This article compares the efficacy between regorafenib and trifluridine/tipiracil in patients with metastatic colorectal cancer refractory to standard chemotherapy, who had access to both drugs, to determine whether a further prospective comparative trial should be conducted.
Keywords: Colorectal cancer, Propensity score, Regorafenib, TAS‐102, Trifluridine/tipiracil
Abstract
Background.
This study compared the efficacy of regorafenib and trifluridine/tipiracil (TFTD) in patients with metastatic colorectal cancer (mCRC) who are refractory to standard chemotherapy, because despite their clinical approval, it still remains unclear which of these two drugs should be used as initial treatment.
Materials and Methods.
The clinical data of patients with mCRC who were treated with regorafenib or TFTD and those of drug‐naive patients, between June 2014 and September 2015, were retrospectively collected from 24 institutions in Japan. Overall survival (OS) was evaluated using the Cox's proportional hazard models based on propensity score adjustment for baseline characteristics.
Results.
A total of 550 patients (223 patients in the regorafenib group and 327 patients in the TFTD group) met all criteria. The median OS was 7.9 months (95% confidence interval [CI], 6.8–9.2) in the regorafenib group and 7.4 months (95% CI, 6.6–8.3) in the TFTD group. The propensity score adjusted analysis showed that OS was similar between the two groups (adjusted hazard ratio [HR], 0.96; 95% CI, 0.78–1.18). In the subgroup analysis, a significant interaction with age was observed. Regorafenib showed favorable survival in patients aged <65 years (HR, 1.29; 95% CI, 0.98–1.69), whereas TFTD was favored in patients aged ≥65 years (HR, 0.78; 95% CI, 0.59–1.03).
Conclusion.
No significant difference in OS between regorafenib and TFTD was observed in patients with mCRC. Although the choice of the drug by age might affect survival, a clearly predictive biomarker to distinguish the two drugs should be identified in further studies.
Implications for Practice.
Previous studies of patients with metastatic colorectal cancer refractory to standard chemotherapy had demonstrated that both regorafenib and trifluridine/tipiracil could result in increased overall survival compared with placebo, but there are no head‐to‐head trials. This large, multicenter, observational study retrospectively compared the efficacy of regorafenib and trifluridine/tipiracil in 550 patients with metastatic colorectal cancer refractory to standard chemotherapy who had access to both drugs. Although no difference in overall survival was found between the two drugs in adjusted analysis using propensity score, regorafenib showed favorable survival in patients aged <65 years, whereas trifluridine/tipiracil was favored in patients aged ≥65 years in the subgroup analysis.
摘要
背景.本研究比较瑞戈非尼与曲氟尿苷/Tipiracil(TFTD)治疗接受标准化疗无效的转移性结直肠癌(mCRC)患者的疗效, 尽管这两种药物已经获批准用于临床用途, 但尚不清楚应将哪种药物用于初始治疗。
材料与方法.从日本的24家机构回顾性地收集了2014年6月至2015年9月间接受瑞戈非尼或TFTD治疗的mCRC患者和未接受药物治疗患者的临床数据。对倾向评分进行基线特征校正, 使用Cox比例风险模型, 评价总生存期(OS)。
结果.共550例患者(瑞戈非尼组223例, TFTD组327例)满足所有标准。瑞戈非尼组与TFTD组的中位OS分别为7.9个月[95%置信区间(CI)为6.8‐9.2]和7.4个月(95% CI, 6.6‐8.3)。校正后的倾向评分分析表明, 两组间的OS相似[校正后的风险比(HR)为0.96, 95% CI, 0.78–1.18]。在亚组分析中观察到与年龄间有显著相互作用。瑞戈非尼显示在年龄<65岁患者中的生存率更高(HR, 1.29;95%CI, 0.98–1.69), 而TFTD显示年龄≥65岁患者中的生存率更高(HR, 0.78;95%CI, 0.59–1.03)。
结论.瑞戈非尼组与TFTD组mCRC患者间的OS没有显著差异。按年龄选择药物可能影响生存率, 在将来的研究中应确定区分这两种药物的明确预测性生物标志物。
对临床实践的启示:关于接受标准化疗无效的转移性结直肠癌患者的既往研究证实, 与安慰剂相比, 瑞戈非尼和曲氟尿苷/Tipiracil均可提高总生存率, 但尚未开展头对头试验。本项大型、多中心、观察性研究回顾性地比较了瑞戈非尼与曲氟尿苷/Tipiracil治疗550例接受标准化疗无效的转移性结直肠癌(mCRC)患者的疗效, 这些患者均可以获得这两种药物。虽然在校正倾向评分的分析中未发现这两种药物的总生存率存在差异, 但亚组分析中瑞戈非尼组显示年龄<65岁的患者的生存率更高, 而曲氟尿苷/Tipiracil组显示年龄≥65岁的患者的生存率更高。
Introduction
The development of novel drugs for metastatic colorectal cancer (mCRC) has progressed, and the median overall survival (OS) from first‐line chemotherapy has reached 30 months [1], [2], [3]. Advances in later‐line chemotherapy, as well as upfront chemotherapies with oxaliplatin‐containing and irinotecan‐containing regimens in combination with angiogenesis inhibitors or anti‐epidermal growth factor receptor (anti‐EGFR) antibody in patients with wild‐type RAS, have significantly contributed to the improvement of the OS duration [4].
