Abstract
Background
Cure rates for chronic hepatitis C have improved dramatically with direct-acting antivirals (DAAs), but treatment barriers remain. We aimed to compare treatment initiation rates and barriers across both interferon-based and DAA-based eras.
Methods
We conducted a retrospective cohort study of all patients with chronic hepatitis C seen at an academic hepatology clinic from 1999 to 2016. Patients were identified to have chronic hepatitis C by the International Classification of Diseases, Ninth Revision codes, and the diagnosis was validated by chart review. Patients were excluded if they did not have at least one visit in hepatology clinic, were under 18 years old or had prior treatment with DAA therapy. Patients were placed in the DAA group if they were seen after 1 January 2014 and had not yet achieved virological cure with prior treatment. All others were considered in the interferon group.
Results
3202 patients were included (interferon era: n=2688; DAA era: n=514). Despite higher rates of decompensated cirrhosis and medical comorbidities in the DAA era, treatment and sustained virological response rates increased significantly when compared with the interferon era (76.7% vs 22.3%, P<0.001; 88.8% vs 55%, P<0.001, respectively). Lack of follow-up remained a significant reason for non-treatment in both groups (DAA era=24% and interferon era=45%). An additional 8% of patients in the DAA era were not treated due to insurance or issues with cost. In the DAA era, African-Americans, compared with Caucasians, had significantly lower odds of being treated (OR=0.37, P=0.02).
Conclusions
Despite higher rates of medical comorbidities in the DAA era, considerable treatment challenges remain including cost, loss to follow-up and ethnic disparities.
Keywords: chronic viral hepatitis, hepatitis c, health service research
Summary box.
What is already known about this subject?
It is important to understand treatment barriers and issues in our linkage to care with newer highly efficacious direct-acting antiviral (DAA) therapies.
While there are several published papers on barriers and treatment rates for interferon (IFN)-based therapies, there is a lack of data on treatment barriers and reasons for non-treatment in this DAA era.
What are the new findings?
Treatment rates with the newer DAA therapies more than tripled as compared with rates in the IFN era.
However, despite this dramatic increase in treatment rates with DAAs, there remains considerable treatment challenges including both systems-level and patient-level factors including loss to follow-up and insurance/cost issues.
In addition, there were significant healthcare disparities: African-Americans, compared with Caucasians, had significantly lower odds of being treated.
How might it impact on clinical practice in the foreseeable future?
Improving patient-level and systems-level factors to better identify patients with chronic hepatitis C (CHC) and ensuring patient follow-up is now even more important to extend the impact of DAA therapies and eradicate CHC.
Introduction
Prior to December 2013, the only approved therapies for the treatment of chronic hepatitis C (CHC) in the USA consisted of interferon (IFN)-based regimens. Treatment rates with these IFN-based therapies were low due to the considerable side effects, prolonged treatment durations of up to 48 weeks, delivery via subcutaneous injection and relatively poor sustained virological response (SVR) rates.1–5 The overall impact of interferon on the US CHC population was low: in large population-based studies of patients with CHC during the IFN era, only 10%–12% were treated and 3.5% achieved virological cure.4 5
In 2014, an all-oral regimen with the direct-acting antivirals (DAAs) simeprevir and sofosbuvir became available for use in the USA.6 These therapies were all-oral, well-tolerated and achieved SVR rates of over 90% in clinical trials. In addition to these increased response rates, the number of prescriptions for antiviral therapies had increased dramatically as well. One study of patients seen at the Veteran’s Affairs (VA) reported that the number of yearly antiviral prescriptions had increased up to 21-fold that of IFN-based numbers within 2 years after the introduction of these DAAs.7
However, as these more efficacious treatments become the standard of care, it is increasingly important to study treatment barriers and issues in our linkage to care. While there are several published papers on barriers and treatment rates for IFN-based therapies, there is a lack of data on treatment barriers and reasons for non-treatment in this DAA era.1 4 5 8–11 Thus, we conducted a longitudinal retrospective cohort study of patients seen at an academic hepatology clinic to determine how treatment rates and barriers have changed as we transitioned from hepatitis C IFN-based to all oral DAA-based therapies.
Materials and methods
Study design and patients
We studied consecutive patients with CHC seen at a subspecialty hepatology clinic affiliated with Stanford University Medical Center from 1999 to 2016. Patients were electronically identified to have CHC via International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code query. The codes used included: 070.44, 070.54, 070.70, 070.71, 070.41 and 070.51. Manual chart review was then performed to validate CHC diagnosis, and a patient was considered to have CHC by laboratory report of positive HCV antibody or HCV RNA and/or a documented history of CHC by a physician. Patients were excluded if they did not have a history of at least one visit in hepatology clinic, were under 18 years of age or had received prior treatment with DAA therapies.
