Abstract
Background
Roux-en-Y gastric bypass (RYGB) accelerates gastric pouch emptying, enhances postprandial glucagon-like peptide 1 (GLP-1) and insulin, and lowers glucose concentrations. To prevent discomfort and reactive hypoglycemia, it is recommended that post-RYGB patients eat small, frequent meals and avoid caloric drinks. However, the effect of meal size and texture on GLP-1 and metabolic response has not been studied.
Objectives
To demonstrate that frequent minimeals and solid meals (S) elicit less GLP-1 and insulin release and less reactive hypoglycemia and are better tolerated compared with a single isocaloric liquid meal (L).
Setting
A university hospital.
Methods
In this prospective study, 32 RYGB candidates were enrolled and randomized to L or S groups before gastric bypass. Each subject received an L or S 600-kcal single meal (SM) administered at hour 0 or three 200-kcal minimeals administered at hours 0, 2, and 4 on 2 separate days. Twenty-one patients were retested 1 year after RYGB. Blood and visual analogue scale measurements were collected up to 6 hours postprandially. Outcome measures included gastric pouch emptying, glucose, insulin, and GLP-1; hunger, fullness, and stomach discomfort were measured by visual analogue scale.
Results
Twenty-one were patients retested after RYGB (L: n = 12; S: n = 9). Meal texture had a significant effect on peak GLP-1 (L-SM: 106.1 ± 67.2 versus S-SM: 45.3 ± 25.2 pM, P = .004), peak insulin, and postprandial glucose. Hypoglycemia was more frequent after the L-SM meal compared with the S-SM. Gastric pouch emptying was 2.4 times faster after RYGB but was not affected by texture.
Conclusions
Meal texture and size have significant impact on tolerance and metabolic response after RYGB.
Keywords: GLP-1, Gastric bypass, Meal texture, Meal size, Gastric emptying
Roux-en-Y gastric bypass surgery (RYGB) results in 30% weight loss at 1 year and resolution or improvement of obesity-related co-morbidities [1]. RYGB accelerates gastric pouch emptying (GPE) and stimulates release of incretins and satiety gut peptides [2,3]. The elevated postprandial release of satiety peptides may contribute to the decreased food intake after RYGB [4]. The enhanced incretin secretion post-RYGB stimulates postprandial insulin secretion and lowers postprandial glucose [5,6]. However, after RYGB, some patients experience reactive hypoglycemia after meals [7–9]. To prevent hypoglycemia, patients are counseled to consume small, frequent meals; avoid simple carbohydrates; and consume solid foods [10]. However, no data currently exist to support this widespread clinical practice.
The goal of this study was to investigate the effect of meal texture and size on GPE, glucagon-like peptide 1 (GLP-1), insulin, glucose, and tolerability in individuals before and 1 year after RYGB. We hypothesized that (1) RYGB would accelerate GPE, elevate GLP-1 and insulin, and decrease postprandial glucose, regardless of meal texture and size; (2) GLP-1 release will vary according to meal texture after RYGB; and (3) the tolerance to large liquid meals will decrease after RYGB.
Methods
Patients
Patients scheduled for RYGB surgery at Mount Sinai St. Luke’s Hospital, aged 20 to 60 years with absence of or diet-controlled type 2 diabetes of <1-year duration and no gastrointestinal disorders, provided written consent before participating. The study was registered at clinicaltrial.gov (NCT02929212) and was approved by the institutional review board.
Study design
This was a prospective study of individuals randomized to either the solid meal texture group (S-group) or the liquid meal texture group (L-group) before and 1 year after RYGB. Patients were tested under 2 conditions, on separate days, with either a single 600-kcal meal (SM) at hour 0 or three 200-kcal isocaloric multiple minimeals (MM) given at hours 0, 2, and 4. The order of SM and MM were randomly assigned (L-SM = liquid single meal, L-MM = liquid multiple meal; S-SM = solid single meal; S-MM = solid multiple meal).
Meal composition
The total calorie amount and the nutrient composition were the same between groups (S or L) and regardless of conditions (SM and MM) (Supplementary Table 1).
Roux-en-Y gastric bypass
RYGB was performed laparoscopically with a 30-mL gastric pouch, 40-cm afferent limb, 150-cm Roux limb, and 12-mm gastrojejunostomy, as described previously [6].
Meal tests
Patients arrived at the Clinical Research Center after an overnight fast and remained sitting at a 45°, with loose-fitting clothing. An intravenous catheter was inserted in 1 forearm and kept warm with a heating pad. After the fasted sample was obtained, patients ingested either a 600-kcal meal over 15 minutes or three 200-kcal meals, each ingested over 5 minutes, every 2 hours. The timing of meal ingestion was controlled for pre- and postsurgery. The flavor of the meals, chocolate or vanilla according to subject preference, was kept consistent during the entire study. Blood was collected in chilled EDTA tubes with aprotinin (500 kallikrein inhibitory units/mL of blood) and dipeptidyl peptidase-4 inhibitor (10 μL/mL of blood; Millipore, St. Charles, MO), at −15, 0, 15, 30, 60, 90, 120, 180, 240, 300, and 360 minutes after meal ingestion during the SM, and at −15, 0, 15, 30, 60, 90, 120, 135, 150, 180, 210, 240, 255, 270, 300, 330, and 360 minutes during the MM. Samples were centrifuged at 4°C and stored at −80°C.
Gastric pouch emptying
GPE was assessed by the acetaminophen method during the single meal. Acetaminophen (1.5 g) was given at the beginning of the meal with a sip of water. Postprandial plasma acetaminophen concentrations were measured and used to estimate gastric emptying rate [11]. Peak and time-to-peak acetaminophen were used as surrogate for GPE.
