Abstract
Whole exome sequencing (WES) has the potential of identifying secondary findings that are predictive of poor pharmacotherapy outcomes. The purpose of this study was to investigate patients’ wishes regarding the reporting of secondary pharmacogenomic findings. WES results (n = 106 patients) were retrospectively reviewed to determine the number of patients electing to receive secondary pharmacogenomic results. Phenotypes were assigned based on Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines. The percent of patients with a predicted phenotype associated with a gene‐based CPIC dosing recommendation was determined. Ninety‐nine patients (93.4%) elected to receive secondary pharmacogenomic findings. For each gene–drug pair analyzed, the number of patients with an actionable phenotype ranged from two (2%) to 43 patients (43.4%). Combining all gene–drug pairs, 84 unique patients (84.8%) had an actionable phenotype. A prospective multidisciplinary practice model was developed for integrating secondary pharmacogenomic findings into clinical practice. Our model highlights a unique collaboration between physician‐geneticists, pharmacists, and genetic counselors.
Study Highlights
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
✓ Patient perspectives on receiving pharmacogenomic findings from whole exome sequencing have been explored via questionnaires and other survey tools. However, there are limited data from a “real‐world” clinical setting describing patients’ wishes to receive pharmacogenomic secondary findings. Furthermore, there is a lack of guidelines or practice models for addressing secondary pharmacogenomic findings and application to patient care.
WHAT QUESTION DID THIS STUDY ADDRESS?
✓ Our study addressed patient decisions to receive secondary pharmacogenomic findings from whole exome sequencing in a “real‐world” setting. We demonstrated that the majority of patients, if given the opportunity, will elect to receive pharmacogenomic results and that most patients will have at least one actionable finding. Our study also addressed the development of a practice model for patient education and integration of secondary pharmacogenomic results into patient care. Our practice model highlights a unique collaboration between physician‐geneticists, pharmacogenomic trained pharmacists, and genetic counselors.
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE
✓ As clinical sequencing becomes increasingly integrated into patient care, our study demonstrates that patients will desire secondary pharmacogenomic findings. Clinicians will be challenged to educate patients about results and when appropriate apply to patient care. A unique practice model for integrating secondary pharmacogenomic findings into patient care was presented.
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE
✓ Clinician responsibilities in the scenario where a patient elects to receive secondary pharmacogenomic findings have not been clearly described. Our findings support the need to develop guidelines and practice models for the reporting of secondary pharmacogenomic findings.
Advances in next‐generation sequencing technologies along with bioinformatics capabilities are propelling the integration of whole genome and exome sequencing into clinic practice. Genome or exome sequencing is particularly useful for diagnostic purposes in those scenarios where rare or unidentified disease states are suspected to be caused by genomic variation, but the exact gene locus harboring the alteration is unknown. Studies have demonstrated that clinical whole exome sequencing (WES) identifies genetic alterations associated with the phenotype of interest in ∼25% of cases.1 Clinical WES also has the potential of reporting genetic variants that are not related to the phenotype of interest, but may be predictive of disease risk or poor pharmacotherapy outcomes. For certain reference laboratories, patients may have the option of electing to receive secondary WES findings such as pharmacogenomic results. Previous studies have demonstrated that the majority of patients elect to receive secondary WES results predictive of disease, but limited data are available that specifically focuses on patient decisions regarding pharmacogenomic results.2 Furthermore, there has been much debate on what constitutes a medically actionable genomic variant, particularly in the context of preemptive secondary pharmacogenomic findings.
The American College of Medical Genetics and Genomics (ACMG) developed recommendations for the reporting of incidental findings from genome and exome sequencing that includes over 50 genes.3 Among the rationale for selecting these genes is the potential for preventive measures to lessen disease risk and improve clinical outcomes. Only one pharmacogene is included on the ACMG list for reporting incidental findings: RYR1, which is associated with malignant hyperthermia susceptibility. There are additional drug–pharmacogene phenotype combinations that can have a detrimental, but preventable, effect on health.4, 5 Although policies and clinical workflows for returning incidental WES results predictive of disease are available,6, 7 there is a lack of guidelines and practice models regarding practitioner responsibilities for returning secondary pharmacogenomic findings to patients.
