Abstract
Heterogeneity in glucocorticoid response to experimental stress conditions has shown to differentiate individuals with healthy from maladaptive real-life stress responses in a number of distinct domains. However, it is not known if this heterogeneity influences the risk for developing stress related disorders or if it is a biological consequence of the stress response itself. Determining if glucocorticoid response to stress induction prospectively predicts psychological vulnerability to significant real life stressors can adjudicate this issue. To test this relationship, salivary cortisol as well as catecholamine responses to a laboratory stressor during academy training were examined as predictors of empirically identified distress trajectories through the subsequent 4 years of active duty among urban police officers routinely exposed to potentially traumatic events and routine life stressors (N = 234). During training, officers were exposed to a video vignette of police officers exposed to real-life trauma. Changes in salivary 3-methoxy-4-hydroxyphenylglycol (MHPG) and cortisol in response to this video challenge were examined as predictors of trajectory membership while controlling for age, gender, and baseline neuroendocrine levels. Officers who followed trajectories of resilience and recovery over 4 years mounted significant increases in cortisol in response to the experimental stressor, while those following a trajectory of chronic increasing distress had no significant cortisol change in response to the challenge. MHPG responses were not associated with distress trajectories. Cortisol response prospectively differentiated trajectories of distress response suggesting that a blunted cortisol response to a laboratory stressor is a risk factor for later vulnerability to distress following significant life stressors.
Keywords: Resilience, Distress, Police, Cortisol, Neuroendocrine, Prospective
Objectives of the Study and Background
Biological response to a stressor is multifaceted, including processes that rapidly increase preparedness for harm as well as slower processes for regaining homeostasis to prevent adverse biological consequences of a prolonged physiological stress response (Conrad, 2011). Rapid responses to a stressor occur in the sympathetic adrenal medullary pathway (SAM), which activate physiological responses such as increased heart rate and blood pressure in service of defensive behavior such as fight or flight (Schedlowski et al, 1993). However, this level of arousal can have lasting physiological consequences if unchecked. Stressors are also associated with increases in cortisol 15-20 minutes post-stressor. Stressor-related cortisol responses have been shown to aid in braking sympathetic stress responses once the perceived threat has been removed (Munck, Guyre, & Holbrook, 1984), and may be important in regaining physiological homeostasis following a significant stressor (Yehuda & LeDoux, 2007). These neurobiological pathways are thought be highly conserved across species as part of the threat response survival circuitry (LeDoux, 2012). As such, identifying their relationship to heterogeneous stress-response phenotypes may be informative as to how threat circuitry functioning leads to clinically relevant long-term outcomes such as resilience, recovery, and chronic stress.
Heterogeneity in cortisol responses to stressful experimental conditions has been identified in a diverse set of clinical stress reactions. It has been proposed that cortisol plays a regulatory role that may be of broad relevance for understanding the relationship between stressor exposure and pathological stress responses. Specifically, a blunted cortisol response, in which individuals do not increase their secretion of cortisol in response to an experimental stressor, has been observed in a number of clinical contexts. Very low income women who demonstrated a blunted cortisol response to a stressor were more likely to experience elevated depression symptomatology (Burke, Fernald, Gertler, & Adler, 2005). Children who received an initial diagnosis of attention deficit hyperactive disorder (ADHD) who demonstrated this effect were more likely to maintain the diagnosis one year later (King, Barkley, & Barrett, 1998). Alcohol and poly-substance abusing men were more likely to demonstrate a blunted cortisol response to an experimental stressor compared to healthy controls (Lovallo, Dickensheets, Myers, Thomas, & Nixon, 2000), and abstaining alcohol and poly-substance abusers who demonstrated this abnormality have been shown to be more likely to relapse (Junghanns et al., 2003). Individuals with schizophrenia, a neurobiological disorder in which symptoms are exacerbated by stress, have also demonstrated a blunted cortisol response compared to healthy controls (Brenner et al, 2009; Jansen et al, 1998; Jansen, Gispen-de Wied, & Kahn, 2000). This effect has also been observed in other contexts in which stress can exacerbate medical symptomatology including tinnitus (Hébert & Lupien, 2007), fatigue among breast cancer survivors (Bower, Ganz, & Aziz, 2005), and flare-ups of inflammatory disorders such as allergic asthma and atopic dermatitis (Buske-Kirschbaum et al, 1997; Buske-Kirschbaum et al, 2003). In preclinical settings, rodents that vary genetically in the corticosterone (the rodent analogue to cortisol) response varied significantly in prolonged behavior stress responses following exposure to a predator stress condition, with blunted corticosterone responses associated with prolonged stress responses (Cohen et al, 2006). Together, we are presented with a transdiagnostic picture in which the inability to mount an effective cortisol response to an experimental stressor is associated with poorer real-life stress management. In most of these studies, heart rate variability was also examined but not found to be predictive of the outcome of interest. This suggests that the ability to biologically regain homeostasis may be more impactful on longer-term stress adaptation then the initial SAM stress response itself.
While these findings support the hypothesis that the management of stress may have strong biological underpinnings rooted in HPA-axis activity, many of the studies to date have focused on populations who have already expressed pathological stress responses. A number of studies have examined cortisol response immediately following trauma exposure as a prospective predictor of subsequent PTSD development but have not presented with consistent evidence of a relationship between cortisol soon after the trauma and later PTSD (Bonne et al, 2003; Heinrichs et al, 2005; McFarlane, Barton, Yehuda, & Wittert, 2011; Shalev et al, 2008). The cortisol awakening response has been examined prospectively in military populations but failed to predict later PTSD development (van Zuiden et al, 2011). In an earlier report from this cohort, the cortisol awakening response was found to prospectively predict both peri-traumatic reactions as well as acute stress disorder symptom severity 12-months into active police service (Inslicht et al, 2011). As such, controversy remains about the assertion that cortisol response is a dimension underlying diverse clinical outcomes despite theory to support the assertion that the cortisol response to significant life stressors will ultimately impact long term patterns of adaptation (Yehuda & LeDoux, 2007). Determining if cortisol responses to an experimental stressor prospectively predict responses to real-life stressors may be informative as to the relationship between underlying threat circuitry functioning and long term psychological responses. In the current study we examine this relationship in urban police officers, as they represent a generally healthy population at the time of academy training who will encounter repeated duty related stressors in the course of their careers.