Baseline sociodemographic and medical data
The following sociodemographic data were collected: year of birth, race/ethnicity (Caucasian, black, Hispanic, Asian) and gender. Additional medical, psychiatric, and liver-related comorbidities for each patient were queried by ICD-9-CM code and included diabetes mellitus, chronic kidney disease, coronary artery disease, chronic obstructive pulmonary disease, depression, bipolar disorder, schizophrenia, chronic hepatitis B, cirrhosis, decompensated cirrhosis and hepatocellular carcinoma (HCC). All patient medical and psychiatric comorbidities and baseline laboratories were obtained at baseline and up to 1-year postbaseline.
HCV treatment and grouping
Treatment data during both IFN and DAA era were manually abstracted and were defined by the initiation of any therapies approved for antiviral use by the US Food and Drug Administration during the study period. The therapies included: IFN-based therapies, ribavirin (RBV), first-generation DAAs telaprevir and boceprevir and second-generation DAAs including simeprevir, sofosbuvir, daclastavir, ledipasvir, velpatasvir, elbasvir, grazoprevir, ombitasvir, paritaprevir, ritonavir and dasabuvir with or without RBV. The date of 1 January 2014 was chosen for the DAA era as this was the first date that DAAs were available at our medical centre for treatment. Therefore, patients were included in the DAA era if they had at least one visit on or after 1 January 2014 and had no treatment prior to this date or were treatment non-responders or relapsers with IFN-based therapies prior to 1 January 2014. The remaining patients were grouped in the IFN era. SVR was defined as an undetectable HCV viral load 24 weeks post-treatment in the IFN era and an undetectable viral load 12 weeks post-treatment in the DAA one.
HCV treatment eligibility and reasons for lack of treatment
Based on recommendations from the American Association for the Study of Liver Diseases (AASLD), patients were considered to be ineligible for IFN therapy if they had laboratory results indicative of decompensated cirrhosis baseline total bilirubin >1.5 g/dL, international normalised ratio >1.5, albumin <3.4, platelet count <75 000, haemoglobin <13 g/dL for men and 12 g/dL for women, neutrophil count <1500 mm3 or serum creatinine >1.5 mg/dL.12 In order to be considered lab ineligible for IFN therapy, subjects were required to have the abnormal lab value(s) for at least 3 months. Patients were also considered ineligible for IFN-based therapy if they had major uncontrolled depressive illness as documented in the physician notes, a history of solid organ transplant, autoimmune hepatitis, uncontrolled medical comorbidities or a known hypersensitivity to antiviral medications. According to the AASLD guidelines, we considered all patients eligible for therapy in the DAA era except those with a limited life expectancy not correctable by HCV treatment or liver transplantation.13 All data were manually abstracted for patients who were not treated but otherwise were considered treatment eligible. We measured as a secondary endpoint a delay of prescription fill that was defined as greater than 3 months of time between the prescription for HCV treatment and when the patient filled the prescription for the DAA era.
Statistical analysis
Baseline demographics and characteristics were reported as mean and SD for continuous variables and proportions for categorical variables. The χ2 test was used to analyse categorical variables and t-tests for normally distributed continuous variables and Mann-Whitney U test for non-normally distributed continuous variables. Fisher’s exact and the Mann-Whitney U tests were used to compare non-parametric categorical and continuous variables. A two-tailed P value of ≤0.05 was determined to be statistically significant. Univariate and multivariable logistic regression analyses were used to determine predictors for treatment. Two separate regressions were performed: one for the patients of the IFN era and one for those of the DAA era. All analyses were performed using STATA V.14.
Results
Patient demographics and comorbidities
We queried a total of 12 486 patients with CHC by ICD-9-CM coding who were seen at Stanford University Medical Center (figure 1). Based on chart review, 2998 patients were excluded as they did not have documented CHC, and an additional 6261 patients were excluded as they were not evaluated in the hepatology clinic. Five patients were excluded who were under the age of 18 years, and 20 patients were excluded due to prior treatment with all-oral DAA therapies at other institutions. A total of 3202 were included in this study: 2688 patients in the IFN era and 514 patients in the DAA era. Patients in the IFN era were followed in clinic for a median of 54 (range 0–82) months and in the DAA era 49 (range 0–223) months.
Figure 1.
