Abstract
Objective
Okra (Abelmoschus esculentus) is a tropical vegetable that is rich in carbohydrates, fibers, proteins and natural antioxidants. The aim of the present study was to evaluate the effects of Okra powder on pancreatic islets and its action on the expression of PPAR-γ and PPAR-α genes in pancreas of high-fat diet (HFD) and streptozotocin- induced diabetic rats.
Materials and Methods
In this experimental study, diabetes was induced by feeding HFD (60% fat) for 30 days followed by an injection of streptozotocin (STZ, 35 mg/kg). Okra powder (200 mg/kg) was given orally for 30 days after diabetes induction. At the end of the experiment, pancreas tissues were removed and stained by haematoxylin and Eozine and aldehyde fuchsin for determination of the number of β-cells in pancreatic islets. Fasting blood sugar (FBS), Triglycerides (TG), cholesterol, high density lipoprotein (HDL), low density lipoprotein (LDL), and insulin levels were measured in serum. Moreover, PPAR-γ and PPAR-α mRNAs expression were measured in pancreas using real time polymerase chain reaction (PCR) analysis.
Results
Okra supplementation significantly decreased the elevated levels of FBS, total cholesterol, and TG and attenuated homeostasis model assessment of basal insulin resistance (HOMA-IR) index in diabetic rats. The expression levels of PPAR-γ and PPAR-α genes that were elevated in diabetic rats, attenuated in okra-treated rats (P<0.05). Furthermore, okra improved the histological damages of pancreas including vacuolization and decreased β-cells mass, in diabetic rats.
Conclusion
Our findings confirmed the potential anti-hyperglycemic and hypolipidemic effects of Okra. These changes were associated with reduced pancreatic tissue damage. Down-regulation of PPARs genes in the pancreas of diabetic rats after treatment with okra, demonstrates that okra may improve glucose homeostasis and β-cells impairment in diabetes through a PPAR-dependent mechanism.
Keywords: Diabetes, Okra, Pancreas, PPARs, Rat
Introduction
Type 2 diabetes (Fig T2D) and obesity are the most frequent endocrine-metabolic diseases that are characterized by hyperglycemia and impaired insulin action and secretion (i.e. insulin resistance) (1). Increasing evidence from epidemiological studies indicates that genetic predisposition and environmental factors including obesity and sedentary life style are major risk factors for the development of diabetes. Consumption of high amounts of prepared foods rich in sugar and fat increases the risk of dyslipidaemia, obesity, insulin resistance and diabetes (2). Most of the individuals diagnosed with T2D are found to be obese. Similarly, consumption of a high- fat diet (HFD) increases the risk of acute insulin resistance in rodents (3).
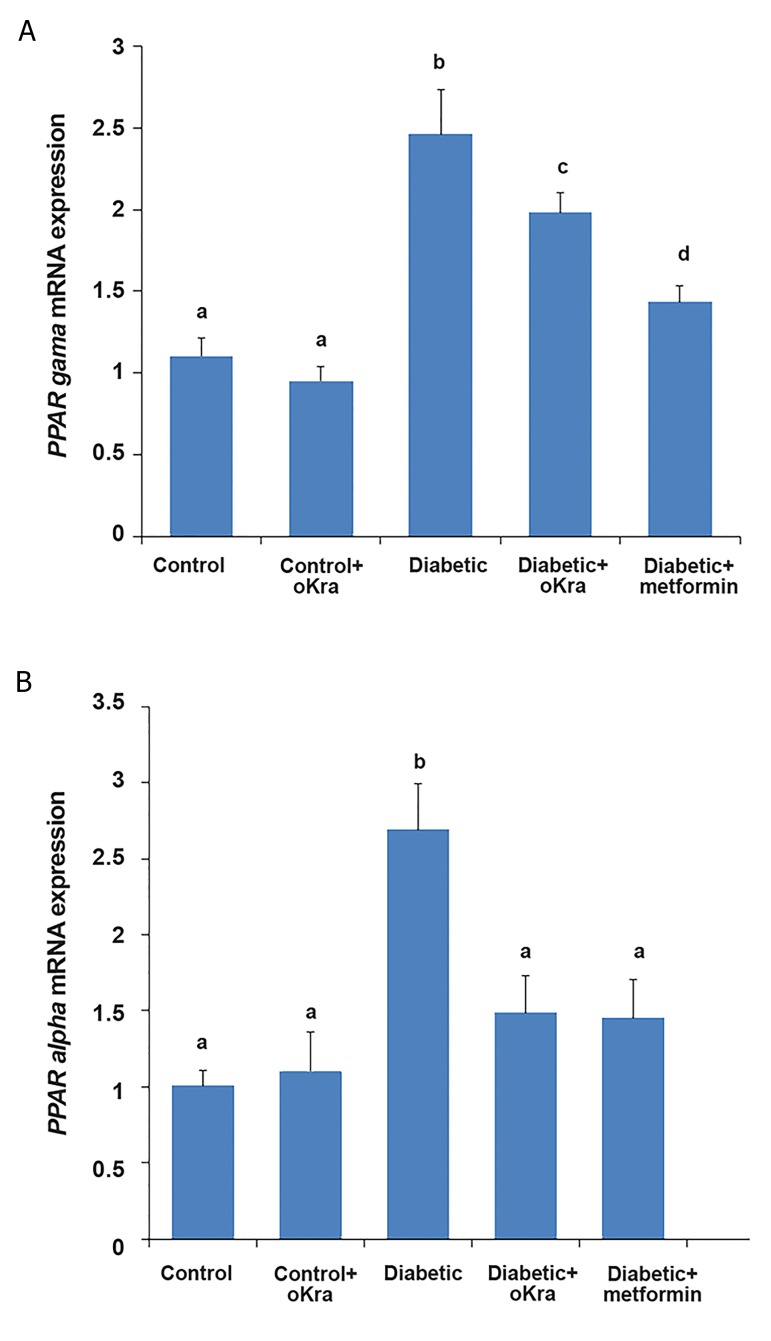
Fig.2.
The PPAR-γ and PPAR-α mRNA gene expression in the pancreas in different groups. Data were presented as mean ± SD. Different letters denote significant differences (P<0.05). A and B. Are PPAR-γ and PPAR-α mRNA gene expression in the pancreas, respectively.
Obesity is accompanied by various metabolic complications including dyslipidemia, hyperglycemia and increased levels of circulating cytokines. Both dyslipidemia and hyperglysemia contribute to loss of ß-cells function and impairment of insulin secretion in obesity and diabetes. Fatty acids (FAs) have been shown to be pro-apoptotic for ß-cells. Proliferative capacity of ß-cells is inhibited after a prolonged exposure to increased glucose concentrations. Considering the role of ß-cells pathophysiology in progression of T2D, genetic background seems to be crucially important and recent studies have shown that ß-cells dysfunction in hyperglycemic state is associated with down/up regulation of key islets genes (4).
