Abstract
Olives contain 3,4‐dihydroxyphenyl compounds (catechols)—especially 3,4‐dihydroxyphenylethanol (DOPET)—that have therapeutic potential as nutraceuticals. Whether olive ingestion affects plasma levels of free (unconjugated) catechols has been unknown. Arm venous blood was sampled before and 15, 30, 45, 60, 120, 180, and 240 min after six healthy volunteers ate 10 Kalamata olives. Catechols were assayed by alumina extraction followed by liquid chromatography with series electrochemical detection. Plasma DOPET increased to 18.5 times baseline at 30 min (area under the curve (AUC) 39.2 ± 9.2 pmol‐min/mL, P = 0.008). 3,4‐Dihydroxyphenylacetic acid (DOPAC) increased markedly (peak 37.4 times baseline, AUC 23,490 ± 4,151 pmol‐min/mL, P = 0.002). The sum of 10 catechols increased 12‐fold (P < 0.0001). Eating olives produces large‐magnitude increases in plasma levels of catechols, mainly DOPAC. DOPET seems to go undergo extensive hepatic metabolism to DOPAC.
Study Highlights
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
✓ Nutraceuticals might slow the progression of or even prevent neurodegenerative diseases such as Parkinson's disease. 3,4‐Dihydroxyphenylethanol (DOPET, hydroxytyrosol) is the main antioxidant phenol in olives and olive oil. Little is known about the plasma levels of DOPET in humans.
WHAT QUESTION DID THIS STUDY ADDRESS?
✓ We asked whether olive ingestion affects plasma levels of free (unconjugated) catechols in healthy volunteers.
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE
✓ Plasma 3,4‐dihydroxyphenylacetic acid (DOPAC) increases markedly after olive ingestion. Increases in plasma DOPET are much smaller.
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE
✓ Despite the fact that DOPET is the most abundant catechol in olives, after people eat olives there are far larger increases in plasma DOPAC than DOPET levels. There probably is extensive hepatic conversion of DOPET to DOPAC. Efforts to use exogenous DOPET for neuroprotection should be directed to modes of delivery that avoid a first‐pass effect.
Nutraceuticals are constituents of foodstuffs that might provide clinical therapeutic benefits beyond the nutrient value of the food itself. There is growing interest currently in assessing whether nutraceuticals can slow the progression of, delay the onset of, or even prevent neurodegenerative diseases such as Parkinson's disease.1 It has been proposed that the catechol hydroxytyrosol (3,4‐dihydroxyphenylethanol, DOPET), which is abundant in olives and olive oil, contributes to the health benefits of the Mediterranean diet.2, 3 Plasma DOPET levels increase between ingestion of olive oil with vs. without added hydroxytyrosol.4
DOPET is not only a nutraceutical but also a minor metabolite of endogenous dopamine (DA); however, plasma DOPET in fasting humans has been below the detection limit of available assays. It is suspected that the bioavailability of ingested DOPET is poor,5 due to metabolic breakdown by multiple enzymes in the gut and liver (Figure 1).
Figure 1.
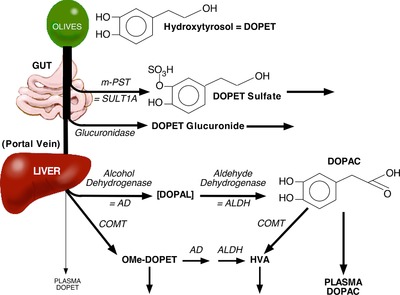
Concept diagram showing the metabolic fate of hydroxytyrosol (DOPET) after olive ingestion. DOPET is broken down by at least four enzymes—monoamine‐preferring sulfotransferase (mPST), glucuronidase, catechol‐O‐methyltransferase (COMT), and alcohol dehydrogenase (AD). ALDH = aldehyde dehydrogenase; HVA = homovanillic acid; OMe‐DOPET = O‐methyl‐DOPET. We predicted relatively small increases in plasma DOPET because of a substantial first‐pass effect.