Survival benefits of salvage chemotherapy have been demonstrated by both regorafenib and trifluridine/tipiracil (TFTD) treatments. Regorafenib, which is a multimolecular targeted drug inhibiting angiogenesis and apoptosis [5], has shown to improve OS compared with placebo in patients with mCRC refractory to standard chemotherapy in a randomized phase III trial (CORRECT) [6]. The median OS was 6.4 months in the regorafenib group and 5.0 months in the placebo group (hazard ratio [HR] 0.77; 95% confidence interval [CI], 0.64–0.94; p = .0052). The improvement of OS after treatment with TFTD, a thymidine‐based nucleic acid analogue and tipiracil hydrochloride [7], compared with placebo, has been confirmed in a global randomized phase III trial (RECOURSE) including patients with mCRC refractory to standard chemotherapy [8]. The median OS was 7.1 months in the TFTD group and 5.3 months in the placebo group (HR 0.68; 95% CI, 0.58–0.81; p < .001). Based on the results of these pivotal trials, the usage of regorafenib and TFTD was approved in Japan in March 2013 and 2014, respectively. Although the eligible patients can receive both drugs individually, it remains unclear which drug should be used first because of a lack of head‐to‐head randomized trials.
The aim of this study was to compare the efficacy between regorafenib and TFTD in patients with mCRC refractory to standard chemotherapy, who had access to both drugs, to determine whether a further prospective comparative trial should be conducted.
Materials and Methods
Patient Population
This study was registered with the University Hospital Medical Information Network (number UMIN000020416). With approval from the Ethics Committee of each participating institution, we retrospectively collected the clinical data of patients with mCRC who received either regorafenib or TFTD between June 2014 and November 2015. The requirement for informed consent was waived because of the retrospective design of this study. The patients’ follow‐up was until September 2016.
Main eligibility criteria were as follows: (a) histologically confirmed colorectal adenocarcinoma, (b) no prior treatment with regorafenib and TFTD, (c) previous treatment with fluoropyrimidine, oxaliplatin, irinotecan, bevacizumab, and anti‐EGFR antibody (if the patients had tumor with wild‐type KRAS/NRAS), (d) Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–2, and (f) adequate organ function. After clinical data collection and blinded assessment, we excluded patients who could receive only a specific drug treatment, either regorafenib or TFTD, because of comorbidity and/or medical history.
Endpoints and Statistical Analysis
The primary endpoint was OS, defined as the time from the start of study treatment to death from any cause. Secondary endpoints included best response rate and disease control rate according to the Response Evaluation Criteria in Solid Tumors version 1.1; progression‐free survival (PFS), defined as the time from the start of study treatment to disease progression or death from any cause; time to treatment failure (TTF), defined as the time from the start of study treatment to the termination from any cause or disease progression; time to ECOG PS ≥2, defined as the time from the start of study treatment to decision of an ECOG PS ≥2; and safety according to the Common Terminology Criteria for Adverse Events version 4.0.
The primary analysis was performed using the Cox's proportional hazard model including treatment group and propensity score for all patients (the observational dataset). A 1:1 matching using the propensity score (propensity score‐matched dataset) was performed as a sensitivity analysis. Patients in the two groups were matched by a difference of propensity score within 0.05. Propensity score was calculated with a multivariable logistic regression model including 20 prognostic variables (supplemental online Table 1). The predictive factor for OS was explored using subgroup analyses and interaction tests. The clinical outcomes, including OS, PFS, TTF, and time to ECOG PS ≥2, were evaluated using the Kaplan‐Meier method. Continuous and categorical variables were presented as median (interquartile range: 25%–75%) and number (proportion) of patients, respectively. Statistical tests were two‐sided with 5% significant level. All analyses were performed using the SAS software version 9.4 (SAS Institute, Cary, NC, https://www.sas.com/en_us/home.html).
