Abstract
Background
New hepatitis C virus (HCV) treatments deliver higher cure rates with fewer contraindications, increasing demand for treatment and healthcare costs. The cost-effectiveness of new treatments is unknown.
Methods
We conducted a microsimulation of guideline testing followed by alternative treatment regimens for HCV among the US population aged 20 and older to estimate cases identified, treated, sustained viral response, deaths, medical costs, quality-adjusted life-years (QALYs), and the incremental cost-effectiveness ratio (ICER) of different treatment options expressed as discounted lifetime costs and benefits from the healthcare perspective.
Results
Compared to treatment with pegylated interferon and ribavirin (PR), and a protease inhibitor for HCV genotype (G) 1 and PR alone for G2/3, treatment with PR and Sofosbuvir (PRS) for G1/4 and treatment with Sofosbuvir and ribavirin (SR) for G2/3 increased QALYs by 555 226, reduced deaths by 80 682, and increased costs by $26.2 billion at an ICER of $47 304 per QALY gained. As compared to PRS/SR, treating with an all oral regimen of Sofosbuvir and Simeprevir (SS) for G1/4 and SR for G2/3, increased QALYs by 1 110 451 and reduced deaths by an additional 164 540 at an incremental cost of $80.1 billion and an ICER of $72 169. In sensitivity analysis, where treatment with SS effectiveness was set to the list price of Viekira Pak and then Harvoni, treatment cost $24 921 and $25 405 per QALY gained as compared to PRS/SR.
Conclusions
New treatments are cost-effectiveness per person treated, but pent-up demand for treatment may create challenges for financing.
Keywords: hepatitis C, cost-effectivenes, pharmacoeconomics, public health, antiviral treatment
In 2012, the US Centers for Disease Control and Prevention (CDC) recommended that Americans born during 1945–1965 receive a 1-time antibody test to identify hepatitis C virus (HCV) infection (birth-cohort testing) [1–3]. In 2013, this recommendation was affirmed by the United States Preventive Services Task Force citing the large health benefits of birth-cohort testing predicted by modeling studies [2-6]. From 2011 to 2013, at least 6 published studies found HCV testing and treatment to be cost-effectiveness, using different parameters and assumption [2,4,6-9]. Adjusting the aggregate results from these studies into per person incremental costs and quality-adjusted life-years (QALYs) allows for the visual comparison of their results (Figure 1).
Figure 1.
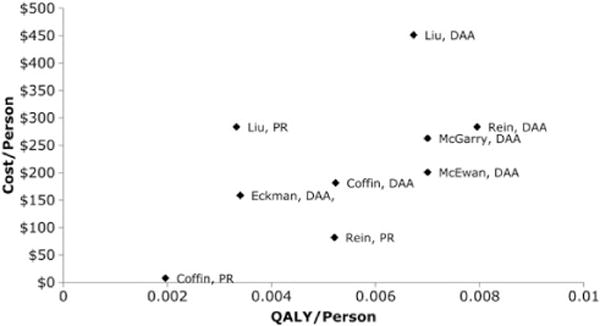
Estimated incremental change in per person costs and per person quality-adjusted life-years estimated across 9 published scenarios that tested population hepatitis C virus testing followed by treatment. Abbreviations: PR, pegylated interferon and ribavirin; QALY, quality-adjusted life-year.
Since publication of the birth-cohort testing recommendations, new highly effective drugs have been released, and clinical treatment recommendations have been updated to incorporate their use [10]. In this article, we modified a previously published model of the cost-effectiveness of birth-cohort testing to assess the cost-effectiveness, financial impacts, and health benefits of birth-cohort testing using new treatments under the assumption of broad population-based implementation [2].
METHODS
Decision Analytic Model
We programmed (Microsoft Visual Studio 2010, Redmond, Washington) a Monte Carlo simulation model of the natural history of hepatitis C with antibody prevalence estimates stratified by age, gender, race/ethnicity, and history of injecting drugs. The model’s natural history, validation, and economic parameters have been previously described, and revisions to the model’s parameters are included in Tables 1 and 2 and technical documentation [2, 52]. Compared to previous versions, the model’s structure now assumes that a sustained viral response (SVR) results in a reduced risk of hepatocellular carcinoma (HCC) instead of risk elimination. Additional information is included in the technical report, available as Supplementary materials.
Table 1.
Treatment Parameters and Costs
| Parameter | Value | 95% Interval Used in Simulation | Source | Distribution |
|---|---|---|---|---|
| Genotype 1&4: Peglyated Interferon + Ribavirin (48 wks) | ||||
| Applicable Scenarios | 2 | |||
| Probability of SVR | 0.358 | 32.5%–39.0% | [11] | Beta |
| Cost of treatment | $61 224 | $47 525–$78 870 | [12] | Lognormal |
| Treatment year utility | 0.882 | 0.852–0.912 | [13] | Uniform |
| Proportion Treated | 0.24 | 0.228–0.251 | [14–25] | Beta |
| Genotype 1&4: Protease Inhibitor (12 wks) + Peglyated Interferon + Ribavirin (24 wks) | ||||
| Applicable Scenarios | 3 | |||
| Probability of SVR | 0.665 | 0.607–0.724 | [11] | Beta |
| Cost of treatment | $78 812 | $61 178–$101 528 | [12, 26] | Lognormal |
| Treatment year utility | 0.968 | 0.853–0.912 | [13] | Uniform |
| Proportion Treated | 0.24 | 0.228–0.251 | [14–25] | Beta |
| Genotype 1&4: Sofosbuvir + Peglyated Interferon + Ribavirin (12 wks) | ||||
| Applicable Scenarios | 4 | |||
| Probability of SVR | 0.902 | 0.856–0.926 | [27] | Beta |
| Cost of treatment | $99 306 | $77 087–$127 929 | [12, 28] | Lognormal |
| Treatment year utility | 0.966 | 0.957–0.974 | [13] | Uniform |
| Proportion Treated | 0.24 | 0.228–0.251 | [14–25] | Beta |
| Genotype 1&4: Sofosbuvir+ Simeprevir (12 wks) | ||||
| Applicable Scenarios | 5 | |||
| Probability of SVR | 0.963 | 0.869–0.998 | [29] | Beta |
| Cost of treatment | $150 360 | $116 718–$193 698 | [28, 30] | Lognormal |
| Treatment year utility | 1.00 | … | Assumption | NA |
| Proportion Treated | 0.72 | 0.66–0.77 | [14–25] and assumptions | Beta |
| Genotype 2: Peglyated Interferon + Ribavirin (24 Wks) | ||||
| Applicable Scenarios | 1, 2 | |||
| Probability of SVR | 0.67 | 0.607–0.724 | [27] | Beta |
| Cost of treatment | $30 612 | $23 723–$39 435 | [12] | Lognormal |
| Treatment year utility | 0.968 | 0.960–0.975 | [13] | Uniform |
| Proportion Treated | 0.24 | 0.228–0.251 | [14–25] | Beta |
| Genotype 2: Sofosbuvir + Ribavirin (12 wks) | ||||
| Applicable Scenarios | 3, 4, 5 | |||
| Probability of SVR | 0.971 | 0.922–0.996 | [27] | Beta |
| Cost of treatment | $88 158 | $68 443–$113 568 | [12, 28] | Lognormal |
| Treatment year utility | 1.00 | … | Assumption | NA |
| Proportion Treated | 0.72 | 0.66–0.77 | [14–25] and assumptions | Beta |
| Genotype 3: Peglyated Interferon + Ribavirin (24 Wks) | ||||
| Applicable Scenarios | 1, 2 | |||
| Probability of SVR | 0.67 | 0.607–0.724 | [27] | Beta |
| Cost of treatment | $30 612 | $23 723–$39 435 | [12] | Lognormal |
| Treatment year utility | 0.9655 | 0.960–0.975 | [13] | Uniform |
| Proportion Treated | 0.24 | 0.228–0.251 | [14–25] | Beta |
| Genotype 3: Sofosbuvir + Ribavirin (24 wks) | ||||
| Applicable Scenarios | 3, 4, 5 | |||
| Probability of SVR | 0.848 | 0.801–0.889 | [31] | Beta |
| Cost of treatment | $176 316 | $136 866–$227 135 | [12, 28] | Lognormal |
| Treatment year utility | 1.00 | … | Assumption | NA |
| Proportion Treated | 0.72 | 0.66–0.77 | [14–25] and assumptions | Beta |
Abbreviations: NA, not applicable; SVR, sustained viral response.