Numerous healthcare systems have implemented clinical pharmacogenomics to mitigate risk of adverse drug events by simply avoiding gene–drug interactions.8, 9 Because of efforts from the Clinical Pharmacogenetics Implementation Consortium (CPIC) and other organizations, there are a growing number of high‐quality evidence‐based recommendations to guide gene‐based drug prescribing.4, 5, 10 Pharmacogenomic implementation models and gene‐based dosing guidelines support the feasibility of reporting secondary pharmacogene findings and incorporation into patient care.11 For those instances where patients or guardians elect to receive secondary pharmacogenomic findings, systematic practice models are needed for patient education and integration into clinical care.
To investigate patients’ wishes to receive secondary pharmacogenomic data, we retrospectively quantitated the number of patients undergoing WES who elected to receive pharmacogenomic findings. The percentage of patients who had an actionable pharmacogenomic result (i.e., a result where a change in drug prescribing may be considered) was determined. Because the majority of patients elected to receive secondary pharmacogenomic findings and most individuals had at least one actionable result, a multidisciplinary practice model was developed for integrating secondary pharmacogenomic findings into clinical practice. Our model is a collaborative effort between physician‐geneticists, pharmacogenomic‐trained pharmacists, and genetic counselors.
METHODS
Cleveland Clinic's Genomic Medicine Institute's clinical arm, the Center for Personalized Genetic Healthcare, offers clinical WES to qualifying patients in the setting of pre‐ and posttest genetic counseling. For this study, those undergoing WES had clinical phenotypes that qualified the patient for testing. A Clinical Laboratory Improvements Amendments and College of American Pathologists certified reference laboratory utilized for WES, Baylor College of Medicine Genetics Laboratories (Houston, TX), offers patients the opportunity of electing to receive secondary pharmacogenomic results. A genetic counselor performs a genetic risk assessment consisting of family history and pedigree, educates each patient about the benefits, risks, and limitations of WES, the possibility of incidental findings, then consents the patient to WES testing along with the opportunity to receive pharmacogenomic results. A legal guardian completes the consent for those patients younger than 18 years of age as well as for those over 18 who are unable to legally provide their own consent. This Institutional Review Board for Human Subjects Protection‐approved study was designed as a single‐center retrospective review of patients undergoing WES at the Cleveland Clinic (Cleveland, OH) from 1 April 2012 through 1 February 2016. All patients who had results returned from the Baylor Genetics Laboratories were eligible for study inclusion. WES results for those instances that involved fetal testing were excluded from this study. Patient characteristics including age, sex, and indication for WES were recorded for each patient.
Baylor WES results were reviewed to determine the number of patients electing to receive pharmacogenomic findings. Consent to receive secondary pharmacogenomic findings was determined by the presence of pharmacogenomic data in the WES report. If pharmacogenomic data were absent from the WES report, the patient was assumed to have not given consent to receive pharmacogenomic findings. The reference laboratory provided a focused interpretation of WES results per ACMG guidelines that included genes related to the patient's clinical phenotype. Illumina HiSeq was utilized for sequencing with a single‐nucleotide polymorphism (SNP)‐array (Illumina HumanExome‐12v1 array) as a quality control measure. Pharmacogenomic variants were limited to CYP2C9 (CYP2C9*1, *2, *5, and *6), CYP2C19 (CYP2C19*1, *2, *3, *4, *5, *8, *10, and *17), and VKORC1 (VKORC1‐1639 G>A). Pharmacogenomic findings were reported as phased alleles with single‐nucleotide variants not provided by the reference laboratory.