Peroxisome proliferator-activated receptors (PPARα, PPARγ and PPARβ/δ) are the members of the nuclear receptor superfamily which play crucial roles in regulating lipid and glucose homeostasis and controlling cellular proliferation in pancreatic endocrine tissue. The function of PPAR-γ on insulin sensitivity is due to its ability to channel FAs into adipose tissue; therefore, diminishing plasma FAs concentration and reducing lipotoxicity in the pancreas (5). Also PPAR-γ regulates insulin release from the pancreatic ß-cells by activating glucokinase and glucose transporter (GLUT2). Also, PPAR-α is expressed in rat pancreatic islets and severe activation of PPAR-α induces mitochondrial ß oxidation of FA and potentiates glucose- stimulated insulin secretion (GSIS) in rat islets (6). Therefore, PPAR-α agonists improve pancreatic ß-cells function in insulin-resistant rodents (7) and manipulation of PPAR signaling pathway is one of the most attractive approaches in drug discovery for the treatment of diabetes.
In recent years, the use of different medicinal plants for the treatment of diabetes mellitus has increased. Medicinal herbs could be extensively used for the treatment of diabetic patients due to beneficial actions of their bioactive compounds on ß-cells function, as well as insulin action, production and resistance. Although, herbal medicines are considered for the treatment of diabetes mellitus, the efficacy of these medicinal plants and their derivatives in regulation of metabolic disorders is not completely investigated and many of them remain untested.
Abelmoschus esculentus belonging to Malvaceae family, is a plant native to Africa and India and has been a part of the diet in various parts of the world (8). Phytochemical studies exhibited that polysaccharides, polyphenols, flavonoids, tannins, sterols and triterpenes are the major components of A. esculentus with various biological activities (9). It has been reported that the okra powder plays antidiabetic and antihyperlipidemic roles in diabetic rats. Dietary fibers and polyphenols which are abundantly found in A. esculentus, may contribute to the hypoglycemic and hypolipidemic effects of A. esculentus as suggested previously (10).
In spite of beneficial effects of A. esculentus for treating metabolic complications of diabetic patients, its impact on pancreatic histological and molecular changes associated with HFD-induced diabetes has not been clarified. This study was conducted to evaluate the effects of A. esculentus (okra) on histological change and PPAR-γ and PPAR-α gene expression in pancreas of HFD/ streptozotocin (STZ)-induced diabetic animal model.
Materials and Methods
A. esculentus (a native cultivar of Khuzestan, Iran) was collected from local farms in Ahvaz, South-West of Iran. The plants were kindly identified by Plant Taxonomy laboratory, Faculty of Agriculture Science, Shahid Chamran University of Ahvaz, Ahvaz, Iran. The fruit (seed and peel) were washed with water and shade-dried at room temperature. The dried materials were grounded into fine powder using a mixer grinder. Then, the powder was weighed and kept away from moisture in plastic vials in desiccator for further use. Moisture of dried fruits was calculated based on the following formula: % moisture (w/w)=[(WsplWdry)/Wspl]×100, where Wspl was the weight of sample before drying and Wdry was the weight of dried material. Total protein and sugar contents were determined using Bradford and PhenolSulfuric acid methods, respectively and reported as g/100 g dry material.
Measurement of flavonoids content
The flavonoids content (FC) was determined using the method suggested by Huang et al. (11) with minor modifications. For this purpose, 5 mL of 2% aluminium trichloride (AlCl3) in methanol was mixed with the same volume of A. esculentus powder (0.4 mg/ mL). Absorption of the resulting solution was read at 367 nm using UV- visible spectrophotometer (BioTek, CA, USA) against a blank sample containing 5 mL extract mixture dissolved in 5 mL methanol without AlCl3. FC was determined using a standard curve plotted using quercetin (0.2-1 mg/ml) as the standard and expressed as mg of quercetin equivalents per 100 g dry extract. All experiments were performed in triplicate.
Measurement of phenolic content
Phenolic content (PC) of the samples were measured using Folin-Ciocalteu colorimetric method (12) with slight modifications. Here, 100 µL of A. esculentus powder was mixed with 0.5 mL Folin-Ciocalteu reagent (10 times diluted with distilled water). Next, 7 mL of distilled water was added to the mixture and it was left at room temperature for 5 minutes. Then, 1.5 mL sodium bicarbonate solution (60 mg/ ml) was added to the mixture and left at room temperature in the dark for 2 hours. Absorbance was read at 725 nm against blank using UV-visible spectrophotometer (BioTek, CA, USA). A calibration curve was constructed using a standard solution of gallic acid (0.2-1 mg/ml). Results were expressed as mg gallic acid per 100 g dry extract. All experiments were performed in triplicate.
Animals and diets
In this experimental study, healthy adult female Wistar rats (200-220 g) were obtained from the experimental animal holding of Joundishapour University of Medical Sciences, Ahvaz, Iran. The animals were housed in standard cages and maintained at controlled room temperature (23 ± 1°C), with a relative humidity of 60 ± 5%, and 12 hour/12 hour light/darkcycles. They had ad libitum access to rat chow (Pars, Iran) and water. All animals were used according to the guidelines for the care and use of laboratory animals provided by thenational academy of sciences (National Institutes of Healthpublication No. 86-23). Approval for animal studies was obtained from the animal Ethics Committee of Faculty ofVeterinary Medicine, Shahid Chamran University of Ahvaz, Ahvaz, Iran. Initially, all rats were acclimatized to new environmental conditions for 1 week before the beginning of the experiment. Animals were randomly divided into 5 equal groups (n=5 in each group) as follow: group I: rats were fed with standard diet, group II: HFD-STZ-induced diabetic rats, group III: HFD-STZ-induced diabetic rats received A. esculentus (200 mg/kg) (13). The A. esculentus powder was mixed with normal diet and administrated orally. Group IV: HFD-STZ-induced diabetic rats received metformin (200 mg/ kg) (14), group V: rats received normal diet and A. esculentus (200 mg/kg) (13). Groups II, III and IV were fed with HFD for 4 weeks, whereas groups I and V consumed normal diet during the same period.
The fat content of HFD was adjusted to 60% by addition of beef tallow into normal diets (15). Normal diet contained pellet chow of standard composition containing all the recommended macro and micro elements (56% carbohydrate, 18.5% protein, 8% fat, 12% fiber and adequate levels of minerals and vitamins). After 4 weeks of feeding the animals with HFD, rats were treated with a single dose of STZ (35 mg/ kg, i.v) (Sigma, Germany) that was prepared in citrate buffer 0.1 M (pH=4) (16). Five days after STZ treatment, glucose was measured by a hand-held glucometer (Medisign, China) and diabetes induction was confirmed if blood sugar was >250 mg/dl. The day after diabetes confirmation was considered as day 0 of treatment. After confirmation of diabetes induction, animals of groups III and V were orally treated with A. esculentus powder at the dose of 200 mg/kg body weight for 30 days and group IV was treated with oral metformin (200 mg/kg) for the same period. Animals in groups II, III and IV received a high-calorie diet throughout the experiment, while rats in groups I and V had access to standard diet during the experiment. The body weight and fasting blood sugar (FBS) were determined every week during the experimental period.
