Because of the rarity of alveolar soft part sarcoma and the lack of prospective trials, a retrospective, multi‐institutional, caseseries analysis was conducted among reference centers for treatment of soft tissue sarcoma.With the support of the World Sarcoma Network, the activity and efficacy of trabectedin and pazopanib in advanced alveolar soft part sarcoma patients was investigated. The results are presented here.
Keywords: Sarcoma, Alveolar soft part sarcoma, Trabectedin, Pazopanib, Chemotherapy
Abstract
Background.
Alveolar soft part sarcoma (ASPS) is an exceedingly rare and orphan disease, without active drugs approved in the front line. Pazopanib and trabectedin are licensed for sarcoma treatment from second‐line, but very little and contradictory data are available on their activity in ASPS. Lacking ongoing and/or planned clinical trials, we conducted a multi‐institutional study involving the reference sites for sarcoma in Europe, U.S., and Japan, within the World Sarcoma Network, to investigate the efficacy of pazopanib and trabectedin.
Materials and Methods.
From May 2007, 14 of the 27 centers that were asked to retrospectively review their databases had identified 44 advanced ASPS patients treated with pazopanib and/or trabectedin. Response was evaluated by Response Evaluation Criteria in Solid Tumors 1.1. Progression‐free survival (PFS) and overall survival (OS) were computed by Kaplan‐Meier method.
Results.
Among 30 patients who received pazopanib, 18 were pretreated (13 with other antiangiogenics). Response was evaluable in 29/30 patients. Best responses were 1 complete response, 7 partial response (PR), 17 stable disease (SD), and 4 progressions. At a 19‐month median follow‐up, median PFS was 13.6 months (range: 1.6–32.2+), with 59% of patients progression‐free at 1 year. Median OS was not reached.
Among 23 patients treated with trabectedin, all were pretreated and evaluable for response. Best responses were 1 PR, 13 SD, and 9 progressions. At a 27‐month median follow‐up, median PFS was 3.7 months (range: 0.7–109), with 13% of patients progression‐free at 1 year. Median OS was 9.1 months.
Conclusion.
The value of pazopanib in advanced ASPS is confirmed, with durable responses, whereas the value of trabectedin appears limited. These results are relevant to defining the best approach to advanced ASPS.
Implications for Practice.
This retrospective study, conducted among the world reference centers for treatment of sarcoma, confirms the value of pazopanib in patients with advanced alveolar soft part sarcoma (ASPS), with dimensional and durable responses, whereas trabectedin shows a limited activity. Alveolar soft part sarcoma is resistant to conventional cytotoxic chemotherapy. Pazopanib and trabectedin are licensed for treatment of sarcoma from second line; in the lack of prospective clinical trials, these results are relevant to defining ASPS best management and strongly support initiatives aimed at obtaining the approval of pazopanib in the front line of the disease.
Introduction
Alveolar soft part sarcoma (ASPS) is a rare soft tissue sarcoma (STS) that carries a specific t(X;17)(p11;q25), involving a ASPSCR1‐transcription factor E 3 (TFE3) fusion [1], [2]. The natural history of this tumor is characterized by an indolent behavior, coupled with a paradoxical high metastatic rate. As a result, most patients eventually require medical therapy. Although conventional anthracycline‐based chemotherapy is inactive in this disease [1], [3], [4], [5], ASPS was reported to be sensitive to antiangiogenic agents like sunitinib, cediranib, and, more recently, anlotinib [6], [7], [8], [9], [10]. These antiangiogenic agents can induce dimensional responses and durable disease control in 35% and 60% of patients, respectively, across different published studies. Unfortunately, none of these drugs are approved for the treatment of ASPS, and no active agents have regulatory approval in the front line.
Trabectedin and pazopanib are among the medical agents licensed for treatment of STS from second line after failure to anthracyclines, but data on their activity in patients affected by ASPS are scant. Trabectedin is a marine‐derived product characterized by a complex and unique mechanism of action, affecting multiple key processes in tumor cell replication and death [11], and targeting tumor microenvironment [12], [13]. Trabectedin approval in STS is based on the results of two trials carried out in adult patients with a diagnosis of liposarcoma or leiomyosarcoma after failure of prior conventional chemotherapy [14], [15]. In the first study, one trabectedin regimen was superior to another (with evidence of a longer progression‐free survival [PFS] in favor of a 24‐hour infusion regimen, as compared with a 3‐hour infusion schedule) [14]. In the other study, trabectedin was superior to dacarbazine in the same histologies in terms of PFS, although not in terms of overall survival (OS) [15].
Pazopanib is an antiangiogenic agent, inhibiting vascular endothelial growth factor receptors (VEGFR) 1–3, that was initially investigated in sarcoma within a phase 2 trial published by Sleijfer and colleagues in 2009 [16], [17]. In the phase 3 study in nonadipocytic STS that followed the phase 2 trial, the median PFS was 4.6 months for pazopanib, compared with 1.6 months for placebo, with an OS of 12.5 months versus 10.7 months [18].
In patients affected by ASPS, there are few case reports stating that trabectedin can achieve the stabilization of the tumor without evidence of tumor shrinkage. Unfortunately, those reports did not detail if treated patients were progressive or not before starting the drug [19], [20], [21]. Conversely, Nakamura and colleagues recently described 4 Response Evaluation Criteria in Solid Tumors (RECIST) responses of 12 (33%) ASPS patients treated with pazopanib [22], while a study from Korea noted 1 response among 6 ASPS patients treated with the drug within a prospective trial that was unfortunately closed early due to slow accrual (Clinicaltrials.gov reference NCT02113826) [23].
