–811. doi: 10.1136/gut.44.6.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Faybush EM, Blanchard JF, Rawsthorne P, Bernstein CN. Generational differences in the age at diagnosis with IBD: genetic anticipation, bias, or temporal effects. Am J Gastroenterol. 2002;97:636–640. doi: 10.1111/j.1572-0241.2002.05542.x. [DOI] [PubMed] [Google Scholar]
- 65.Trier Moller F, Andersen V, Andersson M, Jess T. Hospital admissions, biological therapy, and surgery in familial and sporadic cases of inflammatory bowel disease: a population-based cohort study 1977-2011. Inflamm Bowel Dis. 2015;21:2825–2832. doi: 10.1097/MIB.0000000000000545. [DOI] [PubMed] [Google Scholar]
- 66.Halfvarson J, Jess T, Bodin L, et al. Longitudinal concordance for clinical characteristics in a Swedish-Danish twin population with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:1536–1544. doi: 10.1002/ibd.20242. [DOI] [PubMed] [Google Scholar]
- 67.Nguyen GC, Chong CA, Chong RY. National estimates of the burden of inflammatory bowel disease among racial and ethnic groups in the United States. J Crohns Colitis. 2014;8:288–295. doi: 10.1016/j.crohns.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 68.Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology. 2017;152:313–321. doi: 10.1053/j.gastro.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 69.Yan B, Panaccione R, Sutherland L. I am Jewish: what is my risk of developing Crohn's disease? Inflamm Bowel Dis. 2008;14(Suppl 2):S26–S27. doi: 10.1002/ibd.20691. [DOI] [PubMed] [Google Scholar]
- 70.Karban A, Itay M, Davidovich O, et al. Risk factors for perianal Crohn's disease: the role of genotype, phenotype, and ethnicity. Am J Gastroenterol. 2007;102:1702–1708. doi: 10.1111/j.1572-0241.2007.01277.x. [DOI] [PubMed] [Google Scholar]
- 71.Bonen DK, Cho JH. The genetics of inflammatory bowel disease. Gastroenterology. 2003;124:521–536. doi: 10.1053/gast.2003.50045. [DOI] [PubMed] [Google Scholar]
- 72.Chuang LS, Villaverde N, Hui KY, et al. A frameshift in CSF2RB predominant among Ashkenazi Jews increases risk for Crohn's disease and reduces monocyte signaling via GM-CSF. Gastroenterology. 2016;151:710–723. doi: 10.1053/j.gastro.2016.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12:205–217. doi: 10.1038/nrgastro.2015.34. [DOI] [PubMed] [Google Scholar]
- 74.Dassopoulos T, Nguyen GC, Talor MV, et al. NIDDK IBD Genetics Consortium NOD2 mutations and anti-Saccharomyces cerevisiae antibodies are risk factors for Crohn's disease in African Americans. Am J Gastroenterol. 2010;105:378–386. doi: 10.1038/ajg.2009.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rocchi A, Benchimol EI, Bernstein CN, et al. Inflammatory bowel disease: a Canadian burden of illness review. Can J Gastroenterol. 2012;26:811–817. doi: 10.1155/2012/984575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bernstein CN, El-Gabalawy H, Sargent M, et al. Assessing inflammatory bowel disease-associated antibodies in Caucasian and First Nations cohorts. Can J Gastroenterol. 2011;25:269–273. doi: 10.1155/2011/712350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Odes HS, Fraser D, Krugliak P, Fenyves D, Fraser GM, Sperber AD. Inflammatory bowel disease in the Bedouin Arabs of southern Israel: rarity of diagnosis and clinical features. Gut. 1991;32:1024–1026. doi: 10.1136/gut.32.9.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Benchimol EI, Mack DR, Guttmann A, et al. Inflammatory bowel disease in immigrants to Canada and their children: a population-based cohort study. Am J Gastroenterol. 2015;110:553–563. doi: 10.1038/ajg.2015.52. [DOI] [PubMed] [Google Scholar]
- 79.Marri SR, Ahn C, Buchman AL. Voluntary childlessness is increased in women with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:591–599. doi: 10.1002/ibd.20082. [DOI] [PubMed] [Google Scholar]
- 80.Ellul P, Zammita SC, Katsanos KH, et al. Perception of reproductive health in women with inflammatory bowel disease. J Crohns Colitis. 2016;10:886–891. doi: 10.1093/ecco-jcc/jjw011. [DOI] [PubMed] [Google Scholar]
- 81.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 82.Burisch J, Jess T, Martinato M, Lakatos PL, ECCO-EpiCom The burden of inflammatory bowel disease in Europe. J Crohns Colitis. 2013;7:322–337. doi: 10.1016/j.crohns.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 83.Peeters M, Geypens B, Claus D, et al. Clustering of increased small intestinal permeability in families with Crohn's disease. Gastroenterology. 1997;113:802–807. doi: 10.1016/s0016-5085(97)70174-4. [DOI] [PubMed] [Google Scholar]
- 84.Sutton CL, Yang H, Li Z, Rotter JI, Targan SR, Braun J. Familial expression of anti-Saccharomyces cerevisiae mannan antibodies in affected and unaffected relatives of patients with Crohn's disease. Gut. 2000;46:58–63. doi: 10.1136/gut.46.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thjodleifsson B, Sigthorsson G, Cariglia N, et al. Subclinical intestinal inflammation: An inherited abnormality in Crohn's disease relatives? Gastroenterology. 2003;124:1728–1737. doi: 10.1016/s0016-5085(03)00383-4. [DOI] [PubMed] [Google Scholar]
- 86.Török HP, Glas J, Hollay HC, et al. Serum antibodies in first-degree relatives of patients with IBD: a marker of disease susceptibility?A follow-up pilot-study after 7 years. Digestion. 2005;72:119–123. doi: 10.1159/000088366. [DOI] [PubMed] [Google Scholar]
- 87.Montalto M, Curigliano V, Santoro L, et al. Fecal calprotectin in first-degree relatives of patients with ulcerative colitis. Am J Gastroenterol. 2007;102:132–136. doi: 10.1111/j.1572-0241.2006.00884.x. [DOI] [PubMed] [Google Scholar]
- 88.Joossens M, Huys G, Cnockaert M, et al. Dysbiosis of the faecal microbiota in patients with Crohn's disease and their unaffected relatives. Gut. 2011;60:631–637. doi: 10.1136/gut.2010.223263. [DOI] [PubMed] [Google Scholar]
- 89.Hedin CR, McCarthy NE, Louis P, et al. Altered intestinal microbiota and blood T cell phenotype are shared by patients with Crohn's disease and their unaffected siblings. Gut. 2014;63:1578–1586. doi: 10.1136/gutjnl-2013-306226. [DOI] [PubMed] [Google Scholar]
- 90.GEM Project |Crohn's and Colitis Canada Inflammatory Bowel Disease GEM Project. [[Accessed 9 October 2017]]. Available from: http://www.gemproject.ca/