Results
Patients
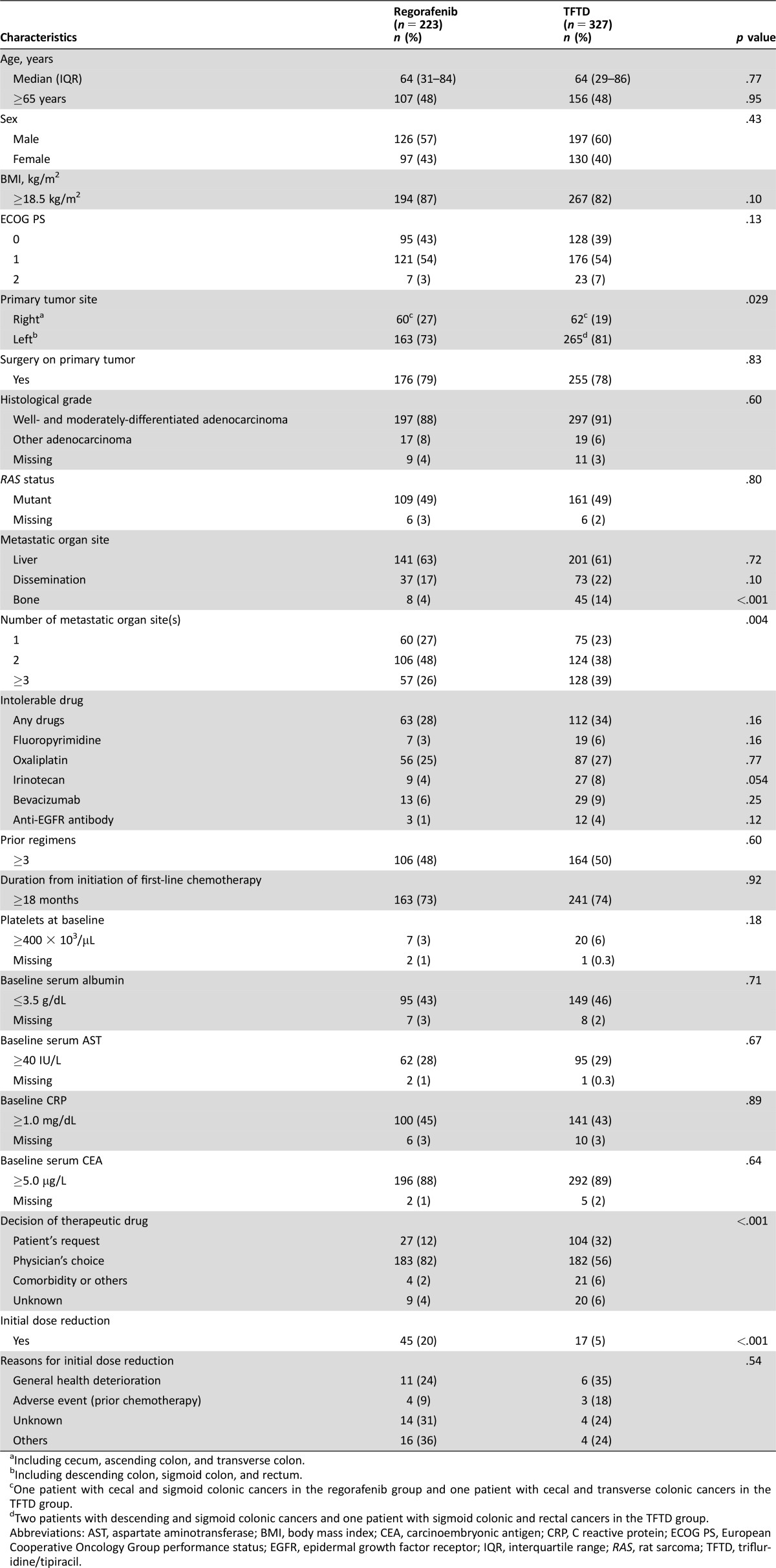
The number of patients who met all criteria for inclusion in the analysis was 550, including 223 patients in the regorafenib group and 327 patients in the TFTD group (Fig. 1). Thirty‐four patients among excluded patients had comorbidity or medical history, such as thrombosis, hemorrhage, and cardiac events. Several characteristics, including primary tumor site, bone metastasis, number of metastatic organ sites, and initial dose reduction, were imbalanced between the two groups (Table 1). Regarding the decision of the therapeutic drug, the physician's choice was more frequent in the regorafenib group, whereas the patient's request was more frequent in the TFTD group (p < .001). The rate of initial dose reduction was higher in the regorafenib group than in the TFTD group (20% vs. 5%; p < .001). The median follow‐up time was 17.6 months in the regorafenib group and 17.3 months in the TFTD group.
Figure 1.
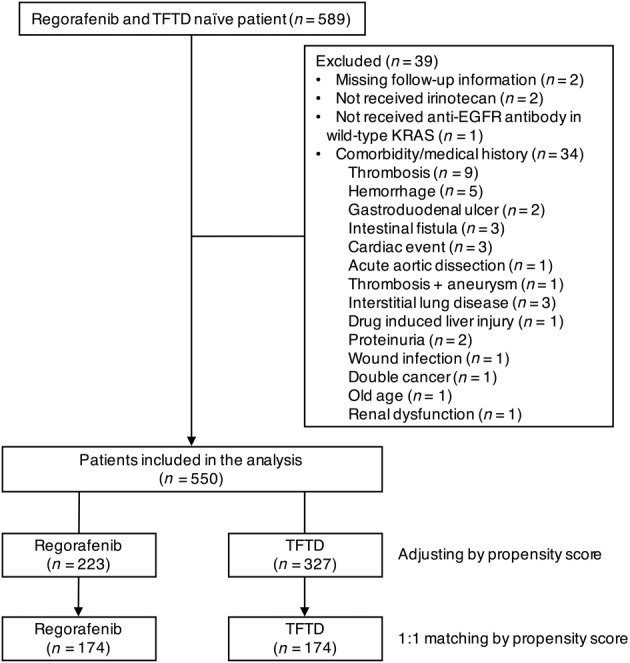
Patient selection flow diagram.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; KRAS, Kirsten rat sarcoma; TFTD, trifluridine/tipiracil.
Table 1. Comparison of patients’ characteristics between regorafenib and TFTD groups in the observational dataset.
Including cecum, ascending colon, and transverse colon.
Including descending colon, sigmoid colon, and rectum.
One patient with cecal and sigmoid colonic cancers in the regorafenib group and one patient with cecal and transverse colonic cancers in the TFTD group.
Two patients with descending and sigmoid colonic cancers and one patient with sigmoid colonic and rectal cancers in the TFTD group.
Abbreviations: AST, aspartate aminotransferase; BMI, body mass index; CEA, carcinoembryonic antigen; CRP, C reactive protein; ECOG PS, European Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; IQR, interquartile range; RAS, rat sarcoma; TFTD, trifluridine/tipiracil.