Table 2.
Key Nontreatment Parameters
| Parameter | Value | 95% Interval Used in Simulation | Source | Distribution |
|---|---|---|---|---|
| Population Size (ages 20 to 90) | 229 185 985 | … | US Census 2012 age 20 + population estimate | … |
| Proportion reachable through health system | 0.75 | … | Assumption | … |
| Screening and Treatment Probabilities | ||||
| Screening probability if screening intervention is not offered | 0.18 | … | [32] | Beta |
| Ribonucleic acid test acceptance probability | 1 | … | Assumption | … |
| Return for anti-HCV results probability | 0.9 | … | [33] | Beta |
| Probability of viral clearance rate given antibody positive status | 0.22 | … | [34] | Beta |
| Probability of being considered for treatment | 1 | … | Assumption | … |
| Proportion treated for regimens including Pegylated Interferon | 0.24 | 0.23–0.25 | [14–21, 35–38] | Beta |
| Proportion treated for regimens excluding Pegylated Interferon | 0.72 | 0.66–0.77 | [14–25] and assumptions | Beta |
| Proportion of Background QALYs retained at Each Disease Stage | ||||
| No HCV | 1 | … | See text | … |
| SVR | 0.93 | 0.91–0.95 | Uniform | |
| Chronic HCV METAVIR—0 | 0.93 | 0.91–0.95 | Uniform | |
| Chronic HCV METAVIR—1–2 | 0.86 | 0.83–0.90 | Uniform | |
| Chronic HCV METAVIR—2–3 | 0.83 | 0.79–0.87 | Uniform | |
| Compensated cirrhosis | 0.81 | 0.77–0.85 | Uniform | |
| Decompensated cirrhosis | 0.7 | 0.63–0.78 | Uniform | |
| HCC | 0.67 | 0.60–0.74 | Uniform | |
| Prior transplant | 0.78 | 0.73–0.83 | Uniform | |
| Annual Probability of Complications from Cirrhosis | ||||
| HCC | 0.025 | 0.022–0.028 | [39] | Beta |
| Decompensated cirrhosis | 0.039 | 0.035–0.043 | [40] | Beta |
| Transplant given HCC or decompensated cirrhosis 0.031 | 0.029–0.033 | [41, 42] | Beta | |
| Relative risk of HCC after SVR | 0.24 | 0.183–0.315 | [43] | Lognormal |
| Annual Probability of Death from Complications | ||||
| HCC | 0.409 | 0.368–0.450 | [44] | Beta |
| Decompensated cirrhosis | 0.135 | 0.122–0.149 | [45] | Beta |
| Transplant first year | 0.14 | 0.126–0.154 | [47] | Beta |
| Transplant years 2–4 | 0.038 | 0.035–0.042 | [47] | Beta |
| Transplant years 5–15 | 0.025 | 0.023–0.027 | [48] | Beta |
| Transplant years 16–18 | 0.014 | 0.012–0.015 | [48] | Beta |
| Relative annual risk of mortality for IDUs—20–39 y old | 2.13 | … | [49] | Lognormal |
| Relative annual risk of mortality for IDUs—40 and older | 1.42 | … | [49] | Lognormal |
| Costs | ||||
| Screening | ||||
| Antibody Testing | $24.65 | $19.09–$31.82 | Unpublished CDC Data | Lognormal |
| Cost of RNA testing | $58.88 | $45.61-$76.01 | [46] | Lognormal |
| Medical | ||||
| Cost of post-diagnostic evaluation, if coordinated $831.63 with treatment | $644.18–1,073.63 | [4, 7–9, 50, 51] | Lognormal | |
| Antiviral treatment See Table 1 | ||||
| Non-antiviral Medical Care | ||||
| Cost of post-diagnostic evaluation after diagnosis if not treated | $869.19 | $673.27–1,122.11 | [4, 7–9, 50, 51] | Lognormal |
| HCV costs for METAVIR stages 0–4, w/out antiviral treatment | $753 | $583.27-$972.11 | [6, 52–57] | Lognormal |
| HCV cost of compensated cirrhosis w/out antiviral treatment | $1433 | $1,110.00–$1,849.99 | [6, 52–57] | Lognormal |
| HCV cost of decompensated cirrhosis w/out antiviral treatment | $19 317 | $11,152.79-$33,457.69 | [6, 52–57] | Lognormal |
| Cost of HCC | $40 663 | $23,477.03-$70,429.67 | [6, 52–57] | Lognormal |
| Cost in Years After SVR | $224.88 | $174.19–$290.32 | [4, 7–9, 50, 51] | Lognormal |
| Cost of liver transplant (year of) | $190 301 | $109,871.44–$329,607.67 | [4, 6–9, 52, 53] | Lognormal |
| Cost of liver transplant (subsequent years) | $34 369 | $19,843.15-$59,528.25 | [4, 6–9, 52, 53] | Lognormal |
| Prevalence rates | ||||
| Hepatitis C infection | Varies by Birth Decade, Race, and Sex. See Technical Report | [58] | Lognormal | |
| Heavy alcohol use (>4 drinks/day) | 0.089 | 0.089–0.090 | [59] | Beta |
| HIV+ | 0.0205 | 0.020–0.021 | [59] | Beta |
| Viral Type 1, black race | 0.900 | 0.794–0.970 | [60] | Beta |
| Viral Type 1, race other than black | 0.700 | 0.628–0.768 | [60] | Beta |
| Prevalence of IDU | Varies by Birth Decade. See Technical Report | [58] | Lognormal | |
| METAVIR level at diagnosis | ||||
| METAVIR 0–1 | 0.107 | … | Unpublished CDC Data | … |
| METAVIR 1–2 | 0.357 | … | Unpublished CDC Data | … |
| METAVIR 2–3 | 0.232 | … | Unpublished CDC Data | … |
| METAVIR 3–4 | 0.143 | … | Unpublished CDC Data | … |
| METAVIR 4+ | 0.161 | … | Unpublished CDC Data | … |
| Annual Incremental Increase in METAVIR Score Units | ||||
| Relative METAVIR rate increase for patients infected with HIV, regardless of age, gender, or alcohol use status | 2.00 | … | [39] | … |
| Infected under age 40 | ||||
| Male, alcohol | 0.154 | 0.125–0.167 | [61] | Lognormal |
| Male, no alcohol | 0.111 | 0.091–0.130 | [61] | Lognormal |
| Female, alcohol* | 0.095 | 0.088–0.100 | [61] | Lognormal |
| Female, no alcohol | 0.095 | 0.088–0.100 | [61] | Lognormal |
| Infected age 40 or older | [61] | |||
| Male, alcohol | 0.267 | 0.200–0.0500 | [61] | Lognormal |
| Male, no alcohol | 0.301 | 0.235–0.333 | [61] | Lognormal |
| Female, alcohol | 0.267 | 0.200–0.0500 | [61] | Lognormal |
| Female, no alcohol | 0.200 | 0.167–0.250 | [61] | Lognormal |
| Annual discount rate | 0.03 | Not applicable | Assumed | Did not vary |
Abbreviations: HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IDU, injection drug use; QALY, quality-adjusted life-year; SVR, sustained viral response.
