The Nutrition and Exercise during Adjuvant Treatment (NExT) study was designed to assess the effectiveness of a supervised exercise and healthy eating program offered as part of supportive care in a real‐world setting for breast cancer patients receiving adjuvant chemotherapy. Reach, effectiveness, maintenance, and implementation were assessed, and the results are reported here.
Keywords: Breast neoplasms, Exercise, Drug therapy, Quality of life
Abstract
Background.
Randomized trials have established efficacy of supervised exercise training during chemotherapy for breast cancer for numerous health outcomes. The purpose of this study was to assess reach, effectiveness, maintenance, and implementation of an evidence‐based exercise and healthy eating program offered within an adjuvant care setting.
Subjects, Materials, and Methods.
Women receiving adjuvant chemotherapy for breast cancer were given a prescription by their oncologist to participate in the Nutrition and Exercise during Adjuvant Treatment (NExT) program. The NExT program consisted of supervised, moderate‐intensity, aerobic and resistance exercise three times a week during adjuvant therapy, followed by a step‐down in supervised sessions per week for 20 additional weeks, plus one group‐based healthy eating session. Usual moderate‐to‐vigorous physical activity (MVPA) and health‐related quality of life (HRQoL) were assessed by questionnaire at baseline, program completion, and one year later, along with measures of satisfaction and safety.
Results.
Program reach encompassed referral of 53% of eligible patients, 78% uptake (n = 73 enrolled), and 78% retention for the 45.0 ± 8.3‐week program. During the program, MVPA increased (116 ± 14 to 154 ± 14 minutes per week, p = .014) and HRQoL did not change. One year later, MVPA (171 ± 24 minutes per week, p = .014) and HRQoL (44 ± 1 to 49 ± 1, p < .001) were significantly higher than baseline. Exercise adherence was 60% ± 26% to three sessions per week during treatment. No major adverse events occurred and injury prevalence did not change relative to baseline. Participants were highly satisfied.
Conclusion.
This oncologist‐referred exercise and healthy eating supportive‐care program for breast cancer patients receiving chemotherapy was safe, successful in reaching oncologists and patients, and effective for improving MVPA and maintaining HRQoL.
Implications for Practice.
Despite evidence that exercise is both safe and efficacious at improving physical fitness, quality of life, and treatment side effects for individuals with cancer, lifestyle programming is not offered as standard of cancer care. This study describes an oncologist‐referred, evidence‐based exercise and healthy eating program offered in collaboration with a university as supportive care to women with breast cancer receiving chemotherapy. The program was well received by oncologists and patients, safe, and relatively inexpensive to operate. Importantly, there was a significant positive impact on physical activity levels and health‐related quality of life lasting for 2 years after initiation of therapy.
Introduction
There is compelling evidence from randomized controlled trials that exercise is safe for women diagnosed with breast cancer during treatment and that it improves the side effects of cancer treatment, quality of life, and overall health of cancer survivors [1], [2], [3], [4], [5]. However, cancer survivors report unique barriers to exercise adoption and maintenance, including concerns about safety, desire for guidance from trained professionals with experience working with cancer survivors, and physical limitations related to treatment side effects [6], [7], [8]. In addition, it is now advocated that support for healthy eating and weight management is integrated into clinical oncology care [9]. Currently, however, access to registered dietitians is limited within cancer care, and resources are typically prioritized to tumor sites at high risk for malnutrition and for management of acute symptoms (e.g., head and neck, gastrointestinal).
Women with newly diagnosed breast cancer may increase exercise when referred by their oncologist [10], [11]. However, cancer survivors in the U.S. and Canada have little access to appropriate exercise programming outside of research studies, especially during adjuvant treatment when the need may be greatest. There exists a gap between the scientific evidence and clinical practice in the management of breast and other cancers with respect to exercise and healthy eating programming [12]. Potential reasons for this gap in knowledge translation include concern among clinicians regarding safety, lack of clinician exercise experience/education [13], and lack of established referral pathways to exercise professionals with cancer experience, as well as inadequate registered dietitian staffing.
With efficacy and safety established by randomized trials, the Nutrition and Exercise during Adjuvant Treatment (NExT) study was designed to assess effectiveness of a supervised exercise and healthy eating program offered as part of supportive care in a real‐world setting. New breast cancer patients who were receiving adjuvant chemotherapy were referred to the program by medical oncologists using a prescription to facilitate with patient screening and enhance enrollment. The program was evaluated using the RE‐AIM framework to report on reach, effectiveness, maintenance, and implementation [14]. Our primary aim was to assess the reach of the program (referral rate, uptake, and retention). The secondary aim was to assess effectiveness of the program on physical activity levels and health‐related quality of life (HRQoL), and maintenance of these changes. The tertiary aim was to assess implementation (adherence, cost, participant satisfaction, and safety). Goals for successful referral rate (50%), uptake (70%), retention (70%), and adherence (70%) were chosen a priori by study authors. Based on previous efficacy trials, we hypothesized that physical activity levels and HRQoL would improve by the completion of the program, and that given the length of the program, changes would be maintained 1 year later.
Subjects, Materials, and Methods
Design
The NExT study was a prospective, single‐arm intervention program. As a study assessing effectiveness, it focused on a design that would be feasible to put into practice, in contrast to previous randomized trials that tested a similar intervention under the ideal circumstances of a well‐controlled research environment with an expectation of adherence to the protocol (i.e., an efficacy trial) [15], [16], [17], [18]. The British Columbia Cancer Agency (BCCA) Research Ethics Board approved the study. Participants provided written informed consent.
Patients
English‐speaking adult women with newly diagnosed stage I–IIIA breast cancer who were scheduled to receive adjuvant chemotherapy (with or without radiation) were invited to enroll within the first half of their chemotherapy treatments. Exclusion criteria were conditions requiring closer monitoring of exercise supervision (e.g., uncontrolled or unstable cardiovascular disease or diabetes mellitus), body mass index >40 kg/m2, use of mobility aids, and stage IV/metastatic disease.