For those electing to receive results, pharmacogenomic phenotypes were assigned based on CPIC guidelines. Assigned phenotypes were used to determine the percentage of patients who had an evidence‐based clinically significant actionable result. A result was considered actionable if the CYP2C9‐phenytoin,12 CYP2C19‐clopidogrel,13 CYP2C19‐tricyclic antidepressants,14 CYP2C19‐selective serotonin reuptake inhibitors,15 CYP2C19‐voriconazole,16 or CYP2C9/VKORC1‐warfarin17 CPIC guideline recommended a therapeutic action (i.e., dose modification or selection of a different drug). The total percentage of patients with an actionable phenotype was calculated by determining if each unique patient had a CYP2C9, CYP2C19, and/or warfarin‐sensitivity phenotype that would result in a therapeutic action per CPIC guidelines.
A prospective practice model for review of secondary pharmacogenomic results and integration into patient care was developed. Clinical workflows consist of a physician‐geneticist interpreting WES results and when warranted providing a diagnosis. A pharmacogenomics‐trained pharmacist assigns predicted pharmacogene phenotypes and reviews medication profiles for potential gene–drug interactions. Pharmacogenomic results, including phenotypes and potential impact on patient care, are discussed with a genetic counselor. During the post‐WES counseling session, a genetic counselor educates the patients about the pharmacogenomic findings. Pharmacogenomic results are eligible for discrete entry into the electronic medical record, with clinical decision support providing gene‐based prescribing recommendations.
RESULTS
A total of 106 unique patients were eligible for study inclusion (Table 1). The percent of males and females was similar among the study population, although a greater percentage of patients (67%) were less than 18 years of age. Neurological and cardiovascular phenotypes were the most common indications for exome sequencing. Ninety‐nine (93.4%) patients elected to receive pharmacogenomic results. A slightly higher percentage of patients 18 years of age and older (34 of 35 patients, 97.1%) elected to receive pharmacogenomic findings when compared with those less than 18 years of age (65 of 71 patients, 91.5%). Multiple patients self‐declared mixed ancestry; therefore, calculation of expected allele frequencies based on population genetics for the study cohort was challenging. CYP2C9, CYP2C19, and VKORC1 allele frequencies (Supplemental Table 1) are similar to the allele frequencies observed in those of Caucasian ancestry (www.pharmgkb.org allele frequency tables), which is generally representative of our patient population.
Table 1.
Patient characteristics (n = 106)
| Age | |
|---|---|
| Median | 9 years |
| Range | 1 day to 60 years |
| Patients <18 years of age | 71 (67%) |
| Patients ≥18 years of age | 35 (33%) |
| Sex | n (%) |
| Female | 55 (51.9) |
| Male | 51 (48.1) |
| Indication for exome sequencing | n (%) |
| Hematologic disorder | 1 (0.9) |
| Hereditary hemorrhagic telangiectasia | 1 (0.9) |
| Pulmonary disorder | 1 (0.9) |
| Autoimmune disorder | 2 (1.9) |
| Decreased growth velocity | 2 (1.9) |
| Dermatology disorder | 2 (1.9) |
| Dysmorphic features | 2 (1.9) |
| Renal disorder | 2 (1.9) |
| Cardiovascular disorder | 8 (7.5) |
| Cardiovascular & neurological disorder | 14 (13.2) |
| Neurological disorder | 71 (67) |
| Elected to receive pharmacogenomic results | n (%) |
| No | 7 (6.6) |
| Yes | 99 (93.4) |
| CYP2C9 phenotypes | n (%) |
| CYP2C9 normal metabolizer | 69 (69.7) |
| CYP2C9 intermediate metabolizer | 25 (25.3) |
| CYP2C9 poor metabolizer | 5 (5) |
| CYP2C19 phenotypes | n (%) |
| CYP2C19 ultrarapid metabolizer | 5 (5) |
| CYP2C19 rapid metabolizer | 36 (36.4) |
| CYP2C19 normal metabolizer | 37 (37.4) |
| CYP2C19 intermediate metabolizer | 19 (19.2) |
| CYP2C19 poor metabolizer | 2 (2) |
| Warfarin sensitivity | n (%) |
| Normal sensitivity | 60 (60.6) |
| Increased sensitivity | 39 (39.4) |
| Actionable pharmacogenomic phenotypes | n (%) |
| No actionablea phenotype | 15 (15.2) |
| One actionablea phenotype | 44 (44.4) |
| Two actionablea phenotypes | 33 (33.3) |
| Three actionablea phenotypes | 7 (7.1) |
Actionable phenotype is defined by whether a therapeutic action per CPIC guidelines is recommended.
