This article provides an overview of basic human hair follicle biology, with a focus on those events most relevant to chemotherapy‐induced alopecia and the processes that occur during hair loss. This includes a description of the known mechanisms by which anticancer agents cause chemotherapy‐induced alopecia, as well as preventative strategies, with a focus on the most effective therapy currently available, scalp cooling.
Keywords: Chemotherapy‐induced alopecia, Hair loss, Chemotherapy, Scalp cooling, Side effects, Toxicity, Hair follicle, Cell models, Prevention, Safety
Abstract
Chemotherapy‐induced alopecia (CIA) is the most visibly distressing side effect of commonly administered chemotherapeutic agents. Because psychological health has huge relevance to lifestyle, diet, and self‐esteem, it is important for clinicians to fully appreciate the psychological burden that CIA can place on patients. Here, for the first time to our knowledge, we provide a comprehensive review encompassing the molecular characteristics of the human hair follicle (HF), how different anticancer agents damage the HF to cause CIA, and subsequent HF pathophysiology, and we assess known and emerging prevention modalities that have aimed to reduce or prevent CIA. We argue that, at present, scalp cooling is the only safe and U.S. Food and Drug Administration‐cleared modality available, and we highlight the extensive available clinical and experimental (biological) evidence for its efficacy. The likelihood of a patient that uses scalp cooling during chemotherapy maintaining enough hair to not require a wig is approximately 50%. This is despite different types of chemotherapy regimens, patient‐specific differences, and possible lack of staff experience in effectively delivering scalp cooling. The increased use of scalp cooling and an understanding of how to deliver it most effectively to patients has enormous potential to ease the psychological burden of CIA, until other, more efficacious, equally safe treatments become available.
Implications for Practice.
Chemotherapy‐induced alopecia (CIA) represents perhaps the most distressing side effect of chemotherapeutic agents and is of huge concern to the majority of patients. Scalp cooling is currently the only safe option to combat CIA. Clinical and biological evidence suggests improvements can be made, including efficacy in delivering adequately low temperature to the scalp and patient‐specific cap design. The increased use of scalp cooling, an understanding of how to deliver it most effectively, and biological evidence‐based approaches to improve its efficacy have enormous potential to ease the psychological burden of CIA, as this could lead to improvements in treatment and patient quality‐of‐life.
Introduction
Chemotherapy‐induced alopecia (CIA) is an acquired form of hair loss that affects patient quality of life, negatively impacts body image, sexuality, and self‐esteem, and provides a strong indication of the individual's health status, with most people associating it with cancer [1], [2]. Increasing use of polytherapies, high‐dose taxane administration, and an associated increase in cases of permanent CIA are being reported. Although a non‐life‐threatening condition, CIA is of huge concern to most patients, yet is often viewed as being of minor clinical importance, when the focus is understandably on the treatment of a potentially fatal malignancy. Equally, whereas considerable efforts have been expended in the attempt to ameliorate other side effects of chemotherapy, the pathobiology of CIA has been heavily overlooked [3].
Chemotherapy‐induced alopecia is often a particular burden for those with young children who report this as the most traumatizing aspect of treatment, because the child becomes emotionally confused and concerned [4]. Consequently, CIA can be one of the most emotionally difficult side effects, with feedback from female patients showing that losing hair is/would be more difficult to live with than the loss of a breast [5]. Social media and the increased pressure on appearance means patients are likely to feel that losing their hair is detrimental to their self‐esteem, while dealing with a possibly life‐threatening disease. These factors could negatively impact therapeutic outcome, because severe stress and depression [2] are linked to a weakened immune system, an instrumental factor in cancer prognosis [6]. Although most of the research on the emotional effects of CIA has been conducted on females, the available research indicates that, at least for younger males, the impact of CIA is the same as that experienced by females [7]. Chemotherapy‐induced alopecia on females portrays that they have cancer, because most women maintain their hair throughout life. Men commonly undergo androgenic alopecia; however, most young males do not, thus males may also be stigmatized as cancer sufferers when CIA occurs.
It is important for clinicians and even patients to fully appreciate the possible psychological burden of this side effect and to have a clear understanding of ways available to prevent it [8]. To this end, here we provide an overview of basic human hair follicle (HF) biology, with a focus on those events most relevant to CIA and the processes that occur during hair loss. This includes a description of the known mechanisms by which anticancer agents cause CIA. We discuss the various preventative strategies that have been investigated both in the lab and the clinic, ultimately focusing on the most effective therapy currently available: scalp cooling.
The Hair Follicle
Structure and Function
Hair is a skin appendage with diverse functions, being important for thermoregulation, protection from solar radiation, and sexual dimorphism [9]. In humans, scalp and facial hair is associated with general well‐being, strong social status, and sexual attraction, and is often used to make a fashion statement or even demonstrate political affiliations [10], [11].
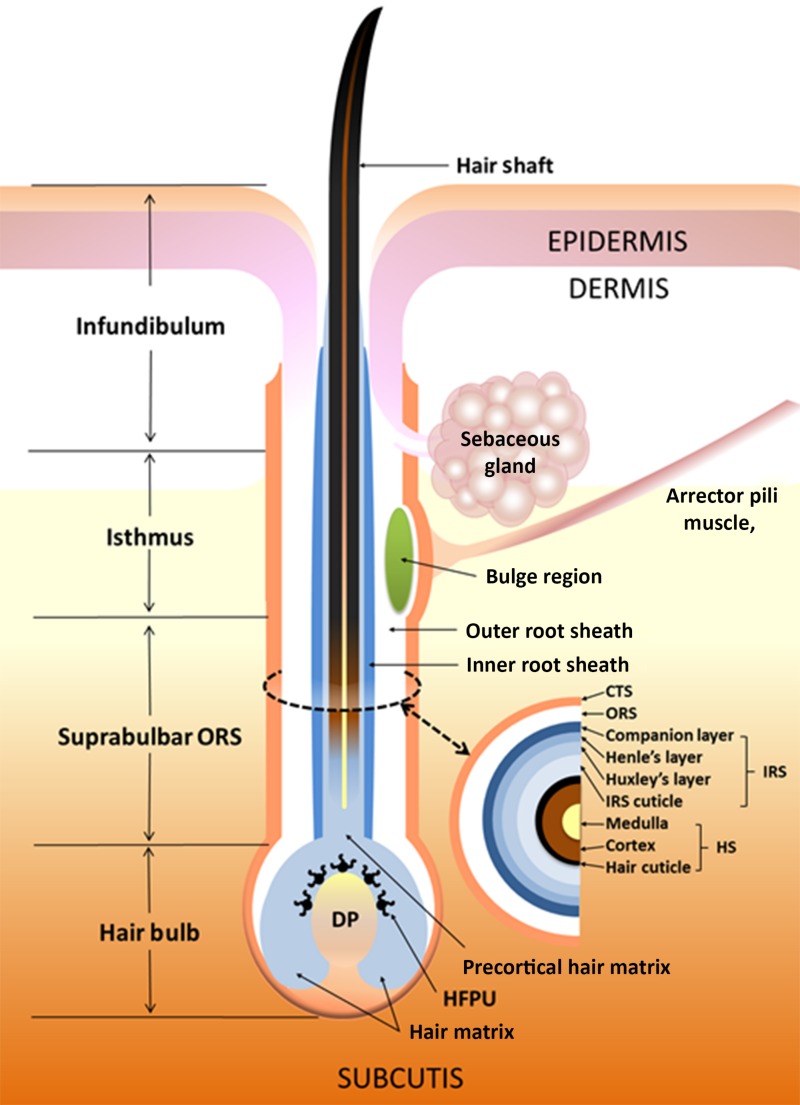
The HF is a mini‐organ and skin appendage; its primary function is to produce the visible hair shaft [12], [13]. The HF is divided into distinct sections, as detailed in Figure 1. The upper sections of the HF are permanent, with the infundibulum running from the opening of the sebaceous gland (SG) duct to the point where the HF meets the epidermis, providing a funnel‐shaped cavity through the epidermis and offering an opening for the hair shaft. The isthmus is located at the lower boundary of the SG at the insertion point for the arrector pili muscle. This region is also commonly described as the bulge, and contains a population of epithelial HF stem cells, the identity of which has recently been reviewed [14]. The progeny of these stem cells produce the hair bulb matrix keratinocytes, as well as contributing to the formation of the epidermis, particularly during wound healing, and it is damage to these cells that severely impairs long‐term hair shaft production [15]. The suprabulbar region contains multiple layers of the outer root sheath (ORS) and inner root sheath (IRS), which form concentric cylinders wrapping the hair shaft itself (Fig. 1). Each of these layers has a unique expression of structural and adhesion proteins [16]. The hair bulb contains the matrix keratinocytes, a population of rapidly‐dividing progenitor cells that differentiate (specialize) to form the IRS and hair shaft. Matrix cells in the lower part of the hair bulb have a higher mitotic (proliferation) rate than those of the upper part and migrate upwards while differentiating [15]. The bulb also contains the HF pigmentary unit, within which are found the melanocytes responsible for hair color.
Figure 1.
Structure of the hair follicle (HF). The schematic illustrates the organization and structure of the human HF, including key areas of the organ, such as the bulge region, the ORS and IRS, and the hair bulb that includes the hair matrix keratinocyte compartment and the dermal papilla region.
Abbreviations: DP, dermal papilla; HFPU, hair follicle pigmentary unit; HS, hair shaft; IRS, inner route sheath; ORS, outer route sheath. Reprinted with permission from [121].
The HF is primarily epithelial in origin, with the exception of the dermal papilla (DP) and connective tissue sheath (CTS), which are mesenchymal. Inductive signals for HF growth and cycling originate from the DP, an oval mass of specialized fibroblasts embedded in an extracellular matrix with extensive vascularization [12], [17], [18]. There is a close relationship between the size of the DP and HF, with a larger DP creating a larger HF capable of generating a thicker hair shaft [19]. The CTS surrounds the HF, separating it from the rest of the dermis, and contains nerve endings, vasculature, and immune cells (such as mast cells).