Model Cohorts
We modeled the US population aged 20 or older, totaling 229 185 985 in 2012 [62]. We stratified the population based on age, sex, and lifetime risk of injecting drugs [63].We further stratified these cohorts into those with and without antibody to HCV (based on year of birth), and those with antibodies into those with chronic (78%) and cleared (22%) infections [64]. We assumed 25% of chronically infected patients were not interested in treatment or were not reachable by the healthcare system and assumed the remainder would be offered testing [35, 36, 40, 65].
We estimated starting fibrosis rates using data from biopsy results of newly diagnosed patients observed in the retrospective component of the Birth-cohort Evaluation to Advance Screening and Testing for Hepatitis C (BEST-C) study [66]. We used census life tables to calculate the annual probability of mortality from nonhepatic causes and assigned a relative risk of mortality of 1.42 for individuals who reported ever injecting drugs [2, 67].
Screening and Treatment Scenarios
For the purpose of our simulation, we assumed that 18.5% of those outside the 1945 to 1965 birth-cohort would be offered testing and that 100% of those in the birth-cohort would be offered testing if they could be reached through the health system. Of those who accepted testing and tested positive for HCV RNA, we compared the cost-effectiveness and health impacts of 5 treatment alternatives: (1) No treatment (NT); (2) Pegylated interferon and ribavirin (PR) for 48 weeks for genotypes 1 and 4, and for 24 weeks for genotypes 2 and 3; (3) PR for 24 weeks plus an additional protease inhibitor (PRPI) for 12 weeks for genotypes 1 and 4 or PR for 24 weeks for genotypes 2 and 3; (4) PR plus Sofosbuvir (PRS) for 12 weeks for genotypes 1 and 4, Sofosbuvir plus ribavirin (SR) for 12 weeks for genotype 2, and SR for 24 weeks for genotype 3; or (5) Simeprevir and Sofosbuvir (SS) for 12 weeks for genotypes 1 and 4, SR for 12 weeks for genotype 2, and SR for 24 weeks for genotype 3. We assumed all treatments occurred in the first year of the simulation. These treatments are consistent with those evaluated by major medical societies in creating their HCV treatment guidelines [10]. Although guidelines discourage the use of older line treatments, we include them to facilitate comparisons with other studies. We also separately report preliminary incremental cost-effectiveness ratio (ICER) estimates for interferon-free combination of ledipasvir and sofosbuvir and the drugs in viekira pak, which were approved after initial submission of this article.
Screening, Contraindication, and Antiviral Initiation
We assumed that 91% of those offered testing would accept and 90% of those who tested positive would receive their result and be evaluated for treatment [37]. To estimate the proportion of patients who would receive treatment we conducted a meta-analysis of rates of treatment found across 12 published studies of community treatment of patients with HCV infection only [14–25]. We estimated the proportion who would be treated with pegylated interferon based treatments (0.242) and its credible interval (0.228–0.251) using Monte Carlo Markov Chain (MCMC) simulation methods programmed with Proc MCMC of the SAS 9.2 Software (SAS Institute, Cary, North Carolina) [68]. We also estimated the proportion of persons who would be treated (0.719) with nonpegylated interferon-based treatments and its credible interval (0.66–0.77).
Effectiveness, Cost, and Benefit of Antiviral Therapy
Older forms of treatment have exhibited lower rates of real world effectiveness and cost than in clinical trial data, but real-world data are not yet available for newer treatments. To enable equivalent comparisons we used clinical trial estimates of efficacy and published package estimates of cost for all treatments. The benefit of successful treatment was an SVR that varied with treatment type and virus genotype. For pegylated interferon based treatments, we also assumed a quality adjusted life year decrement that varied with the duration of treatment. We assumed an SVR eliminated fibrosis progression associated with chronic HCV infection. For patients with cirrhosis, we assumed an SVR was also associated with a relative risk of HCC of 0.24 [34].
Testing and Medical Treatment Costs
We set the cost of testing via routine risk-based assessments to $24.65 per person tested, equal to the incremental costs of testing using an electronic health record prompt system in an unpublished CDC study. Diagnosed patients who did not undergo antiviral therapy or achieve an SVR were assumed to receive HCV-related medical management, with costs per stage estimated as the average costs used across seven previously published cost-effectiveness studies [2, 4, 6–9, 53]. Patients who achieved an SVR accrued annual monitoring costs. Nontreatment clinical management increased costs without increasing benefits.
Utility Losses
Uninfected persons were assigned annual QALY values that decreased with age to account for other health conditions [69]. For persons with HCV, we collected utility losses from 5 studies across 7 HCV states: SVR, METAVIR 0–1, METAVIR 2–3, compensated cirrhosis, DCC, HCC, and post-liver transplant then summarized the scores as reported elsewhere [2, 69–74]. Annual QALYs for patients on pegylated interferon-based therapy were multiplied by 0.85 adjusting for treatment duration [13].
Simulation, Outcomes, and Sensitivity Analysis
We estimated medical outcomes, costs, and QALYs associated with each scenario accounting for uncertainty in each of the model’s key parameters using probabilistic sensitivity analysis, reporting the mean and the empirical 95% credible interval for each outcome. We estimated the ICER for routine and birth-cohort testing combined followed by each treatment scenario as compared to the next most costly alternative. For PRS/SR and for SS/SR, we estimated the ICER of immediate treatment compared to no treatment (NT; scenario 1) for people in METAVIR stages F0, F1, F2, F3, and F4. For PRS/SR compared to PRPI and for SS/SR compared to PRS/SR we tested the univariate sensitivity of the ICER to uncertainty in the model’s key parameters by evaluating results based on the upper and lower bounds of the 95% confidence interval of each parameter included in Tables 1 and 2.
We estimated the cost of treatment for SS/SR at which the ICER was equal to $50 000 per QALY gained compared to PRS/SR and compared to NT. Compared to NT, we estimated the treatment cost at which the ICER of PRS/SR and SS/SR was equal to $50 000 per QALY gained for patients treated at stages F0 and F1.