The plasma of humans normally contains the catecholamines DA, norepinephrine (NE), and epinephrine (EPI, synonymous with adrenaline), the catecholamine precursor, DOPA, and in addition the catecholamine metabolites 3,4‐dihydroxyphenylacetic acid (DOPAC), which is the main neuronal metabolite of DA, and 3,4‐dihydroxyphenylglycol (DHPG), which is the main neuronal metabolite of NE.6 As shown here, human plasma contains at least four other catechols—5‐S‐cysteinylDOPA (Cys‐DOPA), 5‐S‐cysteinylDA (Cys‐DA), 3,4‐dihydroxyhydrocinnamic acid (DHHC, also known as dihydrocaffeic acid), and DOPET. Cys‐DOPA is formed from the enzymatic oxidation of tyrosine or DOPA by tyrosinase and from the spontaneous oxidation of DOPA; plasma Cys‐DOPA has been used as a marker of malignant melanoma.7 Cys‐DA is formed from spontaneous oxidation of DA. Cys‐DA has been detected in human cerebrospinal fluid8, 9 and in the plasma of patients undergoing intravenous DA infusion,10 but has not been described as an endogenous compound in humans. DHHC is present in the plasma of coffee drinkers,11 but plasma DHHC has also not been reported previously in fasting humans.
Although DOPET is abundant in olives, ingested DOPET is likely to be subject to a substantial first‐pass effect (Figure 1). In this study we exploited a highly sensitive method based on batch alumina extraction followed by liquid chromatography with series electrochemical detection to measure plasma levels of DOPET and other catechols in healthy volunteers after eating olives.
MATERIALS AND METHODS
Subjects
The subjects in this study were six healthy adult volunteers (mean age 34 ± 5 (SEM) years, four men and two women). Of the six subjects, five were of western European and one Greek ethnic origin. The subjects were studied as outpatients in the National Institutes of Health (NIH) Clinical Center in a dedicated Patient Observation Room. The clinical study was approved by the Institutional Review Board (IRB) of the National Institute of Neurological Disorders and Stroke (NINDS). All the subjects gave informed written consent to participate in the IRB‐approved protocol before any experimental procedures were done.
Experimental procedures
Each subject reported to a dedicated patient testing room in the morning after having fasted (except for water) overnight. Room temperature was 22–25°C.
The subject urinated to empty the bladder, and the urine was transferred to a preweighed container that had 10 mL of 6N HCl in it. The subject then lay down on a padded testing table with the head on a pillow. An intravenous catheter (i.v.) was placed in an arm vein. Electrocardiographic leads were placed for monitoring heart rate. Blood pressure was measured continuously noninvasively using an automated finger cuff device (Nexfin, bmeye, Amsterdam, the Netherlands). Physiological signals were recorded using a PowerLab system (ADInstruments, Colorado Springs, CO) and analyzed by LabChart software bundled with the PowerLab system.
After at least 15 min with the subject at supine rest, a baseline blood sample (about 5 mL) was drawn through the i.v. Across the six subjects, the baseline samples were obtained at 9:22–10:26 AM.
Each subject then ate 10 Kalamata olives over about 1–2 min. If sitting to eat the olives, the subject was returned immediately to the supine position. Blood was drawn through the i.v. at 15, 30, 45, and 60 min. The subject was then allowed to ambulate. Any excreted urine was transferred to a second preweighed container that had 10 mL of 6N HCl in it. Additional blood samples were obtained at 120, 180, and 240 min, with the subject supine for at least 15 min before the sampling. After the testing, a postingestion urine sample was obtained and transferred to the second urine container. Urine volume was quantified from the net added weight of the containers.
Data analysis and statistics
Plasma levels of catechols were assayed by batch alumina extraction followed by liquid chromatography with series electrochemical detection, as described previously,12 except that additional standards (DOPET, DHHC, Cys‐DOPA, and Cys‐DA) were included, and two different volumes of the same specimen were assayed to ensure chromatographic peaks were on scale. Urinary catechols were assayed by the same method. Our laboratory has published previously on simultaneous measurements of levels of all the catechols reported here in human biological fluids.9, 11, 12, 13, 14
Mean values are expressed ±1 standard error of the mean (SEM). Data for plasma levels of catechols were analyzed by analyses of variance for repeated measures, with post‐hoc comparisons vs. baseline by Dunnett's test, using KaleidaGraph 4.5 (Synergy Software, Reading, PA). Because of skewed distributions of plasma levels of catechols (higher concentrations associated with greater interindividual variability), all the neurochemical data were log‐transformed before statistical analyses. A P value less than 0.05 defined statistical significance.