Visual analogue scale
Assessment of hunger, fullness, stomach discomfort, stomach cramps, intestinal cramps, bloating, feeling of vomiting/nausea, and upset stomach were measured on a 150-mm visual analogue scale with the words “extremely” and “not at all” anchored at each end [12,13]. Visual analogue scale data were collected during each meal at the same times of blood sampling.
Biochemical assays
Glucose concentrations were measured by the glucose oxidase method (Analox Instruments, Lunenburg, MA). Insulin and total GLP-1 were measured by radioimmuno-assay (Millipore). Acetaminophen levels were measured using the ELISA assay (Abbot Laboratories, Chicago, IL), at the Hormone and Metabolite Core Laboratory of the Diabetes Research Center.
Calculations
The homeostasis model assessment of insulin resistance was calculated as: (fasting-insulin μU/mL × fasting-glucose mg/dL)/405. Total area under the curve was calculated using the trapezoidal method. Peak glucose, insulin, and GLP-1, defined as the highest concentrations at any time point after meal ingestion, were measured using data from 0 to 360 minutes for SM and MM. The nadir was defined as the lowest concentration after the meal ingestion, regardless of the time at which it occurred. The delta peak-nadir was used to determine the postprandial drop of glucose levels. Postprandial hypoglycemia was arbitrarily defined as glucose level <70 mg/dL at any set time for blood draw, regardless of symptoms [14]. The total number of documented post-prandial episodes of glucose concentrations ≤55mg/dL was also recorded.
Statistical analyses
Analyses were performed with the Statistical Package for the Social Sciences (SPSS), version 20.0, with level of significance at P < .05. Data were examined for normality using the Shapiro-Wilk test, variables log transformed if necessary, and nonparametric tests used if needed. Independent t tests were used for analyses between groups and paired t tests for within group changes. General Linear Model (GLM) with repeated measure was used to compare difference of the change of glucose or hormones during the meal between conditions and between groups. Pearson’s correlation coefficient, or Spearman’s correlation for data not normally distributed, were used. Sample size was calculated to achieve at least 80% statistical power to test the expected effect size, assuming an α error of .05, assuming a difference in the GLP-1 response between meals of different sizes of at least 40% [15].
Results
Subject characteristics
Of the 32 patients enrolled, 21 patients were retested 1 year after RYGB, 9 in the S-group and 12 in the L-group (1 male in each group). One subject in the L-group completed only the SM. Participant’s age (S: 34.8 ± 9.2, L: 35.3 ± 10.5 yr, P = .901), weight, body mass index, fasted glucose, insulin, and GLP-1, HbA1c (S: 5.72 ± 0.51%, L: 5.65 ± 0.30%, P = .691) at presurgery, and percentage of body mass index loss or percentage of total weight loss (S: 36.04 ± 9.09%, L: 37.85 ± 6.97%, P = .610) were not significantly different between groups (Table 1). There was no order effect of SM and MM conditions on any outcome variables.
Table 1.
Metabolic changes with the single meal (SM) of solid (S) and liquid (L) texture pre- and 1-year post RYGB
| Solid group S-SM (n = 9) | Liquid group L-SM (n = 12) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Pre | Post | Δ | Pre | Post | Δ | |
