Abstract
Men and women exhibit significant differences in obesity, cardiovascular disease and diabetes. To provide better diagnosis and treatment for both sexes, it is important to identify factors that underlie the observed sex differences. Traditionally, sex differences have been attributed to the differential effects of male and female gonadal secretions (commonly referred to as “sex hormones”), which do substantially influence many aspects of metabolism and related diseases. Less appreciated as a contributor to sex differences are the fundamental genetic differences between males and females, which are ultimately determined by the presence of an XX or XY sex chromosome complement. Here we review the mechanisms by which gonadal hormones and sex chromosome complement each contribute to lipid metabolism and associated diseases, and the current approaches that are used to study them. We focus particularly on genetic approaches including genome-wide association studies in humans and mice, “–omics” and “systems genetics” approaches, and unique experimental mouse models that allow distinction between gonadal and sex chromosome effects.
Keywords: gonadal hormones, sex chromosome complement, metabolic syndrome, adipose tissue, glucose metabolism, microbiome
I. Sex and metabolism
a. Why is it important to understand sex differences in metabolism?
The most prevalent diseases in developed countries are cardiovascular disease, diabetes, and some forms of cancer (35, 95). The incidence of these diseases increases in the presence of a group of risk factors known as the Metabolic Syndrome (MetSyn). MetSyn is defined by the presence of three of the following five risk factors: abdominal obesity, elevated triglyceride levels, low high density lipoprotein (HDL) cholesterol levels, high blood pressure (hypertension), and elevated fasting blood glucose levels (73). Sex differences exist in nearly all of the components of MetSyn (Figure 1). For example, females have evolved specific mechanisms to favor adipose tissue storage, whereas mobilization of fat stores tends to be more efficient in males (40, 60, 77, 90). Compared to men, women tend to have greater insulin sensitivity, and the sexes differ in lipoprotein profiles (30, 85). It has become clear that it is critical to understand sex differences in metabolism to optimize prevention, diagnosis, and therapeutic intervention for both sexes.
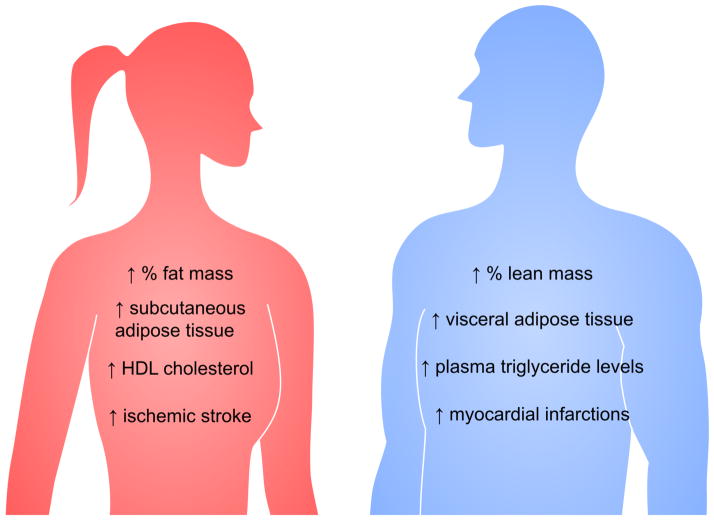
Figure 1. Sex differences in metabolic syndrome (MetSyn) components.
Risk factors for the MetSyn include visceral obesity, elevated triglyceride levels, low high density lipoprotein levels, hypertension, and elevated fasting glucose levels. Sex differences occur in most of these traits. Women tend to have increased fat mass proportional to their body weight, increased subcutaneous adipose tissue, and elevated HDL cholesterol levels. Men generally have greater proportional lean mass, increased visceral adipose tissue, and elevated plasma triglyceride levels. Cardiovascular disease incidence also differs by sex, with women having higher incidence of ischemic stroke, and men higher incidence of myocardial infarction.
Here we review sex differences in obesity, other components of the MetSyn, and related factors such as the gut microbiome, with an emphasis on the genetic mechanisms underlying these differences. In the sections below, we first discuss the components of sex and approaches used to study sex effects. We then review the sex differences in obesity and other MetSyn components in humans, and highlight studies in experimental models that shed light on the mechanisms involved.
b. Components of sex: gonadal hormones and sex chromosome complement
An understanding of why one sex is more susceptible to specific disease processes requires analysis of the component parts of sex. Differences between men and women can be influenced by sex and gender. Sex refers to the biological attributes of males and females that result from the presence of female (XX) or male (XY) sex chromosomes, which ultimately determine the levels and types of gonadal hormones (Figure 2). Gender refers to characteristics that a society or culture defines as masculine or feminine. In humans in particular, gender may influence behaviors that affect metabolism and related diseases. However, gender effects are challenging to assess experimentally and data are lacking on their effects on metabolism, and we will therefore limit our review to the biological components of sex differences.
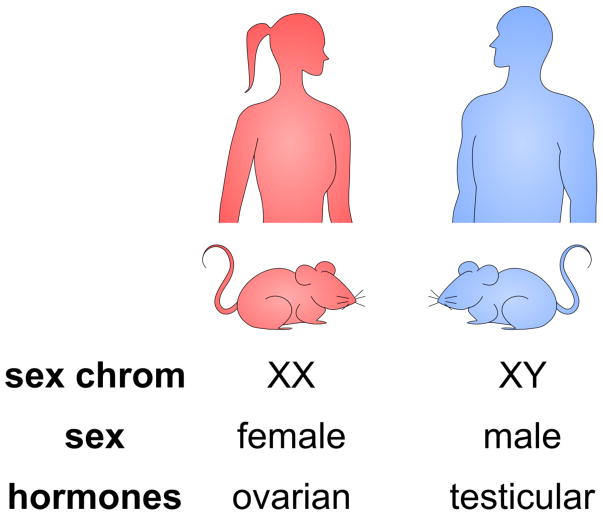
Figure 2. Genetic and hormonal components of sex.
Male and female sex differences may result from genetic or hormonal components. Normal females and males differ in their sex chromosome complement of XX or XY, respectively. They also differ in the presence of ovarian or testicular gonadal hormones. In standard humans and mouse models, the genetic and gonadal contributions to sex differences cannot be easily distinguished.
The most widely studied sex difference between males and females has been the effect of gonadal hormones. In human studies, the contribution of gonadal hormones to a trait of interest is often addressed by comparing women before and after the age of menopause, with differences between the two attributed to the reduced levels of ovarian hormones after menopause. Studies of this sort have demonstrated that post-menopausal women have altered body fat distribution and increased incidence of cardiovascular disease, hypertension, diabetes and other disorders (41, 68, 74, 122). The levels of androgens also influence metabolic disease. Testosterone levels typically diminish in men with age, and low testosterone levels in men are associated with increased body fat and cardiovascular disease (3, 12, 99). However, these studies are confounded by the fact that the pre- and post-menopausal (or high and low testosterone) groups usually constitute different individuals due to the difficulty of performing longitudinal studies for several years in humans, and comparisons between groups could therefore be influenced by genetic and environmental heterogeneity. Studies in humans are also complicated by the fact that reductions in gonadal hormone levels in older men and women cannot necessarily be distinguished from independent changes associated with aging.
In addition to gonadal hormones, sex chromosome complement could contribute to sex differences in metabolic traits (8, 69). It has been difficult to address whether hormone-independent effects of gonadal sex occur in humans because, typically, XX chromosomes and ovaries occur together, and XY chromosomes and testes occur together (Figure 2). Some studies of metabolic factors have been performed on individuals with sex chromosome anomalies (Turner syndrome or Klinefelter syndrome), and have reported increased adiposity and other features of MetSyn (13, 14, 19). However, it is difficult to tease apart the role of sex chromosome complement from gonadal hormones in these individuals because the alterations in chromosome complement occur in concert with abnormal hormones levels. Individuals with Turner syndrome (45, XO) have low endogenous levels of ovarian hormones such that it is impossible to distinguish the effects of the single X chromosome from those resulting from abnormal ovarian hormone levels (44, 48). Furthermore, Turner syndrome subjects often have congenital heart defects, which lead to heart disease independent of MetSyn (31). Individuals with Klinefelter syndrome (47, XXY) have reduced endogenous androgen levels and are often treated with exogenous androgens, which makes it difficult to distinguish effects of the XXY chromosome complement from those of the altered androgen levels (53). In addition to the factors outlined above, the interpretation of metabolic disease traits in Turner and Klinefelter subjects has been limited by small available cohort sizes that preclude meaningful conclusions about metabolic parameters.
c. Approaches to study sex differences
To overcome some of the limitations in studying the components of sex effects in humans, experimental animal models are valuable; unique genetic tools in the mouse make it a particularly useful animal model. Unlike humans, inbred mice allow an analysis of high and low gonadal hormone levels on the identical genetic background. The action of gonadal hormones can be classified into two temporal classes: the permanent effects of gonadal hormones that lead to the development of sex differences during fetal and neonatal development, and the acute actions of gonadal hormones that cause specific responses on numerous processes throughout life. The acute effects of gonadal hormones are reversible, and a standard way to identify acute hormone effects is to compare adult mice with intact gonads to those from which gonads were removed in adulthood. Those sex differences that are caused by acute hormonal effects will be abolished in gonadectomized mice, whereas sex differences resulting from the permanent effects of gonads during development will remain.