Due to the rarity of ASPS and the lack of prospective trials, we conducted a retrospective multi‐institutional case‐series analysis among reference centers for treatment of STS, with the support of the World Sarcoma Network, to investigate the activity and the efficacy of trabectedin and pazopanib in advanced ASPS patients. The results presented herein are important to refine the best clinical management of the disease in the metastatic setting.
Materials and Methods
We considered all consecutive patients with locally advanced and/or metastatic ASPS treated with trabectedin (group A) and/or pazopanib (group B), in front or further lines, outside clinical trials, from May 2007 to November 2016. Patients were treated at 14 international reference centers for treatment of STS from Europe, U.S., and Japan, selected among 27 institutions that were asked to review their databases within the World Sarcoma Network effort. Only cases in which diagnosis of ASPS was histologically proven and confirmed by each institutional pathologist were included in the study. All patients provided a written informed consent to the treatment with trabectedin and/or pazopanib. Approval by each Institutional Review Board was also required.
Treatment
Patients in group A received intravenous trabectedin, at a starting dose ranging from 1.3–1.5 mg/m2 ‐ (at investigator's discretion, with a maximum dose of 2.6 total mg per cycle), given in 24‐hour continuous infusion, with glucocorticoid premedication, every 3 weeks, until progression or toxicity.
Patients in group B were administered pazopanib orally, at the dose of 800 mg per day (either 800 mg oral daily or 400 mg twice a day), continuously, until progression or toxicity.
Clinical Assessment
Performance status, biochemistry, and blood count were evaluated at baseline and monitored throughout the study period. Adverse events were recorded. Disease status was assessed by whole body computed tomography (CT) scan and/or a CT/magnetic resonance imaging (MRI) of the sites of disease at baseline and then repeated every 2–3 months, at the discretion of the treating physician.
Efficacy Assessment
Response to treatment was assessed by RECIST, version 1.1 [24]. Overall survival and PFS were estimated on SAS University Edition software (SAS Institute, Cary, NC, https://www.sas.com/en_us/home.html) and survival analyses were conducted by the Kaplan‐Meier method. Patients without evidence of progression and interrupting treatment with trabectedin (group A) and with pazopanib (group B) for any reason were censored at the last follow‐up. Patients alive or lost to follow‐up were censored at the last contact.
Results
We retrospectively identified 23 patients treated with trabectedin (group A) and 30 patients treated with pazopanib (group B). Four patients were treated sequentially with trabectedin and pazopanib and are included in both groups. Tables 1 and 2 summarize patient characteristics and clinical findings.
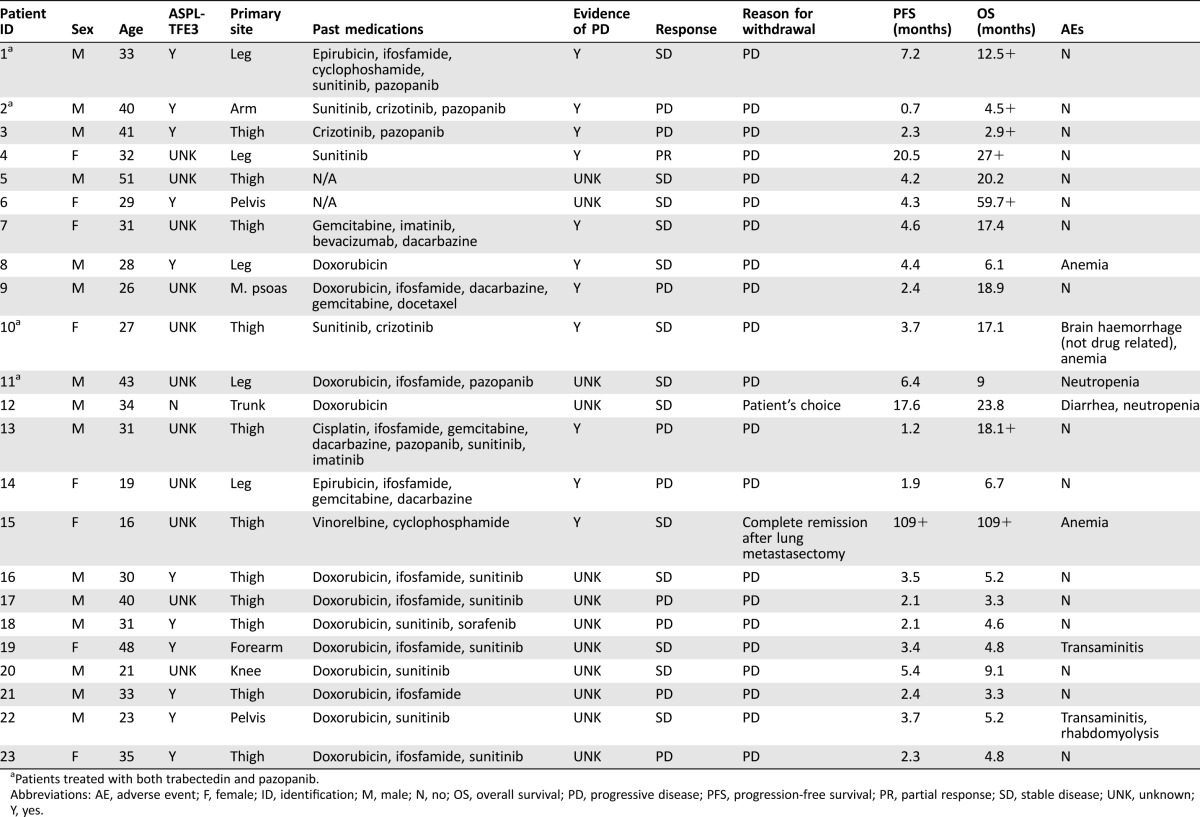
Table 1. Patient characteristics and clinical findings for patients treated with trabectedin (group A).