| BMI, kg·m−2 | 48.02 ± 9.28 | 30.47 ± 5.72* | −17.55 ± 6.17 | 47.40 ± 6.62 | 29.60 ± 6.14* | −17.80 ± 3.47 |
| Weight, kg | 143.0 ± 35.62 | 90.82 ± 23.34 | −52.18 ± 19.02 | 128.9 ± 22.58 | 80.37 ± 18.17* | −48.53 ± 11.53 |
| Fasted glucose, mg·dL−1 | 97.5 ± 14.69 | 88.93 ± 5.50 | −8.57 ± 12.62 | 98.25 ± 13.12 | 86.23 ± 9.49* | −12.02 ± 10.55 |
| Glucose 120′, mg·dL−1 | 111.9 ± 18.68 | 81.92 ± 17.86* | −30.0 ± 18.1 | 119.6 ± 20.12 | 71.39 ± 16.77* | −48.19 ± 19.92‡ |
| Peak glucose, mg·dL−1 | 125.3 ± 21.78 | 173.2 ± 29.54* | 47.86 ± 37.73 | 132.8 ± 17.96 | 171.5 ± 37.28* | 38.71 ± 37.57 |
| Glucose AUC, mg·dL−1·min−1 | 98.26 ± 14.91 | 96.61 ± 7.12 | −1.64 ± 14.26 | 102.4 ± 12.67 | 92.96 ± 9.72* | −9.40 ± 12.61 |
| Glucose AUC 0–90, mg·dL−1·min−1 | 112.7 ± 18.46 | 131.7 ± 24.32 | 18.97 ± 28.20 | 115.0 ± 21.98 | 129.4 ± 23.26 | 14.39 ± 27.70 |
| Fasted insulin, μU·mL−1 | 24.28 ± 10.09 | 6.07 ± 3.15* | −18.21 ± 11.18 | 22.44 ± 6.52 | 5.72 ± 3.20* | −16.71 ± 6.23 |
| Peak insulin, μU·mL−1 | 168.2 ± 75.32 | 286.3 ± 176.1 | 118.1 ± 175.3 | 175.8 ± 107.0 | 309.1 ± 202.9* | 133.3 ± 180.4 |
| Insulin AUC, μU·mL−1·min−1 | 60.65 ± 20.64 | 49.06 ± 26.55 | −11.59 ± 33.23 | 58.36 ± 20.58 | 55.73 ± 41.98 | −2.63 ± 38.52 |
| Insulin iAUC, μU·mL−1·min−1 | 36.37 ± 13.90 | 43.46 ± 23.97 | −7.09 ± 27.73 | 35.93 ± 15.75 | 50.0 ± 40.74 | 14.08 ± 38.46 |
| Insulin AUC 0–90, μU·mL−1·min−1 | 105.4 ± 43.43 | 161.0 ± 89.90 | 55.61 ± 93.30 | 108.9 ± 50.50 | 179.1 ± 134.5* | 70.14 ± 108.3 |
| HOMA-IR | 5.79 ± 2.48 | 1.36 ± 0.74* | −4.43 ± 2.70 | 5.48 ± 1.82 | 1.26 ± 0.73* | −4.22 ± 1.69 |
| GLP-1 peak, pmol·L−1 | 23.69 ± 19.00 | 45.29 ± 25.23* | 21.60 ± 30.10 | 19.87 ± 9.26 | 106.1 ± 67.22*,† | 86.20 ± 71.18‡ |
| GLP-1 AUC, pmol·L−1·min−1 | 14.79 ± 13.78 | 17.26 ± 8.55 | 2.47 ± 9.25 | 10.76 ± 4.07 | 29.96 ± 12.47*,† | 19.2 ± 12.85‡ |
| GLP-1 AUC 0–90, pmol·L−1·min−1 | 16.0 ± 13.83 | 30.54 ± 14.55* | 14.54 ± 16.72 | 13.97 ± 5.53 | 64.98 ± 32.64*,† | 51.01 ± 35.34‡ |
| Acetaminophen time-to-peak, min | 135.0 ± 77.94 | 57.86 ± 59.64 | −77.14 ± 105.9 | 180.0 ± 88.99 | 69.55 ± 66.12* | −110.5 ± 115.7 |
| Peak acetaminophen, mg·dL | 1.40 ± 0.63 | 2.23 ± 0.90 | 0.83 ± 1.31 | 1.27 ± 0.44 | 2.24 ± 0.71 | 0.97 ± 0.70 |
| Nadir glucose, mg·dL−1 | 81.46 ± 12.49 | 71.31 ± 9.32 | −10.16 ± 16.68 | 81.67 ± 12.93 | 64.46 ± 14.58* | −17.21 ± 17.17 |
| Time-to-nadir glucose, min | 320.0 ± 67.08 | 170.0 ± 94.87* | −150.0 ± 90.0 | 238.8 ± 116.62 | 145.0 ± 76.57* | −93.75 ± 146.7 |
| Peak-nadir glucose, mg·dL−1 | 43.85 ± 15.65 | 101.9 ± 33.32* | 58.02 ± 36.92 | 51.15 ± 15.65 | 107.1 ± 33.38* | 55.92 ± 37.66 |
RYGB = Roux-en-Y gastric bypass; BMI = body mass index; AUC = area under the curve; iAUC = incremental area under the curve; HOMA-IR = homeostasis model assessment of insulin resistance; GLP-1 = glucagon-like peptide 1.
Mean ± standard deviation. Delta (Δ) changes with intervention.
P < .05 pre- versus post-intervention within each group.
P < .05 difference between S versus L-group post-RYGB.
Difference between Δ S-SM versus Δ L-SM.
Effects of meal texture
Prior RYGB
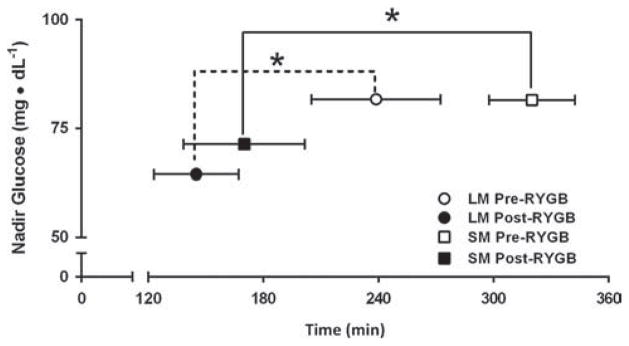
Before surgery, meal texture had no effect on gastric emptying, GLP-1, insulin, or glucose, in either condition (Table 1 and 2). There was little stomach discomfort during all of the meals before surgery (data not shown). There was no significant effect of texture on the time to reach nadir glucose between L-SM and S-SM (Table 1, Fig. 1).
Table 2.