RESULTS
The liquid chromatographic‐electrochemical assay method successfully resolved and quantified 10 catechols of interest—DHPG, NE, DOPA, EPI, Cys‐DOPA, DA, DOPET, Cys‐DA, DOPAC, and DHHC—as well the internal standard (I.S.), 3,4‐dihydroxybenzylamine (Figure 2).
Figure 2.
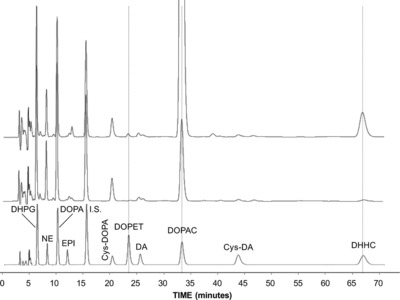
Chromatographs of extracted catechol standards (bottom) and plasma catechols before (middle) and after (top) olive ingestion in a healthy volunteer. The bottom panel shows a chromatograph after batch alumina extraction of 1,000 pg of 3,4‐dihydroxyphenyglycol (DHPG); 250 pg of norepinephrine (NE); 1,000 pg of DOPA; 250 pg of epinephrine (EPI); 1,000 pg of 5‐S‐cysteinyl‐DOPA (Cys‐DOPA); 1,000 pg of 3,4‐dhydroxyphenylethanol (DOPET); 250 pg of dopamine (DA); 1,000 pg of 3,4‐dihydroxyehphenylacetic acid (DOPAC); 1,000 pg of 5‐S‐cysteinyl‐dopamine (Cys‐DA); and 1,000 pg of dihydroxyhydrocinnamic acid (DHHC). The middle panel shows a chromatograph after alumina adsorption of plasma from a healthy subject during supine rest. The top panel shows a chromatograph from the same subject at 60 min after olive ingestion. Note marked increase in plasma DOPAC after olive ingestion.
The catechol concentrations in Kalamata olives (and total consumed catechols assuming the mass of a single olive 25 g), were, in descending order of concentrations, DOPET > DHHC >> caffeic acid > DOPAC = DHPG > DA. Norepinephrine, epinephrine, and DOPA were not detected (Table 1). The active entities in the olives therefore were DOPET and DHHC.
Table 1.
Catechol contents in Kalamata olives
| Olive contents | pg/mg | nmol/g | nmol/10 olives |
|---|---|---|---|
| DOPET | 398,871 | 2,590 | 64,752 |
| DHHC | 49,513 | 294.7 | 7,368 |
| Caffeic acid | 827 | 4.92 | 123 |
| DOPAC | 640 | 3.81 | 95 |
| DHPG | 635 | 3.78 | 94 |
| DA | 43 | 0.26 | 6 |
Norepinephrine, epinephrine, and DOPA were not detected. The mass of a single olive was assumed to be 25 g. DOPET = 3,4‐dihydroxyphenylethanol; DHHC = dihydroxyhydrocinnamic acid; DOPAC = 3,4‐dihydroxyphenylacetic acid; DHPG = 3,4‐dihydroxyphenylglycol; DA = dopamine.
Plasma levels of catechols at baseline (after fasting overnight) varied over a wide range, with the highest levels those of DOPAC, followed by DOPA, Cys‐DOPA, DHHC, NE, DA, Cys‐DA, and EPI (Table 2). The lowest level was that of DOPET. In two subjects DOPET at baseline was below the limit of detection of the assay (about 0.01 pmol/mL).
Table 2.