Efficacy
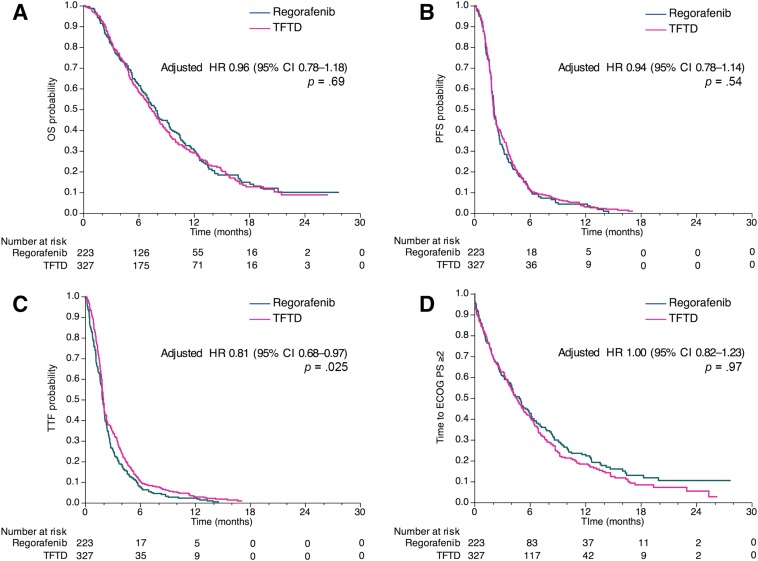
Events of death were observed in 171 patients (77%) in the regorafenib group and 247 patients (76%) in the TFTD group. The median OS was 7.9 months (95% CI, 6.8–9.2) in the regorafenib group and 7.4 months (95% CI, 6.6–8.3) in the TFTD group (Fig. 2A and supplemental online Table 2). There was no significant difference between the two groups (unadjusted HR of TFTD to regorafenib, 1.03; 95% CI, 0.85–1.26; p = .75). In the propensity score adjusted analysis for OS, similar results were observed between the two groups (adjusted HR, 0.96; 95% CI, 0.78–1.18; p = .69). Moreover, the PFS and time to ECOG PS ≥2 were similar between the two groups (adjusted HR, 0.94 and 1.00, respectively), although the TTF was longer in the TFTD group than in the regorafenib group (adjusted HR, 0.81; 95% CI, 0.68–0.97; p = .025; Fig. 1B–1D and supplemental online Table 2). Among patients with target lesions (212 patients in the regorafenib group and 307 patients in the TFTD group), no complete responses were observed and partial response was found in 3 patients (1%) who received TFTD. Lastly, the disease control rate was similar between the two groups (32.1% in the regorafenib group vs. 29.6% in the TFTD group, p = .56).
Figure 2.
OS, PFS, TTF, and time to ECOG PS ≥2 for patients who were treated with regorafenib versus TFTD in the observational dataset. Kaplan‐Meier curves for OS (A), PFS (B), TTF (C), and time to ECOG PS ≥2 (D). Adjusted HRs were calculated using the propensity score.
Abbreviations: CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; OS, overall survival; PFS, progression‐free survival; TFTD, trifluridine/tipiracil; TTF, time to treatment failure.
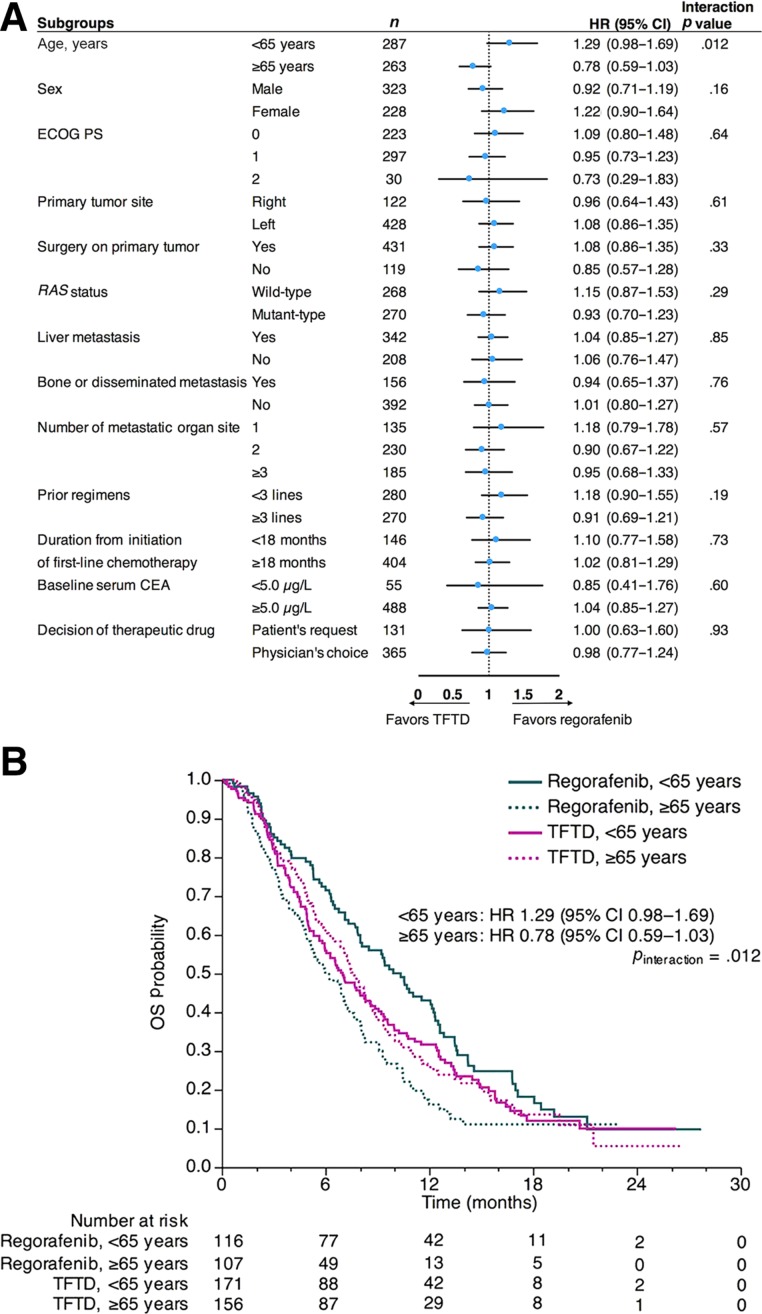
Subgroup Analyses
In the observational dataset, statistical significance was observed only between the interaction of treatment and patient's age (p value for interaction = .012; Fig. 3A). Specifically, regorafenib showed favorable survival in patients aged <65 years (HR, 1.29; 95% CI, 0.98–1.69), whereas TFTD was favored in patients aged ≥65 years (HR, 0.78; 95% CI, 0.59–1.03; Fig. 3B). The median OS in the patients aged <65 years and patients aged ≥65 years was 10.4 months (95% CI, 8.0–12.3) and 6.2 months (95%, CI 4.9–7.4) among the regorafenib group, respectively. The median OS in those patients was 7.0 months (95% CI, 5.8–8.6) and 7.7 months (95% CI, 6.5–8.6) among the TFTD group, respectively.
Figure 3.
Subgroup analyses of OS in the observational dataset. Forest plots with HRs for overall survival (A). Kaplan‐Meier curves for OS according to age <65 years and ≥65 years (B).
Abbreviations: CEA, carcinoembryonic antigen; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; OS, overall survival; RAS, rat sarcoma; TFTD, trifluridine/tipiracil.
Safety and Toxicity
Incidence of grade 3 or more hematologic toxicities was higher in the TFTD group than in the regorafenib group (39% vs. 13%; p < .001), particularly the incidence of neutropenia (33% vs. 3%; p < .001; Table 2). In contrast, incidence of grade 3 or more nonhematologic toxicities was higher in the regorafenib group than in the TFTD group (47% vs. 13%: p < .001), particularly the incidence of hand‐foot skin reaction (20% vs. 0%; p < .001). Liver dysfunction was observed in 12% of patients in the regorafenib group, and one of them died because of liver failure. Treatment‐related death was observed in four patients (2%) of the regorafenib group and two patients (1%) of the TFTD group.