The origin of male and female gonads and the hormones that they produce traces back to the presence of either XX or XY sex chromosomes (Figure 2). Furthermore, at a more fundamental level than hormone action, simply the presence of either XX or XY chromosomes leads to sex differences at the cellular level because of the differential action of genes on the X and Y chromosomes (7). For example, during early embryonic growth prior to the presence of gonads or gonadal hormones, male embryos are larger than female embryos in mice, humans, and several other mammals (16). In the mouse, these differences have been attributed to a combination of effects from the presence of the Y chromosome in male embryos and the sexual imbalance between the number of X chromosomes in males vs. females (17, 18). Following differentiation of the gonads, it is difficult to cleanly separate effects due to hormones from those resulting from the sex chromosome complement. This led to the generation of mouse models that allow the independent segregation of gonadal type from sex chromosome complement.
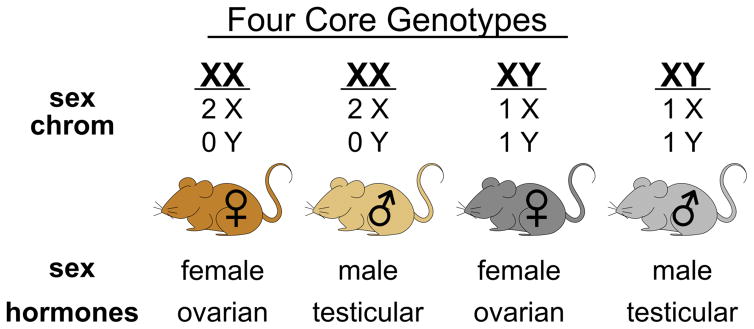
The most widely used model to distinguish gonadal and chromosome sex effects is known as the Four Core Genotypes (FCG) mouse model (Figure 3). This model generates mice having XX chromosomes on both male and female gonadal backgrounds and XY mice on male and female gonadal backgrounds (4–6). This is accomplished by using a Y chromosome from which the testis-determining Sry gene has been deleted. Inheritance of the Sry-deleted Y chromosome fails to specify male gonads, and female gonads develop instead, allowing production of XY female mice. In addition, an Sry transgene is inserted into chromosome 3, such that it segregates independently of the sex chromosomes (56). Inheritance of the Sry transgene, even in the presence of XX sex chromosomes, results in development of male gonads. The FCG model is valuable to identify whether sex differences in a trait of interest are associated with gonadal type or sex chromosome complement (Figure 4). For example, if a trait is influenced by gonadal sex, male mice with XX or XY chromosomes will be similar, but differ from female mice with XX or XY chromosomes. However, if a trait is influenced by sex chromosome complement, XX female and male mice will be similar to one another, but differ from XY female and male mice (Figure 4). This model also allows the detection of interactions between gonadal hormones and chromosomal sex, and the effects of acute hormones can be assessed by comparing gonadally intact with gonadectomized mice, as described above. This model has revealed that sex differences in obesity, lipid levels, fatty liver, glucose homeostasis and other metabolic traits are influenced by XX vs. XY chromosome complement (Figure 5; described in later sections).
Figure 3. Distinction of gonadal and chromosomal contributions to sex differences using the Four Core Genotypes mouse model.
The Four Core Genotypes mouse model breaks sex into gonadal type and sex chromosome complement as independent determinants. The four genotypes can be used to study the contribution of gonadal and chromosomal sex to traits of interest.
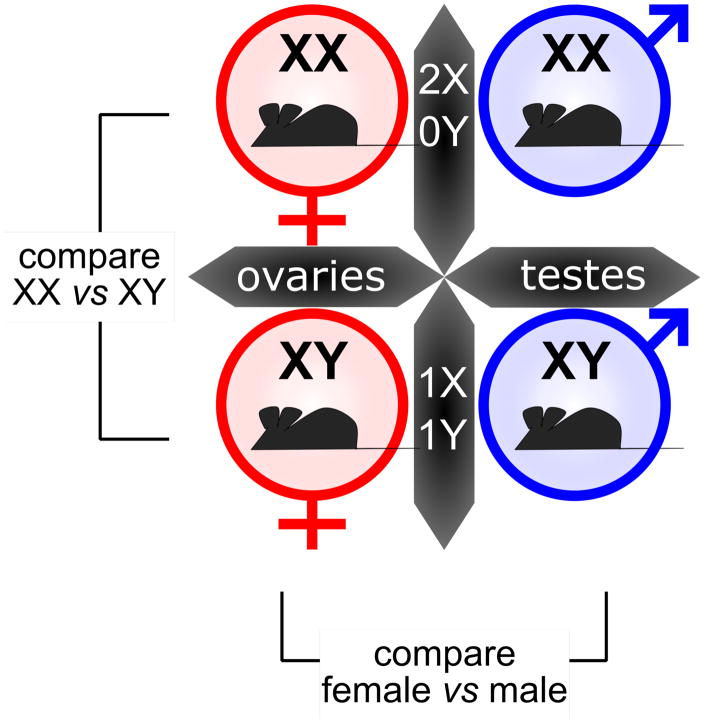
Figure 4. Using the Four Core Genotypes mouse model to study sex differences.
Studies with the Four Core Genotypes are performed in a 2 x 2 comparison, using gonadal type (ovaries vs. testes) and chromosomal type (XX vs. XY) as the categories. A comparison of XX vs. XY mice (on both gonadal backgrounds) allows detection of effects due to sex chromosome complement. A comparison of female vs. male mice (with both sex chromosome genotypes) allows detection of effects due to gonadal type.
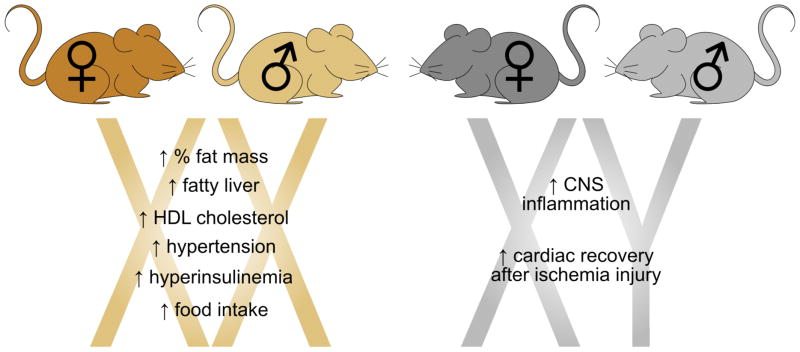
Figure 5. Sex chromosome complement confers sex differences in MetSyn traits.
Studies using the Four Core Genotypes mouse model have revealed that sex differences in some traits associated with MetSyn are influenced by the sex chromosome complement. For example, XX mice, regardless of having ovaries or testes, have increased fat mass relative to body weight, are susceptible to fatty liver, and exhibit increased HDL cholesterol. XY mice have been shown to have greater inflammation in the central nervous system (33) and improved cardiac recovery after ischemia injury (67).
II. Genetic contributions to sex differences in adiposity and obesity
a. Sex-specific obesity loci
The heritability of obesity is estimated to be about 50–70%, indicating that genetics play a large role in fat accumulation (reviewed in 38). Recently, large-scale genome-wide association studies (GWAS) have identified loci linked to fat accumulation and distribution. A large meta-analysis of 32 GWAS, with a follow-up of 16 loci in 29 additional studies, identified 14 genome-wide significant loci that were associated with waist-to-hip ratio, a proxy for adipose distribution (50). In sex-specific meta-analyses, 12 of these 14 single-nucleotide polymorphisms (SNPs) were significant in women, while only three SNPs were significant in men. The 14 loci collectively explained 1.34% of the waist-to-hip ratio variance in women and 0.46% of the variance in men. Subsequent studies confirmed these loci in genome-wide associations with subcutaneous and visceral adipose tissue measured by CT scan (37, 104). When sex-stratified studies were performed, the reported loci had statistically significant associations with fat distribution in women, but not in men. Concordant with these findings, another large meta-analysis of nearly 225,000 individuals reported 49 loci associated with waist-hip ratio adjusted for body mass index, and 20 of these 49 loci had significant sexual dimorphism (103). 19 out of 20 sexually dimorphic loci had a larger effect in women than in men. Taken together, these GWAS findings highlight the importance of performing sex-stratified GWAS to better understand genetic contributors of adiposity. These findings also emphasize that a large proportion of the genetic determinants of obesity in both sexes remain to be identified.
b. Sex differences in regional adipose tissue deposition
It is well appreciated that there are inherent differences between men and women in fat distribution and properties of fat cells within anatomical depots (Figure 1). When normalized to waist circumference or to total body fat, men have more visceral adipose tissue (fat within the abdominopelvic cavity) than pre-menopausal women (64, 66). Men also tend to lose relatively more visceral adipose tissue due to calorie restriction than women (63). By contrast, women typically have more subcutaneous adipose tissue (fat underlying the dermis) (66). Numerous epidemiological studies have linked increased visceral fat to MetSyn, and others have suggested that subcutaneous adipose tissue may even play a protective role against glucose dysregulation and other components of the MetSyn (38, 71, 96, 108). Thus, in the basal state, the greater subcutaneous to visceral adipose tissue composition in women may be a beneficial trait relative to MetSyn development.