Metabolic changes with the multiple mini meals (MM) of solid (S) and liquid (L) texture pre and 1-year post-RYGB
| S-MM (n = 9) | L-MM (n = 11) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Pre | Post | Δ | Pre | Post | Δ | |
| Fasted glucose, mg·dL−1 | 104.1 ± 13.68 | 88.28 ± 11.96* | −15.82 ± 12.46 | 103.8 ± 16.46 | 86.82 ± 8.18* | −16.97 ± 13.88 |
| Glucose 120′, mg·dL−1 | 99.49 ± 14.93 | 86.41 ± 10.20* | −13.08 ± 13.90 | 102.2 ± 11.47 | 83.15 ± 8.44* | −19.06 ± 12.40 |
| Peak glucose, mg·dL−1 | 120.2 ± 15.75 | 129.6 ± 22.56 | 9.35 ± 21.37 | 126.6 ± 15.95 | 140.6 ± 20.83 | 14.01 ± 25.69 |
| Glucose AUC, mg·dL−1·min−1 | 103.6 ± 13.93 | 92.93 ± 13.05 | −10.71 ± 14.03 | 106.1 ± 8.85 | 97.92 ± 10.41 | −8.13 ± 13.60 |
| Fasted insulin, μU·mL−1 | 26.09 ± 10.29 | 6.84 ± 4.25* | −19.25 ± 10.37 | 33.15 ± 38.91 | 6.84 ± 2.73* | −26.31 ± 38.99 |
| Peak insulin, μU·mL−1 | 109.5 ± 38.93 | 105.6 ± 34.64 | −3.90 ± 52.84 | 113.8 ± 70.34 | 98.71 ± 49.62 | −15.11 ± 70.73 |
| Insulin AUC, μU·mL−1·min−1 | 53.55 ± 17.54 | 28.90 ± 12.56* | −24.65 ± 19.72 | 54.78 ± 27.98 | 28.57 ± 11.26* | −26.22 ± 26.11 |
| Insulin iAUC, μU·mL−1·min−1 | 27.46 ± 10.65 | 22.06 ± 9.05 | −5.40 ± 12.74 | 21.63 ± 20.01 | 21.73 ± 10.27 | .10 ± 20.63 |
| GLP-1 peak, pmol·L−1 | 20.98 ± 23.23 | 43.84 ± 31.66* | 22.87 ± 11.57 | 16.12 ± 6.00 | 61.62 ± 38.91* | 45.50 ± 40.02 |
| GLP-1 AUC, pmol·L−1 ·min−1 | 11.59 ± 10.27 | 20.03 ± 14.68* | 8.43 ± 4.87 | 9.87 ± 3.56 | 24.80 ± 12.26* | 14.93 ± 11.87 |
| Nadir glucose, mg·dL−1 | 88.87 ± 14.27 | 75.43 ± 9.20* | −13.44 ± 16.65 | 87.39 ± 7.68 | 75.29 ± 9.15* | −12.10 ± 11.94 |
| Time to nadir glucose, min | 253.3 ± 67.27 | 150.0 ± 110.2* | −103.3 ± 93.81 | 249.6 ± 93.98 | 174.6 ± 108.02* | −75.00 ± 73.18 |
| Peak-nadir glucose, mg·dL−1 | 31.34 ± 4.30 | 54.14 ± 18.15* | 22.79 ± 15.54 | 39.89 ± 16.37 | 65.80 ± 15.35* | 25.91 ± 24.24 |
RYGB = Roux-en-Y gastric bypass; AUC = area under the curve; iAUC = incremental area under the curve; GLP-1 = glucagon-like peptide 1.
Mean ± standard deviation. Δ change with intervention.
P < .05 pre- versus post-intervention.
Fig. 1.
Effect of meal texture on level and time-to-reach nadir glucose during the single 600-kcal solid meal (SM, square) and liquid meal (LM, circle) before (open symbols) and after (closed symbols) Roux-en-Y gastric bypass surgery. Mean ± standard error of the mean (SEM); Statistical significance for time-to-nadir are reported as *P < .05 versus presurgery within each group (n = 12 for LM, n = 9 for SM).
After RYGB: effect of meal texture during the 600-kcal meal.
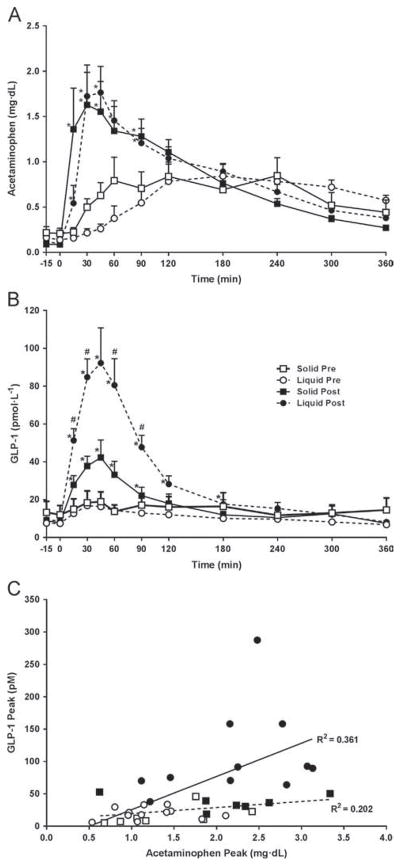
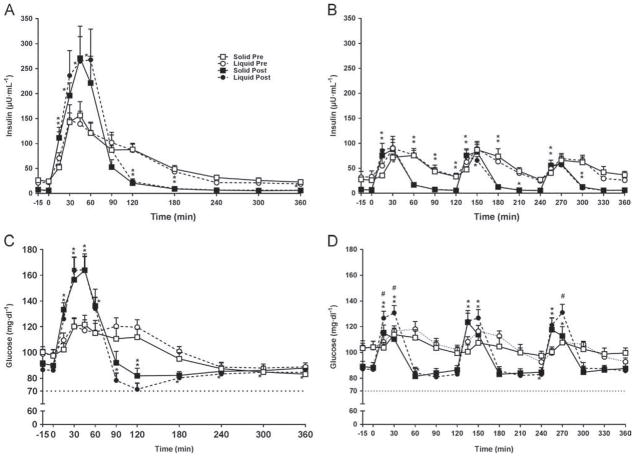
Fasting glucose, fasting insulin, and homeostasis model assessment of insulin resistance decreased after RYGB. The GPE time was 2.5 times faster after RYGB, but this was significant only after L-SM (Table 1; Fig. 2A). GLP-1 increased after RYGB in both groups but the magnitude of enhanced GLP-1 concentration was greater after the liquid (5× versus pre-RYGB) than after the solid meal (2× versus pre-RYGB) (P = .002; Table 1; Fig. 2B). Overall, peak GLP-1 correlated with GPE (peak acetaminophen) (r = .389, P = .14) only after the L-SM (r = .601, P < .002). No significant relationship was observed after the S-SM (r = .39, P = .14) (Fig. 2C). Peak insulin and insulin area under the curve0–90 increased by 1.7- and approximately 1.5-fold, respectively, after RYGB regardless of texture, but reached significance only after the L-SM (Table 1, Fig. 3A). After RYGB the glucose curves were shifted to the left and glucose peaked earlier; postprandial glucose (both 120′ and nadir) decreased in both groups, more after the liquid than the solid meal (Table 1, Fig. 3B). The drop in glucose (peak-nadir) doubled after RYGB, without a texture effect (Table 1). The time to reach nadir glucose was shorter after RYGB, regardless of meal texture, but was not different between L-SM and S-SM (Table 1, Fig. 1).