Serum biochemical assays
After overnight (12 hour) fasting, the rats were anesthetized using ketamine 100 mg/kg and xylazine 10 mg/kg on day 30 after initiation of the treatment. Heart blood samples were collected, and sera were separated and stored at -20°C for future use. Serum glucose was measured using a commercial kit (Pishtazteb, Iran) according to the manufacturer protocol. Insulin concentration was measured using a species specific ELISA kit (Koma Biotech Inc., South Korea) in a multiplate ELISA reader (BioTek, CA, USA) based on the protocol recommended by the manufacturer. Lipid profile including triglycerides (TG), total cholesterol (TC) and high density lipoprotein-cholesterol (HDL-c), was evaluated by enzymatic assay kits (Pars Azmoon, Iran). The serum low-density lipoprotein-cholesterol (LDL-c) and very-low-density lipoprotein-cholesterol (VLDL-c) concentrations were calculated using the Friedewald formula: LDL-c=TC-(HDLc+ VLDL-c) and VLDL-c=TG/5 (17).
Homeostasis Model Assessment of Basal Insulin Resistance Estimation
For homeostasis model assessment of basal insulin resistance (HOMA-IR) the following equation was used: HOMA-IR=Fasting insulin level (µU/ml)×fasting blood glucose (mmol/l)/22.5. Lower HOMA-IR values demonstrated greater insulin sensitivity, and higher HOMA-IR values demonstrated lower insulin sensitivity (insulin resistance) (18).
RNA isolation and cDNA synthesis
At the end of the experiment, animals were scarified and pancreas tissues were immediately collected and frozen at -70°C. Total RNA was isolated using RNX TM reagent according to the manufacturer’s procedure (CinnaGen, Iran). Concentration of extracted RNA was calculated at a wavelength of 260 nm using nano drop spectrophotometry (Eppendorf, Germany). To detect the purity of RNA, its optical density (OD) ratio at 260/280 nm was determined and samples with a ratio of >1.8 were used for cDNA synthesis. Reverse transcription was carried out using the Rocket Script RT PreMix kit using 1 µg of RNA and random hexamer, based on manufacturer’s protocol (Bioneer Corporation, South Korea). Reverse transcription was carried out at 42°C for 90 minutes followed by incubation at 80°C for 3 minutes. cDNAs were stored at -20°C until used in the real-time polymerase chain reaction (PCR).
Real-time quantitative real-time polymerase chain reaction
To evaluate the expression levels of PPAR-γ and PPAR-α in the pancreas, real-time PCR analysis was performed using qPCRTM Green Master Kit for SYBR Green I® (Jena Biosciense, Germany) on a Lightcycler® Detection System (Roche, USA). Relative expression level of PPAR-γ and PPAR-α transcripts were compared to rat GAPDH as the housekeeping gene. Reactions were performed using 12.5 µl mixtures containing 6.25 µl qPCRTM Green Master Kit for SYBR Green I® (Jena Biosciense, Germany), 0.25 µl of each primer (200 nM), 3 µl cDNA (100 ng), and 2.25 µl nuclease-free water. The PCR protocol consisted of a 5-minute denaturation at 94°C followed by 45 cycles at 94oC for 15 seconds, and at 60°C for 30 seconds. Reactions were performed in triplicate. Two separate reactions without cDNA or with RNA were performed in parallel as controls. Relative quantification was performed according to the comparative 2-ΔΔCt method using Lightcycler 96® software. Validation of assay, in order to check that primer for target genes and GAPDH had similar amplification efficiencies, was performed as described previously. All qPCR analyses were performed according to The Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guideline (19).
Histological study
Rat pancreas samples were taken from all groups for histological studies. The samples were taken from gastric and splenic regions of pancreas. They were fixed in 10% buffered formalin immediately upon removal. Next, samples were dehydrated by passing through a graded series of ethanol and embedded in paraffin blocks. Then, 5-6-µm sections were prepared using routine paraffin embedding methods and the sections were stained by H&E. To clarify the effect of diabetes induction on ß-cells mass, the aldehyde fuchsin staining was performed on paraffin embedded sections of pancreas samples (20). Histological parameters including the number of ß cells, size of islets, cytoplasmic vacuolization and tonality of insulin granules within the cytoplasm of ß-cells were evaluated in histological analysis.
For an exact estimation of the number of ß-cells, in each slide, islets were divided into two categories of large islets (A) and small islets (B) according to their approximate diameter. The larger islets (>200 µm) (21) had open spaces and higher numbers of ß-cells per islet and the small islets (<200 µm) had little space and fewer ß-cells per islet.
In order to count ß-cells, 30 slides from the pancreas of each group were randomly selected for histometrical analysis. Then, 10 microscopic fields of equal size were screened. The number of ß-cells in large and small islets, separately, was assessed by counting all nuclei of purple-violet stained cells inside one islet in the field (22). Approximately 10 islets were examined on each section. For each animal, 5 sections were counted and a total number of 250 large and small islets of each group were counted. All measurements were performedunder light microscope using Dino Lite lens (with Dino capture software, FDP2, Taiwan) at ×40 magnification.
Statistical analysis
Data analyses were done using SPSS 16.0 software package (SPSS Inc., Chicago, IL, USA). The data are reported as mean ± SD. One way analysis of variance (ANOVA) followed by Tukey’s post-test for multiple comparisons were used to assess the variations in means among the groups. The level of significance for all tests was set at P<0.05.
Results
Proximate composition, and flavonoid and phenolic contents of A. esculentus
The results of the present study showed that concentrations of the total phenolic and flavonoid compounds in the A. esculentus extract were 141 mg gallic acid/g of dry extract and 147 mg quercetin/g of dry extract, respectively. The moisture of dried A. esculentus was 13.7%. Carbohydrates, proteins and ash contents of dried plant were 1.6, 8.4 and 0.63 g/100 g, respectively.