Table 2. Comparison of the frequency of treatment‐related grade ≥3 adverse events in ≥3% of patients in the observational dataset.

Including AST increase, ALT increase, total‐bilirubin increase, and ALP increase.
Including erythema multiforme and Stevens‐Johnson syndrome.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate aminotransferase; TFTD, trifluridine/tipiracil.
Discontinuation of Study Treatment and Post‐Treatment
Discontinuation of study treatment because of treatment‐related toxicities was higher in the regorafenib group than in the TFTD group (24% vs. 7%; p < .001), whereas the proportion of patients who had ECOG PS ≥2 at discontinuation was similar between the two groups (p = .93; Table 3). The crossover rate was higher in the regorafenib group than in the TFTD group (60% vs. 40%; p < .001). The proportion of patients who were treated with any other chemotherapies except regorafenib and TFTD was higher in the TFTD group than in the regorafenib group (12% vs. 5%; p = .004). In a post hoc analysis, the median OS in patients who received the two drugs was 10.5 months (95% CI, 9.2–12.2) in the regorafenib group and 9.4 months (95% CI, 8.3–10.7) in the TFTD group (p = .53).
Table 3. Comparison of discontinuation of study treatment and post‐study treatment outcomes between regorafenib and TFTD in the observational dataset.

Crossover includes patients who were treated with both regorafenib and TFTD in any lines.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; NE, not evaluated; TFTD, trifluridine/tipiracil.
Sensitivity Analysis
One hundred seventy‐four patients in each group were matched by propensity score. Patients’ characteristics were well‐balanced between the two groups, except the initial dose reduction (p < .0001; supplemental online Table 3), and no significant difference in OS was observed between the two groups (adjusted HR, 1.02; 95% CI, 0.81–1.30; p = .85; supplemental online Fig. 1A and supplemental online Table 4). Progression‐free survival, TTF, and time to ECOG PS ≥2 were also similar to those in the observational dataset (supplemental online Fig. 1B–1D and supplemental online Table 3). The HRs for PFS, TTF, and time to ECOG PS ≥2 were 0.92 (p = .47), 0.80 (p = .036), and 1.02 (p = .85), respectively. In the subgroup analysis, HRs by age were similar to those in the observational dataset, although they were not statistically significant (p value for interaction = .18; supplemental online Fig. 2 and supplemental online Table 5). Incidence of grade 3 or more toxicities and the details of discontinuation of study treatment and post‐treatment were also similar to those in the observational dataset (supplemental online Tables 6 and 7). In a post hoc analysis, the median OS in patients who received the two drugs was 10.8 months (95% CI, 9.3–12.6) in the regorafenib group and 9.5 months (95% CI, 9.2–12.1) in the TFTD group (p = .53).