Fig. 2.
Change of acetaminophen (A) and glucagon-like peptide 1 (GLP-1) (B) levels during the single 600-kcal solid (square) and liquid (circle) meals before (open symbols) and after (closed symbols) gastric bypass surgery. Meal was given at 0 minutes. Data are mean ± SEM; with repeated measure (A and B) *P < .05 post- versus presurgery within each group; #P < .05 liquid versus solid meal texture postsurgery. Correlation between the GLP-1 peak and acetaminophen in solid group (dashed line) and liquid group (solid line), during the single 600-kcal meal (C) (n = 12 for L, n = 9 for S).
Fig. 3.
Insulin and glucose circulating concentrations during the single 600-kcal (A and B) (n = 12 for L-SM, n = 9 for S-SM) and multiple 200-kcal (C and D) (n = 11 for L-MM, n = 9 for S-MM) solid (square) and liquid (circle) meals pre- (open symbols) and post- (closed symbols) Roux-en-Y gastric bypass surgery. Data are mean ± standard error of the mean (SEM); with repeated measure *P < .05 post- versus presurgery within each group; #P < .05 liquid versus solid meal texture postsurgery.
After surgery, more patients had documented hypoglycemia after the L-SM (n = 7) than after the S-SM (n = 1) for glucose <70 mg/dL while only 3 patients had glucose <55 mg/dL (range from 35–55 mg/dL, n = 2 in L-SM and 1 in S-SM), although none had severe symptoms. In addition, the total number of recorded episodes (some patients had >1) of glucose <70 mg/dL increased after the L-SM from 5 pre-RYGB to 14 after RYGB. Few episodes of postprandial glucose <55 mg/dL were recorded after surgery (2 after the L-SM and 1 after the S-SM).
The insulin surge (fasting to peak) correlated with 120′ glucose (r2 = .168 P < .001) and nadir glucose (r2 = .145, P < .001) and very strongly with the drop in glucose (peak-to-nadir glucose) (r2 = 0.394, P < .001) (Supplemental Fig. 1). These correlations were significant either when calculated with the entire data set (i.e., both groups), meal conditions, and time points, or when considering only the solid and liquid single meals postsurgery.
Effect of RYGB and meal texture during the 3, 200-kcal meals
Although there were expected changes in glucose, insulin, and GLP-1 after RYGB in response to the three 200-kcal meals, there was no significant effect of texture on glucose, insulin, or GLP-1 (Table 2). Only 3 patients had documented hypoglycemia (>55 and <70 mg/dL) during the L-MM and 2 during the S-MM after RYGB, none symptomatic.
Effects of meal size before and after RYGB
Pre-RYGB
Meal size had the expected effect on postprandial insulin, glucose, and fullness levels—that is, larger meal elicited greater insulin, glucose, and fullness than smaller meals with no effect of texture (data not shown).
After RYGB
Patients took a longer time to ingest the 600-kcal meal than the 3 isocaloric smaller meals after the surgery, regardless of texture (Table 3).
Table 3.
Effect of single 600 kcal (SM) versus multiple 200 kcal (MM) meals in solid and liquid group 1 year after RYGB
| Solid | Liquid | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| SM (n = 9) | MM (n = 9) | P | SM (n = 12) | MM (n = 11) | P | |
| Meal Duration, min | 17.83 ± 8.03 | 12.78 ± 5.67 | .012 | 16.93 ± 3.99 | 10.9 ± 3.5 | .007 |
| Glucose 120′, mg·dL−1 | 81.92 ± 17.86 | 86.41 ± 10.20 | NS | 71.39 ± 16.77 | 83.15 ± 8.44 | NS |
| Peak glucose, mg·dL−1 | 173.2 ± 29.54 | 129.6 ± 22.56 | .000 | 171.5 ± 37.28 | 140.6 ± 20.83 | .001 |
| Glucose AUC, mg·dL−1·min−1 | 96.61 ± 7.12 | 92.93 ± 13.05 | NS | 92.96 ± 9.72 | 97.92 ± 10.41 | NS |
| Peak insulin, μU·mL−1 | 286.3 ± 176.1 | 105.6 ± 34.64 | .010 | 309.1 ± 202.9 | 98.71 ± 49.62 | .002 |
| Insulin AUC, μU·mL−1·min−1 | 49.06 ± 26.55 | 28.90 ± 12.56 | .016 | 55.73 ± 41.98 | 28.57 ± 11.26 | .027 |
| Insulin iAUC, μU·mL−1·min−1 | 43.46 ± 23.97 | 22.06 ± 9.05 | .012 | 50.0 ± 40.74 | 21.73 ± 10.27 | .021 |
| HOMA-IR | 1.36 ± 0.74 | 1.57 ± 1.07 | NS | 1.26 ± 0.73 | 1.49 ± 0.66 | NS |
| GLP-1 peak, pmol·L−1 | 45.29 ± 25.23 | 43.84 ± 31.66 | NS | 106.1 ± 67.22 | 61.62 ± 38.91 | .042 |
| GLP-1 AUC, pmol·L−1·min−1 | 17.26 ± 8.55 | 20.03 ± 14.68 | NS | 29.96 ± 12.47 | 24.80 ± 12.26 | NS |
| Nadir glucose, mg·dL−1 | 71.31 ± 9.32 | 75.43 ± 9.20 | NS | 64.46 ± 14.58 | 74.79 ± 9.25 | NS |
| Time to reach nadir glucose, min | 170.0 ± 94.87 | 150.0 ± 110.2 | NS | 145.0 ± 76.57 | 174.6 ± 108.0 | NS |
RYGB = Roux-en-Y gastric bypass; NS = not significant; AUC = area under the curve; iAUC = incremental area under the curve; GLP-1 = glucagon-like peptide 1; HOMA-IR = homeostasis model assessment of insulin resistance.