Table 1 and Supplemental Table 2 summarize the predicted phenotypes for the study population. The majority of patients (69.7%) were predicted to have normal CYP2C9 metabolism, with only 37.4% of patients predicated to have normal CYP2C19 metabolism. Seventy‐two percent of patients were heterozygous or homozygous for the VKORC1‐1639 A allele, which is associated with decreased VKORC1 expression. CPIC guidelines were utilized to determine the percent of patients who may have an actionable result in the context of being prescribed a particular drug (Table 2). Dependent on the CPIC gene–drug pair, a range of two patients to 43 patients had a pharmacogenomic result that may require a therapeutic action. If all patients included in this study were prescribed sertraline, only two patients (2%) would be recommended to have a pharmacotherapy modification per CPIC guidelines. However, if all patients in this study were prescribed citalopram, escitalopram, amitriptyline, or voriconazole, 43 patients (43.4%) would be recommended to have a pharmacotherapy modification. In the context of CYP2C9/VKORC1 and assumption of Caucasian ancestry, 39 patients (39.4%) would be recommended to have a dose modification if prescribed warfarin (Table 2, Supplemental Table 3). Three patients were predicted to be greatly sensitive to warfarin with a gene‐based recommended starting dose of 0.5–2 mg warfarin instead of the usual 5–7 mg dose. CYP2C9 genetic variants (e.g., CYP2C9*8) that are important for warfarin dosing in those of African descent were not reported by the reference laboratory.
Table 2.
Percent of patients with an actionablea result based on CPIC guidelines
| CPIC guideline | Actionable result, n (%) |
|---|---|
| CYP2C9‐Phenytoin | 30 (30.3) |
| CYP2C19‐Citalopram/Escitalopram | 43 (43.4) |
| CYP2C19‐Clopidogrel | 21 (21.2) |
| CYP2C19‐Sertraline | 2 (2) |
| CYP2C19‐Tricyclic antidepressants | 43 (43.4) |
| CYP2C19‐Voriconazole | 43 (43.4) |
| CYP2C9/VKORC1‐Warfarin | 39 (39.4) |
Actionable is defined by whether a change in drug therapy may be recommended based on the genotype result.
Combining CYP2C9, CYP2C19, and warfarin‐sensitivity phenotypes, 84 patients (84.8%) have an actionable phenotype. One actionable phenotype was observed in 44 patients (44.4%), with 33 patients (33.3%) having two actionable phenotypes. Seven patients (7.1%) were found to have an actionable CYP2C9, CYP2C19, and warfarin‐sensitivity phenotype (Table 1).
A prospective practice model consisting of a unique collaboration between physician‐geneticists, pharmacogenomic‐pharmacists, and genetic counselors was developed to review WES secondary pharmacogenomic findings, educate patients about results, and incorporate gene‐based recommendations into the drug prescribing process (Figure 1). The multidisciplinary team reviews WES results, and assigns predicted pharmacogenomic phenotypes along with reviewing medication profiles for potential gene–drug interactions. As part of the post‐WES genetic counseling session, genetic counselors educate the patient about pharmacogenomic findings. If requested, a pharmacogenomic summary document is provided to the patient that can be shared with other healthcare professionals to guide future drug prescribing. Because preserving paper documents can be cumbersome, an electronic health record (EHR) patient portal and messaging system is also available for commutating pharmacogenomic information when needed. For those patients medically managed at Cleveland Clinic, pharmacogenomic results can be discretely curated in the EHR with both active and passive clinical decision support providing clinicians with gene‐based drug prescribing recommendations.8 An ambulatory pharmacogenomics clinic or online pharmacogenomics consultation is available to provide clinical services including additional pharmacogenomic testing.8 A patient or physician can also request a pharmacogenomics consultation at the time of drug prescribing. As part of the pharmacogenomic clinical offerings, a note is entered into the EHR that can be accessed through the patient portal and shared with other health providers.