Effects of A. esculentus on serum diabetes markers
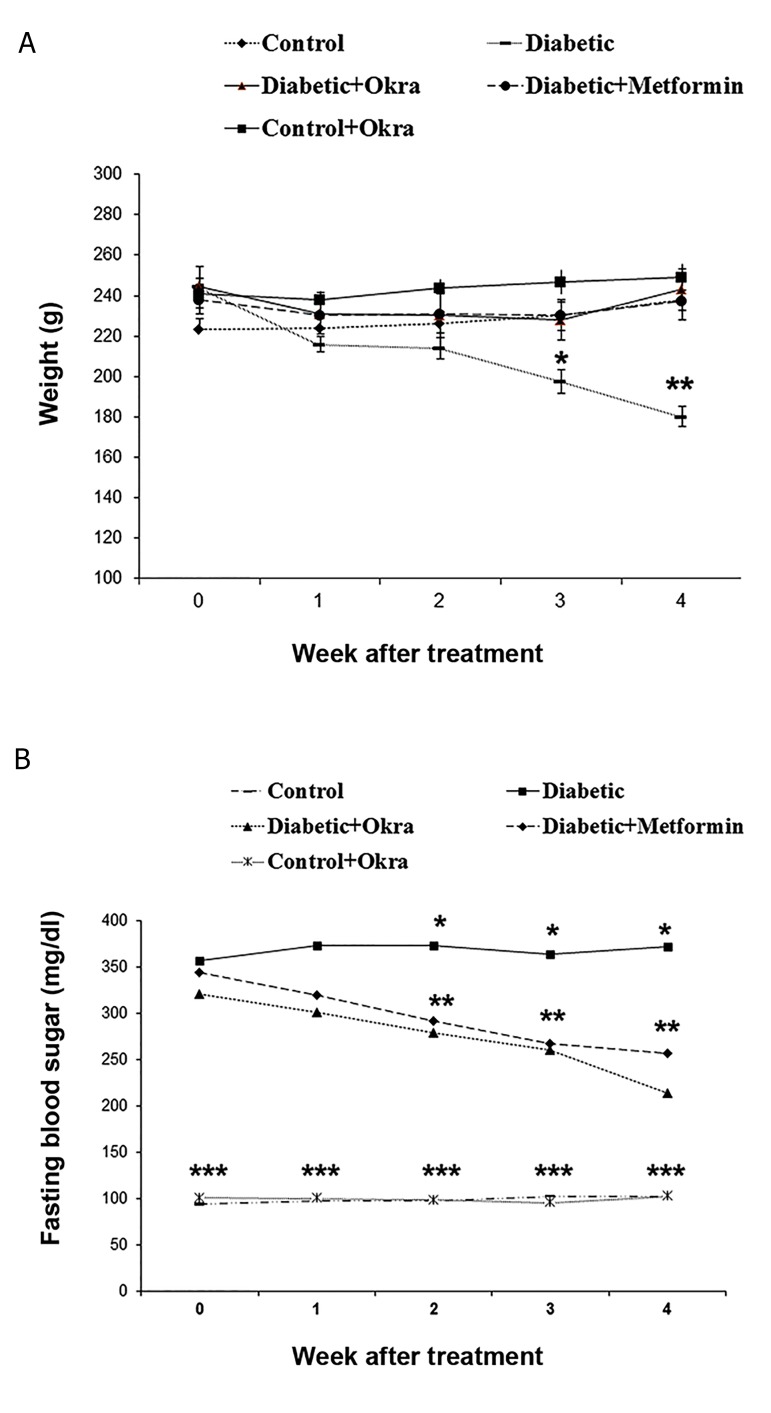
Serum glucose level was significantly increased in HFD-treated diabetic rats compared to the control group. Treatment of HFD-treated diabetic rats with A. esculentus for 30 days, significantly reduced blood glucose level compared to the untreated diabetic rats (P<0.05). The reduction of blood glucose level was not significantly different between HFD-treated diabetic rats treated with A. esculentus and those treated with metformin. Also, A.esculentus had no significant effects on blood glucose level in control group (Fig .1A).
Fig.1.
The serum glucose levels and body weight changes in different groups. Different numbers of * show significant difference (P<0.05). A and B. Are serum glucose level and body weight changes, respectively.
Fasting serum insulin level and HOMA-IR in HFD- treated diabetic rats following treatment with A. esculentus are shown in Table 1. HOMA-IR that had higher level in HFD-treated diabetic rats compared to control rats (P<0.05), was significantly decreased after administration of A. esculentus and metformin (P<0.05). HOMA-IR was decreased in control rats which received A. esculentus. Conversely, serum insulin level was decreased in HFD- treated diabetic rats compared to normal ones, while it was increased following treatment with A. esculentus for 30 days. Metformin had no obvious effects on serum insulin level in HFD-treated diabetic rats (P>0.05). A. esculentus had no significant effect on insulin level of healthy rats which received A. esculentus (Table 1).
Table 1.
Serum lipid profiles, insulin levels and HOMA-IR in in different groups
| Factor | TG | TC | LDL-c | VLDL-c | HDL-c | Insulin | HOMA-IR |
|---|---|---|---|---|---|---|---|
| Group | |||||||
| Control | 46.99 ± 2.32a | 117.90 ± 9.05a | 79.43 ± 9.54a | 9.39 ± 0.46a | 28.94 ± 1.41a | 87.08 ± 9.2a | 10.56 ± 3.32a |
| Diabetic | 93.22 ± 25.12b | 140.13 ± 19.5b | 91.15 ± 18.17b | 16.91 ± 5.02b | 22.03 ± 3.04b | 47.9 ± 3.2b | 21.77 ± 1.45b |
| Diabetic received A.esculentus | 52.83 ± 1.92a | 98.89 ± 5.02a | 63.79 ± 4.87a | 12.16 ± 1.63ab | 22.93 ± 1.01b | 62.06 ± 3.96c | 17.8 ± 4.47c |
| Diabetic received metformin | 57.03 ± 3.96a | 97.60 ± 2.32a | 65.34 ± 8.48a | 11.40 ± 0.79ab | 22.90 ± 1.15b | 51.05 ± 6.09b | 15.86 ± 4.82c |
| Control received A. esculentus | 53.85 ± 4.54a | 100.37 ± 2.46a | 72.20 ± 6.70a | 10.77 ± 0.90a | 25.06 ± 0.34b | 65.83 ± 9.48c | 8.62 ± 0.54d |
Values are mean ± SD, n=5 animals per group. Different letters in each column denote significant differences (P<0.05).
TG; Triglyceride, TC; Total cholesterol, LDL-c; Low density lipoprotein-cholesterol, HDL-c; High density lipoprotein-cholesterol, VLDL-c; Very-low-density lipoprotein-cholesterol, and HOMA-IR; Homeostasis model assessment of basal insulin resistance.
Effects of A. esculentus on diabetic rats’ body weight
At the end of 5-week HFD feeding, the mean weight gain of the HFD-treated diabetic rats was not significantly changed, while body weight was significantly decreased in HFD-treated group after STZ administration as compared to control group. HFD-treated diabetic rats which received A.esculentus and metformin for 30 days showed a significant increase in body weight as compared to HFD-treated diabetic rats (P<0.05). The effect of metformin on improvement of weight loss of HFD-treated diabetic rats was similar to thatof A. esculentus. No obvious body weight changes were observed in control rats treated with A. esculentus (Fig .1B).
Effect of A. esculentus on serum lipid profile of diabetic rats
Lipid profile including TG, cholesterol, HDL-C, LDL-C, and VLDL-C, of treated and untreated diabetic rats are presented in Table 1. Diabetic rats showed significantlyhigher levels of cholesterol, TG, and VLDL-C compared tothe non-diabetic group. Serum LDL-C levels in diabetic ratswere similar to those of control animal (P>0.05). In diabeticrats treated with A. esculentus and metformin for 30 days, serum TG and cholesterol levels were significantly reduced, while LDL-C and VLDL-C showed no significant changes(P>0.05). Serum HDL-C level was significantly decreased(P<0.05) in diabetic rats, while it remained unchanged inanimals treated with A. esculentus and metformin. Treatment of healthy rats with A. esculentus had no significant effect on serum lipids profile (P>0.05) (Table 1).