Mean ± standard deviation.
The magnitude of peak GLP-1 was 3 times greater after the L-SM than for any of the L-MM; interestingly the S-SM did not trigger a larger GLP-1 peak release than the S-MM (Table 3). As expected, glucose and insulin release were proportional to meal caloric load, regardless of texture. Interestingly, although insulin release was significantly greater during the SM versus MM, regardless of texture, nadir and 120′ glucose were not significantly different between the SM and the MM in both texture groups (Table 3). Meal size affected meal duration but not the time to reach nadir glucose (Table 3).
Effect of RYGB on meal tolerance
Visual analogue scale data were highly variable. In spite of large weight loss after RYGB, fasted hunger scores were not different than presurgery. In general, solid food was more efficient at suppressing hunger than liquid food, as were large meals (SM) compared with smaller meals (MM) post-RYGB; however, this did not reach significance. Fullness tended to be greater and peaked 4.3 times faster after RYGB during the L-SM (Supplemental Table 2). Discomfort was significantly greater, occurred sooner after RYGB during meals, and was more pronounced with larger liquid meals (data not shown).
Discussion
The main finding of this study is that meal texture and meal size have a significant impact on meal tolerance and postprandial GLP-1, insulin, and glucose levels after RYGB. After RYGB, smaller meals and meals of solid texture are better tolerated and generate less hypoglycemia than large meals and/or liquid meals. These data bring evidence to support current dietary recommendation for patients after RYGB.
The robust effect of RYGB to enhance GLP-1 concentration after a mixed meal or oral glucose load has been described extensively [2,5,16,17]. Postprandial release of GLP-1 varies based on nutrient types [18], fiber content, and caloric load [15,19] in nonoperated individuals. Meal size remains an important determinant of GLP-1 concentration after RYGB, and the 600-kcal meal released more GLP-1 than one 200-kcal meal both before and after RYGB. The alignment of GLP-1 response to the caloric load persists after bypass. The total GLP-1 output (total area under the curve) was identical for the 600-kcal meal versus the three 200-kcal meal within each time point (pre- or postsurgery) or condition (L or S).
Interestingly, in addition to responding to caloric content of the meal, GLP-1 concentration varies with meal texture. After RYGB, the liquid meal (600 or 200 kcal) released more GLP-1 than the isocaloric solid meal of similar nutrient composition. We hypothesized that the difference in GLP-1 concentration between isocaloric meals of different textures, apparent only after RYGB, could be accounted for by variable GPE time. Many factors contribute to the drastically altered emptying of nutrients from the gastric pouch after RYGB, including the reduced gastric pouch volume, removal of the pylorus, and absent gastric relaxation phase, liquids empty faster than solids [20], and meals of larger size, or volume, also tend to empty faster than smaller meals [21]. Our data show that GLP-1 concentration correlates with GPE only after the liquid meal, in agreement with previous data [2,22–25]. However, the appearance and the peak of acetaminophen, surrogates of GPE, did not significantly differ between the liquid and solid meal. The absence of difference in GPE between meals of different texture is not easily explained. This could be due to methodologic issues, such as absence of very early blood draws (first 5–10 min) after the meal ingestion, and/or possible difference in acetaminophen absorption and pharmacokinetics after RYGB. However, it is unlikely that a small difference in pouch emptying between textures, not picked up by the acetaminophen method, could explain such large difference in GLP-1 concentrations observed between meals of different texture. Therefore, we must hypothesize that factors other than GPE, such as osmolarity or nutrient forms [26–28], may have contributed to the greater release of GLP-1 after the liquid meal. The GLP-1 response dependency on the meal texture after RYGB may result from a more rapid and larger area of the gut exposed to the liquid meal, thereby increasing the number of L-cells being exposed [29].
RYGB increased the peak insulin concentration, but only in response to the larger meal. Meal texture had no effect on insulin release either pre- or post-RYGB. Interestingly, after RYGB, in spite of a significantly larger GLP-1 concentration in response to the L-SM, compared with the isocaloric S-SM, insulin concentration did not differ between textures. Therefore, while GLP-1 concentration post-RYGB depends on GPE, caloric load, and meal texture, the enhanced insulin response after RYGB seems to vary only with caloric load, independent of meal texture and GPE. Insulin concentration at 120′ was lower after RYGB, likely as a result of improved insulin clearance previously described after RYGB, but not measured in this study [30]. The magnitude of the insulin surge, increased 7-fold from fasted to peak levels presurgery, and increased approximately 46-fold after RYGB after both the S-SM and the L-SM. The meal-induced insulin response may have reached a ceiling during the 600-kcal meals and could not be further increased, in spite of greater GLP-1 release during the L-SM compared with the S-SM. This does not preclude a strong incretin effect of GLP-1 on insulin release, as previously demonstrated [31,32].
The incidence of reactive hypoglycemia after RYGB is reported to be 9.1% 1 year after surgery and between 6.6% and 7.9% 5 years after RYGB [33,34]. However, some studies show reported symptoms without documented hypoglycemia [33], and others report documented low glucose values during an oral glucose tolerance test, but without concomitant symptoms [34]; therefore, the true prevalence of symptomatic hypoglycemia is difficult to establish. In addition, there is overlap between symptoms of dumping and of hypoglycemia [33,34]. As hypothesized, large meals and liquid meals resulted in more frequent reactive hypoglycemia than smaller and/or meals of solid texture. However, the overall number of documented hypoglycemic episode (<70 mg/dL) was low, only 3 patients had documented glucose levels ≤55 mg/dL, and none of the participants developed neurologic symptoms.