Patients treated with both trabectedin and pazopanib.
Abbreviations: AE, adverse event; F, female; ID, identification; M, male; N, no; OS, overall survival; PD, progressive disease; PFS, progression‐free survival; PR, partial response; SD, stable disease; UNK, unknown; Y, yes.
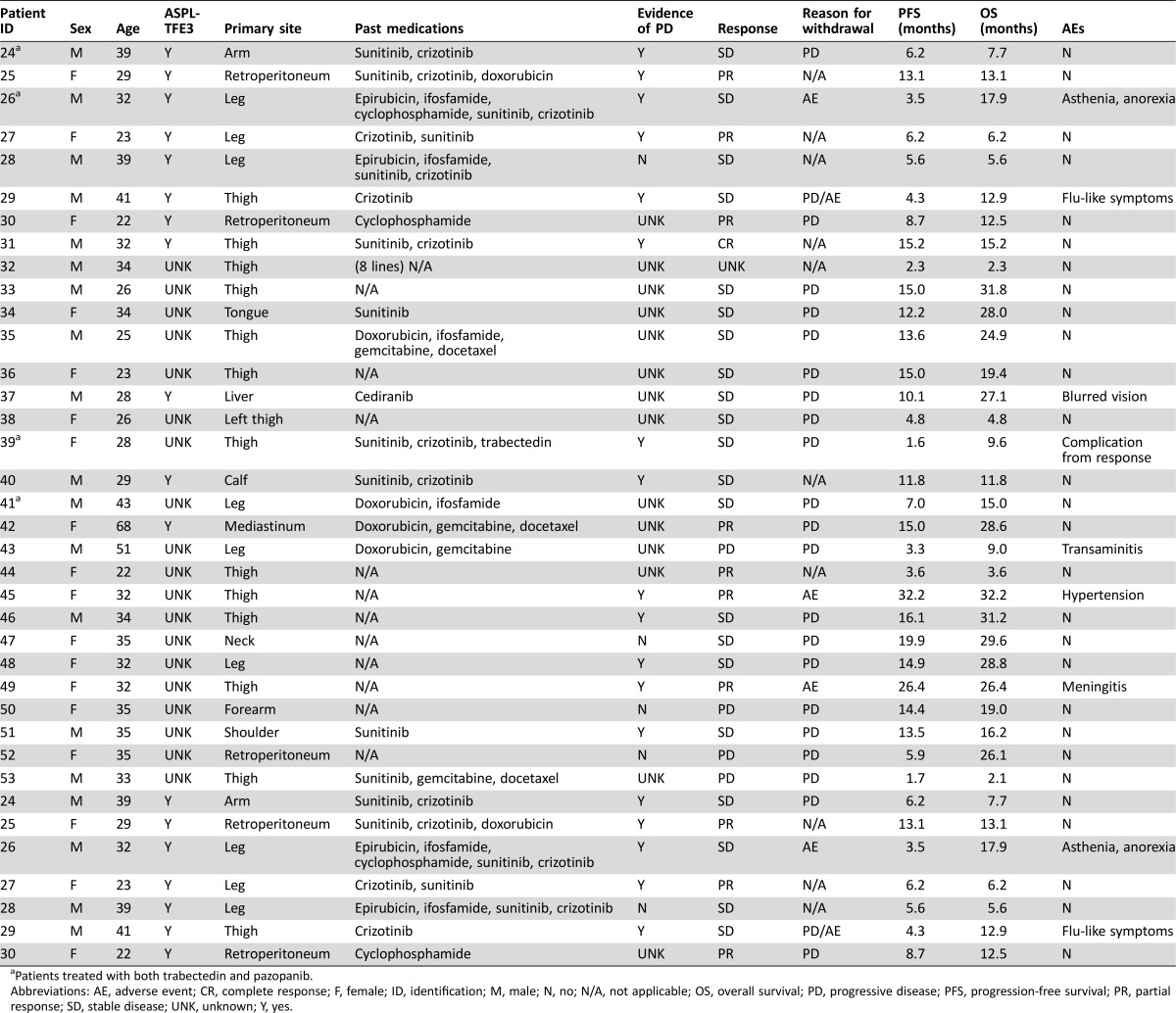
Table 2. Patient characteristics and clinical findings for patients treated with pazopanib (group B).
Patients treated with both trabectedin and pazopanib.
Abbreviations: AE, adverse event; CR, complete response; F, female; ID, identification; M, male; N, no; N/A, not applicable; OS, overall survival; PD, progressive disease; PFS, progression‐free survival; PR, partial response; SD, stable disease; UNK, unknown; Y, yes.