Figure 1.
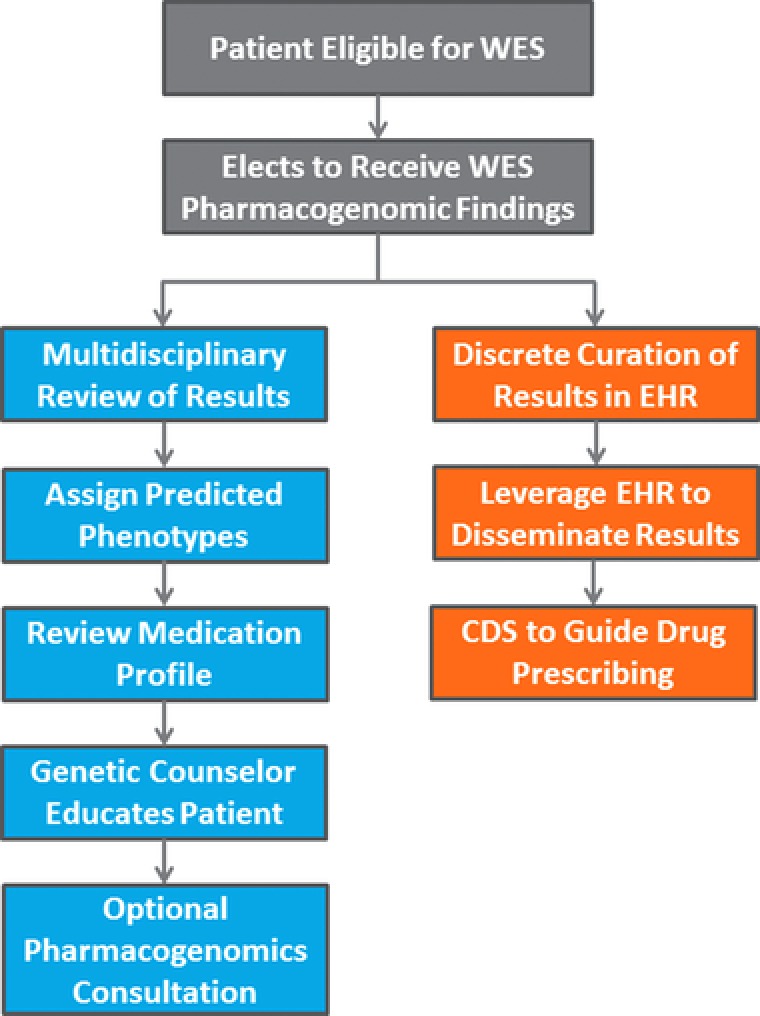
Clinical workflow for reviewing WES secondary pharmacogenomic results. A multidisciplinary team consisting of a physician‐geneticist, pharmacist, and genetic counselor review WES results. A genetic counselor educates the patient about the results. An ambulatory Pharmacogenomics Clinic is available to provide further clinical services including additional pharmacogenomic testing, education, and review of pharmacotherapy. Results can be discretely entered into the electronic health record (EHR) with clinical decision support‐provided gene‐based drug dosing recommendations.