Police officers have been shown to be susceptible to adverse consequences of both routine work stressors and exposure to potentially traumatic events (PTEs) including the development of posttraumatic stress disorder (PTSD) (Marmar et al, 2006), depression (Wang et al., 2010), pathological sleep disturbances (Neylan et al., 2002), anxiety, somatization, alcohol abuse, and aggressive behavior (Gershon, Lin, & Li, 2002). Despite the significant psychological risks associated with police work, evidence indicates that there is significant and meaningful heterogeneity in distress responses among officers exposed to similar conditions. Empirical studies have demonstrated that police officers, along with others who are similarly exposed such as military personnel and first responders, follow a limited set of trajectories of symptom or distress responses including Resilience (little or no long term emotional distress), Recovery (significant distress followed by remission), Chronic Distress (high levels of distress or pathology that do not abate and may increase over time) and, when the stressor is anticipated Anticipatory Distress characterized by elevated pre-event stress that declines following the event is also commonly observed (Bonanno, 2004b; Bonanno, Kennedy, Galatzer-Levy, Lude, & Elfstom, 2012; Bonanno, Mancini, et al, 2012; Galatzer-Levy & Bonanno, 2012; Galatzer-Levy et al, 2013; Galatzer-Levy, Burton, & Bonanno, 2012). These patterns have been found in response to events as varied as terrorist attacks (Bonanno, Rennicke, & Dekel, 2005), disease epidemics (Bonanno et al, 2008), traumatic injury (deRoon-Cassini, Mancini, Rusch, & Bonanno, 2010), deployment to warzones (Dickstein, Suvak, Litz, & Adler, 2010), and traumatic loss (Bonanno, 2004a), and have been observed in response to life stressors including breast cancer diagnosis (Lam et al, 2010), job loss (Galatzer-Levy, Bonanno, & Mancini, 2010), and even childbirth (Galatzer-Levy, Mazursky, Mancini, & Bonanno, 2011). When the population is repeatedly exposed to significant stressors, the chronic stress trajectory shows progressive growth in symptomatology (Reactive-Worsening) rather than a chronic stable elevated trajectory (Galatzer-Levy, Madan, Neylan, Henn-Haase, & Marmar, 2011).
Our previous work has demonstrated that heterogeneous patterns of stress response among police officers do not demonstrate a linear relationship with either routine work stress or PTE exposure (Galatzer-Levy et al, 2013). Specifically, the previous work found that continuous counts of routine work stress and PTEs were non-significant in association with the trajectories of general distress under study in the current work. This does not indicate that such events are not impactful but rather that stress responses among officers, a routinely exposed population, are not characterized by a positive linear increase in association with the number of routine work stressors or PTEs. As such, stressor exposure may be a necessary condition for the emergence of adaptive or maladaptive responses rather than a driving force behind the pattern of response. Neurobiological responses are a strong candidate as a factor impacting individual differences as there is consistent evidence for heterogeneity in the neurobiological (Cohen et al, 2006) and behavioral response (Galatzer-Levy, Bush, Bonanno, & LeDoux, 2012) to identical stress conditions.
Using a prospective longitudinal design in which police officers were followed from academy training through 4 years of active duty service, we tested the hypothesis that a blunted cortisol response to an ecologically valid stress induction during academy training would prospectively predict membership in the Reactive-Worsening distress trajectory. Secondarily, we examined if catecholamine responses to the same experimental condition predicted membership in the Reactive-Worsening trajectory to test the hypothesis that the initial adrenergic response would predict outcomes.
Materials and Methods
Participants and Procedures
Officer recruits from 4 urban police departments (New York City, San Francisco, Oakland, San Jose) were recruited into a large prospective study of bio-psycho-social predictors of stress responses to critical incident exposure. Trainees who had previously served in the military, law enforcement, or emergency services were excluded. Procedures were approved by the University of California, San Francisco Institutional Review Board and a Federal Certificate of Confidentiality was obtained. Participants were evaluated on general distress and cortisol and catecholamine reactivity at baseline (academy training), and general distress, PTSD symptoms, and depression symptoms at 12, 24, 36, and 48 months after commencement of active duty police service. Prior to the initial assessment, study procedures were described in detail and written informed consent was obtained (for a full description of recruitment procedures see (McCaslin, 2008).
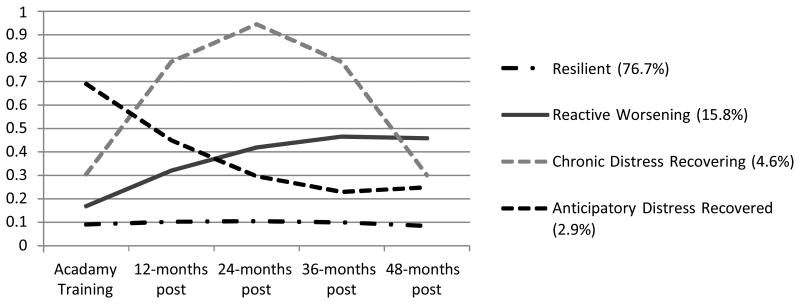
This study utilized a subsample of officers from the parent study who had data on general emotional distress available on at least 3 of 5 time points, including during training and across 48 months of active duty service (N = 234) drawn from a larger cohort of police officers followed longitudinally from academy training (N = 400). Of the 234 participants, n=166 reported exposure to a PTE at least once by 6 months, n = 208 by 12 months, n = 221 by 24 months, n = 224 by 36 months, and n = 227 by 48 months. We previously identified empirical trajectories of general distress using Latent Class Growth Analysis (LCGA). The previously identified trajectories included Resilience, characterized by healthy adaptation from baseline to 4 years into active duty (76.7%), Reactive-Worsening (15.8%), characterized by consistent growth in distress across all 4 years, Recovering (4.6%), demonstrating sharp growth in distress from baseline though 2 years of service that remitted at roughly the same rate from 2 years to 4 years of service, and Anticipatory Distress (2.9%), characterized by high distress at baseline prior to initiation of active duty that diminishes in distress over time (Figure 2). We previously demonstrated that individuals in these trajectories were significantly different with regard to PTSD and depression symptomatology (Galatzer-Levy et al., 2013). For a full description of modeling and sampling procedures see (Galatzer-Levy et al, 2013).