Group A: Trabectedin
All patients completed their treatment (22 progressive; 1 other) and all were evaluable for response. Mean age was 32 (range: 19–48) years. Performance status was ≤ 3 in all cases. All patients, except for one who was treated for a locally advanced tumor, had metastatic disease. Twenty‐one of 23 patients had received at least 1 prior line of medical therapy before starting trabectedin >1 line in 17 patients). The information on the evidence of progression before trabectedin initiation was available in 11 cases, all progressive.
Starting dose of trabectedin was 1.2, 1.3, and 1.5 mg/m2 in 4, 5, and 14 cases, respectively, administered every 3 weeks. Median number of cycles administered was four. Overall, trabectedin toxicity was as expected and was fairly well tolerated [grade (G) ≥ 3 non‐hematologic toxicity 4; G ≥ 3 hematologic toxicity 5]. None of the patients had to definitively stop their treatment due to toxicity. In all patients, toxicity resolved upon holding trabectedin.
Response.
Best RECIST responses with trabectedin were 1 partial response (PR), 13 stable disease (SD), and 9 progressive disease (PD), for an overall response rate (ORR) of 4.3%.
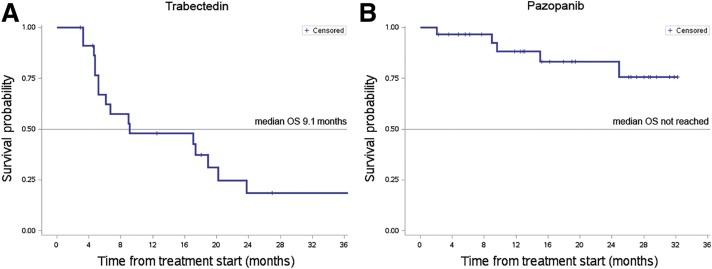
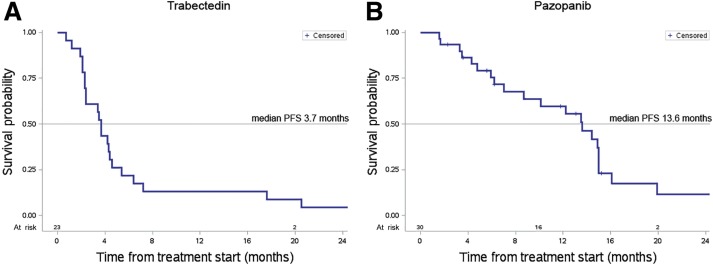
At a 27‐month median follow‐up, the median OS was 9.1 months, with 16 patients dead at the time of the present analysis (Fig. 1A). Median PFS by RECIST was 3.7 months (range: 0.7–109; Fig. 2A), with 13% of patients progression‐free at 12 months.
Figure 1.
Overall survival curve of patients treated with trabectedin and with pazopanib. At a 27‐month median follow‐up, the median OS of patients treated with trabectedin (A) was 9.1 months (range: 2.9–109). At a 19‐month median follow‐up, the median OS of patients treated with pazopanib (B) was not reached (range: 2.1–32.2).
Abbreviation: OS, overall survival.
Figure 2.
Progression‐free survival curve of patients treated with trabectedin and with pazopanib. At a 27‐month median follow‐up, the median PFS by Response Evaluation Criteria in Solid Tumors (RECIST) was 3.7 months (range: 0.7–109) for patients treated with trabectedin (A). In the group of patients treated with pazopanib (B), the median PFS by RECIST was 13.6 months (range: 1.6–32.2) as evaluable at a median follow‐up of 19 months.
Abbreviation: PFS, progression‐free survival.
Group B: Pazopanib
At the time of the present analysis, 23 patients had completed the treatment with pazopanib (19 progression; 4 toxicity), while 7 (23%) were still on therapy. Twenty‐nine of 30 patients were evaluable for response.
Mean age was 33 (range: 22–68) years. Performance status was 0–2 in all patients. All patients had metastatic disease. Twelve patients were treatment‐naïve at the time of starting pazopanib, and 18 were pretreated with at least 1 previous regimen. Thirteen (43%) patients had already received another antiangiogenic drug before starting pazopanib. The evidence of progression before starting pazopanib was documented in 13 out of 17 cases for which this information was available, and the remaining 4 patients were stable.
All patients started pazopanib at the dose of 800 mg/day, except for three patients who received 600 mg/day. Median number of cycles was 12 (range: 2–33). Pazopanib was fairly well tolerated (G ≥ 3 non‐hematologic toxicity: 6; G ≥ 3 hematologic toxicity: 0). Four patients definitively stopped pazopanib due to side effects. In all patients, toxicity resolved upon pazopanib discontinuation.
Response.
Best response to pazopanib by RECIST was: 1 complete response (CR), 7 PR, 17 SD, and 4 PD (Fig. 3), for an ORR of 27%. Notably, three patients responded to pazopanib after failure of another antiangiogenic agent (Fig. 4).
Figure 3.
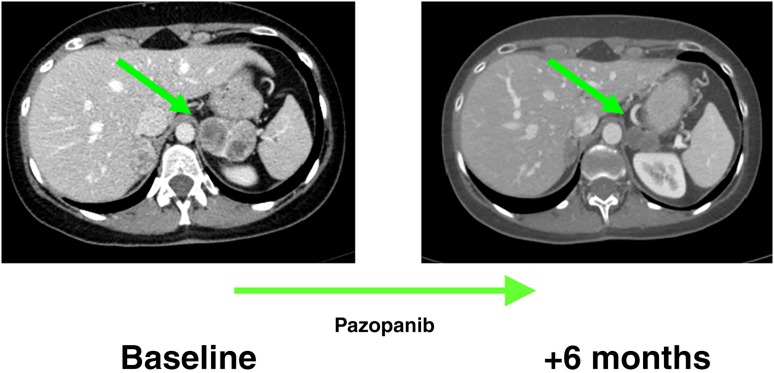
Response to pazopanib. Axial, contrast‐enhanced computed tomography scans in a patient affected by intra‐abdominal metastasis from an alveolar soft part sarcoma of the lower limb, at baseline and after six cycles of treatment with pazopanib, when a Response Evaluation Criteria in Solid Tumors partial response was achieved.