For all patients, we estimated the cost-effectiveness of SS/SR compared to PRS/SR and to NT when the cost of SS was set to the list price of Viekira Pak ($83 319) and the list price of Harvoni ($94 500). We provide only limited results for these scenarios, because these treatments were released during this manuscript’s review process.
RESULTS
Of the 229.2 million Americans aged ≥20 years in 2012, we estimated 3.7 million were antibody positive for HCV, 2.9 million were chronically infected, and that 1.5 million would be identified through testing prior to the development of end-stage liver disease or death from other causes. With no testing or treatment (scenario 1), we estimated that 1.18 million of those chronically infected (41.1%) would develop DCC or HCC and die in those states prior to model termination at age 100 (Table 3). For comparison to other studies, the model’s 45-year mortality rate was 18.7% assuming age of infection of 25 years and a starting fibrosis state of F0. With no testing or treatment, currently infected patients were expected to generate $100.3 billion in discounted hepatitis C medical costs during their lifetimes.
Table 3.
HCV Cumulative Deaths, Costs, and QALYs per Person Associated With the United State Age 20+ United States Populationa, and Incremental Results, Assuming CDC Recommended Testing Followed by Treatment With Different Treatment Modalities
| Tx by Genotype | Cumulative HCV Deaths | Costs per Person | QALYs per Person | Incremental Deaths | Incremental Costs | Incremental QALYs | ICER |
|---|---|---|---|---|---|---|---|
| No Treatment | 1 181 554 | $437 | 15.656 | n/a | n/a | n/a | n/a |
| (1 088 653–1 270 476) | ($344-$561) | (15.652–15.661) | |||||
| G1/4 - PR, 48 wks | 1 131 638 | $517 | 15.658 | −49 916 | $80.0 | 0.0013 | $59 792b |
| G2/3 - PR, 24 wks, 12 Wks | (1 045 343–1 216 951) | ($422-$638) | (15.653–15.662) | (−43 311–−53 526) | ($76-$78) | (0.0011–0.0015) | (-$205 950–$213 295) |
| G1/4 - PRPI, 24 wks, 12 Wks | 1 106 130 | $528 | 15.658 | −75 424 | $91 | 0.0021 | $43 530c |
| G2/3 - PR, 24 wks | (1 022 050–1 190 781) | ($433–$648) | (15.654–15.663) | (−66 603–−79 696) | ($90–$86) | (0.0018–0.0023) | (-$238 295–$227 433) |
| G1/4 - PR+ Sofosbuvir, 12 Wks | 1 025 448 | $642 | 15.661 | −80 682 | $114 | 0.0024 | $47 237 |
| G2 - Sofosbuvir + Ribavirin, 12 wks | (945 291–1 106 558) | ($542–$758) | (15.657–15.665) | (−76 759–−84 223) | ($108–$110) | (0.0021–0.0028) | ($34 058–$63 969) |
| G1/4 – Sofosbuvir + Simeprevir, 12 wks | 860 908 | $992 | 15.666 | −164 540 | $350 | 0.0048 | $72 169 |
| G2 - Sofosbuvir + Ribavirin, 12 wks G3 - Sofosbuvir + Ribavirin, 24 wks | (781 473–936 340) | ($835–$1167) | (15.662–15.669) | (−163 818–−170 218) | ($294-$410) | (0.0044–0.0053) | ($50 931–$102 196) |
Abbreviations: CDC, Centers for Disease Control and Prevention; G, genotype; HCV, hepatitis C virus; ICER, incremental cost-effectiveness ratio; PR, pegylated interferon and ribavirin; PRPI, PR + a protease inhibitor such as telaprevir or boceprevir; QALY, quality-adjusted life-year; Tx, Treatment.
United States Population total used in year 1 of the model = 229 185 985.
Extendedly dominated by PRPI.
Compared to no treatment.
The Health Benefits and Cost Impacts of Treatment Scenarios
With testing and PR treatment (scenario 2), 356 657 patients were treated of whom 156 880 achieved an SVR reducing the number of HCV-associated deaths from 1 181 554 to 1 131 638, a reduction of 49 916 deaths compared to NT. Compared to NT, testing followed by PR treatment increased QALYs by 306 537 and medical costs by $18.3 billion. With the same number of patients treated as compared to NT, PRPI (scenario 3) increased patients achieving an SVR by 237 618 and reduced the number of deaths from HCV to 1 106 130, a reduction of 75 424 deaths. Compared to NT, PRPI increased QALYs by 477 066 and increased medical costs by $20.8 billion. With testing and PRS/SR treatment (scenario 4), 541 136 patients were treated of whom 489 573 achieved an SVR reducing the number of deaths from HCV by 156 106 compared to NT. Compared to NT, PRS/SR increased QALYs by 1 032 292 and increased and medical costs by $47.0 billion. Finally, with testing and SS/SR treatment (scenario 5), 1 057 148 patients were treated of whom 1 010 225 achieved an SVR reducing the number of deaths from HCV by 320 646 compared to NT. Compared to NT, SS/SR increased QALYs by 2 142 743 and medical costs by $127.1 billion.
Incremental Cost-effectiveness
The ICER of PR vs NT was $59 792 per QALY gained (Table 3). PR was extendedly dominated by PRPI. Compared to NT, the ICER of PRPI was $43 530 per QALY gained, PRS/SR cost $47 237 per QALY gained compared to PRPI, and SS/SR cost $72 169 per QALY gained compared to PRS/SR. Compared to NT, the incremental cost per QALY gained was $59 792 for PR, $43 530 for PRPI, $45 524 for PRS/SR, and $59 333 for SS/SR.
SENSITIVITY ANALYSES
Compared to NT, the ICER of both PRS/SR and SS/SR was sensitive to the fibrosis stage at the time of treatment, from $173 800 per QALY gained for SS/SR at stage F0 to $13 000 per QALY gained for PRS/SR for patients with cirrhosis (Figure 2). The ICER of PRS/SR compared to PRPI was most sensitive to the cost of PRS/SR treatment, QALY improvements assumed to occur after an SVR, the speed of fibrosis progression, QALY losses associated with moderate fibrosis (F2, F3) and cirrhosis (F4), the medical cost of DCC, the probability of an SVR for PRS/SR, and the risk reduction of HCC among people with cirrhosis who had achieved an SVR (Figure 3A and 3B). No other parameter in the model changed the ICER by more than 5% when set to the bounds of its 95% confidence interval. The ICER of SS/SR compared to PRS/SR was sensitive to similar variables (cost of treatment, QALY losses associated with infection prior to end stage disease, the probability of an SVR, and the impact of an SVR on reducing HCC).
Figure 2.
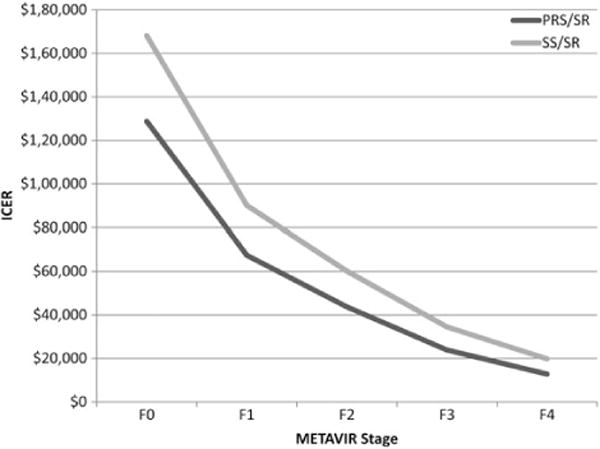
Incremental cost-effectiveness by liver fibrosis score as measured by METAVIR score. Abbreviations: ICER, incremental cost-effectiveness ratio; PRS/SR, pegylated interferon, ribavirin and sofosbuvir for genotypes 1 and 4, and sofosbuvir and ribavirin for genotypes 2 and 3; SS/SR, sofosbuvir and simeprevir for genotypes 1 and 4, and sofosbuvir and ribavirin for genotypes 2 and 3.