Recruitment
Recruitment took place via medical oncologist referral at the Vancouver center of the BCCA. Referrals via word of mouth were also accepted for patients treated at this center. A core team of eight medical oncologists completed and signed a prescription for eligible patients to participate in NExT (Fig. 1). The oncologist gave one copy to their patient and another copy to study staff for referral. The prescription form included documentation of comorbid health conditions and medications and provided clearance to exercise (Fig. 1). The Physical Activity Readiness Questionnaire [19] was administered by study staff via telephone to identify any additional concerns, which were discussed with the oncologist via e‐mail. If an individual contacted the study directly, a prescription was requested from their oncologist.
Figure 1.
Exercise and nutrition program prescription form.
Abbreviations: COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; NExT, Nutrition and Exercise during Adjuvant Treatment.
Sample Size
The goal was to open recruitment for 12 months as a measure of yearly intake for a potential clinical program. However, additional funding allowed recruitment to be extended to a total of 15 months, from August 5, 2013, to October 31, 2014.
Exercise Intervention
The exercise component was managed by a local university, and included aerobic and resistance training based on the benefits established in past trials [15], [16] and recommendations for cancer survivors [20]. Supervised exercise took place at a stand‐alone fitness facility used for individuals with cancer or other chronic diseases located near the cancer treatment center. The goal of the combined supervised and home‐based prescription was to meet the recommendations for cancer survivors of 150 weekly minutes of moderate‐intensity aerobic exercise, and whole‐body resistance training two to three times per week [20]. The exercise program was divided into three phases: treatment (length of chemotherapy, plus radiation if received), post‐treatment (10 weeks), and maintenance (10 weeks). The latter two phases were designed to step‐down the amount of supervised sessions offered, and increase the amount of home‐based exercise encouraged (Table 1).
Table 1. Summary of exercise and healthy eating programming offered in the Nutrition and Exercise during Adjuvant Treatment trial.

Weights were progressed by the minimal possible amount (i.e., 10 lbs for lower body, 2–5 lbs for upper body).
Abbreviations: 1‐RM, one repetition‐maximum; APMHR, age‐predicted maximal heart rate; HRR, heart rate reserve; min, minute; RPE, rating of perceived exertion (Borg 6–20 scale); VO2R, volume of oxygen consumption reserve; wk, week; x, times (number of sessions).
Nutrition Intervention
The healthy eating component consisted of a singular 2‐hour, group‐based healthy eating education session led by a registered dietitian with breast cancer experience; topics are described in Table 1. The session was offered monthly within the cancer treatment center, and the goal was to attend at least once near the start of the program.
Outcome Measures
Reach.
A comprehensive electronic master list of patients scheduled for breast medical oncology consultation appointments at the Vancouver BCCA during the study recruitment dates was used to accurately assess referral rate. A trained staff member identified patients who were referred to the study, then carefully reviewed the medical records of patients who were not referred to ascertain eligibility and specific reasons for noneligibility. Referral rate was calculated as the percentage of eligible patients included in the master list that were referred to the program. Uptake was calculated as the percentage of eligible, referred patients who enrolled. Retention was calculated as the percentage of participants who did not request withdrawal from the study. Representativeness was assessed by age of the study sample relative to those who were eligible but were not referred. This was the only characteristic available for comparison in the electronic list of new patients.
Effectiveness.
Physical activity levels were assessed using a modified version of the Minnesota Leisure Time Physical Activity Questionnaire [21] administered by interview with reference to the 6 months prior to baseline, the end of the program (including supervised and home‐based exercise), and at 1 year after program completion. A metabolic equivalent (MET) score [22] was used to estimate the intensity of each activity. Average moderate‐to‐vigorous physical activity (MVPA) was calculated as the average weekly minutes of aerobic activities with a MET score ≥3.0. The average resistance training performed was calculated as the average weekly minutes of “weight lifting” reported.
Exercise behavior correlates were assessed by questionnaires, including a single rating on a 5‐point Likert scale for exercise enjoyment [23], a 5‐item, 11‐point Likert scale for exercise self‐efficacy [24], and a 21‐item, 5‐point Likert scale for perceived barriers to exercise [23].
Health‐related quality of life was assessed by the physical component summary of the Medical Outcomes Survey RAND‐36, the higher‐ordered cluster based on the physical health variance in common among the eight multi‐item scales [25]. This scale relating to the physical components of quality of life was chosen because it is most likely to respond to interventions that change physical morbidity like exercise [26]. The minimally clinically important difference (MCID) is considered 3–5 points [27].
All of the above measures were assessed at baseline, end of program, and 1‐year follow‐up to end of program, and correlates of exercise behavior and HRQoL were also assessed following completion of treatment.
Implementation.
Adherence was calculated as the number of exercise and healthy eating sessions attended out of the number of prescribed sessions for all participants who started the program. Participants were encouraged to attend as many exercise sessions as they could, and were asked to call or e‐mail if they could not attend. If no notice was provided, or if 2–4 consecutive sessions were missed (determined at the discretion of the gym staff on a case‐by‐case basis), the participant was called or e‐mailed and encouraged to return. The gym was open Monday, Wednesday, and Friday mornings and afternoons except for statutory holidays.
Cost was estimated for running the NExT trial as a clinical program affiliated with a university, and does not include research costs. The personnel costs allow exercise supervision by one lead trainer with a bachelor's degree in Kinesiology to oversee the program, with additional supervision by graduate students in kinesiology or physical therapy, and both paid and volunteer undergraduate kinesiology students to maintain a 1:4 staff to participant ratio. The dietitian costs are to deliver the group session once monthly. The costs do not include leasing of space or major exercise equipment because these costs will vary by location, and there is opportunity to run this program out of an existing public or clinical (e.g., cardiac rehabilitation) facility.