Figure 4.
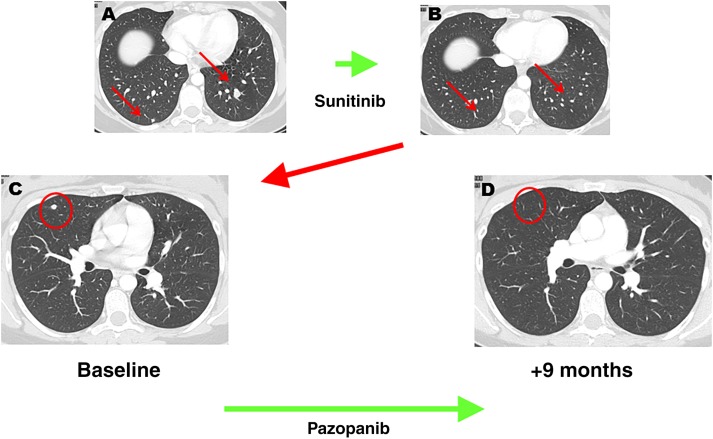
Response to pazopanib after secondary resistance to sunitinib. Axial, contrast‐enhanced computed tomography scans in a patient affected by lung metastases from a retroperitoneal alveolar soft part sarcoma. (A, B): The initial response to sunitinib followed by a secondary progression marked by the appearance of new lung lesions (C, red circle). (D): Afterward, patient was started on pazopanib with a new response.
The median OS was not reached at the time of this analysis (Fig. 1B). At a 19‐month median follow‐up, the median PFS by RECIST was 13.6 months (range: 1.6–32.2+). The PFS at 12 months was 59% (Fig. 2B).
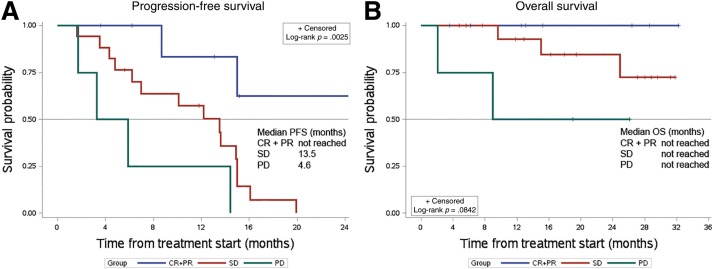
Progression‐free survival analysis stratified by the extent of the response showed a significantly better outcome in patients achieving a RECIST CR and/or PR (8 cases) in comparison with those who had only an SD (17 cases; median PFS: not reached vs. 13.5 months, respectively; median OS: not reached) or a PD (4 cases; median PFS: 4.6 months; median OS: not reached; Fig. 5). A comparison between patients naïve to and pretreated with other antiangiogenics showed a trend towards a better PFS in untreated patients (median PFS: 14.4 vs. 12.2 months, respectively; supplemental online Fig. 1).
Figure 5.
Progression‐free survival and OS analysis stratified by the extent of Response Evaluation Criteria in Solid Tumors (RECIST) response. Patients whose best response was a CR or a PR by RECIST were grouped together (group A, CR/PR patients: 8, blue line in the graph) and compared with those who showed an SD (group B, SD patients: 17, red line) and those who progressed (group C, PD patients: 4, green line). (A): Median PFS (range) was not reached (3.6–32.2), 13.5 months (1.6–19.9), and 4.6 months (1.7–14.4) in group A, B, and C, respectively. (B): Median OS was not reached in all groups, with all patients of group A alive at the time of the present analysis (blue line).
Abbreviations: CR, complete response; OS, overall survival; PD, progressive disease; PFS, progression‐free survival; PR, partial response; SD, stable disease.
Discussion
Our multi‐institutional retrospective study collected the two largest series currently available of patients affected by advanced and/or metastatic ASPS and treated with trabectedin or pazopanib, including 30 and 23 cases, respectively. This study confirms the activity of pazopanib in patients with advanced ASPS, with 27% ORR by RECIST, a median PFS of 13.6 months, and 59% of patients progression‐free at 12 months. Responses to pazopanib were also detectable in 3 of 13 patients with secondary resistance to other antiangiogenics. By contrast, the value of trabectedin in this series looked limited, with only a single RECIST response (ORR 4.3%), a 3.7‐month median PFS, and 13% of patients free from progression at 12 months.
Alveolar soft part sarcoma is an extremely rare disease. Its rarity makes it challenging to conduct prospective clinical trials, as the premature closure of a Korean study on pazopanib due to lack of recruitment recently demonstrated [23]. This confirms that collaborative efforts are of major importance. With all the limitations of a retrospective study, our case‐series analysis provides new information on the activity of two agents that are available for STS in daily clinical practice.