Because a drop in glucose levels can trigger adrenergic symptoms, even in the absence of true hypoglycemia, we also calculated the delta change from peak-nadir glucose. The drop in glucose, from peak to nadir, strongly correlated with the insulin surge. It was greater after RYGB and after large meals compared with small meals, but it was not affected by meal texture. The nadir glucose was lower and glucose levels were more likely to be in the hypoglycemic range after the L-SM than after S-SM. Therefore, texture had an effect on GLP-1 and on glucose in spite of its absence of effect on insulin. It is possible that the suppression of glucagon release by GLP-1 [35] proportional to the magnitude of the GLP-1 release, and/or a defect in other counter regulatory hormones [36], favored lower blood glucose levels after the liquid meal. However, these counter-regulatory hormones were not measured in this study.
In spite of approximately 36% total weight loss, participants did not report being hungrier after RYGB, as reported previously [2]. Peak fullness was higher and the time to reach peak fullness significantly shorter after surgery. These data attest to the powerful effect of the surgery on hunger drive and satiety, possibly contributing to lesser food intake and weight loss. Overall, the large and liquid meals were poorly tolerated, and all participants experienced some degree of dumping after RYGB (SM > MM; L > S).
This study had several strengths. This is a longitudinal design, with patients studied before and 1 year after surgery, in a randomized assignment to 2 different meal textures, tested with 2 different meal patterns; moreover, the results are directly applicable to real-world scenarios. However, the study had some limitations, including a high attrition rate, lack of measure of counter-regulatory hormones, indirect measurement of GPE, and lack of markers of intestinal transit time. In addition, a study design with each subject undergoing the 4 conditions would have been more optimal, although difficult to implement due to the extensive time commitment and the poor tolerance of the test meals after surgery.
Conclusion
The physiology of GLP-1 after RYGB is not fully elucidated. Our data show a partial dissociation among calorie intake, GLP-1 and insulin concentration, and postprandial glucose during large meals after RYGB. Furthermore, our data also suggest dissociation between meal texture, GPE rate, and GLP-1 concentration. Nutritional advice to patients after RYGB should take into account meal size and meal texture to improve meal tolerance and prevent reactive hypoglycemia.
Acknowledgments
The study was supported by grants from the NIH (R01 DK067561, P30 DK26687-30, P30 DK063608) and by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1 TR000040; R.D. was supported by NIH 7 T32 DK007559-25.
The authors thank Toni Colarusso, who helped design the meal tests; Fatima Rimawi, Daniel Boron-Brenner, and Carolina Espinosa, who helped with recruitment and some data collection; the Mount Sinai St Luke’s bariatric surgical team; and Ping Zhou, who ran the hormonal assays.
Appendix. Supplementary data
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.soard.2017.09.004.
Footnotes
Part of the data were presented as a poster at the 2014 Obesity meeting.
Disclosures
The authors have no commercial associations that might be a conflict of interest in relation to this article.
References
- 1.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 2.Morinigo R, Moize V, Musri M, et al. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2006;91(5):1735–40. doi: 10.1210/jc.2005-0904. [DOI] [PubMed] [Google Scholar]
- 3.Vidal J, Nicolau J, Romero F, et al. Long-term effects of Roux-en-Y gastric bypass surgery on plasma glucagon-like peptide-1 and islet function in morbidly obese subjects. J Clin Endocrinol Metab. 2009;94(3):884–91. doi: 10.1210/jc.2008-1620. [DOI] [PubMed] [Google Scholar]
- 4.Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG, Aylwin SJ. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg. 2006;93(2):210–5. doi: 10.1002/bjs.5227. [DOI] [PubMed] [Google Scholar]
- 5.Laferrère B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30(7):1709–16. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laferrère B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93(7):2479–85. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bantle JP, Ikramuddin S, Kellogg TA, Buchwald H. Hyperinsulinemic hypoglycemia developing late after gastric bypass. Obes Surg. 2007;17(5):592–4. doi: 10.1007/s11695-007-9102-6. [DOI] [PubMed] [Google Scholar]
- 8.Marsk R, Jonas E, Rasmussen F, Naslund E. Nationwide cohort study of post-gastric bypass hypoglycaemia including 5,040 patients undergoing surgery for obesity in 1986–2006 in Sweden. Diabetologia. 2010;53(11):2307–11. doi: 10.1007/s00125-010-1798-5. [DOI] [PubMed] [Google Scholar]
- 9.Sarwar H, Chapman WH, III, Pender JR, et al. Hypoglycemia after Roux-en-Y gastric bypass: the BOLD experience. Obes Surg. 2014;24(7):1120–4. doi: 10.1007/s11695-014-1260-8. [DOI] [PubMed] [Google Scholar]
- 10.Allied Health Sciences Section Ad Hoc Nutrition Committee. Aills L, Blankenship J, Buffington C, Furtado M, Parrott J. ASMBS Allied Health Nutritional Guidelines for the Surgical Weight Loss Patient. Surg Obes Relat Dis. 2008;4(5 Suppl):S73–108. doi: 10.1016/j.soard.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Willems M, Quartero AO, Numans ME. How useful is paracetamol absorption as a marker of gastric emptying? A systematic literature study. Dig Dis Sci. 2001;46(10):2256–62. doi: 10.1023/a:1011935603893. [DOI] [PubMed] [Google Scholar]