Figure 3.
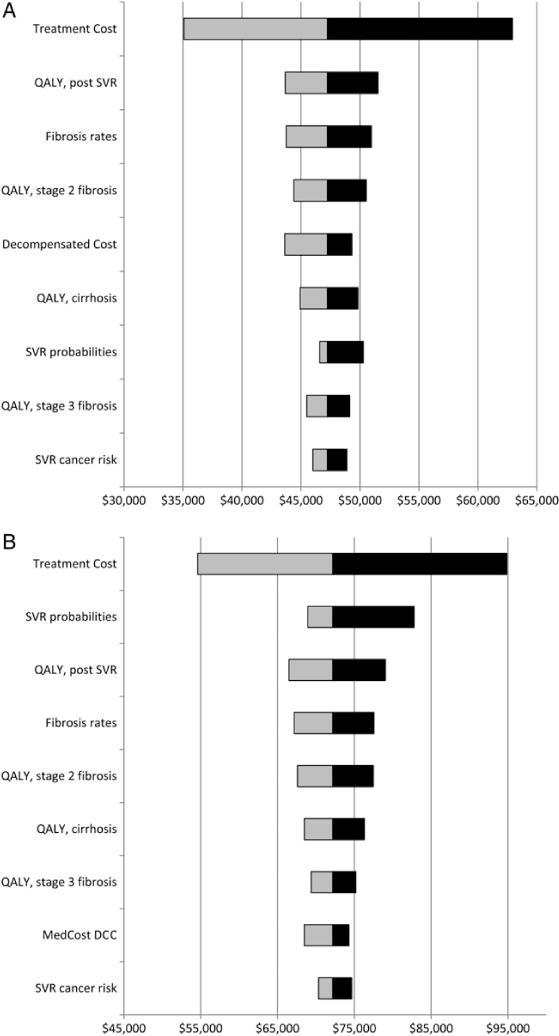
A, Univariate sensitivity to changes in key model parameters of pegylated interferon, ribavirin, and sofosbuvir treatment for G1 and sofosbuvir/ribavirin treatment for G2 and 3 compared to pegylated interferon, ribavirin, and protease inhibitor treatment for G1 and pegylated interferon and ribavirin for G2 and 3. B, Univariate sensitivity to changes in key model parameters of sofosbuvir and simeprevir treatment for G1 and sofosbuvir/ribavirin treatment for G2 and 3 compared to pegylated interferon, ribavirin, and sofosbuvir treatment for G1 and sofosbuvir/ribavirin treatment for G2 and 3. Univariate sensitivity analysis included all parameters from Tables 1 and 2. Tested ranges based on the upper and lower 95% confidence interval bound for each parameter. Only parameters with a >5% impact on ICER are shown. Assumes birth cohort testing is implemented. Abbreviations: DCC, decompensated cirrhosis; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year; SVR, sustained viral response.
The ICER of SS/SR compared to PRS/SR fell to $50 000 per QALY gained at a treatment cost of $136 000. Compared to NT, the ICER of SS/SR was equal to $50 000 per QALY gained at a treatment cost of $139 000. Assuming the same level of effectiveness, SS/SR cost $24 921 per QALY gained compared to PRS/SR and $31 828 compared to NT at the price of Viekira Pak, and $25 405 per QALY gained compared to PRS/SR and $35 100 compared to NT at the list price of Harvoni.
Compared to NT, treating patients at stage F0 with PRS/SR would need to cost $37 600 to achieve an ICER of $50 000 per QALY; $47 000 for treatment with SS/SR. Also as compared to NT, treating patients at stage F1 with PRS/SR would need to cost $73 000 to achieve an ICER of $50 000 per QALY; $82 000 for treatment with SS/SR.
CONCLUSIONS
Our estimates indicate that the treatment alternatives for HCV of pegylated interferon combined with ribavirin and Sofosbuvir, and the all-oral combinations of Sofosbuvir and Simeprevir increase QALYs compared to their alternatives at a cost of $47 237 per QALY gained for PRS/SR and $72 169 per QALY gained for SS/SR. During review of this article, two interferon-free combination treatments were approved for the treatment of genotype 1 HCV patients (Harvoni and Viekira Pak) with lower list prices ($94 500 and $83 319) compared to SS/SR. Assuming an equal effectiveness for these combinations as for SS, the lower prices would result in cost-effectiveness of approximately $25 000 per QALY gained for new treatments compared to PRS/SR, and of approximately $32 000 to $35 000 per QALY gained compared to NT. Potentially lower prices would improve treatment cost-effectiveness further.
However, financing the treatment of all Americans who could benefit from antiviral therapy will be a continuing challenge given the number of individuals who are undiagnosed, untreated, or failed to respond to older treatment regimens. In addition, simply linking diagnosed patients to clinical settings in which they can be evaluated for treatment remains an ongoing challenge that is likely to reduce the potential benefits and costs of new treatments for the foreseeable future [75, 76].
Still our estimates indicate achieving modest identification and treatment benchmarks (1.06 million chronically infected individuals) could increase QALYs by over 2.1 million, decrease deaths from HCV by over 320 000, but also increase lifetime costs. Increased costs are a function of both the unit costs of new treatments that are declining as new drugs enter the market, and also the greater number of individuals that can tolerate all-oral regimens. Given the current difficulties of linking patients to care, the incremental costs of new treatments are likely to accrue over time and may be reduced as more treatments are approved for use and insurers negotiate discounts for their plan members. Our sensitivity analyses indicate that ICER of PRS/SR compared to PRPI and of SS/SR were highly sensitive to the costs of treatment. Lower costs (especially for all-oral regimens) would increase their cost-effectiveness and alleviate financing pressures.
Our sensitivity analyses also indicate that cost-effectiveness is sensitive to the stage at which a patient is treated. Treating with SS/SR costs $173 796 per QALY gained for people with a current fibrosis status of F0 compared to only $35 884 for patients in F3. However, this finding must be understood in context of our lack of knowledge of the health and cost impacts of chronic infection prior to the development of end-stage liver disease and the limited ability to identify patients’ stage of liver fibrosis without the use of biopsy.
Limitations
Our study is limited by at least the following factors. First, we made a number of assumptions regarding the utilization of new treatments. Because the number of people who will seek care is unknown, we assumed that 25% of the population would be beyond the reach of the healthcare system. Given the current difficulties of linking identified individuals to clinical care, this number may be optimistic. To simplify estimation, we further assumed that all patients who received treatment would do so in the base year of the simulation. Compared to an alternative that treats all patients over time and assumes no missed opportunities to prevent disease, this limitation has the effect of making treatment appear more costly and less cost-effective as NT costs are discounted, and NT is averted due to death from non-HCV causes. Finally, we estimated the rates of interferon-based treatment uptake using data from studies prior to the inclusion of more effective agents, and made assumptions about how treatment rates would increase with interferon-free treatment. Sensitivity analyses indicate these assumptions do not have a large impact on cost-effectiveness; however, lower treatment uptake will lower the aggregate health benefits and costs reported for each scenario.