The activity of trabectedin appeared limited, once more underscoring the low sensitivity of ASPS to cytotoxics. Compared with available reports [19], [20], [21], in our series trabectedin did not achieve prolonged disease control in most patients, with only three cases remaining stable while under treatment for > 12 months. Of course, one should consider the high proportion of heavily pretreated patients.
By contrast, our study confirms the role of antiangiogenics in ASPS. Of note, unfortunately, none of the agents with antiangiogenic activity tested in ASPS so far are approved for treatment of the disease in the front line, and pazopanib is the only antiangiogenic drug formally registered for treatment of STS, after failure on anthracycline. The results of a randomized trial on cediranib in ASPS will be available soon and, if positive, will possibly pave the way to the registration of another active drug for this tumor. The antitumor effect of pazopanib in this series was somewhat inferior to what has been reported on sunitinib and cediranib, both in terms of ORR (27% for pazopanib vs. 35%–60% for cediranib and sunitinib) and duration of response (13‐month median PFS in case of pazopanib vs. 15–19 months with sunitinib in different series) [6], [7], [8], [9], [26]. One can provide only tentative explanations. Forty percent of patients in our series had been previously treated with another antiangiogenic agent and this may have limited, at least in part, the effect of pazopanib, although mechanisms of resistance to antiangiogenics in ASPS are unknown and could not be investigated retrospectively in this multi‐institutional series. In fact, a trend towards a better outcome was detected in our study when the PFS of patients pretreated with pazopanib before starting pazopanib was compared with the PFS of those naïve to antiangiogenic agents (median PFS 12.2 vs. 14.4 months). On the other hand, three patients included in our study responded to pazopanib after failure on other antiangiogenic agents. This observation confirms that, at least in some cases, the evidence of resistance to a given antiangiogenic does not prevent the activity of another drug of the same class. Conversely, the different spectrum of targets inhibited by various antiangiogenics could be responsible for a different degree of antitumor effect. In particular, sunitinib has a broader kinase profile compared with pazopanib, which is a “purer” antiangiogenic drug. Sunitinib has activity against platelet derived growth factor receptor (PDGFR)a, PDGFRb, KIT, VEGF receptors 1–3 (VEGFR1–3), fms‐like tyrosine kinase 3 (FLT3), and, notably, macrophage colony‐stimulating factor receptor 1 (MCSFR1) [26], whereas pazopanib is a more selective VEGF‐signaling inhibitor [16]. Overall, the mechanisms underlying the response to sunitinib, cediranib, anlotinib, and pazopanib in ASPS remain under evaluation. The antiangiogenic effect of this class of agents may well target the peculiar vasculature of ASPS [1], sustained by the translocation‐related activation of the lactate pathway, as described by Goodwin et al. [27]. In addition to their antiangiogenic impact, a direct antitumor effect due to the inhibition of VEGFR, PDGFR, and rearranged during transfect (RET) expressed by the tumor cells was described [8]. Finally, Kummar et al. intriguingly showed on tumor biopsies from eight patients treated with cediranib that the antitumor effect of this agent induced the downregulation of genes related to myeloid infiltrate and vasculogenesis [7]. Myeloid infiltration has also been proved by another group, which showed the presence of myeloid cells both along the tumor vessels and interspersed within the tumor cells [28], [29], [30]. The role of tumor‐infiltrating myeloid cells in the formation and maintenance of abnormal blood vessels in tumors is well known [31]. These observations suggest an immunomodulatory role of this class of drugs in addition to the antiangiogenic and direct antitumor effect that could be enhanced when agents with a greater activity against MCSFR1, such as sunitinib, are administered. These data also suggest that immunomodulation could play a role in this sarcoma type, and that combination of immunotherapeutic agents such as immune checkpoint inhibitors and antiangiogenic agents may be worth exploring, as is being done in ongoing clinical trials.
Conclusion
These data confirm that pazopanib is active in patients with advanced ASPS, whereas the value of trabectedin seems to be limited. Collaborative efforts are of major importance in improving the management of people with very rare sarcoma subtypes. In an extremely rare disease where cytotoxics are clearly ineffective, we believe that antiangiogenics should be available for clinical use as first‐line medical options.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
Presented at the 21st Connective Tissue Oncology Society (CTOS) annual meeting, Lisbon, Portugal, November 2016, abs 2569540.