Beyond sex differences in fat mass and distribution, metabolic differences have been identified in adipocyte biology between men and women that ultimately may contribute to the likelihood for abdominal obesity that contributes to MetSyn. For example, a comparison of adipocytes isolated from the subcutaneous and visceral compartments of abdominal fat in healthy women showed that the intraperitoneal adipocytes were 20–30% smaller than those from the subcutaneous depot, whereas men exhibit a similar adipocyte size from these two areas of abdominal fat (106). This is relevant because larger adipocytes have higher rates of lipolysis, are correlated with insulin resistance, and have increased expression of proinflammatory adipokines. It is possible that changes in the basal characteristics of adipocytes in women with abdominal obesity are highly detrimental and contribute to the development of MetSyn.
c. Gonadal hormone signaling effects on adiposity and potential effectors
The mechanisms underlying sex differences in adiposity have been investigated extensively in experimental models by manipulating receptors for gonadal hormone signaling. In particular, ablation of estrogen and androgen receptors in the brain and peripheral tissues in the mouse results in striking metabolic outcomes. For example, knockout of estrogen receptor α (ERα) resulted in increased adiposity in both male and female mice (51, 87). Knockout of ERα in hypothalamic neurons also lead to dysregulation of energy balance, resulting in increased abdominal fat (84, 117). While deficiency of ERα affects both sexes, deficiency of androgen receptor results in late-onset obesity only in males (36). Interestingly, adipose-specific knockout of androgen receptor causes hyperleptinemia, but not increased adiposity. This suggests that androgen receptor is required in adipocytes for normal levels of leptin, but not for expansion of fat tissue.
While gonadal hormones have an irrefutable role in shaping and distributing fat, the specific mechanisms have not been fully elucidated. One possible mechanism is a role for gonadal hormones, along with nutrients and other signals, as cues to signal adipocyte progenitor cells to proliferate and/or differentiate. Indeed, ovarian hormones appear to influence fad pad expansion in a sex-specific manner (57). In mice fed a high fat diet, adipocyte precursor cells proliferate and differentiate into mature adipocytes to a much greater degree than in mice fed a standard chow diet, as would be expected. Interestingly, the removal of acute hormonal effects in females by ovariectomy blunts the proliferative capacity of preadipocytes in the subcutaneous fat pad, but not the visceral fat pad. These results suggest that gonadal hormones affect precursor cells differentially in distinct fat depots. In addition, elegant adipose tissue transplant studies revealed that adipocyte precursor cell behavior is dictated by the recipient depot, implicating environment rather than cell-intrinsic properties as a determinant of fat tissue hyperplasia (57).
Another potential effector of sex hormone effects on adipose tissue depot expansion may be the enzyme aldehyde dehydrogenase 1 (Aldh1a1) (119). Deletion of Aldh1a1 abrogated diet-induced expansion of subcutaneous fat in male and female mice compared to wild-type animals. However, compared to the corresponding wild-type mice, visceral fat mass was reduced in female but not male Aldh1a1−/− mice. The female-specific reduction in visceral fat of Aldh1a1−/− mice was attributed to differential expression of adipose triglyceride lipase, which was likely induced by a buildup of retinaldehyde in Aldh1a1−/− females. The authors postulate that the retinoic acid produced by Aldh1a1 action may activate retinoic acid receptor response elements (such as in the promoter of the PPARγ gene) to a greater degree in female compared to male visceral adipose tissue. While this study identified an interesting potential role of retinoic acid in mediating sex differences in fat depots, more research is needed to understand how sex-biasing factors, such as sex hormones and sex chromosomes, interact with retinaldehyde and retinoic acid signaling pathways.
Sex-biasing factors, such as sex hormones and sex chromosomes, likely mediate sexually dimorphic traits via changes in gene expression. Thousands of sexually dimorphic genes have been identified in adipose tissue by global gene expression analysis in mice (45, 118). These genes were enriched in functional categories such as immune response, lipid metabolism, and insulin signaling. The mice used in these studies were fed a high fat diet (45–47% calories from fat) to increase adiposity, such that some of the observed sexually dimorphic gene expression may reflect sex-dependent responses to nutritional excess and the ensuing metabolic dysregulation. Interestingly, only about 100 genes were differentially expressed between ovariectomized females and gonadally intact females, suggesting that most sexually dimorphic genes are not regulated by circulating gonadal hormones (45).
One factor that may contribute to sexually dimorphic gene expression is regulator of sex-limitation 1 (RSL1), a zinc finger protein that affects the expression of many transcripts in liver and adipose tissue. Deletion of Rsl1 in mice results in sex-specific weight gain (61). Rsl1−/− females gain significantly more weight compared to wild-type females, while Rsl1−/− and wild-type males have equal weight gain on a high fat diet. In addition, Rsl deficiency enhanced sexual dimorphism in adipose tissue gene expression compared to wild-type mice, suggesting that RSL1 normally suppresses sex differences in adipose tissue.
d. Sex chromosome complement influences adiposity
The obesity loci discovered in GWAS studies and the studies from experimental models discussed above account for a small proportion of the estimated genetic heritability of obesity. The missing heritability may arise from a number of factors, including a lack of power to detect combined effects of numerous loci, which individually, may have insignificant associations with adiposity traits. In addition, few GWAS reports stratify associations by sex, thus potentially obscuring our understanding of complex diseases. To determine genetic causes of sex differences in adiposity, one must consider the fundamental definition of sex in a cell: the sex chromosome complement.
In many cases, the X chromosome is not included in GWAS studies, because arrays do not have enough X chromosome variants, or because imputation is needed for combined-sex studies (116). This practice impedes discovery of novel associations, especially with sexually dimorphic traits such as adiposity. There are more than 1,600 coding and hundreds of noncoding genes on the X chromosome, which represent greater than 5% of all human genes. The number of X chromosomes, the ability to randomly inactivate an X chromosome, and X chromosome imprinting are sources of sex differences between females and males that reside in each nucleated cell of every tissue.
Mouse models have been used to demonstrate an effect of XX vs. XY chromosome complement on fat accumulation and obesity co-morbidities. Chen et al. used the Four Core Genotypes mouse model (Figure 3) to distinguish effects of sex hormones from effects of sex chromosomes on obesity (21). All genotypes were on an inbred C57BL/6 background, ruling out genetic differences besides sex chromosome complement. To remove acute effects of circulating hormones, mice were gonadectomized in early adulthood. Mice with two X chromosomes, regardless of whether they originally had ovaries or testes, gained nearly twice as much fat as mice with X and Y chromosomes (Figure 5). Remarkably, the enhanced weight gain in XX mice was observed on a standard chow diet. The weight difference between XX and XY mice was amplified by a high fat diet, becoming statistically significant after merely 3 days on the diet. The increased obesity in XX mice was associated with increased food intake during the light (inactive) phase of the circadian cycle (21, 22). Along with increased adiposity, XX mice developed fatty liver and had elevated levels of fasting insulin (Figure 5), both of which are co-morbidities of obesity in humans.
The provocative finding that XX mice have greater adiposity than XY mice raised the question of whether the effect was due to the presence of two X chromosomes, or to the absence of a Y chromosome. To address this, investigators generated mice with XX, XY, XO and XXY sex chromosome complements. When body weight of these mice was followed for several months following gonadectomy, the XX and XXY mice had greater body weight than XY and XO mice (21). Thus, it was the presence of two X chromosomes that drove increased body weight, irrespective of inclusion of a Y chromosome. Interestingly, another mouse model of altered sex chromosome number also showed increased body weight in mice with two X chromosomes compared to those with one X, regardless of the presence of a Y chromosome (23).
The studies described above implicate X chromosome dosage as a risk factor for obesity. It will be important to further define the mechanisms involved. One hypothesis is that increased expression of genes escaping X chromosome inactivation in XX mice may cause phenotypic differences between XX and XY mice (21, 69, 70). This can be investigated by modulating the dosage of individual genes that escape X chromosome inactivation in cell or mouse models to determine effects on downstream gene expression and physiology.