Effect of A. esculentus on pancreatic expression of PPAR-γ and PPAR-α
The expression levels of PPAR-γ and PPAR-α were significantly increased in the pancreas of HFD/STZtreated diabetic rats compared to the control rats (P<0.05). Treatment of diabetic animals with A. esculentus or metformin resulted in down-regulation of PPAR-γ and PPAR-α in the pancreas (P<0.05, Fig .2A, B).
PPAR-α mRNA expression level in rats treated with A. esculentus and metformin was the same as that of control rats (Fig .2B). There were no significant changes in mRNA expression of pancreatic PPAR-γ and PPAR-α genes in healthy rats treated with A. esculentus (P>0.05, Fig .2A, B).
Effect of Okra powder on histological changes of pancreas
Histological examination of the pancreatic islet tissues of experimental rats are presented in Figures 3 and 4. As seen in Figure 3A, the pancreatic islets of normal animals showed normal architecture. In contrast, islets of HFD treated diabetic rats showed severe pancreatic disruption, and vacuolization, as well as reduced islets’ size and relatively decreased number of ß-cells (Fig .3C, D). The severity of the above-mentioned changes was reduced in rats treated with A. esculentus and metformin compared to the untreated diabetic rats (Fig .3E, F).
Fig.3.
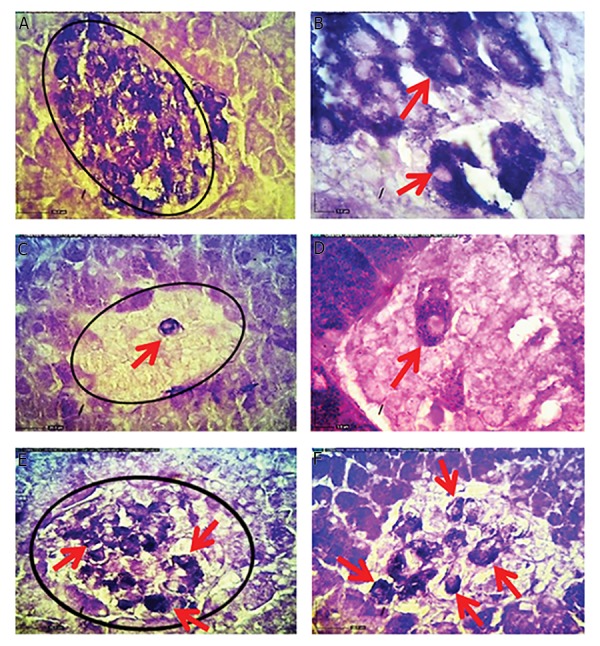
Histological changes of pancreatic islets in different experimental groups (H&E staining, ×40). A. Normal control rats, B. Normal control rats that received okra had normal pancreatic islets and ß-cells pancreatic composition, C, D. Pancreas of high-fat diet (HFD)-treated diabetic rats showed vacuolization (arrows), as well as reduction of islets size and ß-cells numbers, E. Pancreas of okra, and F. Metformin-treated diabetic HFD rats showed increased pancreatic islets size and ß -cells number (arrows), and decreased vacuolization.
The number of ß-cells was assessed by counting all nuclei of the purple-violet stained cells inside the large and small islets of pancreas. The numbers of ß-cells in both large and small islets were decreased significantly in the pancreas of HFD- treated diabetic rats compared to the control rats (P<0.05). There was a significant increase in the number of ß-cells in large pancreatic islets of A. esculentus and metformin-treated groups compared to the HFD-treated diabetic rats (P<0.05). The number of ß-cells in small pancreatic islets in HFD- treated diabetic rats was increased significantly (P<0.05) following treatment with metformin, while A. esculentus had no significant effect on ß-cell mass of small pancreatic islets in HFD-treated diabetic rats (P>0.05). Treatment of healthy rats with A. esculentus caused no significant changes in the number of ß-cells in both large and small islets (P>0.05, Table 2).
Table 2.
The number of β-cells in large and small islets in the pancreas in different groups
| Factor | Large islets (A) | Small islets (B) |
|---|---|---|
| Group | ||
| Control | 264.91 ± 16.92a | 44.5 ± 2.13a |
| Diabetic | 119.43 ± 15.15b | 22.08 ± 1.89b |
| Diabetic received A. esculentus | 172.6111 ± 14.91c | 25.27 ± 0.7bc |
| Diabetic received metformin | 192.29 ± 16.55c | 30.41 ± 3.01c |
| Control received A. esculentus | 253.91 ± 13.078a | 47.16 ± 2.11a |
Values are presented as mean ± SD, n=5 animals per group. Different letters in each column denote significant differences (P<0.05).
The results of the aldehyde fuchsin staining are shown in Figure 4. The normal cells in the islets of Langerhans showed distinct granules that were strongly stained in purple (Fig .4A, B). Diabetic rats (Fig .4C, D) demonstrated significant reductions in ß-cells and few surviving ß-cells were observed in the islets of Langerhans (P<0.05). Analyses of the pancreas of rats treated with A. esculentus and metformin (Fig .4E, F) showed remarkable increases in ß-cell, as reflected by purple granules (P<0.05).
Fig.4.
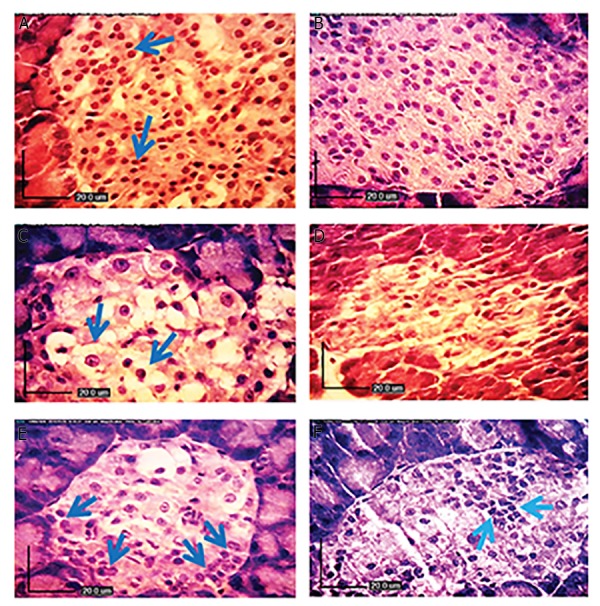
Aldehyde fuchsin staining of pancreatic islets in different experimental groups. A. Normal control rats (×40), B. (×100) showing normal ß-cells in the islets of Langerhans as well as distinct insulin granules filling the entire islets of Langerhans that are strongly stained in deep purple violet. High-fat diet (HFD)-treated diabetic rats, C. (×40), D. (×100) showing few surviving ß-cells in the islets of Langerhans and deficiency of their cytoplasmic tonality compared to control rats. The decreases in the reaction and the number of ß-cells are considerable (arrows, ×40), E. diabetic HFD rats treated with metformin, and F. diabetic HFD rats treated with okra showing remarkable increases in ß-cells mass, with heavily stained insulin granules.