Author Contributions
Conception/design: Silvia Stacchiotti, Axel Le Cesne, Robert G. Maki, Shreyaskumar Patel, Jean Y. Blay, Hans Gelderblom, Javier Martin‐Broto, Piotr Rutkowski, Paolo G. Casali, Akira Kawai
Provision of study material or patients: Silvia Stacchiotti, Olivier Mir, Axel Le Cesne, Bruno Vincenzi, Alexander Fedenko, Robert G. Maki, Neeta Somaiah, Shreyaskumar Patel, Mehedi Brahmi, Jean Y. Blay, Kjetil Boye, Kirsten Sundby Hall, Hans Gelderblom, Nadia Hindi, Javier Martin‐Broto, Hanna Kosela, Piotr Rutkowski, Antoine Italiano, Florence Duffaud, Eisuke Kobayashi, Paolo G. Casali, Salvatore Provenzano, Akira Kawai
Collection and/or assembly of data: Silvia Stacchiotti, Olivier Mir, Axel Le Cesne, Bruno Vincenzi, Alexander Fedenko, Robert G. Maki, Neeta Somaiah, Shreyaskumar Patel, Mehedi Brahmi, Jean Y. Blay, Kjetil Boye, Kirsten Sundby Hall, Hans Gelderblom, Nadia Hindi, Javier Martin‐Broto, Hanna Kosela, Piotr Rutkowski, Antoine Italiano, Florence Duffaud, Eisuke Kobayashi, Paolo G. Casali, Salvatore Provenzano, Akira Kawai
Data analysis and interpretation: Silvia Stacchiotti, Olivier Mir, Axel Le Cesne, Bruno Vincenzi, Alexander Fedenko, Robert G. Maki, Neeta Somaiah, Shreyaskumar Patel, Mehedi Brahmi, Jean Y. Blay, Kjetil Boye, Kirsten Sundby Hall, Hans Gelderblom, Nadia Hindi, Javier Martin‐Broto, Hanna Kosela, Piotr Rutkowski, Antoine Italiano, Florence Duffaud, Eisuke Kobayashi, Paolo G. Casali, Salvatore Provenzano, Akira Kawai
Manuscript writing: Silvia Stacchiotti, Olivier Mir, Axel Le Cesne, Bruno Vincenzi, Alexander Fedenko, Robert G. Maki, Neeta Somaiah, Shreyaskumar Patel, Mehedi Brahmi, Jean Y. Blay, Kjetil Boye, Kirsten Sundby Hall, Hans Gelderblom, Nadia Hindi, Javier Martin‐Broto, Hanna Kosela, Piotr Rutkowski, Antoine Italiano, Florence Duffaud, Eisuke Kobayashi, Paolo G. Casali, Salvatore Provenzano, Akira Kawai
Final approval of manuscript: Silvia Stacchiotti, Olivier Mir, Axel Le Cesne, Bruno Vincenzi, Alexander Fedenko, Robert G. Maki, Neeta Somaiah, Shreyaskumar Patel, Mehedi Brahmi, Jean Y. Blay, Kjetil Boye, Kirsten Sundby Hall, Hans Gelderblom, Nadia Hindi, Javier Martin‐Broto, Hanna Kosela, Piotr Rutkowski, Antoine Italiano, Florence Duffaud, Eisuke Kobayashi, Paolo G. Casali, Salvatore Provenzano, Akira Kawai
Disclosures
Silvia Stacchiotti: PharmaMar (C/A), GlaxoSmithKline, Novartis, Pfizer (RF); Olivier Mir: Amgen, AstraZeneca, Bayer, Bristol‐Meyer Squibb, Lilly, Novartis, Pfizer, Roche (C/A); Axel Le Cesne: Novartis, GlaxoSmithKline, Pfizer, PharmaMar (C/A); Bruno Vincenzi: PharmaMar, GlaxoSmithKline, Novartis (SAB); Alexander Fedenko: GlaxoSmithKline, Johnson & Johnson, Novartis (C/A); Robert G. Maki: PharmaMar, Novartis (C/A); Jean Y. Blay: GlaxoSmithKline, PharmaMar (H, RF); Hans Gelderblom: PharmaMar, Novartis (C/A, RF); Neeta Hindi: PharmaMar, Novartis (travel support); Javier Martin‐Broto: PharmaMar, Novartis, Eisai (C/A, RF), Lilly, Amgen (C/A); Piotr Rutkowski: Novartis (C/A), GlaxoSmithKline, Pfizer (H); Florence Duffaud: Novartis, Pfizer, PharmaMar (C/A); Paolo G. Casali: Bayer, Eisai, Eli Lilly, Novartis, PharmaMar (C/A, RF), Blueprint Medicines, Deciphera Pharmaceuticals, Nektar Therapeutics, Pfizer (C/A), Amgen Dompé, Arog Pharmaceuticals, Daiichi Sankyo Pharma, Epizyme (RF); Akira Kawai: GlaxoSmithKline, Novartis, Taiho Pharmaceutical (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Fletcher CDM, Bridge JA, Hogendoorn PCW et al. World Health Organization (WHO) Classification of Tumours of Soft tissue and Bone. Pathology and Genetics. Lyon, France: IARC Press, 2013:80–82. [Google Scholar]
- 2. Ladanyi M, Lui MY, Antonescu CR et al. The der(17)t(X;17)(p11;q25) of human alveolar soft part sarcoma fuses the TFE3 transcription factor gene to ASPL, a novel gene at 17q25. Oncogene 2001;20:48–57. [DOI] [PubMed] [Google Scholar]
- 3. Pennacchioli E, Fiore M, Collini P et al. Alveolar soft part sarcoma: Clinical presentation, treatment, and outcome in a series of 33 patients at a single institution. Ann Surg Oncol 2010;17:3229–3233. [DOI] [PubMed] [Google Scholar]
- 4. Ogose A, Yazawa Y, Ueda T et al. Alveolar soft part sarcoma in Japan: Multi‐institutional study of 57 patients from the Japanese Musculoskeletal Oncology Group. Oncology 2003;65:7–13. [DOI] [PubMed] [Google Scholar]
- 5. Reichardt P, Lindner T, Pink D et al. Chemotherapy in alveolar soft part sarcomas. What do we know? Eur J Cancer 2003;39:1511–1516. [DOI] [PubMed] [Google Scholar]
- 6. Stacchiotti S, Tamborini E, Marrari A et al. Response to sunitinib malate in advanced alveolar soft part sarcoma. Clin Cancer Res 2009;15:1096–1104. [DOI] [PubMed] [Google Scholar]
- 7. Kummar S, Allen D, Monks A et al. Cediranib for metastatic alveolar soft part sarcoma. J Clin Oncol 2013;31:2296–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stacchiotti S, Negri T, Zaffaroni N et al. Sunitinib in advanced alveolar soft part sarcoma: Evidence of a direct antitumor effect. Ann Oncol 2011;22:1682–1690. [DOI] [PubMed] [Google Scholar]