- 12.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24(1):38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 13.Stubbs RJ, Hughes DA, Johnstone AM, et al. The use of visual analogue scales to assess motivation to eat in human subjects: a review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br J Nutr. 2000;84(4):405–15. doi: 10.1017/s0007114500001719. [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association. Standards of medical care in diabetes–2014. Diabetes Care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 15.Alsalim W, Omar B, Pacini G, Bizzotto R, Mari A, Ahren B. Incretin and islet hormone responses to meals of increasing size in healthy subjects. J Clin Endocrinol Metab. 2015;100(2):561–8. doi: 10.1210/jc.2014-2865. [DOI] [PubMed] [Google Scholar]
- 16.Falken Y, Hellstrom PM, Holst JJ, Naslund E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab. 2011;96(7):2227–35. doi: 10.1210/jc.2010-2876. [DOI] [PubMed] [Google Scholar]
- 17.Laferrère B. Effect of gastric bypass surgery on the incretins. Diabetes Metab. 2009;35(6 Pt 2):513–7. doi: 10.1016/S1262-3636(09)73458-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah M, Franklin B, Adams-Huet B, et al. Effect of meal composition on postprandial glucagon-like peptide-1, insulin, glucagon, C-peptide, and glucose responses in overweight/obese subjects. Eur J Nutr. 2017;56(3):1053–62. doi: 10.1007/s00394-016-1154-8. [DOI] [PubMed] [Google Scholar]
- 19.Vilsboll T, Krarup T, Sonne J, et al. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2003;88(6):2706–13. doi: 10.1210/jc.2002-021873. [DOI] [PubMed] [Google Scholar]
- 20.Horowitz M, Collins PJ, Harding PE, Shearman DJ. Gastric emptying after gastric bypass. Int J Obes. 1986;10(2):117–21. [PubMed] [Google Scholar]
- 21.Nguyen NQ, Debreceni TL, Burgstad CM, et al. Effects of posture and meal volume on gastric emptying, intestinal transit, oral glucose tolerance, blood pressure and gastrointestinal symptoms after Roux-en-Y gastric bypass. Obes Surg. 2015;25(8):1392–400. doi: 10.1007/s11695-014-1531-4. [DOI] [PubMed] [Google Scholar]
- 22.Wang G, Agenor K, Pizot J, et al. Accelerated gastric emptying but no carbohydrate malabsorption 1 year after gastric bypass surgery (GBP) Obes Surg. 2012;22(8):1263–7. doi: 10.1007/s11695-012-0656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLaughlin T, Peck M, Holst J, Deacon C. Reversible hyperinsulinemic hypoglycemia after gastric bypass: a consequence of altered nutrient delivery. J Clin Endocrinol Metab. 2010;95(4):1851–5. doi: 10.1210/jc.2009-1628. [DOI] [PubMed] [Google Scholar]
- 24.Horowitz M, Cook DJ, Collins PJ, et al. Measurement of gastric emptying after gastric bypass surgery using radionuclides. Br J Surg. 1982;69(11):655–7. doi: 10.1002/bjs.1800691108. [DOI] [PubMed] [Google Scholar]
- 25.Naslund I, Beckman KW. Gastric emptying rate after gastric bypass and gastroplasty. Scand J Gastroenterol. 1987;22(2):193–201. doi: 10.3109/00365528708991879. [DOI] [PubMed] [Google Scholar]
- 26.Petersen B, Christiansen J, Holst JJ. A glucose-dependent mechanism in jejunum inhibits gastric acid secretion: a response mediated through enteroglucagon? Scand J Gastroenterol. 2009;20(2):193–7. doi: 10.3109/00365528509089656. [DOI] [PubMed] [Google Scholar]
- 27.Brown RJ, Walter M, Rother KI. Ingestion of diet soda before a glucose load augments glucagon-like peptide-1 secretion. Diabetes Care. 2009;32(12):2184–6. doi: 10.2337/dc09-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Wielen N, Ten Klooster JP, Muckenschnabl S, et al. The noncaloric sweetener rebaudioside a stimulates glucagon-like peptide 1 release and increases enteroendocrine cell numbers in 2-dimensional mouse organoids derived from different locations of the intestine. J Nutr. 2016;146(12):2429–35. doi: 10.3945/jn.116.232678. [DOI] [PubMed] [Google Scholar]
- 29.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–39. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 30.Bojsen-Moller KN, Dirksen C, Jorgensen NB, et al. Increased hepatic insulin clearance after Roux-en-Y gastric bypass. J Clin Endocrinol Metab. 2013;98(6):E1066–71. doi: 10.1210/jc.2013-1286. [DOI] [PubMed] [Google Scholar]
- 31.Jorgensen NB, Dirksen C, Bojsen-Moller KN, et al. Exaggerated glucagon-like peptide 1 response is important for improved beta-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes. 2013;62(9):3044–52. doi: 10.2337/db13-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salehi M, Prigeon RL, D’Alessio DA. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes. 2011;60(9):2308–14. doi: 10.2337/db11-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen JB, Pedersen AM, Gribsholt SB, Svensson E, Richelsen B. Prevalence, severity, and predictors of symptoms of dumping and hypoglycemia after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2016;12(8):1562–8. doi: 10.1016/j.soard.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 34.Raverdy V, Baud G, Pigeyre M, et al. Incidence and predictive factors of postprandial hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass: a five year longitudinal study. Ann Surg. 2016;264(5):878–85. doi: 10.1097/SLA.0000000000001915. [DOI] [PubMed] [Google Scholar]
- 35.Hare KJ. Role of GLP-1 induced glucagon suppression in type 2 diabetes mellitus. Dan Med Bull. 2010;57(9):B4181. [PubMed] [Google Scholar]
- 36.Abrahamsson N, Borjesson JL, Sundbom M, Wiklund U, Karlsson FA, Eriksson JW. Gastric bypass reduces symptoms and hormonal responses in hypoglycemia. Diabetes. 2016;65(9):2667–75. doi: 10.2337/db16-0341. [DOI] [PubMed] [Google Scholar]