Discussion
T2D is associated with decreased pancreatic ß-cells mass and function. Recently, focus on plant research has increased all over the world and a large body of evidence has shown the beneficial effects of medicinal plants on pancreatic dysfunction in diabetic patients (23). Recent findings have shown that A. esculentus can attenuate metabolic disturbances and insulin resistance related to diabetes in experimental animals (18), but its impact on pancreas histology and PPARs-dependent regulation in diabetes has not been clarified. In the present study, the effect of administration of A. esculentus on insulin resistance markers, serum lipid profile, pancreas structure and pancreatic expression of PPARs genes was determined in HFD/STZ-induced diabetic rats.
In the present study, HFD/STZ-induced diabetic rats displayed elevated fasting blood glucose and HOMA-IR, accompanied by decreased serum insulin levels confirming the induction of diabetes in these animals. A. esculentus administration decreased blood glucose levels and HOMAIR and improved insulin resistance in HFD/STZ-treated rats. In accordance with our findings, Ramachandran et al. (24) have reported anti-diabetic activity of A. esculentus in alloxan-induced diabetic rats. Moreover, Sabitha et al. (25) have reported antidiabetic and antihyperlipidemic potential of okra peel and seed powder in STZ-induced diabetic rats. It has been found that administration of peel and seed powder of okra to diabetic rats reduces blood glucose level and increases body weight as compared to diabetic control (13). Various mechanisms have been proposed for antidiabetic action of A. esculentus. High concentrations of fiber and polysaccharides in fruits of A. esculentus can stabilize blood sugar by curbing the rate at which sugar is absorbed from intestinal tract (26). Khatun et al. (27) have also shown that water-soluble fraction of A. esculentus reduced the absorption of glucose from the intestine.
Previous studies have shown that the extract of A. esculentus contains quercetin and its analogues. Recently, it has been found that quercetin decreases blood glucose levels in HFD/STZ-induced diabetic rats (18). Furthermore, quercetin and its analogues ameliorate insulin resistance in diabetic mice (28). Therefore, the phytoconstituents of okra might be responsible for antidiabetic property of this plant in diabetic rats.
One major finding of our study was contrasting effects of A. esculentus on serum insulin level in diabetic and control animals. In accordance with previous works, our results showed that diabetes was associated with decreased pancreatic ß-cells mass and reduced serum insulin level. Therefore, we propose the hypothesis that A. esculentus ability to increase ß-cells mass was the key factor in restoration of insulin production and secretion in diabetic animals. ß-cells mass of control rats that received A. esculentus, had no significant difference with that of control, untreated rats, while serum insulin was obviously reduced in healthy A. esculentus-treated rats. Although the mechanism of insulin-lowering effect of A. esculentus in healthy animals is unknown, it may be indirectly related to increased ß-cells responsiveness to glucose as manifested by reduced HOMA-IR and insulin secretion. Thus, as insulin sensitivity increases in healthy A. esculentus treated rats, first-phase of insulin release may decrease proportionately to maintain normal glucose levels. However, further studies are needed to confirm this hypothesis.
In the present study, diabetes was accompanied by an increase in TC, LDL-C, and TG and a reduction in HDL-C in HFD/STZ-induced diabetic rats. Treatment of HFD/STZ-induced diabetic rats with okra powder could profoundly improve lipid disturbances. Treatment of diabetic rats with A. esculentus notably reduced serum TG and TC levels while HDL-C levels remained unchanged. These results are in agreement with previous studies (29) that have shown that treatment with A. esculentus can reduce TC, total lipids and TG levels in rats fed with a HFD. Different mechanisms may underlie the improvement of lipid disturbances in diabetic rats after treatment with A. esculentus. Previous studies have shown that addition of A. esculentus to diet decreases the gene expression of SREBP1c and FAS (two key modulator of FA and cholesterol biosynthesis), which may finally reduce serum levels of TG and TC. Abundant dietary fibers present in A. esculentus are also capable of binding to bile acids consequently lowering TC through interfering with bile acids reabsorption (29). Roy et al. (30) reported that A. esculentus polysaccharides could decrease blood glucose levels in normal mice.
PPAR-α and PPAR-γ are nuclear hormone receptors that maintain homeostasis of glucose in the pancreas. PPARs exhibit beneficial effects on metabolic abnormalities associated with T2D and also control the expression of various genes that are important for lipid and glucose metabolism (31). Studies have indicated strong correlations between PPARs activation and anti-diabetic effects of many herbal plants (23). More than 200 natural compounds, especially flavonoids, have been identified as agonist or antagonist of PPAR-γ receptors and may play roles in the prevention and treatment of metabolic disorders. In this regard, recent data has shown that A. esculentus can activate PPAR-γ in the liver of HFD- induced obese C57BL/6 mice. Because PPARs have insulin-sensitizing effects in peripheral tissues as well as the ability to sense blood glucose in pancreatic ß-cells, we attempted to evaluate whether okra may affect PPARs gene expression in the pancreas of diabetic rats.
Quantitative real time PCR data showed that mRNA levels of PPAR-γ and PPAR-α were increased in HFD/ STZ-induced diabetic rats compared to control group rats. In accordance with our results, Zhou et al. (32) reported that, PPAR-γ expression is increased >5-fold in islets from Zucker diabetic fatty (ZDF) rats. In T2D, blood glucose and free FA levels are elevated, resulting in intracellular accumulation of TG within the pancreatic islets (33) and ß-cells secretory failure. Intracellular TG accumulation and overabundance of islet lipid, induce apoptosis in ß-cells by increasing free radicals formation. Over expression or activation of PPAR-γ and PPAR-α, up-regulates of key enzymes of mitochondrial and peroxisomal ß-oxidation and enhances FAs oxidation. It is reported that forced activation of PPAR-γ in the islets leads to stimulation of multiple metabolic pathways that help the disposal of FAs (34). Kakuma et al. (35) reported that long-term activation of PPAR-γ can reduce the lipid content of ZDF rat islets. Also, PPAR-α regulates the expression of genes involved in FAs and lipid metabolism (36). Studies performed in rodent models of insulin resistance indicated that PPAR-α activation by natural (FAs) or synthetic (fibrates) ligands, enhances insulin sensitivity by decreasing the lipid content of adipose and nonadipose tissues (7) or decreasing the endogenous glucose production (33). Based on these findings, we concluded that over-expression of PPARs in the pancreas of diabetic rats may be a compensatory mechanism for improvement of glucose sensitivity and ß-cells function. To confirm this hypothesis, previous studies have demonstrated that PPARγ over-expression can protect ß-cells function, morphology, and mass in rodent models of diabetes (34).
