Rare diseases by definition are conditions that affect small numbers of people. The prevalence threshold that designates a disease as rare varies in different countries. Generally, diseases with prevalence fewer than 5 in 10,000 people are considered rare.1 Many rare diseases affect far fewer people worldwide, with some having a single identified case and others with cases numbering from tens to low hundreds of people.2 However, collectively, they are common. There are ∼6,000–8,000 rare diseases, with 250–280 new diseases described annually, affecting an estimated 6–8% of the human population.3, 4, 5, 6 The rarity of these diseases individually creates significant challenges for affected patients, their families, and for clinicians attempting to achieve a confirmed diagnosis and implement best care.7, 8 Obtaining a correct diagnosis is frequently a difficult and lengthy process, as physicians and caregivers often lack appropriate expertise in a disease that they rarely encounter.4 Effective therapeutics are lacking for the large majority of these diseases, and when they exist, they are often very expensive because of scientific and manufacturing challenges, and small potential markets for such products; this creates barriers to access that are frequently difficult to resolve.3
Rare diseases are chronic and often severely disabling, thus treating these patients places a substantial burden on healthcare budgets. For example, one recent study from Western Australia concluded that in 2010 the state population affected by a limited cohort of only 467 rare diseases represented 2% of the population but 10.5% of in‐patient hospital costs.9 Therefore, improved diagnostics and targeted therapeutics that keep these patients healthier and reduce their time in medical facilities would be highly beneficial. Many rare diseases resemble common ones and involve the same genetic pathways, but are generally more aggressive or severe in their presentation. Improved understanding of these diseases may therefore be relevant to improving or developing diagnostics and therapeutics for their more common counterparts.
Most rare diseases are genetic in their etiology, thus research addressing them systematically begins with efforts to identify genetic variants that are causative for particular diseases.1, 5 Such research has been under way for decades and has been successful, with many links between genetic mutations and diseases identified. However, by the mid‐2000s it became increasingly apparent that several factors were limiting success and needed to be addressed systematically on an international scale. Linking a genetic variant with a particular disease requires finding two or, preferably, more unrelated individuals who have a genetic variant in common, as well as exhibiting similar phenotypic characteristics. Identifying such matches can be facilitated by data sharing, but due to at times competitive academic framework and limitations in the consent obtained from research participants, data sharing among research groups working on the same disease was often limited or nonexistent. An additional problem was the lack of a standardized vocabulary to describe phenotypes, further complicating the identification of patients with similar symptoms.10 At the same time, the advent of next‐generation genomic techniques, which dramatically increased throughput and lowered cost, provided the opportunity to drastically accelerate the discovery of gene–disease connections.11 However, with this promise came the additional technical challenges of storing, analyzing, sending, and comparing large sets of data.
The introduction of regulatory and economic incentives (e.g., market exclusivity, fee waivers, protocol assistance) was also successful in attracting the biopharmaceutical industry, which was previously reluctant to invest in rare disease therapeutic development with little or limited return on investment.5 Prior to the introduction of the Orphan Drug Act in the US in 1983, only 10 orphan drugs were approved in a decade, compared with 247 drugs for over 200 rare diseases in the course of the 25 years postlegislation.12 In Europe, following the adoption of the European Commission (EC) Regulation 141/2000, 63 orphan medicinal products received marketing authorization by 2010, a decade after implementation.13 While a large increase in the number of orphan designations was seen on both sides of the Atlantic, the average combined number of orphan‐designated products approved was about 15 per year and they addressed only a handful of rare diseases. By 2010, ∼400 orphan medicinal products were available for less than 300 rare diseases. Additionally, observations by the US Food and Drug Administration (FDA) of a slowdown instead of the expected acceleration in innovative medical therapies reaching patients were also reported in the mid‐2000s.14 Attrition rate aside, therapy development for rare diseases faces a number of specific challenges, including small populations for clinical studies, difficulty in determining relevant outcome measures and endpoints, and poorly understood natural histories.
The heterogeneous pathophysiology and dispersed nature of rare diseases means research and development efforts and patient populations are scattered around the world. A scarcity of expertise poses a huge challenge to patients who seek access to diagnostic testing and appropriate treatment. In this overall setting, following initial discussions between Dr Ruxandra Draghia‐Akli of the Directorate‐General for Research and Innovation (DG RTD) of the European Commission (EC) and Dr Francis Collins of the US National Institutes of Health (NIH), the International Rare Diseases Research Consortium (IRDiRC) was formed.15 A consortium that unites public and private sector funders of research, patient advocacy groups, and scientific researchers, IRDiRC is committed to a global partnership to advance rare diseases research. This is delivered by attracting further resources to the domain and facilitating better international collaboration and coordination among research groups. In 2011, at its inauguration, IRDiRC set two ambitious goals for the rare diseases research collective—to develop 200 new therapies and the means to diagnose most rare genetic diseases by the year 2020. Six years after its official launch, IRDiRC is reflecting on its progress and achievements toward these goals, and considers how best to move forward even more ambitiously in the coming years. In this context, we review the history and the key success factors, as they provide the best markers for our future success and overcoming the apparently insurmountable issues ahead for people living with rare diseases, rare diseases stakeholders, industry, and policy makers.
HISTORY
The EC's DG RTD and the US NIH met in 2009 to discuss the need for expanded and further integrated efforts to address the global imperative for governments to collect public health data and provide solutions for patients affected with rare diseases. A preparatory workshop held in Reykjavík, Iceland, in October 2010 led to the creation of IRDiRC.15 Formally launched in 2011 to foster international research collaboration and investment in the field, IRDiRC had two aspirational goals: i) to contribute to the development of 200 new therapies, and ii) to develop the means to diagnose most rare diseases by the year 2020.