Discussion
We demonstrated that regorafenib and TFTD have similar efficacy in patients with mCRC refractory to standard chemotherapy using propensity score analysis. These drugs have been approved for clinical use in the U.S., Europe, and Japan. However, limited data comparing the efficacy and safety of regorafenib and TFTD are available in patients with mCRC refractory to standard chemotherapy [9], [10]. We performed a large observational study to determine the necessity of randomized trials in comparing the efficiency of two regimens; similar OS between regorafenib and TFTD has been observed in a cross‐trial comparison [6], [8].
To reduce the bias for a retrospective study, we used propensity score analysis. Nevertheless, no significant differences in OS between the two groups were observed, either in the propensity score‐adjustment or propensity score‐matching analyses (HR, 0.96 and 1.02, respectively). The HR for OS was consistently close to 1.00 in all analyses. Although there were no significant differences in the PFS and tumor response between the two groups, the TTF was shorter in the regorafenib group than in the TFTD group because the termination by treatment‐related toxicities was more frequent in the regorafenib group. In fact, incidence of grade 3 or more nonhematologic toxicities associated with regorafenib was consistent with the results of the CORRECT trial [6]. We adopted time to ECOG PS ≥2 as a surrogate of quality of life assessment because regorafenib‐related toxicities might be associated with decreased quality of life. However, time to ECOG PS ≥2 was similar between the two groups. In addition, similar proportion of patients with ECOG PS ≥2 at the study treatment discontinuation was observed between the groups. These results suggest that regorafenib‐related toxicities did not affect progression of their conditions during treatment and at the discontinuation.
The efficacy outcomes of the two drugs reproduced the results of previous respective pivotal trials, despite the real‐world setting of this study, because the participants had access to both drugs [6], [8]. In fact, the proportion of patients who received subsequent chemotherapies after regorafenib or TFTD was higher than that in the pivotal trials (65% vs. 26% in the CORRECT trial and 50% vs. 42% in the RECOURSE trial). However, one fourth of those patients, who were treated with TFTD, received any other chemotherapy except regorafenib. The majority of the subsequent chemotherapies were oxaliplatin‐containing or anti‐EGFR antibody‐containing regimens (data not shown), which have been conducted in previous phase II trials; these regimens were used following a rechallenge strategy [11], [12]. The efficacy of regorafenib after failure of TFTD or vice versa is uncertain and should be determined in a future trial.
In the subgroup analysis for OS, including propensity score‐adjustment and propensity score‐matching analysis, regorafenib was a favorable trend of OS in the younger patients, whereas TFTD was in the elderly patients. The reasons for this difference are unclear, but similar trends were observed in the subgroup analysis of the pivotal trials. Although the OS was significantly longer in the regorafenib group patients than in the placebo group patients among those aged <65 years (HR, 0.72), no significant difference was observed in the OS between the two groups among patients aged ≥65 years (HR, 0.86) [13]. In contrast, the OS was significantly longer in the TFTD group than in the placebo group, both among patients aged <65 years (HR, 0.74) and those aged ≥65 years (HR, 0.62) [14]. In our study, the difference in OS seemed to be higher in the regorafenib group than in the TFTD group. It might be that regorafenib tolerance decreased in elderly patients compared with younger patients, whereas TFTD tolerance was similar between the two age groups examined. These results are consistent with clinical impression; however, they should be confirmed in a prospective trial because the subgroup analysis has a bias.
This study has several limitations. Firstly, as a retrospective observational study, it is characterized by bias. To reduce it, patients were enrolled after the two drugs were approved in Japan, and an adjusted analysis using propensity score was established for the patients without comorbidity and/or medical history who had to receive a specific drug treatment. Secondly, all patients who were enrolled in our study were Japanese. However, no ethnic differences between Japanese and Western patients were observed in either of the pivotal trials [6], [8], [15]. Finally, the patients whose dosage was reduced at the initiation dose were included. The initial dose reduction of regorafenib was reported as one of the prognostic factors in a previous prospective observational study [16]. Nevertheless, in this study, the initial dose reduction was not included as a propensity score because no variability was observed before the treatment. A post hoc analysis was established using the propensity score, including the initial dose reduction; however, the results were similar to those in the primary analysis (data not shown).
Clinical predictive markers to distinguish the two drugs were not identified in our study. Because clinical outcomes did not differ between unadjusted and adjusted populations in an analysis adjusted for patients’ characteristics, it is premature to conduct a superiority randomized trial. Novel genetic or metabolic predictive biomarkers will be needed for a physician to decide the appropriate drug for initiating treatment patients with mCRC. Respective predictive biomarkers have been analyzed in previous reports [17], [18]; however, no clear biomarkers that could distinguish the two drugs have been found.