Figure 2. 4-class conditional model of General Distress (N = 234).
Note: Scores on the y-axis indicate estimated marginal means on the Symptom Checklist-90 General Symptom Index.
Measures
Critical Incident History Questionnaire (CIHQ)
The Critical Incident History Questionnaire (CIHQ) is a 39-item self-report measure designed to assess exposure to PTSD criterion A events typically encountered by police officers in the line of duty [for a full description see Weiss et al, 2010). The CIHQ was administered at 12, 24, 36, and 48 months either in-person or through the mail. Mean life threat through 48 months was 11.58 incidents (SD = 13.98). In the current analysis we utilized a dichotomous variable indicating exposure to a life-threatening event in the first 12 months as a covariate, as this may also predict stress response patterns.
Hopkins Symptom Checklist 90-R Global Severity Index (SCL-90-R GSI)
Self-reported distress from psychological symptoms was measured using a combination of 29 items from multiple scales of the Symptom Checklist-90-R that make up the Global Severity Index (GSI) (Derogatis & Melisaratos, 1983). The SCL-90-R was administered at baseline, 12, 24, 36, and 48 months either in-person or through the mail.
Salivary Cortisol and Salivary 3-Methoxy-4-hydroxyphenylglycol (MHPG)
The details of the critical incident video have been described in an earlier report from this study (Apfel et al., 2011). Briefly, participants observed an innocuous travelogue video for 10 minutes, followed by a 20-minute critical incident depiction video, then a 20-minute travelogue video again during the response period. The critical incident video contained real-life footage of 14 incidents involving police officers that were edited into one continuous 20-minute segment depicting police-related scenes including an officer being hit by a car, an officer being mauled by a dog, and an officer being killed while attempting to defuse a bomb. Saliva was collected at three time points; T1 (Baseline) was a baseline measure obtained immediately following the first travelogue video and just prior to the viewing the video stressor, T2 (Initial Cortisol Response) was a measure obtained immediately after the conclusion of the 20-minute video stressor, and T3 (Final Cortisol Response) which was obtained at the conclusion of the 20-minute response period (see Figure 1). There was no food intake, exercise, or smoking for a minimum of 2 hours before the stress challenge, and use of prescription and over-the-counter medication was systematically assessed (for a complete description of sample collection, processing, and analysis of samples see (Otte et al., 2005). Because of significant evidence that the cortisol response to a laboratory stressor peaks between 20 and 40 minutes after stress onset (Dickerson & Kemeny, 2004), as well as evidence for greatest MHPG change in the same timeframe (Otte et al., 2005), we computed change scores as T2 cortisol and MHPG measurements minus T3 measurements. MHPG and cortisol levels at T1 were used as covariates to capture variance associated with individual differences in MHPG and cortisol that are not associated with the video challenge stress response. We measured MHPG in saliva because it is less invasive than plasma measures and correlates with plasma MHPG, which increases in response to acute stressors and is unaffected by beta-blockade, suggesting that it is a measure of central noradrenergic activity (Hamer, Tanaka, Okamura, Tsuda, & Steptoe, 2007). Salivary MHPG also correlates strongly with MHPG in cerebrospinal fluid, providing further support for its use as a proxy for central noradrenergic metabolism (Reuster, Rilke, & Oehler, 2002). All values were log-transformed to address significant skewness and kurtosis in both. Transformations normalized the distributions of all measures.
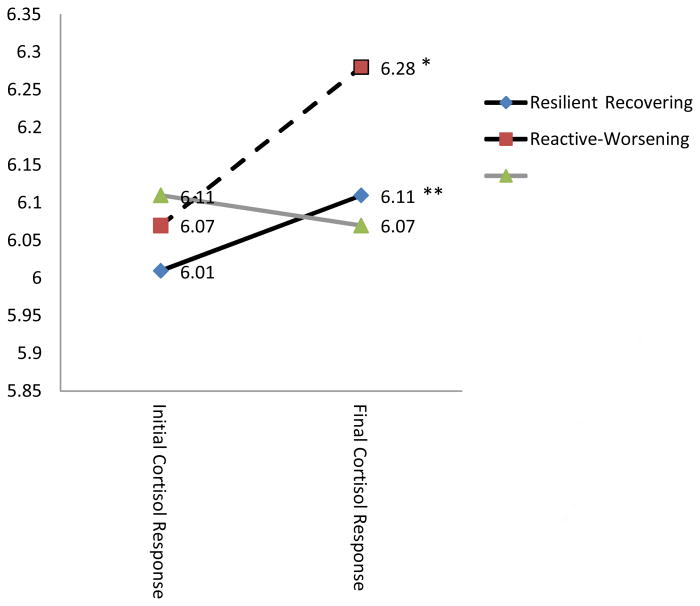
Figure 1. Mean cortisol change across video vignette response period by class.