At its launch, five public research funders committed to the IRDiRC mission. By the end of 2011, IRDiRC had expanded to 18 public funding members in addition to three international umbrella patient groups. The first private funders, i.e., companies, joined the effort in 2012. The Consortium has since expanded globally to include nearly 50 members across Europe, North America, Asia, Australia, and the Middle East (Figure 1), each—excepting the umbrella patient groups—committed to invest a minimum of USD 10 million in rare diseases research funding over 5 years.16 Through this global reach, with the cooperation of its public and private funders and patient groups, IRDiRC began to develop common scientific and policy frameworks to guide research and development in rare diseases under the leadership of Dr Ruxandra Draghia‐Akli (Chair, 2011–2012), Dr Paul Lasko (Chair, 2013–2015), and Dr Christopher Austin (Chair, 2016–present). The work of IRDiRC and its committees is critical for the development of new rare diseases knowledge, which is in turn vital for governments to develop informed, collaborative, and evidence‐based policy, and for academics and industry to be guided in the development of new diagnostics and therapies for rare diseases.
Figure 1.
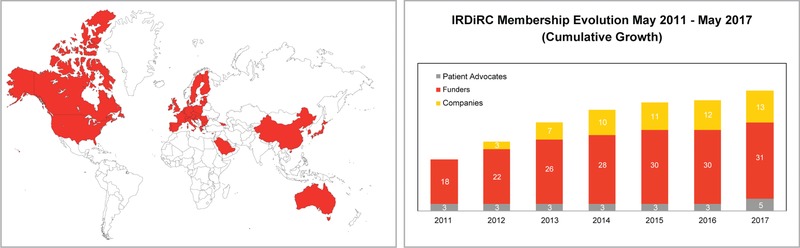
IRDiRC member institutions are found across Europe, North America, Middle East, Asia, and Australia. Its membership has grown steadily over the years, with participation from research funders, companies, and patient advocates.
While IRDiRC has a wide range of members, it is recognized that expertise in rare diseases, just as patients, is distributed around the world. In order to engage with international stakeholders in rare diseases research inside and outside of the Consortium, to assess the progress on the IRDiRC objectives, and to share acquired knowledge and competences, three international IRDiRC Conferences have been organized, to date. These conferences, held in Dublin, Ireland; Shenzhen, China; and Paris, France, respectively, advanced discussions on rare diseases research, and emphasized the utmost importance of collaborations among the global rare disease community to progress and success.
GOVERNANCE OF IRDiRC
IRDiRC is currently governed through a Consortium Assembly (formerly Executive Committee), three Scientific Committees, three Constituent Committees, an Operating Committee, ad hoc Task Forces, and a Scientific Secretariat (Figure 2). The Consortium Assembly is composed of one representative per IRDiRC member, and the Chairs and Vice Chairs of each of the three Scientific Committees. The Consortium Assembly acts as the primary forum to exchange information on issues that influence IRDiRC goals and activities, coordinates scientific and policy efforts in addressing research priorities to advance IRDiRC goals, adopts policies and guidelines, and provides strategic vision to foster rare diseases research. IRDiRC members of the Consortium Assembly further subdivide into three Constituent Committees, one each for Funders, Companies, and Patient Advocates, which each identify common priorities, roadblocks, and gaps across their respective constituent space, and coordinate activities to address those needs. The three Scientific Committees, one each for Diagnostics, Interdisciplinary, and Therapies, advise the Consortium Assembly on research priorities and progress made from a scientific viewpoint, identify bottlenecks and funding gaps in rare diseases research, and agree on actions in their scientific areas that will bring IRDiRC closer to its goals. The Operating Committee—consisting of the Chairs and Vice Chairs of the Consortium Assembly, the Constituent Committees and the Scientific Committees, and the Scientific Secretariat—is the key working committee that monitors and manages progress of IRDiRC activities and processes, provides information to the Consortium Assembly, and enables a more effective management of the Consortium. In order to push policy changes forward and provide technical solutions to specific, prioritized topics, ad hoc Task Forces—each consisting of topically relevant experts—are proposed and set up by the Scientific and Constituent Committees. The organizational, secretarial, reporting, and communications support to IRDiRC and its various members are provided by the Scientific Secretariat. Together, the Consortium contributes to the acceleration of rare diseases research, diagnostics and therapeutics development, reinforces international research cooperation and coordination, and maximizes the impact of investments made in the field of rare diseases research, all in order to bring diagnoses and therapies to rare disease patients throughout the world.
Figure 2.
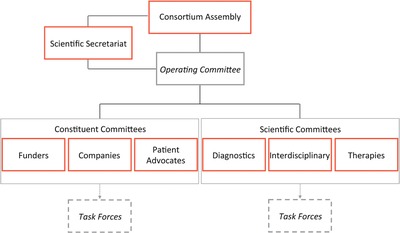
IRDiRC is composed of a Consortium Assembly (formerly Executive Committee), an Operating Committee, three Constituent Committees, three Scientific Committees, a number of Task Forces, and a Scientific Secretariat.
OBJECTIVES AND PROGRESS ON OBJECTIVES 2011–2020, NOW 2017
To meet IRDiRC's goals, collaborative actions and overarching policies are needed to address the challenges encountered as well as streamlining access to relevant data and patient populations.
Funding
Research in rare diseases is urgently needed, as many rare disease patients still lack an accurate and confirmed diagnosis and appropriate treatment. Funding of rare diseases research should not be carried out in isolation; appropriate coordinated effort through a network of funders will ensure that the impact of research investment is maximized through deduplication of research projects, generation of knowledge in lesser studied diseases, creation of common frameworks and infrastructures to facilitate data and patient sample sharing, and translation of basic research into preclinical and clinical studies. Collaborative funding mechanisms also enable the financing of large, transnational projects that require resources beyond what can be provided by individual, national research programs.