Plasma levels (pmol/mL) and urinary excretion rates (nmol/min) of catechols before and after eating olives
| Time (min) | |||||||
|---|---|---|---|---|---|---|---|
| BL | 15’ | 30’ | 45’ | 60’ | 120’ | 180’ | 240’ |
| Catechol | |||||||
| DHPG | |||||||
| 5.6 ± 0.4 | 5.6 ± 0.5 | 7.5 ± 1.0 | 7.7 ± 0.9 | 6.9 ± 0.6 | 5.7 ± 0.4 | 6.0 ± 0.3 | 6.2 ± 0.3 |
| NE | |||||||
| 1.3 ± 0.3 | 1.1 ± 0.2 | 1.4 ± 0.4 | 1.3 ± 0.3 | 1.2 ± 0.2 | 1.4 ± 0.2 | 1.2 ± 0.3 | 1.2 ± 0.2 |
| DOPA | |||||||
| 7.8 ± 0.7 | 6.9 ± 0.6 | 7.5 ± 0.9 | 9.2 ± 0.9 | 7.4 ± 0.7 | 7.4 ± 0.6 | 7.6 ± 0.6 | 7.6 ± 0.6 |
| EPI | |||||||
| 0.05 ± 0.01 | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.07 ± 0.02 |
| Cys‐DOPA | |||||||
| 5.7 ± 1.5 | 5.2 ± 1.5 | 5.4 ± 1.6 | 5.3 ± 1.6 | 5.6 ± 1.5 | 5.1 ± 0.9 | 5.2 ± 1.1 | 5.1 ± 1.1 |
| DOPET | |||||||
| 0.04 ± 0.01 | 0.40 ± 0.19 | 0.64 ± 0.17 | 0.40 ± 0.09 | 0.32 ± 0.04 | 0.11 ± 0.04 | 0.07 ± 0.01 | 0.05 ± 0.01 |
| DA | |||||||
| 0.09 ± 0.03 | 0.13 ± 0.07 | 0.12 ± 0.05 | 0.12 ± 0.04 | 0.12 ± 0.04 | 0.10 ± 0.04 | 0.07 ± 0.02 | 0.07 ± 0.02 |
| DOPAC | |||||||
| 8.7 ± 1.2 | 60 ± 23 | 256 ± 63 | 326 ± 74 | 290 ± 44 | 124 ± 27 | 37 ± 7 | 21 ± 3 |
| Cys‐DA | |||||||
| 0.08 ± 0.04 | 0.08 ± 0.05 | 0.07 ± 0.05 | 0.08 ± 0.05 | 0.08 ± 0.06 | 0.08 ± 0.05 | 0.08 ± 0.05 | 0.06 ± 0.04 |
| Catechol | |||||||
| DHHC | |||||||
| 1.9 ± 1.4 | 3.4 ± 1.2 | 9.2 ± 1.9 | 10.3 ± 2.0 | 8.9 ± 1.6 | 3.9 ± 1.5 | 2.1 ± 1.0 | 1.5 ± 0.9 |
| Urinary excretion rates (nmol/min) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Condition | DHPG | NE | DOPA | EPI | Cys‐DOPA | DOPET | DA | DOPAC | Cys‐DA | DHHC |
| BL | 0.31±0.10 | 0.19±0.07 | 0.06±0.01 | 0.04±0.01 | 0.56±0.21 | 0.02±0.01 | 0.97±0.23 | 2.27±0.66 | 0.03±0.01 | 0.56±0.23 |
| Post | 0.46±0.12 | 0.13±0.04 | 0.08±0.02 | 0.03±0.01 | 0.48±0.15 | 0.25±0.07 | 1.02±0.31 | 17.3±5.9 | 0.02±0.01 | 1.88±0.59 |
Mean values expressed ±SEM. For plasma catechols, items in bold indicate significant differences (P < 0.05) from baseline (BL) by Dunnett's post‐hoc test after repeated measures analysis of variance. Urinary excretion rates before olive ingestion were based on a presumed 94‐min interval from the previous void to the BL sample collection.
Olives ingestion did not affect systolic or diastolic blood pressure or heart rate (Supplementary Table).
Plasma DOPET levels increased rapidly after olives ingestion, followed soon after by DOPAC and DHHC (Figures 3 , 4). The peak mean increase in plasma DOPET occurred at 30 min, and the mean peak increases in plasma DOPAC and DHHC occurred at 45 min.
Figure 3.
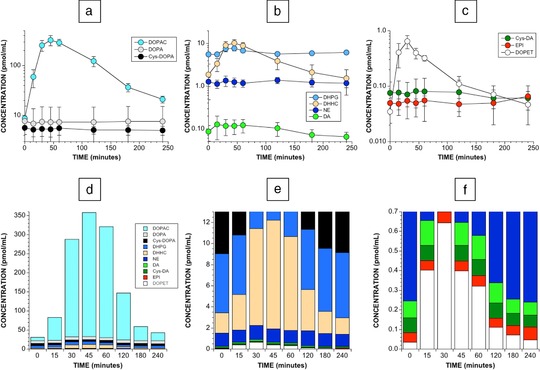
Plasma catechols after olives ingestion. Shown are mean (±SEM) plasma levels of (a) DOPA, DOPAC, and Cys‐DOPA; (b) DHPG, DHCA, NE, and DA; and (c) Cys‐DA, EPI, DOPET as a function of time after eating 10 Kalamata olives in six healthy fasting volunteers. Panels d–f show stack diagrams of the same data but progressively more sensitive y‐axis scales. Note marked proportionate increases in DOPAC, DHHC, and DOPET. As illustrated in panel d, the sum of 10 assayed catechols increased by up to about 12‐fold after olives ingestion.