Note: *p<=05; **p<=001; Results reveal that those in the Resilient and the Recovering class demonstrated cortisol change across the response period while those in the Reactive-Worsening class did not.
Data Analytic Plan
Our data analysis plan consisted of two steps. Step 1 aimed to determine if changes in cortisol and catecholamines in response to the video stressor predicted trajectories of general distress through 48-months of active duty. To test this both catecholamine and cortisol change scores across the response period (T3-T2) were used as predictors of previously identified trajectories of general distress (Galatzer-Levy et al, 2013) using a multinomial logistic regression while controlling for baseline levels of both hormones as well as other possible confounding variables. Change scores only provide a gross estimate of the magnitude of change. However, they do not provide evidence of significant levels of change. To determine if significant levels of change occurred during the response period, those change scores that demonstrate significance as predictors of trajectories were examined using a repeated measures ANOVA with T2 and T3 scores entered as a within subjects factor and class membership entered as a between subjects factor. This analysis would determine if there was significant change in stress hormone levels in response to the video challenge task.
We tested the hypothesis that a blunted cortisol response to an ecologically valid stress induction during academy training would prospectively predict membership in the Reactive-Worsening distress trajectory. Secondarily, we examined if catecholamine responses to the same experimental condition predicted membership in the Reactive-Worsening trajectory to test the hypothesis that the initial adrenergic response would predict outcomes.
Data Analysis
Using Mplus 6.12 (Muthen & Muthen, 2006), Latent Class Growth Analysis (LCGA) was previously employed to identify trajectories of general distress from baseline during training to 48 months of active duty police service. Model selection procedures are more fully described in Galatzer-Levy et al, (2013). The best fitting model solution revealed four classes including Resilience (76.7%), Reactive-Worsening (15.8%), Recovering (4.6%) and Anticipatory Distress (2.9%).
In the current analysis, we assessed differences in levels of cortisol and MHPG change by class while controlling for background variables by regressing class membership on the covariates (age, gender, personal life threat exposure by 12 months, cortisol change scores, MHPG change scores, T1 cortisol, T1 MHPG) in a multinomial logistic regression nested in the unconditional LCGA using the Auxiliary option, which estimates differences on covariates based on posterior probabilities of latent class membership. Because the multinomial logistic regression only provides group comparisons on mean level change but is not informative about the degree of change occurring within each class, change in cortisol scores from immediately following the video stressor to 20 minutes later were examined using a repeated measures ANOVA with trajectory membership as a between subjects factor to examine the significance of cortisol change by trajectory membership. In this analysis log transformed values of T2 and T3 cortisol were tested as a within subjects factor. Corrections were made for multiple comparisons using Least Squared Differences. Exposure at 12-months was examined as a dichotomous variable to determine if individuals exposed earlier in police service would fall into distress trajectories. Total critical incident exposure and work stress exposure across the 48-months has already been examined and found to be non-significant in association with the trajectories (Galatzer-Levy et al., 2013).
Results
In the previous trajectory analysis, we compared solutions with linear parameters only and linear and quadratic parameters to assess which parameters best fit the data. Ultimately a four-class solution with linear and quadratic parameters best fit the data based on a significant reduction the information criteria, entropy, the BLRT, and conformity with theory and parsimony (see Figure 1 for the classes and their proportions). Though reductions in the information criteria continued to be observed through five classes with linear and quadratic weights (reduction in AIC = 43.34, BIC = 29.52, SSBIC = 42.15), the BLRT was significant at p<=.05 for a four vs. three class solution but not a five vs. four class solution. This was true both when applying linear weights only (p = .95) and linear + quadratic weights (p = .36). Further, the addition of a fifth class served to split a small class into two parallel classes, and as such was a less parsimonious and less interpretable solution (Galatzer-Levy et al, 2013).
Next, we explored the data descriptively by generating means and standard deviations or frequencies for our variables of interest, including gender and exposure, age, baseline cortisol and MHPG levels, cortisol and MHPG levels at T2 and T3. Both log-transformed values of cortisol and MHPG that are used in analyses are presented as well as raw scores on these variables for comparison to other studies (See Table 1). Descriptive statistics indicated that there was an increase in cortisol among those in the Resilient, Recovery, and Anticipatory Distress classes and a decrease among those in the Reactive-Worsening class. Similar trends were observed for MHPG with the exception of the Anticipatory Distress class which showed a decrease.
Table 1. Descriptive for predictor variables by class.
| Trajectory Membership | ||||
|---|---|---|---|---|
|
| ||||
| Variables in Model | Resilient (n=178) Mean (SD)/% | Recovering (n=11) Mean (SD)/% | Reactive-Worsening (n=38) Mean (SD)/% | Anticipatory Distress (n =7) Mean (SD)/% |
| Gender (male) | 91.5% | 85.7% | 88.9% | 100.0% |
| Age | 27.34(4.92) | 32.00 (6.35) | 27.33 (4.47) | 25.75 (5.55) |
| Exposure | 80.9% | 77.8% | 80.6% | 100.0% |
| Baseline Cortisol | 6.17 (0.51) | 5.83 (0.85) | 6.15 (0.43) | 6.43 (0.59) |
| Time 2 Cortisol | 6.01 (0.52) | 6.07 (1.06) | 6.11 (0.52) | 6.19 (0.65) |
| Time 3 Cortisol | 6.11 (0.60) | 6.28 (1.01) | 6.07 (0.54) | 6.30 (0.69) |
| Baseline MHPG | 1.63 (0.43) | 1.74 (0.41) | 1.62 (0.40) | 1.83 (0.23) |
| Time 2 MHPG | 1.63 (0.43) | 1.84 (0.42) | 1.69 (0.34) | 1.87 (0.38) |
| Time 3 MHPG | 1.66 (0.42) | 1.86 (0.54) | 1.65 (0.40) | 1.86 (0.39) |
| Baseline Cortisol Raw | 5.60 (2.90) | 5.60 (2.90) | 5.43 (2.07) | 6.37 (1.34) |
| Score | ||||
| Time 2 Cortisol Raw | 5.78 (3.31) | 5.78 (3.31) | 5.74 (1.85) | 6.85 (2.38) |
| Score | ||||
| Time 3 Cortisol Raw | 5.77 (3.05) | 5.77 (3.05) | 5.63 (2.18) | 6.78 (2.10) |
| Score | ||||
| Baseline MHPG Raw | 540.13 (277.60) | 540.13 (277.60) | 509.89 (207.29) | 721.38 (429.33) |
| Score | ||||
| Time 2 MHPG Raw Score | 478.63 (414.32) | 478.63 (414.32) | 517.88 (298.01) | 594.20 (439.03) |
| Time 3 MHPG Raw Score | 578.70 (805.53) | 578.70 (805.53) | 503.77 (295.48) | 660.92 (465.92) |
Note: Exposure is a dichotomous item indicating presence/absence of exposure to a life threatening event in the first year of police duty; All cortisol and MHPG values are log-transformed unless labeled as Raw Scores.