The IRDiRC Scientific Secretariat, in collaboration with Orphanet,17 collects information pertaining to the projects funded by IRDiRC members. Data from IRDiRC public research funding members (excluding data from the NIH National Cancer Institute, from which data collection is ongoing) illustrate the financing of over 3,000 projects since 2010. A majority of them are basic and preclinical research projects, accounting for about 87% of funded projects, while clinical research (including observational studies and clinical trials) represents ∼8% of the total number of awarded projects, and epidemiological, health economics and social sciences studies account for 4% of funded research (Figure 3). Collectively, the basic and preclinical research projects covered nearly 1,200 rare diseases, while the clinical trials covered ∼220 rare diseases. The former mainly focused on rare neurological diseases (including neuromuscular diseases) and developmental disorders (including conditions associated with dysmorphology and congenital anomalies), whereas clinical trials were mainly oriented towards rare cancers and rare neurological diseases (Figures 4, 5). The prevalence and intersection of these disorder groupings in diagnostic and therapeutic domains reflect the biological and clinical overlaps between developmental disorders and cancer.18 This also reflects the reciprocal opportunities between and within rare syndromic diseases, rare cancers, and their intersections for insights into new therapies and for drug repurposing.19 Drug clinical trials constitute the overwhelming majority of trials (Figure 6), with more than half of them in earlier phases of development (phases I to II), 31% in phase III and 5% in phase IV (Figure 7). Approximately half of all clinical trials were funded by companies (48%), the others supported by public institutions. These data serve as the foundation for much of the new work of the Constituent Committees aimed at identifying areas of need, prioritizing, and addressing those gaps via coordinated implementation activities,20 while taking into consideration the promising21 bedside‐to‐bench translational approach to increase the likelihood of success in drug development and clinical translation.
Figure 3.
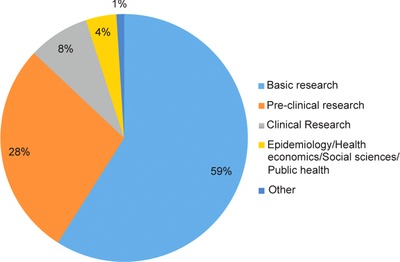
Distribution of funded projects and trials by type of research.
Figure 4.
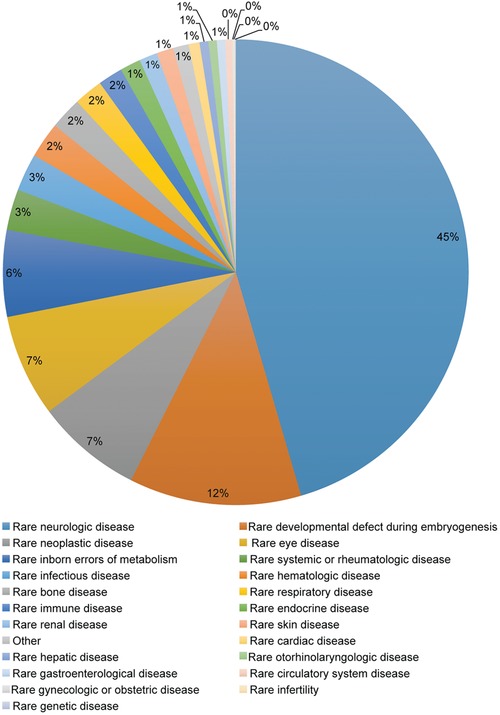
Distribution of research projects by medical domain.
Figure 5.
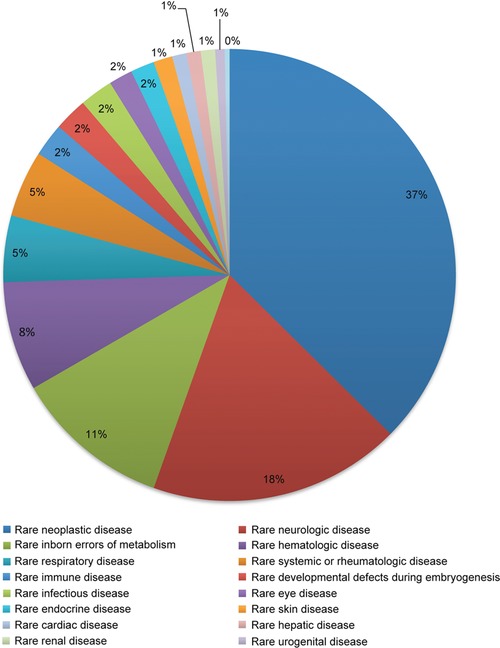
Distribution of clinical trials by medical domain.
Figure 6.
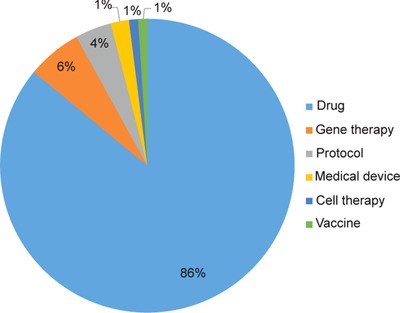
Distribution of clinical trials by modality.
Figure 7.
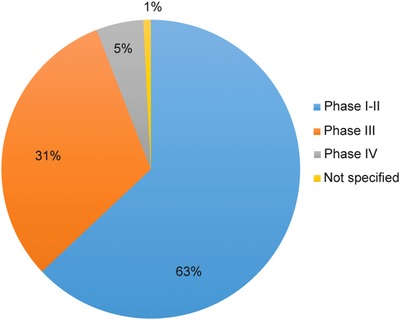
Distribution of drug clinical trials by phase.