The Hair Cycle
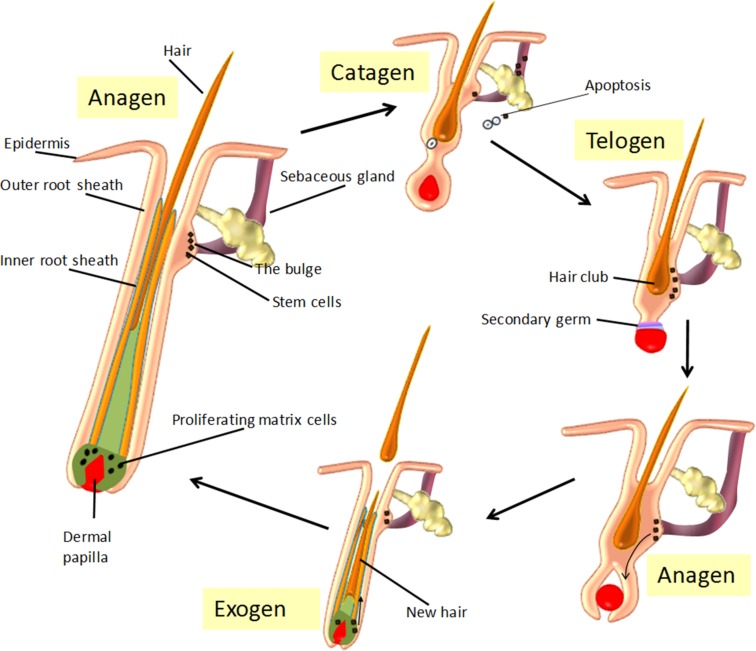
Hair follicle morphogenesis (original/new HF development) occurs antenatally, with the HF beginning a postnatal, life‐long cycle through three distinct phases. Following morphogenesis, this hair cycle begins with a regression phase (catagen), followed by a period of relative quiescence (telogen) and finally a long growth phase (anagen). The hair cycle is summarized in Figure 2 and described in more detail below.
Figure 2.
The hair “cycle.” Schematic diagram of the three main phases of hair cycle: the growth phase (anagen), the dystrophic phase (catagen), an extremely shortened resting phase (telogen), and the ‘shedding’ of the hair (exogen). In anagen, the hair bulb is located deep inside the skin and hair grows towards the skin surface. The dermal papilla survives catagen and moves upward to the lowermost portion of the bulge, which then forms the secondary germ at its base during telogen. In telogen, the hair falls out and the hair bulb relocates down again as the new hair grows. At their cycle end, telogen HFs can be activated through mechanical depilation, pharmacologically, and by specific signaling factors (e.g., Wnt signaling), which stimulates a return to anagen and the generation of the new lower follicle and hair shaft. As the new hair grows in, the old hair is shed during exogen. The duration of each phase depends on the type, site and specific genetic programming of the follicle.
Catagen.
During the regressive catagen phase, extensive cell death (apoptosis) occurs in the hair matrix keratinocytes, IRS and ORS, greatly reducing the HF volume, with the remnants of the ORS forming the epithelial strand [9]. Structurally, an apoptotic cell undergoes DNA condensation and fragmentation, cytoplasmic condensation, membrane blebbing, and formation of apoptotic bodies, and is removed in a controlled manner by immunocytes [20]. Apoptosis is crucial in long‐term regulation of tissue maintenance, which particularly applies to the HF and its cycling/regeneration, yet exogenous agents can inadvertently induce excessive apoptosis. Many factors can stimulate apoptosis in the HF, including UV radiation, x‐rays, extreme temperature, pathogenic toxins, lytic viruses, toxic chemicals, and chemotherapeutic drugs [21]. This stimulation of apoptosis can ultimately drive the HF into the regressive catagen phase, which stops hair production.
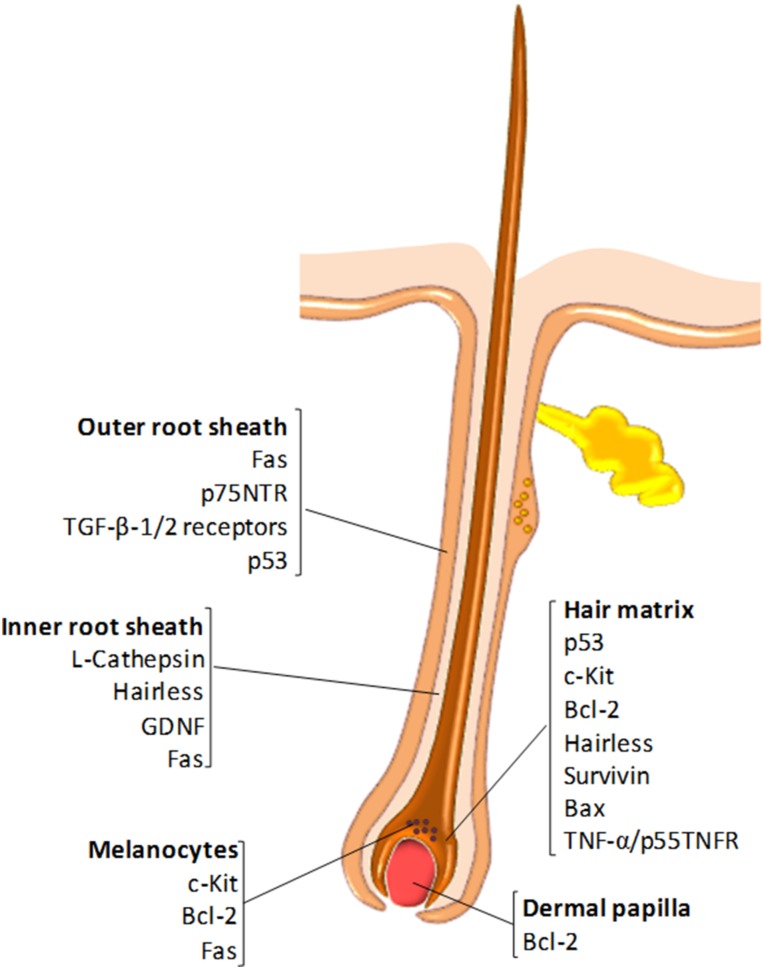
Growth factor‐mediated signaling between epithelial and mesenchymal cells orchestrates the creation of the connective tissue that comprises a developing HF and involves diverse signaling pathways, including Wnt, transforming growth factor beta (TGF‐β)/bone morphogenetic protein (BMP), Hedgehog, epidermal growth factor (EGF), fibroblast growth factor (FGF), and Notch [22], [23], as well as tumour necrosis factor (TNF)‐related signaling events [24], [25]. Catagen‐associated apoptosis primarily occurs in the hair matrix keratinocytes, the proximal and central ORS but generally not in the dermal papilla, which expresses high levels of antiapoptotic Bcl‐2 [21]. The compartmentalized expression of pro‐ and antiapoptotic factors in the HF is shown in Figure 3. A diverse array of additional molecules have been found to play a role in catagen induction, including FGF‐5 [26], [27], interferon (IFN)‐γ [28], substance P [29], and estrogens [30]. The apoptotic processes within the HF are also controlled by caspases −1, −3, −4, and −7 [21], [31], [32], and can also be triggered by the withdrawal of DP‐derived growth factors or by apoptotic signals produced by mast cells located within the CTS [29], [33], [34].
Figure 3.
Molecular regulators of apoptosis in the hair follicle (HF). The diagram illustrates the expression pattern of proapoptotic (e.g., Fas, p53, Bax) and antiapoptotic (Bcl‐2, survivin) molecules in the different HF compartments.
In addition to apoptosis, other events occur during catagen. In particular, the termination of melanogenesis is one of the earliest events and results in the hair shaft becoming less pigmented. The DP becomes condensed and ball‐shaped, detaching from the surrounding matrix keratinocytes [35]. The old hair shaft forms the club hair, which comes to reside entirely in the dermis. Overall, catagen lasts for 7–14 days, with ∼2% of scalp HFs estimated to be in catagen at any one time [15].
Telogen.
Although traditionally described as a quiescent or resting phase of the hair cycle [16], recent evidence has shown that the HF is highly metabolically and transcriptionally active during telogen [36]. Telogen is referred to as either “refractory” or “competent” [37]. In the first state, high levels of DP‐derived BMPs, FGF18, and Wnt antagonists prevent any response to anagen‐inducing signals. As the levels of these molecules fall, the telogen HF becomes primed to enter anagen, which is described as competent telogen. During telogen, the DP is in close contact with the HF bulge (stem cell region), separated by a shortened epithelial strand known as the secondary hair germ [9]. An estimated 10%–15% of HFs are in the telogen phase, which lasts approximately 3–4 months [15], [38].
Anagen.
With stimulation of a new anagen phase, the more distal cycling portions of the HF are gradually renewed, the hair bulb ultimately reaches the dermal adipose layer, and melanogenesis is at its highest level [35]. Hair follicles remain in anagen for approximately 2–6 years [16], with ∼80%–85% of scalp HFs in this phase at any given time [15].
Chemotherapy Drugs and CIA
Anticancer Chemotherapy Agents and Their Action
Since the U.S. Food and Drug Administration (FDA) approved mechlorethamine in 1949 for the treatment of non‐small cell lung cancer, >100 chemotherapy agents have been approved for cancer treatment in the U.S. alone [39]. In contrast to surgery and radiotherapy, which target the primary tumor, chemotherapy is a systemic treatment and therefore targets both primary and metastasized tumor cells [40]. The principle behind infusing chemotherapeutic drugs is that because a greater number of malignant cells are in the cell cycle (are dividing) at any given time compared with healthy cells, the drug should have a greater impact on malignant cells (by stimulating higher levels of apoptosis). Table 1 provides a list of the main categories of commonly used anticancer compounds as well as their point of action in the mammalian cell cycle. Chemotherapy agents are routinely administered intravenously but some may be oral or even topical, with their distribution depending on a number of factors, such as blood flow, drug diffusion, protein binding, tissue penetration, and lipid solubility. Generally, drugs with extensive tissue penetration or high lipid solubility will tend to exhibit prolonged elimination phases due to slower tissue release [41].
Table 1. List of the main categories of commonly used anticancer compounds.