III. Sex differences in obesity co-morbidities
In this section, we will review sex differences in co-morbidities of obesity that are also components of MetSyn. The prevalence of MetSyn varies across world populations, and it is clear that it is influenced by ethnicity, sex, age and socioeconomic status (47, 97, 98). The prevalence of MetSyn in the U.S. population is similar in age-adjusted men and women (34.9% of men, 33.3% of women) (82), but this may differ in other countries (98). The combination of components that most often lead to a diagnosis of MetSyn differ between the sexes (97, 98). For example, in women, the predominant component of MetSyn is abdominal obesity (often measured as waistline circumference), whereas in men, the predominant component is hypertension (62). In both men and women, these two components occur most frequently in combination with low HDL cholesterol levels and elevated triglyceride levels to form the triad of factors used to define MetSyn. Additional components of the MetSyn and other obesity co-morbidities have also been found to exhibit substantial sex differences in both humans and mouse models, as reviewed below and summarized in Figure 1.
a. Plasma lipid levels
Dyslipidemia is a key component of the MetSyn and often occurs together with obesity. A widely held generalization regarding lipid levels and cardiovascular disease is that elevated levels of low density lipoproteins (LDL) are detrimental, whereas elevated high density lipoproteins (HDL) are beneficial (20). However, the reality is more complex than this. Recent work indicates that the context in which HDL levels occur (i.e., levels of LDL and triglycerides) influences the utility of HDL as a predictor of cardiovascular disease risk (9). Furthermore, specific subspecies of each of the lipoprotein classes appear to be more relevant to cardiovascular disease prediction than others. In the broadest terms, pre-menopausal women are found to have higher levels of HDL cholesterol than men, and men have higher LDL cholesterol levels (11, 39, 43, 58, 75). Within the lipoprotein classes are subclasses that differ in size, lipid and protein composition, and potentially, function. An examination of the lipoprotein subclasses reveals that men tend to have a profile that includes several characteristics associated with cardiovascular disease risk (small LDL, small HDL, large very low density lipoproteins (VLDL)), whereas women are likely to likely to have higher concentrations of large HDL particles, which are associated with athero-protection (58).
It is clear that gonadal hormones (either directly or indirectly) influence the lipoprotein profile. Lipoprotein profiles are altered in women after menopause, and this can be partially reversed by exogenous hormone replacement therapy (11, 24, 29, 113). Androgen levels in men also appear to influence lipid levels and cardiovascular disease risk. Conflicting data exist, but at least some data indicate that androgen action is not likely the major determinant of LDL cholesterol levels (54, 81, 113, 115). Much work remains to be done to determine the relevance of specific lipid profile characteristics on cardiovascular disease risk in men and women. Understanding these mechanisms is important, however, as it will inform therapeutic approaches and management of cardiovascular disease.
Toward understanding basic mechanisms that influence lipid levels differentially in males and females, animal models may provide some insight. For example, a study performed in the Four Core Genotype mouse model identified some aspects of lipid levels that are determined predominantly by presence of male or female gonads, and other aspects that are determined by the presence of 2 X chromosomes (as found in XX animals) compared to 1 X chromosome (as found in XY animals) (70). While humans transport the majority of cholesterol in the form of LDL particles, mice transport the majority of cholesterol in the form of HDL particles. However, when fed a cholesterol-enriched diet, mice increase LDL cholesterol levels substantially to levels resembling humans. Studies of lipid levels in mice of the Four Core Genotypes (XX female, XX male, XY female, and XY male mice) revealed that under basal conditions in which the mice were fed a chow diet, plasma HDL cholesterol levels were higher in animals with XX vs. XY chromosome complement, and also in mice with male vs. female gonads (Figure 5). LDL cholesterol levels, which were only about one-fifth of HDL levels on the chow diet, were similar across the four genotypes, whereas triglyceride and free fatty acid levels were higher in males compared to females, regardless of the sex chromosome complement. To determine the contribution of acute-acting gonadal hormones, adult mice were gonadectomized. This led to a loss of male/female differences, and accentuated the effects of XX chromosome complement on promoting higher HDL cholesterol and free fatty acid levels. When fed a cholesterol-enriched diet, LDL cholesterol levels increased and were similar among the four genotypes. However, XX mice had higher levels of HDL cholesterol, whereas XY mice had higher levels of triglycerides and free fatty acids. Importantly, using a separate mouse model with XX, XXY and XY genotypes, it was demonstrated that HDL cholesterol levels are elevated in XX and XXY mice compared to XY mice, indicating that the presence of two X chromosomes, rather than absence of a Y chromosome, confers the high HDL phenotype (70). These results reveal that specific components of the lipid profile may be influenced independently by gonadal hormones and/or sex chromosomes, and that the underlying sex chromosome complement, rather than sex hormones, may be a key determinant of HDL cholesterol levels.
b. Glucose dysregulation
A common co-morbidity of obesity is impaired glucose homeostasis and insulin resistance. The standard measures of glucose homeostasis are determinations of fasting glucose levels or glucose tolerance, the latter measured as the kinetics of glucose clearance from the blood following an acute glucose load. Abnormal values for either of these traits may indicate impaired glucose metabolism, but the mechanisms underlying each are distinct. Indeed, only a minority of individuals with dysregulated glucose metabolism (about one-fourth) exhibit impairment in both fasting glucose and glucose tolerance (49). Elevated fasting glucose levels could result from inadequate levels of insulin secretion and/or impaired hepatic insulin sensitivity, leading to dysregulated glucose production by the liver. By contrast, impaired glucose tolerance is more likely a result of peripheral insulin resistance at the sites of post-prandial glucose disposal, especially skeletal muscle. Notably, men are more likely to develop elevated fasting glucose levels, whereas women are more likely to develop impaired glucose tolerance, as shown in a meta-analysis of more than 20 studies from European and Asian populations (111). Assessments of insulin resistance show greater occurrence in men than women, even after adjusting for age and body mass index (42, 59, 80, 86). Key mechanisms that have been implicated include effects of gonadal hormone-dependent and -independent sex differences in regional adipose tissue distribution, production of cytokines and adipokines, hepatic gluconeogenesis and glycogenolysis, and glucose uptake by skeletal muscle (1, 86, 102). Sex differences in the mechanisms of dysglycemia raise a concern that diagnosis of MetSyn based on fasting glucose levels may not reliably identify women that have MetSyn and should undergo some intervention.
Sex differences in glucose metabolism have also been described in rodent models. In a screen of ~100 inbred mouse strains, Park et al. compared males and females for the development of insulin resistance as assessed by HOMA-IR after feeding a high fat/high carbohydrate diet (93). In nearly all strains, males were more prone to insulin resistance than females. HOMA-IR was correlated with total body fat and mesenteric fat pad weights in both males and females, but only females showed a strong correlation with additional visceral fat pads (gonadal and retroperitoneal fat depots). A set of three inbred mouse strains were studied with intact gonads or gonadectomy to determine the impact of gonadal hormones on the development of diet-induced insulin resistance. Ovariectomy improved insulin sensitivity in females of all three strains examined, but gonadectomy in male mice improved insulin sensitivity only in one of the three strains, demonstrating a hormone-gene interaction (93). Genetic association analyses identified distinct loci in males and females for plasma insulin and glucose levels (93), underscoring that mechanistic differences exist in the regulation of glucose metabolism between males and females.
Another approach to identify mechanisms underlying sex differences in the regulation of glucose homeostasis is the comparison of sex chromosome and gonadal sex contributions to glucose metabolism parameters. In most cases in mice and humans, increased adiposity correlates with insulin resistance. In the Four Core Genotypes mouse model, glucose and insulin levels were analyzed in mice that were gonadectomized to remove effects of acute gonadal hormones. As described in an earlier section, XX mice (with either male or female original gonads) gained more fat than XY mice (of either gonadal type) (21). Despite this, the XX mice had similar glucose and insulin levels and glucose tolerance as XY mice. The ability to maintain glucose homeostasis in XX mice despite higher body fat could indicate that compensatory changes occur in XX mice, as suggested by enhanced fatty acid oxidation gene expression profiles in muscle and liver (21). When animals are made obese by feeding a high fat-high carbohydrate diet, the XX mice appear to be more prone to insulin resistance (Figure 5), as they have two-fold higher plasma insulin levels to maintain similar plasma glucose levels as XY mice (21). This effect may be secondary to increased obesity in XX mice.
c. Non-alcoholic fatty liver
A common co-morbidity of obesity and insulin resistance is nonalcoholic fatty liver disease (NAFLD). This condition is characterized by the accumulation of neutral lipids in hepatocytes and represents the initial stage of a continuum of conditions that can progress to include severe inflammation (steatohepatitis), and further, to fibrosis and cirrhosis (2, 28). Data show increased cardiovascular disease mortality and overall mortality in individuals with NAFLD/steatohepatitis (34, 88), but it is difficult to disentangle this risk from that due to conditions that typically occur in tandem, such as obesity and MetSyn (120). Sex differences have been noted in the prevalence of NAFLD, but these vary depending on the criteria used to define NAFLD, ethnicity, and other clinical characteristics of the subjects (91). In population-based studies, which may have less ascertainment bias than case-control studies, men have been found to have higher incidence of NAFLD than women (reviewed in 68). In some, but not all cases, the greater occurrence of NAFLD in men remained after adjustment for body weight and insulin resistance (15, 25). Interestingly, in a study where individuals are segregated into lean and overweight/obese groups, lean women had a higher incidence of NAFLD than lean men (121). There may be distinct mechanistic etiologies for fatty liver that occurs in the context of obesity compared to that occurring in lean individuals.