Data received from public funders also demonstrated strong research investments and increasing number of collaborative calls on rare diseases, a sign of global commitment toward advancing rare diseases research and thus a commitment to bring diagnoses and treatments to rare disease patients. The EC, an IRDiRC cofounder, through its Framework Programme 7 (2007–2013) and Horizon 2020 Programme (2014–2020), invested around €762M in 135 rare diseases projects between 2010 and 2015,22 and expect to finance more projects in the coming years. The US NIH, another IRDiRC cofounder, has invested around US$10B between 2011 and 2016 via seven institutes and centers that are member organizations of IRDiRC, and the National Center for Advancing Translational Sciences’ Office of Rare Diseases Research (ORDR) alone has invested over US$100M in around 180 rare diseases projects between 2010 and 2015. E‐Rare, a joint transnational funding network, grew from 15 funding partners in 2010 to 26 funding partners in 2017.23 Between 2011 and 2016, E‐Rare funded 77 projects with a budget of €67.5M;24 the largest research funding call took place in 2015 when 19 projects were financed through an investment of €19.2M. Funders at the national level play an equally important role in financing rare diseases research. As an example, in Spain, the National Institute of Health Carlos III spent a total of €43.4M in the period of 2010–2016 to finance around 45–60 projects each year, while additionally earmarking an average of €5M per year to support the Centre of Biomedical Research Network for Rare Diseases (CIBERER). The Japan Agency for Medical Research and Development (AMED), established in April 2015, set up a dedicated Division of Rare/Intractable Disease Research. In 2 years, AMED has disbursed ¥20B (€153M/US$176M) and funded over 350 research projects. The Canadian Institutes of Health Research's (CIHR) Institute of Genetics has invested over CA$110M between 2011 and 2016. In recent years, patient organizations have also been making impressive investments in rare diseases research in order to develop diagnostic tools and therapeutics for their patients. Telethon Italia, for example, funded 337 extramural research projects in 2010–2016, as well as other intramural research activities and support initiatives, for a total budget close to €235M.
A comprehensive data collection and analysis of rare diseases research funding, including the number and types of projects, and number and categories of rare diseases studied, for 2010–2016, is currently under way; the report is expected to be published in late 2017 to complement the State of Art of Research in Rare Diseases report.
Progress in Diagnostics and Therapeutics
Advances in diagnostics and therapeutics in the rare disease space over the past 6 years are attributed to a combination of factors and many players. IRDiRC monitors a number of metrics to quantify the progress made towards its key objectives.
Progress in the diagnostics field relies on the identification of rare diseases and their underlying cause, often genetic in origin, and the development and availability of associated analytical tests. In this setting, the IRDiRC Diagnostics and Interdisciplinary Scientific Committees, in collaboration with Orphanet, analyzed the number of diseases for which a clinical test is available; at present, a genetic test is available for around 3,600 rare diseases, compared with 2,200 in 2010. Based on data curated by Orphanet and Online Mendelian Inheritance in Man (OMIM), an average of about 260–280 rare genetic diseases are discovered per year.4 In recent years, the majority of novel genes were discovered through exome sequencing approaches, with decreased use of previous discovery methods such as positional cloning. However, the discovery rate of exome sequencing over the past couple of years appears to be declining—this may be attributed to the fact that most of the simpler, monogenic rare diseases that are tractable to current techniques have been discovered, leaving the more complex cases to be solved. Nonetheless, in the last 5 years diagnostic efficiency has increased from ∼10% to 30–50%, a considerable step forward for rare disease patients.25 Yet, a number of bottlenecks remain to be tackled to continue the pace of discoveries and classification of disease‐related genes and variants.
In the past two decades, several countries have introduced policies dedicated to orphan drug development and others are currently following suit with specific policies for their jurisdictions.26 To better grasp the number of therapies available for rare disease patients, IRDiRC tracks the cumulative number of approvals of medicinal products with a marketing authorization and orphan designation since 2010 via data from the European Medicines Agency (EMA) and the FDA. An increase has been observed in the number of drug approvals that reach the market in both Europe and the United States, and more are expected to come, as the number of orphan designations has steadily risen, as well. Between 2010 and 2016, over 220 new drugs have reached the market, covering over 170 diseases. The observed rise in the number of orphan drug designations holds a tremendous promise for the future of rare disease drug development, and ultimately for rare disease patients. With the addition of new members, and in particular Japan in 2015, IRDiRC aims to capture data from specific geographic‐regulatory regions. Thus, from 2017 onward, IRDiRC will be tracking approvals by the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan and more globally as other significant regulatory jurisdictions are added to the Consortium.
IRDiRC Policies and Guidelines
In order to i) improve collaborations in rare diseases research, ii) increase the involvement of patients and their representatives in all relevant aspects of research, and iii) improve the sharing of data and resources, IRDiRC has developed a set of principles that address such common roadblocks. The “IRDiRC Policies and Guidelines” were developed by the Scientific Committees to serve as recommendations for research funders and researchers to provide guidance on key topics that include ontologies, diagnostics, biomarkers, patient registries, biobanks, natural history studies, therapeutics, models, publications, intellectual property, and communication.27 These policies and guidelines have been adopted by the IRDiRC members themselves through their own intramural rare diseases research and extramural rare diseases research funding programs, and provide guidelines for the entire rare diseases research community.27, 28
Addressing Barriers and Bottlenecks
Barriers and bottlenecks in diagnostic and therapeutic research prolong the odyssey faced by rare diseases patients. The Constituent and Scientific Committees identify and propose actionable topics for Task Forces to tackle, and issue recommendations to drive policy and systemic changes.
Obtaining a molecularly‐confirmed diagnosis for rare disease patients is indispensable, in order to assure optimal disease management, lessen complications of the disease, advance genetic counseling, and provide important psychosocial benefits to patients and their families. At present, the causative gene is still unknown for approximately half of all rare diseases and only 15% of rare genetic diseases have an easily available single‐gene diagnostic test in the 40 countries of the Orphanet consortium.29, 30 The Diagnostic and Interdisciplinary Scientific Committees investigated the issues surrounding gene discovery and diagnostics of rare genetic diseases, and developed areas and strategies for improvement, including: the use of ontologies for exchanging clinical data; the development of interoperable tools and resources to facilitate genomic and phenotypic data ascertainment and analyses; the development of best and ethical practices in data discovery and sharing; the use of genetic and functional evidence for gene discovery; and the increased investigation of novel disease mechanisms.4 Every successful gene discovery will not only unlock a potential diagnostic opportunity, but also has the potential to contribute to preventive and therapeutic opportunities for the corresponding rare disease, enabling precision medicine approaches for this patient population. Therefore, such genetic linkages to rare diseases are extremely important and have incredible potential for rare disease patients. A further consideration is that equitable diagnostic, preventative, therapeutic innovation, and global scope also require the generation, detailed understanding, and implementation of normative genomic and phenotypic data, especially for those currently underrepresented in such efforts, including across Europe, Asia, the Americas, and in Australia—Aboriginal and Torres Strait Islanders.31, 32 It is likely that this new knowledge will also unlock insights for currently more well‐studied populations.