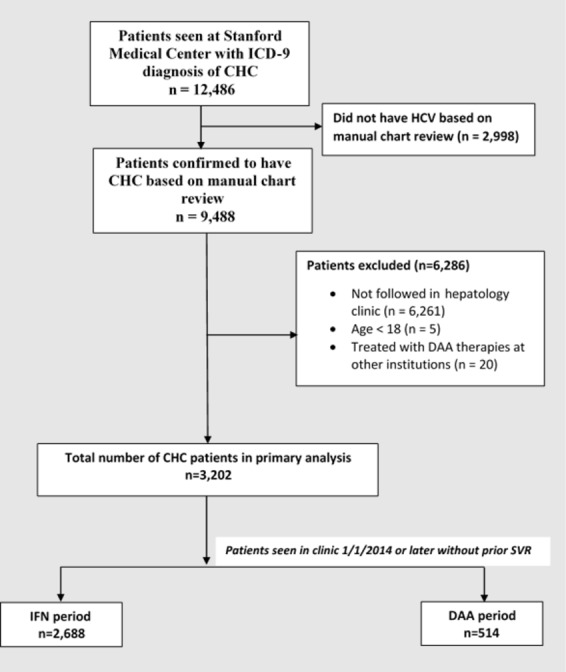
Study flow diagram. A total of 3202 patients were seen in subspecialty hepatology clinic: 2688 patients in the IFN era and 514 in the DAA era. CHC, chronic hepatitis C; DAA, direct-acting antiviral; ICD-9, International Classification of Diseases, Ninth Revision; IFN, interferon; SVR, sustained virological response.
Compared with patients in the IFN era, those in the DAA era trended towards older age (mean age 55.7±8.3 years in the DAA era vs 54.9±10.8 years in the IFN era, P=0.1). In the total cohort, 52.6% were Caucasian, 21.8% were Hispanic, 14.3% were Asian, 6.2% were African-American and 5.1% were of other ethnicity. Patients in the DAA era had higher rates of medical comorbidities including coronary artery disease (14% vs 9.2%, P<0.001), chronic kidney disease (25.5% vs 12%, P<0.001), chronic obstructive lung disease (5.3% vs 2.6%, P=0.001), diabetes mellitus (37% vs 19.7%, P<0.001) and psychiatric disorders (47.7% vs 34.4%, P<0.001). Patients in the DAA era also had higher rates of cirrhosis (84.8% vs 63.8%, P<0.001), and higher rates of decompensation (75.7% vs 45.1%, P<0.001) including HCC (41.3% vs 18.8%, P<0.001). There were similar rates of hepatitis B coinfection in both groups (4.5% in the DAA-era vs 4.7% in the IFN era, P=0.15). Most patients had HCV genotype 1 (69.5%), 2 (12%) or 3 (13.1%). A smaller proportion of patients had HCV genotype 4 (2%) or 6 (3.3%).
In the DAA era, when stratifying patients by ethnicity (n=485 with known ethnicity), African-Americans had the lowest rates of decompensated cirrhosis (44% of African-Americans compared with 71.6% of Caucasians, 82.1% of Asians and 90.9% of Hispanics) (online supplementary table 1). Rates of HCC were highest in Hispanics (40.4%) and African-Americans (24%) and lower in Asians (20.5%) and Caucasians (12.7%). Rates of medical comorbidities were similar between ethnicities.
bmjgast-2017-000181supp001.docx (16.5KB, docx)
Treatment rates, characteristics and efficacy
Both treatment and SVR rates were significantly higher in the DAA era compared with that of the IFN era (treatment rate: 77% vs 22.3%, P<0.001; SVR rate: 88.8% vs 55%, P<0.001) (table 1). In the DAA era, most patients received combination treatments with ledipasvir and sofosbuvir (43.4%), simeprevir and sofosbuvir (24.6%) or sofosbuvir and RBV (16.2%). About one-third of patients (125 of 394 patients, 31.7%) received RBV as part of their treatment course. By ethnicity, in the IFN era, treatment rates were highest in Asian Americans (25%) and Caucasians (24%), lower in African-Americans (21%) and lowest in Hispanics (16%, P=0.004) (online supplementary figure 1). In the DAA era, where treatment rates increased for all ethnicities overall, rates for African-Americans (60%) remained lower than that of other ethnicities (81% for Caucasians, 78% for Asians, 74% for Hispanics, P=0.065).
Table 1.