To test if the observed change in cortisol and MHPG significantly differed by class, we regressed class membership on our covariates in a multinomial logistic regression using the Auxiliary option in MPlus 6.12 on the modeled classes. This analysis revealed no significant differences by age, gender, or trajectory class in the probability of exposure at 12-months. Further, no significant difference was observed between the classes on T1 cortisol or T1 MHPG levels or MHPG change from T2 to T3. However, individuals in the Reactive-Worsening class demonstrated significantly less change in cortisol response from T2 to T3 when the Resilient class was used as the references class (Est/SE = -1.95; p <05) and significantly less change compared to the Recovery class when the Reactive-Worsening class was set as the reference class (Est/SE = 2.14; p <05); see Table 2 for complete results). Post-hoc Analyses
Table 2. Multinomial Logistic Regression for Predictors of Class Membership (N = 234).
| Resilient vs. | Reactive Worsening vs. | Recovering vs. | ||||
|---|---|---|---|---|---|---|
| Recovering | Anticipatory Distress | Recovering | Anticipatory Distress | Resilient | Anticipatory Distress | |
|
|
||||||
| Est./S.E | Est./S.E | Est./S.E | Est./S.E | Est./S.E | Est./S.E | |
| Age | 1.53 | -0.79 | 1.37 | -0.64 | .32 | -1.31 |
| Gender | 0.39 | -0.01 | 0.01 | -0.01 | 0.01 | -0.01 |
| Exposure | 0.16 | 0.01 | -0.89 | 0.01 | -1.51 | 0.01 |
| Cortisol ∆ | 1.03 | -0.88 | 2.14* | 0.18 | 1.95* | -1.30 |
| MHPG ∆ | 0.72 | -1.20 | 1.45 | -0.64 | 1.27 | 1.40 |
| Cortisol | -1.04 | 0.78 | -0.37 | 1.15 | 0.93 | 1.28 |
| Baseline | ||||||
| MHPG Baseline | 1.11 | 0.82 | 0.91 | 0.85 | 0.29 | 0.25 |
Note: Vs. = versus; Est/S.E. = Estimate/Standard Error of the Estimate; *p < .05; Exposure = exposure to a life threatening event in the first 12 months of active duty; Cortisol ∆ = Change in Cortisol during recovery period in response to video stressor; MHPG ∆= Change in MPHG during recovery period in response to video stressor.
To determine if there was significant change in cortisol levels separately by class in response to the video stressor, a Repeated Measures Analysis of Variance (ANOVA) was conducted to test for mean level change in cortisol from T2 to T3 with probable class membership as a fixed effect. In this analysis, log transformed cortisol scores at T2 and T3 were used as a within subjects factor. This analysis revealed that those in the Resilient and Recovery classes demonstrated significant increases in cortisol across these time points [Resilient: F (1,106) = 11.59; p <=001; Recovery F (1, 8) = 6.03; p <05] while those in the Reactive-Worsening distress class did not show a significant increase in cortisol response from T2 to T3 [F (1,30) = 0.11; p = 74; figure 2].
Discussion
In the current investigation we tested the hypothesis that a blunted cortisol response would prospectively predict long-term stress response patterns. Secondarily, we examined whether catecholamine responses during the same time frame would predict these patterns. We tested this hypothesis by examining if trajectories were predicted by change scores in cortisol and catecholamine levels from immediately following a video stressor to 20 minutes later (capturing the cortisol response as well as a sensitive period of catecholamine change). Because cortisol and MHPG levels can be affected by a number of individual characteristics, we included gender, age, and baseline cortisol and MHPG levels as covariates. Further, because patterns of stress response can potentially be explained by initial exposure rather than cortisol and MHPG, exposure by 12-months was also included as a covariate.
Compared to those who demonstrated Resilient and Recovering trajectories, those who demonstrated consistent growth in stress (Reactive-Worsening class) demonstrated significantly lower levels of cortisol change in response to the video challenge stressor prior to exposure to real life stressors. MHPG responses to the video challenge and levels of MHPG and cortisol just prior to the video challenge did not differentiate classes. Further, post-hoc analyses revealed that individuals in both the Recovering and Resilient classes demonstrated significant increases in cortisol in response to the video stress challenge while those in the Reactive-Worsening class did not demonstrate a significant change, indicating that they did not mount a cortisol response to the video challenge while the others did.