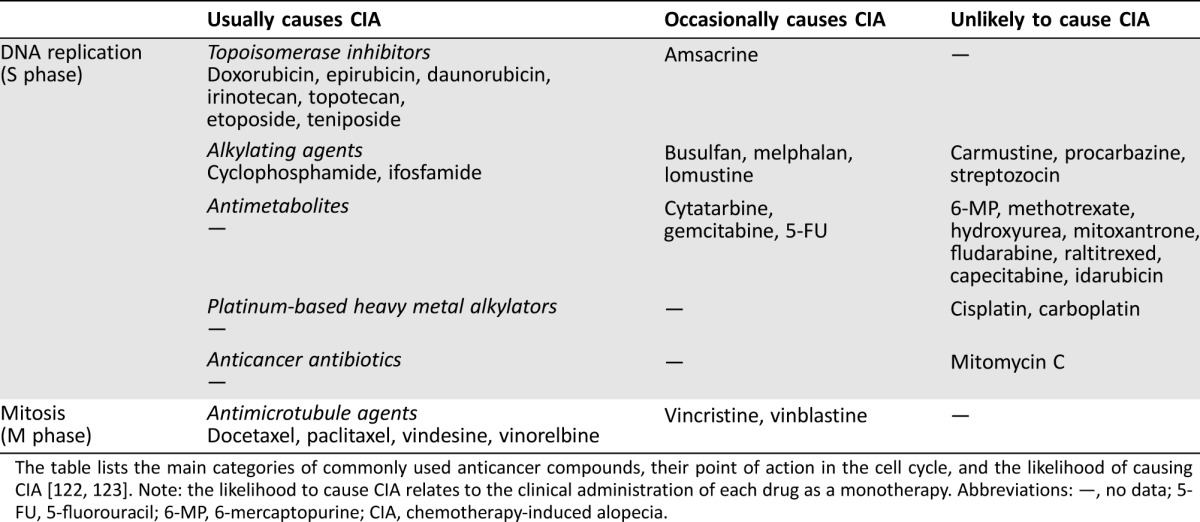
The table lists the main categories of commonly used anticancer compounds, their point of action in the cell cycle, and the likelihood of causing CIA [122], [123]. Note: the likelihood to cause CIA relates to the clinical administration of each drug as a monotherapy. Abbreviations: —, no data; 5‐FU, 5‐fluorouracil; 6‐MP, 6‐mercaptopurine; CIA, chemotherapy‐induced alopecia.
Most agents are administered close to the maximum tolerated dose (MTD) which is quantified relatively to the individual's body surface area; this normalizes the dosage, accounting for physiological factors such as cardiac output, body fat, and size, and is expressed as units of mg/m2 [41]. The frequency and intervals between treatments depend on the cancer type and the treatment regimen and thus are quite variable. Clinical evidence demonstrates that most cancers are unlikely to be managed with a single chemotherapy agent and that combinations are more efficient in disease eradication [42]. The advantages of combinations are believed to be that (a) they provide maximal malignant cell death within the range of tolerated toxicity, (b) malignant cells in different phases of the cell cycle are targeted (discussed below), and (c) there is a reduced risk of malignant cell drug resistance development [43]. Chemotherapy is administered in cycles that include rest periods, so that the body has a chance to recover from side effects (outlined below).
Cellular and Molecular Effects of Chemotherapy Drugs
Cells such as HF matrix keratinocytes, intestinal epithelial cells, and bone marrow cells also divide rapidly, and thus chemotherapy drugs cause side effects in healthy tissues. Bone marrow toxicity causes neutropenia, thrombocytopenia, and anaemia, and damage to the digestive tract results in mucositis, nausea, vomiting, and diarrhea. Induction of apoptosis in keratinocytes can cause nail bed damage, changes in skin integrity, and CIA [40].
Although constant division/cell cycling is one reason why chemotherapy affects cancer cells more than normal cells, cancer cells are also more susceptible to lethal oxidation/reactive oxygen species (ROS). Due to their excessive metabolic rates and abnormally high energy demands, cancer cells operate under conditions of high ROS levels, a state also referred to as oxidative stress; this may in fact represent their “Achilles heel,” because agents that enhance ROS production can selectively trigger more cancer cell death [44]. Many anticancer drugs can increase ROS levels in cancer cells (examples provided below), thus causing them to cross a “lethal proapoptotic threshold.” A range of chemotherapeutic drugs have been shown to induce ROS via various mechanisms, such as phosphorylation of NADPH oxidase family members and by directly impacting on the mitochondria, the main site of production of ROS in cells [45].
Agents shown to augment ROS production to apoptotic levels include anthracyclines (e.g., doxorubicin, epirubicin), alkylating agents (e.g., cyclophosphamide), and platinum‐based drugs (e.g., cisplatin, carboplatin, and oxaliplatin) [46]. Interestingly, it is such agents that induce HF apoptosis at a greater frequency/severity than most other drugs, suggesting a possible relationship between ROS production and stimulation of HF catagen [47]. Indeed, the high mitotic and melanogenic activity in the hair bulb ensures a high basal level of ROS within this compartment. Although the HF is well‐equipped to deal with high levels of reactive moieties, it has recently been shown that exogenous sources of ROS will result in hair matrix apoptosis, lipid peroxidation, and induction of catagen [48]. Moreover, it has been suggested that oxidative damage of mitochondrial DNA [49] and inhibition of endothelial proliferation in the vascular network surrounding the HF can contribute to CIA [50].
Chemotherapy‐Induced HF Pathophysiology.
The HF is particularly sensitive to chemotherapy‐induced apoptosis because >80% of scalp HFs are anagen‐phased at any one time [51]. Strikingly, the division rate displayed by HF matrix keratinocytes during anagen can be greater than that of malignant cells [11], thus resulting in susceptibility to chemotherapy agents. High levels of perfusion around the hair bulb by the DP may also make this region of the HF more susceptible to drug damage.
The severity of CIA depends on the chemotherapy drug, its dose, administration route, and treatment schedule. A list of drugs likely to cause CIA and relative severity is provided in Table 1. High intravenous doses usually cause more rapid and extensive hair loss, whereas oral therapy (despite administration at a higher total dosage) is likely to cause less alopecia [52]. CIA extent can be classified using a World Health Organization (WHO) classification system as “grade 0” implying no CIA, “grade 1” minor, “grade 2” moderate with wig proposal, “grade 3” severe but reversible with wig proposal, and “grade 4” complete irreversible CIA with wig proposal [53], although other scores/scales are available, such as Dean's scale [54]. The estimated incidence of CIA is >60% for alkylating agents, >80% for antimicrotubular agents, 60%–100% for topoisomerase inhibitors, and 10%–50% for antimetabolites [55]. Although even just a single drug treatment can significantly reduce hair density [56], polytherapies (consisting of two or more drugs) produce higher incidence and more severe CIA compared with single administrations [53].
In most cases, HF stem cells appear to be largely unaffected by chemotherapy agents because hair regenerates 3–6 months after treatment [51], [57]. Although permanent CIA or incomplete regrowth is rare, an increasing number of cases are being reported, and this is more common in children, thus suggesting that acute damage to HF stem cells may occur [58], [59], [60]. In the case of children, permanent diffuse alopecia has been associated with hematopoietic stem cell transplantation [61]. In permanent CIA, there is a large decrease in the total number of HFs, but this is not associated with inflammation or fibrosis/scarring [62]. In a study of permanent alopecia, biopsies of the frontal scalp were assessed and showed a reduction in anagen‐phase terminal HFs [63]. Instead, permanent alopecia may be associated with an increase in miniaturized vellus hair [63].
Although permanent CIA or incomplete regrowth is rare, an increasing number of cases are being reported, and this is more common in children, thus suggesting that acute damage to HF stem cells may occur.
Experimental Models for the Study of CIA.
Because CIA remains an important unmet clinical challenge, and because scalp biopsies from patients are difficult to access, there is a clear need to develop robust experimental models to both understand its pathophysiology and to generate avenues for the development of new treatment strategies [11]. Currently available models for studying and understanding CIA together with their advantages and disadvantages are outlined in Table 2. These include animal models (mainly involving the use of newborn rodents), as well as in vitro models.
Table 2. Currently available models for studying chemotherapy‐induced alopecia.
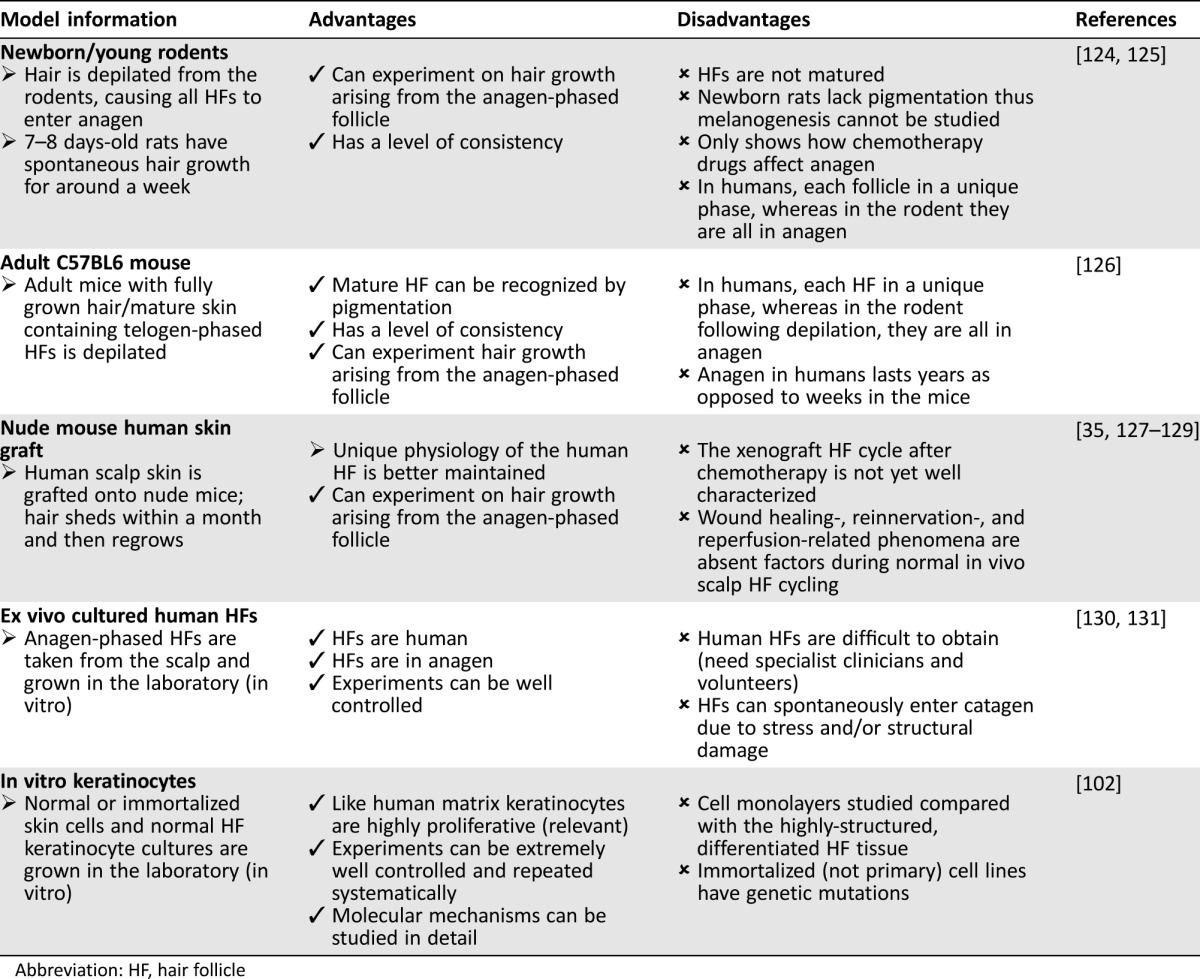
Abbreviation: HF, hair follicle
Prevention Modalities Against CIA
Pharmacological and Biological Interventions
Since the 1970s, there have been numerous attempts to prevent CIA by means of mechanical, physical, and pharmacological interventions [64], [65], [66], [67], [68], [69]. Moreover, several classes of biological and mainly pharmacological agents with different mechanisms of action have been evaluated in animal models of CIA as discussed below.