Baseline demographics and treatment data for the IFN and DAA era
| Characteristic | IFN era (n=2688) | DAA era (n=514) | ||||
| Treated (n=599) % |
Untreated (n=2089) |
P value | Treated (n=394) % |
Untreated (n=120) % |
P value | |
| Age (years) | 52.3±10.8 | 55.7±10.7 | <0.001 | 56±8.2 | 57.1±8.9 | 0.3 |
| Male gender (%) | 56.6 | 59.6 | 0.19 | 69.3 | 61.7 | 0.12 |
| Medical comorbidities | ||||||
| Coronary artery disease | 9 | 9.3 | 0.84 | 15.2 | 10 | 0.15 |
| Chronic kidney disease | 9.7 | 12.7 | 0.05 | 26.9 | 20.8 | 0.18 |
| Chronic obstructive pulmonary disease | 2.7 | 2.5 | 0.9 | 5.1 | 5.8 | 0.75 |
| Diabetes mellitus | 19.5 | 19.7 | 0.92 | 38.6 | 32.7 | 0.17 |
| Liver-related comorbidities | ||||||
| Cirrhosis | 59.9 | 64.9 | 0.03 | 85.3 | 83.3 | 0.6 |
| Decompensated cirrhosis | 34.6 | 48.1 | < 0.001 | 76.4 | 73.3 | 0.49 |
| Hepatitis B coinfection | 4.2 | 4.9 | 0.5 | 4.3 | 5 | 0.75 |
| Hepatocellular carcinoma | 12.7 | 20.5 | < 0.001 | 40.4 | 44.5 | 0.42 |
| Psychiatric disorder* | 34.2 | 34.4 | 0.93 | 48.2 | 45.8 | 0.65 |
| Ethnicity (%) | 0.004 | 0.07 | ||||
| Caucasian | 57.6 | 52.2 | 50.9 | 39.5 | ||
| Asian | 16.4 | 13.9 | 13.7 | 13.2 | ||
| African-American | 6% | 6.5 | 3.9 | 8.8 | ||
| Hispanic | 14.9 | 21.6 | 29.9 | 35.1 | ||
| Others | 5 | 5.8 | 1.6 | 3.5 | ||
| HCV genotype (n=490/1227/330/80) |
0.1 | 0.7 | ||||
| 1 | 66.1 | 71.9 | 67.9 | 60 | ||
| 2 | 15.5 | 11.3 | 9.4 | 12.5 | ||
| 3 | 13.9 | 12 | 14.9 | 20 | ||
| 4 | 1.6 | 1.9 | 2.7 | 0 | ||
| 6 | 2.9 | 2.9 | 5.2 | 5 | ||
| Sustained virological response rate | 55 | – | – | 88.8 | – | – |
| Antiviral regimen (DAA era only) | – | – | – | – | – | – |
| Ledipasvir/sofosbuvir | 43.4 | |||||
| Simeprevir/sofosbuvir | 24.6 | |||||
| Sofosbuvir/ribavirin | 16.2 | |||||
| Daclatasvir/sofosbuvir | 5.1 | |||||
| Other regimen | 10.7 | |||||
*Psychiatric disorders include depression, bipolar disorder and schizophrenia.
DAA, direct-acting antiviral; IFN, interferon.
bmjgast-2017-000181supp002.jpg (99.8KB, jpg)
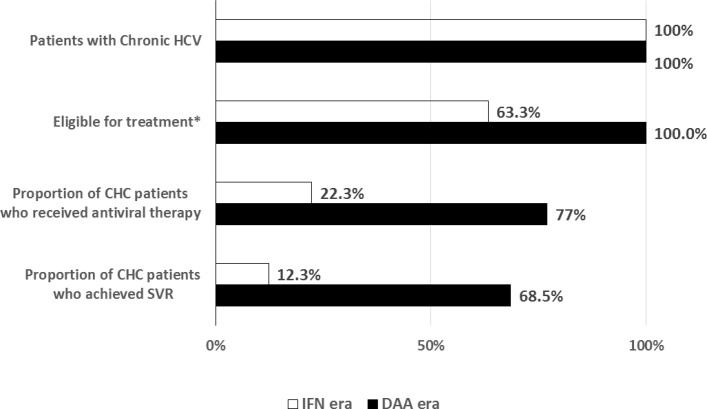
The overall effectiveness of the antiviral therapies increased significantly with the introduction of DAAs (figure 2). Of all patients with CHC seen in hepatology clinic, 12.3% in the IFN era and 68% in the DAA era achieved SVR.
Figure 2.
Treatment effectiveness by era. Treatment effectiveness by time period. Of all patients seen in a subspecialty hepatology clinic, 22.3% (white bar) of patients in the IFN era were treated and 12.3% achieved SVR. Seventy-seven per cent (black bar) of patients were treated and 68.5% achieved SVR in the DAA era. CHC, chronic hepatitis C; DAA, direct-acting antiviral; IFN, interferon; SVR, sustained virological response.
Reasons for lack of treatment in otherwise eligible patients by treatment period
Within the IFN era, the major contributing reasons for lack of treatment in otherwise treatment eligible patients were loss to follow-up (45.1%) and patient choice not to be treated (41.2%) (online supplementary figure 2). A smaller proportion of patients were not treated due to insurance or financial difficulties (11.8%).
bmjgast-2017-000181supp003.jpg (66.3KB, jpg)
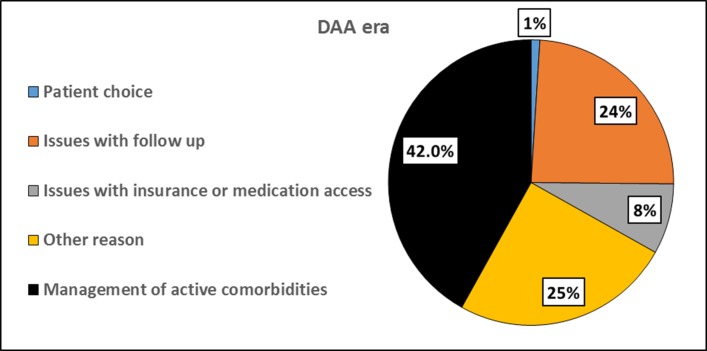
In the DAA era, the most common reason for lack of treatment was management of other active, potentially life-limiting comorbidities (42%) (figure 3). These comorbidities included both medical (active malignancies, depression, end stage renal disease, and recurrent pneumonia) and liver-related (HCC and severely decompensated cirrhosis) reasons. Approximately one-quarter of untreated patients were not treated due to issues with follow-up (24%). Eight per cent were not treated due to insurance or financial difficulties.