As with most metabolic phenotypes discussed here, gonadal hormones likely contribute to differences in fatty liver development, either directly through estrogen and androgen receptor signaling, or indirectly through effects on adiposity, insulin resistance, etc. Studies in male rats that were gonadectomized and treated with estradiol or dihydrotestosterone showed that both of these hormones decreased lipid accumulation in the liver (124). Estradiol appeared to reduce fatty acid synthesis, and testosterone increased cholesterol synthesis, each in specific hepatic zones. Studies using the Four Core Genotypes mouse model indicate that gonad-independent mechanisms also influence fatty liver development. Mice that were gonadectomized as adults and then fed a high fat/high carbohydrate diet for 12 weeks gained substantial body weight and fat, regardless of gonadal and chromosomal sex (21). Interestingly, only XX mice developed pronounced fatty liver with large triglyceride droplets, whereas XY mice accumulated much less triglyceride and only very small lipid droplets (Figure 5). The XX vs. XY effect occurred regardless of whether the original gonads were male or female, and occurred in the absence of gonadal secretions, indicating that gonad-independent sex differences are involved. However, although both XX and XY mice were obese, the XX mice had greater adiposity and hyperinsulinemia, making it difficult to determine whether the fatty liver in XX mice was independent or secondary to these other metabolic effects.
IV. The gut microbiome and sex differences in metabolic syndrome
In the past several years, there has been a surge in studies investigating the connection between the gut microbiome and human health. Perturbations in the microbial community are related to a wide range of diseases, including bowel disorders, autoimmune diseases, and metabolic disorders (27). Indeed, a direct effect of gut microbiota on obesity was elegantly demonstrated by transplanting gut microbiota from lean or obese mice into germ-free mice. Mice that received microbiota from diet-induced or genetically obese mice gained weight compared to recipients of microbiota from lean donors (109, 110). These findings suggest that gut flora have the capacity to dramatically influence host metabolism, including effects on energy acquisition and storage.
Although the field is still young, studies demonstrate that diet, sex, and genetic background may each influence the composition of the gut microflora, and that the microbial community in turn impacts hormone levels, immunity, and metabolic homeostasis in the host. Relevant to obesity, mice fed a high fat/high sucrose diet for 8 weeks dramatically changed their microbial makeup compared to chow fed mice, particularly the levels of Bacteroidetes and Firmicutes (92). These changes were dependent on the specific mouse strain, indicating that genetic background influences the gut microbiota composition. Furthermore, some genera (Akkermansia, Lactococcus, and Allobaculum) were correlated with the gain in body fat due to diet, highlighting the connection of gut microbes with metabolic health. In addition to diet, the gut microbiota respond to the diurnal cycle, with fluctuations in major phyla such as Firmicutes, Bacteroidetes, and Verrucomicrobia (123). Feeding a high fat diet (61% calories from fat) for 8 weeks suppressed the diurnal fluctuations in microbial composition and reduced species, which may be detrimental to overall health (72). One hypothesis is that metabolites produced by the gut flora, including catabolized complex carbohydrates and bile acids, may signal to and alter host metabolism.
A recent study investigated the effects of sex on gut microbial communities (89). Of 89 strains of mice examined, 7 strains had significant sex differences in abundance of microbial taxa. To determine how these sex differences were affected by nutritional excess, the authors examined male and female C57BL/6J, C3H/HeJ, and DBA/2J mice on a high fat/high sucrose diet for 8 weeks. They identified a sex-by-diet interaction in microbiota composition, suggesting that specific taxa respond to diet in a sex-dependent manner. For example, in DBA/2J mice, members of the Firmicutes phylum increased in females but decreased in males in response to high fat diet. In addition, gonadectomy revealed that circulating sex hormones regulate the gut microbiome in a strain- and diet-dependent manner.
While many factors influence microbial composition, the gut flora itself remarkably affects testosterone levels in both males and females (76). Germ-free females had higher levels of testosterone compared to females housed in specific pathogen-free facilities, while germ-free males had reduced levels of testosterone compared to males housed under specific pathogen-free conditions. Perhaps most strikingly, transplantation of microbiota from adult male cecum to female weanlings resulted in a significant increase of testosterone at 7 and 14 weeks of age (76). At 34 weeks of age, the microbial community reverted to a population resembling female gut flora, and testosterone changes were no longer apparent. The implications of this manipulation were reflected in protection of type 1 diabetes in nonobese diabetic (NOD) mice. Female mice that had received male cecal bacteria were protected from autoimmune destruction of pancreatic β cells, while those that had received female cecal bacteria were not. In addition, treatment of an androgen receptor antagonist abrogated the protective effects of male cecal bacteria, suggesting that the transfer-mediated enhanced testosterone levels were required for protection from type 1 diabetes (76). While these and other data showcase a critical role of the gut microbiome in regulating sex hormones and diabetes, future studies are needed to fully understand the underlying mechanisms (94).
Sex differences inherent in the host may also affect processing of metabolites generated by the gut microbiome. The hepatic enzyme flavin monooxygenase 3 (FMO3) is expressed over 1000 times higher in females compared to males (114). FMO3 catalyzes the last step in the gut-flora-dependent metabolism of dietary phosphatidylcholine to trimethylamine N-oxide (TMAO), which is a significant contributor to atherosclerosis (65, 107, 114). Aortic lesion size was positively correlated with Fmo3 expression levels in Apoe−/− mice (114), and increased levels of TMAO were associated with thrombosis risk and with a 4-fold increased mortality risk (101, 125). As more attention is focused on the impact of TMAO on cardiovascular disease risk, investigators should keep in mind the innate sex difference in TMAO levels and Fmo3 expression (105). Taken together, these studies indicate that complex relationships exist between sex, gut microbiota, and metabolic health, and underscore the importance of further mechanistic studies.
V. Future Perspectives
a. Increased emphasis on studying sex differences
Given that sex is one of the most profound biological determinants, it is unfortunate that it has not always received attention in studies of normo- and pathophysiology. Both pre-clinical and clinical studies have traditionally focused on a single sex, and more often than not, this has been male (for examples, see 65, 69, 96). In the case of studies in experimental animals, a frequent justification was the notion that measurements made in male animals are less variable due to the hormonal fluctuations that occur during the female reproductive cycle. These concerns about variation in female rodent models have been refuted by analysis of published data involving behavior, electrophysiology, neurochemistry, histology and gene expression (10, 55). In recent years, the need for inclusion of both sexes in pre-clinical studies has been recognized and publicized (26, 32, 79, 100). It is hoped that these discussions will lead to a greater transparency in reporting the sexes used in pre-clinical studies, and increased inclusion of both sexes in both pre-clinical and clinical studies.
b. Tools to investigate sex differences
To enhance our understanding of sex differences, it is imperative to continue to develop experimental tools and strategies. As described here, tools such as mouse models that disentangle the genetic basis of sex determination from the gonadal type are valuable to begin to interrogate the mechanisms that underlie observed sex differences. Additionally, genetic manipulation of genes that respond to estrogens and androgens has provided insights into the many facets of gonadal hormone action in metabolism. In the future, greater exploitation of this technology to dissect tissue- and temporal-specific effects of estrogen and androgen signaling would be valuable. A resource to explore sex-specific gene expression in humans is now available in the form of the Genotype-Tissue Expression (GTEx) dataset (46). GTEx was developed primarily to identify loci that regulate gene expression in specific tissues. This has wide applicability in the interpretation of variants that are identified in GWAS studies and understanding disease mechanisms. GTEx includes RNA-seq data from approximately 50 human tissues, with hundreds of individual samples for many of the tissues. Fortunately, the architects of the GTEx project strived to include tissues from both men and women, such that these data will be useful to compare gene expression levels and signals for expression quantitative trait loci (eQTL) in men and women. These data have not yet been tapped extensively to analyze sex differences, but will undoubtedly be a valuable resource in the future. Along the same lines, it is hoped that more investigators will include the X chromosome in GWAS analyses, as tools are available for these analyses and recommendations have been made to promote their use (116).
VI. Summary
Despite decades of recognizing sex differences in obesity and cardiovascular disease, emphasis has seldom been placed on rigorously investigating regulation, interaction, and effects of sex differences on cellular mechanisms and whole-body physiology. In this review, we highlighted several key studies that investigate how sex affects obesity and its co-morbidities, including insulin resistance, dyslipidemia, and fatty liver. For many years, sex differences were attributed to gonadal secretions. We and others have begun to reveal the sex chromosome complement as another determinant of sex differences in health and disease (Figure 5). However, gonadal hormones and sex chromosome complement are not mutually exclusive and do not act in isolation. Other factors, including X chromosome imprinting and inactivation, environmental stimulants, and the gut microbiome may interact with gonadal hormones and the sex chromosome complement to exert changes in gene expression and signaling pathways (Figure 6). Diet and circadian rhythms are two of the many external cues that influence metabolic homeostasis, and have been shown to interact with the gut microbiome. Although X chromosome imprinting and inactivation and other environmental factors were beyond the scope of this review, they may have important roles in determining sex differences in metabolism. There is undoubtedly a complex network that involves many components of sex differences and metabolism, which will be an exciting area of research in the future.