Drug‐Specific Antibodies.
To reduce the severity of doxorubicin‐induced alopecia in the newborn rat model, the use of a monoclonal antibody (MAD11) incorporated in liposomes has been explored to neutralize doxorubicin activity. Topical administration of these antianthracyclines prevented doxorubicin‐induced CIA [70]. Further work explored the antibody's ability to prevent the bone marrow [71], gastrointestinal [72], and mucosal [73] toxicity of doxorubicin with positive outcomes in rats; however, no clinical trials to assess this approach for CIA prevention have been reported.
Vasoconstrictors.
Because changes in DP blood flow inevitably correlate with the diffusion gradient of drug delivered to the HF, superficial application of topical vasoconstrictors epinephrine or norepinephrine for prevention of CIA was studied in female Sprague‐Dawley (albino) adult rats treated with Cytoxan or 1‐methyl‐1‐nitroso‐urea (MNU). Vasoconstriction proved highly effective with MNU, which has a shorter half‐life than Cytoxan, demonstrating the effectiveness of preventing drug entry to the HF. The effect of lack of blood flow to the human scalp, patient response variability, and other possible contraindications are yet to be clinically resolved and there is no evidence, as yet, that this would be advantageous over other approaches (e.g., scalp cooling); however, if effective, it could be better tolerated [65].
ROS Inhibitors/Antioxidants.
The antioxidant N‐acetyl cysteine, when applied topically in liposomes, protected newborn rats against cyclophosphamide‐induced CIA, suggesting that cyclophosphamide stimulates ROS to drive HF apoptosis in matrix keratinocytes [74]. Furthermore, topical application of antioxidants resveratrol or aminothiol PrC‐210 reduced CIA in newborn mice treated with Cytoxan [65]. Clinical trials utilizing antioxidants for prevention of CIA have not yet been performed.
Hair Growth Cycle Modifiers.
Immunosuppressive immunophilin ligands, such as cyclosporine A (CSA), are used in the treatment of autoimmune disease and after organ transplantation; however, these drugs also prolong anagen and inhibit the catagen entry of the hair cycle, resulting in enhanced hair growth in several normal and pathogenic alopecia conditions [75], [76]. Neonatal rats and mice have been used to investigate the effects of CSA on CIA. Topical CSA application locally protected from alopecia induced by cyclophosphamide, cytosine arabinoside, and etoposide [77]. Another immunomodulator, AS101, has been shown to reduce the severity of alopecia in patients treated with a combination of carboplatin and etoposide [68]. Given the strong immunosuppressive nature of CSA, it cannot be developed as an effective CIA treatment, yet enhanced understanding of its mechanism of action may yield information that could lead to development of novel therapies.
Topical minoxidil is used for the treatment of male pattern baldness (androgenetic alopecia); minoxidil modifies hair cycle dynamics by shortening the telogen phase, thus facilitating anagen and encouraging hair growth [78]. In the newborn rat model, local application of minoxidil protected against CIA induced by arabinosyl‐cytosine, but showed no protection to doxorubicin and cyclophosphamide‐induced CIA [79]. In a clinical study in breast cancer patients, minoxidil was shown to accelerate recovery from CIA, but did not prevent the initial hair loss [78]. Minoxidil appears to be most beneficial for men suffering with androgenetic alopecia, for which it accelerates hair regrowth [80]. Overall, it helps regrowth following CIA, but currently there is no evidence supporting its use in CIA prevention [64].
Cytokines and Growth Factors.
Interleukin 1 (IL‐1), which plays a role in the regulation of inflammatory and immune responses to infections, and imuvert, a biological response modifier with immune stimulatory properties derived from the bacterium S. marcescens, have both been reported to protect newborn rats from CIA induced by cell cycle‐specific agents, namely cytosine arabinoside and doxorubicin, but not from cell cycle‐nonspecific agents such as cyclophosphamide [81]. Both imuvert and IL‐1 induce the release of multiple cytokines or growth factors and it was suggested that the action of imuvert is via IL‐1 [82]. There is also evidence that acidic FGF and EGF protect from CIA, but again only if CIA is caused by cell cycle‐specific agents [81]. Despite the promise of these agents in newborn rat experimentation models, they have not yet been tested in the clinic for CIA prevention.
Cell Cycle or Proliferation Modifiers.
As discussed above, rapid cell proliferation in HF matrix keratinocytes during anagen and lack of selectivity in anticancer agents is a primary factor in the pathogenesis of CIA. Hence, one approach to protect against the CIA is to inhibit HF cellular proliferation in order to decrease sensitivity to chemotherapy [83]. An example of this “protective preconditioning” approach is the administration of calcitriol (1,25‐dihydroxyvitamin D3) which has multiple effects on keratinocytes, including stimulation of cell differentiation, inhibition of DNA synthesis and G0/G1 cell cycle arrest [84], [85]. Therefore, it is possible that calcitriol, by stimulating terminal keratinocyte differentiation, may alter cell susceptibility to apoptosis. Calcitriol can protect newborn rats from CIA induced by cyclophosphamide, etoposide, and combination of cyclophosphamide and doxorubicin [86]. In addition, in the adult mouse model, calcitriol could enhance normal pigmented hair shaft regrowth and reduce apoptosis in the hair bulb; however, it failed to prevent or retard hair loss after administration of cyclophosphamide [87], [88]. A phase I study showed that calcitriol was well tolerated and 21 subjects showed improved hair retention when treated with taxane therapy [64], but its beneficial effects are most likely limited to taxanes due to the previously mentioned mechanisms of action for calcitriol.
Finally, inhibitors of cyclin‐dependent kinase 2 (CDK2), which plays a key role in the transition from G1 to late G2 of the cell cycle, can block progression from late G1 phase into S phase, reduce the sensitivity of HFs to chemotherapy agents, and inhibit apoptosis induced by etoposide, 5‐fluorouracil, taxol, cisplatin, and doxorubicin. In newborn rats, topical application of a CDK2 inhibitor reduced etoposide‐mediated hair loss by 50% at the site of application and by 33% in CIA induced by combination of doxorubicin and cyclophosphamide [83]. Despite the promise of these findings, such modifiers have not been clinically tested yet.
Inhibitors of Apoptosis.
Caspase‐3 is a key mediator of apoptosis, and pathways leading to its activation can be stimulated by a number of chemotherapy agents [89]. Tsuda et al. showed that a topical administration of M50054, an inhibitor of caspase‐3, reduced CIA induced by etoposide in the newborn rat model [90]. Further experiments have not elucidated whether this would protect against other drugs, and no clinical trials have been reported.
Parathyroid Hormones.
Parathyroid hormone receptor (PPR) ligands have been shown to have a potential role in the hair cycle by inducing hair regrowth following CIA [91]. The best results have been obtained using cyclophosphamide in mice in which it was found that CIA could be reduced, hair regrowth improved, and repigmentation promoted. This suggests that PPR ligands can be potentially useful as a topical application for preventing/treating CIA; however, this may rely on follicles that have not undergone permanent alopecia [92]. Despite initial promise, clinical trial results were disappointing and the first trial was terminated [92]. Understanding the potential issues with pharmacokinetics has led to improved PPR ligands; however, there is no information available on the clinical success of these agents to date.
Physical Interventions/Non‐Drug Therapies
Scalp Tourniquets.
Scalp tourniquets are special bands that tightly fit the scalp region to occlude the superficial blood flow and thus reduce the amount of drug delivered to the HFs [93]. Scalp tourniquets are applied when the plasma drug levels are at their peak, that is, from the last 10 minutes of infusion to 10 minutes after the cessation of drug administration [94]. Tourniquets have achieved a small to moderate degree of rescue from CIA induced by vincristine, cyclophosphamide, and doxorubicin. However, it is no longer recommended due to the high pressure applied causing patient discomfort [85], [94].
Scalp Cooling.
Scalp cooling was introduced in the 1970s [67], with application of cooling throughout the administration of chemotherapy in most cases reducing CIA in patients [95].
A number of hypotheses have been proposed to explain how scalp cooling reduces CIA. Firstly, cooling causes rapid vasoconstriction, which has been shown to significantly reduce blood flow in the scalp. In fact, perfusion can be reduced to 20%–40% of normal levels [96], and this should result in reduced chemotherapeutic drug perfusion through the vasculature of the DP [97]. A second hypothesis is that the rate of drug diffusion across a plasma membrane is reduced at low temperatures due to lower kinetic energy, and membrane lipid fluidity is also lower, which will impact on passive diffusion; together, these would result in a low proportion of drugs entering HF cells [98]. Thirdly, because cell division is an energy‐dependent metabolic process, it is likely that cooling abrogates enzyme‐dependent reactions. It has been reported that temperature can particularly affect the G1 and S phases of the cell cycle [99], and this could be especially important for drugs that target specific phases of the cell cycle, such as mitosis‐targeting microtubule‐destructive drugs. Fourthly, some drugs (e.g., doxorubicin) may enter cells via active transport mechanisms, and this would be reduced by cooling. In support of this hypothesis, it has been shown in cell models that doxorubicin‐induced damage to DNA is reduced at lower temperatures [100]. Fifthly, a general decrease in the metabolic activity of the cells in the HF could cause a reduction in the cytotoxicity of chemotherapy drugs as a range of cellular processes (such as oxidation) decelerate [97]. In practice, it is likely that a combination of these mechanisms play a role in reducing CIA upon cooling, and this may explain the reported efficacy of scalp cooling.
It has been reported that the scalp temperature achieved by cooling is a critical factor in preventing CIA, and dampening the scalp with water improves heat transfer from the head to the cooling source [101]. It has previously been reported that a subcutaneous temperature of 22°C was a “threshold” temperature necessary for effective cooling, and a close relationship exists between epicutaneous and subcutaneous temperatures during cooling, with 22°C subcutaneous corresponding to an epicutaneous temperature of 19°C [97]. More recently, Komen et al. (2016) found that breast cancer patients whose scalp temperature was reduced to 18°C were the least likely to require a wig following anthracycline treatment; the study also raised the important issue of device fitting, to ensure that all areas of the scalp are cooled effectively, so that adequately low temperatures are achieved [56].