Figure 4.
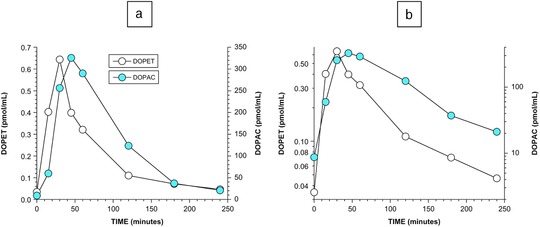
Time courses of plasma DOPET and DOPAC after olives ingestion. (a) Plasma DOPET and DOPAC, linear scale; (b) plasma DOPET and DOPAC, log scale.
Plasma DOPET seemed to decline in a biexponential manner from the peak attained mean value (Figure 4), with effective rate constants of 0.02575 min−1 (r = –0.98, half‐time 27 min) between 30 and 120 min and 0.007214 min−1 (r = 1.00, half‐time 96 min) between 120 and 240 min. Plasma DOPAC seemed to decline in a monoexponential manner, with an effective rate constant of 0.01485 min−1 (r = 0.995, half‐time 47 min) between 45 and 240 min.
Plasma DHPG increased transiently (Table 2), while plasma levels of endogenous catecholamines were unaffected. As indicated by the stack diagram in Figure 3, the total amount of assayed catechols after olive ingestion increased to a peak of about 12 times baseline.
Comparing urinary excretion rates of catechols before vs. after olives ingestion required estimation of the time between the baseline urine collection and the previous void. Since olive flesh contains very low concentrations of DOPA, NE, EPI, DA, and Cys‐DA (Table 1), this was accomplished by assuming that excretion rates of these endogenous catechols would not be affected by ingesting olives. From the mean excretion of the endogenous catechols before olives ingestion (24.23 nmol), divided by the mean excretion of the same catechols during 240 min after olives ingestion (61.80 nmol), the time between the baseline urine collection and the previous void was estimated to be 240 min * 24.23 nmol / 61.80 nmol = 94 min.
Using a 94‐min time interval between the baseline urine collection and the previous void, urinary excretion of DOPAC increased to 8 times baseline (Table 2), DHHC to 3 times baseline, and DOPET to 12 times baseline. The increment in the urinary excretion rate of DOPAC averaged 65 times the increment in the urinary excretion rate of DOPET.
DISCUSSION
It has been proposed that DOPET (hydroxytyrosol) derived from olives and olive oil contributes to the health benefits of the Mediterranean diet.15, 16 The results of this study show that although there is a large proportionate increase in plasma levels of DOPET after people eat olives, the absolute increment in plasma DOPET is very small (area under the curve (AUC) 39 pmol‐min/mL).
In marked contrast, there was a huge increment in plasma DOPAC (AUC 23,490 pmol‐min/mL, about 600 times the AUC for DOPET). Given the low content of DOPAC in olive flesh, this result implies substantial production of DOPAC in the body after olives ingestion.
The large amount of DOPAC found in the plasma after eating olives could be the result of substantial hepatic enzymatic conversion DOPET to DOPAC (Figure 1). Alcohol dehydrogenase converts DOPET to the catecholaldehyde 3,4‐dihydroxyphenylacetaldehyde, which is efficiently oxidized to DOPAC by aldehyde dehydrogenase. In keeping with this proposal, ingestion of ethanol, which competitively inhibits alcohol dehydrogenase, shifts the metabolism of endogenous DA toward DOPET17 and increases the bioavailability of ingested DOPET.18 DOPET is present in red wine, and urinary DOPET excretion increases with ethanol ingestion, increases more with ingestion of de‐ethanolized red wine, and increases further with ingestion of red wine.19
DHHC was detected at baseline in all the plasma samples, despite the subjects having fasted overnight. Whether DHHC is an endogenous compound in humans or is produced over relatively long time periods by colonic bacteria20 remains to be determined.