Participant satisfaction was assessed by a researcher‐developed questionnaire. Safety was assessed by tracking injuries and adverse events using a musculoskeletal injury questionnaire [28] completed by the participants at baseline, end of treatment, and end of study, and case reporting forms, respectively. Adverse events were defined as serious medical events (i.e., cardiac, fractures) that occurred at the exercise facility or were attributed to the intervention, and were monitored on an ongoing basis.
Statistical Analysis
A generalized linear mixed model was used to analyze the questionnaire data due to missing data at various time points, and non‐normal distribution of residuals. A random intercept was used with participant as the random factor to control for the nonindependence of measurements within a participant, and time point was included as a repeated and fixed effect. Main effects for time with p ≤ .05 were further interpreted with contrasts between time points using a Bonferroni correction for multiple pairwise comparisons. The assumption of normality of residuals was not met regardless of the model/method used for resistance training; the consistent general findings with all models tested are reported. The McNemar's test was used to compare the prevalence of participants meeting the MVPA guidelines (≥150 minutes per week) and injuries at baseline relative to end of study, as well as MVPA maintenance between end of study and 1‐year follow‐up. A p value of .05 was used to interpret the McNemar's test results.
Results
Reach
The program referral rate was 53% (82 of 154). The master list generated 938 patients who attended a medical oncology consultation during the study recruitment dates, of whom 772 (82%) were ineligible and 154 were eligible (16%) for the study. Reasons for ineligibility included the following: not receiving adjuvant chemotherapy (n = 620), living outside of Vancouver lower mainland (n = 45), non‐English speaking (n = 37), noninvasive breast cancer (n = 34), having stage IV or metastatic breast cancer (n = 17), not having decided on adjuvant treatment plan prior to study closure (n = 8), disability/mobility issues (n = 6), multiple comorbidities and safety concern for group‐based exercise (n = 3), or male gender (n = 2). Medical oncologists referred 82 eligible patients and 12 noneligible patients who were part of this master list. An additional 15 patients were referred who were not part of this list (n = 109 referrals total) due to having their consultation prior to the start of program recruitment (n = 5) and unknown reasons (n = 10).
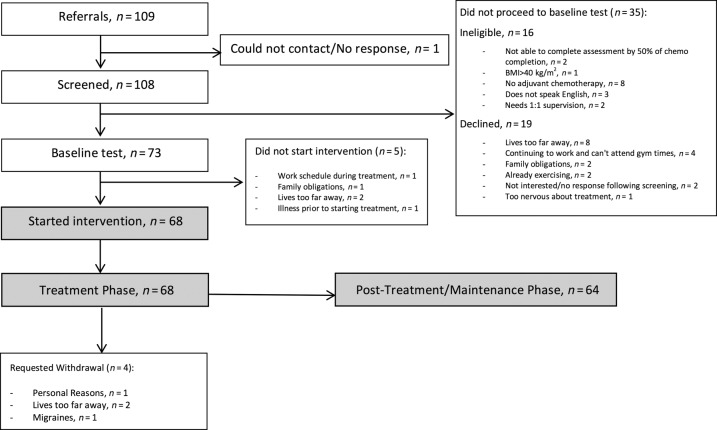
Uptake of the program was 78% (73 of 93). Sixteen of the 109 patients referred were ineligible, and 20 declined participation (Fig. 2). Of the 93 who were referred and eligible, 73 enrolled in the program (Table 2). Nine participants requested withdrawal from the study between enrollment and completion of chemotherapy due to living too far from the gym (n = 4), illness prior to starting chemotherapy (n = 1), migraines (n = 1), work schedule (n = 1), family obligations (n = 1), and personal reasons (n = 1); therefore, retention was 88% for the treatment phase. Five of these participants withdrew prior to starting the program (or completing any exercise sessions). Following treatment completion, an additional seven participants did not attend any further sessions due to moving away (n = 2), returning to work (n = 2), treatment symptoms (n = 1), mental health (n = 1), and unknown reasons (n = 1). Therefore, retention for the entire program duration was 78%. The program length was 45.0 ± 8.3 (27.3–64.9) weeks. Regarding representativeness, participants in the program were significantly younger (50.8 ± 10.6 years) than eligible women who were not referred (55.6 ± 10.6 years, p < .01).
Figure 2.
Flow through study.
Abbreviation: BMI, body mass index.
Table 2. Participant baseline characteristics.
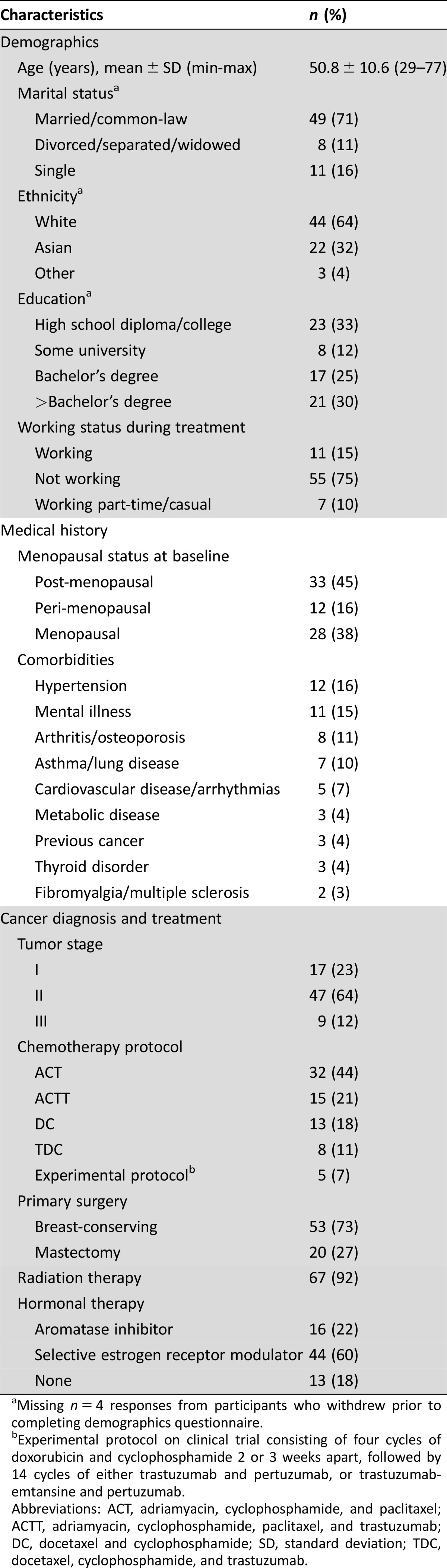
Missing n = 4 responses from participants who withdrew prior to completing demographics questionnaire.