Interestingly, recent laboratory studies have provided support for these clinical observations. It was shown, using a range of in vitro models, that cooling can efficiently protect human keratinocytes from chemotherapy drug‐induced toxicity [102]. Equally importantly, it was shown that the cooling conditions (temperature) used were also a critical factor in preventing cytotoxicity. These experiments provided for the first time biological evidence that progressive reduction of temperature (26°C, 22°C, 18°C, and 14°C) positively correlated with better protection (rescue) of keratinocytes from drug‐induced cell death [102]. It is possible that cooling may have direct cytoprotective effects and at the same time may reduce drug diffusion that renders cells less susceptible to drug toxicity. This is supported by the finding that reducing the scalp temperature below 22°C does not further decrease blood flow [96], thus any increased protection by cooler scalp temperatures may not be a result of reduced scalp perfusion. Interestingly, this “cutoff” point in the protective effect of cooling has been shown to occur for doxorubicin both at the level of the cell membrane permeability [98] and subsequent DNA damage [100].
Practically, a marked reduction in scalp temperature may lead to an increase in patient discomfort and therefore intolerance, so although “more cold” is beneficial, it may not always be feasible. Furthermore, the amount of temperature reduction possible for each person is likely to vary quite considerably due to individual physiological differences/variability [56]; however, in most cases, “the colder the better.”
Scalp Cooling Using Cool Caps.
Initially, scalp cooling was achieved using crushed ice in plastic bags fixed into position with elasticated bandages [103]. Because heat from the head rapidly warmed the ice packs, these needed to be replaced regularly; this was time‐consuming and also meant that temperature increased between replacements [104], [105]. The number of countries and hospitals using scalp cooling increased dramatically following introduction of improved commercially available products. This involved a refrigerated cryogel cap, which is placed in a freezer at −25°C before being fitted to the head (e.g., Penguin cold cap [Penguin Cold Caps, London, U.K., https://penguincoldcaps.com]) [104]. However, because of the very low initial temperature, these gel caps are reported to be uncomfortable, and although better than ice packs, they still thaw rapidly and must be changed regularly to maintain reduced scalp temperature. Thus, several changes are required during chemotherapy perfusion protocols [104], and between replacements, scalp temperature unavoidably increases [105].
Modern Scalp‐Cooling Devices.
Refrigeration unit‐fitted devices designed to circulate liquid refrigerant through a cooling cap are the modern‐day choice for scalp cooling. These caps, such as the Paxman (Paxman, West Yorkshire, U.K., https://paxmanscalpcooling.com) and Dignicap (Dignitana, Lund, Sweden, http://www.dignitana.se/eng) systems, are available in a range of sizes to ensure a suitable fit, because head sizes and shapes vary [106]. The advantage of these systems is that the coolant achieves a constant, reduced scalp temperature throughout drug infusion without the need for cap replacement. This reduces medical staff time investment, and because the caps are not cooled to such initially low temperatures (and are not as heavy), they are reported to be more comfortable. Recent studies by Komen et al. (2016) have shown that 18°C can be reached at the scalp of patients throughout the course of chemotherapy infusion, and most patients tolerate this intervention very well, with the majority indicating either low or moderate levels of discomfort. Only 1 of 62 patients actually reported a mild headache, even when the scalp cooling device could reduce temperatures down to 10°C within 30 minutes [56]. Other studies have shown that the dropout rate due to intolerance is around 3.3% [107]; however, tolerability varies.
Clinical Evidence for the Efficacy of Scalp Cooling in Cancer Patients.
Scalp cooling is the only FDA‐cleared technique supported by statistically significant and clinical evidence‐based efficacy for CIA reduction. Numerous studies have demonstrated that its clinical efficiency can reach ∼90% depending on the chemotherapy agent and/or cooling technique used [64], [67].
Auvinen et al. showed that scalp cooling resulted in a significant reduction in CIA, with 100% of patients maintaining their hair after doxorubicin treatment, 83.3% after docetaxel, 76.5% after 5‐fluorouracil, epirubicin, and cyclophosphamide (FEC), and 78% after docetaxel or FEC [108].
A larger and prospective multicenter study conducted by van den Hurk et al. (2012) explored the effect of scalp cooling on hair preservation in 1,411 chemotherapy patients between 2006 and 2009 [53]. The data were collected by the Dutch scalp‐cooling registry; the mean age of the subjects was 53, with 86% having treatment for breast cancer and 96% of these being female. Treatments varied depending on the stage of the cancer and consisted of the following: five combinatorial regimens of FEC or docetaxel, doxorubicin, and cyclophosphamide (TAC), plus several monotherapies (single dose of anthracyclines and taxanes). Patients in the study used the Paxman PSC‐1, PSC‐2, or ORBIS scalp‐cooling devices, and the median number of chemotherapy and cooling sessions was four [53]. The results were evaluated by questionnaires, with patients scoring their own hair loss according to the WHO scale. The best results were obtained following monotherapy treatments, for instance, taxanes such as docetaxel (75 mg/cm2) or paclitaxel (70–90 mg/cm2), with 94% and 81% of patients, respectively, not requiring a wig. The results were less impressive in the case of the TAC combo therapy, even when used at low doses; only 8% of patients did not require a wig. Overall, 50% of all 1,411 patients surveyed did not use head covering at the time of their last treatment. van den Hurk et al. (2010) reported that besides the specific chemotherapy protocol, other factors can have an influence on the use of head cover, such as patient age (generally it is higher in those over 50), gender, ethnicity, and wetting before scalp cooling [53].
Schaffrin‐Nabe et al. found that of 226 patients with variable chemotherapy regimens, 146 (88%) had positive results from scalp cooling and did not require a head cover. The worst results were obtained with the highest anthracyline doses or polytherapies or when TAC was administered. Documentation of other variables, however, identified some of the factors other than high drug dose that affect the success of cooling, and these included comorbidity, current medications, age, menopause, hair thickness, and nicotine intake [107]. Moreover, Komen et al. (2016) showed that of 62 breast cancer patients (median age 60) treated with up to six cycles (median three cycles) of anthracycline (epirubicin or adriamycin) chemotherapy, 13 (12%) did not require a wig [56]. Cigler et al (2015) evaluated the effects of scalp cooling on 20 patients receiving docetaxel and cyclophosphamide with a total of four cycles over 3‐week intervals. Scalps were cooled 50 minutes before administration and for 4 hours afterwards. Upon follow‐up, only 2 of 20 patients felt the need to wear a wig, whereas normally the vast majority undergo complete alopecia [54]. Ibrahim et al. found that scalp cooling prevented up to 96% of patients from requiring a wig after repeated cycles of taxanes or anthracycline, and for those who did, it was due to higher doses of anthracycline treatment [109].
More recently, Nangia and colleagues reported the results of the Scalp Cooling Alopecia Prevention clinical trial [110]. This is the first randomized, multicenter trial (RCT) on scalp cooling (and the first RCT using scalp‐cooling devices) and was performed from 2013 to 2016. It tested the efficacy of cooling on 192 patients, with 119 patients receiving anthracycline or taxane treatment versus 63 receiving no intervention (controls). All patients in the control group needed a wig, whereas 50% of patients receiving scalp cooling did not. This study was terminated on ethical grounds because the chance of preventing CIA using scalp cooling was so significant [110].
In most studies, the precooling period has been between 5 and 30 minutes to ensure that the scalp is cooled when the drugs reach the HFs [111], [112], [113]; however, recent evidence suggests that it should be around 30 minutes [56], [105]. Another equally important consideration during scalp cooling is the period of time necessary to maintain cooling following completion of drug administration (infusion). Routinely, the cap remains in place during the administration of the chemotherapy drugs and for a period after this, referred to as the postinfusion cooling time (PICT), which allows the drug concentration to drop below toxic levels before the HFs warm up. Although until recently a 90‐minute PICT was recommended, van den Hurk et al. (2012) specifically examined the effect of PICT in reducing CIA after docetaxel treatment and found that better results were obtained by reducing PICT from 90 minutes to 45 minutes [114]. This is presumably because once the plasma concentration of docetaxel drops below toxic levels, the warming of the scalp allows any drug that has accumulated during the course of chemotherapy to be more rapidly “flushed out” of the scalp. This study indicated that some optimization of cooling protocols might be required to improve the efficacy for different chemotherapy regimens [114], [115]. In line with this, Komen et al. (2016) reported that even a 20‐minute PICT is as effective as the 45‐minute period [116]. Therefore, both of these studies represent potentially significant improvements in scalp‐cooling protocols. Shortening the PICT has the additional advantage of reducing the time that patients would be required to spend in the treatment environment.
Although some concerns have been raised as to whether scalp cooling could be associated with a higher incidence of scalp metastasis, there appears to be no evidence for a link between metastasis and scalp cooling [117]. Studies that have been conducted to specifically address this issue in patients with breast cancer confirmed that scalp metastasis occurs very rarely, with an incidence between 0.03% and 3% in individuals who did not receive cooling, and this incidence is no different than that for individuals who received scalp cooling, for whom the incidence was 0.04%–4% [118]. In most cases reported so far, scalp metastases after scalp cooling was not the first metastatic site and thus any that occurred were part of a widespread metastatic disease and not related to scalp cooling. These observations are in accordance with recent studies demonstrating that use of scalp cooling has no effect on the breast cancer patient survival [119]. The lack of any association of scalp cooling with breast cancer metastasis is further supported by a recent, comprehensive systematic review and meta‐analysis reporting that scalp cooling does not increase the incidence of these rare scalp metastases [120]. Moreover, throughout application of scalp cooling, only the outer part of the scalp to a depth of 2 cm is affected, with no alteration of core temperature, excluding any risk of hypothermia [101]. However, patients who are at risk of cold‐induced urticaria, cold agglutinin disease, cryoglobulinemia, and post‐traumatic cold dystrophy should be excluded from scalp cooling [109].
In most cases reported so far, scalp metastases after scalp cooling was not the first metastatic site and thus any that occurred were part of a widespread metastatic disease and not related to scalp cooling. These observations are in accordance with recent studies demonstrating that use of scalp cooling has no effect on the breast cancer patient survival.