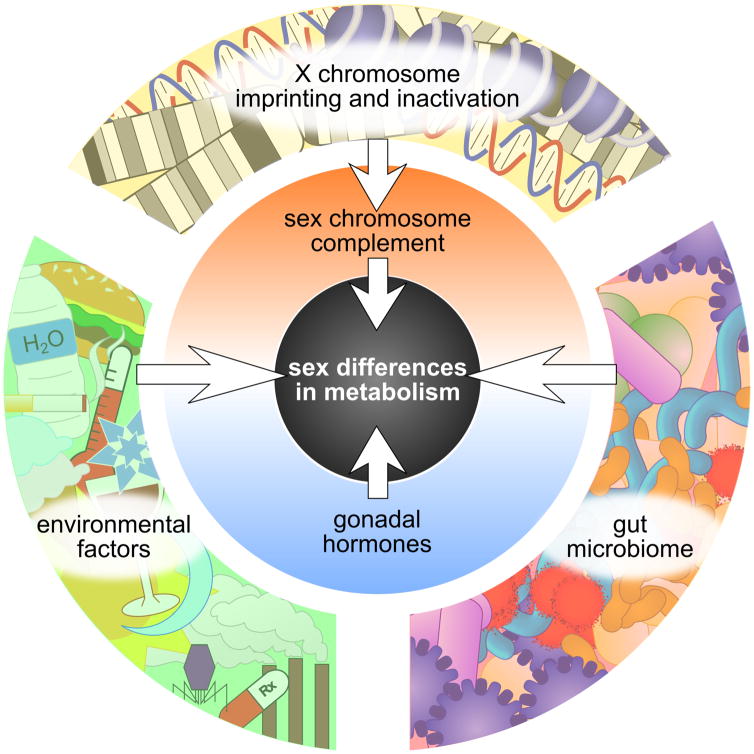
Figure 6. Sex differences in metabolism are influenced by many factors.
Two primary sex-biasing sources are the sex chromosome complement and gonadal hormones, which in turn are influenced by other factors. The white arrows represent mediators of regulation, including gene expression changes and altered protein signaling pathways. In addition to the number of X chromosomes and the presence of a Y chromosome, XX and XY cells differ by the occurrence of X chromosome inactivation (exclusively in XX cells) and by the parent-of-origin X chromosome imprinting (only XX cells have X chromosome imprints from both parents). The gut microbiome and environmental factors such as diet, pollutants, and circadian cycle may interact with the sex chromosome complement and gonadal hormones to affect metabolism differently between males and females.
Acknowledgments
We gratefully acknowledge support from the National Institutes of Health (DK08351 and HL090553).
LITERATURE CITED
- 1.Ahmed-Sorour H, Bailey CJ. Role of ovarian hormones in the long-term control of glucose homeostasis, glycogen formation and gluconeogenesis. Ann Nutr Metab. 1981;25(4):208–12. doi: 10.1159/000176496. [DOI] [PubMed] [Google Scholar]
- 2.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346(16):1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 3.Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96(10):3007–19. doi: 10.1210/jc.2011-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold AP. Mouse models for evaluating sex chromosome effects that cause sex differences in non-gonadal tissues. J Neuroendocrinol. 2009;21(4):377–86. doi: 10.1111/j.1365-2826.2009.01831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold AP, Burgoyne PS. Are XX and XY brain cells intrinsically different? Trends Endocrinol Metab. 2004;15(1):6–11. doi: 10.1016/j.tem.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30(1):1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold AP, Chen X, Link JC, Itoh Y, Reue K. Cell-autonomous sex determination outside of the gonad. Dev Dyn. 2013;242(4):371–79. doi: 10.1002/dvdy.23936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold AP, Reue K, Eghbali M, Vilain E, Chen X, et al. The importance of having two X chromosomes. Philos Trans R Soc B Biol Sci. 2016;371(1688):20150113. doi: 10.1098/rstb.2015.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartlett J, Predazzi IM, Williams SM, Bush WS, Kim Y, et al. Is isolated low high-density lipoprotein cholesterol a cardiovascular disease risk factor? New insights from the Framingham offspring study. Circ Cardiovasc Qual Outcomes. 2016;9(3):206–12. doi: 10.1161/CIRCOUTCOMES.115.002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becker JB, Prendergast BJ, Liang JW. Female rats are not more variable than male rats: a meta-analysis of neuroscience studies. Biol Sex Differ. 2016;7:34. doi: 10.1186/s13293-016-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bittner V. Perspectives on dyslipidemia and coronary heart disease in women. J Am Coll Cardiol. 2005;46(9):1628–35. doi: 10.1016/j.jacc.2005.05.089. [DOI] [PubMed] [Google Scholar]
- 12.Blouin K, Boivin A, Tchernof A. Androgens and body fat distribution. J Steroid Biochem Mol Biol. 2008;108(3–5):272–80. doi: 10.1016/j.jsbmb.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Bojesen A, Høst C, Gravholt CH. Klinefelter’s syndrome, type 2 diabetes and the metabolic syndrome: the impact of body composition. Mol Hum Reprod. 2010;16(6):396–401. doi: 10.1093/molehr/gaq016. [DOI] [PubMed] [Google Scholar]
- 14.Bojesen A, Kristensen K, Birkebaek NH, Fedder J, Mosekilde L, et al. The metabolic syndrome is frequent in Klinefelter’s syndrome and is associated with abdominal obesity and hypogonadism. Diabetes Care. 2006;29(7):1591–98. doi: 10.2337/dc06-0145. [DOI] [PubMed] [Google Scholar]
- 15.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 16.Bukowski R, Smith GCS, Malone FD, Ball RH, Nyberg DA, et al. Human sexual size dimorphism in early pregnancy. Am J Epidemiol. 2007;165(10):1216–18. doi: 10.1093/aje/kwm024. [DOI] [PubMed] [Google Scholar]
- 17.Burgoyne PS. A Y-chromosomal effect on blastocyst cell number in mice. Development. 1993;117(1):341–45. doi: 10.1242/dev.117.1.341. [DOI] [PubMed] [Google Scholar]
- 18.Burgoyne PS, Thornhill AR, Boudrean SK, Darling SM, Bishop CE, Evans EP. The genetic basis of XX-XY differences present before gonadal sex differentiation in the mouse. Philos Trans R Soc Lond B Biol Sci. 1995;350(1333):253–61. doi: 10.1098/rstb.1995.0159. [DOI] [PubMed] [Google Scholar]
- 19.Calcaterra V, Brambilla P, Maffè GC, Klersy C, Albertini R, et al. Metabolic syndrome in Turner syndrome and relation between body composition and clinical, genetic, and ultrasonographic characteristics. Metab Syndr Relat Disord. 2014;12(3):159–64. doi: 10.1089/met.2013.0075. [DOI] [PubMed] [Google Scholar]
- 20.Castelli WP, Anderson K, Wilson PW, Levy D. Lipids and risk of coronary heart disease. The Framingham study. Ann Epidemiol. 2(1–2):23–28. doi: 10.1016/1047-2797(92)90033-m. [DOI] [PubMed] [Google Scholar]
- 21.Chen X, McClusky R, Chen J, Beaven SW, Tontonoz P, et al. The number of X chromosomes causes sex differences in adiposity in mice. PLoS Genet. 2012;8(5):e1002709. doi: 10.1371/journal.pgen.1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X, Wang L, Loh DH, Colwell CS, Taché Y, et al. Sex differences in diurnal rhythms of food intake in mice caused by gonadal hormones and complement of sex chromosomes. Horm Behav. 2015;75:55–63. doi: 10.1016/j.yhbeh.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, Williams-Burris SM, McClusky R, Ngun TC, Ghahramani N, et al. The sex chromosome trisomy mouse model of XXY and XYY: metabolism and motor performance. Biol Sex Differ. 2013;4(1):15. doi: 10.1186/2042-6410-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cífková R, Krajčoviechová A. Dyslipidemia and cardiovascular disease in women. Curr Cardiol Rep. 2015;17(7):609. doi: 10.1007/s11886-015-0609-5. [DOI] [PubMed] [Google Scholar]
- 25.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98(5):960–67. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 26.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509(7500):282–83. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258–70. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332(6037):1519–23. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins P. HDL-C in post-menopausal women: an important therapeutic target. Int J Cardiol. 2008;124(3):275–82. doi: 10.1016/j.ijcard.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292–95. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 31.Czyżyk A, Męczekalski B. Cardiovascular and metabolic problems in Turner’s syndrome patients. Arch Perinat Med. 2012;18(1):47–52. [Google Scholar]
- 32.Danska JS. Sex matters for mechanism. Sci Transl Med. 2014;6(258):258fs40. doi: 10.1126/scitranslmed.3009859. [DOI] [PubMed] [Google Scholar]
- 33.Du S, Itoh N, Askarinam S, Hill H, Arnold AP, Voskuhl RR. Xy sex chromosome complement, compared with xx, in the cns confers greater neurodegeneration during experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2014;111(7):2806–11. doi: 10.1073/pnas.1307091111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunn W, Xu R, Wingard DL, Rogers C, Angulo P, et al. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am J Gastroenterol. 2008;103(9):2263–71. doi: 10.1111/j.1572-0241.2008.02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eyre H, Kahn R, Robertson RM American Cancer Society, the American Diabetes Association, and the American Heart Association Collaborative Writing Committee. Preventing cancer, cardiovascular disease, and diabetes: a common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Diabetes Care. 2004;27(7):1812–24. doi: 10.2337/diacare.27.7.1812. [DOI] [PubMed] [Google Scholar]
- 36.Fan WQ, Yanase T, Nomura M, Okabe T, Goto K, et al. Androgen receptor null male mice develop late-onset obesity caused by decreased energy expenditure and lipolytic activity but show normal insulin sensitivity with high adiponectin secretion. Diabetes. 2005;54(4):1000–1008. doi: 10.2337/diabetes.54.4.1000. [DOI] [PubMed] [Google Scholar]
- 37.Fox CS, Liu Y, White CC, Feitosa M, Smith AV, et al. Genome-wide association for abdominal subcutaneous and visceral adipose reveals a novel locus for visceral fat in women. PLoS Genet. 2012;8(5):e1002695. doi: 10.1371/journal.pgen.1002695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 39.Freedman DS, Otvos JD, Jeyarajah EJ, Shalaurova I, Cupples LA, et al. Sex and age differences in lipoprotein subclasses measured by nuclear magnetic resonance spectroscopy: the Framingham study. Clin Chem. 2004;50(7):1189–1200. doi: 10.1373/clinchem.2004.032763. [DOI] [PubMed] [Google Scholar]