Conclusion
Regorafenib and TFTD showed a similar effect on the OS of patients with mCRC refractory to standard chemotherapy in the real‐world setting, on both unadjusted and adjusted analyses. Although the choice of the drug by age might affect survival, a clearly predictive biomarker to distinguish the two drugs should be identified in further studies.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
We thank all of the participating patients and all of the investigators at the 24 institutions in this study. Study investigators: Kohei Shitara (National Cancer Center Hospital East), Kei Muro (Aichi Cancer Center Hospital), Tetsuya Hamaguchi (National Cancer Center Hospital), Akitaka Makiyama (Japan Community Healthcare Organization Kyushu Hospital), Yukimasa Hatachi (Kobe City Medical Center General Hospital), Kenji Katsumata (Tokyo Medical University), Hiroyuki Uetake (Tokyo Medical and Dental University, Graduate School of Medicine and Dentistry), Yoshihiko Maehara and Eiji Oki (Graduate School of Medical Sciences, Kyushu University), Satoshi Yuki (Hokkaido University Hospital), Akihito Tsuji (Kagawa University, Faculty of Medicine), Shuji Arita (Kyushu University Hospital), Taroh Satoh (Osaka University Graduate School of Medicine), Kazuo Hase (National Defense Medical College), Yoshihiro Kakeji (Graduate School of Medicine, Kobe University). This work was funded by the Japanese Society for Cancer of the Colon and Rectum (JSCCR). We would like to thank Editage (www.editage.jp) for English language editing.
Footnotes
For Further Reading: Martha M. Kirstein, Ansgar Lange, Anne Prenzler et al. Targeted Therapies in Metastatic Colorectal Cancer: A Systematic Review and Assessment of Currently Available Data. The Oncologist 2014;19:1156–1168.
Implications for Practice: Introduction of targeted agents in treatment algorithms of patients with metastatic colorectal cancer (mCRC) has significantly improved median overall survival. Emerging therapeutic options are available for patients with mCRC in 2014. This article reviews and assesses the available phase II and III data in order to elucidate the best combination and sequence modalities of targeted therapies in patients with mCRC.
Author Contributions
Concept/design: Toshikazu Moriwaki, Masahiko Gosho, Yasuhiro Shimada
Provision of study material or patients: Toshikazu Moriwaki, Shota Fukuoka, Hiroya Taniguchi, Atsuo Takashima, Yusuke Kumekawa, Takeshi Kajiwara, Kentaro Yamazaki, Taito Esaki, Chinatsu Makiyama, Tadamichi Denda, Hironaga Satake, Takeshi Suto, Naotoshi Sugimoto, Masanobu Enomoto, Toshiaki Ishikawa, Tomomi Kashiwada, Masahiko Sugiyama, Yoshito Komatsu, Hiroyuki Okuyama, Eishi Baba, Daisuke Sakai, Tomoki Watanabe, Takao Tamura, Kimihiro Yamashita, Yasuhiro Shimada
Collection and/or assembly of data: Toshikazu Moriwaki, Masahiko Gosho
Data analysis and interpretation: Toshikazu Moriwaki, Hiroya Taniguchi, Atsuo Takashima, Kentaro Yamazaki, Masahiko Gosho, Yasuhiro Shimada
Manuscript writing: Toshikazu Moriwaki, Shota Fukuoka, Hiroya Taniguchi, Atsuo Takashima, Hironaga Satake, Masahiko Gosho, Yasuhiro Shimada
Final approval of manuscript: Toshikazu Moriwaki, Shota Fukuoka, Hiroya Taniguchi, Atsuo Takashima, Yusuke Kumekawa, Takeshi Kajiwara, Kentaro Yamazaki, Taito Esaki, Chinatsu Makiyama, Tadamichi Denda, Hironaga Satake, Takeshi Suto, Naotoshi Sugimoto, Masanobu Enomoto, Toshiaki Ishikawa, Tomomi Kashiwada, Masahiko Sugiyama, Yoshito Komatsu, Hiroyuki Okuyama, Eishi Baba, Daisuke Sakai, Tomoki Watanabe, Takao Tamura, Kimihiro Yamashita, Masahiko Gosho, Yasuhiro Shimada
Disclosures
Toshikazu Moriwaki: Chugai Pharma, Takeda, Taiho Pharmaceutical, Yakult Honsha (H, RF), Bayer, Merck Serono, Novelpharma (H), Boehringer Ingelheim, Merck Sharp and Dohme Oncology, Sanofi‐Aventis (RF); Hiroya Taniguchi: Bayer, Chugai Pharma, Eli Lilly Japan, Merck Serono, Taiho Pharmaceutical, Yakult Honsha (H), Boehringer Ingelheim, Merck Sharp and Dohme Oncology, Otsuka, Takeda (RF); Atsuo Takashima: Chugai Pharma, Merck Serono, Taiho Pharmaceutical (H, RF), Takeda (H), Gilead Sciences (RF); Takeshi Kajiwara: Bayer, Taiho Pharmaceutical (H); Kentaro Yamazaki: Bristol‐Myers Squibb Japan (H, RF), Bayer, Chugai Pharma, Daiichi Sankyo, Eli Lilly Japan, Merck Serono, Takeda, Taiho Pharmaceutical, Yakult Honsha (H), Sanofi (RF); Taito Esaki: Eli Lilly Japan, Merck Serono, Taiho Pharmaceutical (H, RF), Chugai Pharma, Yakult Honsha (H), Astellas Oncology, AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Merck Sharp and Dohme Oncology, Novartis Pharma, Ono Pharmaceutical (RF); Tadamichi Denda: Sanofi (RF); Hironaga Satake: Bayer, Chugai Pharma, Eli Lilly Japan, Merck Serono, Takeda, Taiho Pharmaceutical, Yakult Honsha (H); Toshiaki Ishikawa: Chugai Pharma, Merck