Conclusion
Despite the success of adjunct chemotherapy in improving the outcome of cancers such as breast cancer, hair loss still represents a very significant psychological burden for cancer patients. Any intervention that could reduce the side effects of chemotherapy would be expected to lead to improvements in both the initiation and completion of therapy, in patient quality of life, and possibly survival outcomes. Having provided a review of several biological and clinical aspects of CIA, here we ultimately focused on research demonstrating that scalp cooling is currently the only available safe and effective option for CIA reduction/prevention. Despite the well‐established ∼50% success rate of scalp cooling, clinical and biological evidence suggests that further improvement can be made. Improvements relating to changes in PICT have clearly demonstrated this. Another important aspect is the efficacy in delivering adequately low temperature to the scalp, and improving clinical staff expertise in fitting the cap, as well as the possibility of patient‐specific cap design, could prove important in increasing the currently reported efficacy of scalp cooling. Finally, an improved understanding of the biological mechanisms of cooling may not only inform the cap design or temperature of choice, but also provide novel avenues for enhancing the capacity of scalp cooling to protect from CIA.
Acknowledgments
The authors would like to thank Paxman Coolers Ltd. for useful discussions. I.S.H. and N.T.G were joint senior authors on this work.
Footnotes
For Further Reading: Manon M.C. Komen, Carolien H. Smorenburg, Corina J.G. van den Hurk et al. Factors Influencing the Effectiveness of Scalp Cooling in the Prevention of Chemotherapy‐Induced Alopecia. The Oncologist 2013;18:885–891.
Implications for Practice: Despite the continuous development of cytotoxics and new targeted therapies, chemotherapy‐induced alopecia (CIA) remains a major problem. The ongoing underestimation by medical professionals of the high impact of CIA for patients and their relatives has resolved in minimal efforts to prevent CIA. The literature is mainly restricted to patient series evaluating the effectiveness. Future research should focus on determinants of the result, in particular, scalp cooling temperature and time. Reviews on scalp cooling clearly show that scalp cooling is an effective method to prevent CIA. Scalp cooling should therefore be available in every hospital and health care professionals should offer the possibility of scalp cooling to all eligible patients.
Author Contributions
Conception/design: Christopher John Dunnill, Iain Stuart Haslam, Nikolaos Theodoros Georgopoulos
Manuscript writing: Christopher John Dunnill, Wafaa Al‐Tameemi, Andrew Collett, Iain Stuart Haslam, Nikolaos Theodoros Georgopoulos
Final approval of manuscript: NT Georgopoulos
Disclosures
The authors indicated no financial relationships.
References
- 1. Hesketh PJ, Batchelor D, Golant M et al. Chemotherapy‐induced alopecia: Psychosocial impact and therapeutic approaches. Support Care Cancer 2004;12:543–549. [DOI] [PubMed] [Google Scholar]
- 2. Choi EK, Kim IR, Chang O et al. Impact of chemotherapy‐induced alopecia distress on body image, psychosocial well‐being, and depression in breast cancer patients. Psychooncology 2014;23:1103–1110. [DOI] [PubMed] [Google Scholar]
- 3. Paus R. Therapeutic strategies for treating hair loss. Drug Discov Today Ther Strateg 2006;3:101–110. [Google Scholar]
- 4. Forrest G, Plumb C, Ziebland S et al. Breast cancer in the family–Children's perceptions of their mother's cancer and its initial treatment: Qualitative study. BMJ 2006;332:998–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pickard‐Holley S. The symptom experience of alopecia. Semin Oncol Nurs 1995;11:235–238. [DOI] [PubMed] [Google Scholar]
- 6. Spiegel D, Giese‐Davis J. Depression and cancer: Mechanisms and disease progression. Biol Psychiatry 2003;54:269–282. [DOI] [PubMed] [Google Scholar]
- 7. Hilton S, Hunt K, Emslie C et al. Have men been overlooked? A comparison of young men and women's experiences of chemotherapy‐induced alopecia. Psychooncology 2008;17:577–583. [DOI] [PubMed] [Google Scholar]
- 8. Peerbooms M, van den Hurk CJ, Breed WP. Familiarity, opinions, experiences and knowledge about scalp cooling: A dutch survey among breast cancer patients and oncological professionals. Asia Pac J Oncol Nurs 2015;2:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schneider MR, Schmidt‐Ullrich R, Paus R. The hair follicle as a dynamic miniorgan. Curr Biol 2009;19:R132–R142. [DOI] [PubMed] [Google Scholar]
- 10. Hadshiew IM, Foitzik K, Arck PC et al. Burden of hair loss: Stress and the underestimated psychosocial impact of telogen effluvium and androgenetic alopecia. J Invest Dermatol 2004;123:455–457. [DOI] [PubMed] [Google Scholar]
- 11. Paus R, Haslam IS, Sharov AA et al. Pathobiology of chemotherapy‐induced hair loss. Lancet Oncol 2013;14:e50–e59. [DOI] [PubMed] [Google Scholar]
- 12. Whiting DA. The structure of the human hair follicle. Fairfield, NJ: Canfield Publishing, 2004.
- 13. Paus R, Muller‐Rover S, Van Der Veen C et al. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol 1999;113:523–532. [DOI] [PubMed] [Google Scholar]
- 14. Purba TS, Haslam IS, Poblet E et al. Human epithelial hair follicle stem cells and their progeny: Current state of knowledge, the widening gap in translational research and future challenges. Bioessays 2014;36:513–525. [DOI] [PubMed] [Google Scholar]
- 15. Randall VA, Botchkareva NV. The biology of hair growth. In: Ahluwalia FS, ed. Cosmetic applications of laser and light based systems. Amsterdam, Netherlands: Elsevier Inc, 2008:3–35.
- 16. Paus R, Foitzik K. In search of the “hair cycle clock”: A guided tour. Differentiation 2004;72:489–511. [DOI] [PubMed] [Google Scholar]
- 17. Stenn K, Parimoo S, Prouty S. Growth of the hair follicle: A cycling and regenerating biological system. Molecular basis of epithelial appendage morphogenesis. Austin, TX: R.G. Landes, 1998:111–130.
- 18. Tobin DJ, Magerl M, Gunin A et al. Plasticity and cytokinetic dynamics of the hair follicle mesenchyme: Implications for hair growth control. J Invest Dermatol 2003;120:895–904. [DOI] [PubMed] [Google Scholar]
- 19. Legue E, Nicolas JF. Hair follicle renewal: Organization of stem cells in the matrix and the role of stereotyped lineages and behaviors. Development 2005;132:4143–4154. [DOI] [PubMed] [Google Scholar]
- 20. Lockshin RA, Zakeri Z. Apoptosis, autophagy, and more. Int J Biochem Cell Biol 2004;36:2405–2419. [DOI] [PubMed] [Google Scholar]
- 21. Lindner G, Botchkarev VA, Botchkareva NV et al. Analysis of apoptosis during hair follicle regression (catagen). Am J Pathol 1997;151:1601–1617. [PMC free article] [PubMed] [Google Scholar]
- 22. Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol 2002;118:216–225. [DOI] [PubMed] [Google Scholar]
- 23. Xie G, Wang H, Yan Z et al. Testing chemotherapeutic agents in the feather follicle identifies a selective blockade of cell proliferation and a key role for sonic hedgehog signaling in chemotherapy‐induced tissue damage. J Invest Dermatol 2015;135:690–700. [DOI] [PubMed] [Google Scholar]
- 24. Epstein EH Jr, Lutzner MA. Folliculitis induced by actinomycin D. N Engl J Med 1969;281:1094–1096. [DOI] [PubMed] [Google Scholar]
- 25. Tong X, Coulombe PA. Keratin 17 modulates hair follicle cycling in a tnfalpha‐dependent fashion. Genes Dev 2006;20:1353–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hébert JM, Rosenquist T, Götz J et al. FGF5 as a regulator of the hair growth cycle: Evidence from targeted and spontaneous mutations. Cell 1994;78:1017–1025. [DOI] [PubMed] [Google Scholar]
- 27. Higgins CA, Christiano AM. Regenerative medicine and hair loss: How hair follicle culture has advanced our understanding of treatment options for androgenetic alopecia. Regen Med 2014;9:101–111. [DOI] [PubMed] [Google Scholar]
- 28. Ito T, Ito N, Saathoff M et al. Interferon‐gamma is a potent inducer of catagen‐like changes in cultured human anagen hair follicles. Br J Dermatol 2005;152:623–631. [DOI] [PubMed] [Google Scholar]
- 29. Peters EM, Liotiri S, Bodo E et al. Probing the effects of stress mediators on the human hair follicle: Substance P holds central position. Am J Pathol 2007;171:1872–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ohnemus U, Uenalan M, Conrad F et al. Hair cycle control by estrogens: Catagen induction via estrogen receptor (ER)‐alpha is checked by ER beta signaling. Endocrinology 2005;146:1214–1225. [DOI] [PubMed] [Google Scholar]
- 31. Soma T, Ogo M, Suzuki J et al. Analysis of apoptotic cell death in human hair follicles in vivo and in vitro. J Invest Dermatol 1998;111:948–954. [DOI] [PubMed] [Google Scholar]
- 32. Botchkareva NV, Ahluwalia G, Shander D. Apoptosis in the hair follicle. J Invest Dermatol 2006;126:258–264. [DOI] [PubMed] [Google Scholar]
- 33. Brajac I, Tkalčić M, Dragojević DM et al. Roles of stress, stress perception and trait‐anxiety in the onset and course of alopecia areata. J Dermatol 2003;30:871–878. [DOI] [PubMed] [Google Scholar]
- 34. O'Shaughnessy RF, Christiano AM. Inherited disorders of the skin in human and mouse: From development to differentiation. Int J Dev Biol 2004;48:171–179. [DOI] [PubMed] [Google Scholar]
- 35. Oh JW, Kloepper J, Langan EA et al. A guide to studying human hair follicle cycling in vivo. J Invest Dermatol 2016;136:34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Geyfman M, Plikus MV, Treffeisen E et al. Resting no more: Re‐defining telogen, the maintenance stage of the hair growth cycle. Biol Rev Camb Philos Soc 2015;90:1179–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Plikus MV, Chuong CM. Macroenvironmental regulation of hair cycling and collective regenerative behavior. Cold Spring Harb Perspect Med 2014;4:015198a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Botchkareva NV, Khlgatian M, Longley BJ et al. SCF/c‐kit signaling is required for cyclic regeneration of the hair pigmentation unit. FASEB J 2001;15:645–658. [DOI] [PubMed] [Google Scholar]
- 39. Payne AS, James WD, Weiss RB. Dermatologic toxicity of chemotherapeutic agents. Semin Oncol 2006;33:86–97. [DOI] [PubMed] [Google Scholar]
- 40. Symonds RP, Foweraker K. Principles of chemotherapy and radiotherapy. Curr Obstet Gynecol 2006;16:100–106. [Google Scholar]
- 41. Tannock IF, Hill RP, Bristow RG, Harrington L. The Basic Science Of Oncology. Fifth edition. NY, USA: McGraw-Hill education, 2013. ISBN: 9780071745208 [Google Scholar]
- 42. Trigg ME, Flanigan‐Minnick A. Mechanisms of action of commonly used drugs to treat cancer. Community Oncol 2011;8:357–369. [Google Scholar]
- 43. Lilenbaum RC, Herndon JE 2nd, List MA et al. Single‐agent versus combination chemotherapy in advanced non‐small‐cell lung cancer: The cancer and leukemia group B (study 9730). J Clin Oncol 2005;23:190–196. [DOI] [PubMed] [Google Scholar]
- 44. Panieri E, Santoro MM. ROS homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death Dis 2016;7:e2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gupta SC, Hevia D, Patchva S et al. Upsides and downsides of reactive oxygen species for cancer: The roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid Redox Signal 2012;16:1295–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nicolson GL, Conklin KA. Reversing mitochondrial dysfunction, fatigue and the adverse effects of chemotherapy of metastatic disease by molecular replacement therapy. Clin Exp Metastasis 2008;25:161–169. [DOI] [PubMed] [Google Scholar]
- 47. Simon HU, Haj‐Yehia A, Levi‐Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000;5:415–418. [DOI] [PubMed] [Google Scholar]
- 48. Haslam IS, Jadkauskaite L, Szabo IL et al. Oxidative damage control in a human (mini‐) organ: Nrf2 activation protects against oxidative stress‐induced hair growth inhibition. J Invest Dermatol 2017;137:295–304. [DOI] [PubMed] [Google Scholar]
- 49. Bodo E, van Beek N, Naumann V et al. Modulation of chemotherapy‐induced human hair follicle damage by 17‐beta estradiol and prednisolone: Potential stimulators of normal hair regrowth by “dystrophic catagen” promotion? J Invest Dermatol 2009;129:506–509. [DOI] [PubMed] [Google Scholar]
- 50. Amoh Y, Li L, Katsuoka K et al. Chemotherapy targets the hair‐follicle vascular network but not the stem cells. J Invest Dermatol 2007;127:11–15. [DOI] [PubMed] [Google Scholar]
- 51. Batchelor D. Hair and cancer chemotherapy: Consequences and nursing care–A literature study. Eur J Cancer Care (Engl) 2001;10:147–163. [DOI] [PubMed] [Google Scholar]
- 52. Wilkes G. Potential toxicities and nursing management. In: Cancer chemotherapy: A nursing process approach. Boston, MA: Jones & Barlett, 1996.