Experimental protocol on clinical trial consisting of four cycles of doxorubicin and cyclophosphamide 2 or 3 weeks apart, followed by 14 cycles of either trastuzumab and pertuzumab, or trastuzumab‐emtansine and pertuzumab.
Abbreviations: ACT, adriamyacin, cyclophosphamide, and paclitaxel; ACTT, adriamyacin, cyclophosphamide, paclitaxel, and trastuzumab; DC, docetaxel and cyclophosphamide; SD, standard deviation; TDC, docetaxel, cyclophosphamide, and trastuzumab.
Effectiveness
Regarding physical activity levels over the previous 6 months, MVPA significantly increased from baseline to end of program (p = .008), was maintained (i.e., did not change) between end of program and 1‐year follow‐up (p = .465), and remained significantly higher than baseline at 1‐year follow‐up (p = .009; Table 3). The pattern for proportion of participants meeting or exceeding the guidelines of 150 minutes of MVPA per week was similar, with an increase from 44% to 65% at end of program (p = .034). This increase was maintained at 1‐year follow‐up (55%, p = .302 relative to end of study).
Table 3. Effectiveness and maintenance of physical activity behavior and health‐related quality of life.
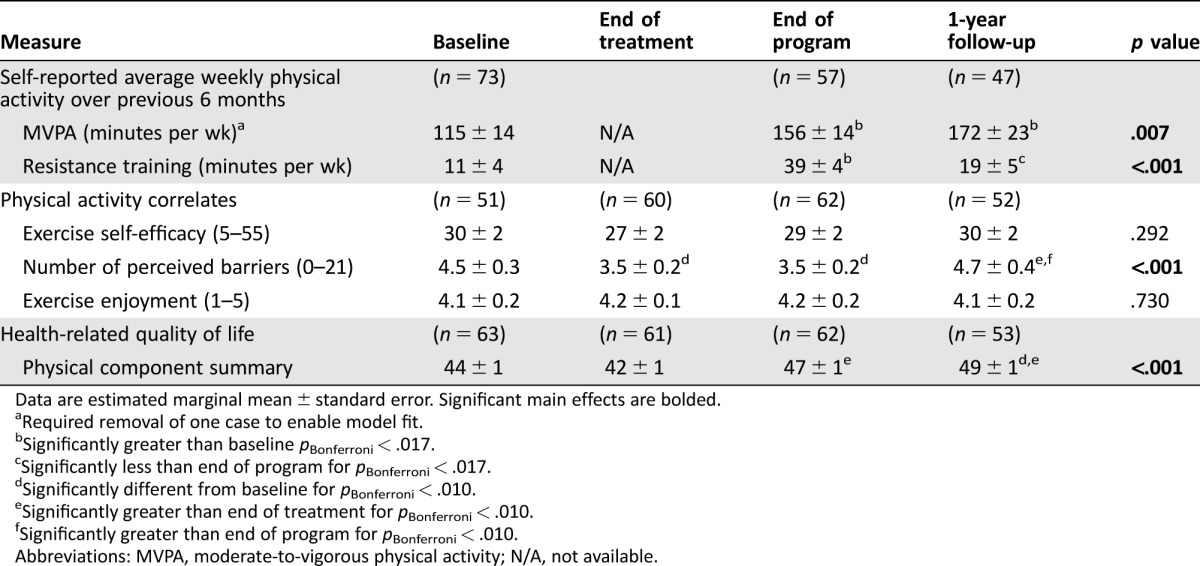
Data are estimated marginal mean ± standard error. Significant main effects are bolded.
Required removal of one case to enable model fit.
Significantly greater than baseline pBonferroni < .017.
Significantly less than end of program for pBonferroni < .017.
Significantly different from baseline for pBonferroni < .010.
Significantly greater than end of treatment for pBonferroni < .010.
Significantly greater than end of program for pBonferroni < .010.
Abbreviations: MVPA, moderate‐to‐vigorous physical activity; N/A, not available.
From baseline to end of program, average minutes of resistance training increased (for all models, p < .017), and decreased between end of study and 1‐year follow‐up (for all models, p < .017). At 1‐year follow‐up, resistance training was not significantly higher than baseline (for all models, p > .017; Table 3).
Exercise self‐efficacy and exercise enjoyment did not change over time (p = .292, p = .730, respectively; Table 3). The number of perceived barriers was reduced from baseline to end of treatment (p = .001), and did not change further from end of treatment at end of program (p = .810). However, from end of program to 1‐year follow‐up, perceived barriers increased (p = .002), and it was no longer different from baseline (p = .769; Table 3).
Health‐related quality of life did not change between baseline and end of treatment (p = .214) nor end of study (p = .106), although the latter mean change exceeded the MCID. Health‐related quality of life significantly increased between end of treatment and end of study (p < .001). At 1‐year follow‐up, HRQoL was not different from end of study (p = .113), but was significantly higher than baseline (p < .001; Table 3).