Interestingly, PPAR-γ antagonists may ameliorate metabolic disorders such as obesity, insulin resistance and dyslipidemia, by inhibition of adipocyte differentiation. PPAR-γ antagonists, tanshinone IIAand ß-cryptoxanthine, have been reported to reduce body weight, blood glucose and serum TG in HFD-induced obese mice (37). Rieusset reported that dimethyl a-(dimethoxyphosphinyl)pchlorobenzyl phosphate (SR-202) as a selective synthetic inhibitor of PPAR-γ inhibits adipocyte differentiation and improves insulin sensitivity in diabetic ob/ob mice (38). Thus, PPARγ antagonists may be useful for the treatment of obesity-related insulin resistance. Our data showed that PPAR-γ and PPAR-α mRNA levels declined in diabetic animals treated with A. esculentus powder, confirming the PPAR antagonistic effect of A. esculentus. These results were in accordance with the findings of Fan et al. (18) that showed that okra consumption inhibits PPAR-γ and PPAR-α transcription in the liver of HFD-induced obese C57BL/6.
Together, we suggest that okra may improve metabolic disorders related to diabetes through suppression of PPARs signaling. In addition to the above-described mechanisms, it seems that reduction of PPAR-γ and PPAR-α expression might be a consequence of improvement of hyperinsulinemia. In other words, increased PPARs expression during insulin resistance state, can improve insulin resistance, attenuate hyperlipidemia and increase overall glucose utilization, while improvement of insulin resistance after A. esculentus treatment results in down-regulation of PPARs in the pancreas. Based on these observations, it has been suggested that an increase in PPARs expression may induce a compensatory mechanism against progression of insulin resistance in obese patients (7, 18, 34).
Additionally, our histological data showed that the size of islets and population of insulin-producing ß-cells were reduced in the pancreas of HFD/STZ-induced diabetic rats. Aldehyde fuchsin staining also demonstrated that A. esculentus powder could restore pancreatic ß-cells mass and reverse the ß-cells damage caused by HFD/STZ treatment. These results were in accordance with previous studies that demonstrated that hyperglycemia leads to a progressive decline in ß-cells function, the insufficiency of insulin secretion by the pancreatic ß -cells (39) and increased apoptosis in pancreatic islets (40).
Conclusion
Based on our data, okra could improve metabolic complications in an animal model of diabetes. Our results revealed that A. esculentus had beneficial effect on the pancreas of diabetic rats by restoration of ß-cell mass and modulation of PPAR-dependent pathways. The results of the present study provide new scientific evidence about therapeutic benefits of A. esculentus in diabetes.
Acknowledgments
Thanks for the financial support of Shahid Chamran University of Ahvaz. The authors declare that there is no conflicts of interest.
Author’s Contributions
N.E.M.; Was supervisors and collaborated in histological studies and wrote the manuscript. M.R.T.; Was advisor and collaborated in molecular study. A.Sh.; Was supervisors and collaborated in biochemistry study. Z.S.; Contributed for her thesis. All authors read and approved the final study.
References
- 1.Bermúdez-Pirela VJ, Cano C, Medina MT, Souki A, Lemus MA, Leal EM, et al. Metformin plus low-dose glimeperide significantly improves homeostasis model assessment for insulin resistance (HOMA(IR)) and beta-cell function (HOMA(beta-cell)) without hyperinsulinemia in patients with type 2 diabetes mellitus. Am J Ther. 2007;14(2):194–202. doi: 10.1097/01.pap.0000249909.54047.0e. [DOI] [PubMed] [Google Scholar]
- 2.Ble-Castillo JL, Aparicio-Trapala MA, Juárez-Rojop IE, Torres-Lopez JE, Mendez JD, Aguilar-Mariscal H, et al. Differential effects of high-carbohydrate and high-fat diet composition on metabolic control and insulin resistance in normal rats. Int J Environ Res Public Health. 2012;9:1663–1676. doi: 10.3390/ijerph9051663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chun MR, Lee YJ, Kim KH, Kim YW, Park SY, Lee KM, et al. Differential effects of high-carbohydrate and high-fat diet composition on muscle insulin resistance in rats. J Korean Med Sci. 2010;25(7):1053–1059. doi: 10.3346/jkms.2010.25.7.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donath MY, Halban PA. Decreased beta-cell mass in diabetes: significance, mechanisms and therapeutic implications. Diabetologia. 2004;47(3):581–589. doi: 10.1007/s00125-004-1336-4. [DOI] [PubMed] [Google Scholar]
- 5.Fie´vet C, Fruchart JC, Staels B. PPARalpha and PPARgamma dual agonists for the treatment of type 2 diabetes and the metabolic syndrome. Curr Opin Pharmacol. 2006;6(6):606–614. doi: 10.1016/j.coph.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Wein S, Behm N, Petersen RK, Kristiansen K, Wolffram S. Quercetin enhances adiponectin secretion by a PPAR gamma independent mechanism. Eur J Pharm Sci. 2010;41(1):16–22. doi: 10.1016/j.ejps.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Koh EH, Kim MS, Park JY, Kim HS, Youn JY, Park HS, et al. Peroxisome proliferator-activated receptor (PPAR)-alpha activation prevents diabetes in OLETF rats: comparison with PPAR-gamma activation. Diabetes. 2003;52(9):2331–2337. doi: 10.2337/diabetes.52.9.2331. [DOI] [PubMed] [Google Scholar]
- 8.Sheu SC, Lai MH. Composition analysis and immuno-modulatory effect of Okra (Abelmoschus esculentus L.) extract.Food Chem.9.Arapitsas P.Identification and quantification of polyphenolic compounds from okra seeds and skins. Food Chem. 2008;110(4):1041–1045. doi: 10.1016/j.foodchem.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Arapitsas P. Identification and quantification of polyphenolic compounds from okra seeds and skins. Food Chem. 2008;110(4):1041–1045. doi: 10.1016/j.foodchem.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Gunness P, Gidley MJ. Mechanisms underlying the cholesterollowering properties of soluble dietary fibre polysaccharides. Food Funct. 2010;1(2):149–155. doi: 10.1039/c0fo00080a. [DOI] [PubMed] [Google Scholar]
- 11.Huang DJ, Lin CD, Chen HJ, Lin YH. Antioxidant and anti proliferative activities of sweet potato (Ipomoea batatas [L.] LamTainong 57’) constituents. Bot Bull Acad Sinica. 2004;45:179–186. [Google Scholar]