- 53. van den Hurk CJ, Peerbooms M, van de Poll‐Franse LV et al. Scalp cooling for hair preservation and associated characteristics in 1411 chemotherapy patients ‐ Results of the Dutch Scalp Cooling Registry. Acta Oncol 2012;51:497–504. [DOI] [PubMed] [Google Scholar]
- 54. Cigler T, Isseroff D, Fiederlein B et al. Efficacy of scalp cooling in preventing chemotherapy‐induced alopecia in breast cancer patients receiving adjuvant docetaxel and cyclophosphamide chemotherapy. Clin Breast Cancer 2015;15:332–334. [DOI] [PubMed] [Google Scholar]
- 55. Trueb RM. Chemotherapy‐induced hair loss. Skin Therapy Lett 2010;15:5–7. [PubMed] [Google Scholar]
- 56. Komen MM, Smorenburg CH, Nortier JW et al. Results of scalp cooling during anthracycline containing chemotherapy depend on scalp skin temperature. Breast 2016;30:105–110. [DOI] [PubMed] [Google Scholar]
- 57. Oshima H, Rochat A, Kedzia C et al. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell 2001;104:233–245. [DOI] [PubMed] [Google Scholar]
- 58. Tosti A, Piraccini BM, Vincenzi C et al. Permanent alopecia after busulfan chemotherapy. Br J Dermatol 2005;152:1056–1058. [DOI] [PubMed] [Google Scholar]
- 59. Cotsarelis G. Epithelial stem cells: A folliculocentric view. J Invest Dermatol 2006;126:1459–1468. [DOI] [PubMed] [Google Scholar]
- 60. Tran D, Sinclair RD, Schwarer AP et al. Permanent alopecia following chemotherapy and bone marrow transplantation. Australas J Dermatol 2000;41:106–108. [DOI] [PubMed] [Google Scholar]
- 61. Bresters D, Wanders DCM, Louwerens M et al. Permanent diffuse alopecia after haematopoietic stem cell transplantation in childhood. Bone Marrow Transplant 2017;52:984–988. [DOI] [PubMed] [Google Scholar]
- 62. Tallon B, Blanchard E, Goldberg LJ. Permanent chemotherapy‐induced alopecia: Case report and review of the literature. J Am Acad Dermatol 2010;63:333–336. [DOI] [PubMed] [Google Scholar]
- 63. Prevezas C, Matard B, Pinquier L et al. Irreversible and severe alopecia following docetaxel or paclitaxel cytotoxic therapy for breast cancer. Br J Dermatol 2009;160:883–885. [DOI] [PubMed] [Google Scholar]
- 64. Shin H, Jo SJ, Kim DH et al. Efficacy of interventions for prevention of chemotherapy‐induced alopecia: A systematic review and meta‐analysis. Int J Cancer 2015;136:E442–E454. [DOI] [PubMed] [Google Scholar]
- 65. Soref CM, Fahl WE. A new strategy to prevent chemotherapy and radiotherapy‐induced alopecia using topically applied vasoconstrictor. Int J Cancer 2015;136:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bohm M, Bodo E, Funk W et al. Alpha‐melanocyte‐stimulating hormone: A protective peptide against chemotherapy‐induced hair follicle damage? Br J Dermatol 2014;170:956–960. [DOI] [PubMed] [Google Scholar]
- 67. Grevelman EG, Breed WP. Prevention of chemotherapy‐induced hair loss by scalp cooling. Ann Oncol 2005;16:352–358. [DOI] [PubMed] [Google Scholar]
- 68. Sredni B, Xu RH, Albeck M et al. The protective role of the immunomodulator AS101 against chemotherapy‐induced alopecia studies on human and animal models. Int J Cancer 1996;65:97–103. [DOI] [PubMed] [Google Scholar]
- 69. Young A, Arif A. The use of scalp cooling for chemotherapy‐induced hair loss. Br J Nurs 2016;25:S22, S24–S27. [DOI] [PubMed] [Google Scholar]
- 70. Balsari AL, Morelli D, Menard S et al. Protection against doxorubicin‐induced alopecia in rats by liposome‐entrapped monoclonal antibodies. FASEB J 1994;8:226–230. [DOI] [PubMed] [Google Scholar]
- 71. Morelli D, Menard S, Cazzaniga S et al. Intratibial injection of an anti‐doxorubicin monoclonal antibody prevents drug‐induced myelotoxicity in mice. Br J Cancer 1997;75:656–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Morelli D, Menard S, Colnaghi MI et al. Oral administration of anti‐doxorubicin monoclonal antibody prevents chemotherapy‐induced gastrointestinal toxicity in mice. Cancer Res 1996;56:2082–2085. [PubMed] [Google Scholar]
- 73. Balsari A, Rumio C, Morelli D et al. Topical administration of a doxorubicin‐specific monoclonal antibody prevents drug‐induced mouth apoptosis in mice. Br J Cancer 2001;85:1964–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jimenez JJ, Huang HS, Yunis AA. Treatment with ImuVert/N‐acetylcysteine protects rats from cyclophosphamide/cytarabine‐induced alopecia. Cancer Invest 1992;10:271–276. [DOI] [PubMed] [Google Scholar]
- 75. Taylor M, Ashcroft AT, Messenger AG. Cyclosporin a prolongs human hair growth in vitro. J Invest Dermatol 1993;100:237–239. [DOI] [PubMed] [Google Scholar]
- 76. Hawkshaw NJ, Haslam IS, Ansell DM et al. Re‐evaluating cyclosporine a as a hair growth‐promoting agent in human scalp hair follicles. J Invest Dermatol 2015;135:2129–2132. [DOI] [PubMed] [Google Scholar]
- 77. Hussein A, Stuart A, Peters W. Protection against chemotherapy‐induced alopecia by cyclosporin a in the newborn rat animal model. Dermatology 2009;190:192–196. [DOI] [PubMed] [Google Scholar]
- 78. Duvic M, Lemak NA, Valero V et al. A randomized trial of minoxidil in chemotherapy‐induced alopecia. J Am Acad Dermatol 1996;35:74–78. [DOI] [PubMed] [Google Scholar]
- 79. Hussein AM. Protection against cytosine arabinoside‐induced alopecia by minoxidil in a rat animal model. Int J Dermatol 1995;34:470–473. [DOI] [PubMed] [Google Scholar]
- 80. Olsen EA, Whiting D, Bergfeld W et al. A multicenter, randomized, placebo‐controlled, double‐blind clinical trial of a novel formulation of 5% minoxidil topical foam versus placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol 2007;57:767–774. [DOI] [PubMed] [Google Scholar]
- 81. Jimenez JJ, Yunis AA. Protection from 1‐beta‐d‐arabinofuranosylcytosine‐induced alopecia by epidermal growth factor and fibroblast growth factor in the rat model. Cancer Res 1992;52:413–415. [PubMed] [Google Scholar]
- 82. Hussein AM. Chemotherapy‐induced alopecia: New developments. South Med J 1993;86:489–496. [DOI] [PubMed] [Google Scholar]
- 83. Knockaert M, Greengard P, Meijer L. Pharmacological inhibitors of cyclin-dependent kinases. Trends in pharmacological sciences 2002;23:417–425. [DOI] [PubMed] [Google Scholar]
- 84. Kobayashi T, Okumura H, Hashimoto K et al. Synchronization of normal human keratinocyte in culture: Its application to the analysis of 1,25‐dihydroxyvitamin D3 effects on cell cycle. J Dermatol Sci 1998;17:108–114. [DOI] [PubMed] [Google Scholar]
- 85. Wang J, Lu Z, Au JL. Protection against chemotherapy‐induced alopecia. Pharm Res 2006;23:2505–2514. [DOI] [PubMed] [Google Scholar]
- 86. Jimenez JJ, Yunis AA. Protection from chemotherapy‐induced alopecia by 1,25‐dihydroxyvitamin D3. Cancer Res 1992;52:5123–5125. [PubMed] [Google Scholar]
- 87. Paus R, Schilli MB, Handjiski B et al. Topical calcitriol enhances normal hair regrowth but does not prevent chemotherapy‐induced alopecia in mice. Cancer Res 1996;56:4438–4443. [PubMed] [Google Scholar]
- 88. Schilli MB, Paus R, Menrad A. Reduction of intrafollicular apoptosis in chemotherapy‐induced alopecia by topical calcitriol‐analogs. J Invest Dermatol 1998;111:598–604. [DOI] [PubMed] [Google Scholar]
- 89. Porter AG, Janicke RU. Emerging roles of caspase‐3 in apoptosis. Cell Death Differ 1999;6:99–104. [DOI] [PubMed] [Google Scholar]
- 90. Tsuda T, Ohmori Y, Muramatsu H et al. Inhibitory effect of M50054, a novel inhibitor of apoptosis, on anti‐Fas‐antibody‐induced hepatitis and chemotherapy‐induced alopecia. Eur J Pharmacol 2001;433:37–45. [DOI] [PubMed] [Google Scholar]
- 91. Gensure RC. Parathyroid hormone‐related peptide and the hair cycle ‐ Is it the agonists or the antagonists that cause hair growth? Exp Dermatol 2014;23:865–867. [DOI] [PubMed] [Google Scholar]
- 92. Skrok A, Bednarczuk T, Skwarek A et al. The effect of parathyroid hormones on hair follicle physiology: Implications for treatment of chemotherapy‐induced alopecia. Skin Pharmacol Physiol 2015;28:213–225. [DOI] [PubMed] [Google Scholar]