DISCUSSION
The vast majority of patients in our “real‐world” clinical setting elected to receive secondary pharmacogenomic findings. This observation is similar to other studies, which focused on disease predictive genes, showing that when given the opportunity most patients elect to receive secondary findings.2, 18 Our study suggests that as genome and exome sequencing becomes increasingly integrated into medical practice, clinicians will be challenged to meet patients’ desire for information regarding secondary findings and potential integration into care. Application of secondary pharmacogenomic findings to clinical practice is currently being debated. There are strong associations between drug‐response and genomic variations, along with guidelines for clinical implementation.12, 13, 14, 15, 16, 17 The clinical utility of pharmacogenomics has been criticized, however, due to a lack of large prospective randomized studies demonstrating improved clinical outcomes. Irrespective of opinions regarding the clinical utility of pharmacogenomics, a reasonable assumption is that patients who elect to receive secondary pharmacogenomic findings will want an explanation of results. Furthermore, clinician responsibilities (e.g., review of medication profiles, discrete entry of actionable results into the medical record, dissemination of results to primary care providers, patient education) in the scenario where a patient elects to receive secondary pharmacogenomic findings have not been clearly described.19 Confounding this issue is that the majority of patients who elect to receive pharmacogenomic results will have at least one actionable result.
In this study, pharmacogenomic results were returned for only three genes (CYP2C9, CYP2C19, and VKORC1). Over 80% of patients who elected to receive pharmacogenomic findings had at least one actionable result. An investigation of ∼1,100 individuals utilizing data obtained from WES and SNP genotyping found that all individuals had an incidental pharmacogenomic finding; however, the majority of the findings were not clinically significant.20 Our analysis focused only on gene–drug pairs with evidence‐based guidelines for clinical implementation. The percent of patients with actionable results in this investigation is similar to other studies that found greater than 90% of individuals undergoing pharmacogenomic panel testing had at least one clinically actionable result.21 Patients are likely to be prescribed at least one drug influenced by a pharmacogene over their lifetime.22 Utilizing secondary pharmacogenomic findings to guide drug prescribing has the potential to reduce the risk of experiencing a gene–drug interaction. Outcomes studies are needed to determine if anticipated clinical benefits of integrating secondary pharmacogenomic results into clinical practice are actualized.
Our retrospective evaluation showing greater than 90% of patients elected to receive secondary pharmacogenomic findings combined with the majority of patients having at least one actionable result prompted the development of clinical workflows to address the future reporting of secondary pharmacogenomic results. Our practice model consists of a unique collaboration between physician‐geneticists, pharmacogenomic‐trained pharmacists, and genetic counselors. A pharmacogeneticist interprets pharmacogene results and reviews medication profiles. When warranted, physicians and genetic counselors review the pharmacogenomic results with the pharmacogeneticist. Genetic counselors provide education to the patient, including the need to share pharmacogenomic results with their primary care physician and pharmacist. A barrier to incorporating secondary pharmacogenomic findings into patient care is the discrete curation of results in the EHR so that clinically actionable phenotypes are easily accessible to clinicians and can be data‐mined for clinical decision support purposes. There are implementation models describing the development and dissemination of EHR clinical decision support alerts for actionable pharmacogenomic results reported by WES.11 For our patients who elect to receive secondary pharmacogenomic findings, pharmacogenomic clinical decision support alerts are available to remind clinicians of important results.8
A concern for returning pharmacogenomic results from WES is robust call rates for pharmacogenes. Recent studies have demonstrated that for most clinically relevant pharmacogene loci, call rates are similar between clinical sequencing and array‐based genotyping.23, 24 For genes such as CYP2D6, pseudogenes (CYP2D7 and CYP2D8) and copy number variants present challenges for interpreting sequencing data. Software tools are being developed to overcome these barriers and translate clinical sequencing pharmacogene data into predicted phenotypes.25, 26 As such tools are refined over time, the reporting of pharmacogenomic data and predicated phenotypes from clinical sequencing will likely become more commonplace. Patients’ desire for pharmacogenomic results, availability of gene‐based drug prescribing guidelines, robust pharmacogene call rates from clinical sequencing, and the potential to preemptively prevent serious gene–drug interactions will further stimulate the debate of whether incidental or secondary pharmacogenomic findings should be routinely evaluated by reference laboratories with results returned to patients.