- 40.Fried SK, Lee MJ, Karastergiou K. Shaping fat distribution: New insights into the molecular determinants of depot- and sex-dependent adipose biology. Obesity. 2015;23(7):1345–52. doi: 10.1002/oby.21133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garaulet M, Pérez-Llamas F, Baraza JC, Garcia-Prieto MD, Fardy PS, et al. Body fat distribution in pre-and post-menopausal women: metabolic and anthropometric variables. J Nutr Health Aging. 2002;6(2):123–26. [PubMed] [Google Scholar]
- 42.Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med. 2009;6(Suppl 1):60–75. doi: 10.1016/j.genm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goff DC, Bertoni AG, Kramer H, Bonds D, Blumenthal RS, et al. Dyslipidemia prevalence, treatment, and control in the Multi-Ethnic Study of Atherosclerosis (MESA): gender, ethnicity, and coronary artery calcium. Circulation. 2006;113(5):647–56. doi: 10.1161/CIRCULATIONAHA.105.552737. [DOI] [PubMed] [Google Scholar]
- 44.Gravholt CH. Epidemiological, endocrine and metabolic features in Turner syndrome. Eur J Endocrinol. 2004;151(6):657–87. doi: 10.1530/eje.0.1510657. [DOI] [PubMed] [Google Scholar]
- 45.Grove KL, Fried SK, Greenberg AS, Xiao XQ, Clegg DJ. A microarray analysis of sexual dimorphism of adipose tissues in high-fat-diet-induced obese mice. Int J Obes. 2010;34(6):989–1000. doi: 10.1038/ijo.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.GTEx Consortium. Human genomics. The genotype-tissue expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015;348(6235):648–60. doi: 10.1126/science.1262110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gurka MJ, Lilly CL, Oliver MN, DeBoer MD. An examination of sex and racial/ethnic differences in the metabolic syndrome among adults: a confirmatory factor analysis and a resulting continuous severity score. Metabolism. 2014;63(2):218–25. doi: 10.1016/j.metabol.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hagen CP, Main KM, Kjaergaard S, Juul A. FSH, LH, inhibin B and estradiol levels in Turner syndrome depend on age and karyotype: longitudinal study of 70 Turner girls with or without spontaneous puberty. Hum Reprod. 2010;25(12):3134–41. doi: 10.1093/humrep/deq291. [DOI] [PubMed] [Google Scholar]
- 49.Harris MI, Eastman RC, Cowie CC, Flegal KM, Eberhardt MS. Comparison of diabetes diagnostic categories in the U.S. population according to the 1997 American Diabetes Association and 1980–1985 World Health Organization diagnostic criteria. Diabetes Care. 1997;20(12):1859–62. doi: 10.2337/diacare.20.12.1859. [DOI] [PubMed] [Google Scholar]
- 50.Heid IM, Jackson AU, Randall JC, Winkler TW, Qi L, et al. Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat Genet. 2010;42(11):949–60. doi: 10.1038/ng.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97(23):12729–34. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herrera BM, Lindgren CM. The genetics of obesity. Curr Diab Rep. 2010;10(6):498–505. doi: 10.1007/s11892-010-0153-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Høst C, Skakkebæk A, Groth KA, Bojesen A. The role of hypogonadism in Klinefelter syndrome. Asian J Androl. 2014;16(2):185–91. doi: 10.4103/1008-682X.122201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Isidori AM, Giannetta E, Greco EA, Gianfrilli D, Bonifacio V, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol. 2005;63(3):280–93. doi: 10.1111/j.1365-2265.2005.02339.x. [DOI] [PubMed] [Google Scholar]
- 55.Itoh Y, Arnold AP. Are females more variable than males in gene expression? Meta-analysis of microarray datasets. Biol Sex Differ. 2015;6:18. doi: 10.1186/s13293-015-0036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Itoh Y, Mackie R, Kampf K, Domadia S, Brown JD, et al. Four core genotypes mouse model: localization of the Sry transgene and bioassay for testicular hormone levels. BMC Res Notes. 2015;8(1):69. doi: 10.1186/s13104-015-0986-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jeffery E, Wing A, Holtrup B, Sebo Z, Kaplan JL, et al. The adipose tissue microenvironment regulates depot-specific adipogenesis in obesity. Cell Metab. 2016;24(1):142–50. doi: 10.1016/j.cmet.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson JL, Slentz CA, Duscha BD, Samsa GP, McCartney JS, et al. Gender and racial differences in lipoprotein subclass distributions: the STRRIDE study. Atherosclerosis. 2004;176(2):371–77. doi: 10.1016/j.atherosclerosis.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 59.Karakelides H, Irving BA, Short KR, O’Brien P, Nair KS. Age, obesity, and sex effects on insulin sensitivity and skeletal muscle mitochondrial function. Diabetes. 2010;59(1):89–97. doi: 10.2337/db09-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karastergiou K, Fried SK. Sex differences in human adipose tissues - the biology of pear shape. Biol Sex Differ. 2012;3(1):13. doi: 10.1186/2042-6410-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krebs CJ, Zhang D, Yin L, Robins DM. The KRAB zinc finger protein RSL1 modulates sex-biased gene expression in liver and adipose tissue to maintain metabolic homeostasis. Mol Cell Biol. 2014;34(2):221–32. doi: 10.1128/MCB.00875-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuk JL, Ardern CI. Age and sex differences in the clustering of metabolic syndrome factors: association with mortality risk. Diabetes Care. 2010;33(11):2457–61. doi: 10.2337/dc10-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuk JL, Ross R. Influence of sex on total and regional fat loss in overweight and obese men and women. Int J Obes. 2009;33(6):629–34. doi: 10.1038/ijo.2009.48. [DOI] [PubMed] [Google Scholar]
- 64.Kvist H, Chowdhury B, Grangård U, Tylén U, Sjöström L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: predictive equations. Am J Clin Nutr. 1988;48(6):1351–61. doi: 10.1093/ajcn/48.6.1351. [DOI] [PubMed] [Google Scholar]
- 65.Lang DH, Yeung CK, Peter RM, Ibarra C, Gasser R, et al. Isoform specificity of trimethylamine N-oxygenation by human flavin-containing monooxygenase (FMO) and p450 enzymes: selective catalysis by FMO3. Biochem Pharmacol. 1998;56(8):1005–12. doi: 10.1016/s0006-2952(98)00218-4. [DOI] [PubMed] [Google Scholar]
- 66.Lemieux S, Prud’homme D, Bouchard C, Tremblay A, Després JP. Sex differences in the relation of visceral adipose tissue accumulation to total body fatness. Am J Clin Nutr. 1993;58(4):463–67. doi: 10.1093/ajcn/58.4.463. [DOI] [PubMed] [Google Scholar]
- 67.Li J, Chen X, McClusky R, Ruiz-Sundstrom M, Itoh Y, et al. The number of x chromosomes influences protection from cardiac ischaemia/reperfusion injury in mice: one x is better than two. Cardiovasc Res. 2014;102(3):375–84. doi: 10.1093/cvr/cvu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lima R, Wofford M, Reckelhoff JF. Hypertension in postmenopausal women. Curr Hypertens Rep. 2012;14(3):254–60. doi: 10.1007/s11906-012-0260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Link JC, Chen X, Arnold AP, Reue K. Metabolic impact of sex chromosomes. Adipocyte. 2013;2(2):74–79. doi: 10.4161/adip.23320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Link JC, Chen X, Prien C, Borja MS, Hammerson B, et al. Increased high-density lipoprotein cholesterol levels in mice with XX versus XY sex chromosomes. Arterioscler Thromb Vasc Biol. 2015;35(8):1778–86. doi: 10.1161/ATVBAHA.115.305460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu J, Fox CS, Hickson DA, May WD, Hairston KG, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab. 2010;95(12):5419–26. doi: 10.1210/jc.2010-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lozupone C, Stombaugh J, Gordon J, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220–30. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lusis AJ, Attie AD, Reue K. Metabolic syndrome: from epidemiology to systems biology. Nat Rev Genet. 2008;9(11):819–30. doi: 10.1038/nrg2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maas AHEM, Appelman YEA. Gender differences in coronary heart disease. Neth Heart J. 2010;18(12):598–602. doi: 10.1007/s12471-010-0841-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Magkos F, Mohammed BS, Mittendorfer B. Effect of obesity on the plasma lipoprotein subclass profile in normoglycemic and normolipidemic men and women. Int J Obes. 2008;32(11):1655–64. doi: 10.1038/ijo.2008.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Markle JGM, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339(6123):1084–88. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 77.Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol Sex Differ. 2015;6:14. doi: 10.1186/s13293-015-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Melloni C, Berger JS, Wang TY, Gunes F, Stebbins A, et al. Representation of women in randomized clinical trials of cardiovascular disease prevention. Circ Cardiovasc Qual Outcomes. 2010;3(2):135–42. doi: 10.1161/CIRCOUTCOMES.110.868307. [DOI] [PubMed] [Google Scholar]