Figure 3.
Reasons for lack of treatment in the DAA era. In the DAA era, the most common reasons for lack of treatment were the management of other life-limiting comorbidities (42%) and issues with follow up (24%). DAA, direct-acting antiviral.
Reasons for delay in prescription fill during the DAA era
In the DAA era, 63 of 394 (16%) treated patients had substantial treatment delays as defined by a time period of 3 months or longer from the prescription to the fill date (online supplementary figure 3). The most common reason for treatment delay was insurance related (52%), followed by delays due to active comorbidities and concurrent medication use (13%). A smaller proportion of patients had treatment delays due to a lack of financial stability (3%). We were unable to ascertain the reason for treatment delay in 22% of patients.
bmjgast-2017-000181supp004.jpg (65.8KB, jpg)
HCV treatment predictors
Supplementary table 2 describes a multivariable logistic regression analysis for predicting treatment in the IFN era. Older age was a significant negative predictor for treatment with an OR of 0.97 (95% CI 0.96 to 0.98, P<0.001) per increase in year. Compared with Caucasians, Asian and African-American ethnicities were not associated with treatment; however, Hispanic ethnicity had lower odds of receiving treatment (OR=0.61, 95% CI 0.46 to 0.82, P<0.001). Compared with patients without decompensated cirrhosis, those with decompensated cirrhosis also had lower odds of receiving treatment (OR=0.59, 95% CI 0.47 to 0.73, P<0.001). The presence of two or more medical comorbidities was a predictor on univariate analysis (OR=0.71, 95% CI 0.51 to 0.98, P=0.039) but was non-significant when controlling for other factors on multivariable analysis (OR=0.9, 95% CI 0.82 to 1.2, P=0.54).
Table 2 describes potential predictors of treatment during the DAA era. On univariate analysis, African-American ethnicity, compared with Caucasians as a referent, was a negative predictor of treatment (OR=0.35, 95% CI 0.15 to 0.83, P=0.02). There was a trend towards males, compared with females, predicting treatment as well (OR=1.4, 95% CI 0.92 to 2.1, P=0.1). On multivariable analysis adjusting for gender, African-American ethnicity (OR=0.37, 95% CI 0.15 to 0.88 vs Caucasians, P=0.032) remained a significant negative predictor of treatment. Hispanic ethnicity, compared with Caucasians, also had lower odds of treatment (OR=0.66, 95% CI 0.41 to 1.1); however, this did not reach statistical significance (P=0.09). Unlike the regression analysis for IFN-based therapies, decompensated cirrhosis and medical comorbidities were not significant predictors.
Table 2.
Multivariate and univariate analysis of predictors of treatment in the DAA era
| Variables | OR Univariate | 95% CI | P value | OR Multivariate | 95% CI | P value |
| Older age (per year) | 0.98 | 0.96 to 1 | 0.2 | – | – | – |
| Male versus female | 1.4 | 0.92 to 2.1 | 0.1 | 1.3 | 0.87 to 2.1 | 0.2 |
| Ethnicity | ||||||
| Caucasian | Referent | Referent | ||||
| Asian | 0.8 | 0.42 to 1.6 | 0.4 | 0.8 | 0.41 to 1.5 | 0.5 |
| African-American | 0.35 | 0.15 to 0.83 | 0.02 | 0.37 | 0.15 to 0.88 | 0.02 |
| Hispanic | 0.66 | 0.35 to 0.93 | 0.09 | 0.66 | 0.41 to 1.1 | 0.09 |
| Others | 0.35 | 0.94 to 1.3 | 0.11 | 0.34 | 0.1 to 1.3 | 0.11 |
| Decompensated cirrhosis | 1.2 | 0.74 to 1.9 | 0.49 | – | – | – |
| ≥2 versus <2 medical comorbidities* | 1.33 | 0.78 to 2.3 | 0.31 | – | – | – |
| History of psychiatric disorder† | 1.1 | 0.73 to 1.7 | 0.65 | – | – | – |
*Medical comorbidities include coronary artery disease, chronic obstructive pulmonary disease, chronic kidney disease and diabetes mellitus.
†Psychiatric disorders include depression, bipolar disorder and schizophrenia.