Because this is a retrospective study, there are limitations to our investigation. Patients may have elected to receive secondary pharmacogenomic findings based on the concept that more information is better. We were unable to quantitate if patients considered the returned pharmacogenomic results to be of significant worth. Prospective studies are needed to measure the outcomes (e.g., patient perspective, prevention of gene–drug interactions) of reporting secondary pharmacogenomic findings. Twenty‐two patients had a cardiovascular indication for WES which could potentially place those individuals at a higher probability for being exposed to a drug such as warfarin. Twenty of the patients elected to receive pharmacogenomics results, with eight (40%) patients predicated to have a warfarin‐sensitive phenotype that may require a lower drug dose. However, the impact that secondary pharmacogenomic findings may have on future drug exposure was difficult to quantitate, as most patients received their primary care elsewhere and were referred for the sole purpose of genetic evaluation.
CONCLUSION
Ninety‐nine of the 106 patients included in this study elected to receive incidental pharmacogenomic findings. Approximately 85% of those electing to have pharmacogenomic results returned had at least one actionable result that potentially increases the risk of a gene–drug interaction. Our retrospective evaluation demonstrates the need for practice models to address pharmacogenomic findings. A unique collaboration between physician‐geneticists, pharmacists, and genetic counselors was established to address the reporting of WES secondary pharmacogenomic findings. Based on patients’ desire for pharmacogenomic results, availability of pharmacogenomic implementation tools, and the potential to preemptively prevent detrimental gene–drug interactions, further consideration should be given to returning incidental or secondary pharmacogenomic findings.
Conflict of Interest
The authors declared no conflict of interest.
Supporting information
Supplemental Table 1. Pharmacogene allele frequencies
Supplemental Table 2. Reported Genotypes (n = 99)
Supplemental Table 3. Warfarin sensitivity based on CPIC guidelines
Acknowledgments
We thank Dr. Vickie Zurcher and the Genetic Counselors from Cleveland Clinic's Center for Personalized Genetic Healthcare for their efforts to consent patients to clinical exome sequencing.
Author Contributions
J.K.H. and C.E. wrote the article; J.K.H. and C.E. designed the research; J.K.H., A.Sh., A.Sc., M.C., R.N., M.N., R.M., T.M., A.E., and C.E. performed the research; J.K.H., A.Sh., A.Sc., M.C., R.N., M.N., R.M., T.M., A.E., and C.E. analyzed the data. J.K.H. and C.E. made substantial contributions to conception, design, data interpretation, and drafting of the article. All authors contributed to data acquisition, critical revisions of the article, final approval of publication, and agreement to be accountable for all aspects of the work.
Declarations
Ethics approval and consent to participate: This study was approved by the Cleveland Clinic Institutional Review Board. This IRB Human Subjects Protection‐approved study waived patient consent as this is a retrospective study exploring existing data with minimal risk to patients. Consent for publication: All information is deidentified. This IRB Human Subjects Protection‐approved study waived patient consent as this is a retrospective study exploring existing data with minimal risk to patients. Availability of data and material: The data sets generated and/or analyzed during the current study are not publicly available due to the WES data being protected health information per HIPPA. A deidentified WES data set is available from the corresponding author on reasonable request.