- 12.Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nut Biochem. 2002;13(10):572–584. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 13.Sabitha V, Ramachandran S, Naveen KR, Panneerselvam K. Antidiabetic and antihyperlipidemic potential of Abelmoschus esculentus (L.) Moench.in streptozotocininduced diabetic rats. J Pharm Bioallied Sci. 2011;3(3):397–402. doi: 10.4103/0975-7406.84447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kabiri N, Tabandeh M, Fatemi Tabatabaie SR. Beneficial effects of pioglitazone and metformin in murine model of polycystic ovaries via improvement of chemerin gene up-regulation. Daru. 2014;22(39):2–10. doi: 10.1186/2008-2231-22-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Binh DV, Dung NTK, Thao LTB, Nhi NB, Chi PV. Macroand microvascular complications of diabetes induced by high-fat diet and low-dose streptozotocin injection in rats model. Diabetes. 2013;2(3):50–55. [Google Scholar]
- 16.Kolahian S, Sadri H, Larijani A, Hamidian Gh, Davasaz A. Supplementation of diabetic rats with leucine, zinc, and chromium: effects on function and histological structure of testes.Int J Vitam Nutr Res. Int J Vitam Nutr Res; 2016. pp. 1–11. [DOI] [PubMed] [Google Scholar]
- 17.Friedewald WT. Estimation of concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem. 1972;18(7):499–502. [PubMed] [Google Scholar]
- 18.Fan S, Zhang Y, Sun Q, Yu L, Li M, Zheng B, et al. Extract of Okra lowers blood glucose and serum lipids in high-fat diet-induced obese C57BL/6 mice. J Nutr Biochem. 2014;25(7):702–709. doi: 10.1016/j.jnutbio.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55(4):611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 20.Bancroft JD. Theory and practice of histological techniques. In: Bancroft JD, Gamble M, editors. 5th ed. 5th ed. Edinburgh: Churchill livingstone; 2002. pp. 125, 130, 175, 206-208. [Google Scholar]
- 21.Ramachandran S, Sandeep VS, Srinivas NK, Dhanaraju MD. 2010.Anti-diabetic activity of Abelmoschus esculentus Linn on alloxan-induced diabetic rats. Res Rev Biosci. 2015;4(3):121–123. [Google Scholar]
- 22.Chintinne M, Stange´ G, Denys B, Ling Z, Veld PI, Pipeleers D. Beta cell count instead of beta cell mass to assess and localize growth in beta cell population following pancreatic duct ligation in Mice. PLoS One. 2012;7(8):e43959–e43959. doi: 10.1371/journal.pone.0043959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Assaei R, Mokarram P, Dastghaib S, Darbandi S, Darbandi M, Zal F, et al. Hypoglycemic effect of aquatic extract of stevia in pancreas of diabetic rats: PPARγ-dependent regulation or antioxidant potential. Avicenna J Med Biotechnol. 2016;8(2):65–74. [PMC free article] [PubMed] [Google Scholar]
- 24.Ramachandran K, Huang HH, Stehno-Bittel L. A simple method to replace islet equivalents for Volume Quantification of Human Islets. Cell Transplant. 2015;24(7):1183–1194. doi: 10.3727/096368914X681928. [DOI] [PubMed] [Google Scholar]
- 25.Sabitha V, Ramachandran S, Naveen KR, Panneerselvam K. Antidiabetic and antihyperlipidemic potential of Abelmoschus esculentus (L.) Moench.in streptozotocin induced diabetic rats. J Pharm Bioallied Sci. 2013;3(3):397–402. doi: 10.4103/0975-7406.84447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sreeshma LS, Nair BR. Biochemical changes associated with fruit development in Abelmoschus esculentus cv.Arka Anamika. Journal of Agricultural Technology. 2013;9(2):373–382. [Google Scholar]
- 27.Khatun H, Rahman A, Biswas M, Islam AU. Water-soluble fraction of Abelmoschus esculentus L interacts with glucose and metformin hydrochloride and alters their absorption kinetics after coadministration in rats. ISRN Pharm. 2011;2011:260537–260537. doi: 10.5402/2011/260537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai X, Ding Y, Zhang Z, Cai X, Bao L, Li Y. Quercetin but not quercitrin ameliorates tumor necrosis factor-alpha-induced insulin resistance in C2C12 skeletal muscle cells. Biol Pharm Bull. 2013;36(5):788–795. doi: 10.1248/bpb.b12-00947. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Chen G, Ren D, Yang ST. Hypolipidemic activity of Okra is mediated through inhibition of lipogenesis and upregulation of cholesterol degradation. Phytother Res. 2014;28(2):268–273. doi: 10.1002/ptr.4998. [DOI] [PubMed] [Google Scholar]
- 30.Roy A, Shrivastava ShL, Mandal SM. Functional properties of Okra Abelmoschus esculentus L.(Moench): traditional claims and scientific evidences. Plant Sci Today. 2014;1(3):121–130. [Google Scholar]
- 31.Wahli W, Braissant O, Desvergne B. Peroxisome proliferator activated receptors: transcriptional regulators of adipogenesis, lipid metabolism and more…. Chem Biol. 1995;2(5):261–266. doi: 10.1016/1074-5521(95)90045-4. [DOI] [PubMed] [Google Scholar]
- 32.Zhou YT, Shimabukuro M, Wang MY, Lee Y, Higa M, Milburn JL, et al. Role of peroxisome proliferator-activated receptor alpha in disease of pancreatic beta cells. Proc Natl Acad Sci USA. 1998;95(15):8898–8903. doi: 10.1073/pnas.95.15.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim H, Ahn Y. Role of peroxisome proliferator-activated receptorgamma in the glucose-sensing apparatus of liver and beta-cells. Diabetes. 2004;53(Suppl 1):S60–S65. doi: 10.2337/diabetes.53.2007.s60. [DOI] [PubMed] [Google Scholar]
- 34.Parton LE, Diraison F, Neill SE, Ghosh SK, Rubino MA, Bisi JE, et al. Impact of PPARgamma overexpression and activation on pancreatic islet gene expression profile analyzed with oligonucleotide microarrays. Am J Physiol Endocrinol Metab. 2004;287(3):E390–E404. doi: 10.1152/ajpendo.00016.2004. [DOI] [PubMed] [Google Scholar]
- 35.Kakuma T, Lee Y, Higa M, Wang Z, Pan W, Shimomura I, et al. Leptin, troglitazone, and the expression of sterol regulatory element binding proteins in liver and pancreatic islets. Proc Natl Acad Sci USA. 2000;97(15):8536–8541. doi: 10.1073/pnas.97.15.8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marx N, Duez H, Fruchart JC, Staels B. Peroxisome proliferatoractivated receptors and atherogenesis: regulators of gene expression in vascular cells. Circ Res. 2004;94(9):1168–1178. doi: 10.1161/01.RES.0000127122.22685.0A. [DOI] [PubMed] [Google Scholar]
- 37.Goto T, Kim YI, Takahashi N, Kawada T. Natural compounds regulate energy metabolism by the modulating the activity of lipid-sensing nuclear receptors. Mol Nutr Food Res. 2013;57(1):20–33. doi: 10.1002/mnfr.201200522. [DOI] [PubMed] [Google Scholar]
- 38.Rieusset J, Touri F, Michalik L, Escher P, Desvergne B, Niesor E, et al. A new selective peroxisome proliferator-activated receptor gamma antagonist with antiobesity and antidiabetic activity. Mol Endocrinol. 2002;16(11):2628–2644. doi: 10.1210/me.2002-0036. [DOI] [PubMed] [Google Scholar]
- 39.Bonora E. Protection of pancreatic beta-cells: is it feasible? Nutr Metab Cardiovasc Dis. 2008;18(1):74–83. doi: 10.1016/j.numecd.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Federici M, Hribal M, Perego L, Ranalli M, Caradonna Z, Perego C, et al. High glucose causes apoptosis in cultured human pancreatic islets of Langerhans: a potential role for regulation of specific Bcl family genes toward an apoptotic death program.Diabetes. Diabetes; 2001. [DOI] [PubMed] [Google Scholar]