Second, our cost-effectiveness results are partially determined by the model’s distribution of starting fibrosis rates which were derived from primary biopsy data from newly diagnosed patients. While, we believed these are superior to previously used simulated estimates, data on this parameter are sparse, and treatment will be less cost-effective if undiagnosed patients have milder progression. However, our sensitivity analyses estimate the cost-effectiveness of treatment at different stages of progression and indicate that treatment at earlier fibrosis stages is still moderately cost-effective compared to NT (at F1, $73 906 per QALY gained for PRS/SR, and $93 236 for SS/SR compared to NT). Updates to medical treatment guidelines call for prioritizing treatment in patients who are F1 or higher.
Our article reports an overall mortality rate of 41% among prevalent hepatitis C cases given NT, a rate higher than reported in earlier model publications [2, 21]. This higher rate of mortality results from the use of a longer time horizon in this paper (until age 100). Our model’s 45-year mortality rate is identical to that from previous work [2].
Our model excludes the treatment benefits of averting secondary transmissions. Although such benefits remain hypothetical, modeling studies suggest that treatment reduces transmission especially among people who inject drugs [77]. The limitation results in a less favorable ICER than had these benefits been included.
Finally, ICER by fibrosis stage estimates assumes that fibrosis level can be reliably ascertained in clinical settings, although performing biopsies among all patients is likely unethical. Although nonbiopsy ascertainment methods like AST/Platelet Ratio Index (APRI), Fibrosis-4 scoring, and elastography are improving, they cannot yet reliably differentiate between pre-cirrhosis fibrosis stages.
Implications
New treatments for HCV infection have the potential to provide substantial public health benefits at a reasonable cost per patient treated. However, the high number of untreated hepatitis C patients creates financing challenges that need to be overcome.
Supplementary Material
Acknowledgments
Financial support. This research was supported by a contract award from the National Foundation for the Centers for Disease Control and Prevention, Inc. MOU # 527-11 SC. Through an award to NORC that did not provide support for this analysis or manuscript, D. B. R., J. S. W., and D. K. L. have received unrestricted research funding related to hepatitis C from Gilead Sciences, Inc.
Footnotes
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.CDC Division of Viral Hepatitis. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012;61(RR-4):1–32. [PubMed] [Google Scholar]
- 2.Rein DB, Smith BD, Wittenborn JS, et al. The cost-effectiveness of birth-cohort screening for hepatitis C antibody in U.S. primary care settings. Ann Intern Med. 2012;156:263–70. doi: 10.7326/0003-4819-156-4-201202210-00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moyer VA. Screening for hepatitis C virus infection in adults: US Preventive services task force recommendation statement. Ann Intern Med. 2013;159:349–57. doi: 10.7326/0003-4819-159-5-201309030-00672. [DOI] [PubMed] [Google Scholar]
- 4.Liu S, Cipriano LE, Holodniy M, Goldhaber-Fiebert JD. Cost-effectiveness analysis of risk-factor guided and birth-cohort screening for chronic hepatitis C infection in the United States. PloS One. 2013;8:e58975. doi: 10.1371/journal.pone.0058975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng W, Yankey D, Ganesh N, Pineau V, Smith PJ. Using soft refusal status in the cell-phone nonresponse adjustment in the National Immunization Survey. American Statistical Association. Joint Statistical Conference. 2012 [Google Scholar]
- 6.McGarry LJ, Pawar VS, Panchmatia HR, et al. Economic model of a birth cohort screening program for hepatitis C virus. Hepatology. 2012;55:1344–55. doi: 10.1002/hep.25510. [DOI] [PubMed] [Google Scholar]
- 7.Coffin PO, Scott JD, Golden MR, Sullivan SD. Cost-effectiveness and population outcomes of general population screening for hepatitis C. Clin Infect Dis. 2012;54:1259–71. doi: 10.1093/cid/cis011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckman MH, Talal AH, Gordon SC, Schiff E, Sherman KE. Cost-effectiveness of screening for chronic hepatitis C infection in the United States. Clin Infect Dis. 2013;56:1382–93. doi: 10.1093/cid/cit069. [DOI] [PubMed] [Google Scholar]
- 9.McEwan P, Ward T, Yuan Y, Kim R, L’Italien G. The impact of timing and prioritization on the cost-effectiveness of birth cohort testing and treatment for hepatitis C virus in the United States. Hepatology. 2013;58:54–64. doi: 10.1002/hep.26304. [DOI] [PubMed] [Google Scholar]
- 10.AASLD, IDSA. Recommendations for testing, managing, and treating hepatitis C. Available at: http://www.hcvguidelines.org/full-report/hcv-testing-and-linkage-care. Accessed 20 March 2014.
- 11.Yang D, Liang HJ, Li D, Wei X, Ma L, Jia Z. The efficacy and safety of telaprevir-based regimens for treating chronic hepatitis C virus genotype 1 infection: a meta-analysis of randomized trials. Intern Med. 2013;52:653–60. doi: 10.2169/internalmedicine.52.8156. [DOI] [PubMed] [Google Scholar]
- 12.Consumer Reports. Best buy drugs, the pegylated interferons: treating hepatitis C, comparing effectiveness, safety, and price. Available at: http://www.consumerreports.org/health/resources/pdf/best-buy-drugs/BBD_Hepatitis_C.pdf. Accessed 25 March.