Therapeutic development is a time‐ and resource‐intense process, and often rare disease patients do not have the luxury to wait nor the means to access therapies, if available. The Therapies Scientific Committee explored the steps along the drug development process, from target identification to drug approval by the regulatory agencies, and developed a set of recommendations that further foster therapeutic development of orphan medicinal products aligned with scientific and regulatory guidance.33 Three key points were emphasized as important to speeding up the process: soliciting protocol assistance from regulators at every stage of product development; enhancing early and continuous dialog with all stakeholders to ensure patients’ needs are addressed; and collaborating with health technology assessment bodies and payers to ensure that therapies brought to market are accessible to patients. The Therapies Scientific Committee is also cognizant of the need to harmonize best practices among the major regulatory agencies in order to bring about a transformational boost in the development of new rare disease therapies. Efforts by regulators to align systems and practices are ongoing and IRDiRC aims to enhance and support such cooperative and collaborative actions between researchers and regulators.19
Task Forces
In order to tackle specific topics of rare diseases research, and to further contribute to the IRDiRC objectives, topic‐specific time‐limited Task Forces are established. Each Task Force reviews current impediments to efficient and effective research and proposes solutions through policy recommendations and/or technical applications including platforms, tools, and standards. These Task Forces may operate solely as IRDiRC initiatives, or jointly with partner groups that wish to collaborate and address similar issues. They are also comprised mostly of external expert members to ensure that different perspectives are cultivated to drive innovation and new approaches.
Matchmaker Exchange, a federated network of genotype and phenotype databases which allows for rare disease gene discovery,34 is a collaborative action between IRDiRC, the Global Alliance for Genomics and Health (GA4GH),35 and other organizations. IRDiRC also partners with GA4GH on the Automatable Discovery and Access Task Force to develop a standardized way to represent consent and other conditions of clinical data use, making the information computer‐readable and available for automated search and sharing activities36; and on the Privacy‐Preserving Record Linkage Task Force, which was set up to develop guidelines on the ethical, legal, and technical requirements of participant identifiers in rare diseases research, and investigate a technical solution for deduplication of research participants in data sets without knowledge of their identities.37 Other IRDiRC Task Forces include: i) the International Consortium of Human Phenotype Terminologies, which provided the community with standards to achieve interoperability between databases by enabling the linkage of phenotype and genotype databases for rare diseases38; ii) the Patient‐Centered Outcome Measures Task Force, which made recommendations on methods to support the development of patient‐relevant outcome measures for rare diseases in order to improve the quality of future trials and to provide data of relevance to the patient community39; iii) the Small Population Clinical Trials Task Force, which produced recommendations for efficient and innovative clinical trial designs relevant to small populations, often the focus of rare disease clinical trials, using scientific advice from regulators40; and iv) the Data Mining and Repurposing Task Force, which identified opportunities to leverage the existing research and patient data to realize the full potential of data mining and drug repurposing.41 As new issues and areas of improvement are identified, Task Forces are set up, aiming to further contribute to diagnostic, therapeutic, and interdisciplinary development, and optimize the global rare diseases research enterprise.
“IRDiRC Recognized Resources”
Just like rare disease patients, the researchers who study rare diseases are often few and geographically spread. As such, resources and tools available to further their work are not always known to them. To address this need, IRDiRC set up an indicator called “IRDiRC Recognized Resources” to highlight publicly available resources that researchers in the rare diseases community have found useful and, if were to be used more broadly, may accelerate the pace of rare diseases research.42 To receive this designation, resources are evaluated via a peer‐review process by IRDiRC Scientific Committee members and experts in the field; applications are accepted on a rolling basis. To date, 17 resources have received the label: five guidelines, five platforms, three reference databases, two standards, and an advisory committee.
Partner Initiatives
IRDiRC successfully collaborates with several partner initiatives to develop global policies and recommendations, build technical solutions for specific rare disease issues, or plan joint projects and events. These initiatives include, but are not limited to, Orphanet,17 GA4GH,35 the Global Genomic Medicine Collaborative (G2MC),43 RD‐Connect,44 TREAT‐NMD Alliance,45 RARE Bestpractices,46 RD‐Action,47 the European Reference Networks (ERNs),48 the US Office of Rare Diseases Research (ORDR) with its Rare Diseases Clinical Research Network (RDCRN) at the NIH's National Center for Advancing Translational Sciences,49 and the Undiagnosed Diseases Network International (UDNI).50
Through these collaborative efforts, there have been marked improvements in disease classification and coding, with a growing acceptance of standard nomenclature and development of policies for ethical and secure data sharing for rare and genetic diseases.51, 52, 53, 54 This is being complemented by significant developments in genomics knowledge and technologies, which are driving faster and more accurate diagnoses25, 55 and the development of specific treatments, labeled as “precision medicine” or “personalized medicine.” While the benefits to individuals of such targeted approaches are clear, the same knowledge and technologies are providing opportunities to better understand and assess the impact of disease at a population level. In line with this, an emerging precision public health paradigm is leading to the development of policies and programs targeted to at‐risk groups, in order to improve the overall health of the population.56 The use of genomics in such public health approaches is key to driving improvements in healthcare delivery for people living with rare diseases.
The catalytic drive for this research is patients living with rare diseases and their families. They are the single most transformative force of the rare diseases sector, in areas that include, for example, research and clinical networks, new approaches to therapies and clinical trials, gene and disease pathway discoveries that unlock new knowledge, data sharing and creation of matchmaking platforms, and the translation of this new knowledge into the public health setting for the benefit of all. In partnerships around the globe, patients and patient organizations have joined with governments, industry, academia, regulators, healthcare professionals, and philanthropic organizations to speak with one voice. People living with rare diseases and their families seek a diagnosis that will enable doctors and other professionals to provide the best care in their setting, and improve their journey.