Measuring plasma levels of catechols is useful for clinical diagnosis,21 evaluating drug effects,22 identifying pathophysiologic mechanisms,23, 24 tracking the course of diseases,25 and establishing prognosis26 in a wide variety of disorders. The present results show that a seemingly trivial dietary manipulation can markedly affect plasma levels of catechols.
Estimated changes in urinary excretion rates of catechols were generally consistent with the alterations in the plasma levels. In particular, the increment in the urinary excretion rate of DOPAC averaged 65 times that of DOPET.
In summary, despite the fact that DOPET is the most abundant catechol in olives, after people eat olives there are very small increases in plasma DOPET concentrations. There are much larger increases in plasma levels of DOPAC. It seems that conversion of DOPET to DOPAC in the liver substantially limits the plasma bioavailability of dietary DOPET. If so, then efforts to use exogenous DOPET as a neuroprotective agent should be directed to modes of delivery that avoid a first‐pass effect, such as via a transdermal patch.27
Conflict of Interest
The authors have no conflicts of interest to disclose.
Supporting information
Table S1: Systemic hemodynamics after olive ingestion
Acknowledgments
The research reported here was supported by the Division of Intramural Research of the National Institute of Neurological Disorders and Stroke.
Author Contributions
D.S.G. and Y.S. wrote the article; D.S.G. designed the research; D.S.G., C.H., and J.C. performed the research; D.S.G., C.H., J.C., and Y.S. analyzed the data.
Financial Support
Division of Intramural Research, NINDS, NIH.
References
- 1. Dadhania, V.P. , Trivedi, P.P. , Vikram, A. & Tripathi, D.N. Nutraceuticals against neurodegeneration: A mechanistic insight. Curr Neuropharmacol. 14: 627–640 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rodriguez‐Morato, J. , Xicota, L. , Fito, M. , Farre, M. , Dierssen, M. & de la Torre, R. Potential role of olive oil phenolic compounds in the prevention of neurodegenerative diseases. Molecules. 20, 4655–4680 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu, T. , He, X.W. , Jiang, J.G. & Xu, X.L. Hydroxytyrosol and its potential therapeutic effects. J. Agric. Food Chem. 62, 1449–1455 (2014). [DOI] [PubMed] [Google Scholar]
- 4. Pastor, A. , et al Analysis of free hydroxytyrosol in human plasma following the administration of olive oil. J. Chromatogr. A. 1437, 183–190 (2016). [DOI] [PubMed] [Google Scholar]
- 5. de la Torre, R . Bioavailability of olive oil phenolic compounds in humans. Inflammopharmacology. 16, 245–247 (2008). [DOI] [PubMed] [Google Scholar]
- 6. Goldstein, D.S. , Eisenhofer, G. & Kopin, I.J. Sources and significance of plasma levels of catechols and their metabolites in humans. J. Pharmacol. Exp. Ther. 305, 800–811 (2003). [DOI] [PubMed] [Google Scholar]
- 7. Meyer, T. , et al Clinical evaluation of 5‐S‐cysteinyldopa testing using a new and optimized detection system as a tumour marker for malignant melanoma. Melanoma Res. 12, 471–477 (2002). [DOI] [PubMed] [Google Scholar]
- 8. Cheng, F.C. , Kuo, J.S. , Chia, L.G. & Dryhurst, G. Elevated 5‐S‐cysteinyldopamine/homovanillic acid ratio and reduced homovanillic acid in cerebrospinal fluid: possible markers for and potential insights into the pathoetiology of Parkinson's disease. J. Neural Transm. 103, 433–446 (1996). [DOI] [PubMed] [Google Scholar]
- 9. Goldstein, D.S. , Holmes, C. , Sullivan, P. , Jinsmaa, Y. , Kopin, I.J. & Sharabi, Y. Elevated cerebrospinal fluid ratios of cysteinyl‐dopamine/3,4‐dihydroxyphenylacetic acid in parkinsonian synucleinopathies. Parkinsonism Relat. Disord. 31, 79–86 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Magnay, J.L. , Tong, J. , Drangova, R. & Baines, A.D. Production of cysteinyl‐dopamine during intravenous dopamine therapy. Kidney Int. 59, 1891–1898 (2001). [DOI] [PubMed] [Google Scholar]