- 13.Wright M, Grieve R, Roberts J, Main J, Thomas HC. Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation. Health Technol Assess. 2006;10:1–113. iii. doi: 10.3310/hta10210. [DOI] [PubMed] [Google Scholar]
- 14.Adeyemi OM, Jensen D, Attar B, et al. Hepatitis C treatment eligibility in an urban population with and without HIV coinfection. AIDS Patient Care STDs. 2004;18:239–45. doi: 10.1089/108729104323038919. [DOI] [PubMed] [Google Scholar]
- 15.Alfandre D, Gardenier D, Federman A, McGinn T. Hepatitis C in an urban cohort: who’s not being treated? J Health Care Poor Underserved. 2009;20:1068–78. doi: 10.1353/hpu.0.0203. [DOI] [PubMed] [Google Scholar]
- 16.Cheung RC, Cunningham BA, Cooper AD. Effectiveness of a screening program for hepatitis C. Dig Dis Sci. 2006;51:976–81. doi: 10.1007/s10620-006-9100-x. [DOI] [PubMed] [Google Scholar]
- 17.Delwaide J, El Saouda R, Gerard C, Belaiche J, Groupe Liegeois d’Etude des Virus H Hepatitis C infection: eligibility for antiviral therapies. Eur J Gastroenterol Hepatol. 2005;17:1185–9. doi: 10.1097/00042737-200511000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Evon DM, Verma A, Dougherty KA, et al. High deferral rates and poorer treatment outcomes for HCV patients with psychiatric and substance use comorbidities. Dig Dis Sci. 2007;52:3251–8. doi: 10.1007/s10620-006-9669-0. [DOI] [PubMed] [Google Scholar]
- 19.Falck-Ytter Y, Kale H, Mullen KD, Sarbah SA, Sorescu L, McCullough AJ. Surprisingly small effect of antiviral treatment in patients with hepatitis C. Ann Intern Med. 2002;136:288–92. doi: 10.7326/0003-4819-136-4-200202190-00008. [DOI] [PubMed] [Google Scholar]
- 20.Moirand R, Bilodeau M, Brissette S, Bruneau J. Determinants of antiviral treatment initiation in a hepatitis C-infected population benefiting from universal health care coverage. Can J Gastroenterol. 2007;21:355–61. doi: 10.1155/2007/576765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrill JA, Shrestha M, Grant RW. Barriers to the treatment of hepatitis C. Patient, provider, and system factors. J Gen Intern Med. 2005;20:754–8. doi: 10.1111/j.1525-1497.2005.0161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narasimhan G, Sargios TN, Kalakuntla R, et al. Treatment rates in patients with chronic hepatitis C after liver biopsy. J Viral Hepat. 2006;13:783–6. doi: 10.1111/j.1365-2893.2006.00763.x. [DOI] [PubMed] [Google Scholar]
- 23.Rifai MA, Moles JK, Lehman LP, Van der Linden BJ. Hepatitis C screening and treatment outcomes in patients with substance use/dependence disorders. Psychosomatics. 2006;47:112–21. doi: 10.1176/appi.psy.47.2.112. [DOI] [PubMed] [Google Scholar]
- 24.Rowan PJ, Tabasi S, Abdul-Latif M, Kunik ME, El-Serag HB. Psychosocial factors are the most common contraindications for antiviral therapy at initial evaluation in veterans with chronic hepatitis C. J Clin Gastroenterol. 2004;38:530–4. doi: 10.1097/01.mcg.0000123203.36471.70. [DOI] [PubMed] [Google Scholar]
- 25.Rocca LG, Yawn BP, Wollan P, Kim WR. Management of patients with hepatitis C in a community population: diagnosis, discussions, and decisions to treat. Ann Fam Med. 2004;2:116–24. doi: 10.1370/afm.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.AIDSMEDS. Fair Pricing Coalition says Vertex’s Incivek price ‘outrageous’. Available at: http://www.aidsmeds.com/articles/incivek_price_advocacy_1667_20508.shtml. Accessed 25 March 2015.
- 27.Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–87. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 28.Gilead. US Food and Drug Administration approves Gilead’s Sovaldi (Sofosbuvir) for the treatment of chronic Hepatitis C. Available at: http://www.gilead.com/news/press-releases/2013/12/us-food-and-drug-administration-approves-gileads-sovaldi-sofosbuvir-for-the-treatment-of-chronic-hepatitis-c. Accessed 25 March 2015.
- 29.Jacobson IM, Ghalib RH, Rodriguez-Torres M, et al. AASLD. Washington, DC: 2013. Abstract #LB-3: SVR results of a once-daily regimen of simeprevir (TMC435) plus sofosbuvir (GS-7977) with or without ribavirin in cirrhotic and non-cirrhotic HCV genotype 1 treatment-naïve and prior null responder patients: The COSMOS study. [Google Scholar]
- 30.Hepatitis C New Drug Research And Liver Health. OLYSIO Simeprevir Cost? Available at: http://hepatitiscnewdrugresearch.com/-olysiotrade-simeprevir-cost.html. Accessed 25 March 2015.
- 31.Zeuzem S, Dusheiko GM, Salupere R, et al. AASLD. Washington, DC: 2013. Abstract# 1085: Sofosbuvir + Ribavirin for 12 or 24 weeks for patients with HCV genotype 2 or 3: the VALENCE trial. [Google Scholar]
- 32.Bennett WG, Inoue Y, Beck JR, Wong JB, Pauker SG, Davis GL. Estimates of the cost-effectiveness of a single course of interferon-alpha 2b in patients with histologically mild chronic hepatitis C. Ann Intern Med. 1997;127:855–65. doi: 10.7326/0003-4819-127-10-199711150-00001. [DOI] [PubMed] [Google Scholar]
- 33.Solomon L, Flynn C, Muck K, Vertefeuille J. Prevalence of HIV, syphilis, hepatitis B, and hepatitis C among entrants to Maryland correctional facilities. J Urban Health. 2004;81:25–37. doi: 10.1093/jurban/jth085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329–37. doi: 10.7326/0003-4819-158-5-201303050-00005. [DOI] [PubMed] [Google Scholar]
- 35.Hagan H, Campbell J, Thiede H, et al. Self-reported hepatitis C virus antibody status and risk behavior in young injectors. Public Health Rep. 2006;121:710–9. doi: 10.1177/003335490612100611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wasley A, Finelli L, Bell B, Alter M. The knowledge and behaviors of HCV-infected persons identified in a seroprevalence survey, USA, 2001-2002. J Clin Virol. 2006;36(suppl 2):S198–9. [Google Scholar]
- 37.Honeycutt AA, Harris JL, Khavjou O, Buffington J, Jones TS, Rein DB. The costs and impacts of testing for hepatitis C virus antibodies in public STD clinics. Public Health Rep. 2007;122(suppl 2):55–62. doi: 10.1177/00333549071220S211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chou R, Cottrell EB, Wasson N, Rahman B, Guise JM. Screening for hepatitis C virus infection in adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;158:101–8. doi: 10.7326/0003-4819-158-2-201301150-00574. [DOI] [PubMed] [Google Scholar]
- 39.Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Empirically calibrated model of hepatitis C virus infection in the United States. Am J Epidemiol. 2002;156:761–73. doi: 10.1093/aje/kwf100. [DOI] [PubMed] [Google Scholar]
- 40.Culver DH, Alter MJ, Mullan RJ, Margolis HS. Evaluation of the effectiveness of targeted lookback for HCV infection in the United States— interim results. Transfusion. 2000;40:1176–81. doi: 10.1046/j.1537-2995.2000.40101176.x. [DOI] [PubMed] [Google Scholar]
- 41.Planas R, Balleste B, Alvarez MA, et al. Natural history of decompensated hepatitis C virus-related cirrhosis. A study of 200 patients. J Hepatol. 2004;40:823–30. doi: 10.1016/j.jhep.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463–72. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 43.Ortner NJ, Cosway RG. US organ and tissue transplant cost estimates and discussion. Seattle: Milliman Consultants and Actuaries; 2005. [Google Scholar]
- 44.Jain A, Reyes J, Kashyap R, et al. Long-term survival after liver transplantation in 4,000 consecutive patients at a single center. Ann Surg. 2000;232:490–500. doi: 10.1097/00000658-200010000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vlahov D, Wang C, Ompad D, et al. Mortality risk among recent-onset injection drug users in five U.S. cities. Subst Use Misuse. 2008;43:413–28. doi: 10.1080/10826080701203013. [DOI] [PubMed] [Google Scholar]
- 46.Centers for Medicare & Medicaid Services (CMS) Clinical Laboratory Fee Schedule. 2014 Available at: http://www.cms.gov/apps/ama/license.aspfile=/ClinicalLabFeeSched/downloads/14CLAB.zip. Accessed 6 May 2014.