This study has several limitations. Stress hormone levels are variable for a number of reasons including individual differences in age, gender, medication, substance use, psychiatric comorbidity, and time of day that levels are recorded. We attempted to address this limitation by controlling age, gender, and baseline hormone levels which will account for random individual variability, and by examining change scores in response to a standardized task. Further, it remains unclear what the true impact of critical incidents and routine life stressors are on patterns of response. In the current study we found that exposure to a life-threatening event in the first year was not predictive of trajectory, and in the previous study we found that whether individuals were exposed to trauma throughout the 48 months was similarly not predictive of the trajectories (Galatzer-Levy et al, 2013). However, this may be because there is not much variability in dichotomous measures of exposure, as this is a highly exposed population overall. Conversely, continuous measures of exposure may not be predictive in this context as the population overall is highly and consistently exposed leading to truncated variability clustered in the high exposure range. A further limitation is that the relationship between cortisol response and early childhood trauma exposure was not explored. There is evidence that childhood trauma has a significant impact on HPA axis reactivity (Heim et al, 2000) and glucocorticoid receptor sensitivity (Klengel et al, 2013). As such, childhood trauma may explain observed differences by class on cortisol response to the video stressor. Unfortunately, though the Early Trauma Inventory Self Report—Short Form [ETISR-SF,(Bremner, 2007) ] was collected in this sample, significant missingness in >50% of cases precluded these analyses. This represents a significant limitation that should be addressed in future studies. In addition, an alternative explanation of the results is that those in the Reactive-Worsening class simply mounted a more rapid cortisol response and recovery and as such, their response was not captured in the sampling window. This is plausible given that glucocorticoid hypersensitivity as well as glucocorticoid resistance have been identified in PTSD (Bachmann et al, 2005; Yehuda, Giller, Southwick, Lowy, & Mason, 1991).
Finally, in previous work, we demonstrated that individuals in the Recovery class demonstrated both the highest levels of distress and PTSD symptomatology in years 1 through 3 and did not differ from those in the Reactive-Worsening class on levels of PTSD symptomatology at 48-months (Galatzer-Levy et al., 2013). However, PTSD symptom levels were primarily below the clinical cut-off in this sample limiting interpretations as to the role of cortisol or MHPG response in relation to psychopathology. As such, the present results do not clarify how the cortisol response influences patterns PTSD response.
Despite these limitations, the current study provides evidence that a blunted cortisol response prospectively predicts the subsequent development of chronic non-remitting stress responses. This finding has both theoretical and practical relevance. First, such tasks are easy to implement and may provide useful information regarding likely outcomes among populations that are likely to be exposed to PTEs and other significant stressors such as police, firefighters, first responders, and service members. Second, these findings provide evidence that a blunted cortisol response may be a pre-existing risk factor for poor adaptation to stressors. This informs the understanding of the role of individual differences in HPA-axis responses to stress as they relate to the development and long-term course of stress pathology and resilience. It is important to note that post-hoc analyses demonstrated that those on the Recovery and Resilient trajectories both demonstrated significant increases in cortisol response to the video challenge while those in the Reactive-Worsening class demonstrated a non-significant cortisol response. This may indicate that cortisol response influences more than the short term stress response; it also influences the long-term course of adaptation to stress. As such, identification of individuals with a blunted cortisol response may potentially aid in the early identification of those who are likely to follow a chronic course and informs our understanding of the neurobiological role in psychological vulnerability to pathological stress responses.
Acknowledgments
This work was supported by an NIMH R01 5R01MH056350-13 Prospective Study of Traumatic Stress in Police Officers, Charles Marmar P.I.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apfel BA, Otte C, Inslicht SS, McCaslin SE, Henn-Haase C, Metzler TJ, Marmar CR. Pretraumatic prolonged elevation of salivary MHPG predicts peritraumatic distress and symptoms of post-traumatic stress disorder. Journal of psychiatric research. 2011;45(6):735–741. doi: 10.1016/j.jpsychires.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann AW, Sedgley TL, Jackson RV, Gibson JN, Young RM, Torpy DJ. Glucocorticoid receptor polymorphisms and post-traumatic stress disorder. Psychoneuroendocrinology. 2005;30(3):297–306. doi: 10.1016/j.psyneuen.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Bonanno GA. Loss, Trauma, and Human Resilience: Have We Underestimated the Human Capacity to Thrive After Extremely Aversive Events? The American psychologist. 2004a;59(1):20–28. doi: 10.1037/0003-066x.59.1.20.. [DOI] [PubMed] [Google Scholar]
- Bonanno GA. Loss, Trauma, and Human Resilience: Have We Underestimated the Human Capacity to Thrive After Extremely Aversive Events? American Psychologist. 2004b;59(1):20–28. doi: 10.1037/0003-066x59.1.20.. [DOI] [PubMed] [Google Scholar]