DAA, direct-acting antiviral.
Discussion
In this retrospective cohort study of 3236 patients with CHC seen at an academic hepatology clinic, we found that treatment rates dramatically increased with the introduction of DAA therapies. Treatment rates of the DAA era, compared with those of the IFN era, increased over threefold from 22.3% to 76.6% and SVR rates increased from 55% to 88.8% among treated patients. The overall treatment effectiveness within our population increased considerably as well; while only 12 in 100 patients were cured of HCV during the IFN era, 68 of 100 patients were cured during the DAA era.
These increases in both treatment and SVR rates are consistent with those reported from a large population-based study of US veterans. In this study, Moon et al 7 reported higher SVR rates of over 90% and a 21-fold increase in 2015 in the number of antiviral prescriptions compared with those in 2010. This exponential increase was attributed to both the higher tolerability and lower treatment threshold of DAA-based therapies and also the increased US government funding to VA facilities to treat CHC.7
Similarly, we found a number of reasons why treatment and response rates have increased in our population. Cure rates with DAA-based therapies are consistently higher than IFN-based therapies. In our study, over 88% of patients treated during the DAA era were cured of HCV. Our findings may be slightly lower than those from clinical trials due to the small proportion of HCV genotype 3 patients and a small proportion of patients treated with IFN-based therapies (<2% of our DAA population); however, our DAA population was quite sick as over 75% of patients treated were experiencing decompensated cirrhosis during treatment.7 14–17
This is an especially significant finding for the use of DAAs as this is one of the first studies to be published on the use of DAAs in the patients with decompensated cirrhotic in the real-world clinical setting after the AASLD and Infectious Diseases Society of America (IDSA) guidelines recommended treatment should be considered for all patients with CHC. This finding further demonstrates that the recommendation can be followed in a safe and effective manner. In fact, in the IFN era, a history of decompensated cirrhosis decreased the odds of being treated with IFN-based therapy by nearly half (OR=0.59, P<0.001).
In contrast during the DAA era, treatment rates for patients with decompensated cirrhosis were actually slightly higher than that of patients without decompensation (77.4% for decompensated vs 74.4% for non-decompensated), which may represent a higher provider priority in treating this sick population. Recently, a large post hoc analysis of patients from phase 3 clinical trials suggests that treating these patients with advanced liver disease also significantly improves their patient-reported outcomes, which can translate into economic benefits viewed by health gains and improved worker productivity.18
Patients were also much less likely to refuse treatment. During the IFN era, approximately 41.2% of patients otherwise eligible for IFN chose not to be treated possibly owing to an overall lack of symptoms from the CHC combined with high rates of treatment toxicity of IFN-based therapies. It is important to note that in these studies, refusal rates were similar to older studies of IFN-treated populations.10 19 In contrast, only one person (<1%) in the DAA era in our study chose not to be treated.
Despite these optimistic findings, significant barriers and disparities remain from the IFN to DAA era. Issues with patient follow-up remained a significant reason for non-treatment. Although rates appear to have decreased considerably from the IFN period (45.1% vs 28.1% in the DAA era), patient retention becomes much more important now that highly efficacious DAA therapies are available for all CHC patients. Financial or insurance barriers were also present in our study as to why treatment was delayed for the DAA era patients. These are not surprising findings as Younossi et al 20 in a large retrospective observational study, reported that patients’ delay in treatment was related to problems with finances and/or insurance.
In addition, there were considerable ethnic disparities in treatment rates. In particular, African-Americans appeared undertreated compared with other ethnicities. On multivariate logistic regression analysis, compared with Caucasians, African-Americans were approximately 70% less likely to be treated. While this finding may be more institution specific, there are numerous studies that have described treatment discrepancies among ethnic minorities with IFN-based therapies.5 21 22 Lin et al also reported similar findings in a large VA population: African-Americans were significantly less likely to be treated than Caucasians at the VA. This was attributed to higher rates of ongoing drug use and patient reluctance to undergo treatment in the African-American patients.23
In this current study, African-Americans had much lower rates of decompensated cirrhosis than other ethnicities but had a disproportionately higher burden of HCC (supplementary table 1). While rates of decompensated cirrhosis and HCC in Caucasians were 71.6% and 12.7%, respectively, rates in African-Americans were 44% and 24%, respectively. Given their fewer symptoms, patients may have been more hesitant to start antiviral therapy, and providers may have been more reluctant to treat patients with HCC and especially those with more advanced disease.