Implementation
The average exercise adherence across participants was 60% ± 26%, 52% ± 33%, and 50% ± 38% for the treatment, post‐treatment, and maintenance phases, respectively. Eighty‐four percent (57 of 68) of participants attended the group healthy eating session.
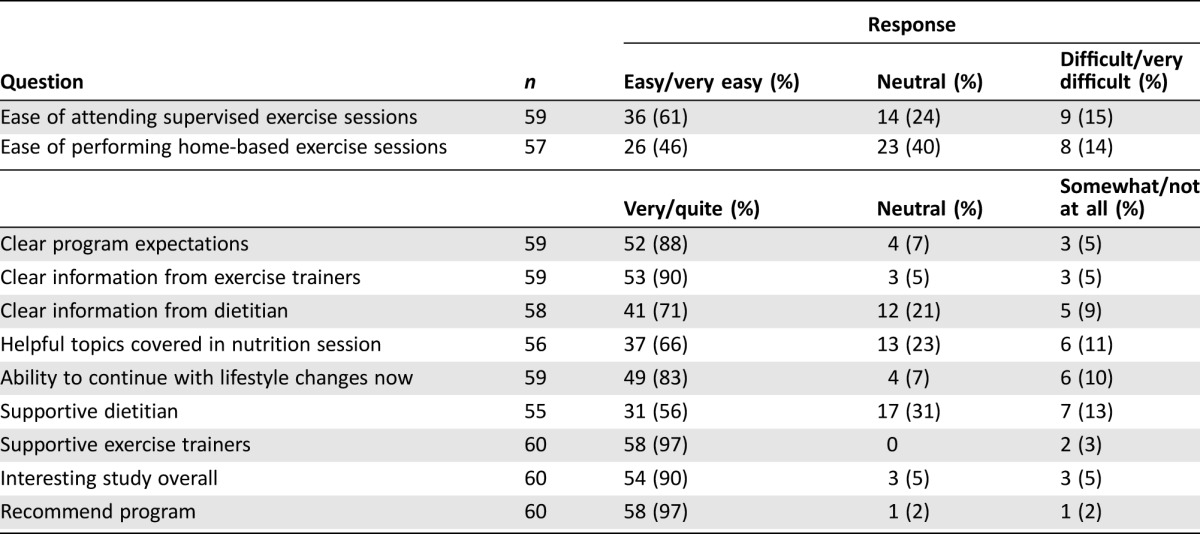
The estimated cost of the NExT program for the first year and for subsequent years is $44,821 and $41,766 USD (Table 4). The estimated average cost per participant starting the program is $1,273, and per exercise session delivered is $22 USD. Participant satisfaction outcomes were available for 87% of participants (Table 5). The majority felt it was easy to attend sessions, thought the study was interesting and the program expectations were clear, and felt supported. Most of the respondents felt that they could continue with their lifestyle changes and would recommend the program to a friend.
Table 4. Estimated startup and minimal annual operating costs (U.S. dollars) for the Nutrition and Exercise during Adjuvant Treatment program.

Table 5. Participant satisfaction survey responses.
Regarding safety, no major adverse events occurred. In the 12 months prior to baseline, injuries preventing the completion of normal daily activities were reported by 32% of participants, and this prevalence was not different during the program (40%, p = .310). Regarding injuries potentially related to the exercise program, new repetitive strain injury of the foot/leg attributed to walking/running or resistance training were reported by 5% during the treatment phase, and 16% during the post‐treatment or maintenance phases. Reported causes for other new injuries occurring during the program included home‐based physical activities (n = 7), falls during daily activities (n = 3), late chemotherapy side effects (n = 2), and other causes (n = 3). There were no diagnosed cases of lymphedema during the program.
Discussion
Clinical experience suggests a high demand and interest among breast cancer survivors for lifestyle‐based programs, and this population consistently reports health goals related to increasing physical activity, eating a healthier diet, and managing body weight [29]. Furthermore, there are calls to action from health care professionals for institutions, policy makers, and other leaders to include exercise and healthy eating in the supportive care services provided for cancer survivors [9], [13], [30]. The NExT study was designed to translate efficacy trials into a clinical care setting and address the reported barriers to delivery of this programming as supportive care for cancer survivors [13].
The NExT program was successful in receiving referrals from a dedicated team of medical oncologists and enrolling patients undergoing adjuvant chemotherapy, and met the benchmarks chosen a priori for program reach. Participants referred to the program were on average younger than those who were not referred. This may suggest a bias of the referring oncologists, and a future opportunity to increase reach by addressing potential barriers to the referral of older age groups. Regardless, program uptake was high, and retention rate exceeded those reported by other community‐based cancer rehabilitation programs [31], [32], [33], suggesting a large demand for programming when available. This is particularly noteworthy considering that the NExT program was substantially longer than previous community programs and randomized trials (45 vs. 12–24 weeks). Opportunities for continued access to dedicated exercise facilities and professionals may be an important consideration following completion of cancer treatment. By design, the study targeted women receiving adjuvant treatment, for whom need for supervised programming may be greatest. However, two‐thirds of patients who had a breast medical oncology consultation during the 15 months of study recruitment did not receive adjuvant chemotherapy, which suggests the need to consider expanding programming eligibility to increase the availability of services for the majority of breast cancer survivors seen by medical oncologists.