- 11. Goldstein, D.S. , Stull, R. , Markey, S.P. , Marks, E.S. & Keiser, H.R. Dihydrocaffeic acid: a common contaminant in the liquid chromatographic‐electrochemical measurement of plasma catecholamines in man. J. Chromatogr. 311, 148–153 (1984). [DOI] [PubMed] [Google Scholar]
- 12. Holmes, C. , Eisenhofer, G. & Goldstein, D.S. Improved assay for plasma dihydroxyphenylacetic acid and other catechols using high‐performance liquid chromatography with electrochemical detection. J. Chromatogr. B Biomed. Appl. 653, 131–138 (1994). [DOI] [PubMed] [Google Scholar]
- 13. Goldstein, D.S. , Stull, R. , Eisenhofer, G. & Gill, J.R., Jr. Urinary excretion of dihydroxyphenylalanine and dopamine during alterations of dietary salt intake in humans. Clin Sci. 76, 517–522 (1989). [DOI] [PubMed] [Google Scholar]
- 14. Eisenhofer, G. , et al Simultaneous liquid‐chromatographic determination of 3,4‐dihydroxyphenylglycol, catecholamines, and 3,4‐dihydroxyphenylalanine in plasma, and their responses to inhibition of monoamine oxidase. Clin. Chem. 32, 2030–2033 (1986). [PubMed] [Google Scholar]
- 15. Owen, R.W. , et al Olive‐oil consumption and health: the possible role of antioxidants. Lancet Oncol. 1, 107–112 (2000). [DOI] [PubMed] [Google Scholar]
- 16. Tuck, K.L. & Hayball, P.J. Major phenolic compounds in olive oil: metabolism and health effects. J. Nutr. Biochem. 13, 636–644 (2002). [DOI] [PubMed] [Google Scholar]
- 17. Tank, A.W. & Weiner, H. Ethanol‐induced alteration of dopamine metabolism in rat liver. Biochem. Pharmacol. 28, 3139–3147 (1979). [DOI] [PubMed] [Google Scholar]
- 18. de la Torre, R. , Covas, M.I., Pujadas, M.A., Fito, M. & Farre, M. Is dopamine behind the health benefits of red wine? Eur. J. Nutr. 45, 307–310 (2006). [DOI] [PubMed] [Google Scholar]
- 19. Perez‐Mana, C. , et al Moderate consumption of wine, through both its phenolic compounds and alcohol content, promotes hydroxytyrosol endogenous generation in humans. A randomized controlled trial. Mol. Nutr. Food Res. 59, 1213–1216 (2015). [DOI] [PubMed] [Google Scholar]
- 20. Ludwig, I.A. , Paz de Pena, M. , Concepcion, C. & Alan, C. Catabolism of coffee chlorogenic acids by human colonic microbiota. Biofactors. 39, 623–632 (2013). [DOI] [PubMed] [Google Scholar]
- 21. Goldstein, D.S. , Holmes, C.S. & Kaler, S.G. Relative efficiencies of plasma catechol levels and ratios for neonatal diagnosis of menkes disease. Neurochem. Res. 34 1464–1468 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nurnberger, J.I., Jr. , et al Separate mechanisms for behavioral, cardiovascular, and hormonal responses to dextroamphetamine in man. Psychopharmacology. 84 200–204 (1984). [DOI] [PubMed] [Google Scholar]
- 23. Goldstein, D.S. & Sharabi, Y. Neurogenic orthostatic hypotension: a pathophysiological approach. Circulation. 119, 139–146 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wittstein, I.S. , et al Neurohumoral features of myocardial stunning due to sudden emotional stress. N. Engl. J. Med. 352, 539–548 (2005). [DOI] [PubMed] [Google Scholar]
- 25. Goldstein, D.S. , Holmes, C. & Axelrod, F.B. Plasma catechols in familial dysautonomia: a long‐term follow‐up study. Neurochem Res. 33, 1889–1893 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cohn, J.N. , Levine, B.T. & Olivari, M.T. Plasma norepinephrine as a guide to prognosis in patients with congestive heart failure. N. Engl. J. Med. 311, 819–823. (1984). [DOI] [PubMed] [Google Scholar]
- 27. Ng, S.F. , Tan, L.S. & Buang, F. Transdermal anti‐inflammatory activity of bilayer film containing olive compound hydroxytyrosol: physical assessment, in vivo dermal safety and efficacy study in Freund's adjuvant‐induced arthritic rat model. Drug Dev. Ind. Pharm. 43, 108–119 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Systemic hemodynamics after olive ingestion