- 93. O'Brien R, Zelson JH, Schwartz AD et al. Scalp tourniquet to lessen alopecia after vincristine. N Engl J Med 1970;283:1469. [DOI] [PubMed] [Google Scholar]
- 94. Maxwell MB. Scalp tourniquets for chemotherapy‐induced alopecia. Am J Nurs 1980;80:900–903. [PubMed] [Google Scholar]
- 95. Protiere C, Evans K, Camerlo J et al. Efficacy and tolerance of a scalp‐cooling system for prevention of hair loss and the experience of breast cancer patients treated by adjuvant chemotherapy. Support Care Cancer 2002;10:529–537. [DOI] [PubMed] [Google Scholar]
- 96. Janssen FP, Rajan V, Steenbergen W et al. The relationship between local scalp skin temperature and cutaneous perfusion during scalp cooling. Physiol Meas 2007;28:829–839. [DOI] [PubMed] [Google Scholar]
- 97. Bülow J, Friberg L, Gaardsting O et al. Frontal subcutaneous blood flow, and epi‐and subcutaneous temperatures during scalp cooling in normal man. Scand J Clin Lab Invest 1985;45:505–508. [DOI] [PubMed] [Google Scholar]
- 98. Lane P, Vichi P, Bain DL et al. Temperature dependence studies of adriamycin uptake and cytotoxicity. Cancer Res 1987;47:4038–4042. [PubMed] [Google Scholar]
- 99. Watanabe I, Okada S. Effects of temperature on growth rate of cultured mammalian cells (L5178Y). J Cell Biol 1967;32:309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Vichi P, Robison S, Tritton TR. Temperature dependence of adriamycin‐induced DNA damage in L1210 cells. Cancer Res 1989;49:5575–5580. [PubMed] [Google Scholar]
- 101. Janssen FE, Van Leeuwen GM, Van Steenhoven AA. Modelling of temperature and perfusion during scalp cooling. Phys Med Biol 2005;50:4065–4073. [DOI] [PubMed] [Google Scholar]
- 102. Al‐Tameemi W, Dunnill C, Hussain O et al. Use of in vitro human keratinocyte models to study the effect of cooling on chemotherapy drug‐induced cytotoxicity. Toxicol In Vitro 2014;28:1366–1376. [DOI] [PubMed] [Google Scholar]
- 103. Guy R, Shah S, Parker H et al. Scalp cooling by thermocirculator. Lancet 1982;1:937–938. [DOI] [PubMed] [Google Scholar]
- 104. Katsimbri P, Bamias A, Pavlidis N. Prevention of chemotherapy‐induced alopecia using an effective scalp cooling system. Eur J Cancer 2000;36:766–771. [DOI] [PubMed] [Google Scholar]
- 105. Pliskow B, Mitra K, Kaya M. Simulation of scalp cooling by external devices for prevention of chemotherapy‐induced alopecia. J Therm Biol 2016;56:31–38. [DOI] [PubMed] [Google Scholar]
- 106. Massey CS. A multicentre study to determine the efficacy and patient acceptability of the paxman scalp cooler to prevent hair loss in patients receiving chemotherapy. Eur J Oncol Nurs 2004;8:121–130. [DOI] [PubMed] [Google Scholar]
- 107. Schaffrin‐Nabe D, Schmitz I, Josten‐Nabe A et al. The influence of various parameters on the success of sensor‐controlled scalp cooling in preventing chemotherapy‐induced alopecia. Oncol Res Treat 2015;38:489–495. [DOI] [PubMed] [Google Scholar]
- 108. Auvinen PK, Mahonen UA, Soininen KM et al. The effectiveness of a scalp cooling cap in preventing chemotherapy‐induced alopecia. Tumori 2010;96:271–275. [DOI] [PubMed] [Google Scholar]
- 109. Ibrahim T, Kattan J, Assi T et al. Efficacy of a silicon based continuous scalp cooling system with thermostat on chemotherapy induced alopecia. J Palliat Care Med 2015;05:209. [Google Scholar]
- 110. Nangia J, Wang T, Osborne C et al. Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: The scalp randomized clinical trial. JAMA 2017;317:596–605. [DOI] [PubMed] [Google Scholar]
- 111. Lemenager M, Lecomte S, Bonneterre ME et al. Effectiveness of cold cap in the prevention of docetaxel‐induced alopecia. Eur J Cancer 1997;33:297–300. [DOI] [PubMed] [Google Scholar]
- 112. Anderson JE, Hunt JM, Smith IE. Prevention of doxorubicin‐induced alopecia by scalp cooling in patients with advanced breast cancer. Br Med J (Clin Res Ed) 1981;282:423–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Vendelbo Johansen L. Scalp hypothermia in the prevention of chemotherapy‐induced alopecia. Acta Radiol Oncol 1985;24:113–116. [DOI] [PubMed] [Google Scholar]
- 114. van den Hurk CJ, Breed WP, Nortier JW. Short post‐infusion scalp cooling time in the prevention of docetaxel‐induced alopecia. Support Care Cancer 2012;20:3255–3260. [DOI] [PubMed] [Google Scholar]
- 115. Komen MM, Smorenburg CH, van den Hurk CJ et al. Factors influencing the effectiveness of scalp cooling in the prevention of chemotherapy‐induced alopecia. The Oncologist 2013;18:885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Komen MM, Breed WP, Smorenburg CH et al. Results of 20‐ versus 45‐min post‐infusion scalp cooling time in the prevention of docetaxel‐induced alopecia. Support Care Cancer 2016;24:2735–2741. [DOI] [PubMed] [Google Scholar]
- 117. Lemieux J, Desbiens C, Hogue JC. Breast cancer scalp metastasis as first metastatic site after scalp cooling: Two cases of occurrence after 7‐ and 9‐year follow‐up. Breast Cancer Res Treat 2011;128:563–566. [DOI] [PubMed] [Google Scholar]
- 118. Lemieux J, Amireault C, Provencher L et al. Incidence of scalp metastases in breast cancer: A retrospective cohort study in women who were offered scalp cooling. Breast Cancer Res Treat 2009;118:547–552. [DOI] [PubMed] [Google Scholar]
- 119. Lemieux J, Provencher L, Perron L et al. No effect of scalp cooling on survival among women with breast cancer. Breast Cancer Res Treat 2015;149:263–268. [DOI] [PubMed] [Google Scholar]
- 120. Rugo HS, Melin SA, Voigt J. Scalp cooling with adjuvant/neoadjuvant chemotherapy for breast cancer and the risk of scalp metastases: Systematic review and meta‐analysis. Breast Cancer Res Treat 2017;163:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Haslam IS, El‐Chami C, Faruqi H et al. Differential expression and functionality of atp‐binding cassette transporters in the human hair follicle. Br J Dermatol 2015;172:1562–1572. [DOI] [PubMed] [Google Scholar]
- 122. Payne S, Miles D. Mechanisms of anticancer drugs. In: Gleeson M, ed. Scott‐Brown's Otorhinolaryngology: Head and Neck Surgery. 8th ed. Boca Raton, FL: CRC Press, 2008:34–46.
- 123. Trüeb RM. The difficult hair loss patient. Guide to successful management of alopecia and related conditions. Cham, Switzerland: Springer International Publishing, 2015.
- 124. Wikramanayake TC, Amini S, Simon J et al. A novel rat model for chemotherapy‐induced alopecia. Clin Exp Dermatol 2012;37:284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hussein AM, Jimenez JJ, McCall CA et al. Protection from chemotherapy‐induced alopecia in a rat model. Science 1990;249:1564–1566. [DOI] [PubMed] [Google Scholar]
- 126. Paus R, Handjiski B, Eichmüller S et al. Chemotherapy‐induced alopecia in mice. Induction by cyclophosphamide, inhibition by cyclosporine A, and modulation by dexamethasone. Am J Pathol 1994;144:719. [PMC free article] [PubMed] [Google Scholar]
- 127. Manning DD, Reed ND, Shaffer CF. Maintenance of skin xenografts of widely divergent phylogenetic origin of congenitally athymic (nude) mice. J Exp Med 1973;138:488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Domashenko A, Gupta S, Cotsarelis G. Efficient delivery of transgenes to human hair follicle progenitor cells using topical lipoplex. Nat Biotechnol 2000;18:420–423. [DOI] [PubMed] [Google Scholar]
- 129. Kyoizumi S, Suzuki T, Teraoka S et al. Radiation sensitivity of human hair follicles in SCID‐hu mice. Radiat Res 1998;149:11–18. [PubMed] [Google Scholar]
- 130. Philpott MP, Green MR, Kealey T. Human hair growth in vitro. J Cell Sci 1990;97(Pt 3):463–471. [DOI] [PubMed] [Google Scholar]
- 131. Bodo E, Tobin DJ, Kamenisch Y et al. Dissecting the impact of chemotherapy on the human hair follicle: A pragmatic in vitro assay for studying the pathogenesis and potential management of hair follicle dystrophy. Am J Pathol 2007;171:1153–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]