The NExT exercise program was effective in increasing MVPA and resistance training levels, and maintaining MVPA but not resistance training levels above baseline for an additional year without study contact. In total, the 1‐year follow‐up time point was approximately 2 years after diagnosis. Physical inactivity is a modifiable risk factor for a number of chronic diseases (e.g., cardiovascular disease, diabetes mellitus, obesity, hypertension, arthritis, depression) and all‐cause mortality [34]. Among breast cancer survivors, a reduction in physical activity levels following diagnosis and treatment is common [35], [36]. Importantly, physical activity following a cancer diagnosis is associated with a 38% reduction in breast cancer‐specific mortality [37] and an incremental reduction of cardiovascular events in breast cancer survivors [38]. Therefore, the success of NExT in increasing physical activity levels and maintaining this increase for a period of 2 years after initiating adjuvant treatment has the potential to influence important clinical outcomes. The number of perceived barriers to exercise were reduced from baseline to end of treatment, and were maintained at end of program. Some of the barriers assessed in the questionnaire included access to knowledgeable exercise staff, experience of fatigue, nausea, or pain, costs related to engaging in exercise, and fear of injury [23]. Although neither exercise self‐efficacy nor enjoyment changed at any point, 83% of participants indicated that they were confident in their ability to continue with lifestyle changes on their own at program completion, suggesting that NExT did enhance skills relevant to lifestyle management. However, this could be partially attributed to a relatively high level of exercise self‐efficacy and enjoyment at baseline.
The NExT program was also successful in mitigating the reduction in the physical component of HRQoL that is commonly observed to occur with adjuvant treatment, which is consistent with the effect of exercise during treatment established by randomized controlled trials (RCTs) [39]. At end of program, HRQoL was significantly increased relative to end of treatment, and although not statistically significant relative to baseline, there was a clinically meaningful change. It should be noted that our outcome assessment rates at 1‐year follow‐up for participants who did not withdraw were 73%–83%. It is likely that those who were less healthy and active at follow‐up were less inclined to return, which would positively bias our results.
A key element of effectiveness is intervention adherence in a real‐world setting. Previous efficacy studies utilizing a similar supervised exercise prescription (2–3 moderate‐intensity weekly sessions) in similar populations have reported higher exercise adherence rates (70%–72% during chemotherapy only [15], [16], [17]) relative to NExT (60% throughout chemotherapy and radiation). Because there were less‐stringent enrollment criteria with regard to expectations around adherence [18] and a longer duration relative to previous efficacy trials (i.e., 16–24 weeks), lower adherence was not unexpected for NExT, and provides insight into the adherence pattern that is more reflective of what can be expected in a supportive care program.
Overall, the majority of participants were satisfied with the program. The exercise component included multiple sessions per week, as opposed to the singular nutrition session. This was an improvement upon past trials with no nutrition component, but the addition of more nutrition sessions to support continued healthy eating habits during treatment as well as weight‐reduction strategies following completion of treatment may be warranted.
An important finding regarding safety was the lack of adverse events. A key element of recruitment when assessing effectiveness was expanding eligibility beyond the stringent eligibility criteria that are typically used in efficacy trials. The intention was to offer the program to all newly diagnosed women receiving adjuvant chemotherapy, including those with stable comorbid conditions. The rate of injuries from any cause also did not increase during adjuvant treatment concurrent with the exercise program relative to the year prior to adjuvant treatment. Of note, there were 10 injuries to the lower extremity of a nonacute origin (i.e., repetitive strain) reported by participants that could be attributed to the exercise training performed in the program. This is consistent with injury types and rates commonly observed with initiation of aerobic exercise in a previously sedentary population [28]. Additional strategies for injury prevention, monitoring, and referral for treatment, including for potential cases of lymphedema, are important to consider for future exercise programming for women with breast cancer.
Although comprehensive cost‐effectiveness analyses were not part of the primary trial, the cost for running the NExT program is reported to provide some insights for future program development. The main annual cost is for personnel ($40,906). Personnel are the most critical component of a successful program because they are the primary conduits for delivering a safe, effective, enjoyable, and engaging program. Oncology‐specific training programs or certifications have significantly increased capacity for exercise and nutrition professionals to support the implementation of lifestyle programming for cancer survivors [40], [41], [42]. A smaller but important cost to consider is cleaning supplies, because a strict cleaning protocol is required for individuals with compromised immune systems.
Strengths and Limitations
The NExT study has a number of strengths. The program addressed key knowledge translation gaps identified for including exercise and healthy eating programming as part of supportive care: namely, engagement of the medical oncologist to prescribe the program, and utilization of professionally educated staff for the exercise (bachelor's/master's degree in kinesiology) and healthy eating (registered dietitian) components. Furthermore, NExT took place in a dedicated facility near the cancer treatment center, and utilized exercise and healthy eating content that is evidence‐based and specific to breast cancer. The programming was also delivered to a wider range of women with breast cancer (e.g., one‐third nonwhite ethnicity, wide range of comorbid conditions and age) than is typical in many trials, in a format resembling how a clinical program might operate, thereby producing generalizable results. Another strength was the examination of eligibility of all new patients during study recruitment, which provided helpful information for planning clinical programs and identified reasons for noneligibility that may be able to be addressed in future programs to increase reach.
A limitation of the program was that exercise programming was only offered on three mornings/afternoons, with no opportunities to make up for missed sessions. Although this was purposeful to mirror the format of a potential clinical program offered in an institutional/clinical setting, a balance between personnel costs and allowing more flexible access to improve adherence should be considered. Further, the NExT gym location in a metropolitan center with a large clinical catchment area could have impacted referral rate and uptake. The extra travel required to engage in supervised exercise training on top of medical visits may be difficult for some patients due to time, transportation, and financial constraints. As a lifestyle intervention, there was an imbalance between the amount of time allocated to exercise programming relative to the healthy eating component. In future trials, increased opportunities to meet with dietitians and receive individualized support should be considered, especially if weight management is a key outcome.
Conclusion
The NExT study assessed effectiveness of an exercise and healthy eating program prescribed as a part of supportive care for early breast cancer patients receiving adjuvant chemotherapy. The NExT program reached a broader population of women on chemotherapy than typical efficacy trials and resulted in increased self‐reported physical activity levels and a maintenance of HRQoL during and following adjuvant chemotherapy. Given the significance of physical activity levels for prevention of comorbid conditions and mortality, the maintenance of MVPA levels above baseline 1 year after NExT program completion represents an important outcome for women receiving adjuvant chemotherapy for early breast cancer. The NExT program is a model of an effective, safe, and low‐cost approach that provides a strong rationale for the provision of funded lifestyle programming as a standard part of supportive care within a clinical oncology setting.