- 9. Judson I, Scurr M, Gardner K et al. Phase II study of cediranib in patients with advanced gastrointestinal stromal tumors or soft‐tissue sarcoma. Clin Cancer Res 2014;20:3603–3612. [DOI] [PubMed] [Google Scholar]
- 10. Sun Y, Niu W, Du F et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi‐target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol 2016;9:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Larsen AK, Galmarini CM, D'Incalci M. Unique features of trabectedin mechanism of action. Cancer Chemother Pharmacol 2016;77:663–671. [DOI] [PubMed] [Google Scholar]
- 12. Germano G, Frapolli R, Belgiovine C et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell 2013;23:249–262. [DOI] [PubMed] [Google Scholar]
- 13. Allavena P, Germano G, Belgiovine C et al. Trabectedin: A drug from the sea that strikes tumor‐associated macrophages. Oncoimmunology 2013;2:e24614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Demetri GD, Chawla SP, von Mehren M et al. Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcoma after failure of prior anthracyclines and ifosfamide: Results of a randomized phase II study of two different schedules. J Clin Oncol 2009;27:4188–4196. [DOI] [PubMed] [Google Scholar]
- 15. Demetri GD, von Mehren M, Jones RL et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: Results of a phase III randomized multicenter clinical trial. J Clin Oncol 2016;34:786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schutz FA, Choueiri TK, Sternberg CN. Pazopanib: Clinical development of a potent anti‐angiogenic drug. Crit Rev Oncol Hematol 2011;77:163–171. [DOI] [PubMed] [Google Scholar]
- 17. Sleijfer S, Ray‐Coquard I, Papai Z et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: A phase II study from the European organisation for research and treatment of cancer‐soft tissue and bone sarcoma group (EORTC study 62043). J Clin Oncol 2009;27:3126–3132. [DOI] [PubMed] [Google Scholar]
- 18. Van der Graaf WT, Blay JY, Chawla SP et al. Pazopanib for metastatic soft‐tissue sarcoma (PALETTE): A randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet 2012;379:1879–1886. [DOI] [PubMed] [Google Scholar]
- 19. Pink D, Bertz‐Lepel J, Busemann C et al. Efficacy of trabectedin in patients with advanced or metastatic alveolar soft‐part sarcoma. Onkologie 2012;35:249–252. [DOI] [PubMed] [Google Scholar]
- 20. Le Cesne A, Cresta S, Maki RG et al. A retrospective analysis of antitumour activity with trabectedin in translocation‐related sarcomas. Eur J Cancer 2012;48:3036–3044 [DOI] [PubMed] [Google Scholar]
- 21. Kawai A, Araki N, Sugiura H et al. Trabectedin monotherapy after standard chemotherapy versus best supportive care in patients with advanced, translocation‐related sarcoma: A randomised, open‐label, phase 2 study. Lancet Oncol 2015;16:406–416. [DOI] [PubMed] [Google Scholar]
- 22. Nakamura T, Matsumine A, Kawai A et al. The clinical outcome of pazopanib treatment in Japanese patients with relapsed soft tissue sarcoma: A Japanese Musculoskeletal Oncology Group (JMOG) study. Cancer 2016;122:1408–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim M, Kim TM, Keam B et al. A phase II trial of pazopanib in patients with metastatic alveolar soft part sarcoma. Ann Oncol 2016;27:483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 25. Switaj T, Kozak K, Kosela‐Paterczyk H. Long‐term results of therapy with sunitinib in metastatic alveolar soft part sarcoma (ASPS) patients. Paper presented at: XXI Connective Tissue Oncology Society (CTOS) annual meeting; November 2016; Lisbon, Portugal; 019a.
- 26. Chow LQ, Eckhardt SG. Sunitinib: From rational design to clinical efficacy. J Clin Oncol 2007;25:884–896. [DOI] [PubMed] [Google Scholar]
- 27. Goodwin ML, Jin H, Straessler K et al. Modeling alveolar soft part sarcomagenesis in the mouse: A role for lactate in the tumor microenvironment. Cancer Cell 2014;26:851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Castelli C, Tazzari M, Negri T et al. Structured myeloid cells and anti‐angiogenic therapy in alveolar soft part sarcoma. J Transl Med 2013;11:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stacchiotti S, Tazzari M, Negri T et al. Alveolar soft part sarcoma displays a structurally organized infiltrating myeloid cells. Paper presented at: XXI Connective Tissue Oncology Society (CTOS) annual meeting; November 2016; Lisbon, Portugal; 2570389a.
- 30.Hindi, Martinez‐Tryfero J, Carranza A. Exploratory analysis of immunomodulation and apoptosis factors in alveolar soft part sarcoma: A retrospective study from the Spanish group for research on sarcoma (GEIS). XXI Connective Tissue Oncology Society (CTOS) annual meeting; November 2016; Lisbon, Portugal; 2570689a.
- 31. Bolli E, Movahedi K, Laoui D et al. Novel insights in the regulation and function of macrophages in the tumor microenvironment. Curr Opin Oncol 2017;29:55–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.