- 79.Miller LR, Marks C, Becker JB, Hurn PD, Chen W-J, et al. Considering sex as a biological variable in preclinical research. FASEB J. 2016 doi: 10.1096/fj.201600781R. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mittendorfer B. Insulin resistance: sex matters. Curr Opin Clin Nutr Metab Care. 2005;8(4):367–72. doi: 10.1097/01.mco.0000172574.64019.98. [DOI] [PubMed] [Google Scholar]
- 81.Monroe AK, Dobs AS. The effect of androgens on lipids. Curr Opin Endocrinol Diabetes Obes. 2013;20(2):132–39. doi: 10.1097/MED.0b013e32835edb71. [DOI] [PubMed] [Google Scholar]
- 82.Mozumdar A, Liguori G. Persistent increase of prevalence of metabolic syndrome among U.S. adults: NHANES III to NHANES 1999–2006. Diabetes Care. 2011;34(1):216–19. doi: 10.2337/dc10-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–26. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 84.Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang X-J, et al. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104(7):2501–6. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nishizawa H, Shimomura I, Kishida K, Maeda N, Kuriyama H, et al. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes. 2002;51(9):2734–41. doi: 10.2337/diabetes.51.9.2734. [DOI] [PubMed] [Google Scholar]
- 86.Nuutila P, Knuuti MJ, Mäki M, Laine H, Ruotsalainen U, et al. Gender and insulin sensitivity in the heart and in skeletal muscles. Studies using positron emission tomography. Diabetes. 1995;44(1):31–36. doi: 10.2337/diab.44.1.31. [DOI] [PubMed] [Google Scholar]
- 87.Ohlsson C, Hellberg N, Parini P, Vidal O, Bohlooly-Y M, et al. Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochem Biophys Res Commun. 2000;278(3):640–45. doi: 10.1006/bbrc.2000.3827. [DOI] [PubMed] [Google Scholar]
- 88.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49(4):608–12. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 89.Org E, Mehrabian M, Parks BW, Shipkova P, Liu X, et al. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes. 2016;7(4):313–22. doi: 10.1080/19490976.2016.1203502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol. 2015;402:113–19. doi: 10.1016/j.mce.2014.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pan J-J, Fallon MB. Gender and racial differences in nonalcoholic fatty liver disease. World J Hepatol. 2014;6(5):274–83. doi: 10.4254/wjh.v6.i5.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Parks BW, Nam E, Org E, Kostem E, Norheim F, et al. Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice. Cell Metab. 2013;17(1):141–52. doi: 10.1016/j.cmet.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Parks BW, Sallam T, Mehrabian M, Psychogios N, Hui ST, et al. Genetic architecture of insulin resistance in the mouse. Cell Metab. 2015;21(2):334–46. doi: 10.1016/j.cmet.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Paun A, Danska JS. Modulation of type 1 and type 2 diabetes risk by the intestinal microbiome. Pediatr Diabetes. 2016;17(7):469–77. [Google Scholar]
- 95.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Circulation. 2006;113(6):898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 96.Porter SA, Massaro JM, Hoffmann U, Vasan RS, O’Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care. 2009;32(6):1068–75. doi: 10.2337/dc08-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pradhan AD. Sex differences in the metabolic syndrome: implications for cardiovascular health in women. Clin Chem. 2014;60(1):44–52. doi: 10.1373/clinchem.2013.202549. [DOI] [PubMed] [Google Scholar]
- 98.Rochlani Y, Pothineni NV, Mehta JL. Metabolic syndrome: does it differ between women and men? Cardiovasc Drugs Ther. 2015;29(4):329–38. doi: 10.1007/s10557-015-6593-6. [DOI] [PubMed] [Google Scholar]
- 99.Ruige JB, Mahmoud AM, De Bacquer D, Kaufman J-M. Endogenous testosterone and cardiovascular disease in healthy men: a meta-analysis. Heart. 2011;97(11):870–75. doi: 10.1136/hrt.2010.210757. [DOI] [PubMed] [Google Scholar]
- 100.Sandberg K, Umans JG Georgetown Consensus Conference Work Group. Recommendations concerning the new U.S. National Institutes of Health initiative to balance the sex of cells and animals in preclinical research. FASEB J. 2015;29(5):1646–52. doi: 10.1096/fj.14-269548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Senthong V, Wang Z, Li XS, Fan Y, Wu Y, et al. Intestinal microbiota-generated metabolite trimethylamine-N-oxide and 5-year mortality risk in stable coronary artery disease: the contributory role of intestinal microbiota in a COURAGE-like patient cohort. J Am Heart Assoc. 2016;5(6):e002816. doi: 10.1161/JAHA.115.002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shi H, Seeley RJ, Clegg DJ. Sexual differences in the control of energy homeostasis. Front Neuroendocrinol. 2009;30(3):396–404. doi: 10.1016/j.yfrne.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518(7538):187–96. doi: 10.1038/nature14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sung YJ, Pérusse L, Sarzynski MA, Fornage M, Sidney S, et al. Genome-wide association studies suggest sex-specific loci associated with abdominal and visceral fat. Int J Obes. 2016;40(4):662–74. doi: 10.1038/ijo.2015.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tang WHW, Hazen SL. Microbiome, trimethylamine N-oxide, and cardiometabolic disease. Transl Res. 2016 doi: 10.1016/j.trsl.2016.07.007. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tchernof A, Després J-P. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93(1):359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 107.Tjoa S, Fennessey P. The identification of trimethylamine excess in man: quantitative analysis and biochemical origins. Anal Biochem. 1991;197(1):77–82. doi: 10.1016/0003-2697(91)90358-z. [DOI] [PubMed] [Google Scholar]
- 108.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7(5):410–20. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3(4):213–23. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Turnbaugh PJ, Ley RE, Mahowald Ma, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 111.Unwin N, Shaw J, Zimmet P, Alberti KGMM. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med. 2002;19(9):708–23. doi: 10.1046/j.1464-5491.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- 112.Wald C, Wu C. Of mice and women: the bias in animal models. Science. 2010;327(5973):1571–72. doi: 10.1126/science.327.5973.1571. [DOI] [PubMed] [Google Scholar]
- 113.Wang X, Magkos F, Mittendorfer B. Sex differences in lipid and lipoprotein metabolism: it’s not just about sex hormones. J Clin Endocrinol Metab. 2011;96(4):885–93. doi: 10.1210/jc.2010-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Whitsel EA, Boyko EJ, Matsumoto AM, Anawalt BD, Siscovick DS. Intramuscular testosterone esters and plasma lipids in hypogonadal men: a meta-analysis. Am J Med. 2001;111(4):261–69. doi: 10.1016/s0002-9343(01)00833-6. [DOI] [PubMed] [Google Scholar]
- 116.Wise AL, Gyi L, Manolio TA. eXclusion: toward integrating the X chromosome in genome-wide association analyses. Am J Hum Genet. 2013;92(5):643–47. doi: 10.1016/j.ajhg.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14(4):453–65. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang X, Schadt EE, Wang S, Wang H, Arnold AP, et al. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 2006;16(8):995–1004. doi: 10.1101/gr.5217506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yasmeen R, Reichert B, Deiuliis J, Yang F, Lynch A, et al. Autocrine function of aldehyde dehydrogenase 1 as a determinant of diet- and sex-specific differences in visceral adiposity. Diabetes. 2013;62(1):124–36. doi: 10.2337/db11-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Younossi ZM, Otgonsuren M, Venkatesan C, Mishra A. In patients with non-alcoholic fatty liver disease, metabolically abnormal individuals are at a higher risk for mortality while metabolically normal individuals are not. Metabolism. 2013;62(3):352–60. doi: 10.1016/j.metabol.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 121.Younossi ZM, Stepanova M, Negro F, Hallaji S, Younossi Y, et al. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine. 2012;91(6):319–27. doi: 10.1097/MD.0b013e3182779d49. [DOI] [PubMed] [Google Scholar]
- 122.Zamboni M, Armellini F, Milani MP, De Marchi M, Todesco T, et al. Body fat distribution in pre- and post-menopausal women: metabolic and anthropometric variables and their interrelationships. Int J Obes Relat Metab Disord. 1992;16(7):495–504. [PubMed] [Google Scholar]
- 123.Zarrinpar A, Chaix A, Yooseph S, Panda S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 2014;20(6):1006–17. doi: 10.1016/j.cmet.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang H, Liu Y, Wang L, Li Z, Zhang H, et al. Differential effects of estrogen/androgen on the prevention of nonalcoholic fatty liver disease in the male rat. J Lipid Res. 2013;54(2):345–57. doi: 10.1194/jlr.M028969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165(1):111–24. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]