Acknowledgments
This project was funded by the British Columbia Cancer Foundation. Amy Kirkham was funded by Doctoral Award from the Canadian Institutes of Health Research.
Footnotes
For Further Reading: Jessica M. Scott, Susan Lakoski, John R. Mackey et al. The Potential Role of Aerobic Exercise to Modulate Cardiotoxicity of Molecularly Targeted Cancer Therapeutics. The Oncologist 2013;18:221‐231.
Implications for Practice: Cardiotoxicity, a frequent and devastating adverse complication of some molecularly targeted therapies (MTTs), can lead to potentially life‐threatening cardiovascular complications, therapy discontinuation, and poor quality of life. In non‐cancer patients with left ventricular dysfunction and heart failure, aerobic exercise is one of the mainstay clinical interventions for the prevention and treatment of cardiovascular disease. However, few studies have investigated the efficacy of aerobic exercise in the prevention and/or treatment of MTT‐induced cardiac injury. This topic is of particular importance because cardiac function is a strong predictor of cardiovascular and all‐cause mortality, quality of life, and fatigue, and maybe even cancer‐specific mortality. Here, we provide a comprehensive overview of cardiac molecular and cell‐signaling pathways specific to MTT‐induced cardiac toxicity. This review also outlines many pertinent aerobic exercise‐inducedmolecular signaling pathways that may uniquely prevent and/or treatMTTcardiac injury. Overall, information presented in this review provides critical information for basic scientists, clinicians, and exercise oncology researchers who are investigating the application of exercise in cancer control.
Author Contributions
Conception/design: Amy A. Kirkham, Cheri L. Van Patten, Karen A. Gelmon, Donald C. McKenzie, Kristin L. Campbell
Provision of study material or patients: Karen A. Gelmon
Collection and/or assembly of data: Amy A. Kirkham, Cheri L. Van Patten, Alis Bonsignore, Kelcey A. Bland, Kristin L. Campbell
Data analysis and interpretation: Amy A. Kirkham, Kristin L. Campbell
Manuscript writing: Amy A. Kirkham, Cheri L. Van Patten, Kristin L. Campbell
Final approval of manuscript: Amy A. Kirkham, Cheri L. Van Patten, Karen A. Gelmon, Donald C. McKenzie, Alis Bonsignore, Kelcey A. Bland, Kristin L. Campbell
Disclosures
The authors indicated no financial relationships.
References
- 1. Speck RM, Courneya KS, Mâsse LC et al. An update of controlled physical activity trials in cancer survivors: A systematic review and meta‐analysis. J Cancer Surviv 2010;4:87–100. [DOI] [PubMed] [Google Scholar]
- 2. Fong DY, Ho JW, Hui BP et al. Physical activity for cancer survivors: Meta‐analysis of randomised controlled trials. BMJ 2012;344:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ballard‐Barbash R, Friedenreich CM, Courneya KS et al. Physical activity, biomarkers, and disease outcomes in cancer survivors: A systematic review. J Natl Cancer Inst 2012;104:815–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheema BS, Kilbreath SL, Fahey PP et al. Safety and efficacy of progressive resistance training in breast cancer: A systematic review and meta‐analysis. Breast Cancer Res Treat 2014;148:249–268. [DOI] [PubMed] [Google Scholar]
- 5. Meneses‐Echávez JF, González‐Jiménez E, Ramírez‐Vélez R. Effects of supervised exercise on cancer‐related fatigue in breast cancer survivors: A systematic review and meta‐analysis. BMC Cancer 2015;15:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sander AP, Wilson J, Izzo N et al. Factors that affect decisions about physical activity and exercise in survivors of breast cancer: A qualitative study. Phys Ther 2012;92:525–536. [DOI] [PubMed] [Google Scholar]
- 7. Rogers LQ, Matevey C, Hopkins‐Price P et al. Exploring social cognitive theory constructs for promoting exercise among breast cancer patients. Cancer Nurs 2004;27:462–473 [DOI] [PubMed] [Google Scholar]
- 8. Whitehead S, Lavelle K. Older breast cancer survivors' views and preferences for physical activity. Qual Health Res 2009;19:894–906. [DOI] [PubMed] [Google Scholar]
- 9.NCCN Guidelines Version 1.2017 Panel Members Survivorship. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Survivorship. Version 1.2017. Fort Washington (PA): National Comprehensive Cancer Network.