Serono, Takeda, Taiho Pharmaceutical (H); Yoshito Komatsu: Bayer, Taiho Pharmaceutical (H, RF); Daisuke Sakai: Taiho Pharmaceutical (RF); Masahiko Gosho: Daiichi Sankyo, Ferring Pharmaceuticals, Novartis, Taiho Pharmaceutical (C/A), Takeda, Kowa (H); Yasuhiro Shimada: Eli Lilly Japan, Taiho Pharmaceutical (H, RF), Chugai Pharma, Takeda (H), Merck Serono, Merck Sharp and Dohme Oncology, Yakult Honsha (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Heinemann V, von Weikersthal LF, Decker T et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first‐line treatment for patients with metastatic colorectal cancer (FIRE‐3): A randomised, open‐label, phase 3 trial. Lancet Oncol 2014;15:1065–1075. [DOI] [PubMed] [Google Scholar]
- 2. Loupakis F, Cremolini C, Masi G et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 2014;371:1609–1618. [DOI] [PubMed] [Google Scholar]
- 3. Yamazaki K, Nagase M, Tamagawa H et al. Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first‐line treatment for patients with metastatic colorectal cancer (WJOG4407G). Ann Oncol 2016;27:1539–1546. [DOI] [PubMed] [Google Scholar]
- 4. Sanz‐Garcia E, Grasselli J, Argiles G et al. Current and advancing treatments for metastatic colorectal cancer. Expert Opin Biol Ther 2016;16:93–110. [DOI] [PubMed] [Google Scholar]
- 5. Wilhelm SM, Dumas J, Adnane L et al. Regorafenib (BAY 73‐4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011;129:245–255. [DOI] [PubMed] [Google Scholar]
- 6. Grothey A, Van Cutsem E, Sobrero A et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo‐controlled, phase 3 trial. Lancet 2013;381:303–312. [DOI] [PubMed] [Google Scholar]
- 7. Fukushima M, Suzuki N, Emura T et al. Structure and activity of specific inhibitors of thymidine phosphorylase to potentiate the function of antitumor 2'‐deoxyribonucleosides. Biochem Pharmacol 2000;59:1227–1236. [DOI] [PubMed] [Google Scholar]
- 8. Mayer RJ, Van Cutsem E, Falcone A et al. Randomized trial of TAS‐102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909–1919. [DOI] [PubMed] [Google Scholar]
- 9. Masuishi T, Taniguchi H, Hamauchi S et al. Regorafenib versus trifluridine/tipiracil for refractory metastatic colorectal cancer: A retrospective comparison. Clin Colorectal Cancer 2017;16:e15–e22. [DOI] [PubMed] [Google Scholar]
- 10. Sueda T, Sakai D, Kudo T et al. Efficacy and safety of regorafenib or TAS‐102 in patients with metastatic colorectal cancer refractory to standard therapies. Anticancer Res 2016;36:4299–4306. [PubMed] [Google Scholar]
- 11. Santini D, Vincenzi B, Addeo R et al. Cetuximab rechallenge in metastatic colorectal cancer patients: How to come away from acquired resistance? Ann Oncol 2012;23:2313–2318. [DOI] [PubMed] [Google Scholar]
- 12. Suenaga M, Mizunuma N, Matsusaka S et al. Phase II study of reintroduction of oxaliplatin for advanced colorectal cancer in patients previously treated with oxaliplatin and irinotecan: RE‐OPEN study. Drug Des Devel Ther 2015;9:3099–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Cutsem E, Sobrero A, Siena S et al. Regorafenib (REG) in progressive metastatic colorectal cancer (mCRC): Analysis of age subgroups in the phase III CORRECT trial. Abstract presented at: 2013 ASCO Annual Meeting; May 31 to June 4, 2013; Chicago, IL:3636a.
- 14. Van Cutsem E, Benedetti F, Mizuguchi H et al. TAS‐102 vs placebo (PBO) in patients (pts) ≥65 years (y) with metastatic colorectal cancer (mCRC): An age‐based analysis of the RECOURSE trial. Abstract presented at: 2015 ASCO Annual Meeting; May 29 to June 2, 2015; Chicago, IL:3595a.
- 15. Yoshino T, Komatsu Y, Yamada Y et al. Randomized phase III trial of regorafenib in metastatic colorectal cancer: Analysis of the CORRECT Japanese and non‐Japanese subpopulations. Invest New Drugs 2015;33:740–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Adenis A, de la Fouchardiere C, Paule B et al. Survival, safety, and prognostic factors for outcome with Regorafenib in patients with metastatic colorectal cancer refractory to standard therapies: results from a multicenter study (REBACCA) nested within a compassionate use program. BMC Cancer 2016;16:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suenaga M, Schirripa M, Cao S et al. Genetic variants of DNA repair‐related genes predict efficacy of TAS‐102 in patients with refractory metastatic colorectal cancer. Ann Oncol 2017;28:1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tabernero J, Lenz HJ, Siena S et al. Analysis of circulating DNA and protein biomarkers to predict the clinical activity of regorafenib and assess prognosis in patients with metastatic colorectal cancer: A retrospective, exploratory analysis of the CORRECT trial. Lancet Oncol 2015;16:937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.