Our study did have limitations related to its retrospective design. As we analysed comorbidity and treatment data using electronic querying, there may be some level of measurement error, especially with medical and psychiatric comorbidities as these were queried via ICD-9-CM coding. In addition, our inclusion of IFN-treated patients who did not achieve SVR in our DAA group may have inflated our treatment initiation rates in the DAA era. These IFN-experienced patients were among other ‘warehoused’ patients awaiting DAA therapy approval for treatment initiation. We also did not have certain sociodemographic and consistent viral characteristics including insurance payer and comprehensive data on HCV genotype, both of which have been described in recent reports as significant predictive factors for treatment in the current DAA era.20 24 In fact, Younossi et al’s study found that patients with commercial insurance were 6.5 times as likely to start sofosbuvir-based therapy compared with those with Medicaid.20
As we studied only patients evaluated in a subspecialty hepatology clinic, our findings may also underestimate the full impact of DAAs CHC’s total burden within the USA. First, as was apparent from our study, we excluded 65% of our population from primary analysis because they were not seen in our subspecialty hepatology clinic. Many of these excluded patients were seen for one time encounters (one time subspecialty surgery, single evaluation in the ER or single admissions on inpatient floors) without subsequent outpatient follow-up. Such a finding corroborates other recent studies in which the number of patients seen in a one-time setting such as the emergency room far exceeds the number of CHC patients seen in a subspecialty clinic.25 26 Linkage to care, as also seen in this study, is a large issue and needs further study to overcome this barrier to care.
Second, our inclusion criteria did not capture patients who were at high risk for HCV but went undiagnosed for the presence or absence of disease. Chhatwal et al 27 report in their modelling study based on the National Health and Nutrition Examination Survey data that CHC will exert considerable healthcare and economic burden largely because of the undiagnosed population. As such, they estimate over the next 35 years, 320 000 patients will die, 157 000 patients will develop HCC and 203 000 patients will develop decompensated cirrhosis.
In summary, the introduction of DAAs has significantly increased treatment and efficacy rates for patients seen at an academic hepatology clinic. Many patients considered ineligible for treatment with IFN-based therapies including those with decompensated cirrhosis are now easily treated with DAAs. However, despite the availability of DAAs, there remains considerable treatment challenges including patient retention and ethnic disparities. Improving patient-level and systems-level factors to better identify patients with CHC and ensuring patient follow-up is now even more important to extend the impact of DAA therapies and eradicate CHC.
Footnotes
PN and PV contributed equally.
Contributors: PN, PV and MHN have conceived and designed this study. LAY and SW contributed in acquisition of data, queried the electronic data and identified patients appropriate for chart review. PN, JH, ST and LH collected individual patient information. PN, PV and MHN conducted analysed and interpreted the data. All authors drafted and/or revised the manuscript. All authors have approved this draft of the manuscript for submission.
Competing interests: MHN: grant/research support: Bristol Myers Squibb, Gilead Sciences, Janssen Pharmaceuticals and National Cancer Institute; advisory board/consultant: Anylam Pharmaceutical, Gilead Sciences, Dynax Laboratories and Intercept Pharmaceutical.
Ethics approval: This study was approved by the institutional review board at Stanford University.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Our dataset is available as a supplementary file in Excel format.
References
- 1. Butt AA, Justice AC, Skanderson M, et al. Rate and predictors of treatment prescription for hepatitis C. Gut 2007;56:385–9. doi:10.1136/gut.2006.099150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feillant M, Jézéquel C, Lison H, et al. Chronic hepatitis C: treat or wait? A prospective study on reasons for treatment or nontreatment in the era of first-generation protease inhibitors. Eur J Gastroenterol Hepatol 2016;28:164–72. doi:10.1097/MEG.0000000000000506 [DOI] [PubMed] [Google Scholar]
- 3. Ghany MG, Strader DB, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 2009;49:1335–74. doi:10.1002/hep.22759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kramer JR, Kanwal F, Richardson P, et al. Gaps in the achievement of effectiveness of HCV treatment in national VA practice. J Hepatol 2012;56:320–5. doi:10.1016/j.jhep.2011.05.032 [DOI] [PubMed] [Google Scholar]
- 5. Vutien P, Hoang J, Brooks L, et al. Racial disparities in treatment rates for chronic hepatitis C: analysis of a population-based cohort of 73,665 patients in the United States. Medicine 2016;95:e3719 doi:10.1097/MD.0000000000003719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet 2014;384:1756–65. doi:10.1016/S0140-6736(14)61036-9 [DOI] [PubMed] [Google Scholar]