- 47.American Association for the Study of Liver Diseases: diagnosis, management, and treatment of Hepatitis C. Accessed 24 May 2007.
- 48.Carey WD, Fried MW, Jeffers L, et al. Hepatitis C management. In: The Cleveland Clinic, editor. The Cleveland clinic monograph series. Cleveland: The Cleveland Clinic Foundation; 2007. [Google Scholar]
- 49.Gray L, Parkinson J, editors. The essential RBRVS. Salt Lake City: St. Anthoney’s Publishing; 2003. [Google Scholar]
- 50.Recommended adult immunization schedule - United States, October 2007–September 2008 (Reprinted from MMWR, vol 56, pg Q1-Q4, 2007). JAMA 2007; 298:2477–80.
- 51.Pediatric/VFC Vaccine Price List.
- 52.Rein DB, Wittenborn JS, Weinbaum CM, Sabin M, Smith BD, Lesesne SB. Forecasting the morbidity and mortality associated with prevalent cases of pre-cirrhotic chronic hepatitis C in the United States. Dig Liver Dis. 2011;43:66–72. doi: 10.1016/j.dld.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 53.Chan K, Lai MN, Groessl EJ, et al. Cost effectiveness of direct-acting antiviral therapy for treatment-naive patients with chronic HCV genotype 1 infection in the Veterans Health Administration. Clin Gastroenterol Hepatol. 2013;11:1503–10. doi: 10.1016/j.cgh.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 54.Milliman I. In: Liver transplant costs. Personal Communication RH, editor. Milwaukee, WI: 2007. [Google Scholar]
- 55.Englesbe MJ, Dimick J, Mathur A, et al. Who pays for biliary complications following liver transplant? A business case for quality improvement. Am J Transplant. 2006;6:2978–82. doi: 10.1111/j.1600-6143.2006.01575.x. [DOI] [PubMed] [Google Scholar]
- 56.Showstack J, Katz PP, Lake JR, et al. Resource utilization in liver transplantation: effects of patient characteristics and clinical practice. NIDDK Liver Transplantation Database Group. JAMA. 1999;281:1381–6. doi: 10.1001/jama.281.15.1381. [DOI] [PubMed] [Google Scholar]
- 57.Berge JJ, Drennan DP, Jacobs RJ, et al. The cost of hepatitis A infections in American adolescents and adults in 1997. Hepatology. 2000;31:469–73. doi: 10.1002/hep.510310229. [DOI] [PubMed] [Google Scholar]
- 58.Wong JB, McQuillan GM, McHutchison JG, Poynard T. Estimating future hepatitis C morbidity, mortality, and costs in the United States. Am J Public Health. 2000;90:1562–9. doi: 10.2105/ajph.90.10.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.(CDC) CfDCaP. National Center for Health Statistics (NCHS), National Health and Nutrition Examination Survey (NHANES) Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 1999–2010. [Google Scholar]
- 60.Centers for Disease Control and Prevention. National Center for Health Statistics. National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2001–2006. Vol. Available at: http://www.cdc.gov/nchs/about/major/nhanes/datalink.htm. Accessed 28 April 15. [Google Scholar]
- 61.Nainan OV, Alter MJ, Kruszon-Moran D, et al. Hepatitis C virus genotypes and viral concentrations in participants of a general population survey in the United States. Gastroenterology. 2006;131:478–84. doi: 10.1053/j.gastro.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 62.US Census. PEPASR5H. Annual estimates of the resident population by sex, age, race alone or in combination, and Hispanic origin for the United States and States: April 1, 2010 to July 1, 2012.
- 63.Centers for Disease Control and Prevention. National Center for Health Statistics National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 1999–2010. Vol. Available at: http://www.cdc.gov/nchs/about/major/nhanes/datalink.htm. Accessed 28 April 15. [Google Scholar]
- 64.Chou R, Cottrell EB, Wasson N, Rahman B, Guise JM. Screening for hepatitis C virus infection in adults: a systematic review to update the 2004 U.S. preventive services task force recommendation. Ann Intern Med. 2012;158:101–8. doi: 10.7326/0003-4819-158-2-201301150-00574. [DOI] [PubMed] [Google Scholar]
- 65.Kwiatkowski CF, Fortuin Corsi K, Booth RE. The association between knowledge of hepatitis C virus status and risk behaviors in injection drug users. Addiction. 2002;97:1289–94. doi: 10.1046/j.1360-0443.2002.00208.x. [DOI] [PubMed] [Google Scholar]
- 66.Smith BD, Yartel AK, Krauskopf K, et al. Hepatitis C virus (HCV) antibody positivity and predictors among previously undiagnosed adult primary care outpatients: cross-sectional analysis of a multi-site retrospective cohort study. Clin Infect Dis. 2015;60:1145–52. doi: 10.1093/cid/civ002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arias E. United States life tables, 2003. Natl Vital Stat Rep. 2006;54:1–40. [PubMed] [Google Scholar]
- 68.Chen F. Paper 257-2009: Bayesian modeling using the MCMC procedure. Available at: http://support.sas.com/resources/papers/proceedings09/257-2009.pdf. Accessed 17 April 2014.
- 69.Gold MR, Franks P, McCoy KI, Fryback DG. Toward consistency in cost-utility analyses: using national measures to create condition-specific values. Medical Care. 1998;36:778–92. doi: 10.1097/00005650-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 70.Chong CA, Gulamhussein A, Heathcote EJ, et al. Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterol. 2003;98:630–8. doi: 10.1111/j.1572-0241.2003.07332.x. [DOI] [PubMed] [Google Scholar]
- 71.Siebert U, Sroczynski G. Effectiveness and cost-effectiveness of initial combination therapy with interferon/peginterferon plus ribavirin in patients with chronic hepatitis C in Germany: a health technology assessment commissioned by the German Federal Ministry of Health and Social Security. Int J Technol Assess Health Care. 2005;21:55–65. doi: 10.1017/s0266462305050075. [DOI] [PubMed] [Google Scholar]
- 72.Sherman KE, Sherman SN, Chenier T, Tsevat J. Health values of patients with chronic hepatitis C infection. Arch Intern Med. 2004;164:2377–82. doi: 10.1001/archinte.164.21.2377. [DOI] [PubMed] [Google Scholar]
- 73.Thein HH, Krahn M, Kaldor JM, Dore GJ. Estimation of utilities for chronic hepatitis C from SF-36 scores. Am J Gastroenterol. 2005;100:643–51. doi: 10.1111/j.1572-0241.2005.40976.x. [DOI] [PubMed] [Google Scholar]
- 74.Grieve R, Roberts J, Wright M, et al. Cost effectiveness of interferon alpha or peginterferon alpha with ribavirin for histologically mild chronic hepatitis C. Gut. 2006;55:1332–8. doi: 10.1136/gut.2005.064774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yehia BR, Schranz AJ, Umscheid CA, Lo Re V., III The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PloS one. 2014;9:e101554. doi: 10.1371/journal.pone.0101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med. 2013;368:1859–61. doi: 10.1056/NEJMp1302973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martin NK, Hickman M, Hutchinson SJ, Goldberg DJ, Vickerman P. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin Infecti Dis. 2013;57(suppl 2):S39–45. doi: 10.1093/cid/cit296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