- Bonanno GA, Ho SMY, Chan JCK, Kwong RSY, Cheung CKY, Wong CPY, Wong VCW. Psychological resilience and dysfunction among hospitalized survivors of the SARS epidemic in Hong Kong: A latent class approach. Health Psychology. 2008;27(5):659–667. doi: 10.1037/0278-6133.27.5.659. [DOI] [PubMed] [Google Scholar]
- Bonanno GA, Kennedy P, Galatzer-Levy IR, Lude P, Elfstom ML. Trajectories of resilience, depression, and anxiety following spinal cord injury. Rehabilitation Psychology. 2012 doi: 10.1037/a0029256. [DOI] [PubMed] [Google Scholar]
- Bonanno GA, Mancini AD, Horton JL, Powell TM, LeardMann CA, Boyko EJ, Smith TC. Trajectories of trauma symptoms and resilience in deployed US military service members: prospective cohort study. The British Journal of Psychiatry. 2012;200(4):317–323. doi: 10.1192/bjp.bp.111.096552. [DOI] [PubMed] [Google Scholar]
- Bonanno GA, Rennicke C, Dekel S. Self-Enhancement Among High-Exposure Survivors of the September 11th Terrorist Attack: Resilience or Social Maladjustment? Journal of Personality and Social Psychology. 2005;88(6):984–998. doi: 10.1037/0022-3514.88.6.984. [DOI] [PubMed] [Google Scholar]
- Bonne O, Brandes D. Prospective evaluation of plasma cortisol in recent trauma survivors with posttraumatic stress disorder. Psychiatry Res. 2003;119:171–175. doi: 10.1016/s0165-1781(03)00098-2. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N. Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosomatic Medicine. 2005;67(2):277–280. doi: 10.1097/01.psy.0000155666.55034.c6. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Bolus R, Mayer EA. Psychometric properties of the Early Trauma Inventory-Self Report. J Nerv Ment Dis. 2007;195:211–218. doi: 10.1097/01.nmd.0000243824.84651.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner K, Liu A, Laplante DP, Lupien S, Pruessner JC, Ciampi A, King S. Cortisol response to a psychosocial stressor in schizophrenia: Blunted, delayed, or normal? Psychoneuroendocrinology. 2009;34(6):859–868. doi: 10.1016/j.psyneuen.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Burke HM, Fernald LC, Gertler PJ, Adler NE. Depressive symptoms are associated with blunted cortisol stress responses in very low-income women. Psychosomatic Medicine. 2005;67(2):211–216. doi: 10.1097/01.psy.0000156939.89050.28. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Psych D, Wustmans A, Kirschbaum C, Rauh W, Hellhammer D. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine. 1997;59(4):419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, von Auer K, Krieger S, Weis S, Rauh W, Hellhammer D. Blunted cortisol responses to psychosocial stress in asthmatic children: a general feature of atopic disease? Psychosomatic Medicine. 2003;65(5):806–810. doi: 10.1097/01.psy.0000095916.25975.4f. [DOI] [PubMed] [Google Scholar]
- Cohen H, Zohar J, Gidron Y, Matar MA, Belkind D, Loewenthal U, Kaplan Z. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biological psychiatry. 2006;59(12):1208–1218. doi: 10.1016/j.biopsych.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Conrad CD. The Handbook of Stress: Neuropsychological Effects on the Brain. Vol. 9. Wiley.com; 2011. [Google Scholar]
- Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an Introductory Report. Psychological Medicine. 1983;13(3) [PubMed] [Google Scholar]
- deRoon-Cassini TA, Mancini AD, Rusch MD, Bonanno GA. Psychopathology and resilience following traumatic injury: A latent growth mixture model analysis. Rehabilitation Psychology. 2010;55(1):1–11. doi: 10.1037/a0018601. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dickstein BD, Suvak M, Litz BT, Adler AB. Heterogeneity in the course of posttraumatic stress disorder: Trajectories of symptomatology. Journal of Traumatic Stress. 2010;23(3):331–339. doi: 10.1002/jts.20523. [DOI] [PubMed] [Google Scholar]
- Galatzer-Levy IR, Bonanno GA. Beyond normality in the study of bereavement: Heterogeneity in depression outcomes following loss in older adults. Social Science & Medicine. 2012;74(12):1987–1994. doi: 10.1016/j.socscimed.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galatzer-Levy IR, Bonanno GA, Mancini AD. From marianthal to latent growth mixture modeling: A return to the exploration of individual differences in response to unemployment. Journal of Neuroscience, Psychology, and Economics. 2010;3(2):116–125. [Google Scholar]
- Galatzer-Levy IR, Brown AD, Henn-Haase C, Metzler TJ, Neylan TC, Marmar CR. Positive and Negative Emotion Prospectively Predict Trajectories of Resilience and Distress Among High-Exposure Police Officers. 2013 doi: 10.1037/a0031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galatzer-Levy IR, Burton CL, Bonanno GA. Coping Flexibility, Potentially Traumatic Life Events, and Resilience: A Prospective Study of College Student Adjustment. Journal of Social and Clinical Psychology. 2012;31(6):542–567. doi: 10.1521/jscp.2012.31.6.542. [DOI] [Google Scholar]
- Galatzer-Levy IR, Madan A, Neylan TC, Henn-Haase C, Marmar CR. Peritraumatic and trait dissociation differentiate police officers with resilient versus symptomatic trajectories of posttraumatic stress symptoms. Journal of Traumatic Stress. 2011;24(5):557–565. doi: 10.1002/jts.20684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galatzer-Levy IR, Mazursky H, Mancini AD, Bonanno GA. What we don't expect when expecting: Evidence for heterogeneity in subjective well-being in response to parenthood. Journal of Family Psychology. 2011;25(3):384–392. doi: 10.1037/a0023759. [DOI] [PubMed] [Google Scholar]
- Gershon RRM, Lin S, Li X. Work Stress in Aging Police Officers. Journal of occupational and environmental medicine. 2002;44(2):160–167. doi: 10.1097/00043764-200202000-00011. [DOI] [PubMed] [Google Scholar]