- 7. Moon AM, Green PK, Berry K, et al. Transformation of hepatitis C antiviral treatment in a national healthcare system following the introduction of direct antiviral agents. Aliment Pharmacol Ther 2017;45:1201–12. doi:10.1111/apt.14021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grebely J, Bryant J, Hull P, et al. Factors associated with specialist assessment and treatment for hepatitis C virus infection in New South Wales, Australia. J Viral Hepat 2011;18:e104–116. doi:10.1111/j.1365-2893.2010.01370.x [DOI] [PubMed] [Google Scholar]
- 9. Gupta V, Kumar A, Sharma P, et al. Most patients of hepatitis C virus infection in India present late for interferon-based antiviral treatment: an epidemiological study of 777 patients from a North Indian tertiary care center. J Clin Exp Hepatol 2015;5:134–41. doi:10.1016/j.jceh.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khokhar OS, Lewis JH. Reasons why patients infected with chronic hepatitis C virus choose to defer treatment: do they alter their decision with time? Dig Dis Sci 2007;52:1168–76. doi:10.1007/s10620-006-9579-1 [DOI] [PubMed] [Google Scholar]
- 11. Yan KK, Wong GL, Wong VW, et al. Rate and factors affecting treatment uptake of patients with chronic hepatitis C in a tertiary referral hospital. Dig Dis Sci 2010;55:3541–7. doi:10.1007/s10620-010-1412-1 [DOI] [PubMed] [Google Scholar]
- 12. Strader DB, Wright T, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C. Hepatology 2004;39:1147–71. doi:10.1002/hep.20119 [DOI] [PubMed] [Google Scholar]
- 13. Panel AIHG. AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 2015;62:932–54. doi:10.1002/hep.27950 [DOI] [PubMed] [Google Scholar]
- 14. Gane EJ, Stedman CA, Hyland RH, et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med 2013;368:34–44. doi:10.1056/NEJMoa1208953 [DOI] [PubMed] [Google Scholar]
- 15. Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med Overseas Ed 2013;368:1878–87. doi:10.1056/NEJMoa1214853 [DOI] [PubMed] [Google Scholar]
- 16. Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med 2014;370:211–21. doi:10.1056/NEJMoa1306218 [DOI] [PubMed] [Google Scholar]
- 17. Su F, Beste LA, Green PK, et al. Direct-acting antivirals are effective for chronic hepatitis C treatment in elderly patients: a real-world study of 17 487 patients. Eur J Gastroenterol Hepatol 2017;29:686–93. doi:10.1097/MEG.0000000000000858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Younossi ZM, Stepanova M, Feld J, et al. Sofosbuvir and velpatasvir combination improves patient-reported outcomes for patients with HCV infection, without or with compensated or decompensated cirrhosis. Clin Gastroenterol Hepatol 2017;15:421–30. doi:10.1016/j.cgh.2016.10.037 [DOI] [PubMed] [Google Scholar]
- 19. Kanwal F, Hoang T, Spiegel BM, et al. Predictors of treatment in patients with chronic hepatitis C infection - role of patient versus nonpatient factors. Hepatology 2007;46:1741–9. doi:10.1002/hep.21927 [DOI] [PubMed] [Google Scholar]
- 20. Younossi ZM, Bacon BR, Dieterich DT, et al. Disparate access to treatment regimens in chronic hepatitis C patients: data from the TRIO network. J Viral Hepat 2016;23:447–54. doi:10.1111/jvh.12506 [DOI] [PubMed] [Google Scholar]
- 21. Cheung RC, Currie S, Shen H, et al. Chronic hepatitis C in Latinos: natural history, treatment eligibility, acceptance, and outcomes. Am J Gastroenterol 2005;100:2186–93. doi:10.1111/j.1572-0241.2005.00240.x [DOI] [PubMed] [Google Scholar]
- 22. Rousseau CM, Ioannou GN, Todd-Stenberg JA, et al. Racial differences in the evaluation and treatment of hepatitis C among veterans: a retrospective cohort study. Am J Public Health 2008;98:846–52. doi:10.2105/AJPH.2007.113225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin M, Kramer J, White D, et al. Barriers to hepatitis C treatment in the era of direct-acting anti-viral agents. Aliment Pharmacol Ther 2017;46:992–1000. doi:10.1111/apt.14328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lo Re V, Gowda C, Urick PN, et al. Disparities in absolute denial of modern hepatitis C therapy by type of insurance. Clin Gastroenterol Hepatol 2016;14:1035–43. doi:10.1016/j.cgh.2016.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hsieh YH, Rothman RE, Laeyendecker OB, et al. Evaluation of the centers for disease control and prevention recommendations for hepatitis C virus testing in an urban emergency department. Clin Infect Dis 2016;62:1059–65. doi:10.1093/cid/ciw074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Younossi ZM, LaLuna LL, Santoro JJ, et al. Implementation of baby boomer hepatitis C screening and linking to care in gastroenterology practices: a multi-center pilot study. BMC Gastroenterol 2016;16:45 doi:10.1186/s12876-016-0438-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chhatwal J, Wang X, Ayer T, et al. Hepatitis C Disease Burden in the United States in the era of oral direct-acting antivirals. Hepatology 2016;64:1442–50. doi:10.1002/hep.28571 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgast-2017-000181supp001.docx (16.5KB, docx)
bmjgast-2017-000181supp002.jpg (99.8KB, jpg)
bmjgast-2017-000181supp003.jpg (66.3KB, jpg)
bmjgast-2017-000181supp004.jpg (65.8KB, jpg)