To achieve these outcomes and the equitable care citizens expect of their health system, many rare disease patients willingly make public the details of their lives and the lives of their loved ones. This, in and of itself, might seem to be a higher price to pay than others with a smaller health burden are expected to pay. However, the reality is that the rare diseases community has increasingly become self‐organized through social media networks and patient organizations, many of which are international. A number of organizations are truly global and provide voice to more than 350 million people living with rare diseases worldwide. In this article, the authors are not able to do justice to the phenomenal drive and empowerment derived from the national and international patient organizations and networks. As an example, two recent global developments, the launch of the Rare Diseases International (RDI) and the inauguration of the newly formed nongovernmental organization (NGO) Committee for Rare Diseases, established under the umbrella of the Conference of NGOs with Consultative Status to the United Nations Economic and Social Council (CoNGO), serve as a testament to the escalation in the international networks of patient organizations, and also to the many decades of accumulated and expanding influence of rare diseases patients and their families in driving change.57, 58
Interaction with External Stakeholders
A major tenet of IRDiRC is the promotion of collaboration, and technology and knowledge sharing. To this end, interactions with external stakeholders are of utmost importance to achieving the IRDiRC objectives. In addition to its many partnered initiatives to develop global policies, IRDiRC recruits expert nonmembers to populate its Scientific Committees and Task Forces. For its Scientific Committees, IRDiRC members identify appropriate expert opinion leaders to ensure diverse geographical representation and breadth and depth of scientific expertise. IRDiRC's expertise, knowledge base, and scientific networks are further broadened by the Task Forces, which are assembled with a very specific purpose, with clear time‐delimited aims and milestones. Through this process IRDiRC members and Scientific Committee members engage and involve domain and subject matter experts throughout the world to achieve maximally informed and impactful results. For example, several Task Forces of the IRDiRC Diagnostics Scientific Committee have partnered with the GA4GH (see section above for details about the Task Forces). IRDiRC members give presentations on IRDiRC in a wide range of international settings, while also reaching out to other organizations, networks, and individuals to join the consortium and contribute their insights to achieving the IRDiRC goals. To advance IRDiRC's impact and reduce unintended duplication, the Scientific Committees and Task Forces regularly publish open‐access articles on their work, complemented by recommendations and reports published on the IRDiRC website and promoted via social media. These dissemination efforts are crucial not only to the translation of new knowledge and insights across the scientific and medical communities, but also to the provision of relevant and authoritative information on the state of the rare disease field to our most valuable partners, the people and families living with rare diseases.
CONCLUSION
The past 6 years have been remarkable for the rare diseases research community and for rare disease patients. Tremendous progress has been made toward improved diagnostics and patient care. Major public‐sector research initiatives focused in this area have emerged in many countries, and most notably in the US NIH, the EC, and the AMED. However, the further transformative aspect has been the engagement and collaboration between these public funders, industry, and the people living with rare diseases. This being said, substantial challenges remain. Next‐generation genomics and improved data sharing have resulted in faster discovery of causative genes and consequent development of new diagnostics, although there are signs that the rate of disease gene discovery is now slowing as the remaining unsolved diseases are likely more complex. New approaches, including those better interrogating the nonprotein‐coding majority of the genome will need to be developed to address this next step. The rate of rare disease therapeutic development is increasing and there is substantial cause for optimism with regard to innovative therapies. However, the translational rate of these developments to marketing approvals is still relatively low and it remains the case that over 90% of rare diseases lack a specific treatment. Today, it has been estimated that only 6% of rare diseases currently have available treatment, of which less than 1% are curative, leaving a large majority of patients still awaiting a therapy.59, 60 Parallel to the development of innovative therapies, the potential provided by data‐mining and repurposing approaches has not been fully realized. Serious inequities also exist with respect to patient access for those diseases for which an effective treatment is available. In addition, it is not clear that the present model for recovering drug development costs from market sales can be extrapolated to rare diseases with worldwide patient populations of hundreds or less. Novel approaches to therapeutic discovery, as well as new models both for funding drug discovery and for covering treatment costs, are likely to be necessary for rare diseases to be treated in a comprehensive manner. Informed by the phenomenal progress enabled by the Scientific Committees, the Consortium has recently instituted three sector‐specific Constituent Committees (Funders, Companies, and Patient Advocacy Groups), which are identifying areas of common need and opportunity within their sector, and prioritizing and addressing solutions via coordinated implementation activities.20
IRDiRC has been a major positive factor in raising public awareness of rare diseases, the need for more research to address them, and of collaborative tools that allow ethical data sharing for and with patients. It has clearly led to increased investment of public sector research funds for rare diseases. IRDiRC has helped to catalyze several important initiatives that are improving collaboration among researchers and enhancing the ability of patients to engage as constructive partners in research. While considerable headway has been made, there is a need to accelerate progress, building on the willingness and impetus to collaborate across borders, to ensure that IRDiRC's new goals for the rare diseases community for the next 10 years will not only be achieved, but surpassed.
This time next year,‐ this time ten years,‐ this time one hundred years……I cannot doubt but that these things, which now seem to us so mysterious, will be no mysteries at all; that the scales will fall from our eyes; that we shall learn to look on things in a different way ‐ when that which is now a difficulty will be the only common sense and intelligible way of looking at the subject.
—Sir William Thomson, Baron Kelvin of Largs, 1889
Conflict of Interest
The authors declare no conflicts of interest.
Supporting information
Supporting Material
Acknowledgments
The authors thank the present and former representatives of the IRDiRC Consortium Assembly, and present and former members of the IRDiRC Scientific Committees, Working Groups, Task Forces and Scientific Secretariat for their contribution to this collaborative effort (see Supplementary Document 1). The IRDiRC Scientific Secretariat is supported by the European Commission FP7 contract, “SUPPORT‐IRDiRC” (No 305207) and HL is supported by the European Commission FP7 contract, “RD‐Connect” (No 305444). CPA has contributed to this work in his capacity as Chair of the IRDiRC Consortium Assembly, not in his role as director of the National Center for Advancing Translational Sciences.