- Hamer M, Tanaka G, Okamura H, Tsuda A, Steptoe A. The effects of depressive symptoms on cardiovascular and catecholamine responses to the induction of depressive mood. Biological Psychology. 2007;74(1):20–25. doi: 10.1016/j.biopsycho.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Hébert S, Lupien SJ. The sound of stress: Blunted cortisol reactivity to psychosocial stress in tinnitus sufferers. Neuroscience Letters. 2007;411(2):138–142. doi: 10.1016/j.neulet.2006.10.028. http://dx.doi.org/10.1016/j.neulet.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. Jama. 2000;284(5):592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Wagner D, Schoch W, Soravia LM, Hellhammer DH, Ehlert U. Predicting posttraumatic stress symptoms from pretraumatic risk factors: a 2-year prospective follow-up study in firefighters. Am J Psychiatry. 2005;162(12):2276–2286. doi: 10.1176/appi.ajp.162.12.2276. [DOI] [PubMed] [Google Scholar]
- Inslicht SS, Otte C, McCaslin SE, Apfel BA, Henn-Haase C, Metzler T, Marmar CR. Cortisol awakening response prospectively predicts peritraumatic and acute stress reactions in police officers. Biological psychiatry. 2011;70(11):1055–1062. doi: 10.1016/j.biopsych.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen L, Gispen-de Wied CC, Gademan PJ, De Jonge RCJ, van der Linden JA, Kahn RS. Blunted cortisol response to a psychosocial stressor in schizophrenia. Schizophrenia research. 1998;33(1):87–94. doi: 10.1016/s0920-9964(98)00066-8. [DOI] [PubMed] [Google Scholar]
- Jansen LMC, Gispen-de Wied CC, Kahn RS. Selective impairments in the stress response in schizophrenic patients. Psychopharmacology. 2000;149(3):319–325. doi: 10.1007/s002130000381. [DOI] [PubMed] [Google Scholar]
- Junghanns K, Backhaus J, Tietz U, Lange W, Bernzen J, Wetterling T, Driessen M. Impaired serum cortisol stress response is a predictor of early relapse. Alcohol and Alcoholism. 2003;38(2):189–193. doi: 10.1093/alcalc/agg052. [DOI] [PubMed] [Google Scholar]
- King JA, Barkley RA, Barrett S. Attention-deficit hyperactivity disorder and the stress response. Biological psychiatry. 1998;44(1):72–74. doi: 10.1016/s0006-3223(97)00507-6. http://dx.doi.org/10.1016/S0006-3223(97)00507-6. [DOI] [PubMed] [Google Scholar]
- Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, Bradley B. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nature neuroscience. 2013;16(1):33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam WWT, Bonanno GA, Mancini AD, Ho S, Chan M, Hung WK, Fielding R. Trajectories of psychological distress among Chinese women diagnosed with breast cancer. Psycho-Oncology. 2010;19(10):1044–1051. doi: 10.1002/pon.1658. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Rethinking the emotional brain. Neuron. 2012;73(4):653–676. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, Nixon SJ. Blunted Stress Cortisol Response in Abstinent Alcoholic and Polysubstance-Abusing Men. Alcoholism: Clinical and Experimental Research. 2000;24(5):651–658. [PubMed] [Google Scholar]
- Marmar C, McCaslin SE, Metzler T, Best S, Weiss DS, Fagan J, Neylan T. Predictors of posttraumatic stress in police and other first responders. Annals of the New York Academy of Sciences. 2006;1071:1–18. doi: 10.1196/annals.1364.001. [DOI] [PubMed] [Google Scholar]
- McCaslin SE. Trait Dissociation Predicts Posttraumatic Stress Disorder Symptoms in a Prospective Study of Urban Police Officers. The journal of nervous and mental disease. 2008;196(12):912–918. doi: 10.1097/NMD.0b013e31818ec95d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane AC, Barton CA, Yehuda R, Wittert G. Cortisol response to acute trauma and risk of posttraumatic stress disorder. Psychoneuroendocrinology. 2011;36(5):720–727. doi: 10.1016/j.psyneuen.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocrine Reviews. 1984;5(1):25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen B. Mplus User's Guide Fourth Edition. Los Angeles, CA: Muthen and Muthen; 2006. [Google Scholar]
- Neylan TC, Metzler TJ, Best SR, Weiss DS, Fagan JA, Liberman A, Marmar CR. Critical incident exposure and sleep quality in police officers. Psychosom Med. 2002;64(2):345–352. doi: 10.1097/00006842-200203000-00019. [DOI] [PubMed] [Google Scholar]
- Otte C, Neylan TC, Pole N, Metzler T, Best S, Henn-Haase C, Marmar CR. Association between childhood trauma and catecholamine response to psychological stress in police academy recruits. Biol Psychiatry. 2005;57(1):27–32. doi: 10.1016/j.biopsych.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Reuster T, Rilke O, Oehler J. High correlation between salivary MHPG and CSF MHPG. Psychopharmacology. 2002;162(4):415–418. doi: 10.1007/s00213-002-1125-z. [DOI] [PubMed] [Google Scholar]
- Schedlowski M, Jacobs R, Alker J, Pröhl F, Stratmann G, Richter S, Tewes U. Psychophysiological, neuroendocrine and cellular immune reactions under psychological stress. Neuropsychobiology. 1993;28(1-2):87–90. doi: 10.1159/000119006. [DOI] [PubMed] [Google Scholar]
- Shalev AY, Videlock EJ, Peleg T, Segman R, Pitman RK, Yehuda R. Stress hormones and post-traumatic stress disorder in civilian trauma victims: a longitudinal study. Part I: HPA axis responses. The International Journal of Neuropsychopharmacology. 2008;11(03):365–372. doi: 10.1017/S1461145707008127. [DOI] [PubMed] [Google Scholar]
- van Zuiden M, Kavelaars A, Rademaker AR, Vermetten E, Heijnen CJ, Geuze E. A prospective study on personality and the cortisol awakening response to predict posttraumatic stress symptoms in response to military deployment. Journal of Psychiatric Research. 2011;45(6):713–719. doi: 10.1016/j.jpsychires.2010.11.013. [DOI] [PubMed] [Google Scholar]
- Wang Z, Inslicht SS, Metzler TJ, Henn-Haase C, McCaslin SE, Tong H, Marmar CR. A prospective study of predictors of depression symptoms in police. Psychiatry Res. 2010;175(3):211–216. doi: 10.1016/j.psychres.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Giller EL, Southwick SM, Lowy MT, Mason JW. Hypothalamic-pituitary-adrenal dysfunction in posttraumatic stress disorder. Biological Psychiatry. 1991;30:1031–1048. doi: 10.1016/0006-3223(91)90123-4. [DOI] [PubMed] [Google Scholar]
- Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56(1):19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]