References
- 1. Yang, Y. et al Clinical whole‐exome sequencing for the diagnosis of Mendelian disorders. N. Engl. J. Med. 369, 1502–1511 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shahmirzadi, L. , Chao, E.C. , Palmaer, E. , Parra, M.C. , Tang, S. & Gonzalez, K.D. Patient decisions for disclosure of secondary findings among the first 200 individuals undergoing clinical diagnostic exome sequencing. Genet. Med. 16, 395–399 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Green, R.C. et al ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet. Med. 15, 565–574 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Relling, M.V. & Klein, T.E. CPIC : Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin. Pharmacol. Ther. 89, 464–467 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Swen, J.J. et al Pharmacogenetics: from bench to byte–an update of guidelines. Clin. Pharmacol. Ther. 89, 662–673 (2011). [DOI] [PubMed] [Google Scholar]
- 6. Kalia, S.S. et al Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): a policy statement of the American College of Medical Genetics and Genomics. Genet. Med. 19, 249–255 (2017). [DOI] [PubMed] [Google Scholar]
- 7. Hunter, J.E. et al A standardized, evidence‐based protocol to assess clinical actionability of genetic disorders associated with genomic variation. Genet. Med. 18, 1258–1268 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hicks, J.K. et al Implementation of clinical pharmacogenomics within a large health system: from electronic health record decision support to consultation services. Pharmacotherapy 36, 940–948 (2016). [DOI] [PubMed] [Google Scholar]
- 9. Dunnenberger, H.M. et al Preemptive clinical pharmacogenetics implementation: current programs in five US medical centers. Annu. Rev. Pharmacol. Toxicol. 55, 89–106 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shaw, K. et al Clinical practice recommendations on genetic testing of CYP2C9 and VKORC1 variants in warfarin therapy. Ther. Drug. Monit. 37, 428–436 (2015). [DOI] [PubMed] [Google Scholar]
- 11. Nishimura, A.A. et al Development of clinical decision support alerts for pharmacogenomic incidental findings from exome sequencing. Genet. Med. 17, 939–942 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caudle, K.E. et al Clinical pharmacogenetics implementation consortium guidelines for CYP2C9 and HLA‐B genotypes and phenytoin dosing. Clin. Pharmacol. Ther. 96, 542–548 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scott, S.A. et al Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin. Pharmacol. Ther. 94, 317–323 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hicks, J.K. et al Clinical pharmacogenetics implementation consortium guideline for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants. Clin. Pharmacol. Ther. 93, 402–408 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hicks, J.K. et al Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin. Pharmacol. Ther. 98, 127–134 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moriyama, B. et al Clinical Pharmacogenetics Implementation Consortium (CPIC(R)) Guideline for CYP2C19 and voriconazole therapy. Clin. Pharmacol. Ther., (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johnson, J.A. et al Clinical Pharmacogenetics Implementation Consortium Guidelines for CYP2C9 and VKORC1 genotypes and warfarin dosing. Clin. Pharmacol. Ther. 90, 625–629 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holla, O.L. et al Clinical exome sequencing—Norwegian findings. Tidsskr Nor Laegeforen 135, 1833–1837 (2015). [DOI] [PubMed] [Google Scholar]
- 19. Yu, J.H. , Harrell, T.M. , Jamal, S.M. , Tabor, H.K. & Bamshad, M.J. Attitudes of genetics professionals toward the return of incidental results from exome and whole‐genome sequencing. Am. J. Hum. Genet. 95, 77–84 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee, E.M. et al Pharmacogenomic incidental findings in 308 families: The NIH Undiagnosed Diseases Program experience. Genet. Med. 18, 1303–1307 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Van Driest, S.L. et al Clinically actionable genotypes among 10,000 patients with preemptive pharmacogenomic testing. Clin. Pharmacol. Ther. 95, 423–431 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schildcrout, J.S. et al Optimizing drug outcomes through pharmacogenetics: a case for preemptive genotyping. Clin. Pharmacol. Ther. 92, 235–242 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang, W. et al Comparison of genome sequencing and clinical genotyping for pharmacogenes. Clin. Pharmacol. Ther. 100, 380–388 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qiao, W. et al Long‐read single molecule real‐time full gene sequencing of Cytochrome P450‐2D6. Hum. Mutat. 37, 315–323 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klein, T. , Whirl‐Carrillo, M. , Whaley, M. & et, a. Pharmacogenomics Clinical Annotation Tool (PharmCAT). In: 66th Annual Meeting of The American Society of Human Genetics. Vancouver, Canada: Abstract 1185F (2016). [Google Scholar]
- 26. Twist, G.P. et al Constellation: a tool for rapid, automated phenotype assignment of a highly polymorphic pharmacogene, CYP2D6, from whole‐genome sequences. NPJ Genom. Med. 1, 15007 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Pharmacogene allele frequencies
Supplemental Table 2. Reported Genotypes (n = 99)
Supplemental Table 3. Warfarin sensitivity based on CPIC guidelines