- 10. Jones LW, Courneya KS, Fairey AS et al. Effects of an oncologist's recommendation to exercise on self‐reported exercise behavior in newly diagnosed breast cancer survivors: A single‐blind, randomized controlled trial. Ann Behav Med 2004;28:105–113. [DOI] [PubMed] [Google Scholar]
- 11. Park JH, Lee J, Oh M et al. The effect of oncologists' exercise recommendations on the level of exercise and quality of life in survivors of breast and colorectal cancer: A randomized controlled trial. Cancer 2015;121:2740–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Courneya KS, Rogers LQ, Campbell KL et al. Top 10 research questions related to physical activity and cancer survivorship. Res Q Exerc Sport 2015;86:107–116. [DOI] [PubMed] [Google Scholar]
- 13. Santa Mina D, Alibhai SM, Matthew AG et al. Exercise in clinical cancer care: A call to action and program development description. Curr Oncol 2012;19:e136–e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: The RE‐AIM framework. Am J Public Health 1999;89:1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Courneya KS, Segal RJ, Mackey JR et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. J Clin Oncol 2007;25:4396–4404. [DOI] [PubMed] [Google Scholar]
- 16. Courneya KS, McKenzie DC, Mackey JR et al. Effects of exercise dose and type during breast cancer chemotherapy: Multicenter randomized trial. J Natl Cancer Inst 2013;105:1821–1832. [DOI] [PubMed] [Google Scholar]
- 17. van Waart H, Stuiver MM, van Harten WH et al. Effect of low‐intensity physical activity and moderate‐ to high‐intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: Results of the PACES randomized clinical trial. J Clin Oncol 2015;33:1918–1927. [DOI] [PubMed] [Google Scholar]
- 18. Courneya KS. Efficacy, effectiveness, and behavior change trials in exercise research. Int J Behav Nutr Phys Act 2010;7:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thomas S, Reading J, Shephard RJ. Revision of the Physical Activity Readiness Questionnaire (PAR‐Q). Can J Sport Sci 1992;17:338–345. [PubMed] [Google Scholar]
- 20. Schmitz KH, Courneya KS, Matthews C et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 2010;42:1409–1426. [DOI] [PubMed] [Google Scholar]
- 21. Taylor HL, Jacobs DR Jr, Schucker B et al. A questionnaire for the assessment of leisure time physical activities. J Chron Dis 1978;31:741–755. [DOI] [PubMed] [Google Scholar]
- 22. Ainsworth BE, Haskell WL, Herrmann SD et al. 2011 compendium of physical activities: A second update of codes and MET values. Med Sci Sports Exerc 2011;43:1575–1581. [DOI] [PubMed] [Google Scholar]
- 23. Rogers LQ, McAuley E, Courneya KS et al. Correlates of physical activity self‐efficacy among breast cancer survivors. Am J Health Behav 2008;32:594–603. [DOI] [PubMed] [Google Scholar]
- 24. Marcus BH, Selby VC, Niaura RS et al. Self‐efficacy and the stages of exercise behavior change. Res Q Exerc Sport 1992;63:60–66. [DOI] [PubMed] [Google Scholar]
- 25. Ware JE Jr. SF‐36 health survey update. Spine (Phila Pa 1976) 2000;25:3130–3139. [DOI] [PubMed] [Google Scholar]
- 26. Hemingway H, Stafford M, Stansfeld S et al. Is the SF‐36 a valid measure of change in population health? Results from the Whitehall II Study. BMJ 1997;315:1273–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hays RD, Morales LS. The RAND‐36 measure of health‐related quality of life. Ann Med 2001;33:350–357. [DOI] [PubMed] [Google Scholar]
- 28. Campbell K, Foster‐Schubert K, Xiao L et al. Injuries in sedentary individuals enrolled in a 12‐month, randomized, controlled, exercise trial. J Phys Act Health 2012;9:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lauver D, Connolly‐Nelson K, Vang P. Health‐related goals in female cancer survivors after treatment. Cancer Nurs 2007;30:9–15. [DOI] [PubMed] [Google Scholar]
- 30. Segal R, Zwaal C, Green E et al. Exercise for people with cancer: A clinical practice guideline. Curr Oncol 2017;24:40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Noble M, Russell C, Kraemer L et al. UW WELL‐FIT: The impact of supervised exercise programs on physical capacity and quality of life in individuals receiving treatment for cancer. Support Care Cancer 2012;20:865–873. [DOI] [PubMed] [Google Scholar]
- 32. Haas BK, Kimmel G, Hermanns M et al. Community‐based FitSTEPS for life exercise program for persons with cancer: 5‐year evaluation. J Oncol Pract 2012;8:320–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leach HJ, Danyluk JM, Nishimura KC et al. Evaluation of a community‐based exercise program for breast cancer patients undergoing treatment. Cancer Nurs 2015;38:417–425. [DOI] [PubMed] [Google Scholar]
- 34. Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: The evidence. CMAJ 2006;174:801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kwan ML, Sternfeld B, Ergas IJ et al. Change in physical activity during active treatment in a prospective study of breast cancer survivors. Breast Cancer Res Treat 2012;131:679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Irwin ML, Crumley D, McTiernan A et al. Physical activity levels before and after a diagnosis of breast carcinoma. Cancer 2003;97:1746–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Friedenreich CM, Neilson HK, Farris MS et al. Physical activity and cancer outcomes: A precision medicine approach. Clin Cancer Res 2016;22:4766–4775. [DOI] [PubMed] [Google Scholar]
- 38. Jones LW, Habel LA, Weltzien E et al. Exercise and risk of cardiovascular events in women with nonmetastatic breast cancer. J Clin Oncol 2016;34:2743–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mishra SI, Scherer RW, Geigle PM et al. Exercise interventions on health‐related quality of life for cancer survivors. Cochrane Database Syst Rev 2012;15:CD007566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.American College of Sports Medicine. ACSM/ACS Certified Cancer Exercise Trainer. Available at http://certification.acsm.org/acsm-cancer-exercise-trainer. Accessed July 15, 2015.
- 41. Hayward R, ed. Rocky Mountain Cancer Exercise Specialist Workshop. University of Northern Colorado. Available at http://www.unco.edu/nhs/cancer-rehabilitation-institute/education/workshop/. Accessed Septemper 13, 2017.
- 42.Commission on Dietetic Registration. Board Certification as a Specialist in Oncology Nutrition. Available at https://www.cdrnet.org/certifications/board-certification-as-a-specialist-in-oncology-nutrition. Accessed July 6, 2015.
- 43. Wisløff U, Støylen A, Loennechen JP et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: A randomized study. Circulation 2007;115:3086–3094. [DOI] [PubMed] [Google Scholar]
- 44. Rognmo Ø, Hetland E, Helgerud J et al. High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil 2004;11:216–222. [DOI] [PubMed] [Google Scholar]
- 45. Knutzen KM, Brilla LR, Caine D. Validity of 1RM prediction equations for older adults. J Strength Cond Res 1999;13. [Google Scholar]