Disclaimer
The views expressed in this publication are the sole responsibility of the authors and do not necessarily reflect the views of their affiliated organizations. Neither the European Commission nor any person acting on behalf of the Commission is responsible for the use which might be made of the following information. CPA contributed in his capacity as Chair of the IRDiRC Consortium Assembly, not in his role as the Director of the National Center for Advancing Translational Sciences.
References
- 1. Stolk, P. , Willemen, M.J. & Leufkens, H.G. Rare essentials: drugs for rare diseases as essential medicines. Bull. World Health Organ. 84, 745–751 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prevalence and Incidence of Rare Diseases: Bibliographic Data, in Orphanet Report Series, Rare Diseases Collection (eds. Rath, A. & Nguengang‐Wakap, S.). http://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_alphabetical_list.pdf (2016) Accessed: 29 May 2017.
- 3. Tambuyzer, E. Rare diseases, orphan drugs and their regulation: questions and misconceptions. Nat. Rev. Drug Discov. 9, 921–929 (2010). [DOI] [PubMed] [Google Scholar]
- 4. Boycott, K.M. et al International cooperation to enable the diagnosis of all rare genetic diseases. Am. J. Hum. Genet. 100, 695–705 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Melnikova, I. Rare diseases and orphan drugs. Nat. Rev. Drug Discov. 11, 267–268 (2012). [DOI] [PubMed] [Google Scholar]
- 6. List of Rare Diseases by Orphanet . http://www.orpha.net/orphacom/cahiers/docs/GB/List_of_rare_diseases_in_alphabetical_order.pdf Accessed 21 July 2017.
- 7. Molster, C. et al Survey of healthcare experiences of Australian adults living with rare diseases. Orphanet J. Rare Dis. 11, 30 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. EURORDIS , The Voice of 12,000 Patients: Experiences and Expectations of Rare Disease Patients on Diagnosis and Care in Europe . (2009) EURORDIS‐Rare Diseases Europe.
- 9. Walker, C.E. et al The collective impact of rare diseases in Western Australia: an estimate using a population‐based cohort. Genet. Med. 19, 546–552 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schieppati, A. et al Why rare diseases are an important medical and social issue. Lancet 371, 2039–2041 (2008). [DOI] [PubMed] [Google Scholar]
- 11. Tweardy, D.J. & Belmont, J.W. “Personalizing” academic medicine: opportunities and challenges in implementing genomic profiling. Transl. Res. 154, 288–294 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Braun, M.M. et al Emergence of orphan drugs in the United States: a quantitative assessment of the first 25 years. Nat. Rev. Drug Discov. 9, 519–522 (2010). [DOI] [PubMed] [Google Scholar]
- 13. Committee for Orphan Medicinal, P . et al European regulation on orphan medicinal products: 10 years of experience and future perspectives. Nat. Rev. Drug Discov. 10, 341–349 (2011). [DOI] [PubMed] [Google Scholar]
- 14. Innovation or Stagnation: Challenge and Opportunity on the Critical Path to New Medical Products . https://www.fda.gov/ScienceResearch/SpecialTopics/CriticalPathInitiative/CriticalPathOpportunitiesReports/ucm077262.htm (2004) Accessed: 29 May 2017.
- 15. Abbott, A. Rare‐disease project has global ambitions. Nature 472, 17 (2011). [DOI] [PubMed] [Google Scholar]
- 16. IRDiRC Members. Available from: http://www.irdirc.org/about-us/members/ Accessed: 29 May 2017.
- 17. Orphanet. Available from: http://www.orpha.net/ Accessed: 29 May 2017.
- 18. Bellacosa, A. Developmental disease and cancer: biological and clinical overlaps. Am. J. Med. Genet. A. 161A, 2788–2796 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baynam, G. et al A germline MTOR mutation in Aboriginal Australian siblings with intellectual disability, dysmorphism, macroencephaly and small thoraces. Am. J. Med. Genet. A 167, 1659–1667 (2015). [DOI] [PubMed] [Google Scholar]
- 20. Austin, C.P. et al Future of rare diseases research 2017–2027: an IRDiRC perspective. Clin. Trans. Sci. 11, 21–27 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wagner J.A. & Kroetz D.L. Transforming translation: Impact of Clinical and Translational Science . Clin. Transl. Sci. 9:3–5 (2016). https://doi.org.10.1111/cts.12380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. List of research projects funded by the European Commission. Available from: http://www.irdirc.org/member-funded-research/research-projects/european-commission-european-union/ Accessed 21 July 2017.
- 23. Julkowska, D. et al The importance of international collaboration for rare diseases research‐a European perspective. Gene Ther. (2017) [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. List of research projects funded by the E‐Rare Consortium. Available from: http://www.irdirc.org/member-funded-research/research-projects/e-rare-european-union/ Accessed 21 July 2017.
- 25. Baynam, G. et al The rare and undiagnosed diseases diagnostic service — application of massively parallel sequencing in a state‐wide clinical service. Orphanet J. Rare Dis. 11, 77 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mariz, S. et al Worldwide collaboration for orphan drug designation. Nat. Rev. Drug Discov. 15, 440–441 (2016). [DOI] [PubMed] [Google Scholar]
- 27. IRDiRC Policies and Guidelines . Available from: http://www.irdirc.org/reports-guidelines/policies-guidelines/ (2013) Accessed: 29 May 2017.
- 28. Lochmuller, H. et al The International Rare Diseases Research Consortium: Policies and Guidelines to Maximize Impact (2017) (submitted for publication). [DOI] [PMC free article] [PubMed]
- 29. Orphanet — Diagnostic Test. Available from: http://www.orpha.net/consor/cgi-bin/ClinicalLabs_Search.php?lng=EN Accessed 30 June 2017.
- 30. Orphadata: Free Access Data From Orphanet© Inserm 1997. Available from: http://www.orphadata.org Accessed 30 June 2017.
- 31. Baynam, G. The need for genetic studies of Indegenous Australians. Med. J. Aust. 196, 313 (2012). [DOI] [PubMed] [Google Scholar]
- 32. Hansen, M. et al Prevalence of microencephaly in an Australian population‐based birth defects register, 1980–2015. Med. J. Aust. 206, 351–356 (2017). [DOI] [PubMed] [Google Scholar]
- 33. Recommendations of the IRDiRC Therapies Scientific Committee to implement IRDiRC Policy 2013 and aim of new 200 therapies by 2020 . Available from: http://www.irdirc.org/reports-guidelines/policies-guidelines/tsc-recommendations/ Accessed: 29 May 2017.
- 34. Philippakis, A.A. et al The Matchmaker Exchange: a platform for rare disease gene discovery. Hum. Mutat. 36, 915–921 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Global Alliance for, G. and Health , GENOMICS. A federated ecosystem for sharing genomic, clinical data. Science 352, 1278–1280 (2016). [DOI] [PubMed] [Google Scholar]
- 36. Automatable Discovery and Access Matrix . Available from: https://genomicsandhealth.org/work-products-demonstration-projects/automatable-discovery-and-access-matrix Accessed: 29 May 2017.
- 37. Privacy‐Preserving Record Linkage . Available from: http://www.irdirc.org/activities/current-activities/privacy-preserving-record-linkage/ Accessed: 29 May 2017.
- 38. International Consortium of Human Phenotype Terminologies . Available from: http://www.irdirc.org/activities/current-activities/ichpt/ Accessed: 29 May 2017.
- 39. Patient‐Centered Outcome Measures in the Field of Rare Diseases (eds. Sabino, G. et al) http://www.irdirc.org/activities/current-activities/tf-pcom/ (2016) Accessed: 29 May 2017.
- 40. Small Population Clinical Trials: Challenges in the Field of Rare Diseases (eds. Jonker, A.H. et al). http://www.irdirc.org/activities/current-activities/tf-spct/ (2016) Accessed: 29 May 2017.
- 41. Data Mining and Repurposing . Available from: http://www.irdirc.org/activities/current-activities/data-mining-repurposing/ Accessed: 29 May 2017.
- 42. Lochmuller, H. et al ‘IRDiRC Recognized Resources’: a new mechanism to support scientists to conduct efficient, high‐quality research for rare diseases. Eur. J. Hum. Genet. 25, 162–165 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Global Genomic Medicine Collaborative (G2MC) . Available from: http://www.nationalacademies.org/hmd/Activities/Research/GenomicBasedResearch/Innovation-Collaboratives/Global_Genomic_Medicine_Collaborative.aspx Accessed: 29 May 2017.
- 44. Thompson, R. et al RD‐Connect: an integrated platform connecting databases, registries, biobanks and clinical bioinformatics for rare disease research. J. Gen. Intern. Med. 29 (Suppl 3), S780‐7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bushby, K. et al Collaborating to bring new therapies to the patient–the TREAT‐NMD model. Acta Myol. 28, 12–15 (2009). [PMC free article] [PubMed] [Google Scholar]
- 46. Taruscio, D. et al RARE‐Bestpractices: a platform for sharing best practice for the management of rare diseases. Rare Dis. Orphan Drugs 1, 5–8 (2014). [Google Scholar]
- 47. RD‐Action . Available from: http://www.rd-action.eu Accessed: 29 May 2017.
- 48. European Reference Networks . Available from: http://ec.europa.eu/health/ern/ Accessed: 29 May 2017.
- 49. Krischer, J.P. et al The Rare Diseases Clinical Research Network's organization and approach to observational research and health outcomes research. J. Gen. Intern. Med. 29 (Suppl 3), S739–744 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Taruscio, D. et al Undiagnosed Diseases Network International (UDNI): White paper for global actions to meet patient needs. Mol. Genet. Metab. 116, 223–225 (2015). [DOI] [PubMed] [Google Scholar]
- 51. Rath, A. et al Representation of rare diseases in health information systems: the Orphanet approach to serve a wide range of end users. Hum. Mutat. 33, 803–808 (2012). [DOI] [PubMed] [Google Scholar]
- 52. Ayme, S. , Bellet, B. & Rath, A. Rare diseases in ICD11: making rare diseases visible in health information systems through appropriate coding. Orphanet J. Rare Dis. 10, 35 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mascalzoni, D. et al International Charter of principles for sharing bio‐specimens and data. Eur. J. Hum. Genet. 23, 721–728 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McCormack, P. et al ‘You should at least ask’. The expectations, hopes and fears of rare disease patients on large‐scale data and biomaterial sharing for genomics research. Eur. J. Hum. Genet. 24, 1403–1408 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Beaulieu, C.L. et al FORGE Canada Consortium: outcomes of a 2‐year national rare‐disease gene‐discovery project. Am. J. Hum. Genet. 94, 809–817 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Precision Public Health Summit — Summit Report . https://cvp.ucsf.edu/PPHS-Summit-Report-For-Posting.pdf (2016) Accessed: 29 May 2017.
- 57. Rare Diseases International . Available from: http://www.rarediseasesinternational.org/ Accessed: 29 May 2017.
- 58. NGO Committee for Rare Diseases . Available from: http://www.ngocommitteerarediseases.org/ Accessed: 29 May 2017.
- 59. List of Medicinal Products for Rare Diseases in Europe, in Orphanet Report Series, Orphan Drugs Collection — April 2017 . http://www.orpha.net/orphacom/cahiers/docs/GB/list_of_orphan_drugs_in_europe.pdf Accessed 30 June 2017.
- 60. Orphanet — Orphan Drugs . Available from: http://www.orpha.net/consor/cgi-bin/Drugs_Search.php?lng=EN Accessed 30 June 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Material


